Abstract
In vivo dendritic-cell targeting constitutes a promising strategy for anticancer vaccination. Here, we discuss the usage of multivalent DC-SIGN-targeting glycan platforms that allow for the efficient routing of antigens to the endo-lysosomal pathway as well as to a yet uncharacterized cross-presentation mechanism inducing CD4+ and CD8+ T-cell responses.
Keywords: cell surface molecules, dendritic cells, DC-SIGN, glycans, human, rodent
The past 15 years have witnessed a growing interest in the development of dendritic cell (DC)-targeting vaccines for the treatment of cancer in parallel with the accumulation of knowledge on the biology of C-type lectin receptors (CLRs) and other pattern-recognition receptors on DCs. Among the different modalities of DC targeting, in vivo targeting is the preferred option because vaccines do not need to be tailored to every patient and can be prepared in large standardized batches. Since the pioneering work of Steinman and coworkers on the use of DEC-205 to induce potent antigen-specific CD4+ and CD8+ T cell-mediated immunity,1 several other CLRs2 have been tested for their capacity to induce T-cell responses against cancer in animal models. Most of these strategies are based on the use of anti-CLR monoclonal antibodies conjugated to the antigen of choice. In this setting, glycans, the natural ligands of CLRs, offer several advantages, including a low toxicity and immunogenicity as well as the possibility to be produced in large-scale by chemical methods. DC-SIGN is an ideal CLR for such approach as its ligands allow for specific targeting and are readily available for conjugation to model antigens.
DC-SIGN is a type II transmembrane CLR mostly expressed on myeloid DCs.3 DC-SIGN contains a carbohydrate recognition domain that has specificity for high-mannose-containing structures and Lewis-type blood antigens (i.e., LeX, LeY, Leb and Lea).4 DC-SIGN is organized in tetramers5 that are grouped in randomly distributed nanodomains on the membrane of DCs.6 Both the physical properties and the distribution pattern of DC-SIGN on the membrane of DCs determine the fact that the presentation of glycan ligands in a multivalent form results in an elevated binding affinity. Therefore, for the design of a DC-SIGN-targeting vaccine, it is critical to optimize the ideal multivalent glycan antigen-carrier to achieve efficient antigen presentation while decreasing off-target effects such as those mediated by other fucose/mannose specific lectins (e.g., the mannose receptor). Another advantage of multivalent glycan platforms is that they also allow for the incorporation of multiple antigens or multiple copies of a single antigen within the same macromolecule, as well as for the inclusion of the adjuvants needed to boost the immune response. We have recently focused on two types of multivalent glycan antigen-carrier platforms: dendrimers and liposomes (Fig. 1). Dendrimers are repetitively branched synthetic molecules that carry functional groups allowing for the conjugation of glycans and/or antigens. These structures are highly compact, flexible, soluble, provide a defined geometric orientation of glycans and can be engineered with the desired amount of glycan and/or peptide antigen(s). Compactness and solubility are essential to facilitate the preparation of DC-SIGN-targeting compounds and also contribute to minimize receptor-independent uptake. Another advantage of glycan-modified dendrimers consists in the possibility of adapting their design to the particular orientation of the carbohydrate-recognition domains of DC-SIGN tetramers as well as of the organization of DC-SIGN in nanodomains. In this respect, we have recently demonstrated that spherical targeting molecules are more efficient than linear ones.7 Furthermore, a certain degree of molecular flexibility is expected to facilitate the engagement of multiple DC-SIGN carbohydrate-recognition domains simultaneously, further enhancing receptor clustering and antigen uptake. Finally, the possibility to engineer dendrimers with the desired number of glycan units allows for the preparation of agents that exhibit an optimal level of multivalency. We have used a wide range of polyamido amide dendrimers8 differing in the number of available functional groups (4–512) to characterize the optimal multivalency needed for DC-SIGN targeting. This study demonstrated that third generation Leb-modified glycopeptide dendrimers, carrying up to 32 glycan units are sufficient to increase the affinity of DC-SIGN binding to the nanomolar range and achieve maximal antigen uptake.7 Because of the elevated antigen load of these glycopeptide dendrimers, our compounds induced very strong CD4+ and CD8+ T-cell responses. Similar dendrimers could be prepared carrying more than one antigenic epitope and incorporating adjuvants to simultaneously induce DC maturation and polyclonal responses.
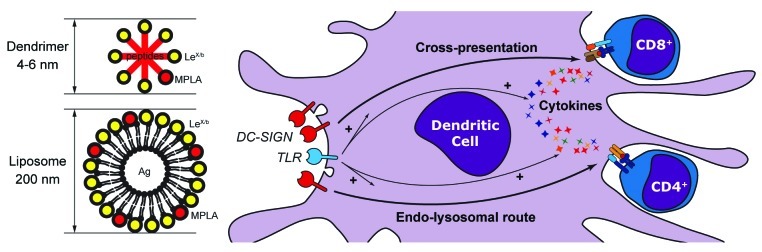
Figure 1. Glycan-modified dendrimers and liposomes enhance antigen presentation. Although dendrimers and liposomes greatly differ in size and molecular properties, both systems allow for the display of DC-SIGN ligands, such as LeX or Leb, in a multivalent form. Also Toll-like receptor (TLR) ligands, such as monophosphoryl lipid A, can be incorporated in dendrimers and liposomes. Dendrimers are prepared using peptide epitopes that require little antigen-processing capacity, while liposomes can encapsulate whole peptides and adjuvants. Both LeX/b-modified dendrimers and liposomes are highly specific for DC-SIGN and induce efficient MHC class I and MHC class II presentation to CD8+ and CD4+ T cells, respectively. Besides providing the co-stimulatory signals required for T-cell priming, TLR activation promotes antigen presentation to CD4+ T cells and a yet uncharacterized cross-presentation mechanism leading to the cross-priming of CD8+ T cells.
When whole antigens or hydrophobic compounds must be employed, liposomes provide some benefits over dendrimers, since (1) they allow for the encapsulation of hydrophilic material and the inclusion of hydrophobic compounds in the lipid bilayer and (2) can be modified with the desired amount of surface glycans (Fig. 1). Nevertheless, liposomes are well tolerated and have low toxicity. We have prepared liposomes modified with DC-SIGN-targeting glycans (LeX) and tested their effects in antigen uptake and T-cell proliferation assays. Our data show that LeX-modified liposomes are efficiently internalized by DCs, leading to a massive potentiation (> 100-fold) in antigen presentation. The large increase in T-cell proliferation that we observed stemmed from two factors: (1) the large amount of antigens that can be encapsulated in liposomes and (2) the simultaneous uptake of multiple liposomes.9 Similarly to dendrimers, liposomes also allow for the incorporation of a Toll-like receptor (TLR) ligand and can therefore be used to simultaneously activate DCs while delivering high amounts of antigen.
DC-SIGN is an internalization receptor that has long been known to facilitate routing to the endo-lysosomal pathway,10 so that antigens targeted to DC-SIGN are directly linked to processing and presentation on MHC class II molecules. This said, both DC-SIGN-targeting dendrimers7 and liposomes9 have been shown to induce extremely robust CD8+ T-cell responses, demonstrating the co-existence of an alternative DC-SIGN-dependent intracellular routing leading to antigen cross-presentation and CD8+ T-cell cross-priming. Our experiments have demonstrated that routing through this pathway is greatly enhanced when TLR4 ligands are combined with DC-SIGN targeting. TLR4 triggering also enhanced antigen presentation to CD4+ T cells, a promising perspective for complementing robust CD8+ T cell responses with an efficient CD4+ T cell help for the design of efficient anticancer immunotherapies.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- CLR
C-type lectin receptor
- DC
dendritic cell
- TLR
Toll-like receptor
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23040
References
- 1.Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 2.Caminschi I, Maraskovsky E, Heath WR. Targeting Dendritic Cells in vivo for Cancer Therapy. Front Immunol. 2012;3:13. doi: 10.3389/fimmu.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GCF, Adema GJ, van Kooyk Y, et al. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–85. doi: 10.1016/S0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 4.Appelmelk BJ, van Die I, van Vliet SJ, Vandenbroucke-Grauls CM, Geijtenbeek TB, van Kooyk Y. Cutting edge: carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J Immunol. 2003;170:1635–9. doi: 10.4049/jimmunol.170.4.1635. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell DA, Fadden AJ, Drickamer K. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. J Biol Chem. 2001;276:28939–45. doi: 10.1074/jbc.M104565200. [DOI] [PubMed] [Google Scholar]
- 6.Cambi A, Lidke DS. Nanoscale membrane organization: where biochemistry meets advanced microscopy. ACS Chem Biol. 2012;7:139–49. doi: 10.1021/cb200326g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.García-Vallejo JJ, Ambrosini M, Overbeek A, van Riel WE, Bloem KE, Unger WW, et al. Multivalent glycopeptide dendrimers for the targeted delivery of antigens to dendritic cells. Mol Immunol. 2013;53:387–97. doi: 10.1016/j.molimm.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Biricova V, Laznickova A. Dendrimers: Analytical characterization and applications. Bioorg Chem. 2009;37:185–92. doi: 10.1016/j.bioorg.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Unger WWJ, van Beelen AJ, Bruijns SC, Joshi M, Fehres CM, van Bloois L, et al. Glycan-modified liposomes boost CD4+ and CD8+ T-cell responses by targeting DC-SIGN on dendritic cells. J Control Release. 2012;160:88–95. doi: 10.1016/j.jconrel.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Engering A, Geijtenbeek TB, van Vliet SJ, Wijers M, van Liempt E, Demaurex N, et al. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J Immunol. 2002;168:2118–26. doi: 10.4049/jimmunol.168.5.2118. [DOI] [PubMed] [Google Scholar]


