Abstract
Regulatory T cells (Tregs) interfere with antitumor immune responses, hence facilitating oncogenesis and tumor progression. Our recent findings indicate that neuropilin 1, which is highly expressed by Tregs, mediates the infiltration of these cells into the tumor in a vascular endothelial growth factor (VEGF)-dependent manner, thereby contributing to cancer-associated immunosuppression.
Keywords: melanoma, regulatory T cells, T-cell migration
Owing to their potent immunosuppressive functions, CD4+CD25+ regulatory T cells (Tregs) are involved in the maintenance of immune homeostasis and control several facets of immunity, including autoimmune, anti-pathogen and antitumor responses.1 High levels of Tregs have been found in neoplastic lesion as well as in the peripheral blood of cancer patients, and such an increase has been shown to correlate with poor prognosis in patients affected by breast, gastric and ovarian carcinoma.1 Murine models of Tregs depletion, for instance obtained with anti-CD25 antibodies or in transgenic DEREG (Depletion of Regulatory T cells) mice, provide further support to the notion that Tregs interfere with effective antitumor immune responses and hence contribute to oncogenesis and tumor progression, as Treg-deficient animals are able to control tumor growth better than their wild-type (WT) littermates.2
CD4+CD25+ Tregs specifically express the forkhead transcription factor FOXP3, which is essential for their development and function, hence representing a useful marker for their identification.3 However, as FOXP3 is expressed within the cell, it is not suitable for the isolation of viable Tregs. We have identified neuropilin 1 (NRP1) as a reliable surface marker of Tregs.4 The majority of Foxp3+ Tregs from naïve WT mice expresses Nrp1, in contrast to other T-cell subsets (Fig. 1). Functional analyses revealed that Cd4+Nrp1+ murine T cells exhibit an immunosuppressive phenotype in vitro.4 Besides the thymus-derived naturally occurring Tregs (nTregs), a heterogeneous population of induced Tregs (iTregs) has been described. Moreover, Yadav et al. and Weiss et al. have recently proposed that the expression of NRP1 may be useful for distinguishing nTregs from iTregs generated in vivo from naïve T cells under several circumstances, including via the physiologically relevant mucosal route.5,6 However, whether and how NRP1 de facto contributes to Treg function remain unclear.
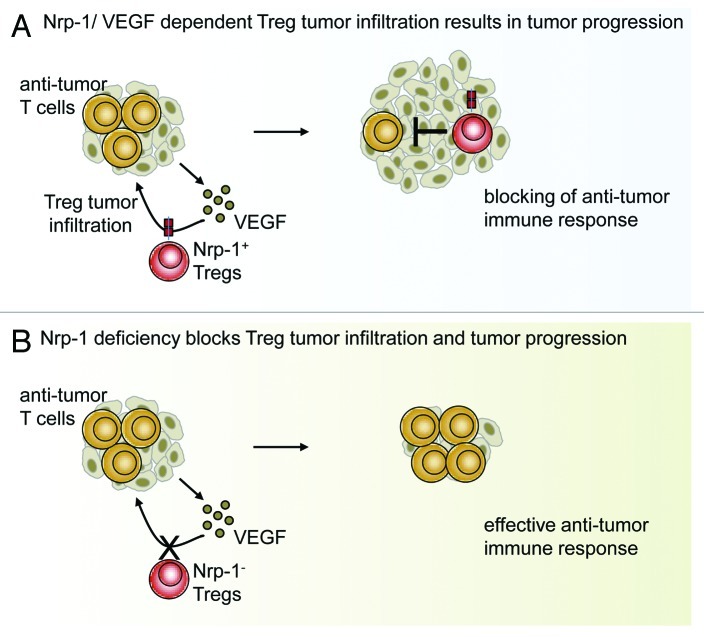
Figure 1. Neuropilin 1 expression by regulatory T cells is required for tumor infiltration. (A) Tumor-produced vascular endothelial growth factor (VEGF) attracts NRP1-expressing regulatory T cells (Tregs), resulting in local immunosuppression accompanied by tumor progression. (B) The T cell-specific ablation of NRP1 expression blocks tumor infiltration by Tregs, enabling the development of effective antitumor immune responses.
In a recent study, we have investigated the role of NRP1 expressed by Tregs on the development and progression of tumors in mice. We observed an impaired tumor growth in mice bearing a T cell-specific ablation of Nrp1.2 This phenotype was accompanied by an increased antitumor CD8+ T-cell response, suggesting that NRP1 is directly involved in the functions of Tregs. Interestingly, in vitro studies revealed that Nrp1-deficient Tregs exhibit a similar inhibitory activity than Nrp1-expressing Tregs obtained from WT mice.2 These results led us to conclude that NRP1 is not involved in the intrinsic immunosuppressive function of Tregs. However, a growing body of evidence indicates that inhibitory molecules expressed by Tregs and the underlying molecular mechanisms are essential, but not necessarily sufficient, for efficient immunosuppressive Treg functions in vivo. Indeed, an effective immunosuppression in vivo requires the appropriate co-localization of suppressor and effector cells.7 Tumors themselves promote their own progression by creating an immunosuppressive environment that facilitates their escape from immunosurveillance. Tregs play a critical role in this process, but it remains controversial whether iTregs are induced within the tumor tissue or whether nTregs are attracted and expanded by tumor-derived cytokines and chemokines.
NRP1 acts as a co-receptor for the vascular endothelial growth factor (VEGF), and NRP1-expressing endothelial cells migrate toward VEGF gradients.8 Tumors produce high amounts of VEGF, and we detected elevated levels of Nrp1-expressing Foxp3+ Tregs within tumors transplanted in WT mice. Hence, we propose that tumor-derived VEGF attracts Tregs via NRP1. Our in vitro studies further corroborate this notion, as Nrp1+ Tregs (but not their Nrp1-deficient counterparts) migrated in response to recombinant VEGF, and the analysis of re-isolated tumor sections revealed that the T cell-specific ablation of Nrp1 results in significantly lower numbers of tumor-infiltrating Foxp3+ Tregs.2 Strikingly, the abrogation of tumor-produced VEGF produced a similar phenotype, featuring reduced tumor growth, a comparatively stronger activation of tumor-infiltrating CD8+ T cells and decreased amounts of tumor-infiltrating Tregs.2 These results clearly demonstrate that NRP1 expression is critically required for tumor infiltration by Tregs as driven by cancer cell-derived VEGF in subcutaneous transplantation models.
As mentioned above, Weiss et al. have recently proposed NRP1 as a marker for distinguishing nTregs from iTregs, in particular mucosa-generated iTregs.6 With our results in mind, this implicates that nTregs are the key player in subcutaneous tumor transplantation models. In line with this notion, Weiss et al. detected high amounts of Nrp1+ Tregs within tumor sections in a subcutaneous transplantation model. In contrast, the majority of Tregs re-isolated from tumors generated by the intraperitonal transplantation of adenocarcinoma cells did not express Nrp1,6 suggesting that the origin of tumor-infiltrating lymphocytes might depend on contextual variables. Tregs isolated from subcutaneously transplanted tumors2,6 and, more importantly, from endogenously driven cutaneous malignant melanoma were found to express high amounts of Nrp1.2 The T cell-specific ablation of Nrp1 significantly reduced the number of tumor-infiltrating Foxp3+ Tregs in subcutaneously transplanted tumors and in spontaneously developing melanoma, resulting in impaired tumor growth.2 Thus, interfering with the NRP1/VEGF interaction may stand out as a new therapeutic strategy that might be superior to Treg depletion with regard to the development of autoimmune side effects, at least for the treatment of melanoma. We assume that blocking the tumor-derived VEGF-dependent trafficking of NRP1+ Tregs would not affect the function and number of Tregs systemically. Indeed, we did not observe any changes in the frequencies of Tregs in tumor-draining lymph nodes or in peripheral lymphoid organs of tumor-bearing mice bearing a T cell-specific Nrp1 ablation or VEGF-deficient tumors.2 However, whether the NRP1/VEGF-mediated trafficking of nTregs is a tumor-specific phenomenon or is also involved in other inflammatory immune responses will have to be carefully determined in future experiments.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- iTreg
induced Treg
- NRP1
neuropilin 1
- nTreg
naturally occurring Treg
- Treg
regulatory T cell
- VEGF
vascular endothelial growth factor
- WT
wild-type
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23039
References
- 1.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–67. doi: 10.1002/ijc.25429. 10.1002.ijc.25429 [DOI] [PubMed] [Google Scholar]
- 2.Hansen W, Hutzler M, Abel S, Alter C, Stockmann C, Kliche S, et al. Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth. J Exp Med. 2012;209:2001–16. doi: 10.1084/jem.20111497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 4.Bruder D, Probst-Kepper M, Westendorf AM, Geffers R, Beissert S, Loser K, et al. Neuropilin-1: a surface marker of regulatory T cells. Eur J Immunol. 2004;34:623–30. doi: 10.1002/eji.200324799. [DOI] [PubMed] [Google Scholar]
- 5.Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209:1713–22, S1-19. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J Exp Med. 2012;209:1723–42, S1. doi: 10.1084/jem.20120914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–44. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Zeng H, Wang P, Soker S, Mukhopadhyay D. Neuropilin-1-mediated vascular permeability factor/vascular endothelial growth factor-dependent endothelial cell migration. J Biol Chem. 2003;278:48848–60. doi: 10.1074/jbc.M310047200. [DOI] [PubMed] [Google Scholar]