Abstract
The systemic unresponsiveness of the immune system to orally administered antigens is known as oral tolerance. Recent findings describe a new step for the induction of oral tolerance, consisting in the homing of FOXP3+ regulatory T cells to the small bowel and the local acquisition of full immunosuppressive capacities, a process in which retinoic acid-producing dendritic cells might play a crucial role.
Keywords: FOXP3, dendritic cells, gut homing, oral tolerance, retinoic acid
Oral tolerance is the process whereby local or systemic pro-inflammatory immune responses against specific antigens are prevented following their oral administration. The induction of this state of unresponsiveness may be crucial for the maintenance of intestinal homeostasis, considering the continuous exposure of the intestine to vast amounts of food antigens and commensal microbes. Furthermore, it has been proposed that defects in the mechanisms underlying oral tolerance contribute to intestinal disorders such as food allergies and inflammatory bowel diseases. Understanding how oral tolerance is established might result in novel therapeutic approaches to such disorders. Although several mechanisms for the induction of oral tolerance have been proposed,1 the prevailing view indicates that oral tolerance relies on the induction of antigen-specific FOXP3+ regulatory T cells (Tregs) in the mesenteric lymph nodes (MLNs). Such Tregs then enter the circulation and eventually reach different peripheral tissues, where they exert their immunosuppressive effect.
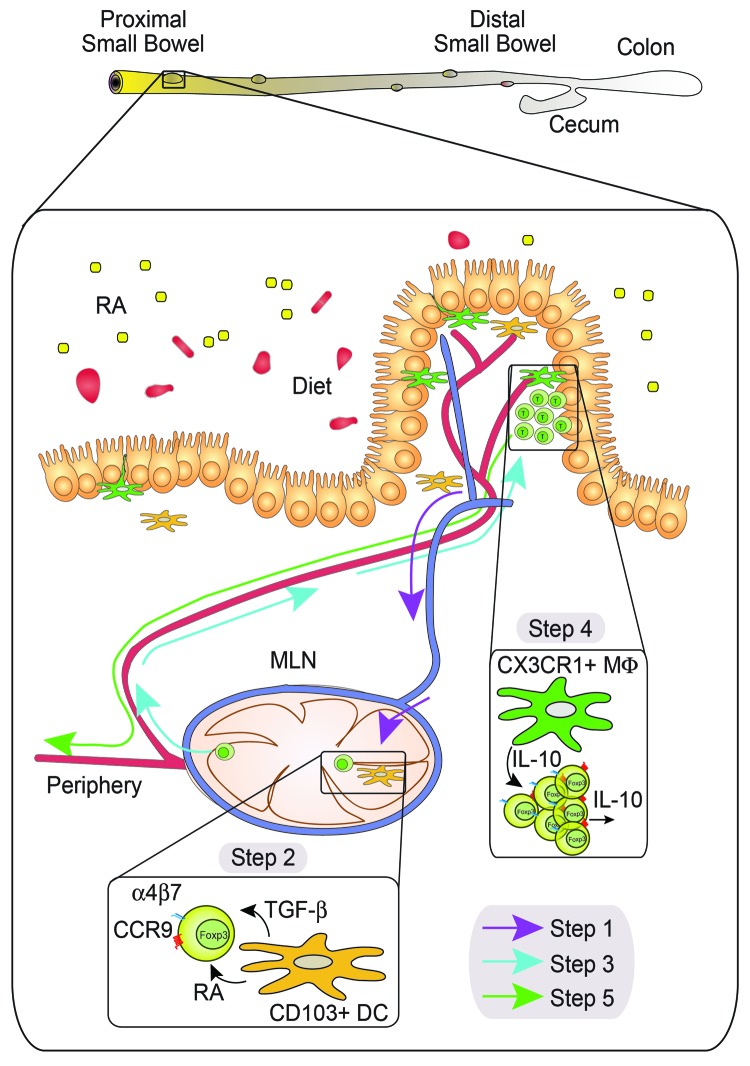
In a recent study published in Gastroenterology,2 we have demonstrated that the establishment of oral tolerance requires an additional step that involves the induction of two gut-homing molecules, namely the chemokine receptor CCR9 and integrin α4β7, on FOXP3+ Tregs. Once gut-tropic FOXP3+ Tregs are induced, they egress the MLNs and home toward the small bowel lamina propria, rather than entering immediately into the bloodstream. In the small bowel, FOXP3+ Tregs are subjected to a secondary expansion3 and acquire the ability to produce interleukin (IL)-10,2 a potent immunosuppressive cytokine. After acquiring the capacity of secreting IL10, FOXP3+ Tregs leave the lamina propria and enter the circulation, either directly or via draining lymphatics. The migration of FOXP3+ Tregs from MLNs to the small bowel requires both CCR9 and α4β7. Consistent with this hypothesis, β7-deficient CD4+ T cells (lacking α4β7) are not able to rescue oral tolerance in CCR9-deficient recipients, whereas wild type CD4+ T cells can do so.2 Moreover, in agreement with the requirement of gut-homing receptors on CD4 T+ cells, oral tolerance was abrogated in animals depleted of retinoic acid (RA, one of the bioactive forms of vitamin A), which is needed to induce the expression of guthoming receptors by T cells. Importantly, the frequencies of FOXP3+ Tregs in vitamin A-depleted and control animals were comparable, and in vitro-generated gut-tropic T cells were able to rescue the establishment of oral tolerance in vitamin A-depleted animals.2 Taken together, these data suggest that RA is required for the induction of gut tropism, rather than for the generation of FOXP3+ Tregs from naïve T cells. In summary, we and others have recently described a new step in which, upon priming in MLNs, FOXP3+ Tregs detour to the small bowel to receive further instructions and gain full immunosuppressive potential (Fig. 1).2,3
Figure 1. The proposed five-step model of oral tolerance. Oral tolerance may occur in the proximal small bowel where a gradient of retinoic acid (RA) imprints lamina propria CD103+ dendritic cells (DCs) to synthesize RA. These RA-producing DCs pick up food antigens and migrate through the lymphatics (blue) toward mesenteric lymph nodes (MLNs) (Step 1). Once in MLNs, CD103+ DCs induce FOXP3+ Treg conversion and gut tropism (Step 2). Gut-tropic FOXP3+ Tregs leave MLNs to migrate toward the small bowel (Step 3), where they expand and gain the ability to produce interleukin (IL)-10, likely by sensing CX3CR1+ macrophage-derived IL-10 (Step 4). Eventually, IL-10-producing FOXP3+ Tregs will leave the small bowel to enter into circulation (red) and reach peripheral tissues (Step 5), where they will exert their immunosuppressive function
Since CCR9 is required for T cells to migrate toward the proximal small bowel,4 tolerance to commensal bacteria, which are predominantly located in the colon and distal small bowel, may not rely on CCR9 induction. Moreover, the unresponsiveness to commensal microbiota is induced locally rather than systemically,5 suggesting that different mechanism(s) underpin tolerance against food as opposed to commensal microbiota.6 If this were the case, the proximal small bowel microenvironment would presumably play a crucial role during the establishment of oral immunological tolerance to food antigens, whereas the colon microenvironment would do so for commensal microbiota. In agreement with this hypothesis, we have recently reported that lamina propria dendritic cells (LP-DCs) isolated from the proximal small bowel induce a higher gut tropism and FOXP3+ Treg conversion than LP-DCs isolated from the distal small bowel or colon.7 Along these lines, we found that LP-DCs isolated from the proximal small bowel possess greater RA-synthesizing ability than their distal counterparts, correlating with the levels of RA found throughout the intestinal tract.7 Importantly, these properties are RA-dependent, since DCs from animals fed on vitamin A-deficient diet lose their capacity to synthesize RA or to induce gut tropism in T cells. Hence, LP-DCs sense local RA (presumably produced by intestinal epithelial cells), inducing their own RA synthesis in a positive feedback loop.7 Although this has not yet been formally demonstrated, our work suggests that the proximal small bowel plays a prominent role in the establishment of oral tolerance. It is possible that, upon food intake, LP-DCs in the proximal small bowel pick up antigens and migrate toward MLNs, where naïve T cells are primed. Since LP-DCs from the proximal small bowel are able to produce high levels of RA, they will efficiently convert naïve T cells into antigen-specific FOXP3+ Tregs expressing the gut-homing receptors CCR9 and α4β7. Such newly generated gut-tropic FOXP3+ Tregs will be able to migrate and reach the small bowel lamina propria, where they will further expand and become able to produce IL-10, hence acquiring full immunosuppressive functions (Fig. 1). Fractalkine receptor CX3CR1+ macrophages seem to play a crucial role in this last step, since mice lacking Cx3cr1 exhibit decreased FOXP3+ Treg expansion in the lamina propria and—consequently—do not develop oral tolerance, which has been correlated with the ability of F4/80+CX3CR1+ LP-macrophages to produce IL-10 3. Thus, based on our data, I propose a model in which small bowel-derived LP-DCs are critical in the establishment of oral tolerance to food antigens. Future work will assess whether oral tolerance to commensal bacteria relies on colon-derived LP-DCs, a colon-specific microenviroment, or perhaps involves direct interactions of immune cells with products of commensal microbiota.8
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22987
References
- 1.Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol Rev. 2011;241:241–59. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cassani B, Villablanca EJ, Quintana FJ, Love PE, Lacy-Hulbert A, Blaner WS, et al. Gut-tropic T cells that express integrin α4β7 and CCR9 are required for induction of oral immune tolerance in mice. Gastroenterology. 2011;141:2109–18. doi: 10.1053/j.gastro.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, et al. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237–46. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Stenstad H, Svensson M, Cucak H, Kotarsky K, Agace WW. Differential homing mechanisms regulate regionalized effector CD8alphabeta+ T cell accumulation within the small intestine. Proc Natl Acad Sci U S A. 2007;104:10122–7. doi: 10.1073/pnas.0700269104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pabst O, Mowat AM. Oral tolerance to food protein. Mucosal Immunol. 2012;5:232–9. doi: 10.1038/mi.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng T, Elson CO. Adaptive immunity in the host-microbiota dialog. Mucosal Immunol. 2011;4:15–21. doi: 10.1038/mi.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villablanca EJ, Wang S, de Calisto J, Gomes DC, Kane MA, Napoli JL, et al. MyD88 and retinoic acid signaling pathways interact to modulate gastrointestinal activities of dendritic cells. Gastroenterology. 2011;141:176–85. doi: 10.1053/j.gastro.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–6. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]