Abstract
Radiotherapy is a frontline treatment for the clinical management of CNS tumors. Though effective in eradicating tumor cells, radiotherapy also depletes neural stem and progenitor cells in the hippocampus that are important for neurogenesis and cognitive function. Consequently, the use of radiation to control primary and metastatic brain tumors often leads to debilitating and progressive cognitive decrements in surviving patients, representing a serious medical condition that to date, has no satisfactory, long-term solutions. As a result, we have explored the use of stem cells as therapeutic agents to improve cognition after radiotherapy. Our past work has demonstrated the capability of cranially transplanted human embryonic (hESCs) and neural (hNSCs) stem cells to functionally restore cognition in rats 1 and 4-months after head-only irradiation. We have now expanded our cognitive analyses with hESCs and quantified both survival and differentiated fates of engrafted cells at 1 and 4 months after irradiation. Our findings indicate the capability of hESC transplantation to ameliorate radiation-induced cognitive dysfunction 1-month following cranial irradiation, using a hippocampal-dependent novel place recognition task. Irradiated animals not engrafted with stem cells experienced prolonged and significant cognitive dysfunction. Stereological estimates indicated that 35% and 17% of the transplanted hESCs survived at 1- and 4-months post-grafting, respectively. One month after irradiation and grafting, phenotypic analyses revealed that 26% and 31% of the hESCs differentiated into neurons and astrocytes, while at the 4-month time, neuronal and astrocytic differentiation was 7% and 46%, respectively. Comparisons between present and past data with hESCs and hNSCs demonstrates equivalent cognitive restoration and a preference of hNSCs to commit to neuronal versus astrocytic lineages over extended engraftment times. Our data demonstrate the functional utility of human stem cell replacement strategies for ameliorating the adverse effects of cranial irradiation on cognition.
Keywords: Human embryonic stem cells, human neural stem cells, transplantation, radiation, cognition, hippocampus, spatial recognition memory
INTRODUCTION
Radiotherapy provides the most effective means for forestalling the progression of primary and secondary malignancies in the brain. While beneficial, cranial irradiation causes an unintended and deleterious decline in cognitive function in cancer survivors, representing a significant clinical problem that remains unresolved with no long-term, satisfactory solutions (1,8). Pediatric cases are a particular concern, as their longer-term survival is with marked cognitive decrements that severely impact quality of life and impose significant socioeconomic burdens (22,31). Despite treatments designed to limit overt morphologic brain injury, variable degrees of cognitive dysfunction that manifest as impaired hippocampal-dependent (and independent) learning and memory occur routinely and persist, and can include disrupted attention, concentration, and impaired executive functions such as planning and multi-tasking (20,28-30). Thus, although advancements in cancer treatment have led to prolonged survival, similar improvements to long-term cognitive function have not been realized.
The mechanisms underlying radiation-induced cognitive dysfunction are complex, but accumulating evidence now indicates that disruption to hippocampal neurogenesis plays a contributory if not causal role (20,36). Ionizing radiation depletes radiosensitive populations of mulitpotent neural stem and progenitor cells in the hippocampus and elicits a persistent oxidative stress that compromises the neurogenic niche and serves to impair neurogenesis long after irradiation (6,14,19-21). The temporal time course of stem cell death, the inhibition of neurogenesis and the onset of hippocampal-dependent learning and memory have suggested the importance of neural stem cells and neurogenesis in the maintenance of cognitive function throughout life (20,21). Such evidence also implies that strategies directed at preserving and/or replenishing stem cell pools in the brain may forestall the development of cognitive impairments that plague those surviving the treatment of brain cancer.
To explore interventions that might provide some clinical recourse to those afflicted with radiation-induced cognitive dysfunction, we have developed an animal model in which we have conducted intrahippocampal transplantation of human stem cells following cranial irradiation (2,3). Our prior work has now established the efficacy of this methodology, whereby human embryonic (hESCs) or neural (hNSCs) stem cells transplanted 2 days after head only irradiation were found to survive, differentiate along neuronal and glial lineages and prevent (or improve) impairments in cognition as long as 1 and 4 months afterwards (2-5). To more completely understand the potential limitations and the underlying mechanisms responsible for the beneficial effects of cranially transplanted stem cells, we have expanded on our studies using hESC significantly. Here we report our findings that hESC transplantation improves cognition at earlier as well as later times post-engraftment, along with the quantified yields of engrafted cell survival and differentiated fate 1 and 4 months later. We also compare the functional consequences and outcomes of transplanted human stem cells in the irradiated brain.
MATERIALS AND METHODS
Animals and irradiation
All animal procedures were performed in accordance with NIH and Institutional IACUC guidelines. As in our previous study (2,3), we employed immuno-compromised athymic nude rats (ATN) to circumvent cross-species immune rejection. A total of 38 young, male (2 month old) ATN rats (strain 02N01 Cr:NIH-rnu; NCI), maintained in sterile conditions, were used in this study. Animal groups included: non-irradiated sham surgery controls (CON; 1-month and 4-month, n=8); irradiated sham surgery, (IRR; 1-month, n=6, 4-month, n=12) and irradiated with engrafted hESCs, (IRR+hESC; 1-month, n=6, 4-month, n=6). Anesthetized rats were exposed to cranial γ-irradiation (10 Gy) using a 137Cs irradiator (J.L. Shepard and Associates Mark I, Glendale, CA) at a dose rate of 2.07 Gy/min, as previously described (2,3).
Human stem cell (hESC and hNSC) transplantation surgery
The use of human stem cells in this study was approved by UCI’s Human Stem Cell Research Oversight Committee (hSCRO). The hESC line H9 (a kind gift from Dr. Peter J. Donovan, UC Irvine) was maintained as monolayer colonies (passages 42-49), as described previously (2). Prior to transplantation, undifferentiated hESCs were labeled with 5-bromo-2′-deoxyuridine (BrdU, Sigma-Aldrich) and BrdU incorporation was detected using immunocytochemistry according to a standard protocol (2,3). The in vitro BrdU index for all transplantation studies was 98 ± 0.75 (Mean ± S.E.M., N=16). The data for hNSCs (ENStem-A cell line, EMD Millipore) described in our previous study (3), has been adapted and re-plotted for comparison with our new hESC data presented in this study.
Irradiated rats received bilateral, intrahippocampal engraftment of BrdU-labeled hESCs two days after cranial irradiation (IRR+hESC). 1.0 × 105 live undifferentiated hESCs were injected per grafting site in 1 μl of the cell suspension using a 33-gauge microsyringe at an injection rate of 0.25 μl/min. Each hippocampus received 4 distinct injections (total 4.0 × 105 live hESCs per hemisphere) using the following stereotaxic coordinates (2): (i) AP: 3.0 mm from bregma, ML: 1.8 mm from midline, and ventral DV: 3.2 mm from the surface of the brain; (ii) AP: 3.6 mm, ML: 2.5 mm, DV: 3.2 mm; (iii) AP: 4.2 mm, ML: 3.2 mm, DV: 3.2 mm and (iv) AP: 4.8 mm, ML: 4.0 mm, DV: 3.2 mm. Control (CON) and Irradiated (IRR) rats receiving sterile vehicle (conditioned medium) at the same stereotaxic coordinates served as sham surgery groups.
Assessment of cognition
CON, IRR and IRR+hESCs groups were tested on a novel place recognition (NPR) task at 1-month or 4-months post-grafting. The NPR task uses spontaneous exploration as a means of assessing spatial recognition memory, which relies on intact hippocampal function (23,32). A standard protocol (2,3) was followed employing Ethovision XT (v6.0; Noldus Information Technology, Inc., Leesburg, VA) software for automated tracking and exploration detection. Two open field white acrylic arenas, each measuring 45 high × 70 × 70 cm were placed next to each other on the floor of a brightly lit, dedicated behavioral testing room. Various large, high contrast posters placed on the walls of the testing rooms served as extramaze spatial cues. The task began with 2 days of habituation during which rats explored the arenas and two toy objects that were not used for subsequent testing for 20min before being returned to their home cages. For all phases, arenas and objects were cleaned with 70% ethanol between trials to minimize odor cues. The familiarization phase and 5-minute test phase were administered the following day. Identical plastic blocks (~8 × 3 × 10 cm high), placed 27 cm from opposing corners of the arena were used as stimuli. Small pieces of white Velcro placed on the undersides of the blocks were used to secure them in place during testing. For the familiarization phase, rats were placed in the arenas to explore for 5 min, and then returned to a holding cage for a 5-minute retention interval. For the 5-minute test phase, one of the stimuli was moved to an open corner at a distance of 18 cm from the arena wall (“novel place”), while the other block remained at its former spatial location (“familiar place”) and rats were returned to the arenas for 3min. Rats were then returned to their home cages for a delay of 24h before being returned to the arenas for the 24-hour test phase. For this phase, the “novel place” block was again moved, this time to the remaining open corner, and the “familiar place” block remained in the same location. For all phases, the “head direction to zone” function in Ethovision XT was used to track exploration of the blocks. Exploration was defined as the animal’s head being directed towards and located within a 2cm radius of the stimulus.
Immunohistochemistry, stereology and confocal microscopy
At the end of behavior testing, animals were euthanized by intracardiac perfusion and tissues were processed as described (2). For stereological quantification, every 10th section through the entire hippocampus was processed for BrdU immunostaining (3). Stereological counting of BrdU positive nuclei was done using an Olympus BX60 microscope (MBF CX9000 color digital camera and 100× oil-immersion lens with 1.30 numerical aperture), 3-axis motorized stage and StereoInvestigator software (v9.0, MBF Biosciences, Williston, VT) using unbiased stereological principles, as described previously (3). The surviving hESCs are reported as the percentage yield of graft derived cells at 1- and 4-months post-grafting and compared to our previous data with hNSCs (3). The Guenderson co-efficient of error (CE) for each hippocampus was <0.038 ± 0.0025 (Mean ± S.E.M., N=8).
Phenotypic fate analysis of hESC graft-derived cells was done using dual immunofluorescence and laser scanning confocal microscopy (Nikon Eclipse TE2000-U, EZ-C1 interface) as described previously (2,3). Standard immunostaining protocols were used for BrdU and neuronal (Tuj1, NeuN), astro-glial (GFAP, S100, O4, NG2) and functional markers (Ki67, Nestin) as described in detail previously (2,3). Z-stack analyses were carried out using 1 μm stack intervals and orthogonal image reconstruction was done using Nikon Elements AR software (v3.2, Nikon Instech Co. Ltd., Japan).
Statistical analyses
Exploration ratio, or the proportion of total time spent exploring the novel spatial location (tnovel/tnovel+tfamiliar), was used as the main dependent measure for statistical analyses. We analyzed the behavior of the animals during minute 1 of the 5-min and 24-hr test phases; previous research has shown that preference for the novel place diminishes after the first minute as the spatial locations become equally familiar to the animals (23). To establish baseline exploratory behavior, we also analyzed exploration of the stimuli during the initial familiarization phase.
Analyses of cognitive data were performed using PASW Statistics version 17.0 (SPSS Inc.). Analyses for the 1-month and 4-month post-transplantation times were conducted separately. Group sample sizes for the statistical analyses were as follows: non-irradiated sham surgery controls (CON; 1-month and 4-month, n=8); irradiated sham surgery, (IRR; 1-month, n=6, 4-month, n=12) and irradiated with engrafted hNSCs, (IRR+hNSC; 1-month, n=6, 4-month, n=6). In all cases, statistical tests were two-tailed, and p-values less than 0.05 were considered statistically significant.
Exploration ratio data were analyzed using univariate ANOVAs for the 5-min and 24-hr test phases. In all cases, we confirmed that the data were normally-distributed using the Kolmogorov-Smirnov test and that the error variances did not differ between groups by using Levene’s test of equality of error variances. When a statistically significant overall group effect was found, multiple comparisons were made using Fisher’s Protected Least Significant Difference (FPLSD) post-hoc tests to compare the individual groups. Additional analyses of recognition memory were conducted using one-sample t-tests to determine if the mean proportion of time spent exploring the novel spatial location for each group differed significantly from chance (i.e. 0.5).
RESULTS
HESC grafting improves cognition
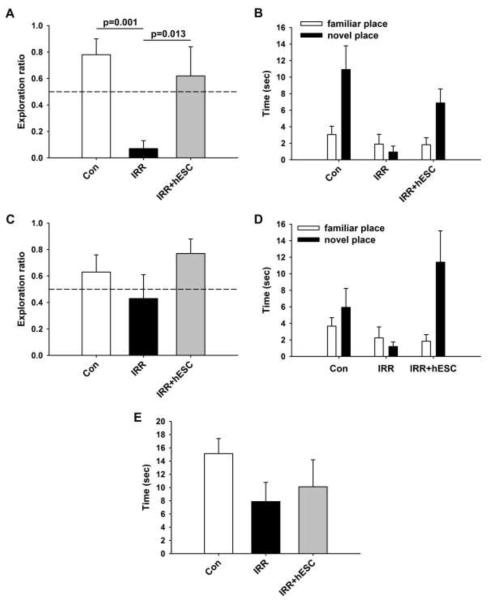
One month post-grafting, rats were habituated and trained on the NPR task. Successful performance of the task has been shown to rely on intact hippocampal function (23,32). As we have demonstrated in past studies (2,3) irradiated rats (IRR) showed impaired novel place recognition at the short, 5-min retention interval. The group means and 95% CI for the 5-min test were: CON (mean=0.7812, 95% CI=0.5174-1.0451); IRR (mean=0.0717, 95% CI=-0.0641-0.2074) and IRR+hESC (mean=0.6150, 95% CI 0.1097-1.1203). We found significant group differences for exploration ratio at the 5-min retention interval [Fig. 1A; F(2,17)=7.912; p=0.004]. Irradiated animals spent significantly less time exploring the novel place compared to both CON (p<0.001) and IRR+hESC (p=0.013). Moreover, IRR animals explored the novel place less than expected by chance (p=0.001). In contrast, after the 5-min retention interval, irradiated, hESC-transplanted rats (IRR+hESC) did not differ from CON animals and like controls spent more time than expected by chance exploring the novel spatial location (indicated by the dashed line in Fig. 1A). Analogous trends were observed in terms of total time spent exploring the novel spatial location (Fig. 1B), and in frequency of visits to the novel spatial location and latency to explore the novel spatial location (data not shown). These results suggest that transplantation of hESC prevented (or improved) post-irradiation cognitive impairments and preserved short-term memory for spatial information in a hippocampal-dependent task up to 1 month later.
Figure 1.
Human embryonic stem cell grafting improves radiation-induced impairments in novel place recognition assessed 1 month post-implantation. (A) and (C) Exploration ratios (i.e. timenovel/timenovel+timefamiliar) for the first minute of the 5min and 24hr test sessions, respectively. (A) Following the 5min retention interval, irradiated animals (IRR) spent a significantly lower proportion of time exploring the novel place vs. controls (Con; p=0.001) and transplanted animals (IRR+hESC; p=0.013). In contrast, irradiated animals that received hESC injections (IRR+hESC) did not differ from controls (p=0.375). (C) After the 24-h retention interval, animals in the IRR group spent a lower proportion of time exploring the novel place, although this difference was not statistically significant. IRR+hESC animals showed a trend toward improved performance compared to the IRR cohort, and spent more time than expected by chance exploring the novel spatial location (p=0.045). Similar trends were observed when total time spent exploring the novel place was assessed after both the 5-min (B) and 24-h (D) retention intervals. Analysis of time spent exploring both objects during the initial familiarization phase revealed no significant group differences, although IRR animals tended to spend less time engaged in exploration than both the Con and IRR+hESC groups (E).
At the 24-h retention interval, IRR rats again exhibited impaired NPR compared to control animals (Fig. 1C). As before, IRR+hESC rats did not differ significantly from CON animals and spent more time than expected by chance exploring the novel spatial location (p=0.045, Fig. 1C). For this longer retention interval, the irradiated group transplanted with stem cells (IRR+hESC) also showed a trend for increased exploration ratio compared to irradiated (IRR alone) animals not given stem cells (Fig. 1C). Again, similar trends were observed when total exploration of the novel spatial location (Fig. 1D), as well as frequency of visits and latency to first explore the novel location was analyzed (data not shown). These results suggest that hESC transplantation may also improve long-term retention of spatial information, although the treatment appeared to be more effective at the shorter, 5-min retention interval.
To analyze further why IRR animals showed impaired short- and long-term NPR behavior, total time spent exploring both objects during the familiarization phase given before the retention intervals was analyzed. IRR animals tended to spend less time exploring during the familiarization phase compared to both the control and IRR+hESC animals (Fig. 1E). This result suggests that the impairments seen in the IRR animals at 5min and 24h post-familiarization might be related to lower exploration during initial exposure to the objects.
Comparing cognitive outcomes following stem cell grafting
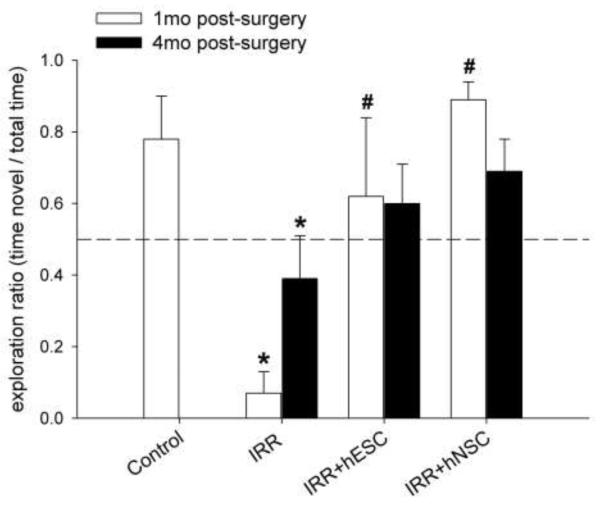
The foregoing results now provide the basis to make a comparison between hESC and hNSC transplantation for the restoration of cognition 1 and 4-months following irradiation. As indicated, IRR animals did not explore the novel spatial location more than expected by chance (dashed line) and showed significant deficits compared to non-irradiated controls (1-month, p=0.001 and 4-month, p=0.014; Fig. 2) following the 5min retention interval. Animals transplanted with hESC or hNSC and tested on the NPR task at a 5min retention interval 1 or 4-months later did not differ from non-irradiated controls at either post-irradiation time and tended to explore the novel spatial location more than expected by chance (Fig. 2). Transplantation of irradiated animals with human stem cells (IRR+hESC or IRR+hNSC) improved cognition significantly at 1 month when compared to their corresponding IRR group (Fig. 2, p<0.05). At 4-months post-irradiation, stem cell-engrafted groups showed a trend toward improved cognition compared to non-transplanted animals tested 4-months post-irradiation (Fig. 2).
Figure 2.
IRR-induced deficits in novel place recognition (NPR) performance are ameliorated in animals receiving hippocampal transplantation of human stem cells 1- and 4-months after irradiation. Irradiated animals did not explore the novel spatial location more than expected by chance (indicated by dashed line) and showed significant deficits compared to non-irradiated controls. By contrast, animals transplanted with either hESCs or hNSCs did not differ from non-irradiated controls on the 5min test and tended to explore the novel spatial location more than chance. At 1-month, transplanted animals showed a significant improvement in NPR performance. Similar trends were observed 4-months post-IRR in both transplanted groups. Graphs show group means + 1 S.E.M. *indicates significant difference versus controls; # indicates significant difference versus corresponding IRR group (i.e. p<0.05 on posthoc Fisher LSD group comparisons).
Stereological enumeration of engrafted cell survival
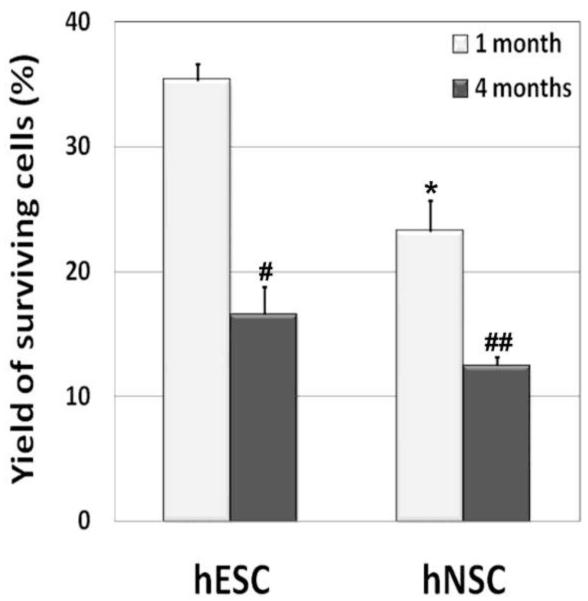
To establish the extent of engrafted cell survival at 1- and 4-months post-irradiation, unbiased stereology was undertaken to quantify the yield of human cells in the host brain. Optical fractionator analysis of brain sections from the 1- and 4-months post-engraftment group using BrdU immunostaining revealed that engrafted cells migrated extensively throughout the hippocampus, populating the dentate gyrus (DG), dentate hilus (DH), CA3 and CA1 subfields, and slightly into the corpus callosum (CC) (data not shown). Past work has shown that engrafted cells preferentially migrated to the dentate subgranular zone (SGZ), the neurogenic niche of the hippocampus, at both the 1- and 4-months post-engraftment time points (2). Stereologic quantification of 1- and 4-months tissues revealed engrafted cell survival levels of 35% and 17%, respectively (Fig 3), whereas, for hNSCs, engrafted cell survival was 23% and 12.5% respectively ((3); Fig 3). These relative yields of hESCs translated to an average of approximately 138,000 and 65,000 new cells added to each hemisphere at 1 and 4 months post-engraftment, respectively. The average yield of new hNSCs added to each hippocampus at 1 and 4-months post-engraftment were approximately 123,000 and 49,000 respectively (3). We did not observe any tumor formation at 1 and 4-months postgrafting with either human stem cell.
Figure 3.
Yield of surviving engrafted human stem cells (hESCs and hNSCs) at 1- and 4-months post-irradiation. Engrafted cells were detected by BrdU immunostaining and quantified by counting BrdU-positive nuclei using an optical fractionator and unbiased stereology. Data is represented as the Mean ± S.E.M. of 4 individual observations. #,p<0.05 and ##, p<0.043 indicates significance between 1 versus 4-month groups. *, p<0.01 denotes significance between hESC versus hNSC-engrafted groups.
Phenotypic fate of engrafted hESCs
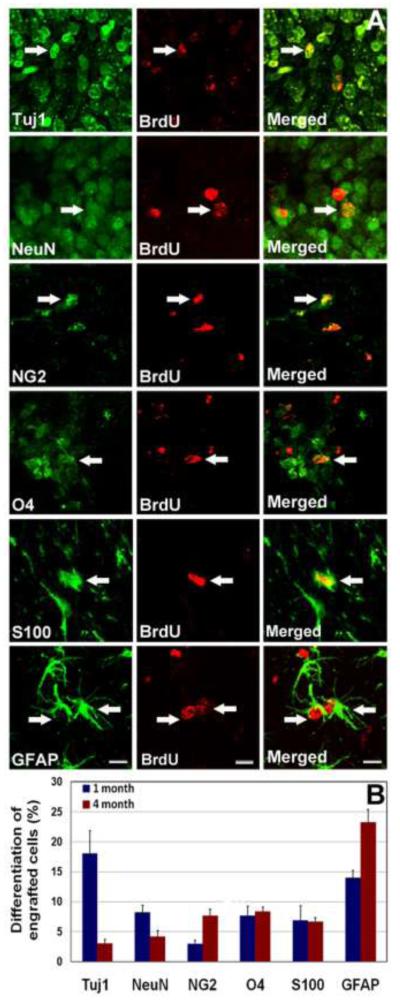
To elucidate the fate of engrafted cells, neuronal and glial markers were assessed in conjunction with BrdU labeling 1 and 4 months after grafting (Fig. 4A). The expression of the functional cell cycle marker (Ki67) was 4% at 1-month and reduced to 2% at 4-month post-grafting (data not shown). By contrast, expression of the neural stem cell marker (nestin) remained steady (1.6-2%) at both post-grafting time points (data not shown). The yield of immature (BrdUrd+/Tuj1+) neurons dropped from 18% to 3% from 1 to 4-months post-grafting, while the yield of mature (BrdU+/NeuN+) neurons reduced from 8% to 4% respectively over this same timeframe (Fig. 4B). The majority of engrafted cells differentiated along astro-glial lineages (Fig. 4A-B). The yield of immature (BrdU+/GFAP+) astrocytes increased from 14% to 23% from 1 to 4-months post-grafting, while the yield (7-8%) of mature (BrdU+/S100+) astrocytes did not differ at either time (Fig. 4B). Although BrdU+/GFAP+ engrafted cells were located throughout the hippocampus, astrocytic differentiation was most predominant in the CC (data not shown). The yield of oligo-progenitor (BrdU+/NG2+) cells increased from ~3% to 8% in the 1 to 4-month groups respectively, while mature oligodendrocytes (BrdU+/O4+) showed little change in yield (8-9%) over these same times (Fig. 4B).
Figure 4.
Differentiation of transplanted hESCs in the irradiated hippocampus. Engrafted hESCs differentiated into neuronal and astro-glial phenotypes at 1- and 4-month post-engraftment. hNSC graft-derived BrdU+ cells differentiated into Tuj1+ immature and NeuN+ mature neurons (A, B). The majority of engrafted hESCs differentiated into S100+ (S100β protein) mature, GFAP+ immature astrocytes (A, B) and O4+ mature and NG2+ oligo-progenitor cells. Arrows indicate dual-labeled engrafted cell. (Scale bars: A, 5 μm).
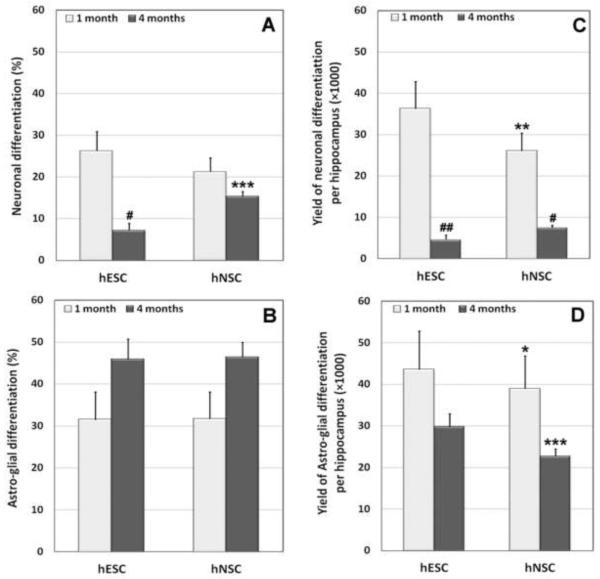
Thus, 1 month after transplantation, 26% of engrafted cells differentiated into neurons and 31% differentiated into glia (Fig. 5A and B). At 4-months the relative yield of neurons dropped to 7% while the yield of glia increased to 46% (Fig. 5A and B). Absolute numbers of newly added neurons and glia per hippocampus were also calculated (Fig.5C and D). Extrapolating these yields of differentiated cell types based on stereology estimates indicates that transplantation of hESCs resulted in the addition of approximately 36,000 neurons and 44,000 astro-glia at 1 month post-engraftment (Fig. 5C and D), whereas approximately 4,700 neurons and 30,000 astro-glia were added at 4 months post-engraftment (Fig. 5C and D) into each hemisphere of irradiated animals.
Figure 5.
Yields of differentiated human stem cells engrafted in the irradiated brain. The phenotypic fate of hESCs and hNSCs expressed as a percentage in each hippocampus of the irradiated rat brain (A, B). The yield of differentiated hESCs and hNSCs in each hippocampus of the irradiated rat brain as calculated from stereological estimates of graft-derived cells (C, D). #,p<0.002 and ##, p<0.001 indicates significant difference between 1 versus 4-month postgrafting group. *, p<0.05; **, p<0.01; ***, p<0.001 denotes significant difference between hESC versus hNSC-engrafted groups.
DISCUSSION
The radio-therapeutic management of brain cancer adversely impacts quality of life in survivors due to the progressive and often debilitating cognitive decrements that develop over time. Our finding that intrahippocampal engraftment of human stem cells prevents the development of radiation-induced cognitive impairment suggests that stem cell replacement strategies might one day be a promising strategy for treating this serious adverse side effect. Our present and past data (2-5) clearly show the benefits of using transplanted stem cells for ameliorating radiation-induced cognitive deficits. Engraftment of either hESCs or hNSCs affords comparable benefits to cognition when analyzed at 1 or 4 months after grafting based on the NPR task (Fig. 2). Unlike hESC-transplanted animals assessed 1 month post-transplantation, those assessed at the 4-month time point did not show statistically significant improvements in NPR performance compared to non-transplanted IRR animals (Fig. 2). This is due to the fact that their performance was compared with IRR animals assessed 4 months after whole-brain irradiation, which showed less impaired performance compared to those assessed 1 month post-irradiation, possibly because the protracted assessment time allowed for some amount of recovery. Further, although several independent studies have now shown the novel place recognition task to be sensitive to radiation-induced deficits as well as improvements following transplantation surgery, this behavioral test paradigm relies on spontaneous exploration, which may be confounded by group differences in locomotor activity or anxiety levels. We assessed locomotor activity (distance traveled and velocity of movement) in our experimental animals during the initial familiarization phase of the NPR task. Irradiated animals, whether they underwent transplantation surgery or not, showed reduced locomotion and velocity compared to non-irradiated controls (data not shown). Thus, additional paradigms that do not rely on spontaneous exploration but are known to engage the hippocampus, such as contextual fear conditioning and water maze spatial learning are needed to confirm and extend the present findings. Further, important decisions regarding the best choice of cell types to use for similar such strategies must also rely on additional data and endpoints, such as longer-term efficacy and safety with regards to teratoma risk. While data indicate hNSC provide slightly better cognitive benefits than hESC based on exploration ratios measured, differences between these cell types for the restoration of cognition was not however found to be statistically significant.
Following irradiation and engraftment, stem cells survived and migrated extensively throughout the hippocampus, expressing neuronal and astrocytic markers (Fig. 4, refs). Engrafted hESC survival quantified by unbiased stereology revealed that 35 and 17% of the transplanted cells survived at 1 and 4 months, respectively. By contrast, our recent findings of hNSC resulted in 23 and 12% of the transplanted cells remaining at these same time points (3). While absolute differences in the yield of surviving cells are to be expected between these cell types under the experimental conditions, it is interesting that the relative decrease in surviving cells from 1 to 4 month was nearly identical (i.e. ~2-fold reduction). Despite the drop in the number of engrafted cells, our data suggest that each stem cell type is subject to and sensitive to similar selective pressures for survival within the irradiated tissue bed.
Precisely how the attrition of engrafted cells impacts endogenous neurogenesis and cognition is unclear at this time. Cell death could cause disruptions to the neurovascular unit by altering the blood brain barrier and neurovascular niche (34,35,38). Alterations to the functional interaction between non-neuronal cells types in close proximity to neurons may modulate paracrine signaling and/or leave an inflammatory footprint for the recruitment and activation of microglia (35,38). Activated microglia increase following irradiation (20), and their role as pro- or anti-neurogenic affectors depends upon the equilibrium between secreted molecules having pro- or anti-inflammatory properties (13). While the role of immune modulation on neurogenesis, learning and memory in our irradiated animal model remains to be clarified, a wealth of evidence clearly suggests that these are important issues to resolve (7,37).
While improved cognition may track with the number of surviving cells (i.e. there was a trend of improved performance at 1-month compared to 4-months), the absolute number of engrafted cells surviving at the times of cognitive testing may have been more than necessary to ameliorate any radiation-induced cognitive decrements. On an average, about 5,000 newly born cells are added to each hippocampus (hemisphere) per day, accounting for approximately 150,000 new cells per month (26). A cranial dose of 10Gy depletes about 90% of newly born and proliferating cells but not more mature neuronal phenotypes (20). Therefore, ~135,000 fewer cells will be added to the hippocampus as a result of the irradiation 1 month prior. Based on stereological estimates, about 140,000 engrafted cells (hESCs) survived 1-month after irradiation. Therefore, this number of engrafted cells compensates for the number of newly born cells that would have survived in the unirradiated brain.
It should also be noted that cognition is a complex and multifaceted endpoint and likely to rely on many factors besides the yield of surviving engrafted cells. Nonetheless, having quantified yields of surviving engrafted cells does provide useful information in defining cellular dose-response thresholds for the improvement of cognition after cranial irradiation. Clearly, the numbers and types of engrafted cells surviving at 1 and 4 months were sufficient to offset the adverse effects of radiation on NPR performance. Whether fewer cells would be equally efficacious is uncertain at this time. In the present study quantification of phenotypic fate of the engrafted hESCs revealed the addition of both immature and mature neurons and astro-glial cell types in the irradiated hippocampus 1 and 4 months afterwards (Fig. 4). The adverse impact of irradiation on cognition was effectively counteracted 1-month later by the presence of ~140,000 newly added cells (~26% neuronal and 31% glial), while at 4-months after irradiation, only ~65,000 newly added cells (7% neuronal and 46% glial) were required to prevent the cognitive deficits observed in irradiated rats receiving no engraftment. While the mechanism(s) of stem cell based cognitive rescue has not been elucidated, it is likely that some combination of functional replacement and/or trophic support is playing a role.
Our past data showing that engrafted neurons express the activity-regulated cytoskeleton-associated protein (Arc), a behaviorally induced immediate early gene in the CNS, suggests that cell replacement may provide certain benefits to cognition (3). Nonetheless, trophic support derived from the engrafted astro-glial cells is likely to impact cognitive processes as well. Engrafted cells secrete a range of beneficial growth factors like GDNF and BDNF (9,11,18). More recent data suggest that graft-host interactions can be facilitated by manipulations to the microvascular niche and maintenance of gap junctions (17,24,25). Astrocytes are also known to modulate nucleoside metabolism, and changes in adenosine levels mediated by adenosine kinase may impact cognition through alterations in the bioenergetics of synaptic networks (10,12). Learning and activity increase levels of basic fibroblast growth factor (bFGF), which in turn increases astrocyte densities in the hippocampus (16), and infusion of bFGF following traumatic brain injury increases neurogensis and enhances cognitive recovery (33). Notwithstanding the importance of neurons, significant work has clearly defined critical roles for astrocytes in a wide spectrum of functions that support the integration of information necessary for learning and the consolidation of memory (15,27).
Collectively our transplantation data indicate the broader applicability of using stem cell-based replacement strategies for the amelioration of radiation-induced cognitive deficits. Somewhat unexpectedly, significant differences between the differentiated fates of engrafted hESC versus hNSC were not found (Fig. 5). Each cell type was found to commit to roughly the same proportion of neuronal and astroglial lineages at each time post-grafting; the only real difference was found in the higher incidence of neuronal differentiation of the hESC at the 1-month post-grafting time. These data suggest that both pluripotent and multipotent cells respond similarly to the same microenvironmental cues persisting in the irradiated host brain, where they survive and differentiate to ameliorate cognitive dysfunction over protracted times after irradiation. While additional studies regarding the persistence and safety of stem cell transplants are still required, the persistent benefits found from a single dose of stem cells in our present and past studies (2-5) suggest that this strategy may provide a satisfactory long-term solution to the cognitive deficits experienced by brain tumor survivors subjected to cranial radiotherapy.
Acknowledgments
We are grateful to Drs. Aileen J. Anderson and Brian J. Cummings for providing access to stereology equipment and Dr. Peter J. Donovan for providing human embryonic stem cells. This work was supported by the California Institute for Regenerative Medicine (CIRM) Seed Grant RS1-00413 (C.L.L.), by the National Institutes of Health NINDS Grant R01 NS074388 (C.L.L.), by the Office of Science (BER), U.S. Department of Energy Grant No. DE-FG02-09ER64798 (C.L.L.) and by a CIRM Training Grant TG2-01152 (M.M.A.).
Footnotes
Authors declare no conflicts of interest.
REFERENCES
- 1.Abayomi OK. Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol. 1996;35(6):659–663. doi: 10.3109/02841869609083995. [DOI] [PubMed] [Google Scholar]
- 2.Acharya MM, Christie LA, Lan ML, Donovan PJ, Cotman CW, Fike JR, Limoli CL. Rescue of radiation-induced cognitive impairment through cranial transplantation of human embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2009;106(45):19150–19155. doi: 10.1073/pnas.0909293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acharya MM, Christie LA, Lan ML, Giedzinski E, Fike JR, Rosi S, Limoli CL. Human neural stem cell transplantation ameliorates radiation-induced cognitive dysfunction. Cancer Res. 2011;71(14):4834–4845. doi: 10.1158/0008-5472.CAN-11-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acharya MM, Christie LA, Lan ML, Giedzinski E, Limoli CL. Human neural stem cell transplantation restores cognitive function following cranial irradiation. Cell Transplant. 2011;20(4):543. [Google Scholar]
- 5.Acharya MM, Christie LA, Lan ML, Limoli CL. Improving cognition following cranial irradiation through human stem cell transplantation. Cell Transplant. 2009;19(3):329–330. [Google Scholar]
- 6.Acharya MM, Lan ML, Kan VH, Patel NH, Giedzinski E, Tseng BP, Limoli CL. Consequences of ionizing radiation-induced damage in human neural stem cells. Free Radic. Biol. Med. 2010;49(12):1846–1855. doi: 10.1016/j.freeradbiomed.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Anderson AJ, Haus DL, Hooshmand MJ, Perez H, Sontag CJ, Cummings BJ. Achieving stable human stem cell engraftment and survival in the CNS: is the future of regenerative medicine immunodeficient? Regen. Med. 2011;6(3):367–406. doi: 10.2217/rme.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson VA, Godber T, Smibert E, Weiskop S, Ekert H. Cognitive and academic outcome following cranial irradiation and chemotherapy in children: a longitudinal study. Br. J. Cancer. 2000;82(2):255–262. doi: 10.1054/bjoc.1999.0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Muller FJ, Loring JF, Yamasaki TR, Poon WW, Green KN, LaFerla FM. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc. Natl. Acad. Sci. USA. 2009;106(32):13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boison D. Modulators of nucleoside metabolism in the therapy of brain diseases. Curr. Top. Med. Chem. 2011;11(8):1068–1086. doi: 10.2174/156802611795347609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen B, Gao XQ, Yang CX, Tan SK, Sun ZL, Yan NH, Pang YG, Yuan M, Chen GJ, Xu GT, Zhang K, Yuan QL. Neuroprotective effect of grafting GDNF gene-modified neural stem cells on cerebral ischemia in rats. Brain Res. 2009;1284:1–11. doi: 10.1016/j.brainres.2009.05.100. [DOI] [PubMed] [Google Scholar]
- 12.de Groot M, Iyer A, Zurolo E, Anink J, Heimans JJ, Boison D, Reijneveld JC, Aronica E. Overexpression of ADK in human astrocytic tumors and peritumoral tissue is related to tumor-associated epilepsy. Epilepsia. 2012;53(1):58–66. doi: 10.1111/j.1528-1167.2011.03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009;158(3):1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 14.Fike JR, Rosi S, Limoli CL. Neural precursor cells and central nervous system radiation sensitivity. Semin. Radiat. Oncol. 2009;19(2):122–132. doi: 10.1016/j.semradonc.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibbs ME, Hutchinson D, Hertz L. Astrocytic involvement in learning and memory consolidation. Neurosci. Biobehav. Rev. 2008;32(5):927–944. doi: 10.1016/j.neubiorev.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Pinilla F, So V, Kesslak JP. Spatial learning and physical activity contribute to the induction of fibroblast growth factor: neural substrates for increased cognition associated with exercise. Neuroscience. 1998;85(1):53–61. doi: 10.1016/s0306-4522(97)00576-9. [DOI] [PubMed] [Google Scholar]
- 17.Jaderstad J, Jaderstad LM, Li J, Chintawar S, Salto C, Pandolfo M, Ourednik V, Teng YD, Sidman RL, Arenas E, Snyder EY, Herlenius E. Communication via gap junctions underlies early functional and beneficial interactions between grafted neural stem cells and the host. Proc. Natl. Acad. Sci. USA. 2010;107(11):5184–5189. doi: 10.1073/pnas.0915134107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kameda M, Shingo T, Takahashi K, Muraoka K, Kurozumi K, Yasuhara T, Maruo T, Tsuboi T, Uozumi T, Matsui T, Miyoshi Y, Hamada H, Date I. Adult neural stem and progenitor cells modified to secrete GDNF can protect, migrate and integrate after intracerebral transplantation in rats with transient forebrain ischemia. Eur. J. Neurosci. 2007;26:1462–1478. doi: 10.1111/j.1460-9568.2007.05776.x. [DOI] [PubMed] [Google Scholar]
- 19.Limoli CL, Giedzinski E, Rola R, Otsuka S, Palmer TD, Fike JR. Radiation response of neural precursor cells: Linking cellular sensitivity to cell cycle checkpoints, apoptosis and oxidative stress. Radiat. Res. 2004;161:17–27. doi: 10.1667/rr3112. [DOI] [PubMed] [Google Scholar]
- 20.Mizumatsu S, Monje M, Morhardt D, Rola R, Palmer T, Fike J. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63(14):4021–4027. [PubMed] [Google Scholar]
- 21.Monje M. Cranial radiation therapy and damage to hippocampal neurogenesis. Dev. Disabil. Res. Rev. 2008;14(3):238–242. doi: 10.1002/ddrr.26. [DOI] [PubMed] [Google Scholar]
- 22.Moore BD, 3rd., Copeland DR, Ried H, Levy B. Neurophysiological basis of cognitive deficits in long-term survivors of childhood cancer. Arch. Neurol. 1992;49(8):809–817. doi: 10.1001/archneur.1992.00530320033009. [DOI] [PubMed] [Google Scholar]
- 23.Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn. Mem. 2002;9(2):49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ourednik J, Ourednik V, Lynch WP, Schachner M, Snyder EY. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat. Biotechnol. 2002;20(11):1103–1110. doi: 10.1038/nbt750. [DOI] [PubMed] [Google Scholar]
- 25.Ourednik V, Ourednik J, Xu Y, Zhang Y, Lynch WP, Snyder EY, Schachner M. Cross-talk between stem cells and the dysfunctional brain is facilitated by manipulating the niche: evidence from an adhesion molecule. Stem Cells. 2009;27(11):2846–2856. doi: 10.1002/stem.227. [DOI] [PubMed] [Google Scholar]
- 26.Parihar VK, Hattiangady B, Kuruba R, Shuai B, Shetty AK. Predictable chronic mild stress improves mood, hippocampal neurogenesis and memory. Mol. Psychiatry. 2011;16(2):171–183. doi: 10.1038/mp.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pereira A, Jr., Furlan FA. Astrocytes and human cognition: modeling information integration and modulation of neuronal activity. Prog. Neurobiol. 2010;92(3):405–420. doi: 10.1016/j.pneurobio.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Raber J. Unintended effects of cranial irradiation on cognitive function. Toxicol. Pathol. 2010;38(1):198–202. doi: 10.1177/0192623309352003. [DOI] [PubMed] [Google Scholar]
- 29.Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat. Res. 2004;162(1):39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 30.Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike JR. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp. Neurol. 2004;188(2):316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Roman DD, Sperduto PW. Neuropsychological effects of cranial radiation: current knowledge and future directions. Int. J. Radiat. Oncol. Biol. Phys. 1995;31(4):983–998. doi: 10.1016/0360-3016(94)00550-8. [DOI] [PubMed] [Google Scholar]
- 32.Save E, Buhot MC, Foreman N, Thinus-Blanc C. Exploratory activity and response to a spatial change in rats with hippocampal or posterior parietal cortical lesions. Behav. Brain Res. 1992;47(2):113–127. doi: 10.1016/s0166-4328(05)80118-4. [DOI] [PubMed] [Google Scholar]
- 33.Sun D, Bullock MR, McGinn MJ, Zhou Z, Altememi N, Hagood S, Hamm R, Colello RJ. Basic fibroblast growth factor-enhanced neurogenesis contributes to cognitive recovery in rats following traumatic brain injury. Exp. Neurol. 2009;216(1):56–65. doi: 10.1016/j.expneurol.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3(3):279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat. Neurosci. 2011;14(11):1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yazlovitskaya EM, Edwards E, Thotala D, Fu A, Osusky KL, Whetsell WO, Jr., Boone B, Shinohara ET, Hallahan DE. Lithium treatment prevents neurocognitive deficit resulting from cranial irradiation. Cancer Res. 2006;66(23):11179–11186. doi: 10.1158/0008-5472.CAN-06-2740. [DOI] [PubMed] [Google Scholar]
- 37.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 2011;25(2):181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 38.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]