Abstract
Human plasmacytoid and myeloid dendritic cells (DCs), when appropriately stimulated, can express the archetypal natural killer (NK)-cell surface marker CD56. In addition to classical DC functions, CD56+ DCs are endowed with an unconventional cytotoxic capacity.
Keywords: CD56, cytotoxicity, dendritic cells, human, myeloid DCs, plasmacytoid DCs
In the human hematopoietic system, CD56 is considered as a classical surface marker of natural killer (NK) cells and it is used to identify the two main NK-cell subsets: CD56bright and CD56dim. Despite its stringent association with NK cells, the expression of CD56 has also been detected on other human lymphoid cells including NKT cells and activated T cells.1 Common to all these CD56-expressing cell types is a cytotoxic function, strongly suggesting that the expression of CD56 in the hematopoietic system is restricted to cytotoxic effector cells.
In two recent papers, we have shown that human plasmacytoid and myeloid dendritic cells (DCs) can also adopt a CD56+ phenotype and acquire cytotoxic functions. In the first study,1 we revealed that the activation of plasmacytoid DCs (pDCs) with the tick-borne encephalitis vaccine FSME, which presumably acts as a Toll-like receptor (TLR) ligand, causes a partial upregulation of CD56 on their cell surface. Despite the expression of CD56, FSME-activated pDCs fulfilled all phenotypic and functional characteristics to be classified as DCs, including a potent allostimulatory ability and the capacity to prime naive antigen-specific T-cell responses. Intriguingly, in addition to these classical DC functions, FSME-activated pDCs were also found to exhibit cytotoxic activity against major histocompatibility complex (MHC) class I-negative tumor cell lines (e.g., K562 cells).1
Similar observations were made for human myeloid DCs (mDCs) in our second paper.2 In this study, we showed that mDCs differentiating from blood monocytes in response to a combination of granulocyte macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-15, partially express CD56 and are lytically active against K562 cells. Detailed phenotypic and functional studies confirmed that these so-called “IL-15 mDCs” are unrelated to NK cells and should be regarded as bona fide DCs endowed with an additional cytotoxic effector function.2
The observation that human DCs can acquire CD56 and act as killer cells illustrates that the phenotypic and functional boundaries between DCs and NK cells are not as sharp as conventionally thought. Since the pioneer report published in 1996 by Suss and Shortman,3 several research groups have independently provided evidence that DCs can indeed play an unanticipated role in innate immunity as cytotoxic effector cells.4 The actual existence of such “killer DCs” has nevertheless been subject of debate. Much of this controversy finds its origin in the confusing literature on the presence within the murine immune system of a so-called natural killer dendritic cell (NKDC) subset (also known as interferon-producing killer DCs, IKDCs). NKDCs were initially erroneously classified as a separate cell type with overlapping features of DCs and NK cells, but it later became evident that these cells in fact represent activated NK cells.5 This has led many researchers to dispute the existence of ”killer DCs” as a distinct DC subset. Nevertheless, a considerable body of evidence has now accumulated, in rodents as well as in humans, to indicate that DCs themselves can have cytotoxic activity.4 This is supported by our results, which establish FSME-activated pDCs and IL-15 mDCs as ‘genuine’ DCs with killing activity, despite their partial phenotypic (CD56 expression) and functional (cytotoxicity against MHC class I-negative target cells) overlap with NK cells.1,2
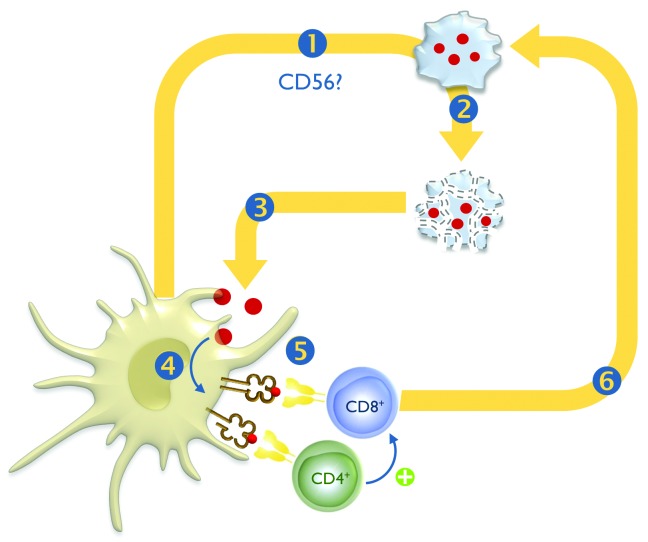
A paramount unanswered question is why DCs can act as killer cells and whether this observation holds relevance in vivo. In vitro, killer DCs appear to have a much lower cytotoxic potential as compared with classical cytotoxic effector cells such as NK cells or cytotoxic T lymphocytes (CTLs), and one can thus wonder whether their lytic activity has any biological significance.2 It has been speculated that killer DCs may kill only a limited number of cancer cells to provide themselves with antigens to subsequently cross-prime tumor antigen-specific CTLs. These CTLs are supposed to kill the bulk of tumor cells (Fig. 1). If this hypothesis held true, killer DCs might thus be of critical importance for antitumor immunity as “amplifiers” of the effector phase of the adaptive immune response (Fig. 1).4
Figure 1. The killing road: postulated mechanism of action of killer dendritic cells. The killer dendritic cell (DC) recognizes (1) and kills (2) tumor cells. Tumor cell-derived fragments are then captured by the killer DC (3), internally processed (4) and subsequently presented on MHC molecules to CD4+ and CD8+ T cells (5). Tumor antigen-specific CD8+ CTLs then lyse the remainder bulk of tumor cells (6).
Another aspect that deserves to be highlighted and that has so far not received much attention from immunologists is that CD56—by many believed to be an exclusive NK cell marker—can also be expressed by DCs. Recent studies have provided evidence for the in vivo existence of CD56-expressing DC subsets within both human pDC and mDC populations,6,7 indicating that the expression of CD56 on FSME-activated pDCs and IL-15 mDCs that we observed was not merely an artifact. The routine use of CD56 as an exclusion marker in DC biology studies explains why the presence of these CD56+ DCs has been largely overlooked.7 As a consequence, at present little is known about the role of CD56 expression on DCs and about the biology of CD56-expressing DC subsets.
Given the strong association between CD56 expression and cytotoxic effector functions, it is tempting to speculate that naturally occurring CD56-expressing DC subsets, similar to their in vitro counterparts, also classify as killer DCs. Whether CD56 directly contributes to the cytotoxic functions of DCs is an interesting question that remains to be clarified. We hypothesize that CD56 on DCs may function as a cell adhesion molecule, allowing them to bind to and interact with their targets (Fig. 1). Support for this hypothesis comes from a previous report showing that CD56 is involved in the cytotoxicity of human NK cells by promoting NK-cell adhesion to target cells.8
In summary, we have demonstrated that human CD56+ pDCs and mDCs, in addition to serving classical DC functions, are endowed with cytotoxic activity. In view of this “killer DC” profile, CD56+ DCs may represent versatile tools for future immunotherapy applications.9,10
Disclosure of Potential Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23037
References
- 1.Tel J, Smits EL, Anguille S, Joshi RN, Figdor CG, de Vries IJ. Human plasmacytoid dendritic cells are equipped with antigen-presenting and tumoricidal capacities. Blood. 2012;120:3936–44. doi: 10.1182/blood-2012-06-435941. [DOI] [PubMed] [Google Scholar]
- 2.Anguille S, Lion E, Tel J, de Vries IJM, Couderé K, Fromm PD, et al. Interleukin-15-induced CD56+ myeloid dendritic cells combine potent tumor antigen presentation with direct tumoricidal potential. PLoS ONE. 2012;7(12):e51851. doi: 10.1371/journal.pone.0051851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Süss G, Shortman K. A subclass of dendritic cells kills CD4 T cells via Fas/Fas-ligand-induced apoptosis. J Exp Med. 1996;183:1789–96. doi: 10.1084/jem.183.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larmonier N, Fraszczak J, Lakomy D, Bonnotte B, Katsanis E. Killer dendritic cells and their potential for cancer immunotherapy. Cancer Immunol Immunother. 2010;59:1–11. doi: 10.1007/s00262-009-0736-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guimont-Desrochers F, Boucher G, Dong Z, Dupuis M, Veillette A, Lesage S. Redefining interferon-producing killer dendritic cells as a novel intermediate in NK-cell differentiation. Blood. 2012;119:4349–57. doi: 10.1182/blood-2011-11-395954. [DOI] [PubMed] [Google Scholar]
- 6.Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, et al. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–46. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 7.Milush JM, Long BR, Snyder-Cappione JE, Cappione AJ, 3rd, York VA, Ndhlovu LC, et al. Functionally distinct subsets of human NK cells and monocyte/DC-like cells identified by coexpression of CD56, CD7, and CD4. Blood. 2009;114:4823–31. doi: 10.1182/blood-2009-04-216374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nitta T, Yagita H, Sato K, Okumura K. Involvement of CD56 (NKH-1/Leu-19 antigen) as an adhesion molecule in natural killer-target cell interaction. J Exp Med. 1989;170:1757–61. doi: 10.1084/jem.170.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anguille S, Lion E, Smits E, Berneman ZN, van Tendeloo VF. Dendritic cell vaccine therapy for acute myeloid leukemia: questions and answers. Hum Vaccin. 2011;7:579–84. doi: 10.4161/hv.7.5.14652. [DOI] [PubMed] [Google Scholar]
- 10.Anguille S, Willemen Y, Lion E, Smits EL, Berneman ZN. Dendritic cell vaccination in acute myeloid leukemia. Cytotherapy. 2012;14:647–56. doi: 10.3109/14653249.2012.693744. [DOI] [PubMed] [Google Scholar]