Abstract
Infection rate among intravenous drug users (IDU) is higher than the general public, and is the major cause of morbidity and hospitalization in the IDU population. Epidemiologic studies provide data on increased prevalence of opportunistic bacterial infections such as TB and pneumonia, and viral infections such as HIV-1 and hepatitis in the IDU population. An important component in the intravenous drug abuse population and in patients receiving medically indicated chronic opioid treatment is opioid withdrawal. Data on bacterial virulence in the context of opioid withdrawal suggest that mice undergoing withdrawal had shortened survival and increased bacterial load in response to Salmonella infection. As the body of evidence in support of opioid dependency and its immunosuppressive effects is growing, it is imperative to understand the mechanisms by which opioids exert these effects and identify the populations at risk that would benefit the most from the interventions to counteract opioid immunosuppressive effects. Thus, it is important to refine the existing animal model to closely match human conditions and to cross-validate these findings through carefully controlled human studies. Better understanding of the mechanisms will facilitate the search for new therapeutic modalities to counteract adverse effects including increased infection rates. This review will summarize the effects of morphine on innate and adaptive immunity, identify the role of the mu opioid receptor in these functions and the signal transduction activated in the process. The role of opioid withdrawal in immunosuppression and the clinical relevance of these findings will also be discussed.
Keywords: Opioid Drug abuse, Morphine, Immune Function, Opportunistic infections
INTRODUCTION
The status of the immune system generally dictates the outcome following microbial infection in an organism. Invasion of a host by pathogenic infectious agents initiates a sequence of immune responses through interactions between a diverse array of pathogen-borne virulence factors and the immune surveillance mechanisms of the host. In human infections, the integrity of the host immune system and an appropriate host response is crucial for pathogen clearance. Primary immune deficiencies predispose to different infections, depending on the nature of the underlying immune defect. An inappropriate immune response may not only result in lack of protection, but even contribute to disease severity. Chronic opioid use and abuse has been documented to severely compromise the immune system and thereby, increase the risk of opportunistic infection (Roy and Loh, 1996; Roy et al., 2006; Friedman and Eisenstein, 2004; Dinda et al., 2005). This is supported by epidemiological studies showing increased prevalence of opportunistic infections such as tuberculosis, HIV infection and pneumonia in opioid addicts (Georges et al., 1999; Nath et al., 2002; Quaglio et al., 2002). Confounding variables such as sharing of unsterilized, contaminated needles, nutritional status, environment influences, history of drug use and genetic variability can contribute to the frequency of infections in these patients, however, epidemiological studies suggest that the immune modulating effects of morphine abuse to be a major factor in disease prevalence (Georges et al., 1999; Nath et al., 2002; Quaglio et al., 2002). Furthermore, in animal studies where these variables can be controlled, morphine treatment (used as a surrogate for heroin which is rapidly converted to morphine following first pass metabolism) results in significant immune detriment with increased susceptibility to opportunistic infections (Roy, et al,1996, 2006). Defense against microbes is mediated by the early reactions of innate immunity and the later response of adaptive immunity. Chronic morphine abuse has been shown to affect both arms of immune defense (Vallejo et al., 2004). This review will summarize the effects of chronic and acute morphine on innate and adaptive immunity, identify the role of the mu opioid receptor and the signal transduction activated in the process. The role of opioid withdrawal in immunosupression and the clinical relevance of these findings will also be discussed.
1. MORPHINE MODULATION OF INNATE IMMUNE FUNCTION
Studies supporting a direct role for opioids on immune system modulation emerged with the discovery of opioid receptors on immune cells. The concept of direct and indirect morphine action was first introduced through work with morphine dependent rodents. Extensive body of literature now supports that morphine-induced immunosuppression is mediated primarily by the mu opioid receptor (MOR), although some functions are amplified following sympathetic activation or in the presence of corticosterone (CORT). The inhibition of IFN-gamma synthesis and activation of macrophage-cytokine synthesis is CORT-independent and only partially dependent on sympathetic activation (Peterson et al., 1987; Bryant et al., 1991; Casellas et al., 1991; Perez-Castrillon et al., 1992). With the discovery of opioid receptors on leukocytes, research focus has shifted to direct effects of opioids on immune cells and will therefore be the topic of discussion of this review.
In this section, we summarize seminal studies and current literature investigating how morphine directly inhibits the innate immune system by modulating macrophage, neutrophil, dendritic, mast and natural killer cell function.
1.1 Macrophage
Macrophages are one of the first lines of innate immune defense against invading pathogens. They play an essential role in pathogen clearance through their phagocytic, chemotactic and bactericidal actions. Therefore, any defects in macrophage function can be detrimental for the host. Macrophages have been at the center of many studies examining morphine mediated immune suppression and the significant role they play as the key factors in the innate immune system. Opioid inhibition of the innate immune system has been observed at several levels. Morphine treatment has been implicated in the inhibition of cytokine secretion, leukocyte recruitment and bacterial clearance. By decreasing the proliferative capacity of macrophage progenitor cells and lymphocytes, morphine treatment reduces the number of macrophages that are available to respond to infection (Roy et al., 2006) thereby compromising the host’s ability to fight off invading pathogens. Furthermore, morphine treatment has been also shown to directly inhibit macrophages’ ability to ingest opsonized pathogens (Casellas et al., 1991; Szabo et al., 1993; Tomei and Renaud, 1997).
Initial opioid induced immunosuppression studies investigated the role of endogenous opioids such as leucine (leu) or methionine (met) enkephalin. It was observed that in vitro administration of endogenous opioid peptides such as leu- and met-enkephalin resulted in inhibition of phagocytosis by murine macrophages of opsonized sheep red blood cells (Casellas et al., 1991). When the field expanded to examine the role of exogenous opioids in this process, findings showed that morphine treatment also led to inhibition of macrophage function. Murine peritoneal macrophages undergoing morphine treatment exhibited suppression of phagocytosis, respiratory burst activity (Menzebach et al., 2004) while in human monocytes morphine has been implicated to inhibit and chemotaxis (Perez-Castrillon et al., 1992). By preventing bacterial internalization morphine leads to inadequate bacterial clearance. Furthermore, through inhibition of respiratory burst activity, morphine attenuates bacterial killing which together with diminished phagocytosis leads to increased bacteremia and bacterial escape from latency in mice (Bhaskaran et al., 2001; Wang et al., 2005; Lugo-Chinchilla et al., 2006). Human studies and rodent models of drug abuse indicate that morphine impairs the host’s ability to eradicate infection by inhibiting phagocytosis. More specifically, in vitro studies have indicated that morphine inhibits Fcγ receptor (FcgR)-mediated phagocytosis which is essential for internalization of extracellular pathogens (Szabo et al., 1993; Tomassini et al., 2004). Literature reports that inhibition of phagocytosis occurs through a classical opioid receptor pathway. In vivo studies of morphine abuse indicate that morphine inhibits the phagocytic ability of non-elicited and elicited macrophages in a naltrexone reversible manner (Rojavin et al., 1993). Furthermore, in vitro studies have confirmed that morphine acts directly through the μ and δ opioid receptors (Szabo et al., 1993; Tomassini et al., 2004) and that morphine mediated inhibition of phagocytosis is abolished in macrophages from MORKO mice, adding further evidence for the role of the MOR in these processes (Roy et al., 1998a).
In addition to inhibiting macrophage phagocytosis, several studies report that morphine attenuates bacterial killing in mice as evident by increased bacterial loads or sepsis (Hilburger et al., 1997). Several microbicidal mechanisms are utilized by macrophages, namely, release of nitric oxide and superoxide intermediates. In mice, chronic morphine has been implicated in modulation of bacterial killing by inhibition of NO release (Menzebach et al., 2004; Bhaskaran et al., 2007). Studies indicate that in rodent models, chronic morphine through inhibition of NO release increases the susceptibility to bacterial infection, resulting in bacteremia, bacterial dissemination and bacterial invasion of the CNS (Wang et al., 2005; Asakura et al., 2006; Bhaskaran et al., 2007). A recent study (Singh and Singal, 2007) notes that morphine administration has a dose-dependent biphasic modulation in Leishmania donovani infected mice and peritoneal macrophages in vitro, via a NO-dependent mechanism. These studies demonstrate that morphine administration in the nanomolar range was protective against L.donovani infection, while morphine concentrations in the micromolar range led to augmented parasite growth in macrophages. Furthermore, micromolar concentrations of morphine has been implicated in the inhibition of superoxide production, however, the mechanisms underlying the protective effects with nanomolar doses are not clear. Additional studies investigating morphine’s effect on infection examined superoxide release as a mechanism of bacterial killing, where it was observed that morphine inhibits superoxide production in neutrophils and macrophages (Sharp et al., 1985; Simpkins et al., 1986; Welters et al., 2000; Welters et al., 2000). These studies were reproduced in human peripheral mononuclear cells, conducted by Peterson et al., which examined respiratory burst activity in response to phorbol myristate acetate (PMA) (Peterson et al., 1987; Peterson et al., 1989).
Along with inhibition of bacterial clearance (Grimm et al., 1998a), morphine treatment leads to inhibition of macrophage recruitment and function following invasion of pathogens. A study carried out by Grimm et al. (1998) showed a significant decrease in macrophage chemotaxis when cells were preincubated with morphine, or met-enkephalin (Grimm et al., 1998a). They concluded that inhibition of subsequent macrophage chemotaxis by morphine occurs upon direct binding of morphine to MOR on macrophage membranes, and that this activation of MOR leads to the phosphorylation and desensitization of chemokine receptors CCR1, CCR2, CXCR1 and CXCR2. The desensitized chemokine receptors are then unable to elicit a response when their ligands are present. In the presence of the endotoxin lipopolysaccharide (LPS), suppression of cytokines IL-6 and TNF-α was seen following morphine treatment (Roy et al., 1998c). The transcription factor NF-κB, responsible for up-regulation of several cytokines including IL-6, TNF-α, NO and IL-10, was also suppressed following morphine treatment. Additionally, murine alveolar macrophages undergoing in vitro morphine treatment show significantly reduced release of macrophage inflammatory protein-2 (MIP-2) and NF-κB-dependent gene transcription following Streptococcus pneumoniae infection. Further studies demonstrated that morphine treatment impairs TLR9 mediated NF-κB signaling leading to diminished bacterial clearance in mice during early stages of S. pneumoniae infection (Wang et al., 2008a). Modulation of TLR expression and activation following morphine treatment will be discussed in section 3.4 as potential mechanism for this effect.
1.2 Neutrophils
Neutrophils, like macrophages are key phagocytes of the innate immune system. Their recruitment, as well as their phagocytic and bactericidal abilities is essential for adequate elimination of invading pathogens.
Early studies of opioid modulation of neutrophil function indicate that both exogenous and endogenous opioids inhibit neutrophil bactericidal function. Endogenous opioids had similar inhibitory effects on human polymorphonuclear leukocytes, where pretreatment with endogenous opioid peptides leu- or met-enkephalins reduced neutrophil’s ability to generate superoxide production in response to the Escherichia coli product, N-formyl methionyl leucyl phenylalanine (FMLP) (Sharp et al., 1985; Simpkins et al., 1986). Inhibition of superoxide production in neutrophils, as seen in macrophages, can lead to inadequate bacterial clearance and thereby increased bacterial persistence in the host. Although it has been observed for some time that chronically administered morphine modulates neutrophil chemotaxis and function, controversy still exists in determining which mechanisms are at play. A growing body of literature supports morphine’s suppressive effects on recruitment and activation of neutrophils during an innate immune response. Exogenous opioid treatment of human blood neutrophils leads to inhibition of IL-8 production (Glattard et al., 2010), IL-8 receptor expression (Yossuck et al., 2008) as well as IL-8-induced chemotaxis (Grimm et al., 1998b). Similar to human models, murine models of opioid abuse show dose dependent inhibition of neutrophil migration into peri-incisional tissue as determined by myeloperoxidase (MPO) assay and immunohistochemistry (Clark et al., 2007). We recently demonstrated in a murine wound healing model that chronic morphine treatment resulted in a significant delay and reduction in both neutrophil and macrophage recruitment to wound sites (Martin et al., 2010b). The delay and reduction in neutrophil recruitment was attributed to altered early expression of keratinocyte derived cytokine (KC) and was independent of MIP-2 expression, whereas suppression of macrophage infiltration was attributed to suppressed levels of the potent macrophage chemoattractant, monocyte chemotactic protein-1 (MCP-1). In more recent murine studies we reported that morphine treatment causes a decrease in S. pneumoniae induced IL-23/IL-17 production by macrophages, leading to delayed and reduced neutrophil migration, resulting in severe lung infection and the initiation of systemic infection (Ma et al., 2010) with similar effects in dendritic cells (discussed below).
Interestingly, unlike exogenous opioids, endogenous opioids enhance neutrophil migration through an increase in human neutrophil chemotaxis following endogenous opioid (β-endorphin) treatment (Simpkins et al., 1984). One explanation for the differences between the effects of endogenous and exogenous opioids on neutrophil recruitment may in part be explained by the differences in affinity of morphine and β-endorphins for the mu opioid receptors on immune cells.
Taken together, there is strong evidence that morphine modulation of neutrophil bactericidal ability via inhibition of superoxide formation, as well as inhibition of recruitment to the site of infection compromises bacterial clearance resulting in greater bacterial dissemination. Therefore further study on the underlying mechanisms involved in opioid induced inhibition of neutrophil function is essential for our understanding of opioid mediated immune suppression.
1.3 Dendritic Cells
Dendritic cells (DCs) play a central role in the initiation and control of the adaptive immune response. Dendritic cells link the innate to the adaptive immune response by detecting, capturing and presenting foreign antigens to T cells (Banchereau and Steinman, 1998). Although dendritic cells play an important role in the innate and adaptive immune system, few studies have examined how morphine modulates their function. Recent findings have shown that morphine inhibits IL-23 production by murine dendritic cells. Using a well-established murine in vitro S. pneumoniae infection model, we demonstrated that morphine impaired S. pneumoniae induced IL-23 production through MyD88-IRAK1/4-dependent TLR2 and Nod2 signaling in DCs (Wang et al., 2011). Implications of IL-23 inhibition are significant in that disruption of the IL-23/IL-17 axis lead to suppression of antimicrobial proteins and further potentiated morphine anti-inflammatory functions (Wang et al., 2011).
1.4 Natural Killer Cells
Natural killer (NK) cells are specialized cytotoxic lymphocytes and are a major component of the innate immune system. Although their role in intracellular pathogen clearance is invaluable, present knowledge in the field on how morphine modulates their function is scarce.
The current body of literature indicates that morphine’s suppression of NK cell activity does not occur as a result of direct interactions with NK cells, but rather as a consequence of opioid receptor activation in the central nervous system (Saurer et al., 2006a). Specifically, in rodent models, mu opioid receptors in the periaqueductal gray matter (PAG) of the mesencephalon have been shown to mediate morphine’s effect (Weber and Pert, 1989; Carr et al., 1993; Lysle et al., 1996; Nelson et al., 2000). Morphine modulation of NK cell activity seems to vary based on the administered dose. At high doses morphine has been shown to inhibit NK cell activity (Yokota et al., 2004; Saurer et al., 2006a; Saurer et al., 2006b). For example, a single administration of morphine in the periaqueductal gray matter (Weber and Pert, 1989), or intrathecal administration (Yokota et al., 2004) diminishes the activity of NK cells in the periphery (spleen or blood). Interestingly, lower doses of morphine have been shown to enhance NK cell cytotoxicity. Similarly, in catheterized Pietrain crossbred pigs, morphine treatment induced a dose- and time-dependent increase in NK cell cytotoxicity in a naltrexone reversible manner. A 0.5 mg/kg (low dose) morphine treatment evoked a four-fold increase in porcine NK cell cytotoxicity compared to saline control, without eliciting changes in the number of circulating large granular lymphocytes or NK cells (Borman et al., 2009), indicating that enhancement of NK cell cytotoxicity occurs independently of NK cell recirculation.
These types of discrepancies are not uncommon in the field of opioid mediated immune suppression, and could be attributed to the potential biphasic effect of morphine on NK cell activity, or simply due to the duration of opioid treatments (acute vs chronic).
1.5 Mast Cells
Studies examining morphine’s effect on mast cell-deficient and reconstituted mice (C57BL6/J and Swiss–Webster), show that resident mast cells mediate selective immunosuppression in the peritoneal cavity following morphine treatment. These experiments (Madera-Salcedo et al., 2011) demonstrate that that acute morphine prevents TNF-α but not CCL2 release after LPS challenge in both strains and that its inhibitory action is mediated by a direct effect on resident intraperitoneal mast cells. These findings were confirmed when the suppressive effects of morphine were restored in animals reconstituted with mast cells, in which the ability to release TNF-α was also repaired. This result strongly suggests that mast cells are the target of the inhibitory actions of morphine and that a negative crosstalk between opioid receptor and TLR-4 signaling pathways occurs in resident peritoneal mast cells (Madera-Salcedo et al., 2011). Interestingly, morphine modulation of mast cell functions seems also to vary in different models. While low doses of morphine (5 mg/kg of body weight) attenuated pain in all the investigated strains of mice, only the high dose of morphine (20mg/kg of body weight) additionally inhibited the early stages of i.p. accumulation of murine exudatory leukocytes in most strains, namely in C57C3H, SWISS, Balb/c, C57BL/6 strain, but not in the CBA strain (Plytycz and Natorska, 2002; Stankiewicz et al., 2004). The authors went on to explore these differences and concluded that morphine enhances histamine release by peritoneal mast cells from CBA mice, contrasting results from SWISS mice where it suppresses histamine release. Inflammation in CBA mouse peritonitis model was exacerbated by morphine induced pro-inflammatory effects; however all other strains examined, supported the concept that morphine let to an overall immunosuppression of mast cell function (Stankiewicz et al., 2004).
Additionally, mast cells have been implicated to play a role in morphine modulation of gut permeability. Harari et al (2006), conducted studies on distal ileal mucosa, where mucosal exposure of intact and isolated ilia to FMLP led to an increase in permeability to dextran, which was completely ablated by morphine. Ilea of mast cell-deficient mice were unresponsive to FMLP compared to their wild type littermates. Therefore the authors concluded that mucosal mast cells play a central role in morphine mediated antagonism of the provocative effect of FMLP on the mucosal barrier to dextran of the ilea of rats and mice (Harari et al., 2006). By inhibiting mast cell functions such as TNF-α production, morphine inhibited permeability of distal ileal mucosa following FMLP stimulation. These findings describe morphine as a profound cause of mast cell dysfunction and their role in modulation of gut permeability.
Summary
In this section we have summarized recent findings examining multiple ways by which morphine modulates the cells of the innate immune system (Table 1). Taken together, the complexity by which morphine acts as a suppressor of recruitment and functional activity of innate immune responders proves detrimental to the hosts’ ability to eradicate pathogens. Investigation of the modulation of innate immune function by morphine has generated some controversy throughout the years. In spite of contradictory responses by a few mouse strains, or variance in opioid doses used, overwhelming evidence supports the inhibitory role of chronic morphine on innate immune function.
Table 1.
Morphine modulation of innate and adaptive immune system

|
Considering the prevalence of opioid use today, consequences of morphine’s inhibition of innate immune function are more relevant than ever. Although great strides have been made in this field, mechanisms by which morphine modulates cells of the innate immune system still require further investigation.
2. MORPHINE MODULATION OF ADAPTIVE IMMUNE FUNCTION
An extensive body of literature, including ours, detailing the immunomodulatory effects of morphine on the components of the adaptive immune system has been published. Early rodent studies showed that morphine induced a decrease in splenic and thymic weight, leading to impaired T cell function (Bryant et al., 1988; Bryant et al., 1991), Additionally, altered cytokine expression, suppressed T cell apoptosis and modified T cell differentiation have been described as a hallmark for morphine mediated immunomodulation (Roy et al., 2001; Wang et al., 2001). Like innate immune cells, T and B cells are known to express the MOR (Beagles et al., 2004; Borner et al., 2009), which has been shown to be inducible by TNFα and IL-4 in T lymphocytes (Borner et al., 2009). Morphine, understandably, has been shown to have direct effects on all major aspects of adaptive immune response, including antigen presentation, T cell activation/differentiation and lymphocyte recirculation in both acute and chronic treatment conditions. In most cases, the effects observed were different in the two treatment conditions. Effects of acute morphine treatment are comparable to morphine withdrawal, while chronic morphine treatment seems to have opposite results. These differences will be discussed in detail in section 7 of this review (Flores et al., 1995; Fecho and Lysle, 1999; Donahoe et al., 2001; Mellon et al., 2001; Wang et al., 2001; Qian et al., 2005; Nugent et al., 2011).
2.1 Antigen Presentation
The professional antigen presenting cells (APCs) serve as a link between the innate and adaptive immune systems. The immunomodulatory effects of morphine on macrophages, monocytes and DCs have been well documented (Wang et al., 2008a), mostly in the context of innate immunity and have been covered in the previous section of this review. In terms of the essential function of the APCs in T cell activation, recent information highlights the role of B cells and acute morphine mediated impaired antigen presentation in these cells (Beagles et al., 2004; Nugent et al., 2011). This becomes important, since a coordinated antigen presentation by the B cells and DCs is an absolute necessity for optimal CD4+ T cell activation (Kleindienst and Brocker, 2005). Antigen presentation by DCs is sufficient for activation of the CD4+ T cells, B cell antigen presentation has been shown to be essential for CD4+ T cell proliferation and clonal expansion (Kleindienst and Brocker, 2005). In this context, the major histocompatibility complex, class II (MHC-II) has emerged as an essential component of antigen presentation. In a recent study, rats treated with morphine (20 mg/kg b.w.) resulted in approximately four-fold reduction in MHC-II levels in terms of message and surface expression within 2 hours, specifically in the B cells (Beagles et al., 2004). Additionally, mice lacking MHC-II presented with coordinated loss of the CD4+ T lymphocytes in thymus and spleen (Madsen et al., 1999). B cell specific MHC-II knockout mice showed impaired T cell proliferation and expansion, coupled with altered levels of TH1 and TH2 cytokines (Crawford et al., 2006). In all these cases, interestingly, the defects in T cell activation, expansion and proliferation, were mainly attributed to impaired MHC-II expression and could be mirrored with morphine treatment. It has been shown that as little as 10 mg/kg b.w. morphine treatment reduced the MHC-II expression on the circulating B cells by almost 33%, which cannot be rescued upon IL-4 administration (Nugent et al., 2011). However, it remains an open argument whether morphine is directly modulating the APCs or indirectly by mediating effects on the central HPA axis. Some studies implicate a central HPA axis mediated changes in antigen presentation, where direct corticosterone treatment was able to reduce MHC-II expression on APCs, irreversible upon stimulation with IL-4 (Schwab et al., 2005; Nugent et al., 2011). In summary, while the effects of morphine on antigen presentation is clear (Wang et al., 2008b), more work is needed to delineate the exact mechanism involved, and to identify the primary target of morphine in these cells or in the central HPA and/or the sympathetic nervous system (if any).
2.2 B and T Cell Dynamics
The findings on the effects of morphine on the two major adaptive immunity cell types, namely B and T cells have been contrasting. While there is evidence of morphine causing direct effects on the various aspects of T cell biology, its effects on B cells have been found to be limited at best. One of the earliest B cell studies reported the reduction in the numbers of antibody-producing cells in mice spleen upon acute morphine administration (Lefkowitz and Chiang, 1975). Similar phenomena was observed in a chronic morphine mouse model where, apart from severe splenic and thymic weight reduction, B cell proliferation in B6C3F1 mice (Bhargava et al., 1994) and antibody response in C3H/HeJ and C57Bl6 mice was found to be suppressed, but not in MOR deficient CxBk/ByJ (Bussiere et al., 1992). Interestingly, however, it was soon discovered that these effects of morphine were reversible upon exogenous administration of macrophage derived IL-1, IL-6 or IFN-γ (Bussiere et al., 1993). Arguably, morphine was seen to effect the cytokine expression and phagocytic profile in the macrophages, whereby the 1μM concentration was demonstrated to be sufficient to bring 41% and 40% reduction in the phagocytic index (average C.albicans cells per macrophage), both in vitro and in vivo respectively (Rojavin, et al, 1993), thereby altering the activation and proliferation profile of the B cells (Weber et al., 1987; Rojavin et al., 1993). In aggregate, literature supports the notion that morphine, through a neuro-immune circuit, depresses the function of macrophages and polymorphonuclear leukocytes, which modulates B cell function, albeit, indirectly (Eisenstein and Hilburger, 1998).
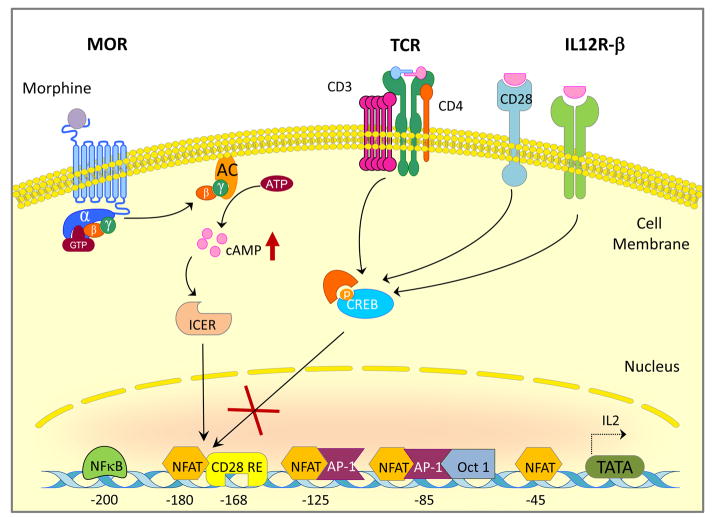
In contrast, morphine mediated immunosuppression of T cells has been relatively well studied and found to be more direct. The potential mechanisms proposed, could be divided into two broad categories: activation/proliferation and differentiation. In the former category, several mouse models have described the morphine-mediated inhibition of IL-2 transcription in activated T lymphocytes (Thomas et al., 1995; Roy et al., 1997; Wang et al., 2003; Wang et al., 2007). In terms of mechanism, it was demonstrated that morphine mediated inhibition of c-fos mRNA (Roy et al., 1997) and modulation of cAMP response element-binding transcription factors CREB/CREM/ICER (Wang et al., 2003; Wang et al., 2007) was primarily responsible for the inhibition of IL-2 expression (Figure 1). In human naïve T cells, similar suppression of IL-2 and its transactivators (AP1, NFAT and NF-κB) was observed and attributed to the pertussis toxin sensitive biphasic hyper-elevation of cAMP (Borner et al., 2009). This is particularly relevant since elevation of cAMP is known to directly inhibit the TCR-activated signaling cascade of T cells via the PKA-Csk-Lck pathway (Mustelin and Tasken, 2003) and indirectly leads to the second mechanistic category, i.e. differentiation. Several studies have focused on the influence of morphine on the T helper lineage bias towards TH2 phenotype (Wang et al., 2003; Roy et al., 2004; Greeneltch et al., 2005; Qian et al., 2005; Roy et al., 2005; Borner et al., 2009). Morphine induced cAMP production has been shown to inhibit TH1 cytokine expression (Borner et al., 2009) and promote IL-4 production in vitro (Roy et al., 2001; Roy et al., 2004; Qian et al., 2005) and in vivo (Greeneltch et al., 2005; Kelschenbach et al., 2005) via multiple mechanisms including cAMP dependent suppression of IFN-γ (Roy et al., 2001; Wang et al., 2003), modulation of the GATA-3/T-bet switch (Figure 2) (Roy et al., 2004) and Fas/Fas ligand dependent activation-induced cell death of the TH1 cells (Greeneltch et al., 2005). Thus, the global effect of morphine on T cells results in a robust inhibition of early pro-inflammatory response to opportunistic infections. The signaling downstream of MOR activation are further described in section 4.
Figure 1. Morphine modulates IL-2 promoter activity.
Binding of Morphine to the mu-opioid receptor results in superactivation of adenylyl cyclase which is mediated by the βγ subunit of heterotrimeric G protein complex. Activation of adenylyl cyclase by the βγ subunit increases intracellular cAMP leading to activation of ICER competing out CREB from the IL-2 promoter, thereby preventing recruitment of NFAT to the CD-28 responsive element.
Figure 2. Morphine skews the lineage bias of CD4+ T cells towards TH2 phenotype.
Morphine leads to superactivation of adenylyl cyclase and increase in intracellular cAMP levels. cAMP activates p38 MAPK, in turn leading to CREB phosphorylation and nuclear translocation. CREB promotes activation of GATA3 and it’s binding to the consensus motifs. GATA3 activation on one hand inhibits activation of T-Bet, inhibiting IFNγ production. On the other hand it enters the positive feedback loop involving IL4 and STAT6 thereby committing itself to the TH2 phenotype.
Summary
Morphine has been shown to suppress the immune system at different stages, starting from innate immune cells through antigen presentation and ending in modulation of the T lymphocyte activation and differentiation. In the realm of adaptive immune response, while there have been conflicting reports on morphine mediated immunomodulation of lymphocyte apoptotic behavior (Suzuki et al., 2003; Ohara et al., 2005), strong evidence exists regarding the modulation of the T cell lineage bias, with its clinical relevance and implications. Although a few in vivo studies initially attributed the altered T cell responsiveness to the global effect of morphine on the HPA axis in rats and rhesus monkeys (Donahoe et al., 2001; Mellon et al., 2001), a consensus seems to be emerging on the TH1 to TH2 switch being responsible for the muted immune response to pathogens across the organisms studied (Figure 2) (Rivera-Amill et al., 2010). This, coupled with defective antigen presentation, contributes to clinically immune-compromised individual’s susceptibility to opportunistic infections.
3. ROLE OF OPIOID RECEPTORS IN MORPHINE MODULATION OF IMMUNE RESPONSE
Modulation of immune function by morphine has been shown to be either direct, via opioid receptors on immune cells, or indirect, when drug exposure takes place in vivo. Indirect effects are mediated by the binding of opioids to classical opioid receptors in the central nervous system (CNS), causing the release of catecholamines and/or steroids, which then affect the functioning of immune cells. When morphine is administered in vivo to mice or humans, numerous complex metabolic pathways lead to the generation of active and inactive metabolites. The two main compounds: morphine-6-glucuronide and morphine-3-glucuronide, have been well described. In most in vitro settings, morphine sulfate is used as a key agonist and any potential cellular metabolism may be cell specific. Studies in human embryonic kidney stem cells investigating the efficacy and potency of morphine and its nine major metabolites show that morphine, morphine-6-glucuronide, normorphine, morphine-6-sulfate, 6-acetylmorphine and 3-acetylmorphine are efficacious receptor activatiors at nanomolar concentrations (Frolich et al., 2011). Weaker metabolites such as: morphine-N-oxide, morphine-3-sulfate, morphine-3-glucuronide and pseudomorphine can activate MOR pathways at micromolar concentrations (Frolich et al., 2011). Other metabolites, such as normorphine, 6-acetylmorphine and morphine-6-glucuronide, exhibit lower potency for G-protein activation but higher potencies and efficacies for β-arrestin recruitment when compared to morphine (Frolich et al., 2011). The existence of such complexities, may account for cell specific signaling and responses. Here we attempt to summarize opioid receptor expression in different immune cells (Table 2) and the associated signal transduction pathways activated following opioid receptor ligation on cells of the immune system. The immunosuppressive effects are unique to morphine, as other MOR ligands such as fentanyl did not inhibit neutrophil and monocyte functional activities.
Table 2.
Opioid Receptor Expression on Immune Cells
| Opioid Receptors Cell Types | μ | κ | Δ | Reference |
|---|---|---|---|---|
| Macropahge/Monocyte | Human monocyte; Murine peritoneal macrophages | Murine peritoneal macrophages | Rat and murine peritoneal macrophages | (Bonnet, et al, 2008) (Stanojevic, et al, 2008) (Sacerdote, 2003) (Tomassini, et al, 2003) |
| Neutrophil | Rat peritoneal lavage neutrophil | Bone marrow neutrophils | Human granulocytes | (Azuma, et al, 2000) (Kulkarni-Narla, et al, 2001) (Rogers, et al, 2000) (Makman, et al, 1995) |
| Dendritic cell (DCs) | Mouse primary DCs; Murine Dcs | Murine DCs | Human and Murine DCs | (Wang, et al, 2011) (Li, et al, 2009) (Kirst, et al, 2002) (Makarenkova, et al, 2001) (Tseng, et al, 2005) |
| Natural killer (NK) cells | Splenic NK cells | Human rheumatoid arthritis NK cell | Splenic NK cell | (Nelson, et al, 2000) (Gunji, et al, 2000) (Boyadjieva, et al, 2004) |
| T cell | Human Jurkat T Cells; CEM ×174 cells | Murine thymocytes | Murine thymocyte | (Kraus, et al, 2010) (Liu, et al, 2010) (Ignatowski, et al, 1998) (Linner, et al, 1995) |
| B cell | CEM ×174 cells | Human B cell; CEM ×174 cells | CEM ×174 cells | (Liu, et al, 2010) (Gunji, et al, 2000) (Suzuki, et al, 2002) |
3.1 Mu-Opioid Receptor on Immune Cells
Three major types of opioid receptors, mu, delta, and kappa, which mediate the pharmacological effects of opioids, have been cloned, and shown to belong to the G protein-coupled receptor superfamily (Mansour et al., 1995). Although primarily expressed in the CNS, expression of functional opioid receptors has been documented in several immune cell types, including human peripheral blood monocytes and neutrophils (Van Epps and Saland, 1984; Ruff et al., 1985; Grimm et al., 1998b) and murine splenocytes (Taub et al., 1991), lymphocytes and macrophages (Bidlack, 2000; Miyagi et al., 2000; McCarthy et al., 2001; Roy et al., 2001; Zhang and Oppenheim, 2005). MOR is considered to be the major molecular target of morphine (Matthes et al., 1996). The existence of mRNA for the MOR was found in rat peritoneal macrophages by reverse transcription with polymerase chain reaction (RT-PCR) (Sedqi et al., 1995; Tomassini et al., 2003; Chang et al., 2007), suggesting regulation of MOR at the transcriptional level. In human TPA-HL-60 cells, which are capable of differentiating into macrophage-like cells by treatment with 12-O-tetradecanoyl-phorbol-13-acetate(TPA), gp120 potentiated LPS-induced up-regulation of MOR expression (Chang et al., 2007).The presence of MOR mRNA in human T- and B-cells, CD4+ T cells, monocytes, macrophages, and granulocytes have also been reported (Chuang et al., 1995).
Activation of MOR by exogenous or endogenous agonists has a pleiotropic action on physiological and immune processes, such as analgesia, respiratory depression, cough suppression, vomiting, constipation, euphoria, addiction and microbial infections (Kieffer and Gaveriaux-Ruff, 2002; Tegeder and Geisslinger, 2004). Additionally, increased MOR expression has been attributed to inflammatory bowel diseases (IBD) through increased migration of circulatory cells to the site of inflammation and/or from enhancement of MOR expression by activated resident mucosal monocytes and T cells (Philippe et al., 2006).
A number of recent studies showed that the MOR can be modulated in both immune cells and neuronal cells by immune-cell derived cytokines. The induction of MOR in primary human T cells and Jurkat cells in response to IL-4 has been reported earlier (Kraus et al., 2001), and has been shown to be mediated through STAT6 and chromatin remodeling (Kraus et al., 2010). Furthermore, treatment of SK-N-SH cells with morphine and interleukin-1beta (IL-1β) produced dual regulation of the mRNA for the human MOR (Mohan et al., 2010).
3.2 Role of Kappa and Delta-Opioid Receptors in Immune Modulation
Kappa-opioid receptor (KOR) has been identified in human and monkey peripheral blood lymphocytes (Chuang et al., 1995), and in immature thymic CD4−, CD8−T-cells. Activation of KOR results in down-regulation of HIV replication in human PBMCs and this effect was, in part, a result of down-regulation of the major HIV-1 coreceptor, CXCR4 (Finley et al., 2011). Activation of KOR suppressed the expression of several proinflammatory cytokines, including IL-1, IL-6, and TNF-α, in primary macrophages and macrophage/monocyte cell lines. Delta-opioid receptor (DOR) transcripts has been detected in monkey peripheral blood mononuclear cells (Chuang et al., 1994). DOR transcripts were found in human T-, B-, and monocyte cell lines and in murine splenocytes (Gaveriaux et al., 1995).
3.3 MOR Mediated Signal Transduction in Immune Cells
Investigations of intracellular signaling molecules upon morphine treatment has been studied in the neuronal cells and more recently begun in immune cells. Our lab has had a long standing interest in T cell function and development. We and others have found that cAMP, a key signaling molecule, increases intracellularly upon chronic morphine treatment in primary mouse T cells and Jurkat T cell lines (Wang et al., 2003; Borner et al., 2007). While chronic morphine treatment differs from accute, chronic morphine treatment of mouse T cells decreases IFN-γ promoter activity and phosphorylation of ERK1/2 and p38 MAPK (Wang et al., 2003). Interleukin-2 (IL-2) is inhibited at both the level of transcription and translation with chronic morphine treatment. IL-2 production is tightly regulated by several transcription factors that bind to the IL-2 promoter. Chronic morphine treatment increased cAMP levels and concurrently up-regulated the inducible cAMP early repressor (ICER)/cAMP response element modulator (CREM), while down-regulating p-cAMP-response element-binding protein (CREB) in activated T cells. ICER competes for p-CREB binding to the cAMP-responsive elements (CREs), thereby, uncoupling CBP/p300 to reduce IL-2 transcription (Figure 1). These effects were further confirmed as over-expression of either antisense CREM or CREB plasmid rescued morphine-induced inhibition of IL-2 promoter activity and protein production (Wang et al., 2007).
Other investigations using primary human T lymphocytes and Jurkat T cells showed that both morphine and its endogenous counterpart, β-endorphin independently inhibit the transactivation of IL-2 transcription through modulation of AP-1, NFAT, and NF-κB in activated human T lymphocytes. The activation of T-Cell Receptor (TCR) induces calcium flux and MAPK activation but is inhibited with opioids. These investigators also reported that T cells treated with opioids results in a marked increase in cAMP which in turn activates protein kinase A (PKA), augments C-terminal Src kinase and enhances leukocyte-specific protein tyrosine kinase (Lck) to block initiation of TCR signaling (Borner et al., 2009).
Similarly, our earlier investigations addressing intracellular mechanisms by which morphine controls CD4+ T cell differentiation demonstrated that in vitro morphine treatment increased IL-4 and GATA-3 mRNA levels, and cAMP concentration through a pertussis toxin-sensitive receptor in CD4+ T cells in a dose-dependent manner. In these studies we also demonstrated that chronic morphine treatment increases anti-CD3/CD28 Ab-induced IL-4 promoter activity and IL-4 protein expression through a p38 MAPK-dependent, but PKA- and Erk1/2-independent signaling pathway (Figure 2) (Roy et al., 2005). In the human B cell line CEMX174, morphine treatment reduces apoptosis and cell infection after short term exposure to simian immunodeficiency virus. In contrast to the observations made in T cells, morphine down regulated CEMX174 B cell cAMP and PKA activity as well as histone H3 phosphorylation to reduce early SIVmac239-induced apoptosis (Hao et al., 2003).
3.4 Morphine Modulation of TLR Signaling
Neutrophils and monocytes express the Toll-Like receptors (TLRs) whose range of ligands includes lipopolysaccharide (LPS), a component of bacterial cell walls. LPS signals the activation of the well studied DNA binding nuclear transcription factor, NF-κB in neutrophils and monocytes through TLRs (Welters et al., 2000). Morphine signals through opioid receptors (naloxone reversible) to induce nitric oxide release and suppress NF-κB mediated transcriptional activation in neutrophils and monocytes derived from human blood (Welters et al., 2000). While no evidence exists for physical interaction between opioid receptors and TLRs, it has been suggested that this effect may be due to downstream intracellular cross talk. Similarly, in vitro studies using human neutrophils show that low concentrations of morphine, acting through the MOR is naloxone reversible and inhibits LPS induced IL-8 release (Glattard et al., 2010). Peptidoglycan (PGN) from Staphylococcus aureus is a specific TLR2 ligand. Morphine, through the MOR, significantly inhibited PGN stimulation of cytokines (TNFα, IL-6) from freshly isolated human monocytes. In contrast to monocytes, morphine significantly inhibited TNF, but not the IL-6 production, in a MOR independent manner in PGN-stimulated peripheral blood mononuclear cells (PBMC) (Bonnet et al., 2008). Thus, it is not clear how morphine modulates TLR signaling based on the differential effect.
In vivo, using a murine incisional model, acute morphine administration (0.1–10 mg/kg b.w) reduced incision related hind paw allodynia, stimulated levels of IL-1 beta, IL-6, TNFα, granulocyte colony stimulating factor (G-CSF) and keratinocyte-derived cytokine (KC). Interestingly, these studies also showed that morphine dose-dependently suppressed the infiltration of neutrophils into incisional sites (Clark et al., 2007). Further studies are need to establish the modulation of TLR signaling being responsible for the defects observed. In our own studies, intranasal inoculation of mice with Streptococcus pneumoniae increased TNF-α, IL-1, IL-6, MIP-2, and KC in broncho-alveolar lavage (BAL) fluids and lung tissue prior to neutrophil infiltration. Chronic morphine treatment significantly decreased cytokine induction, NF-κB activation in lung resident cells, as well disrupted accumulation of galectin-3 in the alveolar space of Streptococcus infected lungs (Wang et al., 2005). These effects were mediated through modulation of TLR signaling pathways since blocking TLR receptors attenuated morphine’s effect (Wang et al., 2005). Similar modulation of TLR2 signaling by morphine is also observed in a study by (Li et al., 2009). This study showed that overexpression of TLR2 caused an increase in apoptosis following morphine treatment. Furthermore, inhibition of MyD88 or overexpression of β-arrestin 2 in TLR2/HEK293 cells attenuated morphine-induced apoptosis. The authors concluded that TLR2 signaling mediates the morphine-induced apoptosis and β-arrestin 2 acts as a negative regulator in morphine-induced, TLR2-mediated apoptosis.
3.5 Morphine Modulation of Chemokine Receptor Function
As described above morphine primarily signals through the MOR, and its interaction with chemokine receptors has been a topic of great interest. Morphine, through the MOR, has been shown to induce the phosphorylation of chemokine receptors CXCR1 and CXCR2, independent of chemokine receptor ligand binding or internalization in neutrophils and monocytes (Grimm et al., 1998a). Similar to MOR, many of the chemokine receptors are G-protein-coupled receptors (GPCRs). Opioid receptor mediated heterologous desensitization of chemokine receptors have been implicated as the key mechanism for inhibition of chemokine-induced chemotaxis in neutrophils and monocytes (Grimm et al., 1998b). Early studies using human neutrophils showed that both morphine and met-enkephalin, can inhibit IL-8-induced chemotaxis of human neutrophils (Grimm et al., 1998b). These studies also reported that morphine and met-enkephalin inhibited macrophage inflammatory protein (MIP)-1α, regulated upon activation, normal T expressed and secreted (RANTES), and MCP 1, but not MIP-1β induced chemotaxis of human monocytes (Grimm et al., 1998b). Studies in human CEM ×174 and monkey lymphocytes showed that CCR5 co-precipitated with all three subtypes (mu, delta, and kappa) of opioid receptors and was localized in close proximity on the cell membrane (Suzuki et al., 2002). Other investigators have proposed the MOR induces cross-desensitization of CCR5, through the activation of PKCζ and phosphoinositol-dependent kinase-1 (PDK1) (Song et al., 2011).
Summary
Morphine through the MOR, DOR and KOR modulates many aspects of immune cell function. These include immuno-suppressive effects of immune cell cytokine release, chemokine receptor activation and cell migration. Depending on the morphine treatment (acute or chronic) or concentration, the primary intracellular effectors, identified to date, following opioid receptor binding include modulation of adenylate cyclase, activation or inhibition of stress activated protein kinases, decrease in calcium flux and recruitment of β-arrestin mediated signaling thus resulting in activation or inhibition of various cellular and nuclear events. Studies investigating morphine-MOR interaction with TLRs and chemokine receptors strengthened the postulated role of opiates as immuno-modulators. The paucity of data surrounding immune cell signaling after opioid receptor activation warrants future research. More research is necessary to form a clearer understanding of the cellular and molecular targets of opioid action, particularly within the immune system.
4. MORPHINE AND SUSCEPTIBILITY TO INFECTION
As described above, there is a wealth of evidence supporting the conclusion that chronic opioid drug abuse and use affects both innate and adaptive immunity contributing to increased host susceptibility to microbial pathogens. Global estimates of injection drug users (IDUs) range from 11 to 21 million with over 10 million (70 %) living in underdeveloped nations (Aceijas et al., 2004). Opportunistic infections have historically been recognized as one of the most serious complications of drug abuse (Hussey and Katz, 1950; Scheidegger and Zimmerli, 1989). Epidemiological studies clearly demonstrate that IDU’s are susceptible to pulmonary, endovascular, skin and soft tissue, bone and joint, and sexually transmitted infections caused by a wide range of bacterial, viral, fungal and protozoan pathogens (Levine & Brown, 2005). In addition, the increased prevalance for parenterally acquired infections such as human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), tetanus and malaria are also significantly greater in this population. It was reported as early as 1987, that higher risk of HIV infection was associated with opioid addiction (Alme et al., 1987; Robinson et al., 1987). Cumulative studies from the past 30 years further establish the correlation between intravenous drug abuse and HIV infection(Kelschenbach et al., 2008; Topp et al., 2008; Wiesli et al., 2006). In addition, the following studies have shown that opioid based drugs may intrinsically promote the pathogenesis of HIV by directly modulating immune function or by directly modifying the CNS response to HIV: Co-treatment of human peripheral blood mononuclear cells (PBMC) with HIV-1 gp120/morphine synergistically induces apoptosis in PBMC (Moorman et al., 2009). HIV-1 patients, who abuse opioid-based drugs, including heroin and morphine, are at a higher risk of developing HIV induced dementia (Turchan-Cholewo et al., 2008). Molecular changes caused by HIV infection and several drugs of abuse may affect different aspects of adult hippocampal neurogenesis (Venkatesan et al., 2007). Morphine can exacerbates HIV-1 viral protein gp120 induced modulation of chemokine gene expression in human brain-derived astrocytoma cells, causing neurotoxicity (Mahajan et al., 2005b).
4.1 Morphine and Viral Infections
Clinical and epidemiologic studies report high incidence of HIV infection in heroin users. These subjects not only demonstrate a greater risk of HIV associated neurological complications but also have accelerated progression of Acquired Immune Deficiency Syndrome (AIDS). However, the molecular mechanism(s) which delineate the complex interactions between opioids and HIV neurotoxicity remain unknown. Opioids can modulate HIV associated neuropathogenesis by regulating pathogen entry in the immune cells, influencing the trafficking of the infected immune cells and viral transmission in CNS. In recent years, emerging studies have investigated the mechanisms through which opioid abuse increases patient susceptibility to HIV infection. The major HIV-1 co-receptors, CCR5 and CXCR4 cooperate sequentially with CD4 to facilitate virus entry into target cells. The tropism of HIV is predominantly determined by the sequential interaction of the surface envelope protein gp120 with these receptors and CD4 (Berger et al., 1999). A potential mechanism for increased susceptibility is provided by studies that show DAMGO, a selective MOR agonist, increases CXCR4 and CCR5 expression in both CD3 (+) lymphoblasts and CD14 (+) monocytes by three to five fold (Guo et al., 2002; Wang et al., 2005). Furthermore, DAMGO-induced elevation of HIV-1 co-receptor expression leads to enhanced replication of both X4 and R5 viral strains of HIV-1. By enhancing CXCR4 and CCR5 expression, opioids increase both X4 and R5 HIV-1 infection (Steele et al., 2003). In addition, several independent studies have shown that morphine and other MOR agonists exacerbates the expression of these receptors (CXCR4 and CCR5) in glia cells (Miyagi et al., 2000; Suzuki et al., 2002; Steele et al., 2003; Mahajan et al., 2005a; Mahajan et al., 2005b; Burbassi et al., 2008; Happel et al., 2008; Bokhari et al., 2009). Presently, it is unclear by which mechanism morphine induces these chemokine receptors, however modulation of cytokines (IL-12 and TNF alpha) which are known to stimulate chemokine receptor expression or, alternatively, inhibition of synthesis of certain chemokines (e.g., RANTES, macrophage inflammatory protein MIP–1a (CCL3), or MIP- 1b (CCL4) that cause internalization of receptors have been postulated as potential mechanisms (Hu et al., 2000).
Various reports document that chronic morphine not only upregulates HIV entry into immune cells but also enhances activation and viral replication in human, and simian PBMCs (Peterson et al., 1990; Suzuki et al., 2002; Kumar et al., 2004). HIV-1 replication in microglial cells has been reported in brain autopsy studies, where HIV-infected opioid users showed severe HIV encephalitis (Anthony et al., 2005). The molecular mechanisms involved in opioid induced HIV replication could be modulated by the inhibition of anti-HIV microRNA (miRNA-28, 125b, 150, and 382) expression (Wang et al., 2011). In addition, the role of Toll-like receptors (TLRs) in viral replication in HIV infection has been widely investigated (Equils et al., 2001; Bafica et al., 2004a; Bafica et al., 2004b; Brichacek et al., 2010). Enhanced TLR2 expression on the surface of monocytes from HIV-infected patients has been reported (Heggelund et al., 2004). Any upregulation of TLR expression in HIV patients by opioids can further induce viral infection, since morphine triggers microglia activation through TLR2 (Zhang et al., 2011). In neurons, on the other hand, TLR1, -3, -6, -7, and -8 were found to be expressed at relatively low levels, TLR2 and -4 at intermediate levels, and TLR5 and -9 at higher levels (Ma et al., 2006; Tang et al., 2007).
Besides HIV infection, mounting evidence also implicates the high prevalence of other virus infections like viral hepatitis A, B, and C, in opiate abusers (Roy et al., 2006). Opioids such as morphine have been shown to enhance HCV replication in HCV replicon-containing cells (Li et al., 2003). Meanwhile, suppressed production of IFN-γ in mice by morphine may also contribute to higher risks of hepatitis virus infection (Moore and Dusheiko, 2005). The effect of morphine on prevalence of HCV infections is the focus of another chapter in this issue and will not be expanded in this review.
4.2 Morphine and Bacterial Infections
In addition to viral infections, opioid abuse is correlated with high risk of bacterial infections as well. Numerous clinical studies demonstrate that opiate abuse-induced immunosuppression contributes to increased risk of various bacterial infections such as skin soft-tissue, respiratory tract, endovascular and musculoskeletal infections (Wang et al., 2008b). Injection drug users often suffer from severe soft tissue infections caused by S. aureus and group A Streptococcus (Guarner et al., 2006). Drug users are also more susceptible to respiratory tract infection caused by S. pneumoniae, S. aureus, K. pneumoniae, Mycobacterium, Haemophilus and many other pathogens (Hind, 1990; Perlman et al., 1995). Injection drug users are easily infected by Staphylococcus aureus, Streptococcus, and Pseudomonas, which may lead to severe conditions such as infective endocarditis, septic thrombophlebitis, mycotic aneurysms, and sepsis (McIlroy et al., 1989; Tomei and Renaud, 1997).
Meanwhile, a large amount of animal studies have been conducted to understand the effects of opiate abuse on susceptibility to infection. Consistent with the clinical observation, opiate treatment leads to increased susceptibility and mortality of mice to S. pneumoniae (Wang et al., 2005; Wang et al., 2008a) and K. pneumoniae infection (Tubaro E, 1987). There are studies showing that morphine treatment can diminish bacterial clearance following S. pneumoniae infection in resident macrophages through impairing TLR9-NF-κB signaling (Wang et al., 2008a). Moreover, morphine treatment has been shown to cause a dysfunction in IL-23-producing dendritic cells and macrophages and IL-17-producing γδT lymphocytes in response to S. pneumoniae lung infection, which leads to subdued release of antimicrobial S100A8/A9 proteins, compromised neutrophil recruitment, and more severe infection. In addition, other studies demonstrated that mice implanted with morphine pellets are sensitized to oral Salmonella enterica, serovar Typhimurium acute infection, where potentiating effect is dependent on the MOR (Feng et al., 2006a; Breslow et al.). Interestingly, some studies also indicated that morphine treatment alone results in a significant increase in the concentration of serum endotoxin and may be able to induce sepsis in mice, which suggests that morphine can modulate the gut-associated immune system (Yukioka et al., 1987). In other animal experimental models, morphine has been shown not only to induce the degradation of the host defense barrier but also synergize with LPS and augment the secretion of both IL-6 and TNF-α (Roy et al., 1998c; Ohara et al., 2005; Ocasio et al., 2004).
4.3 Morphine, Fungal and Parasitic Infections
Opiate abuse also modulates the immune responses to fungal and parasite infections. It was found that exposure of mice to morphine increases lethality to the fungus, Candida albicans (Haverkos and Lange, 1990; Tubaro E, 1987) and depresses phagocytosis and killing of Candida by isolated macrophages and polymorphonuclear leukocytes. Similar, sensitization is also demonstrated with the parasite, Toxoplasma gondi (Chao et al., 1990).
Summary
Based on the studies illustrated above, it is clear that opiate abuse is closely related to increased susceptibility to a variety of infections by interfering with pathogen replication and hosts’ immune responses to infections (Table 3). Therefore, better understanding of the correlation between opiate abuse and susceptibility to infection may provide us with the potential targets for development of new therapeutic strategies in the context of infectious disease.
Table 3.
Morphine enhances susceptibility to infection
| Pathogen | Species | Effects of opioids | References | |
|---|---|---|---|---|
| Virus | HIV | Human | Steele, Henderson et al. 2003 | |
| Enhance virus replication; | Wang, Ye et al. ; Guo, Li et al. 2002 | |||
| Promote virus infection; | Wang, Barke et al. 2003; Wang, Douglas et al. 2006 | |||
| Hepatitis virus | Human | Impair anti-virus mechanism | Moore and Dusheiko 2005 | |
| Li, Zhang et al. 2003 | ||||
| Bacteria | Haemophilus | Human | Guarner, Bartlett et al. 2006; | |
| K. pneumoniae | Human/Mice | Hind 1990; Perlman, Salomon et al. 1995 | ||
| Mycobacterium | Human | Impair clearance mechanism | McIlroy, Reddy et al. 1989; Ting and Cheng 1997 | |
| Pseudomonas | Human | Impair defense barrier | Wang, Barke et al. 2005; Wang, Barke et al. 2008 | |
| S. aureus | Human | Regulate cytokine production | Tubaro, Borelli et al. 1983 | |
| S. pneumoniae | Human/Mice | Breslow, Feng et al. ; Feng, Rahim et al. 2006 | ||
| Streptococcus | Human | Roy, Cain et al. 1998 | ||
| Salmonella enterica | Mice | Ocasio, Jiang et al. 2004 | ||
| Fungus | Candida | In vitro | Impair clearance mechanism | Tubaro E 1987 |
| Parasite | Toxoplasma gondi | Mice | Increases lethality to infection | Chao, Sharp et al. 1990 |
5. OPIOID WITHDRAWAL AND IMMUNE MODULATION
It is well established that morphine addiction leads to immunosuppression and therefore, causes different infectious diseases. Morphine abuse also leads to development of tolerance and physical dependence, which is well characterized by withdrawal syndromes when morphine is terminated. Morphine withdrawal could be either abrupt (abrupt termination of morphine abuse) (Rahim et al., 2002) or precipitated (termination of drug effect by the use of morphine antagonists like Naloxone) (Bhargava et al., 1994). Morphine withdrawal is characterized by behavioral manifestations like teeth chattering, tremors, ptosis, wet dog shakes and diarrhea in rats (Blasig et al., 1973). Morphine withdrawal (both abrupt and precipitated) has been shown to have very strong immunosuppressive effects, where the mechanisms mediating immunosuppression are largely unknown.
5.1 Morphine Withdrawal-Induced Immunosuppression
Immune modulation by morphine withdrawal has recently been widely investigation, yet there are very few reports on the effects of morphine withdrawal on the immune response. Dr. Eisenstein’s group has extensively studied the effect of morphine withdrawal on immune functions. As seen by splenic plaque forming cell (PFC) formation, mice undergoing abrupt and precipitated withdrawal showed different early response, but 24h post pellet removal, both showed almost 80% suppression (Rahim et al., 2002), as well as suppressed splenic macrophage functions (Rahim et al., 2003). This deficit in macrophage function was not due to a change in population of different immune cells but rather due to decreased cytokine production by splenic macrophage populations. Other studies from the same group demonstrated that morphine withdrawn mice, when administered a sub-lethal dose of LPS exhibited a decrease in IL-12 production and 100% mortality (Feng et al., 2005). Abrupt morphine withdrawal decreased LPS induced IL-12p40 production in addition to other cytokines including splenic TNFα (Rahim et al., 2003). Decreased splenic cytotoxic T cell activity (Kelschenbach et al., 2008), B cell proliferation and IL-2 production (Bhargava et al., 1994), suppressed splenic T cell proliferation, decreased IFNγ production, as well as the increased serum TNFα levels were also observed after abrupt morphine withdrawal (West et al., 1999).. Abrupt morphine withdrawal-induced immunosuppression was mediated by activation of population of suppressor macrophages and suppressor B cells (Rahim et al., 2005), decreased IL-12p40 message levels, as well as decreased IFN-γ suppressed macrophage function (Rahim et al., 2003; Rahim et al., 2004; Feng et al., 2005; Rahim et al., 2005).
Macrophages and other APCs produce potent stimulatory cytokines including IL-1β and IL-12 which activate T- and B-cells. Morphine withdrawal has been shown to suppress these cytokines. Morphine withdrawal suppressed LPS-induced IL-12p40 expression thereby skewing T cells towards TH-2 polarization (Kelschenbach et al., 2005). In mice, the message and protein levels of TNF-α were suppressed in morphine withdrawn animals. Decreased levels of IFN-γ were also seen in these mice (Rahim et al., 2003). These decreased cytokine levels were probably responsible for the deficit in macrophage activity in mice undergoing morphine withdrawal. Recent reports from our lab have consistently shown that morphine withdrawal suppresses LPS-induced IL-12p40 expression. This reduced expression was controlled at the transcriptional level where transcription factors like NF-κB, AP-1 and C/EBP binding to consensus binding sequence in IL-12p40 promoter was greatly reduced. This effect was abrogated in MOR knock-out (MORKO) mice clearly indicating involvement of the classical opioid receptor pathway (Subhas et al., 2011).
5.2 Effects of Withdrawal Stress on Immune Response
Stress has been found to play a major role in several aspects of morphine use and abuse. Morphine withdrawal by itself is considered a stress response where it modulates behavioral, neural and endocrine activity (Houshyar et al., 2001). Stress response in any given system is measured as elevations in corticosterone levels. Elevated circulating glucocorticoids have also been shown to cause dysfunction of the immune system (Yang and Glaser, 2002). Although chronic morphine driven elevations of glucocorticoids are mediated by MOR, as evidenced by loss of glucocorticoid increase in MORKO mice (Roy et al., 2001), the role of morphine withdrawal has not been well investigated. Findings from our lab has shown that morphine withdrawal did not increase glucocorticoid receptor translocation to the nucleus of splenic macrophages in WT mice, but significantly decreased the promoter activity of LPS-induced IL-12p40 in the presence of corticosterone treatment, in alveolar and peritoneal macrophage cell lines. These results suggest that morphine withdrawal stress and glucocorticoids follow two independent pathways to modulate LPS-induced IL-12p40 expression (Das et al., 2011). Comparatively, the glucocorticoid receptor antagonist, mifepristone did not block the immunosuppressive effects of morphine (Liang-Suo et al., 2002), supporting independent role of hypothalamic-pituitary-adrenal (HPA) axis.
5.3 Molecular Pathways for Morphine Withdrawal-Induced Immunosuppression
The molecular mechanisms underlying morphine withdrawal induced immunosuppression has not been explored. What we know so far is the role of different cytokines and other stimulatory molecules under morphine withdrawal conditions, but we do not know the precise mechanisms involved in morphine withdrawal induced inhibition of immune response. One report from our lab showed that morphine withdrawal inhibited IL-12p40 expression due to diminished interaction of transcription factors like NF-κB (Kelschenbach et al., 2008). Morphine withdrawal inhibited IκBα degradation therebby reducing the translocation of p65 to the nucleus. At the same time, morphine withdrawal also increased cytosolic levels of cAMP, which inhibited NF-κB pathway but activated PKA pathway. Our data further shows that morphine withdrawal inhibits the interaction of transcription factors like NF-κB, AP-1 and C/EBP, in vivo and in vitro, to their consensus binding motifs, thus, clearly indicate the role of morphine withdrawal at transcriptional level as a repressor (Subhas et al., 2011). Results also show the involvement of NF-κB pathway where morphine withdrawal inhibits IKK thereby inhibiting PP2A, a phosphatase, which interacts with IKK. Inhibition of PP2A leads to hyper-activation of ERK1/2 phosphorylation, showing ERK1/2 negatively modulates IL-12p40 expression under the morphine withdrawn conditions. Inhibited IKK did not release IκBα for degradation, thereby inhibiting p65/p50 translocation to the nucleus. The question still remains how ERK1/2 negatively regulates IL-12p40 under morphine withdrawn conditions.
Therefore, understanding the mechanism of immunomodulation by morphine withdrawal leading to enhanced susceptibility to infection is of utmost necessity. Comprehending the underlying mechanisms could be a driving force to understand the cellular immune functions and host responses in the context of HIV infection, drug abuse and in relapse patients undergoing withdrawal phases.
Summary
Morphine withdrawal-induced immunosuppression can lead to many diseased state biased towards decreased pro-inflammatory cytokine expression by APCs. This immunosuppressive deficit has been shown to be multi-faceted where suppressive effects are mainly active at transcriptional level as well as post-transcriptional level. However, the complete information on interaction between these two levels is lacking and needs to be further investigated in order to develop viable therapies for HIV and drug abuse patients.
6. MORPHINE MODULATION OF IMMUNE FUNCTION - CLINICAL CONSEQUENCES
Opioids are the most potent analgesics and used widely in a variety of clinical settings. In fact, the use of opioids in the United States has dramatically increased over the past several years, making them one of the most common class of prescription medications (Chapman et al., 2010; Ripamonti and Bruera, 1996). Chronic opioid use is common among cancer patients where the incidence of pain depends on the stage of the disease, averaging 51% overall and increases to 71% in advanced stages (Ripamonti and Bruera, 1996). Around the world the incidence of chronic non-cancer pain ranges from 5% to 33% depending on the population (Gureje et al., 1998). Although the use of opioids in non-cancer patients remains controversial, a subgroup of this population with chronic pancreatitis, suffers from unremitting pain in 80–90% of cases, and almost universally ends up on chronic opioid management (Staahl et al., 2007). In addition to medical use, according to a Drug and Alcohol Services Information System (DASIS) report published in 2007, 379,000 Americans ages 12 or older used heroin in 2005, of which, 63% used the injectable form of the drug. The body of evidence in support of chronic opioid use in relation to modulation of the immune system is growing. However, it is also clear from the preclinical studies that not all opioids affect immune function in the same way. Some reviewers divide opioids into two categories: immunosuppressive (codeine, methadone, morphine, fentanyl and remifentanil) and less immunosuppressive (buprenorphine, hydropmorphone, oxycodone and tramadol) (Sacerdote, 2006). Therefore, it is crucial to understand the impact of chronic use of individual opioids and withdrawal mechanisms in the clinical setting, and identify the populations at risk where the deleterious effects may be most pronounced.
A large body of evidence on the immunosuppressive effects of opioids comes from infection susceptibility studies of patients with chronic opioid use and abuse history. As mentioned earlier, infection rate among intravenous drug users (IDU) is higher than the general public, and is a number one cause of morbidity and hospitalization in the IDU population (Scheidegger and Zimmerli, 1989). In vitro studies implicated intravenous opioid abuse with promotion of HIV infection via decreased secretion of α and β chemokines and stimulation of chemokine receptors CCR5 and CCR3 (coreceptors for HIV) (Nath et al., 2002; Vallejo et al., 2004). At the same time, studies on hepatocytes indicated increased hepatitis C virus replication and decreased susceptibility to IFN-α therapy in the setting of chronic morphine treatment (Li et al., 2003; Zhang et al., 2006). Epidemiologic studies provide data on increased prevalence of opportunistic bacterial infections such as TB and pneumonia in IDUs, with a 10-fold increase in the incidence of pneumonia among this population (Hind, 1990; Perlman et al., 1995). A study of 598 IDUs from Vancouver, Canada showed that 73.6% of the patients were seen in emergency departments at least once over three year period, where the two most common reasons for admission were pneumonia (132 admission among 79 patients) and soft-tissue infections (90 admissions among 59 patients) (Palepu et al., 2001). Pathogens that are implicated in pulmonary infections among IDUs are S. pneumoniae, S. aureus, H. influenzae, Klebsiella pneumoniae, Chlamydia pneumoniae, Mycoplasma pneumoniae, Legionella pneumophilia, and HIV associated opportunistic infections such as M. tuberculosis, M. avium, P. aeruginosa, nocardia species and Rhodococcus equi (Gordon and Lowy, 2005). The epidemiological studies on pulmonary infections among IDUs are supported by animal and in vitro data, where chronic morphine treatment increased susceptibility and mortality in murine model of Streptococcus pneumoniae through inhibition of neutrophil migration to the site of the infection and through impairment of alveolar macrophages and decreased bacterial clearance (Wang et al., 2005; Wang et al., 2008a; Wang et al., 2008b). Other investigators reported similar deleterious effects of chronic opioid use in animal models in response to K. pneumonia, Toxoplasma gondii, Salmonella typhimurium, Candida albicans, S. aureus, and Listeria monocytogenes (Tubaro et al., 1983; Koyuncuoglu et al., 1988; Chao et al., 1990; Chao et al., 1991; Szabo et al., 1993; Asakura et al., 2006; Feng et al., 2006a). There is some discordance in clinical and in vitro data in regards to chronic opioid use and M. tuberculosis infection. From the clinical side, opioid use is considered a risk factor for M. tuberculosis infection; however, in vitro and animal studies show stimulatory effect of morphine on innate immune system (Peterson et al., 1995; Singh et al., 2008).
The majority of nonpulmonary infections among IDUs are associated with S. aureus and other Streptococcus species (Gordon and Lowy, 2005; Kaushik et al., 2011). The infection’s presentation ranges from minor skin abscesses and superficial cellulitis to necrotizing fasciitis, distal seeding with CNS abscesses, osteomyelitis, bacteremia, mycotic aneurysms and right sided endocarditis with involvement of the tricuspid valve (Gordon and Lowy, 2005). Although, the above mentioned infectious complications can be partially attributed to unsanitary practices and sharing of drug paraphernalia, given the mounting evidence that implicate chronic opioid use in modulation of innate and adaptive immune response, one cannot neglect the significance of these data and apply the knowledge to deliver the best care to these patient populations. Further, the issue of increased susceptibility to pathogens such as S. aureus among IDU, expands beyond this patient population. Multiple reports link the outbreaks of community associated methicillin resistant S. aureus (MRSA) with IDU, which is rapidly emerging as a public health problem (Fleisch et al., 2001; Gilbert et al., 2006). Lastly, surgical or traumatic wound healing may also be delayed in patients with history of opioid abuse as suggested by recent murine studies where chronic morphine treatment compromised the wound healing by inhibiting immune cell recruitment and angiogenesis at the site of infection (Martin et al., 2010a; Martin et al., 2010b).
Sepsis following the enteric bacterial translocation is another area where chronic opioid use may have a significant impact. According to the American College of Chest Physicians and Society of Critical Care Medicine 1992 consensus statement, sepsis syndrome has been defined as the presence of infection, a systemic inflammatory response, and acute organ dysfunction (Bone et al., 1992). Even with aggressive resuscitation and antibiotic therapy, diagnosis of severe sepsis contributes to 10% of admissions in intensive care units (ICU) and carries an estimated 40% mortality rate (Linde-Zwirble and Angus, 2004). Although, most clinical studies primarily concentrate on acute opioid use, the chronic use of morphine in the ICU and it’s contribution to ICU infections and sepsis has not been systematically investigated. As described earlier, multiple animal studies suggest that chronic morphine use promotes bacterial translocation from the intestinal lumen into the blood stream with dissemination to the liver, spleen and peritoneal cavity (Hilburger et al., 1997). In addition to potentiating the bacterial translocation, in vitro and in vivo studies show that chronic morphine works synergistically with LPS, accelerating the progression to septic shock (Roy et al., 1998b). Thus, it is important to refine the existing animal model to closely match human conditions and to cross-validate these findings through carefully controlled human studies.
7. CONCLUSION
Any pathogenic infection spontaneously activates the host’s innate responses, triggering PAMP mediated cytokine release from macrophages and dendritic cells, concurrent with phagocytosis and antigen presentation. On the other hand, the cytokines mediate the release of defense molecules and neutrophil chemoattractants from the mucosal epithelium, apart from creating a pro-inflammatory environment with the participation of both the resident immune and mucosal cells. The professional APCs carry the pathogenic information to the lymphocytes, thus activating the adaptive immune responses for long-term protection of the host and creating a pathogen-specific molecular imprint (memory) for any subsequent infection. Taken together, the cells of the innate and adaptive immunity, in the face of a hostile pathogen, mount a highly coordinated and disciplined response to identify, process and eliminate the pathogen from the host. Uncontrolled and prolonged infection and classical disease symptoms are a result of a breakdown in this coordination, typically resulting from compromised functioning of one or more cell types participating in the host response.
Opioids represent a cornerstone of modern pain management on one hand, and the popular drug of abuse by the modern society on the other. All opioids, especially morphine, are known to alter or suppress the functionality of the various cell types of both innate and adaptive immunity (Roy and Loh, 1996; Roy et al., 2006; Friedman and Eisenstein, 2004; Dinda et al., 2005). While some of its effects are mediated through the HPA axis (e.g. NK cells and B cells), in majority of the cases, as exemplified throughout the review, the effects of morphine on individual cell types are direct in modulating their functionality. In terms of innate immune cells, morphine has been shown to suppress pro-inflammatory signal in DCs (Wang et al., 2011), suppress activation and phagocytic property of macrophages (Section 1, Table 1 and Figure 3) (Roy et al., 2006; Casellas et al., 1991; Szabo et al., 1993; Tomei and Renaud, 1997) and superoxide production in both macrophages and neutrophils (Sharp et al., 1985; Simpkins et al., 1986). In all cases, there is a major morphine mediated modulation of TLR signaling and suppression of pro-inflammatory cytokine secretion following exposure to pathogens or pathogen signatures, leading to impaired neutrophils recruitment (Section 3, Table 2) (Welters et al., 2000; Glattard et al., 2010). In adaptive immune cells, major effects of morphine on B cells seem to be indirect and carried over from the impaired functioning of APCs and modulation of the HPA axis, resulting in suppressed B cell activation and down-regulation of MHC class II on their surfaces (Table 1 and Figure 3). T cells, on the other hand exhibit direct effects of morphine on activation and differentiation, namely morphine mediated suppression of IL-2 transcription (Table 1 and Figure 1) (Thomas et al., 1995; Roy et al., 1997; Wang et al., 2003; Wang et al., 2007) and lineage bias towards TH2 phenotype (Table 1 and Figure 2) (Wang et al., 2003; Roy et al., 2004; Greeneltch et al., 2005; Qian et al., 2005; Roy et al., 2005; Borner et al., 2009).
Figure 3. Schematic outline illustrating modulation of both innate and adaptive immunity following morphine treatment.
Morphine modulates both branches of the immune system leading to inhibition of host’s defenses and impaired pathogen clearance by binding to the mu-opioid receptor. Morphine treatment inhibits innate immunity by inhibiting macrophage and neutrophil phagocytic, bactericidal, chemotactic and migratory functions. In addition morphine treatment inhibits antigen presentation by dendritic cells and reduces NK cytotoxicity. By reducing mast cell activation morphine affects gut permeability further increasing host’s risk of infection. Morphine mediated modulation of adaptive immune functions is not as well as explored. Chronic morphine treatment leads to a decrease in TH1 cytokine production and T cell activation, and increases TH1 cell death and TH2 differentiation. In B cells, morphine treatment has been implicated to reduce antibody production, MHC II expression and proliferation. In summary, by inhibiting key effectors of both branches of the immune system morphine treatment inhibits pathogen clearance and enhances bacterial dissemination.
Morphine mediated modulation of the key effectors of the immune system leads to a systemic breakdown in their coordination and ultimately, their ability to mount a disciplined response to pathogenic attack. Naturally, this and morphine-withdrawal mediated transcriptional modulation of key cytokines (Section 5), results in robust disease progression and high pathogenic load in the host (Section 4, Table 3 and Figure 3). As the body of evidence in support of opioid immunosuppressive qualities is growing, it is imperative to understand the mechanisms by which opioids exert these effects, and identify the populations at risk that would benefit the most from the interventions to counteract opioid immunosuppressive qualities. Better understanding the mechanisms that are involved in opioid modulation of immunity will facilitate the search for new therapeutic modalities to counteract adverse effects including increased opportunistic infection rates in the chronic opioid using population.
Acknowledgments
This work was supported by National Institutes of Health grants NIH RO1 DA12104, RO1 DA022935, KO2 DA015349, R01 DA031202 and P50 DA11806 (S.R.) K05DA033881,1R01DA034582
Footnotes
No Conflict of Interest
BIBLIOGRAPHY
- Aceijas C, Stimson GV, Hickman M, Rhodes T United Nations Reference Group on HIV/AIDS Prevention and Care among IDU in Developing and Transitional Countries. Global overview of injecting drug use and HIV infection among injecting drug users. AIDS. 2004;18:2295–2303. doi: 10.1097/00002030-200411190-00010. [DOI] [PubMed] [Google Scholar]
- Alme B, Carlsson L, Kanerva P, Lundberg G, Persson E, Rydberg U, Bottiger M, Putkonen PO. HIV infection and narcotic addiction. Lakartidningen. 1987;84:213–6. [PubMed] [Google Scholar]
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Does drug abuse alter microglial phenotype and cell turnover in the context of advancing HIV infection? Neuropathol Appl Neurobiol. 2005;31:325–338. doi: 10.1111/j.1365-2990.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- Asakura H, Kawamoto K, Igimi S, Yamamoto S, Makino S. Enhancement of mice susceptibility to infection with Listeria monocytogenes by the treatment of morphine. Microbiol Immunol. 2006;50:543–547. doi: 10.1111/j.1348-0421.2006.tb03824.x. [DOI] [PubMed] [Google Scholar]
- Bafica A, Scanga CA, Equils O, Sher A. The induction of Toll-like receptor tolerance enhances rather than suppresses HIV-1 gene expression in transgenic mice. J Leukoc Biol. 2004a;75:460–466. doi: 10.1189/jlb.0803388. [DOI] [PubMed] [Google Scholar]
- Bafica A, Scanga CA, Schito M, Chaussabel D, Sher A. Influence of coinfecting pathogens on HIV expression: evidence for a role of Toll-like receptors. J Immunol. 2004b;172:7229–7234. doi: 10.4049/jimmunol.172.12.7229. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Beagles K, Wellstein A, Bayer B. Systemic morphine administration suppresses genes involved in antigen presentation. Mol Pharmacol. 2004;65:437–442. doi: 10.1124/mol.65.2.437. [DOI] [PubMed] [Google Scholar]
- Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- Bhargava HN, Thomas PT, Thorat S, House RV. Effects of morphine tolerance and abstinence on cellular immune function. Brain Res. 1994;642:1–10. doi: 10.1016/0006-8993(94)90899-0. [DOI] [PubMed] [Google Scholar]
- Bhaskaran M, Kapasi AA, Reddy K, Singhal PC. Morphine priming rescues high-dose morphine-induced biological perturbations. J Infect Dis. 2007;195:1860–1869. doi: 10.1086/518039. [DOI] [PubMed] [Google Scholar]
- Bhaskaran M, Reddy K, Sharma S, Singh J, Radhakrishnan N, Kapasi A, Singhal PC. Morphine-induced degradation of the host defense barrier: role of macrophage injury. J Infect Dis. 2001;184:1524–1531. doi: 10.1086/324667. [DOI] [PubMed] [Google Scholar]
- Bidlack JM. Detection and function of opioid receptors on cells from the immune system. Clin Diagn Lab Immunol. 2000;7:719–723. doi: 10.1128/cdli.7.5.719-723.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasig J, Herz A, Reinhold K, Zieglgansberger S. Development of physical dependence on morphine in respect to time and dosage and quantification of the precipitated withdrawal syndrome in rats. Psychopharmacologia. 1973;33:19–38. doi: 10.1007/BF00428791. [DOI] [PubMed] [Google Scholar]
- Bokhari SM, Yao H, Bethel-Brown C, Fuwang P, Williams R, Dhillon NK, Hegde R, Kumar A, Buch SJ. Morphine enhances Tat-induced activation in murine microglia. J Neurovirol. 2009;15:219–228. doi: 10.1080/13550280902913628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- Bonnet MP, Beloeil H, Benhamou D, Mazoit JX, Asehnoune K. The mu opioid receptor mediates morphine-induced tumor necrosis factor and interleukin-6 inhibition in toll-like receptor 2-stimulated monocytes. Anesth Analg. 2008;106:1142–9. doi: 10.1213/ane.0b013e318165de89. table of contents. [DOI] [PubMed] [Google Scholar]
- Borman A, Ciepielewski Z, Wrona D, Stojek W, Glac W, Leszkowicz E, Tokarski J. Small doses of morphine can enhance NK cell cytotoxicity in pigs. Int Immunopharmacol. 2009;9:277–283. doi: 10.1016/j.intimp.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Borner C, Stumm R, Hollt V, Kraus J. Comparative analysis of mu-opioid receptor expression in immune and neuronal cells. J Neuroimmunol. 2007;188:56–63. doi: 10.1016/j.jneuroim.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Borner C, Warnick B, Smida M, Hartig R, Lindquist JA, Schraven B, Hollt V, Kraus J. Mechanisms of opioid-mediated inhibition of human T cell receptor signaling. J Immunol. 2009;183:882–889. doi: 10.4049/jimmunol.0802763. [DOI] [PubMed] [Google Scholar]
- Breslow JM, Feng P, Meissler JJ, Pintar JE, Gaughan J, Adler MW, Eisenstein TK. Potentiating effect of morphine on oral Salmonella enterica serovar Typhimurium infection is mu-opioid receptor-dependent. Microb Pathog. 49:330–5. doi: 10.1016/j.micpath.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brichacek B, Vanpouille C, Kiselyeva Y, Biancotto A, Merbah M, Hirsch I, Lisco A, Grivel JC, Margolis L. Contrasting roles for TLR ligands in HIV-1 pathogenesis. PLoS One. 2010;5:e12831. doi: 10.1371/journal.pone.0012831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant HU, Bernton EW, Holaday JW. Morphine pellet-induced immunomodulation in mice: temporal relationships. J Pharmacol Exp Ther. 1988;245:913–920. [PubMed] [Google Scholar]
- Bryant HU, Bernton EW, Kenner JR, Holaday JW. Role of adrenal cortical activation in the immunosuppressive effects of chronic morphine treatment. Endocrinology. 1991;128:3253–3258. doi: 10.1210/endo-128-6-3253. [DOI] [PubMed] [Google Scholar]
- Burbassi S, Aloyo VJ, Simansky KJ, Meucci O. GTPgammaS incorporation in the rat brain: a study on mu-opioid receptors and CXCR4. J Neuroimmune Pharmacol. 2008;3:26–34. doi: 10.1007/s11481-007-9083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussiere JL, Adler MW, Rogers TJ, Eisenstein TK. Cytokine reversal of morphine-induced suppression of the antibody response. J Pharmacol Exp Ther. 1993;264:591–597. [PubMed] [Google Scholar]
- Bussiere JL, Adler MW, Rogers TJ, Eisenstein TK. Differential effects of morphine and naltrexone on the antibody response in various mouse strains. Immunopharmacol Immunotoxicol. 1992;14:657–673. doi: 10.3109/08923979209005416. [DOI] [PubMed] [Google Scholar]
- Carr DJ, Gebhardt BM, Paul D. Alpha adrenergic and mu-2 opioid receptors are involved in morphine-induced suppression of splenocyte natural killer activity. J Pharmacol Exp Ther. 1993;264:1179–1186. [PubMed] [Google Scholar]
- Casellas AM, Guardiola H, Renaud FL. Inhibition by opioids of phagocytosis in peritoneal macrophages. Neuropeptides. 1991;18:35–40. doi: 10.1016/0143-4179(91)90161-b. [DOI] [PubMed] [Google Scholar]
- Chang SL, Beltran JA, Swarup S. Expression of the mu opioid receptor in the human immunodeficiency virus type 1 transgenic rat model. J Virol. 2007;81:8406–8411. doi: 10.1128/JVI.00155-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, Sharp BM, Pomeroy C, Filice GA, Peterson PK. Effects of morphine addiction on the pathogenesis of murine toxoplasmosis. Adv Exp Med Biol. 1991;288:223–227. doi: 10.1007/978-1-4684-5925-8_25. [DOI] [PubMed] [Google Scholar]
- Chao CC, Sharp BM, Pomeroy C, Filice GA, Peterson PK. Lethality of morphine in mice infected with Toxoplasma gondii. J Pharmacol Exp Ther. 1990;252:605–609. [PubMed] [Google Scholar]
- Chapman CR, Lipschitz DL, Angst MS, Chou R, Denisco RC, Donaldson GW, Fine PG, Foley KM, Gallagher RM, Gilson AM, Haddox JD, Horn SD, Inturrisi CE, Jick SS, Lipman AG, Loeser JD, Noble M, Porter L, Rowbotham MC, Schoelles KM, Turk DC, Volinn E, Von Korff MR, Webster LR, Weisner CM. Opioid pharmacotherapy for chronic non-cancer pain in the United States: a research guideline for developing an evidence-base. J Pain. 2010;11:807–829. doi: 10.1016/j.jpain.2010.02.019. [DOI] [PubMed] [Google Scholar]
- Chuang LF, Chuang TK, Killam KF, Chuang AJ, Jr , Kung HF, Yu L, Chuang RY. Delta opioid receptor gene expression in lymphocytes. Biochem Biophys Res Commun. 1994;202:1291–1299. doi: 10.1006/bbrc.1994.2071. [DOI] [PubMed] [Google Scholar]
- Chuang LF, Chuang TK, Killam KF, Qiu Q, Jr, Wang XR, Lin JJ, Kung HF, Sheng W, Chao C, Yu L. Expression of kappa opioid receptors in human and monkey lymphocytes. Biochem Biophys Res Commun. 1995;209:1003–1010. doi: 10.1006/bbrc.1995.1597. [DOI] [PubMed] [Google Scholar]
- Clark JD, Shi X, Li X, Qiao Y, Liang D, Angst MS, Yeomans DC. Morphine reduces local cytokine expression and neutrophil infiltration after incision. Mol Pain. 2007;3:28. doi: 10.1186/1744-8069-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A, Macleod M, Schumacher T, Corlett L, Gray D. Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J Immunol. 2006;176:3498–3506. doi: 10.4049/jimmunol.176.6.3498. [DOI] [PubMed] [Google Scholar]
- Das S, Kelschenbach J, Cahrboneau R, Barke RA, Roy S. Morphine withdrawal stress and glucocorticoids independently modulate LPS-induced IL-12p40 expression by activating extracellular-signal regulated kinase 1/2. 2011. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinda A, Gitman M, Singhal PC. Immunomodulatory effect of morphine: therapeutic implications. Expert Opin Drug Saf. 2005;4:669–675. doi: 10.1517/14740338.4.4.669. [DOI] [PubMed] [Google Scholar]
- Donahoe RM, Byrd LD, McClure HM, Brantley M, Wenzel D, Ansari AA, Marsteller F. Effects of morphine on T-cell recirculation in rhesus monkeys. Adv Exp Med Biol. 2001;493:89–101. doi: 10.1007/0-306-47611-8_11. [DOI] [PubMed] [Google Scholar]
- Eisenstein TK, Hilburger ME. Opioid modulation of immune responses: effects on phagocyte and lymphoid cell populations. J Neuroimmunol. 1998;83:36–44. doi: 10.1016/s0165-5728(97)00219-1. [DOI] [PubMed] [Google Scholar]
- Equils O, Faure E, Thomas L, Bulut Y, Trushin S, Arditi M. Bacterial lipopolysaccharide activates HIV long terminal repeat through Toll-like receptor 4. J Immunol. 2001;166:2342–2347. doi: 10.4049/jimmunol.166.4.2342. [DOI] [PubMed] [Google Scholar]
- Fecho K, Lysle DT. Phenotypic analysis of splenocyte subsets following acute morphine treatment in the rat. Cell Immunol. 1999;195:137–146. doi: 10.1006/cimm.1999.1534. [DOI] [PubMed] [Google Scholar]
- Feng P, Meissler JJ, Jr, Adler MW, Eisenstein TK. Morphine withdrawal sensitizes mice to lipopolysaccharide: elevated TNF-alpha and nitric oxide with decreased IL-12. J Neuroimmunol. 2005;164:57–65. doi: 10.1016/j.jneuroim.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Feng P, Rahim RT, Cowan A, Liu-Chen LY, Peng X, Gaughan J, JMJ, Adler MW, Jr, Eisenstein TK. Effects of mu, kappa or delta opioids administered by pellet or pump on oral Salmonella infection and gastrointestinal transit. Eur J Pharmacol. 2006;534:250–7. doi: 10.1016/j.ejphar.2006.01.048. [DOI] [PubMed] [Google Scholar]
- Feng P, Wilson QM, Meissler JJ, Jr, Adler MW, Eisenstein TK. Increased sensitivity to Salmonella enterica serovar Typhimurium infection in mice undergoing withdrawal from morphine is associated with suppression of interleukin-12. Infect Immun. 2005;73:7953–7959. doi: 10.1128/IAI.73.12.7953-7959.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley MJ, Steele A, Cornwell WD, Rogers TJ. Transcriptional regulation of the major HIV-1 coreceptor, CXCR4, by the {kappa} opioid receptor. J Leukoc Biol. 2011 doi: 10.1189/jlb.1010546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch F, Zbinden R, Vanoli C, Ruef C. Epidemic spread of a single clone of methicillin-resistant Staphylococcus aureus among injection drug users in Zurich, Switzerland. Clin Infect Dis. 2001;32:581–586. doi: 10.1086/318716. [DOI] [PubMed] [Google Scholar]
- Flores LR, Wahl SM, Bayer BM. Mechanisms of morphine-induced immunosuppression: effect of acute morphine administration on lymphocyte trafficking. J Pharmacol Exp Ther. 1995;272:1246–1251. [PubMed] [Google Scholar]
- Friedman H, Eisenstein TK. Neurological basis of drug dependence and its effects on the immune system. J Neuroimmunol. 2004;147:106–108. doi: 10.1016/j.jneuroim.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Frolich N, Dees C, Paetz C, Ren X, Lohse MJ, Nikolaev VO, Zenk MH. Distinct pharmacological properties of morphine metabolites at G(i)-protein and beta-arrestin signaling pathways activated by the human mu-opioid receptor. Biochem Pharmacol. 2011;81:1248–1254. doi: 10.1016/j.bcp.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Gaveriaux C, Peluso J, Simonin F, Laforet J, Kieffer B. Identification of kappa- and delta-opioid receptor transcripts in immune cells. FEBS Lett. 1995;369:272–276. doi: 10.1016/0014-5793(95)00766-3. [DOI] [PubMed] [Google Scholar]
- Georges H, Leroy O, Vandenbussche C, Guery B, Alfandari S, Tronchon L, Beaucaire G. Epidemiological features and prognosis of severe community-acquired pneumococcal pneumonia. Intensive Care Med. 1999;25:198–206. doi: 10.1007/s001340050816. [DOI] [PubMed] [Google Scholar]
- Gilbert M, MacDonald J, Gregson D, Siushansian J, Zhang K, Elsayed S, Laupland K, Louie T, Hope K, Mulvey M, Gillespie J, Nielsen D, Wheeler V, Louie M, Honish A, Keays G, Conly J. Outbreak in Alberta of community-acquired (USA300) methicillin-resistant Staphylococcus aureus in people with a history of drug use, homelessness or incarceration. CMAJ. 2006;175:149–154. doi: 10.1503/cmaj.051565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glattard E, Welters ID, Lavaux T, Muller AH, Laux A, Zhang D, Schmidt AR, Delalande F, Laventie BJ, Dirrig-Grosch S, Colin DA, Van Dorsselaer A, Aunis D, Metz-Boutigue MH, Schneider F, Goumon Y. Endogenous morphine levels are increased in sepsis: a partial implication of neutrophils. PLoS One. 2010;5:e8791. doi: 10.1371/journal.pone.0008791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon RJ, Lowy FD. Bacterial infections in drug users. N Engl J Med. 2005;353:1945–1954. doi: 10.1056/NEJMra042823. [DOI] [PubMed] [Google Scholar]
- Greeneltch KM, Kelly-Welch AE, Shi Y, Keegan AD. Chronic morphine treatment promotes specific Th2 cytokine production by murine T cells in vitro via a Fas/Fas ligand-dependent mechanism. J Immunol. 2005;175:4999–5005. doi: 10.4049/jimmunol.175.8.4999. [DOI] [PubMed] [Google Scholar]
- Grimm MC, Ben-Baruch A, Taub DD, Howard OM, Resau JH, Wang JM, Ali H, Richardson R, Snyderman R, Oppenheim JJ. Opiates transdeactivate chemokine receptors: delta and mu opiate receptor-mediated heterologous desensitization. J Exp Med. 1998a;188:317–325. doi: 10.1084/jem.188.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm MC, Ben-Baruch A, Taub DD, Howard OM, Wang JM, Oppenheim JJ. Opiate inhibition of chemokine-induced chemotaxis. Ann N Y Acad Sci. 1998b;840:9–20. doi: 10.1111/j.1749-6632.1998.tb09544.x. [DOI] [PubMed] [Google Scholar]
- Guarner J, Bartlett J, Reagan S, Fischer M, Finn S, O’Briain DS, Black M, Hood J, Zaki SR. Immunohistochemical evidence of Clostridium sp, Staphylococcus aureus, and group A Streptococcus in severe soft tissue infections related to injection drug use. Hum Pathol. 2006;37:1482–1488. doi: 10.1016/j.humpath.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Guo CJ, Li Y, Tian S, Wang X, Douglas SD, Ho WZ. Morphine enhances HIV infection of human blood mononuclear phagocytes through modulation of beta-chemokines and CCR5 receptor. J Investig Med. 2002;50:435–42. doi: 10.1136/jim-50-06-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gureje O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being: a World Health Organization Study in Primary Care. JAMA. 1998;280:147–151. doi: 10.1001/jama.280.2.147. [DOI] [PubMed] [Google Scholar]
- Hao YS, Li PF, Zhang FX, Huang DA, Liu XH, Li G. Morphine alleviates early stages of apoptosis in cultured CEM x174 cells infected with simian immunodeficiency virus. Cell Biol Int. 2003;27:719–725. doi: 10.1016/s1065-6995(03)00141-0. [DOI] [PubMed] [Google Scholar]
- Happel C, Steele AD, Finley MJ, Kutzler MA, Rogers TJ. DAMGO-induced expression of chemokines and chemokine receptors: the role of TGF-beta1. J Leukoc Biol. 2008;83:956–963. doi: 10.1189/jlb.1007685. [DOI] [PubMed] [Google Scholar]
- Harari Y, Weisbrodt NW, Moody FG. The effect of morphine on mast cell-mediated mucosal permeability. Surgery. 2006;139:54–60. doi: 10.1016/j.surg.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Haverkos HW, Lange WR. From the Alcohol, Drug Abuse, and Mental Health Administration. Serious infections other than human immunodeficiency virus among intravenous drug abusers. J Infect Dis. 1990;161:894–902. doi: 10.1093/infdis/161.5.894. [DOI] [PubMed] [Google Scholar]
- Heggelund L, Muller F, Lien E, Yndestad A, Ueland T, Kristiansen KI, Espevik T, Aukrust P, Froland SS. Increased expression of toll-like receptor 2 on monocytes in HIV infection: possible roles in inflammation and viral replication. Clin Infect Dis. 2004;39:264–269. doi: 10.1086/421780. [DOI] [PubMed] [Google Scholar]
- Hilburger ME, Adler MW, Truant AL, Meissler JJ, Jr, Satishchandran V, Rogers TJ, Eisenstein TK. Morphine induces sepsis in mice. J Infect Dis. 1997;176:183–188. doi: 10.1086/514021. [DOI] [PubMed] [Google Scholar]
- Hind CR. Pulmonary complications of intravenous drug misuse. 1. Epidemiology and non-infective complications. Thorax. 1990a;45:891–898. doi: 10.1136/thx.45.11.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hind CR. Pulmonary complications of intravenous drug misuse. 2. Infective and HIV related complications. Thorax. 1990b;45:957–961. doi: 10.1136/thx.45.12.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houshyar H, Galigniana MD, Pratt WB, Woods JH. Differential responsivity of the hypothalamic-pituitary-adrenal axis to glucocorticoid negative-feedback and corticotropin releasing hormone in rats undergoing morphine withdrawal: possible mechanisms involved in facilitated and attenuated stress responses. J Neuroendocrinol. 2001;13:875–86. doi: 10.1046/j.1365-2826.2001.00714.x. [DOI] [PubMed] [Google Scholar]
- Hu S, Chao CC, Hegg CC, Thayer S, Peterson PK. Morphine inhibits human microglial cell production of, and migration towards, RANTES. J Psychopharmacol. 2000;14:238–243. doi: 10.1177/026988110001400307. [DOI] [PubMed] [Google Scholar]
- Hussey HH, Katz S. Infections resulting from narcotic addiction; report of 102 cases. Am J Med. 1950;9:186–193. doi: 10.1016/0002-9343(50)90021-0. [DOI] [PubMed] [Google Scholar]
- Kaushik KS, Kapila K, Praharaj AK. Shooting up: the interface of microbial infections and drug abuse. J Med Microbiol. 2011;60:408–422. doi: 10.1099/jmm.0.027540-0. [DOI] [PubMed] [Google Scholar]
- Kelschenbach J, Barke RA, Roy S. Morphine withdrawal contributes to Th cell differentiation by biasing cells toward the Th2 lineage. J Immunol. 2005;175:2655–2665. doi: 10.4049/jimmunol.175.4.2655. [DOI] [PubMed] [Google Scholar]
- Kelschenbach J, Ninkovic J, Wang J, Krishnan A, Charboneau R, Barke RA, Roy S. Morphine withdrawal inhibits IL-12 induction in a macrophage cell line through a mechanism that involves cAMP. J Immunol. 2008;180:3670–9. doi: 10.4049/jimmunol.180.6.3670. [DOI] [PubMed] [Google Scholar]
- Kieffer BL, Gaveriaux-Ruff C. Exploring the opioid system by gene knockout. Prog Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- Kleindienst P, Brocker T. Concerted antigen presentation by dendritic cells and B cells is necessary for optimal CD4 T-cell immunity in vivo. Immunology. 2005;115:556–564. doi: 10.1111/j.1365-2567.2005.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyuncuoglu H, Gungor M, Ang O, Inanc D, Ang-Kucuker M, Sagduyu H, Uysal V. Aggravation by morphine and D-aspartic acid of pyelonephritis induced by i.v. inoculation of Staphylococcus aureus in rats. Infection. 1988;16:42–45. doi: 10.1007/BF01646931. [DOI] [PubMed] [Google Scholar]
- Kraus J, Borner C, Giannini E, Hickfang K, Braun H, Mayer P, Hoehe MR, Ambrosch A, Konig W, Hollt V. Regulation of mu-opioid receptor gene transcription by interleukin-4 and influence of an allelic variation within a STAT6 transcription factor binding site. J Biol Chem. 2001;276:43901–43908. doi: 10.1074/jbc.M107543200. [DOI] [PubMed] [Google Scholar]
- Kraus J, Lehmann L, Borner C, Hollt V. Epigenetic mechanisms involved in the induction of the mu opioid receptor gene in Jurkat T cells in response to interleukin-4. Mol Immunol. 2010;48:257–263. doi: 10.1016/j.molimm.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Kumar R, Torres C, Yamamura Y, Rodriguez I, Martinez M, Staprans S, Donahoe RM, Kraiselburd E, Stephens EB, Kumar A. Modulation by morphine of viral set point in rhesus macaques infected with simian immunodeficiency virus and simian-human immunodeficiency virus. J Virol. 2004;78:11425–11428. doi: 10.1128/JVI.78.20.11425-11428.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz SS, Chiang CY. Effects of certain abused drug s on hemolysin forming cells. Life Sci. 1975;17:1763–1767. doi: 10.1016/0024-3205(75)90458-0. [DOI] [PubMed] [Google Scholar]
- Li Y, Sun X, Zhang Y, Huang J, Hanley G, Ferslew KE, Peng Y, Yin D. Morphine promotes apoptosis via TLR2, and this is negatively regulated by beta-arrestin 2. Biochem Biophys Res Commun. 2009;378:857–861. doi: 10.1016/j.bbrc.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang T, Douglas SD, Lai JP, Xiao WD, Pleasure DE, Ho WZ. Morphine enhances hepatitis C virus (HCV) replicon expression. Am J Pathol. 2003;163:1167–1175. doi: 10.1016/S0002-9440(10)63476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang-Suo J, Gomez-Flores R, Weber RJ. Immunosuppression induced by central action of morphine is not blocked by mifepristone (RU 486) Life Sci. 2002;71:2595–602. doi: 10.1016/s0024-3205(02)02087-8. [DOI] [PubMed] [Google Scholar]
- Linde-Zwirble WT, Angus DC. Severe sepsis epidemiology: sampling, selection, and society. Crit Care. 2004;8:222–226. doi: 10.1186/cc2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo-Chinchilla AM, Baez D, Velez M, Ildefonso C, Renaud FL. Altered subcellular signaling in murine peritoneal macrophages upon chronic morphine exposure. J Neuroimmunol. 2006;176:86–94. doi: 10.1016/j.jneuroim.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Lysle DT, Hoffman KE, Dykstra LA. Evidence for the involvement of the caudal region of the periaqueductal gray in a subset of morphine-induced alterations of immune status. J Pharmacol Exp Ther. 1996;277:1533–1540. [PubMed] [Google Scholar]
- Ma J, Wang J, Wan J, Charboneau R, Chang Y, Barke RA, Roy S. Morphine disrupts interleukin-23 (IL-23)/IL-17-mediated pulmonary mucosal host defense against Streptococcus pneumoniae infection. Infect Immun. 2010;78:830–837. doi: 10.1128/IAI.00914-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Li J, Chiu I, Wang Y, Sloane JA, Lu J, Kosaras B, Sidman RL, Volpe JJ, Vartanian T. Toll-like receptor 8 functions as a negative regulator of neurite outgrowth and inducer of neuronal apoptosis. J Cell Biol. 2006;175:209–215. doi: 10.1083/jcb.200606016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madera-Salcedo IK, Cruz SL, Gonzalez-Espinosa C. Morphine decreases early peritoneal innate immunity responses in Swiss-Webster and C57BL6/J mice through the inhibition of mast cell TNF-alpha release. J Neuroimmunol. 2011;232:101–107. doi: 10.1016/j.jneuroim.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C, Mathis D, Fugger L. Mice lacking all conventional MHC class II genes. Proc Natl Acad Sci U S A. 1999;96:10338–10343. doi: 10.1073/pnas.96.18.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan SD, Aalinkeel R, Reynolds JL, Nair BB, Fernandez SF, Schwartz SA, Nair MP. Morphine exacerbates HIV-1 viral protein gp120 induced modulation of chemokine gene expression in U373 astrocytoma cells. Curr HIV Res. 2005a;3:277–288. doi: 10.2174/1570162054368048. [DOI] [PubMed] [Google Scholar]
- Mahajan SD, Schwartz SA, Aalinkeel R, Chawda RP, Sykes DE, Nair MP. Morphine modulates chemokine gene regulation in normal human astrocytes. Clin Immunol. 2005b;115:323–332. doi: 10.1016/j.clim.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- Martin JL, Charboneau R, Barke RA, Roy S. Chronic morphine treatment inhibits LPS-induced angiogenesis: implications in wound healing. Cell Immunol. 2010a;265:139–145. doi: 10.1016/j.cellimm.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JL, Koodie L, Krishnan AG, Charboneau R, Barke RA, Roy S. Chronic morphine administration delays wound healing by inhibiting immune cell recruitment to the wound site. Am J Pathol. 2010b;176:786–799. doi: 10.2353/ajpath.2010.090457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- McCarthy L, Szabo I, Nitsche JF, Pintar JE, Rogers TJ. Expression of functional μ-opioid receptors during T cell development. J Neuroimmunol. 2001;114:173–180. doi: 10.1016/s0165-5728(01)00248-x. [DOI] [PubMed] [Google Scholar]
- McIlroy MA, Reddy D, Markowitz N, Saravolatz LD. Infected false aneurysms of the femoral artery in intravenous drug addicts. Rev Infect Dis. 1989;11:578–585. doi: 10.1093/clinids/11.4.578. [DOI] [PubMed] [Google Scholar]
- Mellon RD, Noori NE, Hernandez MC, Bayer BM. Altered T-cell responsiveness in morphine “tolerant” rats: evidence for a potential role of the paraventricular nucleus of the hypothalamus. Adv Exp Med Biol. 2001;493:177–185. doi: 10.1007/0-306-47611-8_21. [DOI] [PubMed] [Google Scholar]
- Menzebach A, Hirsch J, Nost R, Mogk M, Hempelmann G, Welters ID. Morphine inhibits complement receptor expression, phagocytosis and oxidative burst by a nitric oxide dependent mechanism. Anasthesiol Intensivmed Notfallmed Schmerzther. 2004;39:204–211. doi: 10.1055/s-2004-814389. [DOI] [PubMed] [Google Scholar]
- Miyagi T, Chuang LF, Doi RH, Carlos MP, Torres JV, Chuang RY. Morphine induces gene expression of CCR5 in human CEMx174 lymphocytes. J Biol Chem. 2000;275:31305–31310. doi: 10.1074/jbc.M001269200. [DOI] [PubMed] [Google Scholar]
- Mohan S, Davis RL, DeSilva U, Stevens CW. Dual regulation of mu opioid receptors in SK-N-SH neuroblastoma cells by morphine and interleukin-1beta: evidence for opioid-immune crosstalk. J Neuroimmunol. 2010;227:26–34. doi: 10.1016/j.jneuroim.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K, Dusheiko G. Opiate abuse and viral replication in hepatitis C. Am J Pathol. 2005;167:1189–91. doi: 10.1016/S0002-9440(10)61207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman J, Zhang Y, Liu B, LeSage G, Chen Y, Stuart C, Prayther D, Yin D. HIV-1 gp120 primes lymphocytes for opioid-induced, beta-arrestin 2-dependent apoptosis. Biochim Biophys Acta. 2009;1793:1366–71. doi: 10.1016/j.bbamcr.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustelin T, Tasken K. Positive and negative regulation of T-cell activation through kinases and phosphatases. Biochem J. 2003;371:15–27. doi: 10.1042/BJ20021637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Hauser KF, Wojna V, Booze RM, Maragos W, Prendergast M, Cass W, Turchan JT. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S62–9. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- Nelson CJ, Schneider GM, Lysle DT. Involvement of Central μ- but Not δ- or κ-Opioid Receptors in Immunomodulation. Brain Behav Immun. 2000;14:170–184. doi: 10.1006/brbi.1999.0575. [DOI] [PubMed] [Google Scholar]
- Nugent AL, Houghtling RA, Bayer BM. Morphine suppresses MHC-II expression on circulating B lymphocytes via activation of the HPA. J Neuroimmune Pharmacol. 2011;6:130–141. doi: 10.1007/s11481-010-9218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocasio FM, Jiang Y, House SD, Chang SL. Chronic morphine accelerates the progression of lipopolysaccharide-induced sepsis to septic shock. Journal of neuroimmunology. 2004;149:90–100. doi: 10.1016/j.jneuroim.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Ohara T, Itoh T, Takahashi M. Immunosuppression by morphine-induced lymphocyte apoptosis: is it a real issue? Anesth Analg. 2005;101:1117–22. doi: 10.1213/01.ane.0000167772.16584.0f. table of contents. [DOI] [PubMed] [Google Scholar]
- Palepu A, Tyndall MW, Leon H, Muller J, O’Shaughnessy MV, Schechter MT, Anis AH. Hospital utilization and costs in a cohort of injection drug users. CMAJ. 2001;165:415–420. [PMC free article] [PubMed] [Google Scholar]
- Perez-Castrillon JL, Perez-Arellano JL, Garcia-Palomo JD, Jimenez-Lopez A, De Castro S. Opioids depress in vitro human monocyte chemotaxis. Immunopharmacology. 1992;23:57–61. doi: 10.1016/0162-3109(92)90009-2. [DOI] [PubMed] [Google Scholar]
- Perlman DC, Salomon N, Perkins MP, Yancovitz S, Paone D, Des Jarlais DC. Tuberculosis in drug users. Clin Infect Dis. 1995;21:1253–1264. doi: 10.1093/clinids/21.5.1253. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Brummitt C, Pentel P, Bullock M, Simpson M, Hitt J, Sharp B. Suppression of human peripheral blood mononuclear cell function by methadone and morphine. J Infect Dis. 1989;159:480–487. doi: 10.1093/infdis/159.3.480. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Hu S, Sheng WS, Molitor TW, Chao CC. Morphine stimulates phagocytosis of Mycobacterium tuberculosis by human microglial cells: involvement of a G protein-coupled opiate receptor. Adv Neuroimmunol. 1995;5:299–309. doi: 10.1016/0960-5428(95)00020-3. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Sharp B, Gekker G, Brummitt C, Keane WF. Opioid-mediated suppression of cultured peripheral blood mononuclear cell respiratory burst activity. J Immunol. 1987;138:3907–3912. [PubMed] [Google Scholar]
- Peterson PK, Sharp BM, Gekker G, Portoghese PS, Sannerud K, Balfour HH., Jr Morphine promotes the growth of HIV-1 in human peripheral blood mononuclear cell cocultures. AIDS. 1990;4:869–873. doi: 10.1097/00002030-199009000-00006. [DOI] [PubMed] [Google Scholar]
- Philippe D, Chakass D, Thuru X, Zerbib P, Tsicopoulos A, Geboes K, Bulois P, Breisse M, Vorng H, Gay J, Colombel JF, Desreumaux P, Chamaillard M. Mu opioid receptor expression is increased in inflammatory bowel diseases: implications for homeostatic intestinal inflammation. Gut. 2006;55:815–823. doi: 10.1136/gut.2005.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plytycz B, Natorska J. Morphine attenuates pain and prevents inflammation in experimental peritonitis. Trends Immunol. 2002;23:345–346. doi: 10.1016/s1471-4906(02)02257-3. [DOI] [PubMed] [Google Scholar]
- Qian YN, Jin WJ, Wang L, Wang HJ. Effect of different concentrations of morphine and tramadol on the differentiation of human helper T cells in vitro. Br J Anaesth. 2005;95:277. doi: 10.1093/bja/aei574. [DOI] [PubMed] [Google Scholar]
- Quaglio G, Lugoboni F, Talamini G, Lechi A, Mezzelani P. Prevalence of tuberculosis infection and comparison of multiple-puncture liquid tuberculin test and Mantoux test among drug users. Scand J Infect Dis. 2002;34:574–576. doi: 10.1080/00365540110080791. [DOI] [PubMed] [Google Scholar]
- Rahim RT, Adler MW, JMJ, Cowan A, Jr, Rogers TJ, Geller EB, Eisenstein TK. Abrupt or precipitated withdrawal from morphine induces immunosuppression. J Neuroimmunol. 2002;127:88–95. doi: 10.1016/s0165-5728(02)00103-0. [DOI] [PubMed] [Google Scholar]
- Rahim RT, Feng P, Meissler JJ, Rogers TJ, Zhang L, Adler MW, Eisenstein TK. Paradoxes of immunosuppression in mouse models of withdrawal. J Neuroimmunol. 2004;147:114–20. doi: 10.1016/j.jneuroim.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Rahim RT, JMJ, Adler MW, Jr, Eisenstein TK. Splenic macrophages and B cells mediate immunosuppression following abrupt withdrawal from morphine. J Leukoc Biol. 2005;78:1185–91. doi: 10.1189/jlb.0304123. [DOI] [PubMed] [Google Scholar]
- Rahim RT, Meissler JJ, Zhang L, Adler MW, Rogers TJ, Eisenstein TK. Withdrawal from morphine in mice suppresses splenic macrophage function, cytokine production, and costimulatory molecules. J Neuroimmunol. 2003;144:16–27. doi: 10.1016/s0165-5728(03)00273-x. [DOI] [PubMed] [Google Scholar]
- Ripamonti C, Bruera E. Pain and Symptom Management in Palliative Care. Cancer Control. 1996;3:204–213. doi: 10.1177/107327489600300302. [DOI] [PubMed] [Google Scholar]
- Rivera-Amill V, Kumar R, Noel RJ, Garcia Y, Jr, Rodriguez IV, Martinez M, Sariol CA, Kraiselburd E, Iszard M, Mukherji M, Kumar S, Giavedoni LD, Kumar A. Short communication: Lack of immune response in rapid progressor morphine-dependent and SIV/SHIV-infected rhesus macaques is correlated with downregulation of TH1 cytokines. AIDS Res Hum Retroviruses. 2010;26:919–922. doi: 10.1089/aid.2010.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GM, Thornton NJ, Rout J, Mackenzie N. AIDS--risk behaviours and AIDS knowledge in intravenous drug users. N Z Med J. 1987;100:209–11. [PubMed] [Google Scholar]
- Rojavin M, Szabo I, Bussiere JL, Rogers TJ, Adler MW, Eisenstein TK. Morphine treatment in vitro or in vivo decreases phagocytic functions of murine macrophages. Life Sci. 1993;53:997–1006. doi: 10.1016/0024-3205(93)90122-j. [DOI] [PubMed] [Google Scholar]
- Roy S, Balasubramanian S, Sumandeep S, Charboneau R, Wang J, Melnyk D, Beilman GJ, Vatassery R, Barke RA. Morphine directs T cells toward T(H2) differentiation. Surgery. 2001;130:304–309. doi: 10.1067/msy.2001.116033. [DOI] [PubMed] [Google Scholar]
- Roy S, Barke RA, Loh HH. MU-opioid receptor-knockout mice: role of mu-opioid receptor in morphine mediated immune functions. Brain Res Mol Brain Res. 1998a;61:190–194. doi: 10.1016/s0169-328x(98)00212-5. [DOI] [PubMed] [Google Scholar]
- Roy S, Cain KJ, Charboneau RG, Barke RA. Morphine accelerates the progression of sepsis in an experimental sepsis model. Adv Exp Med Biol. 1998b;437:21–31. doi: 10.1007/978-1-4615-5347-2_3. [DOI] [PubMed] [Google Scholar]
- Roy S, Chapin RB, Cain KJ, Charboneau RG, Ramakrishnan S, Barke RA. Morphine inhibits transcriptional activation of IL-2 in mouse thymocytes. Cell Immunol. 1997;179:1–9. doi: 10.1006/cimm.1997.1147. [DOI] [PubMed] [Google Scholar]
- Roy S, Loh HH. Effects of opioids on the immune system. Neurochem Res. 1996;21:1375–1386. doi: 10.1007/BF02532379. [DOI] [PubMed] [Google Scholar]
- Roy S, Wang J, Charboneau R, Loh HH, Barke RA. Morphine induces CD4+ T cell IL-4 expression through an adenylyl cyclase mechanism independent of the protein kinase A pathway. J Immunol. 2005;175:6361–6367. doi: 10.4049/jimmunol.175.10.6361. [DOI] [PubMed] [Google Scholar]
- Roy S, Wang J, Gupta S, Charboneau R, Loh HH, Barke RA. Chronic morphine treatment differentiates T helper cells to Th2 effector cells by modulating transcription factors GATA 3 and T-bet. J Neuroimmunol. 2004;147:78–81. doi: 10.1016/j.jneuroim.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Roy S, Wang J, Kelschenbach J, Koodie L, Martin J. Modulation of immune function by morphine: implications for susceptibility to infection. J Neuroimmune Pharmacol. 2006;1:77–89. doi: 10.1007/s11481-005-9009-8. [DOI] [PubMed] [Google Scholar]
- Roy S, Wang JH, Balasubramanian S, Sumandeep Charboneau R, Barke R, Loh HH. Role of hypothalamic-pituitary axis in morphine-induced alteration in thymic cell distribution using mu-opioid receptor knockout mice. J Neuroimmunol. 2001;116:147–55. doi: 10.1016/s0165-5728(01)00299-5. [DOI] [PubMed] [Google Scholar]
- Ruff MR, Wahl SM, Mergenhagen S, Pert CB. Opiate receptor-mediated chemotaxis of human monocytes. Neuropeptides. 1985;5:363–366. doi: 10.1016/0143-4179(85)90029-0. [DOI] [PubMed] [Google Scholar]
- Sacerdote P. Opioids and the immune system. Palliat Med. 2006;20(Suppl 1):s9–15. [PubMed] [Google Scholar]
- Saurer TB, Carrigan KA, Ijames SG, Lysle DT. Suppression of natural killer cell activity by morphine is mediated by the nucleus accumbens shell. J Neuroimmunol. 2006a;173:3–11. doi: 10.1016/j.jneuroim.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Saurer TB, Ijames SG, Lysle DT. Neuropeptide Y Y1 receptors mediate morphine-induced reductions of natural killer cell activity. J Neuroimmunol. 2006b;177:18–26. doi: 10.1016/j.jneuroim.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Scheidegger C, Zimmerli W. Infectious complications in drug addicts: seven-year review of 269 hospitalized narcotics abusers in Switzerland. Rev Infect Dis. 1989;11:486–493. doi: 10.1093/clinids/11.3.486. [DOI] [PubMed] [Google Scholar]
- Schwab CL, Fan R, Zheng Q, Myers LP, Hebert P, Pruett SB. Modeling and predicting stress-induced immunosuppression in mice using blood parameters. Toxicol Sci. 2005;83:101–113. doi: 10.1093/toxsci/kfi014. [DOI] [PubMed] [Google Scholar]
- Sedqi M, Roy S, Ramakrishnan S, Elde R, Loh HH. Complementary DNA cloning of a mu-opioid receptor from rat peritoneal macrophages. Biochem Biophys Res Commun. 1995;209:563–574. doi: 10.1006/bbrc.1995.1538. [DOI] [PubMed] [Google Scholar]
- Sharp BM, Keane WF, Suh HJ, Gekker G, Tsukayama D, Peterson PK. Opioid peptides rapidly stimulate superoxide production by human polymorphonuclear leukocytes and macrophages. Endocrinology. 1985;117:793–795. doi: 10.1210/endo-117-2-793. [DOI] [PubMed] [Google Scholar]
- Simpkins CO, Alailima ST, Tate EA, Johnson M. The effect of enkephalins and prostaglandins on O-2 release by neutrophils. J Surg Res. 1986;41:645–652. doi: 10.1016/0022-4804(86)90090-9. [DOI] [PubMed] [Google Scholar]
- Simpkins CO, Dickey CA, Fink MP. Human neutrophil migration is enhanced by beta-endorphin. Life Sci. 1984;34:2251–2255. doi: 10.1016/0024-3205(84)90213-3. [DOI] [PubMed] [Google Scholar]
- Singh PP, Singal P. Morphine-induced neuroimmunomodulation in murine visceral leishmaniasis: the role(s) of cytokines and nitric oxide. J Neuroimmune Pharmacol. 2007;2:338–351. doi: 10.1007/s11481-007-9094-y. [DOI] [PubMed] [Google Scholar]
- Singh RP, Jhamb SS, Singh PP. Effects of morphine during Mycobacterium tuberculosis H37Rv infection in mice. Life Sci. 2008;82:308–314. doi: 10.1016/j.lfs.2007.11.024. [DOI] [PubMed] [Google Scholar]
- Song C, Rahim RT, Davey PC, Bednar F, Bardi G, Zhang L, Zhang N, Oppenheim JJ, Rogers TJ. PKC{zeta} mediates {micro}-opioid receptor-induced cross-desensitization of CCR5. J Biol Chem. 2011 doi: 10.1074/jbc.M110.177303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staahl C, Dimcevski G, Andersen SD, Thorsgaard N, Christrup LL, Arendt-Nielsen L, Drewes AM. Differential effect of opioids in patients with chronic pancreatitis: an experimental pain study. Scand J Gastroenterol. 2007;42:383–390. doi: 10.1080/00365520601014414. [DOI] [PubMed] [Google Scholar]
- Stankiewicz E, Wypasek E, Plytycz B. Mast cells are responsible for the lack of anti-inflammatory effects of morphine in CBA mice. Mediators Inflamm. 2004;13:365–368. doi: 10.1155/S0962935104000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele AD, Henderson EE, Rogers TJ. Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology. 2003;309:99–107. doi: 10.1016/s0042-6822(03)00015-1. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Chuang AJ, Chuang LF, Doi RH, Chuang RY. Morphine promotes simian acquired immunodeficiency syndrome virus replication in monkey peripheral mononuclear cells: induction of CC chemokine receptor 5 expression for virus entry. J Infect Dis. 2002;185:1826–1829. doi: 10.1086/340816. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Chuang LF, Doi RH, Chuang RY. Morphine suppresses lymphocyte apoptosis by blocking p53-mediated death signaling. Biochem Biophys Res Commun. 2003;308:802–808. doi: 10.1016/s0006-291x(03)01472-4. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Chuang LF, Yau P, Doi RH, Chuang RY. Interactions of opioid and chemokine receptors: oligomerization of mu, kappa, and delta with CCR5 on immune cells. Exp Cell Res. 2002;280:192–200. doi: 10.1006/excr.2002.5638. [DOI] [PubMed] [Google Scholar]
- Szabo I, Rojavin M, Bussiere JL, Eisenstein TK, Adler MW, Rogers TJ. Suppression of peritoneal macrophage phagocytosis of Candida albicans by opioids. J Pharmacol Exp Ther. 1993;267:703–706. [PubMed] [Google Scholar]
- Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, Magnus T, Camandola S, Mattson MP. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub DD, Eisenstein TK, Geller EB, Adler MW, Rogers TJ. Immunomodulatory activity of mu- and kappa-selective opioid agonists. Proc Natl Acad Sci U S A. 1991;88:360–364. doi: 10.1073/pnas.88.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegeder I, Geisslinger G. Opioids as modulators of cell death and survival--unraveling mechanisms and revealing new indications. Pharmacol Rev. 2004;56:351–369. doi: 10.1124/pr.56.3.2. [DOI] [PubMed] [Google Scholar]
- Thomas PT, House RV, Bhargava HN. Direct cellular immunomodulation produced by diacetylmorphine (heroin) or methadone. Gen Pharmacol. 1995;26:123–130. doi: 10.1016/0306-3623(94)00162-g. [DOI] [PubMed] [Google Scholar]
- Tomassini N, Renaud F, Roy S, Loh HH. Morphine inhibits Fc-mediated phagocytosis through mu and delta opioid receptors. J Neuroimmunol. 2004;147:131–133. doi: 10.1016/j.jneuroim.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Tomassini N, Renaud FL, Roy S, Loh HH. Mu and delta receptors mediate morphine effects on phagocytosis by murine peritoneal macrophages. J Neuroimmunol. 2003;136:9–16. doi: 10.1016/s0165-5728(02)00463-0. [DOI] [PubMed] [Google Scholar]
- Tomei EZ, Renaud FL. Effect of morphine on Fc-mediated phagocytosis by murine macrophages in vitro. J Neuroimmunol. 1997;74:111–116. doi: 10.1016/s0165-5728(96)00213-5. [DOI] [PubMed] [Google Scholar]
- Topp L, Iversen J, Wand H, Day C, Kaldor J, Maher L. Representativeness of injecting drug users who participate in HIV surveillance: results from Australia’s Needle and Syringe Program Survey. J Acquir Immune Defic Syndr. 2008;47:632–8. doi: 10.1097/qai.0b013e31816a1d68. [DOI] [PubMed] [Google Scholar]
- Tubaro ESC. Methadone vs morphine: comparison of their effect on phagocytic functions. Int J Immunopharmacol. 1987;1987:79–88. doi: 10.1016/0192-0561(87)90113-5. [DOI] [PubMed] [Google Scholar]
- Tubaro E, Borelli G, Croce C, Cavallo G, Santiangeli C. Effect of morphine on resistance to infection. J Infect Dis. 1983;148:656–666. doi: 10.1093/infdis/148.4.656. [DOI] [PubMed] [Google Scholar]
- Turchan-Cholewo J, Dimayuga FO, Ding Q, Keller JN, Hauser KF, Knapp PE, Bruce-Keller AJ. Cell-specific actions of HIV-Tat and morphine on opioid receptor expression in glia. J Neurosci Res. 2008;86:2100–10. doi: 10.1002/jnr.21653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo R, de Leon-Casasola O, Benyamin R. Opioid therapy and immunosuppression: a review. Am J Ther. 2004;11:354–365. doi: 10.1097/01.mjt.0000132250.95650.85. [DOI] [PubMed] [Google Scholar]
- van Epps DE, Saland L. Beta-endorphin and met-enkephalin stimulate human peripheral blood mononuclear cell chemotaxis. J Immunol. 1984;132:3046–3053. [PubMed] [Google Scholar]
- Venkatesan A, Nath A, Ming GL, Song H. Adult hippocampal neurogenesis: regulation by HIV and drugs of abuse. Cell Mol Life Sci. 2007;64:2120–32. doi: 10.1007/s00018-007-7063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Barke RA, Charboneau R, Loh HH, Roy S. Morphine negatively regulates interferon-gamma promoter activity in activated murine T cells through two distinct cyclic AMP-dependent pathways. J Biol Chem. 2003;278:37622–37631. doi: 10.1074/jbc.M301224200. [DOI] [PubMed] [Google Scholar]
- Wang J, Barke RA, Charboneau R, Roy S. Morphine impairs host innate immune response and increases susceptibility to Streptococcus pneumoniae lung infection. J Immunol. 2005;174:426–434. doi: 10.4049/jimmunol.174.1.426. [DOI] [PubMed] [Google Scholar]
- Wang J, Barke RA, Charboneau R, Schwendener R, Roy S. Morphine induces defects in early response of alveolar macrophages to Streptococcus pneumoniae by modulating TLR9-NF-kappa B signaling. J Immunol. 2008a;180:3594–3600. doi: 10.4049/jimmunol.180.5.3594. [DOI] [PubMed] [Google Scholar]
- Wang J, Barke RA, Ma J, Charboneau R, Roy S. Opiate abuse, innate immunity, and bacterial infectious diseases. Arch Immunol Ther Exp (Warsz) 2008b;56:299–309. doi: 10.1007/s00005-008-0035-0. [DOI] [PubMed] [Google Scholar]
- Wang J, Barke RA, Roy S. Transcriptional and epigenetic regulation of interleukin-2 gene in activated T cells by morphine. J Biol Chem. 2007;282:7164–7171. doi: 10.1074/jbc.M604367200. [DOI] [PubMed] [Google Scholar]
- Wang J, Charboneau R, Balasubramanian S, Barke RA, Loh HH, Roy S. Morphine modulates lymph node-derived T lymphocyte function: role of caspase-3, -8, and nitric oxide. J Leukoc Biol. 2001;70:527–536. [PubMed] [Google Scholar]
- Wang J, Ma J, Charboneau R, Barke R, Roy S. Morphine Inhibits Murine Dendritic Cell IL-23 Production by Modulating Toll-like Receptor 2 and Nod2 Signaling. J Biol Chem. 2011a;286:10225–10232. doi: 10.1074/jbc.M110.188680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ye L, Zhou Y, Liu MQ, Zhou DJ, Ho WZ. Inhibition of anti-HIV microRNA expression: a mechanism for opioid-mediated enhancement of HIV infection of monocytes. Am J Pathol. 2011b;178:41–47. doi: 10.1016/j.ajpath.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber RJ, Ikejiri B, Rice KC, Pert A, Hagan AA. Opiate receptor mediated regulation of the immune response in vivo. NIDA Res Monogr. 1987;76:341–348. [PubMed] [Google Scholar]
- Weber RJ, Pert A. The periaqueductal gray matter mediates opiate-induced immunosuppression. Science. 1989;245:188–190. doi: 10.1126/science.2749256. [DOI] [PubMed] [Google Scholar]
- Welters ID, Menzebach A, Goumon Y, Cadet P, Menges T, Hughes TK, Hempelmann G, Stefano GB. Morphine inhibits NF-kappaB nuclear binding in human neutrophils and monocytes by a nitric oxide-dependent mechanism. Anesthesiology. 2000;92:1677–1684. doi: 10.1097/00000542-200006000-00027. [DOI] [PubMed] [Google Scholar]
- West JP, Dykstra LA, Lysle DT. Immunomodulatory effects of morphine withdrawal in the rat are time dependent and reversible by clonidine. Psychopharmacology (Berl) 1999;146:320–7. doi: 10.1007/s002130051123. [DOI] [PubMed] [Google Scholar]
- Wiesli P, Pavlicek V, Perren A, Baechli E, Pfammatter T, Krahenbuhl L, Schulthess G, Schmid C. Recurrent hypoglycaemia in HIV-positive narcotic addicts. Swiss Med Wkly. 2006;136:805–10. doi: 10.4414/smw.2006.11549. [DOI] [PubMed] [Google Scholar]
- Yang EV, Glaser R. Stress-induced immunomodulation and the implications for health. Int Immunopharmacol. 2002;2:315–24. doi: 10.1016/s1567-5769(01)00182-5. [DOI] [PubMed] [Google Scholar]
- Yokota T, Uehara K, Nomoto Y. Addition of noradrenaline to intrathecal morphine augments the postoperative suppression of natural killer cell activity. J Anesth. 2004;18:190–195. doi: 10.1007/s00540-004-0247-3. [DOI] [PubMed] [Google Scholar]
- Yossuck P, Nightengale BJ, Fortney JE, Gibson LF. Effect of morphine sulfate on neonatal neutrophil chemotaxis. Clin J Pain. 2008;24:76–82. doi: 10.1097/AJP.0b013e3181582c76. [DOI] [PubMed] [Google Scholar]
- Yukioka H, Rosen M, Evans KT, Leach KG, Hayward MW, Saggu GS. Gastric emptying and small bowel transit times in volunteers after intravenous morphine and nalbuphine. Anaesthesia. 1987;42:704–710. doi: 10.1111/j.1365-2044.1987.tb05314.x. [DOI] [PubMed] [Google Scholar]
- Zhang N, Oppenheim JJ. Crosstalk between chemokines and neuronal receptors bridges immune and nervous systems. J Leukoc Biol. 2005;78:1210–1214. doi: 10.1189/jlb.0405224. [DOI] [PubMed] [Google Scholar]
- Zhang T, Li Y, Ho WZ. Drug abuse, innate immunity and hepatitis C virus. Rev Med Virol. 2006;16:311–327. doi: 10.1002/rmv.508. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li H, Li Y, Sun X, Zhu M, Hanley G, Lesage G, Yin D. Essential role of toll-like receptor 2 in morphine-induced microglia activation in mice. Neurosci Lett. 2011;489:43–47. doi: 10.1016/j.neulet.2010.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]