Abstract
Therapeutic strategies to deplete lymphocytes, especially regulatory T cells, in cancer patients have been proposed to increase the benefits of (immuno)chemotherapy. In this study, we explored the influence of temozolomide (TMZ) on different T-cell populations and addressed if the depletion of CD4+ T cells would be associated to the clinical benefits of TMZ. Patients were treated with TMZ (150 mg/m2 daily, every two weeks on a four-week schedule) until disease progression. Changes in T-lymphocyte subsets were characterized by flow cytometry. All patients enrolled in this study had histologically verified unresectable stage IV melanoma. Objective responses were induced in 12.5% of the patients, while 42.5% of them obtained short-term disease stabilization. The median progression-free survival (PFS) of this patient cohort was 8.7 mo. Lymphopenia (< 0.7 × 109 cells/L, grade 2) developed in 71% of the patients after 3 treatment cycles (~100 d). The development of grade 2 lymphopenia after the 3rd cycle of therapy positively correlated with clinical outcome (p = 0.01), and was linked, though non-significantly, to prolonged median PFS (303 vs. 200 d). In addition, significant changes in CD8+ T-cell subgroups were observed, notably a shift from naïve T cells toward more differentiated memory T cells. Finally, we demonstrated that specific CD8+ T-cell responses against selected tumor associated antigens (TAAs) were enhanced by the administration of TMZ (p = 0.04), while virus-specific T-cell responses were stable. Thus, immunological monitoring in the course of TMZ treatment might become an important tool for clinical guidance in the future.
Keywords: lymphocytes, lymphopenia, metastatic melanoma, regulatory T cells, temozolomide
Introduction
The incidence of melanoma has steadily increased over the past 50 y in most fair-skinned populations in Europe, especially among people living in Nordic countries. Though the survival of such melanoma patients has reportedly improved in the last decades, the mortality-dependent mortality rate continues to rise in Europe.1,2 The prognosis of patients affected by metastatic melanoma is poor, with a 5-y survival of approximately 10% and a median survival of 6–9 mo.3
Temozolomide (TMZ) is a cytotoxic alkylating agent used in the treatment of metastatic melanoma. TMZ is advantageous in that it can be administered orally and as it penetrates the blood-brain barrier. Moreover, TMZ has comparable efficacy to dacarbazine (DTIC).4,5 As a standalone intervention against metastatic melanoma, TMZ is associated with an objective response rate of 4–20%.6-9 In Denmark, TMZ is also used as a systemic therapy in selected metastatic melanoma patients.
Different dosing regimens of TMZ have been investigated, including (1) five consecutive days of treatment in a 28-d cycle (5/28-d schedule), (2) seven consecutive days of treatment alternating with 7 drug-free days (14/28-d schedule), (3) 21 consecutive days of treatment in a 28 d cycle (21/28-d schedule), and (4) a six-week continuous, extended dosing followed by a two-week stop, every eight weeks (eight-week schedule).10 An unexpected high incidence of lymphopenia (60%) and occasional opportunistic infections have been retrospectively associated with the eight-week schedule back in 2004.11 This tendency was confirmed prospectively by Rietschel et al. in 2008.12 In our study, 150 mg/m2 TMZ was given as a day 1–7 plus day 15–21 in a 28 d cycle (14/28-d schedule), which is the standard dosing for treatment of melanoma patients in Denmark.
Regulatory T cells (Tregs) are known to participate in the suppression of antitumor immune responses as mediated by multiple mechanisms.13 Increased levels of Tregs in both the peripheral blood and the tumor microenvironment have been shown to negatively correlate with clinical outcome in patients affected by a variety of neoplasms. Thus, Tregs seem to be a major obstacle against the success of immunotherapy, and recent data suggest that Treg-eliminating strategies might constitute an important therapeutic tool.14 Recently, Rosenberg et al. have demonstrated that the addition of lymphodepleting cytotoxic regimens to adoptive T-cell transfer lead to impressive clinical responses in melanoma patients.15 TMZ-induced CD4+ lymphopenia might also be advantageous if the depletion mainly involve Tregs. This study was designed to address how 150 mg/m2 TMZ given in a 14/28 d schedule affects the circulating levels of different T-cell subsets. We assessed changes in frequency and absolute counts of different T-cell subsets, i.e., CD3+ cells, CD4+ cells, Tregs, CD49d- Tregs, CD8+ cells and various CD8+ cell memory subsets including central memory (Tcm), CD45RA- effector memory (Tem), CD45RA+ effector memory (Temra) T cells, and of immunosuppressive myeloid-derived suppressor cells (MDSC), and evaluated if these changes were associated with clinical responses to TMZ treatment. Moreover, we detected CD8+ T-cell responses specific for viral antigens and melanoma-associated antigens in patients before and after TMZ therapy.
Results
Demographics
Forty patients with metastatic melanoma were enrolled in this prospective study. All patients received at least 3 cycles of TMZ and were evaluated for clinical responses using CT scans. Among these 40 patients, 6 patients were excluded from immunological assessments due to concomitant treatment with prednisone. The study population, including 3 patients with uveal melanoma, was constituted of 19 women and 21 men with a median age of 67 y (range 39–85 y). TMZ treatment represented the first line systemic regimen in 73% of the patients. Patients were not eligible for other first line systemic treatments due to age (54%), brain metastases (38%) or co-morbidity (8%). Thus, only 6 patients had formerly been treated with high dose interleukin-2/interferon α (IL-2/IFNα) (decrescendo regimen).16,17 All patients had metastatic stage IV disease, (M1a = 2, M1b = 9 and M1c = 29 patients), and the average number of completed TMZ cycles were 5.3 (range 3–13 cycles).
Clinical efficacy and overall survival time
The overall objective response rate in this study was 12.5%. Complete responses (CRs) were obtained in 2/40 patients and partial responses (PRs) in 3/40 patients. Disease stabilization (SD) for 3 mo was observed in 14/40 (35%) patients and SD for 6 mo in 3/40 (7.5%) patients. The rate of clinical benefit, defined as CR or PR or SD for at least 6 mo was 8/40 (20%), whereas disease progression (PD) at first evaluation was observed in 18/40 (45%) of the patients.
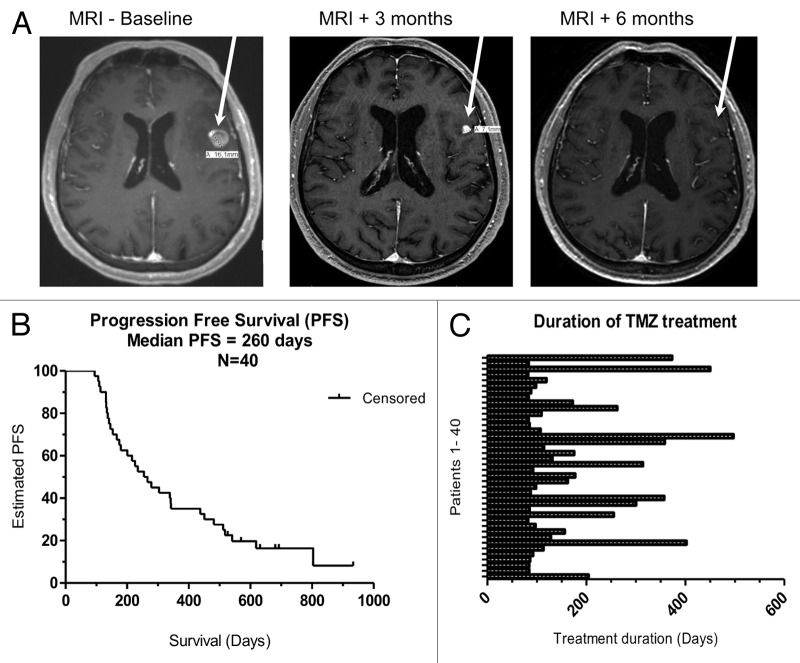
One patient manifesting a CR developed hemolytic anemia, severe lung infection and died shortly thereafter; whereas the other patient developing a CR has never evolved since, nowadays more than 18 mo. Three patients manifested PRs lasting from 2 to 5 mo and one of them had an almost CR in a brain lesion (Fig. 1A). All 3 patients undergoing SD for > 6 mo continue in the same status, nowadays for more than 16, 21 and 7 mo. Median progression-free survival (PFS) was calculated using the Kaplain Meier method18 (Fig. 1B). PFS was defined as the time intervening between the first dose of TMZ and disease progression, death (for any cause) or - if neither of these events - data were censored at the date of last adequate disease assessment. Median PFS was 8.7 mo. The duration of treatment was defined as time intervening between the first and the last dose of TMZ. The median treatment duration was 3.9 mo (range 3.0–16.6 mo) (Fig. 1C).
Figure 1. Clinical efficacy and overall survival of melanoma patients treated with temolozomide. (A) Magnetic resonance imaging (MRI) scans from a patient undergoing a partial response and demonstrating an almost complete response in a brain lesion after 6 mo of temolozomide (TMZ) treatment. (B) Kaplan-Meier plot of progression-free survival (PFS) for all patients included in this study (n = 40). Median PFS was 260 d. PFS was defined as the time intervening between the first dose of TMZ until disease progression or death from any cause. When no progression or death occurred, data were censored at the date of last adequate disease assessment. (C) Duration of TMZ treatment for all enrolled patients. The duration of treatment was defined as time intervening between the first and the last dose of TMZ. The mean treatment duration was 173 d (95% CI = 135.4–210.3), corresponding to ~5 cycles of TMZ treatment.
Immunological assessments
Lymphocyte counts were assessed before treatment and after each TMZ cycle. Lymphopenia was graded according to the Common Toxicity Criteria for Adverse Events (CTCAE). Overall, lymphopenia (< 0.7 × 109 cells/L, CTCAE grade 2) developed in 71% of the patients after 3 TMZ cycles (~100 d). The lymphocyte reduction was highly significant p < 0.001 (Fig. 2).
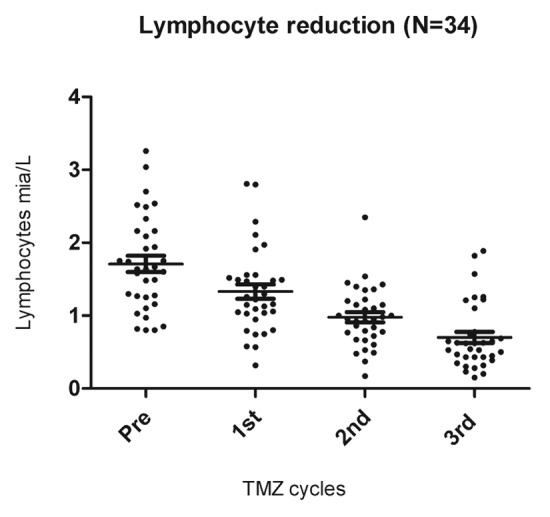
Figure 2. Lymphopenia induced by temolozomide in melanoma patients. Lymphocyte counts on freshly drawn blood samples (as assessed by flow cytometry) before the administration of temolozomide (TMD) and after the first, second and 3rd cycles of chemotherapy. **p < 0.01; ***p < 0.001 (paired Student's t-test).
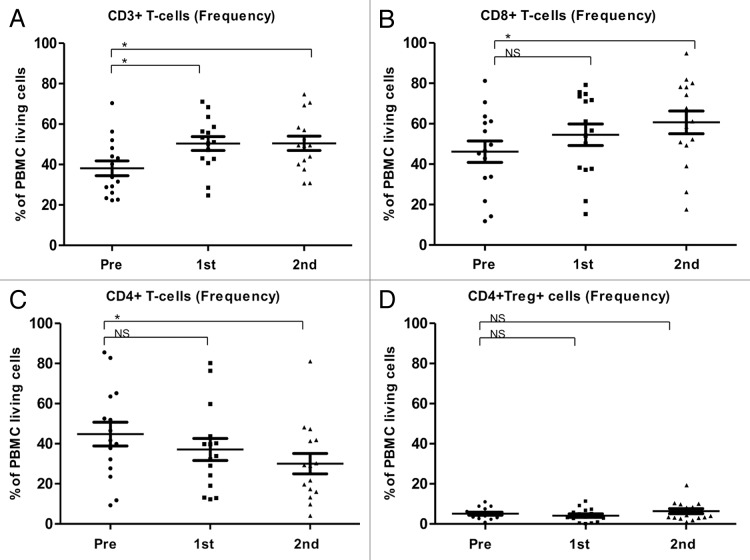
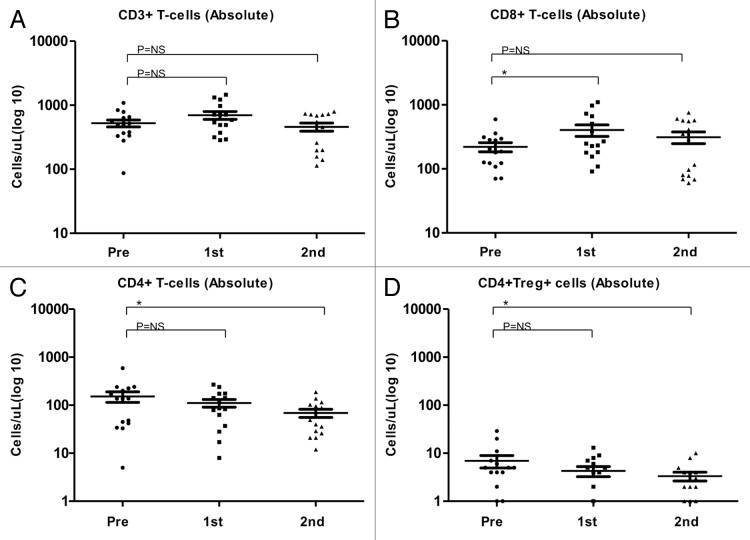
In 15 patients, the mean frequencies (%) (Fig. 3A-D) and absolute counts (cells/µL) (Fig. 4A-D) of CD3+, CD8+, CD4+ and Tregs (defined as CD4+,CD25high, CD127-) before and after the first and second cycles of TMZ were analyzed. After the first TMZ cycle, the frequencies and absolute numbers of CD3+ and CD8+ T cells were increased, whereas the frequencies and absolute counts of CD4+ T cells and Tregs decreased. After the second TMZ cycle, CD8+ cells kept increasing significantly and CD4+ cells kept decreasing significantly. The frequency of Tregs was non-significantly increased after the second TMZ cycle, whereas the absolute numbers of Tregs were lower after the second TMZ cycle than after the first one, though this decrease did not reach the threshold for statistical significance (p = 0.2).
Figure 3. Alterations of the frequency T-cell subsets as induced by temolozomide in melanoma patients. (A–D) Frequency of T cell subpopulations on frozen/thawed peripheral blood mononuclear cells (PBMCs, as assessed by flow cytometry) before the administration of temolozomide (TMD) and after the first and second cycles of chemotherapy: *p < 0.05; NS = non-significant (paired Student's t-test).
Figure 4. Alterations of the absolute abundance of T-cell subsets as induced by temolozomide in melanoma patients. (A–D) Absolute T-cell counts on frozen/thawed peripheral blood mononuclear cells (PBMCs, as assessed by flow cytometry) before the administration of temolozomide (TMD) and after the first and second cycles of chemotherapy. *p < 0.05; NS = non-significant (paired Student's t-test).
Recently, a subpopulation of Tregs that do not express the surface marker CD49 was suggested to exert potent immunosuppressive effects, hence constituting “true Tregs.”19 Thus, to further characterize the Treg population we specifically quantified CD49d- Tregs, finding the percentage of CD49d- Tregs before TMZ treatment to be 3.9% (range 1.9–7.3%), and after TMZ treatment to be 3.6% (range 1.6–7.7%). Our data suggest that CD49d- Tregs undergo a small, non-significant decrease, in melanoma patients receiving TMZ (data not shown).
Another highly potent immunosuppressive cell population is constitute by myeloid-derived suppressor cells (MDSCs), which have been defined as HLA-DR-Lin-(CD3-CD19-CD56-CD33+CD11b+) cells. MDSCs can be further divided into two populations: monocytic MDSCs, which express CD14, and granulocytic MDSCs, which express CD15.20 The percentage of monocytic and granulocytic MDSCs was not altered in our patient cohort by the administration of TMZ (data not shown).
T-cell alterations correlate to clinical response
Thirty-four patients were evaluable for the precise characterization of lymphopenia (i.e., they did not receive prednisone) as well as for clinical responses (by CT scans) after 3rd cycles of TMZ (Fig. 5A). We found a significant positive correlation between lymphocyte reduction and response to treatment (p = 0.01, Fisher exact test). After 3 cycles of TMZ, two patients subgroups, that is, lymphocyte responders (defined as individuals with lymphocyte counts ≤ 0.7 × 109 cells/L; n = 23 patients) and lymphocyte non-responders (defined as subjects with lymphocyte counts > 0.7 × 109 cells/L; n = 11 patients), showed a non-significantly (p = 0.2, Log-rank test) different median PFS (lymphocyte responders vs. non-responders 303 vs. 200 d (7 mo) (Fig. 5B).
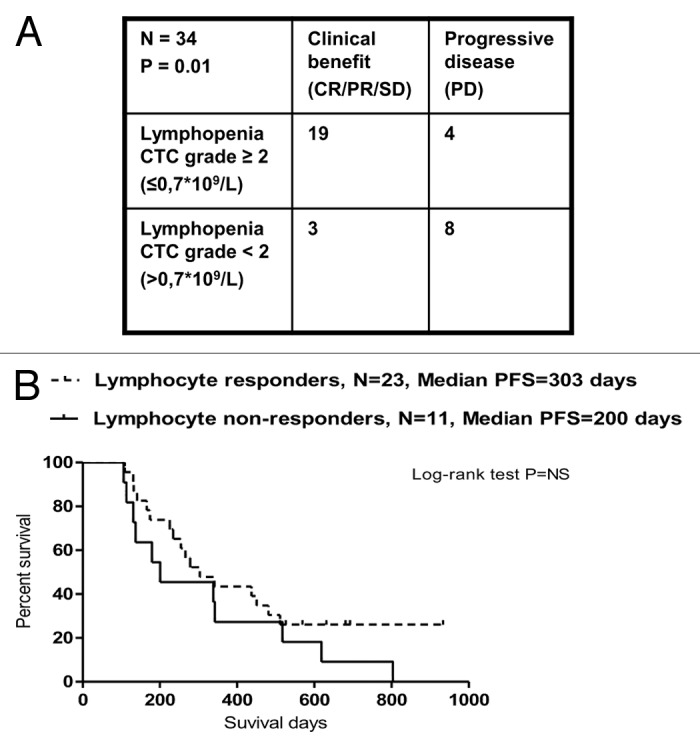
Figure 5. Correlation between temolozomide-induced lymphopenia and clinical parameters. (A) Correlation between the grade of lymphopenia (as determined on freshly drawned blood samples) and the response of n = 34 melanoma patients to 3 cycles of temolozomide (TMZ). p = 0.01 (Fischer’s exact test). Lymphocyte counts were done directly on. (B) Progression-free survival (PFS) of lymphocyte responders (defined as individuals with lymphocyte counts ≤ 0.7 × 109 cells/L, n = 23) and lymphocyte non-responders (defined as individuals with lymphocyte counts > 0.7 × 109 cells/L, n = 11). The Kaplan-Meier estimate demonstrates a non-significantly (p = 0.2, Log-Rank test) prolongation in median PFS in lymphocyte responders (303 d) vs. lymphocyte non-responders (200 d).
To evaluate the impact of Tregs, we compared the Treg/CD4+ T-cell ratio in clinical responders (CR+PR+SD) and non-responders (PD). In responders the pre-treatment ratio was 0.02, after the first cycle 0.03, and after the second cycle 0.04, whereas the ratio of non-responders remained stable at 0.02 throughout TMZ cycles, suggesting perhaps that Tregs decrease in clinical responders. To characterize if clinical responders had a superior CD8+ T-cell profile than non-responders, the CD8+/CD4+ T-cell ratio was calculated during the first 2 cycles of TMZ. The CD8+/CD4+ T-cell ratio in clinical responders before treatment was 1.7, after the first cycle 2.9, and after the second cycle 5.22, whereas non-responders displayed a CD8+/CD4+ T-cell ratio before TMZ of 2.15, after the first cycle of 2.35 and after the second cycle of 3.16, indicating a comparatively stronger CD8+ T-cell profile among clinical responders (Fig. 6A). In addition, we investigated the repertoire of distinct populations of CD8+ T lymphocytes, including naïve T cells as well as antigen-experienced Tcm, Tem and Temra, as described by Sallusto et al.21 The frequencies of CD8+ naïve, Tcm, Tem, Temra T cells were measured in the course of TMZ treatment (n = 15). We found a significant decrease in the frequency of CD8+ naïve T cells (p = 0.05), and a significant increase in the frequency of the differentiated CD8+ T cells (i.e., CD8+ Tcm plus Tem plus Temra T cells; p = 0.02) after 2 cycles of TMZ (Fig. 6B and C).
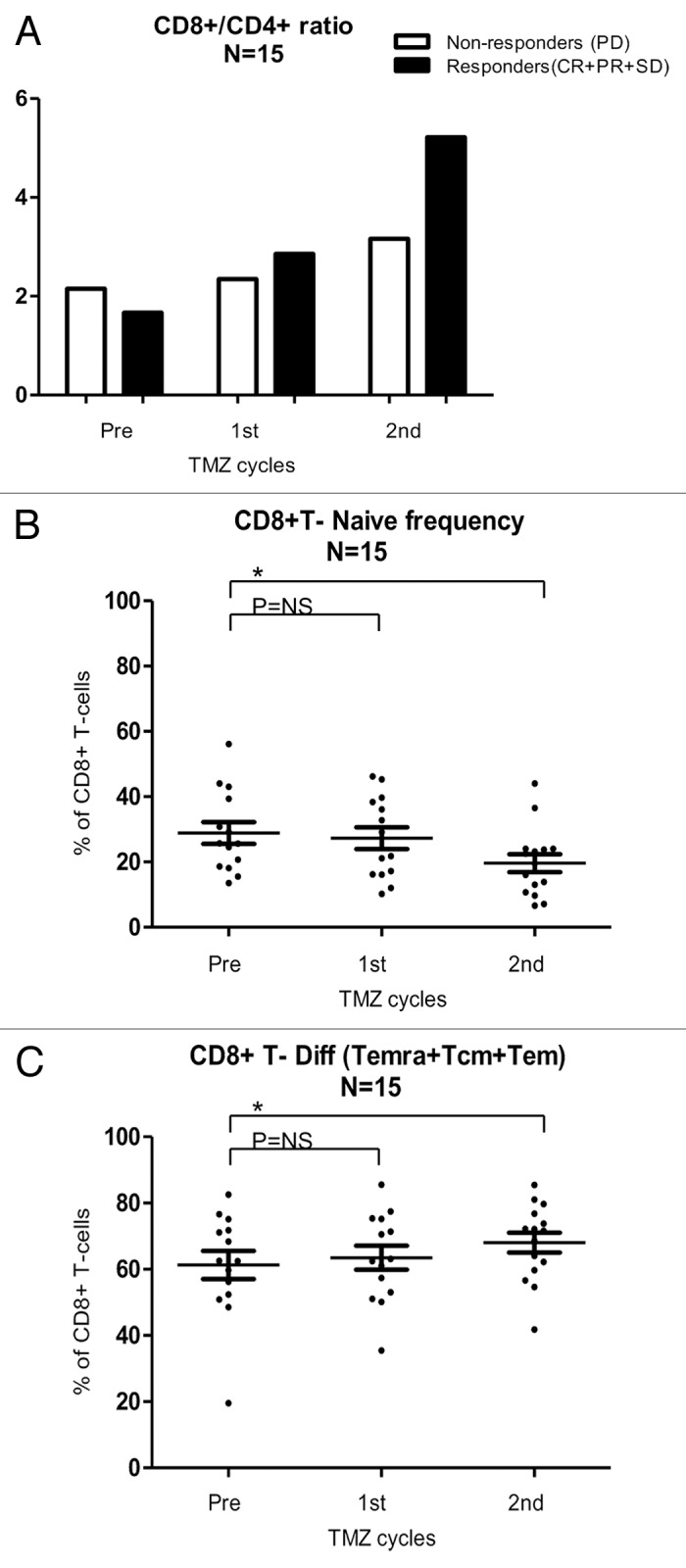
Figure 6. CD8+ T-cell subsets in melanoma patients responding or not to temolozomide. (A) CD8+/CD4+ T-cell ratio (as assessed by flow cytometry) before the administration of temolozomide (TMD) and after the first and second cycles of chemotherapy to clinical responders, exhibiting either complete response (CR), either partial response (PR) or stable disease (SD), and clinical non-responders, exhibiting progressive disease (PD). (B) Frequency of naïve CD8+ T cells (as assessed by flow cytometry) before the administration of temolozomide (TMD) and after the first and second cycles of chemotherapy. *p < 0.05; NS = non-significant (paired Student's t-test). (C) Frequency of differentiated CD8+ T cell (Tcm plus Tem plus Terma) (as assessed by flow cytometry) before the administration of temolozomide (TMD) and after the first and second cycles of chemotherapy. *p = 0.02; NS = non-significant (paired Student's t-test).
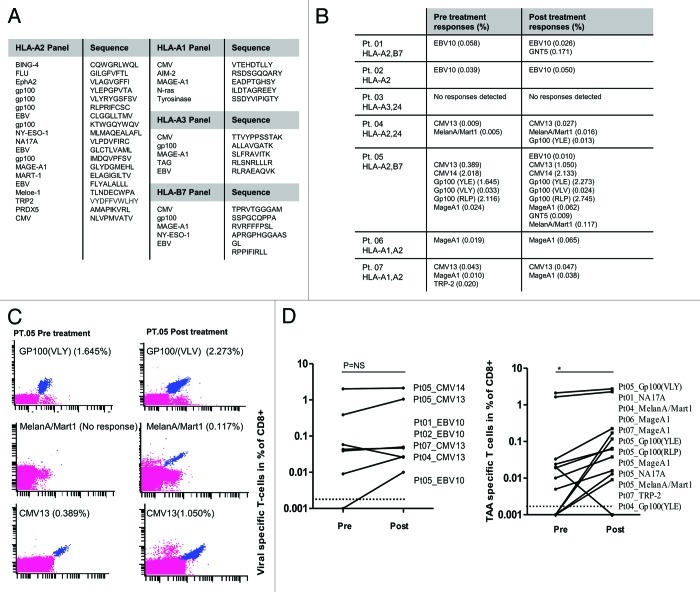
We then analyzed CD8+ T-cell immune responses in melanoma patients before and after chemotherapy, focusing on a panel of 27 peptides that have recently been described as targets for melanoma infiltrating lymphocytes.22,23 Peptides were selected from a library of previously described melanoma-associated antigens, including overexpressed, differentiation-associated and cancer-testis antigens presented on HLA-A1, HLA-A2, HLA-A3 and HLA-B7.23 We also included a number of viral epitopes, to monitor the general status of the immune system. To screen the reactivity against these peptides using minimal amounts of patient material, we took advantage of the recently described technique for the combinatorial encoding of specific T-cells by fluorescent MHC multimers22,24 (Fig. 7A and B). In 7 patients, MHC multimer staining was performed to investigate if CD8+ T-cell responses against the selected peptides were influenced by TMZ. The majority of the patients analyzed (6/7) expressed HLA-A2 molecules, constituting the restriction element for most selected peptides (Fig. 7A). In 5/7 patients, we demonstrated significantly enhanced T-cell responses against different TAAs (p = 0.04), whereas in the remaining 2 patients no responses against TAAs were detected. In 5/7 patients, we also demonstrated preserved T-cell responses against selected viral epitopes after TMZ treatment (Fig. 7B-D). There was no correlation between the capability of patient material from these 7 individuals to recognize TAAs and clinical outcome.
Figure 7. Tumor-associated antigen- and viral antigen-specific T-cell responses in melanoma patients receiving temolozomide. (A) Peptide panel used for screening T-cell responses in patients with tissue types HLA-A1, A2, A3 and B7. (B) Responses to viral and tumor-associated antigens evaluated before and after temolozomide (TMD) chemotherapy in n = 7 melanoma patients. (C) Examples of pre and post-chemotherapy T-cell responses in-patient 05, as detected by flow cytometry with MHC multimers. CD8+ T-cell responses were gated on living single CD8+ T cells (mean number of CD8+ cells for all experiments = 73,655; range = 8056–238,576). MHC multimer positive populations are depicted in blue, from the top recognizing (1) Gp100(VLY), labeled by PE and Q655, (2) MelanA/Mart1, labeled by Q585 and Q605 and (3) CMV13, labeled by Q605 and Pe-Cy7. (D) Viral antigen- and tumor antigen-specific responses pre- and post-TMZ chemotherapy. *p = 0.04; NS = non-significant (paired Student's t-test). The dotted line represents the detection limit = 0.002% CD8+ T cells.
Discussion
To improve the selection of patients that are likely to respond to therapy as well as to combine and develop new therapeutic approaches against melanoma, it is important to identify relevant biomarkers. In this sense, we estimated of value assessing T-cell subpopulations in the course of cytotoxic TMZ treatment. We have formerly shown that the levels of Tregs are lowered in patients receiving TMZ before entering an experimental dendritic cell-based vaccine protocol. In the present study, we have determined changes in the frequency and absolute abundance of different T-cell subsets in the peripheral blood of melanoma patients treated with TMZ (Danish standard doses) and compared these changes to clinical response.
Previously, it has been shown that TMZ given as a continuous dosing (eight-week schedule) induces lymphopenia.11,12 Rietschel et al. found that 75% of these patients develop lymphopenia after the 3rd cycle of TMZ, and that the incidence of lymphopenia increases with additional cycles. The same authors also reported that TMZ induces a steady decline of total CD3+ cells, mainly as a result of CD4+ T-cell depletion, whereas the CD8+ T-cell population seems relatively less affected by TMZ.12
Here, we have demonstrated a statistically significant reduction of T lymphocytes in melanoma patients after 3 cycles of TMZ, which is in concordance with the hypothesis of the study. Moreover, we have shown that the development of ≥ grade 2 lymphopenia after the 3rd TMZ cycle positively correlates with clinical response. Recently, we have shown a similar correlation between lymphopenia and clinical outcome in stage IV melanoma patients receiving both TMZ and thalidomide.25 In the present study, we have also shown that PFS is not correlated with treatment-dependent lymphopenia (< 0.7 × 109 cells/L).
It is generally accepted that Tregs influence oncogenesis and tumor progression, and that the host immune system plays a critical role in therapeutic responses. Treg-depleting strategies to achieve potent anticancer immune responses with therapeutic impact have been investigated by other groups. Targeting CD25 with denileukin has resulted in the partial depletion of circulating Tregs, resulting in an improved stimulation of tumor-specific T cells.26 Rasku et al. have found that denileukin breaks peripheral tolerance and allow for the expansion of the effector T cells.27 Golovina et al. have studied the clinical use of the anti-CD25 antibody daclizumab and demonstrated that Tregs are efficiently eliminated by this approach, suggesting that daclizumab might also be effective in modulating Tregs.28 In our study, we focused on both the frequency and absolute abundance of Tregs and CD4+ T lymphocytes during TMZ treatment. We found decreasing levels of both CD4+ T cells and Tregs after the first cycle of therapy. However, after the second cycle the absolute counts of Tregs kept decreasing whereas their frequency had increased. This increase in frequency may be due to a global depletion of CD4+ T cells or to the re-expansion/re-trafficking of T cells from lymph nodes, as recently suggested by Rasku et al.27 Surprisingly, even though a general depletion of CD4+ T cells and Tregs occurred, we found a Treg/CD4+ T-cell ratio favoring Tregs in clinical responders to TMZ therapy, which was not seen among non-responders.
Kleinewietfield et al. have shown how anti-CD49d antibodies can be an important tool in defining “true Tregs,” as it allows for the removal of CD49d+CD127+ cells to effector cells from Tregs.19 We have previously shown that Tregs with proven inhibitory function can be determined by CD49d expression on the cells surface.29 In the present study, we performed a similar CD49d staining, which revealed only a marginal decline of this Treg population in the course of TMZ treatment.
Furthermore, we found increasing levels of CD8+ T cells. This suggests that the CD8+ T-cell population might be able to expand even though CD4+ cells are reduced. Importantly, we observed that the CD8+/CD4+ T-cell ratio was higher among clinical responders than in non-responders, suggesting that a better CD8+ T-cell profile might be advantageous for therapeutic responses. In this context, the depletion of T cells bearing a non-specific T-cell receptor (TCR) might generate space for development of a better-primed T-cell population bearing tumor-specific TCRs.27
To further investigate if a tumor-specific immune response was induced in the course of TMZ treatment, we analyzed the capacity of patient material to recognize a library of TAAs-derived peptides that are known to be recognized by melanoma-infiltrating lymphocytes.23,30 In 7 patients, we assessed TAA-specific T-cell populations by MHC multimer combinatorial encoding. We found that CD8+ T-cell responses against TAAs are significantly enhanced post TMZ, whereas virus-targeting CD8+ T-cell responses were stable, suggesting that the chemotherapy-induced killing of cancer cells might release TAAs that can be presented by antigen-presenting cells and hence elicit significant T-cell responses. As suggested by Galluzzi et al.,31 classical cytotoxic agents may stimulate tumor-specific immune responses by inducing immunogenic cell death, a peculiar form of apoptosis involving the release of ATP and activating numerous immune effector mechanisms. This lends support to the idea of combining TMZ with anticancer vaccine strategies.
In general, we found that the absolute lymphocyte counts in our patients were rather low, probably due to their age (mean = 67 y). In line with this notion, Rech et al. found that absolute lymphocyte and CD4+ T-cell counts are low (< 200 cells/µL) in cancer patients treated with adjuvant chemotherapy or having metastatic disease as compared with healthy donors.32 We found that the CD4+ T-cell population is more vulnerable to freezing/thawing procedures than its CD8+ counterpart, emphasizing the need for using a marker of cell death when performing T-cell assays. Rasku et al. have also observed that the CD4+ T-cell population is more fragile than CD8+ T cells.27
We also investigated the repertoire of antigen-naïve CD8+ T cells as well as of distinct populations of antigen-experienced CD8+ T lymphocytes, including Tcm, Tem and Temra. We demonstrated a significant decrease of naïve CD8+ T cells along with a significant increase of differentiated CD8+ T cells (Temra plus Tcm plus Tem) after 2 cycles of TMZ, that is, a rise in the mature CD8+ compartment at the expenses of naïve CD8+ cells. It is well known that anti-melanoma CD8+ T cells are primed against cancer during oncogenesis and disease progression. Hence, an increase in these CD8+ cell populations might be critical for therapeutic responses. The naïve CD8+ /differentiated CD8+ T-cell ratio was similarly reduced in the course of treatment in both clinical responders and non-responders. In our experience, the profile of CD8+ memory cells during TMZ treatment has not yet been investigated by others, and additional insights into memory cell subsets, including information on their antigen specificity, is needed to elucidate the importance of our data.
Another cell population that influence the therapeutic response of cancer patients, due to their potent immunosuppressive activity, is constituted by MDSCs. Solito et al. have demonstrated that increased circulating levels of MDSCs correlate with worsened prognosis in patients affected by colorectal cancer,20 though an unambiguous phenotype of MDSCs exerting immunosuppressive functions is intensively discussed. A recent review of murine and human MDSCs suggests that MDSCs mainly consist of a monocytic population characterized by the expression of CD14 but not CD15.33 Finkelstein et al. have investigated the influence of MDSCs in renal cell carcinoma and melanoma patients treated with high-dose IL-2, finding increased levels of MDSCs after treatment even in patients with the most favorable clinical outcome.34 In our study, we observed that the levels of monocytic and granulocytic MDSCs are unaffected in metastatic melanoma patients receiving TMZ.
The clinical efficacy of TMZ in our cohort (PFS = 8.7 mo and objective RR = 12.5%) was in accordance with previous studies in similar clinical settings.12,35 One patient still manifests an ongoing CR, nowadays for more than 18 mo, and all 3 patients exhibiting SD for more than 6 mo have not yet progressed, nowadays exhibiting SD for more than 16, 21 and 7 mo. CRs in stage IV melanoma patients treated with TMZ/DTIC do occur and are occasionally durable, though very infrequent.11,12 Long-term survival after TMZ/DTIC treatment not always is induced by chemotherapy, but rather can results from host factors or from an indolent disease. Presumably, patients bearing indolent disease are those who benefit the most from immunotherapy.4 This suggests that TMZ treatment may be suitable for combination with other immunotherapies, e.g., melanoma antigen-specific vaccines. Kyte et al. have reported that the administration of TMZ together with hTERT-derived peptide vaccine resulted in one long-term survivor (> 5 y, PET-CT scan negative).36 At our institution, we have set up a new clinical trial to investigate the combination of TMZ and a peptide vaccine in melanoma patients (NCT01543464).
TMZ has been shown to exert clinical efficacy in patients affected by aggressive tumors such as stage IV melanoma. Here, we demonstrated that ≥ grade 2 lymphodepletion after the 3rd TMZ cycle correlates with treatment response and that median PFS does not change significantly between lymphocyte responders and non-responders. In clinical responders, TMZ induced a significant increase in CD8+ T-cell memory subsets along with a drop in naïve T cells and a beneficial CD8+ T-cell profile. Furthermore, we proved that CD8+ T-cell responses specific for selected viral antigens and TAAs were preserved or even enhanced after TMZ treatment. Though these data need validation, our study indicates that the modulation of specific differentiated T-lymphocyte subsets could be important for the clinical efficacy of systemic chemotherapies. However, further studies are required to elucidate if CD4+ T-cell depletion in general provides clinical benefits to melanoma patients.
Material and Methods
Patients and blood samples
Patients with stage IV metastatic melanoma planned for TMZ therapy were eligible. All included patients fulfilled the following criteria: ≥ 18 y of age, histological confirmation of unresectable American Joint Committee on Cancer (AJCC) stage IV melanoma with progressive disease according to RECIST v. 1.0, and a World Health Organisation (WHO) performance status of 0–2. Patients were treated daily with 150 mg/m2 TMZ for 7 d every second week in four-week cycles until unacceptable toxicity, progression of disease or patients wish. Peripheral blood samples of 30 mL were collected prospectively before the initiation of the treatment and after the first and second TMZ cycle. The protocol was approved by the Ethics Committee for The Capital Region of Denmark (H-A-2007–0124) and all patients gave written informed consent. The study was conducted in accordance with the Helsinki Declaration of 1975. Project blood samples were drawn simultaneously with routine blood samples during TMZ treatment. For multimer MHC combinatorial coding, tissue typing of 7 patients was performed by flow cytometry and PCR, screening patients for the expression of HLA-A1, 2, 3, 24 and B7. The resultant HLA expression pattern is provided in Figure 7C.
Cytofluorometric analyzes
PBMCs
PBMCs were obtained from the peripheral blood by gradient centrifugation according to the Lymphoprep technique. Isolated cells were frozen immediately with 10% DMSO and 90% humanised AB-serum and stored at -140°C.
CD4+/Treg assays
PBMCs were thawed and stained for cytofluorometric analyzis with a FACSCanto II cytometer (BD bioscience). Briefly, PBMCs were thawed and incubated with mouse serum (Invitrogen) followed by labeling with the following fluorochrome–conjugated antibodies or relevant isotype (surface antigens): AMCYAN-CD3 (BD bioscience), PerCP-CD4 (BD bioscience), APC-CD25 (E-bioscience), FITC-CD27 (E-bioscience). PE-FOXP3 (E-bioscience) and isotype PE rat IgG2a (E-bioscience) were used for intracellular staining. In this context, cells were fixed and permeabilized using a fixation/permeabilization kit (E-bioscience), according to manufactures instruction. The APC-Cy7 near IR (Invitrogen) fluorescent reactive dye was used as a dead-cell marker.
CD8+ naive, Tcm, Tem, and Temra T-cell assays
For surface antigen staining the following antibodies were used: APC-CD3 (BD bioscience), FITC-CD4 (BD bioscience), PE-CD8 (BD bioscience), PECy7-CCR7 (BD PharMingen), HorizonV450-CD45RA (BD Bioscience) and the relevant isotype controls.
Treg CD4+CD49d- assays
For surface antigen staining the following antibodies were used: APC-Cy7-CD3 (BD bioscience), PerCP-CD4 (BD bioscience), Pacific blue-CD8 (DAKO), APC-CD25 (Ebioscience), FITC-CD127 (Ebioscience) and PE-CD49d (BD bioscience) and the relevant isotype controls.
Myeloid-derived suppressor cells (MDSC) assays
Cells were incubated with mouse serum (Invitrogen) to prevent unspecific labeling, and for surface antigen staining the following antibodies were used: PerCP-HLADR lineage (BD Bioscience), Pe-Cy7-CD3 lineage (BD PharMingen), Pe-Cy7-CD19 lineage (BD PharMingen) and Pe-Cy7-CD56 lineage (BD PharMingen), PE-CD33 (DAKO), APC-CD11b (BD PharMingen), APC-Cy7-CD14 (BD PharMingen) and Pacific blue-CD15 (Biolegend). For these assays, 200,000 lymphocytes were collected and gated on forward and side scatter profiles.
Combinatorial encoding with MHC multimers
Cells were thawed in Pulmozyme buffer and washed twice, then resuspended in FACS-PBS buffer and distributed into 96-well plates before centrifugation. A panel of 27 MHC multimers (each specificity having a specific color combination, 2 µL per specificity produced, as indicated below) were mixed with cells and incubated 10 min at 37°C, then the relevant fluorochrome-conjugated antibodies and dead-cell marker were added for further incubation, 30 min at 4°C. We used PerCP-CD8 (Invitrogen) and a dump channel (FITC), including: FITC-CD4 (BD Bioscience), FITC-CD14 (BD Bioscience), FITC-CD19 (BD Bioscience), FITC-CD40 (Serotec) and FITC-CD16 (BD Bioscience). Analyzes were recorded on an LSRII SORP cytometer and data were analyzed with FACSDiva Version 6.1.3 (BD bioscience).
MHC multimer production
UV-sensitive ligands were synthesized, and recombinant human HLA-A1 and -A2 heavy chains as well as the human β2-microglobulin light chain were produced in Escherichia coli, then refolded with the conditional ligand, and purified.37 Specific peptide-MHC complexes were generated using the MHC peptide exchange technology. MHC multimers were produced by multimerization of the generated peptide-MHC complexes using eight different fluorescent-streptavidin (SA) conjugates, SA-PE, SA-APC, and SA-PE-Cy7 (Biolegend), and Qdot 585, 605, 625, 655, and 705 (Invitrogen), as described. MHC multimers were frozen in 0.5% bovine serum albumin (BSA) and 16% glycerol. The final concentration of the MHC multimers was 100 µg/mL.22,24
Clinical assessment of response
Clinical response evaluation was performed by computed axial tomography of the chest and abdomen (CT scans) and evaluated by Response Evaluation Criteria In Solid Tumors (RECIST v. 1.0.). CT scans were performed before treatment start (baseline) and every 3rd month until disease progression or death. In patients with brain metastases, complementary magnetic resonance imaging (MRI scan) of the brain was performed within 4 weeks from the beginning of the treatment and every three treatment cycles thereafter.
Statistical procedures
All data were evaluated using the GraphPad Prism Software version 5. Individually serial samples of T-lymphocyte subsets were assessed using paired Student's t-test and results are given as means with 95% confidence intervals (CI). For the correlation of lymphocyte counts to treatment response, the Fischer exact test was used. Survival statistics for PFS were performed by the Kaplan-Meier method. Survival between lymphocyte responders and non-responders was compared by the Log-Rank test. In all analyzes, a two-sided p value < 0.05 was considered statistically significant.
Acknowledgments
We would like to thank Tobias Wirenfeldt Klausen for statistical assistance. In addition, we would like to thank the Units of Clinical Research at Department of Oncology at Herlev and Odense University Hospitals for assistance in patient registration. Grant support: The Danish Cancer Society, Herlev University Hospital and the Danielsen Foundation. T.Z.I., M.K.B., K.N., R.S.A., S.R.H., M.H.A., L.B. and I.M.S. contributed to patient sampling and data monitoring, designed and performed immunological experiments and interpreted data. T.Z.I. drafted the manuscript; S.R.H., M.H.A., L.B. and I.M.S. designed and supervised the study, interpreted data and co-wrote the paper.
Glossary
Abbreviations:
- CR
complete remission
- DTIC
dacarbazine
- IL-2/IFNα
interleukin-2/interferon α
- MDSC
myeloid-derived suppressor cell
- NE
not evaluable
- PBMC
peripheral blood mononuclear cell
- PD
progressive disease
- PR
partial response
- SD
stable disease
- TMZ
temozolomide
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23288
References
- 1.Erdmann F, Lortet-Tieulent J, Schüz J, Zeeb H, Greinert R, Breitbart EW, et al. International trends in the incidence of malignant melanoma 1953-2008-are recent generations at higher or lower risk? Int J Cancer. 2013;132:385–400. doi: 10.1002/ijc.27616. [DOI] [PubMed] [Google Scholar]
- 2.Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146(Suppl 61):1–6. doi: 10.1046/j.1365-2133.146.s61.2.x. [DOI] [PubMed] [Google Scholar]
- 3.Hauschild A, Agarwala SS, Trefzer U, Hogg D, Robert C, Hersey P, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009;27:2823–30. doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]
- 4.Kim C, Lee CW, Kovacic L, Shah A, Klasa R, Savage KJ. Long-term survival in patients with metastatic melanoma treated with DTIC or temozolomide. Oncologist. 2010;15:765–71. doi: 10.1634/theoncologist.2009-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Middleton MR, Grob JJ, Aaronson N, Fierlbeck G, Tilgen W, Seiter S, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18:158–66. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 6.Amaravadi RK, Schuchter LM, McDermott DF, Kramer A, Giles L, Gramlich K, et al. Phase II Trial of Temozolomide and Sorafenib in Advanced Melanoma Patients with or without Brain Metastases. Clin Cancer Res. 2009;15:7711–8. doi: 10.1158/1078-0432.CCR-09-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gogas HJ, Kirkwood JM, Sondak VK. Chemotherapy for metastatic melanoma: time for a change? Cancer. 2007;109:455–64. doi: 10.1002/cncr.22427. [DOI] [PubMed] [Google Scholar]
- 8.Spieth K, Kaufmann R, Dummer R, Garbe C, Becker JC, Hauschild A, et al. Temozolomide plus pegylated interferon alfa-2b as first-line treatment for stage IV melanoma: a multicenter phase II trial of the Dermatologic Cooperative Oncology Group (DeCOG) Ann Oncol. 2008;19:801–6. doi: 10.1093/annonc/mdm565. [DOI] [PubMed] [Google Scholar]
- 9.Tarhini AA, Kirkwood JM, Gooding WE, Moschos S, Agarwala SS. A phase 2 trial of sequential temozolomide chemotherapy followed by high-dose interleukin 2 immunotherapy for metastatic melanoma. Cancer. 2008;113:1632–40. doi: 10.1002/cncr.23791. [DOI] [PubMed] [Google Scholar]
- 10.Trinh VA, Patel SP, Hwu WJ. The safety of temozolomide in the treatment of malignancies. Expert Opin Drug Saf. 2009;8:493–9. doi: 10.1517/14740330902918281. [DOI] [PubMed] [Google Scholar]
- 11.Su YB, Sohn S, Krown SE, Livingston PO, Wolchok JD, Quinn C, et al. Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J Clin Oncol. 2004;22:610–6. doi: 10.1200/JCO.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 12.Rietschel P, Wolchok JD, Krown S, Gerst S, Jungbluth AA, Busam K, et al. Phase II study of extended-dose temozolomide in patients with melanoma. J Clin Oncol. 2008;26:2299–304. doi: 10.1200/JCO.2007.14.5292. [DOI] [PubMed] [Google Scholar]
- 13.Gajewski TF. Cancer immunotherapy. Mol Oncol. 2012;6:242–50. doi: 10.1016/j.molonc.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–7. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bukowski RM, Tendler C, Cutler D, Rose E, Laughlin MM, Statkevich P. Treating cancer with PEG Intron: pharmacokinetic profile and dosing guidelines for an improved interferon-alpha-2b formulation. Cancer. 2002;95:389–96. doi: 10.1002/cncr.10663. [DOI] [PubMed] [Google Scholar]
- 17.Keilholz U, Scheibenbogen C, Tilgen W, Bergmann L, Weidmann E, Seither E, et al. Interferon-alpha and interleukin-2 in the treatment of metastatic melanoma. Comparison of two phase II trials. Cancer. 1993;72:607–14. doi: 10.1002/1097-0142(19930715)72:2<607::AID-CNCR2820720245>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 18.Harvey Motulsky. 2012. GraphPad Prism, Version 5.0. In Statistical analyses for laboratory and clinical researchers [Google Scholar]
- 19.Kleinewietfeld M, Starke M, Di Mitri D, Borsellino G, Battistini L, Rötzschke O, et al. CD49d provides access to “untouched” human Foxp3+ Treg free of contaminating effector cells. Blood. 2009;113:827–36. doi: 10.1182/blood-2008-04-150524. [DOI] [PubMed] [Google Scholar]
- 20.Solito S, Falisi E, Diaz-Montero CM, Doni A, Pinton L, Rosato A, et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood. 2011;118:2254–65. doi: 10.1182/blood-2010-12-325753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 22.Andersen RS, Kvistborg P, Frøsig TM, Pedersen NW, Lyngaa R, Bakker AH, et al. Parallel detection of antigen-specific T cell responses by combinatorial encoding of MHC multimers. Nat Protoc. 2012;7:891–902. doi: 10.1038/nprot.2012.037. [DOI] [PubMed] [Google Scholar]
- 23.Andersen RS, Thrue CA, Junker N, Lyngaa R, Donia M, Ellebæk E, et al. Dissection of T-cell antigen specificity in human melanoma. Cancer Res. 2012;72:1642–50. doi: 10.1158/0008-5472.CAN-11-2614. [DOI] [PubMed] [Google Scholar]
- 24.Hadrup SR, Bakker AH, Shu CJ, Andersen RS, van Veluw J, Hombrink P, et al. Parallel detection of antigen-specific T-cell responses by multidimensional encoding of MHC multimers. Nat Methods. 2009;6:520–6. doi: 10.1038/nmeth.1345. [DOI] [PubMed] [Google Scholar]
- 25.Vestermark LW, Holtved E, Dahlrot R, Brimnes MK, Svane IM, Bastholt L. A phase II study of thalidomide and temozolomide in patients with brain metastases from malignant melanoma: lymphopenia correlates with response. Ecancermedicalscience. 2008;2:91. doi: 10.3332/ecancer.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–33. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rasku MA, Clem AL, Telang S, Taft B, Gettings K, Gragg H, et al. Transient T cell depletion causes regression of melanoma metastases. J Transl Med. 2008;6:12. doi: 10.1186/1479-5876-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golovina TN, Vonderheide RH. Regulatory T cells: overcoming suppression of T-cell immunity. Cancer J. 2010;16:342–7. doi: 10.1097/PPO.0b013e3181eb336d. [DOI] [PubMed] [Google Scholar]
- 29.Engell-Noerregaard L, Ellebæk E, Iversen TZ, Hansen TH, Brimnes MK, Met O, et al. Analysis of regulatory T cell phenotype, including CD49d, during treatment of melanoma patients with dendritic cell vaccine, Interleukine-2 and/or metronomic Cyclophosphamide. Clinical and Cellular Immunology. 2011 In press. [Google Scholar]
- 30.Kvistborg P, Shu CJ, Heemskerk B, Fankhauser M, Thrue CA, Toebes M, et al. TIL therapy broadens the tumor-reactive CD8(+) T cell compartment in melanoma patients. Oncoimmunology. 2012;1:409–18. doi: 10.4161/onci.18851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov. 2012;11:215–33. doi: 10.1038/nrd3626. [DOI] [PubMed] [Google Scholar]
- 32.Rech AJ, Mick R, Kaplan DE, Chang KM, Domchek SM, Vonderheide RH. Homeostasis of peripheral FoxP3(+) CD4 (+) regulatory T cells in patients with early and late stage breast cancer. Cancer Immunol Immunother. 2010;59:599–607. doi: 10.1007/s00262-009-0780-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montero AJ, Diaz-Montero CM, Kyriakopoulos CE, Bronte V, Mandruzzato S. Myeloid-derived suppressor cells in cancer patients: a clinical perspective. J Immunother. 2012;35:107–15. doi: 10.1097/CJI.0b013e318242169f. [DOI] [PubMed] [Google Scholar]
- 34.Finkelstein SE, Carey T, Fricke I, Yu D, Goetz D, Gratz M, et al. Changes in dendritic cell phenotype after a new high-dose weekly schedule of interleukin-2 therapy for kidney cancer and melanoma. J Immunother. 2010;33:817–27. doi: 10.1097/CJI.0b013e3181ecccad. [DOI] [PubMed] [Google Scholar]
- 35.Kirkwood JM, Bastholt L, Robert C, Sosman J, Larkin J, Hersey P, et al. Phase II, open-label, randomized trial of the MEK1/2 inhibitor selumetinib as monotherapy versus temozolomide in patients with advanced melanoma. Clin Cancer Res. 2012;18:555–67. doi: 10.1158/1078-0432.CCR-11-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kyte JA, Gaudernack G, Dueland S, Trachsel S, Julsrud L, Aamdal S. Telomerase peptide vaccination combined with temozolomide: a clinical trial in stage IV melanoma patients. Clin Cancer Res. 2011;17:4568–80. doi: 10.1158/1078-0432.CCR-11-0184. [DOI] [PubMed] [Google Scholar]
- 37.Rodenko B, Toebes M, Hadrup SR, van Esch WJ, Molenaar AM, Schumacher TN, et al. Generation of peptide-MHC class I complexes through UV-mediated ligand exchange. Nat Protoc. 2006;1:1120–32. doi: 10.1038/nprot.2006.121. [DOI] [PubMed] [Google Scholar]