Abstract
The induction of tumor vasculature, known as the ‘angiogenic switch’, is a rate-limiting step in tumor progression. Normal blood vessels are composed of two distinct cell types: endothelial cells which form the channel through which blood flows, and mural cells, the pericytes and smooth muscle cells which serve to support and stabilize the endothelium. Most functional studies have focused on the responses of endothelial cells to pro-angiogenic stimuli; however, there is mounting evidence that the supporting mural cells, particularly pericytes, may play key regulatory roles in both promoting vessel growth as well as terminating vessel growth to generate a mature, quiescent vasculature. Tumor vessels are characterized by numerous structural and functional abnormalities, including altered association between endothelial cells and pericytes. These dysfunctional, unstable vessels contribute to hypoxia, interstitial fluid pressure, and enhanced susceptibility to metastatic invasion. Increasing evidence points to the pericyte as a critical regulator of endothelial activation and subsequent vessel development, stability, and function. Here we discuss both the stimulatory and inhibitory effects of pericytes on the vasculature and the possible utilization of vessel normalization as a therapeutic strategy to combat cancer.
Keywords: Angiogenesis, Pericytes, Vascular normalization
Introduction
Angiogenesis is a dynamic process that requires coordinated interactions between vascular cells and the extracellular matrix (ECM) to properly regulate the processes of capillary sprouting, lumen formation, and vessel stabilization. Unlike vasculogenesis, where blood vessels form de novo, new blood vessels are formed in angiogenesis by sprouting from the existing vasculature, a process which requires transient phenotypic plasticity of its participating cells. Mature normal vessels are composed of quiescent and stationary endothelial cells (EC), which form the inner vessel wall and conducting tubule of blood vessels, and perivascular mural cells, the pericytes and smooth muscle cells which cover the endothelial tubule and perform support functions necessary to maintain vascular stability and tissue homeostasis. Pericytes are embedded in the basement membrane of arterioles, capillaries, and postcapillary venules, as either single cells or as a discontinuous single cell layer surrounding the endothelial tubule, and coordinate signaling with endothelial cells and other vascular components to help maintain vessel stability. In contrast, vascular smooth muscle cells (vSMC) form multiple concentric layers in association with arteries and veins and mediate vascular tone and contractility.
During the initiation of angiogenesis, the stable association between endothelial cells and pericytes in mature, normal vessels is disrupted, allowing transient phenotypic changes to occur in each cell type [1]. The pericyte coating of the vessel dissociates, followed by matrix degradation, vessel dilation and extracellular deposition of fibrin, to effectively abolish pericyte suppression of endothelial proliferation and migration in response to angiogenic signals. Assembly of a functional vascular sprout requires the selection of endothelial cells, with distinct phenotypic specifications (reviewed in [2] and [3]). Pro-angiogenic signals, such as vascular endothelial growth factor (VEGF), initiate a signaling cascade that enables an endothelial cell to become a tip cell, which guides the leading edge of the emerging sprout, whereas lateral inhibition via Dll4/Notch signaling instructs neighboring cells to become stalk cells, which follow the tip cell and proliferate to form the emerging stalk. The polarization of tip cells enables directed movement, so that the leading edge of the cell extends lamellipodia and filopodia to probe the microenvironment and detect guidance cues and repulsive signals, while the trailing edge maintains contact with stalk cells [3]. During physiological angiogenesis, mural cell precursors are then recruited to the newly developed sprout (discussed in detail below), where contact with endothelial cells results in their differentiation into mature mural cells. In this ‘maturation phase’, cell-cell junctions are established and basement membrane is reconstituted, resulting in quiescence of both cell types.
The Angiogenic Switch
It is well established that tumors must acquire the ability to stimulate capillary formation to progress from a small localized growth with a limited oxygen and nutrient supply to a well-vascularized enlarged tumor [4]. This conversion of the tumor from an avascular state to a vascular state has been termed the ‘angiogenic switch’ and occurs by local alteration of the balance of pro-angiogenic factors and the inhibitor molecules that maintain the quiescence of the vasculature [5]. This dogma has led to numerous investigations which have focused on the endothelial cell, as the main component of the vessel, and targeted its inhibition as a means to prevent pathological angiogenesis. However, the role of the mural cell, the other main component of the blood vessel, has received less attention. The importance of the initial pericyte dissociation step is underscored by the ability of breast cancer cells to increase initiation of angiogenesis without accelerating neovessel growth rate [6]. Hypoxia is thought to be an important early driver of the angiogenic switch (recently reviewed in [7]), likely by its ability to upregulate the vessel destabilizing factors VEGF [8] and Angiopoietin-2 (Ang2) [9]. Interestingly, macrophages have been demonstrated to contribute to the angiogenic switch in tumors [10]; this may result from both the ability of macrophages to produce VEGF [11] and/or Ang2 [12], as well as directly facilitate pericyte detachment from the vessel wall [1].
Abnormalities of Tumor-Associated Vasculature
Tumor-associated blood vessels exhibit structural and functional abnormalities which severely impact disease progression and the efficacies of therapies. Unlike the normal mature vasculature, which has a structural hierarchy of vessels with a characteristic size, shape and vessel wall structure, tumor vasculatures are frequently irregular and disorganized networks lacking conventional hierarchies, with abnormal branching and uneven basement membranes [13, 14]. These vessels are highly dysfunctional and promote the development of a microenvironment that stimulates non-productive angiogenesis and metabolic adaptations by tumor cells that favor metastasis. Oncogenic mutations in tumor cells that stimulate the production of angiogenic factors [15] and tumor co-option of macrophages to facilitate ECM breakdown and angiogenesis [16] can promote hyperactive vessel growth that lacks sufficient functionality to maintain tissue homeostasis. Inadequate or intermittent perfusion and oxygenation of these vessels renders the tumor hypoxic and acidic, which elicits additional pro-angiogenic adaptations, and creates a potentially self-perpetuating cycle of non-productive angiogenesis [17].
The defective normalization, or “abnormalization” (reviewed in [17]) observed in tumor vessels reflects altered associations and functions of both endothelial and mural cells. The endothelial cells of the vessel tubule are often gapped or have loosened junctions, along with increased vascular permeability and significant plasma extravasation (reviewed in [18]), resulting in both the formation of a pro-angiogenic fibrin/fibrinogen provisional matrix [19, 20] and increased interstitial fluid pressure in the tumor microenvironment [21–23]. Interstitial hypertension may cause several problems relating to the progression and treatment of tumors, including 1) the enforced flow of interstitial fluid bearing angiogenic growth factors and possibly metastasizing tumor cells into the surrounding microenvironment; and 2) compromised delivery of therapeutic agents across the vessel wall and into the tumor interstitium [14].
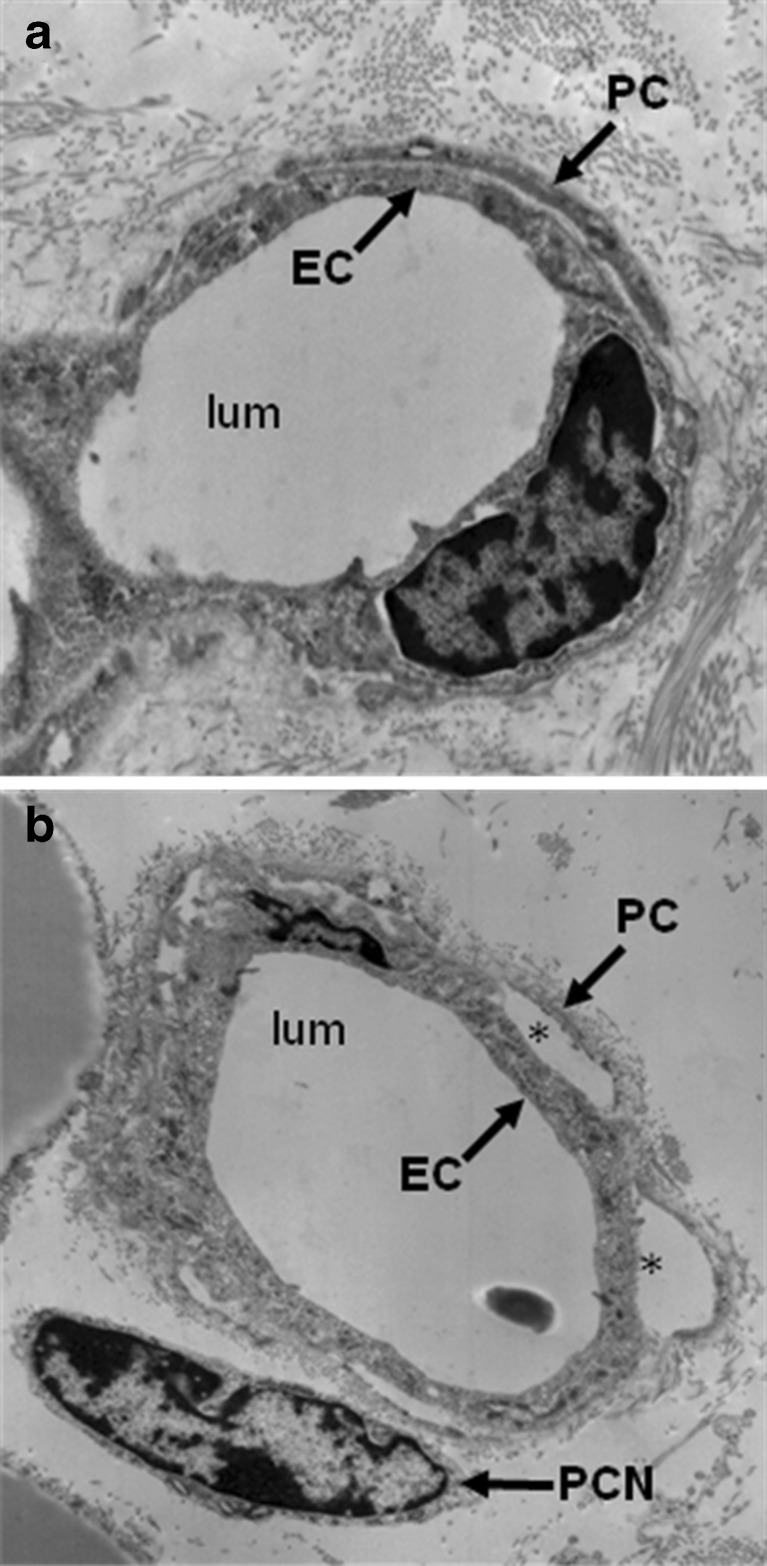
The immature functional state of tumor vasculature is further exemplified by the abnormal mural cell coverage and investment observed on tumor-associated blood vessels. Analysis of vascular morphogenesis and pathological angiogenesis suggest that disruption of pericyte contact is required for initiation of angiogenesis. Studies analyzing mural cell investment of tumor vasculature suggest a decreased detection of pericytes [24]; however, this finding may partially reflect the choice of mural cell markers, as expression of differentiation markers may be altered in tumor-associated pericytes [25]. Studies using confocal or electron microscopy show that mural cells may be present on tumor-associated vessels but exhibit abnormal association with underlying endothelial cells (Fig. 1). In contrast to the tight association observed between pericytes and endothelial cells on normal capillaries, pericytes on tumor capillaries are loosely associated with endothelial cells and exhibit an abnormal shape, sometimes extending their processes away from the endothelium toward the tumor [25–27], as if the tumor is exerting a chemotactic effect that outcompetes physiological pericyte-endothelial interactions. As a result, the overall functional immaturity of these vessels allows for continued angiogenesis.
Fig. 1.
Electron micrograph of endothelial-pericyte associations. a. A capillary of a normal vessel in mouse skin, showing tight association between endothelial cell and pericyte processes. b. A capillary of a tumor-associated vessel in an M1 chloroma tumor, showing dissociation of pericyte from endothelium with gaps (*) between both cell types. PC, pericyte; PCN, pericyte nucleus; EC, endothelial cell; lum, lumen. (Image generated by Suleyman Ergun)
Little is known about the cause of the pericyte investment defect of tumor vessels. Co-injection of mouse embryo fibroblasts (MEF) with tumor cells results in MEF investiture of tumor vessels, suggesting that tumor endothelial cells may retain the ability to recruit mural cell precursors [26]. Thus, the defect may therefore lie in an inhibitory effect on mural cell proliferation or differentiation. It is interesting to note that the presence of the mural cell precursor C3H 10T1/2 can either stimulate endothelial tubule formation in response to VEGF-A, or inhibit tubule formation induced by bFGF [28], whereas differentiated vSMC inhibit endothelial sprouting in response to VEGF-A [29]. Interestingly, VEGF-A downregulates the expression of the differentiation marker smooth muscle actin (α-SMA) on vSMC in vivo [30]. Immature mural cells lacking the differentiation markers calponin and caldesmon do not confer vessel stability in vivo [31], and the mural cells on some tumor vasculature lack calponin expression [32–34]. It is possible that maintenance of mural cells in a less differentiated state may be a means by which tumors not only prevent mural cell-mediated vessel quiescence, but also facilitate angiogenesis.
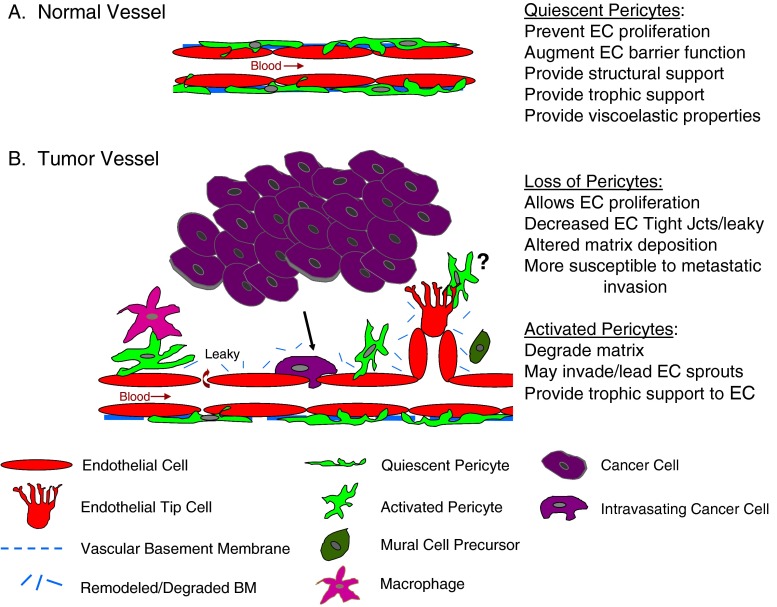
As outlined below, many of the abnormalities of the tumor vasculature may result, either directly or indirectly, from the loss of the inhibitory, stabilizing properties of differentiated pericytes and/or the tumor-induced activation of pericytes (Fig. 2).
Fig. 2.
a. Schematic representation of endothelial cells (EC) and pericytes on a normal quiescent vessel. Pericytes physically encircle the endothelial tubule, extending processes through the vascular basement membrane to physically contact the underlying endothelium. Following contact, pericytes participate in the deposition of the basement membrane (BM), enhance endothelial tight junctions (Jcts) to augment barrier function, prevent endothelial proliferation, provide structural support, and confer viscoelastic properties to the vessel to regulate blood flow. b. Abnormal association of pericytes with endothelium in a tumor vessel. At the initiation of angiogenesis, pericytes dissociate from the endothelial tubule in response to tumor-secreted factors and/or tumor-educated macrophages. The basement membrane undergoes remodeling or degradation, allowing endothelial cell proliferation to ensue. An invading endothelial tip cell leads the growth of the sprout. Alternatively, some evidence suggest pericytes may be present at the leading tip. Mural cell precursors may be recruited toward the new vessel, but in the tumor environment may fail to properly invest the endothelium. Under these conditions, the pericyte may not be able to exert inhibitory effects on endothelial cells, yet still provide trophic signals to facilitate their survival. Vessels that lack sufficient pericyte support are also commonly leaky, have abnormal basement membranes, and are more susceptible to metastatic invasion
Modulators of Pericyte Recruitment and Investiture
Mural cell recruitment and blood vessel stabilization rely on multiple pathways including Angiopoietin/Tie2, Eph-Ephrin, PDGF/PDGFR, and S1P/S1P1/2 signaling, both as independent pathways and modulation via crosstalk between the pathways. The angiopoietin family of growth factors are ligands for the Tie2 receptor. Angiopoietin-1 (Ang1) binds to Tie2, resulting in receptor phosphorylation and downstream signaling [35]. Although Ang2 can also bind Tie2, its canonical role is disruption of Ang1/Tie2 signaling in endothelial cells. However, Ang2 has been shown to stimulate Tie2 activation in a cell type- or context-specific manner [36–38]. The severe vascular defects and embryonic lethality of Tie2 receptor knockout [39, 40], Ang1 knockout [41], and Ang2 overexpressing mice [36] highlight the significance of these factors in proper development of the vasculature. Pericyte coverage of vessels is diminished and their interaction with endothelial cells is weakened in Ang1 knockout mice [41], although loss of Ang1 expression after embryonic day E13.5 did not result in altered pericyte recruitment [42]. Likewise, Ang2 overexpression reduces capillary pericyte coverage during retinal development [43], while tumor models with Ang2 neutralization exhibit increased pericyte coverage of blood vessels [44–46]. Changes in Ang1/2 cytokine ratio alter mural cell recruitment to the vasculature. As proposed by Folkman and D’Amore [47], the recruitment of mural cells may be mediated by Ang1-induced endothelial secretion of mural cell chemoattractants. Indeed, endothelial cells stimulated with Ang1 secrete hepatocyte growth factor (HGF), enhancing mural cell migration towards endothelial cells in co-cultures [48]. Ang1-stimulated endothelial cells also secrete heparin binding EGF-like growth factor (HB-EGF) which activates mural cell ErbB1/ErbB2 receptors to induce mural cell recruitment [49, 50]. Additionally, increased recruitment of mural cells in aortic ring cultures is seen following Ang1 induction of MCP-1 and p38MAPK signaling, although the cellular source of the MCP-1 was not determined [51]. There is also in vivo evidence to suggest that mural cell precursors may express low levels of Tie2 [52], and that direct stimulation of mural cells with Ang1/2 may also influence recruitment. In vivo, the combination of Ang1 and VEGF induces an influx of mural cell precursors that is not observed with either factor alone [53]. In vitro, both Ang1 and Ang2 enhance mural cell precursor migration, with an additive effect rather than antagonism [54], while no significant chemotaxis was seen in differentiated mural cells [55]. Although Tie2 expression appears to be downregulated following mural cell differentiation [52], we and others have observed the induction of Tie2 expression on differentiated mural cells under angiogenic conditions such VEGF stimulation [56, 57] or in the presence of hypoxia [56], which may allow for direct recruitment of mural cells by Ang1. Following recruitment to neovasculature, mural cell secretion of Ang1 induces trans-association of the Tie2 receptors between endothelial cells in close contact and enhances AKT related survival, vessel integrity, and quiescence [58]. Ang1-activated endothelial Tie2 also induces basement membrane deposition though AKT-induced Dll4/Notch signaling which further enhances the vascular barrier [59].
Platelet-derived growth factor (PDGF)/PDGFR is another pathway that strongly influences mural cell recruitment and investiture. Endothelial produced PDGF-B enhances mural cell proliferation, migration, and recruitment to growing vasculature through the mural cell PDGFR-β receptor [60]. An absence of mural cell investiture, hemorrhage, and embryonic lethality are found when either PDGF-B [61] or PDGFR-β [62] is knocked out, indicating their critical roles in angiogenesis. Similarly, reduced PDGFR-β signaling by receptor inhibition by imatinib [50], receptor hypomorphic mutations [63], and adenoviral expression of soluble PDGFR-β as a ligand decoy [64] all significantly decrease pericyte presence in the vasculature. Interestingly, endothelial, and not tumor, production of PDGF-B is needed for proper mural cell investiture, full coverage and formation of intimate mural-endothelial cell contacts. In the absence of the heparin sulfate proteoglycan (HSPG) binding motif of PDGF-B, pericytes are recruited but form only loose associations with endothelial cells and there is persistent microvascular dysfunction [65]. Ectopic expression of PDGF-B in tumor cells enhances pericyte recruitment, but is unable to improve pericyte investiture [66]. The endothelial production and subsequent retention of PDGF-B in the periendothelial space likely creates a gradient that promotes both pericyte recruitment and intimate association with the endothelial abluminal surface [66].
Recent studies suggest that PDGFR-β activity in mural cells may be modulated by VEGF [67]. In the presence of VEGF, VEGF-R2 and PDGFR-β complex, inhibit PDGFR-β phosphorylation, and subsequently reduce pericyte proliferation, migration, and incorporation along vascular sprouts. During angiogenic sprouting when VEGF is highly expressed, the inhibition of mural PDGFR-β may enable continued endothelial proliferation and growth of neovasculature. When angiogenic growth is sufficient in an area and VEGF levels drop, PDGFR-β activation would then be permitted, enabling mural cell recruitment, subsequent endothelial quiescence, and vessel stability. Enhancement of mural cell migration by PDGF-B also occurs indirectly by the stimulation of endothelial secretion of SDF-1α that then binds mural CXCR4 receptors [68]. PDGFR-α may also play a role in mural cell recruitment and maturation. In contrast to PDGFR-β, PDGF-B and PDGF-A can both bind PDGFR-α in mural cells, activating mural cell migration, recruitment to vasculature, and differentiation in a neuropilin-1 and p130Cas-dependant mechanism [69].
Proper mural cell investiture is also influenced by Eph-ephrin interactions. Ephrin-B ligands and their Eph receptor tyrosine kinases are typically both membrane bound, requiring close cell-cell proximity for interaction. Eph-ephrins can signal bidirectionally, “forward” through the receptor and “reverse” through the interacting ligand’s cytoplasmic domain. Similar to the knockouts in the Ang1/Tie2 pathway, knockout of mural cell ephrin-B2 is embryonic lethal, a result of edema and hemorrhage [70]. While total mural cell numbers are not compromised, ephrin-B2 knockout specific mural cells round up and poorly interact with the endothelium [70]. Not surprisingly, ephrin-B2 phosphorylation occurs at points where mural-endothelial contacts occur, and this reverse signaling in both endothelial and mural cells is required for their assembly into cord-like structures [71]. Furthermore, endothelial-EphB4 activation of ephrin-B2 induces mural Ang1 expression and increases Tie2 activation. As a result, pericyte investiture is enhanced and tumor blood vessel leakiness declines [72]. Additional reports suggest a requisite role for ephrin-B2 downstream of PDGF-B/PDGFR-β signaling and recruitment in pericyte-like hepatic stellate cells [73]. A possible role for EphA-ephrinA in pericyte physiology is less clear, although it has been shown that pericyte investiture is defective in EphA2-deficient mice [74].
Sphingosine-1-phosphate (S1P) and its family of membrane-bound G protein-coupled receptors S1P1 – S1P5 [75], previously known as EDG-receptors, also play an integral role in pericyte recruitment. Under varying conditions, mural cells express of all of these receptors, with highest expression of S1P2/3 and lower levels of S1P1 [76–80]. S1P regulates mural cell proliferation [76, 77, 81, 82], migration [77, 83], response to PDGF and EGF [79], and differentiation [82]. Defects in vasculature and discontinuous coverage of mural cells are seen following ablation of sphingosine kinases [84] or knockout of S1P1 [85]. This effect is mediated by mural-endothelial interactions including N-cadherin trafficking [86] and adherens junctions [87] because endothelial-specific knockout mimics the phenotype of the full knockout [88]. In contrast, S1P2 knockout enhances mural cell recruitment to tumor blood vessels, potentially through the production of angiogenic factors that promote vascular maturation like TGFβ or the loss of chemorepulsion mediated by S1P2 on mural cell precursors [89]. While it is known that activation of S1P2 by S1P inhibits mural cell migration and spreading through inhibition of Rac [90, 91], and the use of S1P2 antagonist JTE-013 restores mural cell migration in response to PDGF [78], knockout of S1P2 in mural cells could further aid in clarifying the S1P2 direct versus endothelial-mediated effects. In summary, multiple systems are critical in driving the recruitment of mural cells, formation of tight mural-endothelial contacts, and subsequent stabilization of the vasculature. The mechanisms of these pathways and the factors that modulate their activity during angiogenesis and disease remain an ongoing area of research.
Molecular Mediators of Junctional Interactions Between Pericytes and EC
Although separated by a basement membrane, pericytes and endothelial cells make physical contact with each other by extension of processes through openings in the basement membrane. This physical contact can be mediated by adherens junctions, gap junctions, and peg-and-socket junctions. In the latter, pericyte processes serve as the ‘pegs’ which insert into endothelial cell ‘sockets’ [92]. As these structures have been suggested to both be associated with more mature vasculature [93] and occur during angiogenesis [94, 95], the exact nature or function of these structures remains unclear. However, it has been suggested that these cell-cell contacts are also home to other stabilizing junctional interactions [1]; indeed, one study localized the expression of Ang1 to pericytes and Tie2 to endothelial cell membranes within such interdigitations [96]. Adherens junctions composed of N-cadherin play an important stabilizing role in pericyte-endothelial interactions, as blocking [97, 98] or endothelial loss [86] of N-cadherin results in decreased pericyte adhesion to endothelium.
Gap junctions are composed of proteins known as connexins, which couple with assembled connexins on an adjacent cell membrane to form a functional junction. Gap junction intercellular communication (GJIC) allows for the direct exchange of ions, second messengers, and small hydrophilic molecules (generally under 1 kDa) between neighboring cells to mediate organized growth and adaptive responses within tissues. Functional GJIC between endothelial cells and mural cells has been demonstrated in vitro using electron probe microanalysis [99], electrical resistance [100], and dye coupling studies [101, 102]; ex vivo by dye coupling studies [103] and in vivo by ultrastructural studies [104]. The presence of these gap junctions has been thought to facilitate the conductance of electrical signals along the vessels wall to control vascular tone [105, 106]. These gap junctions also play a critical role in vascular assembly during vessel formation. When mural cell precursors are recruited to a newly-developed vessel, they form Connexin 43 (Cx43)-dependent gap junctions with the endothelium which leads to activation of latent TGF-β to induce full mural cell maturation [107].
Another role for gap junctions in the vasculature has been recently proposed. Normal vessels undergo vascular remodeling in response to environmental, metabolic, and hemodynamic stimuli [108, 109], and proper gap junctions may be required for maintenance of normal responsive vascular structures [110]. Mathematical modeling suggests that, in the absence of properly integrated signaling along the vessels due to alterations in heterocellular gap junction communication, formation of arterio-venous shunts is favored [110]. Interestingly, an inactivating phosphorylation of Cx43, which is thought to disrupt junctional communication, has been reported on the capillaries of breast and other tumors [111]. In addition, VEGF decreases endothelial gap junction communication [112]. A loss of vascular gap junction activity could therefore explain both the decrease in detection of pericyte differentiation markers and the aberrant formation of arterio-venous shunts which plague the tumor vasculature.
Pericytes as Drivers of Angiogenesis
Pericytes May Remodel Matrix
Although the canonical view of pericyte function in angiogenesis holds that pericytes function primarily in the resolution of angiogenesis by contributing to vascular stabilization and maturation, circumstantial evidence suggests that pericytes play a permissive, if not stimulatory, role in early angiogenesis. The vascular basement membrane is a stabilizing physical barrier to soluble molecules and migrating cells that also serves as a scaffold for pericyte-EC interactions. Activation of matrix proteases and degradation of the basement membrane is necessary for angiogenesis to occur (reviewed in [113]) and there is evidence that tumors secrete factors that activate pericytes to degrade the basement membrane and liberate matrix-bound growth factors, thereby contributing to angiogenesis and possibly facilitating tumor growth and invasion. The basement membrane in the vicinity of pericytes is altered following stimulation of muscle angiogenesis in vivo, suggesting that matrix proteases have been activated [114]. Similarly, in hypoxia-induced angiogenesis in the brain, one of the earliest morphological changes observed was thickening of basement membrane between endothelial cells and pericytes, along with disintegration of the basal lamina at the leading edge of migrating cells [115] and upregulation of the urokinase plasminogen activator receptor (uPAR) on pericytes [116]. Immunostaining of sprouting tubules in human fetal brain angiogenesis shows that endothelial cells and pericytes associated with the basement membrane both express activated MMP-2, suggesting that these cells may cooperate to disassemble the basement membrane to allow cell migration and the emergence of a nascent sprout [117]. In breast carcinoma in situ samples, matrix metalloproteinase (MMP)-1 and MMP-9 were detected in both capillary pericytes and fibroblasts, and levels of these proteases were further increased in invasive carcinomas [118]. Increased MMP-9 expression by pericytes has been detected in neuroblastoma xenograft tissues [119], human glioma samples [120], and human breast cancer tissues [121]. In vitro studies have shown that treatment with pro-angiogenic factors is sufficient to stimulate mural cells to secrete MMPs. Vascular smooth muscle cells treated with VEGF, an important angiogenic mediator in may cancers, secrete MMP-9 [122, 123], as well as MMP-1 and MMP-3 [122], resulting in the invasion of vSMC through Matrigel [122, 123] and a mixture of type I and type III collagen [123]. Similarly, MMP-2 and MMP-9 are activated in vSMC in response to bFGF [124] and IL-1β + PDGF-BB [125], respectively. In addition to facilitating sprouting angiogenesis, activated pericytes have also been implicated in the degradation of vascular basement membrane associated with the development of ‘mother’ vessels [126], a pathological subtype of angiogenic venule that divides to give rise to new ‘daughter’ vessels [127]. Interestingly, the degradation of basement membrane during this process results not from MMP production, but from an increase in pericyte-derived cathepsin activity which results in pericyte detachment and vascular enlargement [126]. These data, combined with reports that tumor cells induce invasion and migration of mural cells [128], suggest that tumor stimulation of mural cells may cause them to play an active role in the initiation and propagation of angiogenesis.
Pericytes May Lead Angiogenic Sprouts
Additional observations suggest that pericytes may have non-canonical roles in regulating vascular sprouting in certain tissues, possibly by helping to guide the emerging sprout [129]. Pericytes have been detected with endothelial cells at the growing vascular tip in the mouse retina. Combined immunohistochemistry and electron microscopy studies have detected pericytes during the earliest histological stage in the formation of granulation tissue during wound healing [130] and following the induction of angiogenesis in skeletal muscle by electrical stimulation [114]. Pericytes have similarly been reported to be located on sprouts emerging in mammary and other tumors [25, 131], although the mechanistic implications of this co-localization are not well understood. The guidance molecule Slit3 has been shown to be secreted by both endothelial cells and vSMC and interactions between Slit3 and endothelial Robo4 receptor stimulate endothelial proliferation, motility, chemotaxis, and tubule formation in vitro [132]. Since vSMC similarly express Robo1 and Robo4, the authors proposed that autocrine and paracrine Slit3/Robo signaling may help coordinate endothelial-mural cell interactions in vessel maturation, although additional studies are needed to determine the potential contributions of Slit/Robo signaling to earlier stages of angiogenesis [133].
Studies of angiogenesis in ovulation have observed that pericytes are located at the leading edge of endothelial sprouts in the collapsed follicle [134, 135], but become closely associated with EC in the mature corpus luteum (reviewed in [136]). Endothelial cells in luteal arterioles and capillaries produce nitric oxide and luteal pericytes express VEGF, suggesting that a hypoxia-driven paracrine loop may help coordinate sprouting [136]. In addition to contributing a VEGF gradient, pericytes may also degrade and remodel the ECM to form additional “guiding structures” that aid in the luteal invasion of endothelial sprouts, possibly by laying down a scaffold containing fibronectin that is permissive for endothelial migration [137]. A complementary or possibly alternative guidance structure is suggested by immunohistochemical analyses of growing microvessels in the developing human brain, where it was observed that pericyte markers extended the length of the growing microvessel, while endothelial markers were observed only in the initial segment of the vessel [117]. The immunohistochemical staining pattern was interpreted as illustrating endothelial cell-free segments in which leading pericytes were recruiting EC from the parental vessel, possibly by secreting factors such as VEGF or by cell-cell interactions mediated by molecules such as NG2 proteoglycan. Similar endothelial-free pericyte assemblies have been reported to regulate sprouting in retinal neovascularization, the adult mouse cornea [138], and mouse tumor models [25, 139], suggesting that pericytes may confer guidance to invading endothelial cells in a variety of contexts. Indeed, a clue to the reconciliation of these apparent discrepancies in the role of pericytes in sprouting angiogenesis is suggested by a study indicating that the positioning of pericytes at the leading front versus recruitment following nascent vessel formation is governed by the specific angiogenic stimulus [140]. Additional studies are required to determine (1) the extent to which pericyte assemblies contribute to neovascularization in other tissues and contexts; and (2) whether tumor-induced defects in pericyte functions pertaining to guidance of EC contributes to the altered architecture exhibited by tumor vasculature.
Pericytes Provide Trophic Support for Endothelial Cells
In addition to interactions promoting stability and quiescence, there are several reports indicating that pericytes provide paracrine survival support for endothelial cells. Immature tumor blood vessels which lack pericyte coverage are more vulnerable to VEGF withdrawal than vessels with more extensive pericyte coverage [141]. It has also been suggested that pericytes may play a role in the abundant microvascular proliferation found in glioblastoma [142]. Treatment with IL-1β [143], PDGF-BB, or media conditioned by colon cancer cells [144] induces VEGF production in mural cells, suggesting that mural cells may provide paracrine trophic support in a variety of physiological and pathological contexts. Contact between endothelial cells and multipotent mesenchymal cells is required to induce mural VEGF [145]. Pericyte production of VEGF was observed in the developing retinal vasculature in vivo, further supporting the concept that heterotypic contact-induced pericyte differentiation upregulates local VEGF production as part of the regulatory program that stabilizes newly formed vessels. The functional consequences of trophic cross-talk between endothelial cells and pericytes is demonstrated by the observation that tumor vessels with pericyte coverage do not regress as efficiently following anti-vascular therapy as vessels lacking coverage [146–148], suggesting that a bi-compartmental strategy may be required to more completely target tumor vasculature. The combined use of VEGFR and PDGFR inhibitors has been investigated as a possible strategy to circumvent pericyte-mediated protection of tumor vessels by disrupting signaling pathways mediating functional endothelial-pericyte interactions to promote vessel regression [149–151]. Interestingly, combined inhibition of VEGF and PDGF signaling did not alter normal vessels [150], emphasizing the phenotypic differences between endothelial-pericyte interactions in normal and neoplastic tissues.
Pericytes as Inhibitors of Angiogenesis and Tumor Progression
Mural Cells are Necessary to Maintain a Quiescent Stable Endothelium
Several studies [152, 153] have demonstrated that actively proliferating endothelium lacks coverage by mural cells. In addition, mural cell-endothelial cell interactions are reduced following stimulation of angiogenesis by at least three different methods [154], and the arrival of pericytes coincides with the cessation of vessel growth during wound healing [155], suggesting that contact with mural cells leads to quiescence of endothelial cells. Diminished pericyte coverage in tumor vessels corresponds with increased endothelial proliferation. Whereas quiescent normal endothelia exhibit an average proliferation rate of only 0.1 % [156], breast tumor endothelial proliferation indices are elevated 20 to 50-fold [24, 157]. The negative correlation between mural cell investment and angiogenesis was further corroborated by a study showing that pericyte coverage in low vascular density areas of breast tumors is significantly higher than in areas with high vascular density [158]. A variety of in vitro studies culturing endothelial cells with vSMC or pericytes corroborate the decreased growth of endothelial cells under these conditions [159, 160]. Further, mural cells prevent endothelial cell migration [161] and sprouting [29], and activation of endothelial MT1-MMP [162]. Together, these data underscore the ability of mural cells to limit endothelial cell responsiveness to external angiogenic stimuli when they exhibit a normal, functional association with endothelium.
The molecular mechanisms regulating the functions of pericytes in stabilization and maturation of newly formed vessels are not well understood. Several in vitro studies using 3-dimensional (3D) collagen matrices suggest that crosstalk between endothelial cells and pericytes regulating the balance of MMPs and TIMPs is critical for vessel morphogenesis, sprout stabilization, and vessel regression. When grown in 3D matrices of type I collagen, endothelial cells arrange into networks and undergo lumen formation [163, 164]. During the formation of these networks, endothelial cells secrete high levels of the zymogens pro-MMP-1 and pro-MMP-10 [165, 166]. Activation of MMP-1 and MMP-10 correlated with collagen gel contraction and capillary tube regression. The addition of bovine retinal pericytes to the collagen matrix was sufficient to block MMP-1 and MMP-10-dependent capillary tubule regression in the presence of plasminogen, whereas other cell types were ineffective, suggesting that endothelial contact with pericytes inhibits vessel regression by limiting protease activity and/or function [167]. Endothelial cells were the predominant source of TIMP-1, TIMP2, and PAI-1, whereas pericytes were a strong source of TIMP-3 that was induced by EC-pericyte interactions. Targeting of TIMP-2 in endothelial cells and TIMP-3 in pericytes by siRNA resulted in capillary tube regression in a process dependent on MMPs. These results indicate that pericyte-dependent stabilization of capillary tubules is mediated, at least in part, by protease inhibition. Additional studies are required to determine whether tumors may alter regulation of protease activities contributed by endothelial cells and pericytes to subvert vessel morphogenesis and regression and distort normal vascular architecture.
Restoration of Pericyte Investment of Tumor Blood Vessels Leads to Tumor Inhibition
As discussed, Ang1 and its receptor Tie2 play an important role in mural cell recruitment and vessel maturation [39, 41]. Ectopic expression of Ang1 in breast, colon, and squamous cell carcinoma results in decreased tumor proliferation and angiogenesis in some xenograft models [168–170]. The blood vessels in Ang1-expressing breast [53] and other [170, 171] tumors demonstrated significantly greater association with α-SMA-positive mural cells compared to controls, suggesting that enforced maturation of the blood vessel functionally inhibited tumor angiogenesis. It is noteworthy that Ang1 can also potently stimulate angiogenesis via enhancing the migration, capillary formation, and survival of endothelial cells, which can lead to enhanced tumor growth (reviewed in [172]); however, in xenograft models where Ang1 expression leads to enhanced pericyte investment, tumor growth is decreased [171]. Ang1 also decreased metastasis, tumor growth, and ascites formation in an experimental model of colon peritoneal carcinomatosis [173]. Recently, in an in vivo melanoma model it was shown that vessel stabilization is associated with a reduced tumor vessel density and slows the tumor growth significantly but it also results in a resistance to anti-angiogenic therapy [148]. These data, coupled with aforementioned studies demonstrating the anti-angiogenic effects of mural cell association, suggest that stabilization of tumor blood vessel by mural cells may be a desirable therapeutic goal by which new vessel formation may be inhibited and tumor growth thereby arrested. However, continued therapeutic success may require additional strategies to target a stabilized tumor vasculature.
Mural Cells Stabilize Vessels by Deposition of Matrix
The vascular basement membrane confers an additional level of structural stability to blood vessels. Not surprisingly, abnormal deposition of matrix is commonly observed in the unstable tumor vasculature. This may be manifested as discontinuous [174, 175] basement membrane, or abnormal morphology of the basement membrane including multiple layers and variable increased thickness [131, 175, 176]. Isolated pericytes have been shown to express the basement membrane components collagen IV and various laminin isoforms [177], as well as significant levels of fibronectin [178]. One recent study suggested that in vSMC-endothelial co-cultures the endothelial networks become ensheathed by collagen IV and collagen XVIII, while lamins and fibronectins localize to the vSMC layer [179]. Endostatin, a fragment of collagen XVIII and an endogenous inhibitor of angiogenesis [180] was shown to stabilize newly formed blood vessels by stabilizing the inter-endothelial contacts and by anchoring the nascent endothelial tubule to basement membrane which results in a better integration of pericytes into the capillary wall [181]. Another study demonstrated that while three-dimensional endothelial tubules cultured in the absence of pericytes showed some expression of basement membrane proteins, expression of collagen type IV, laminin, nidogens, perlecan, and fibronectin was substantially increased when pericytes are present [182]. This enrichment of matrix proteins results from the contribution of both pericytes (nidogen-1; laminin α4, α5, β2, and γ1 subunits; perlecan) and endothelial cells (fibronectin and laminin α5). Changes in matrix deposition are associated with corresponding changes in integrin expression in both cell types, resulting in tighter adhesion to matrix and control of lumen diameter and endothelial behavior. Appropriate matrix deposition appears to be critically dependent on both fibronectin and the pericyte expression of TIMP-3 in co-culture, as inhibition of either results in altered collagen IV deposition and loss of tube maturation. Regulation of basement membrane deposition by pericytes appears to play an important role in not just structural support of the vessel but also provides the integrin-mediated cues which help to regulate vessel phenotype.
Mural Cells Enhance Endothelial Tight Junctions/Barrier Activity
The barrier function performed by a normal, mature endothelium results from the formation of tight junctions between adjacent endothelial cells. The abnormal tumor vasculature is hyperpermeable, due to activation of both transcellular and paracellular/intercellular pathways (reviewed in [183]). Increasing evidence suggests that pericytes may participate in regulation of vessel permeability. Pathological ‘mother vessels’, which are notably pericyte poor, are hyperpermeable via transcellular routes of extravasation [127]. In addition, mouse models with vessel maturation defects are reported to be more susceptible to leakiness [184] and/or have altered expression of endothelial tight junction proteins [185], suggesting a role in regulation of intercellular permeability. Studies using membrane co-culture models have demonstrated that mural cells enhance endothelial barrier function, as measured by in vitro permeability and electrical assays [97, 186–188], and heterotypic contact between pericytes and endothelial cells produces an endothelium that is more resistant to hypoxic injury [100]. Part of this may be due to physical location of pericytes, which preferentially situate themselves at endothelial cell junctions and cover gaps between endothelial cells [1]. Secretion of Ang1 by mural cells upregulates endothelial expression of tight junction proteins zona occludens (ZO)-1 and occludin [189, 190], suggesting a molecular mechanism by which mural cells enhance endothelial barrier function. Enhancement of barrier function may also result from pericyte-secreted S1P upregulation of VE-cadherin and downregulation of Ang2 in co-cultures [97]. Some studies suggest that barrier-enhancing effects are not observed in non-contacting co-cultures, leading to the hypothesis that intercellular communication through gap junctions is required for the enhancement of endothelial barrier function by pericytes [100]. Reports demonstrating the interaction of connexins with tight junction proteins ZO-1 and ZO-2 and the ability of ZO-1 to regulate the rate of undocked aggregations of connexins, known as connexons, to assemble into functional gap junctions [191, 192] suggest a potential mechanism by which crosstalk between endothelial cells and pericytes enhances both barrier function and vessel stabilization.
Vessels Lacking Mural Cells are More Susceptible to Tumor Metastasis
Hematogenous metastasis requires that tumor cells invade a blood vessel, spread via the bloodstream to a distal site, and extravasate out of the blood vessel into the new tissue. Several studies suggest that proper association between endothelial cells and mural cells may decrease susceptibility to metastasis. In colorectal cancer, the absence of mural cell coverage on tumor vessels correlates with metastasis [193] . Similarly, in a mouse model of prostate cancer, vessels stabilized by pericytes were less frequently invaded by tumor cells compared to regions with decreased pericyte coverage [194]. In addition, three different knockout animals with phenotypes exhibiting lack of mural cell stabilization of vessels are more susceptible to metastatic invasion [195, 196]. Further, the loss of pericyte-endothelial association observed in tumors overexpressing both bFGF and PDGF-BB is associated with increased metastasis [197], whereas anti-VEGF treatment, which elicits vessel normalization, prevents prostate cancer metastasis in a mouse model [198], suggesting vascular normalization may help prevent metastasis. Interestingly, normal venules display regions with decreased deposition of basement membrane proteins in regions that correspond to gaps between adjacent pericytes, sites which appear to be utilized by transmigrating neutrophils [199, 200]. One may therefore conjecture that the increased susceptibility of destabilized vessels may result, in part, from regions of altered matrix deposition in the basement membrane of tumor vessels.
Vascular Normalization as a Therapeutic Strategy
The dysfunctional tumor vasculature provides multiple barriers to the delivery and response to anti-tumor therapies. Recent work suggests that the use of anti-VEGF therapies may result in what Jain has termed a ‘normalization’ of the tumor vasculature. Some of the most structurally aberrant vasculature that lacks pericyte investment may be ‘pruned’ under these conditions [23], which can lead to increased regions of necrosis in the tumor [201]. Interestingly, the remaining vasculature may undergo a transient normalization period characterized by an Ang1-mediated increase in pericyte investiture and an MMP-mediated reduction of the abnormally thickened basement membrane [176]. During this ‘normalization window’, numerous preclinical models suggest that, due to an increase in vessel perfusion, oxygen to the tumor is increased (reviewed in [202]). Importantly, increased oxygenation allows for more robust response to radiation therapy, and may reduce the hypoxia-driven progression of tumors. The enhanced blood flow and decreased permeability of the ‘normalized’ vessels also results in a decrease in interstitial fluid pressure [23], resulting in enhanced delivery of systemic therapeutic compounds into the tumor (reviewed in [202]). In the brain, normalizing glioma vasculature via use of anti-VEGFR therapies results in decreased edema [203]. Administration of COMP-Ang1, an Ang1 variant, resulted in vessel normalization and enhanced delivery of 5-fluorouracil to achieve an enhanced therapeutic response in a lung cancer xenograft model [204]. Intriguingly, normalization of the vasculature by anti-angiogenic therapies [205] or in RGS5-knockout mice [206] leads to increased infiltration of cytotoxic leukocytes into the tumor, where they may facilitate immune rejection of the tumor [206]. Together these data suggest that vascular normalization may be a desirable therapeutic goal in the treatment of cancer. Ongoing studies aimed at identifying and optimizing the ‘normalization window’ should lead to improved strategies to effect tumor control.
Conclusions
Pericytes are receiving increasing recognition for the pivotal role they play in the development and function of the vasculature. While a primary function of the pericyte is to stabilize a vessel and render it quiescent, tumors can activate pericytes to participate in pathological angiogenesis. Both loss of the stabilizing functions of the pericyte and the acquisition of a pro-angiogenic phenotype facilitate the generation of a vasculature that is proliferative, leaky, and dysfunctional. Modulation of the two faces of the pericyte – the “Jekyll” and “Hyde” – may provide a therapeutic opportunity. The ‘normalization’ of the vasculature in response to anti-VEGF therapies, characterized by restoration of functional pericyte-endothelial associations, reverses the severity of many of the defects of the tumor vasculature. Judicial use of conventional chemo- and radiation therapy concurrent with this ‘normalization window’ shows promise in enhancing tumor response to therapy. A better understanding of the mechanisms by which tumors subvert pericyte function may lead to identification of means to disrupt this process, leading to a more sustained ‘normalization’ state that may be exploited to achieve better tumor control.
Acknowledgements
The work of LJMB is supported by Public Health Service grant CA138727 from the National Institutes of Health. AS was supported by NIH T32 CA113267. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Kalluri and colleagues recently provide evidence that decreased pericyte coverage of the tumor vasculature is associated with distant metastases and shorter disease-free survival of breast cancer patients. Further, they demonstrated that ablation of pericytes is associated with increased metastasis to lung in an orthotopic mouse model of breast cancer. Cooke et al., Cancer Cell 21:66–81, 2012.
References
- 1.Diaz-Flores L, Gutierrez R, Madrid JF, Varela H, Valladares F, Acosta E, Martin-Vasallo P, Diaz-Flores L., Jr Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24:909–969. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- 2.De Smet F, Segura I, De Bock K, Hohensinner PJ, Carmeliet P. Mechanisms of vessel branching: Filopodia on endothelial tip cells lead the way. Arterioscler Thromb Vasc Biol. 2009;29:639–649. doi: 10.1161/ATVBAHA.109.185165. [DOI] [PubMed] [Google Scholar]
- 3.Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell Biol. 2010;22:617–625. doi: 10.1016/j.ceb.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. Tumor angiogenesis: a possible control point in tumor growth. Ann Intern Med. 1975;82:96–100. doi: 10.7326/0003-4819-82-1-96. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 6.Watson JC, Redmann JG, Meyers MO, Alperin-Lea RC, Gebhardt BM, Delcarpio JB, Woltering EA. Breast cancer increases initiation of angiogenesis without accelerating neovessel growth rate. Surgery. 1997;122:508–513. doi: 10.1016/s0039-6060(97)90045-3. [DOI] [PubMed] [Google Scholar]
- 7.Coulon C, Georgiadou M, Roncal C, De Bock K, Langenberg T, Carmeliet P. From vessel sprouting to normalization: role of the prolyl hydroxylase domain protein/hypoxia-inducible factor oxygen-sensing machinery. Arterioscler Thromb Vasc Biol. 2010;30:2331–2336. doi: 10.1161/ATVBAHA.110.214106. [DOI] [PubMed] [Google Scholar]
- 8.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 9.Oh H, Takagi H, Suzuma K, Otani A, Matsumura M, Honda Y. Hypoxia and vascular endothelial growth factor selectively up-regulate angiopoietin-2 in bovine microvascular endothelial cells. J Biol Chem. 1999;274:15732–15739. doi: 10.1074/jbc.274.22.15732. [DOI] [PubMed] [Google Scholar]
- 10.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 11.Berse B, Brown LF, Van de Water L, Dvorak HF, Senger DR. Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol Biol Cell. 1992;3:211–220. doi: 10.1091/mbc.3.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hubbard NE, Lim D, Mukutmoni M, Cai A, Erickson KL. Expression and regulation of murine macrophage angiopoietin-2. Cell Immunol. 2005;234:102–109. doi: 10.1016/j.cellimm.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev. 2005;15:102–111. doi: 10.1016/j.gde.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res. 2007;74:72–84. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rak J, Filmus J, Finkenzeller G, Grugel S, Marme D, Kerbel RS. Oncogenes as inducers of tumor angiogenesis. Cancer Metastasis Rev. 1995;14:263–277. doi: 10.1007/BF00690598. [DOI] [PubMed] [Google Scholar]
- 16.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 17.De Bock K, Cauwenberghs S, Carmeliet P. Vessel abnormalization: another hallmark of cancer? Molecular mechanisms and therapeutic implications. Curr Opin Genet Dev. 2011;21:73–79. doi: 10.1016/j.gde.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Dvorak HF. Vascular permeability to plasma, plasma proteins, and cells: an update. Curr Opin Hematol. 2010;17:225–229. doi: 10.1097/MOH.0b013e3283386638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dvorak HF, Senger DR, Dvorak AM. Fibrin as a component of the tumor stroma: origins and biological significance. Cancer Metastasis Rev. 1983;2:41–73. doi: 10.1007/BF00046905. [DOI] [PubMed] [Google Scholar]
- 20.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 21.Boucher Y, Baxter LT, Jain RK. Interstitial pressure gradients in tissue-isolated and subcutaneous tumors: implications for therapy. Cancer Res. 1990;50:4478–4484. [PubMed] [Google Scholar]
- 22.Stohrer M, Boucher Y, Stangassinger M, Jain RK. Oncotic pressure in solid tumors is elevated. Cancer Res. 2000;60:4251–4255. [PubMed] [Google Scholar]
- 23.Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 24.Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin HG. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer Res. 2000;60:1388–1393. [PubMed] [Google Scholar]
- 25.Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160:985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abramsson A, Berlin O, Papayan H, Paulin D, Shani M, Betsholtz C. Analysis of mural cell recruitment to tumor vessels. Circulation. 2002;105:112–117. doi: 10.1161/hc0102.101437. [DOI] [PubMed] [Google Scholar]
- 27.Ozawa MG, Yao VJ, Chanthery YH, Troncoso P, Uemura A, Varner AS, Kasman IM, Pasqualini R, Arap W, McDonald DM. Angiogenesis with pericyte abnormalities in a transgenic model of prostate carcinoma. Cancer. 2005;104:2104–2115. doi: 10.1002/cncr.21436. [DOI] [PubMed] [Google Scholar]
- 28.Tille JC, Pepper MS. Mesenchymal cells potentiate vascular endothelial growth factor-induced angiogenesis in vitro. Exp Cell Res. 2002;280:179–191. doi: 10.1006/excr.2002.5635. [DOI] [PubMed] [Google Scholar]
- 29.Korff T, Kimmina S, Martiny-Baron G, Augustin HG. Blood vessel maturation in a 3-dimensional spheroidal coculture model: direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates vegf responsiveness. FASEB J. 2001;15:447–457. doi: 10.1096/fj.00-0139com. [DOI] [PubMed] [Google Scholar]
- 30.Witmer AN, van Blijswijk BC, van Noorden CJ, Vrensen GF, Schlingemann RO. In vivo angiogenic phenotype of endothelial cells and pericytes induced by vascular endothelial growth factor-a. J Histochem Cytochem. 2004;52:39–52. doi: 10.1177/002215540405200105. [DOI] [PubMed] [Google Scholar]
- 31.Hughes S, Chan-Ling T. Characterization of smooth muscle cell and pericyte differentiation in the rat retina in vivo. Invest Ophthalmol Vis Sci. 2004;45:2795–2806. doi: 10.1167/iovs.03-1312. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki Y, Yamamura H, Kawakami Y, Yamada T, Hiratsuka M, Kameyama M, Ohigashi H, Ishikawa O, Imaoka S, Ishiguro S, Takahashi K. Expression of smooth muscle calponin in tumor vessels of human hepatocellular carcinoma and its possible association with prognosis. Cancer. 2002;94:1777–1786. doi: 10.1002/cncr.10402. [DOI] [PubMed] [Google Scholar]
- 33.Kinouchi T, Mano M, Matsuoka I, Kodama S, Aoki T, Okamoto M, Yamamura H, Usami M, Takahashi K. Immature tumor angiogenesis in high-grade and high-stage renal cell carcinoma. Urology. 2003;62:765–770. doi: 10.1016/s0090-4295(03)00512-0. [DOI] [PubMed] [Google Scholar]
- 34.Koganehira Y, Takeoka M, Ehara T, Sasaki K, Murata H, Saida T, Taniguchi S. Reduced expression of actin-binding proteins, h-caldesmon and calponin h1, in the vascular smooth muscle inside melanoma lesions: an adverse prognostic factor for malignant melanoma. Br J Dermatol. 2003;148:971–980. doi: 10.1046/j.1365-2133.2003.05238.x. [DOI] [PubMed] [Google Scholar]
- 35.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the tie2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 36.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 37.Gill KA, Brindle NP. Angiopoietin-2 stimulates migration of endothelial progenitors and their interaction with endothelium. Biochem Biophys Res Commun. 2005;336:392–396. doi: 10.1016/j.bbrc.2005.08.097. [DOI] [PubMed] [Google Scholar]
- 38.Teichert-Kuliszewska K, Maisonpierre PC, Jones N, Campbell AIM, Master Z, Bendeck MP, Alitalo K, Dumont DJ, Yancopoulos GD, Steward DJ. Biological action of angiopoietin-2 in a fibrin matrix model of angiogenesis is associated with activation of tie2. Cardiovasc Res. 2001;49:659–670. doi: 10.1016/s0008-6363(00)00231-5. [DOI] [PubMed] [Google Scholar]
- 39.Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- 40.Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases tie-1 and tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 41.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the tie2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 42.Jeansson M, Gawlik A, Anderson G, Li C, Kerjaschki D, Henkelman M, Quaggin SE. Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J Clin Invest. 2011;121:2278–2289. doi: 10.1172/JCI46322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng Y, vom Hagen F, Pfister F, Djokic S, Hoffmann S, Back W, Wagner P, Lin J, Deutsch U, Hammes HP. Impaired pericyte recruitment and abnormal retinal angiogenesis as a result of angiopoietin-2 overexpression. Thromb Haemost. 2007;97:99–108. [PubMed] [Google Scholar]
- 44.Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, Politi LS, Gentner B, Brown JL, Naldini L, De Palma M. Targeting the ang2/tie2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19:512–526. doi: 10.1016/j.ccr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 45.Hashizume H, Falcon BL, Kuroda T, Baluk P, Coxon A, Yu D, Bready JV, Oliner JD, McDonald DM. Complementary actions of inhibitors of angiopoietin-2 and vegf on tumor angiogenesis and growth. Cancer Res. 2010;70:2213–2223. doi: 10.1158/0008-5472.CAN-09-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nasarre P, Thomas M, Kruse K, Helfrich I, Wolter V, Deppermann C, Schadendorf D, Thurston G, Fiedler U, Augustin HG. Host-derived angiopoietin-2 affects early stages of tumor development and vessel maturation but is dispensable for later stages of tumor growth. Cancer Res. 2009;69:1324–1333. doi: 10.1158/0008-5472.CAN-08-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Folkman J, D'Amore PA. Blood vessel formation: What is its molecular basis? Cell. 1996;87:1153–1155. doi: 10.1016/s0092-8674(00)81810-3. [DOI] [PubMed] [Google Scholar]
- 48.Kobayashi H, DeBusk LM, Babichev YO, Dumont DJ, Lin PC. Hepatocyte growth factor mediates angiopoietin-induced smooth muscle cell recruitment. Blood. 2006;108:1260–1266. doi: 10.1182/blood-2005-09-012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iivanainen E, Nelimarkka L, Elenius V, Heikkinen SM, Junttila TT, Sihombing L, Sundvall M, Maatta JA, Laine VJ, Yla-Herttuala S, Higashiyama S, Alitalo K, Elenius K. Angiopoietin-regulated recruitment of vascular smooth muscle cells by endothelial-derived heparin binding egf-like growth factor. FASEB J. 2003;17:1609–1621. doi: 10.1096/fj.02-0939com. [DOI] [PubMed] [Google Scholar]
- 50.Stratman AN, Schwindt AE, Malotte KM, Davis GE. Endothelial-derived pdgf-bb and hb-egf coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood. 2010;116:4720–4730. doi: 10.1182/blood-2010-05-286872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aplin AC, Fogel E, Nicosia RF. Mcp-1 promotes mural cell recruitment during angiogenesis in the aortic ring model. Angiogenesis. 2010;13:219–226. doi: 10.1007/s10456-010-9179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 53.Tian S, Hayes AJ, Metheny-Barlow LJ, Li LY. Stabilization of breast cancer xenograft tumour neovasculature by angiopoietin-1. Br J Cancer. 2002;86:645–651. doi: 10.1038/sj.bjc.6600082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iurlaro M, Scatena M, Zhu WH, Fogel E, Wieting SL, Nicosia RF. Rat aorta-derived mural precursor cells express the tie2 receptor and respond directly to stimulation by angiopoietins. J Cell Sci. 2003;116:3635–3643. doi: 10.1242/jcs.00629. [DOI] [PubMed] [Google Scholar]
- 55.Witzenbichler B, Maisonpierre PC, Jones P, Yancopoulos GD, Isner JM. Chemotactic properties of angiopoietin-1 and −2, ligands for the endothelial-specific receptor tyrosine kinase tie2. J Biol Chem. 1998;273:18514–18521. doi: 10.1074/jbc.273.29.18514. [DOI] [PubMed] [Google Scholar]
- 56.Park YS, Kim NH, Jo I. Hypoxia and vascular endothelial growth factor acutely up-regulate angiopoietin-1 and tie2 mrna in bovine retinal pericytes. Microvasc Res. 2003;65:125–131. doi: 10.1016/s0026-2862(02)00035-3. [DOI] [PubMed] [Google Scholar]
- 57.Metheny-Barlow LJ, Tian S, Hayes AJ, Li LY. Direct chemotactic action of angiopoietin-1 on mesenchymal cells in the presence of vegf. Microvasc Res. 2004;68:221–230. doi: 10.1016/j.mvr.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 58.Fukuhara S, Sako K, Minami T, Noda K, Kim HZ, Kodama T, Shibuya M, Takakura N, Koh GY, Mochizuki N. Differential function of tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat Cell Biol. 2008;10:513–526. doi: 10.1038/ncb1714. [DOI] [PubMed] [Google Scholar]
- 59.Zhang J, Fukuhara S, Sako K, Takenouchi T, Kitani H, Kume T, Koh GY, Mochizuki N. Angiopoietin-1/tie2 signal augments basal notch signal controlling vascular quiescence by inducing delta-like 4 expression through akt-mediated activation of beta-catenin. J Biol Chem. 2011;286:8055–8066. doi: 10.1074/jbc.M110.192641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of pdgf-b and pdgfr-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 61.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in pdgf-b-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 62.Soriano P. Abnormal kidney development and hematological disorders in pdgf beta-receptor mutant mice. Genes Dev. 1994;8:1888–1896. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- 63.Winkler EA, Bell RD, Zlokovic BV. Pericyte-specific expression of pdgf beta receptor in mouse models with normal and deficient pdgf beta receptor signaling. Mol Neurodegener. 2010;5:32. doi: 10.1186/1750-1326-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuhnert F, Tam BY, Sennino B, Gray JT, Yuan J, Jocson A, Nayak NR, Mulligan RC, McDonald DM, Kuo CJ. Soluble receptor-mediated selective inhibition of vegfr and pdgfrbeta signaling during physiologic and tumor angiogenesis. Proc Natl Acad Sci U S A. 2008;105:10185–10190. doi: 10.1073/pnas.0803194105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lindblom P, Gerhardt H, Liebner S, Abramsson A, Enge M, Hellstrom M, Backstrom G, Fredriksson S, Landegren U, Nystrom HC, Bergstrom G, Dejana E, Ostman A, Lindahl P, Betsholtz C. Endothelial pdgf-b retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 2003;17:1835–1840. doi: 10.1101/gad.266803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abramsson A, Lindblom P, Betsholtz C. Endothelial and nonendothelial sources of pdgf-b regulate pericyte recruitment and influence vascular pattern formation in tumors. J Clin Invest. 2003;112:1142–1151. doi: 10.1172/JCI18549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Greenberg JI, Shields DJ, Barillas SG, Acevedo LM, Murphy E, Huang J, Scheppke L, Stockmann C, Johnson RS, Angle N, Cheresh DA. A role for vegf as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456:809–813. doi: 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song N, Huang Y, Shi H, Yuan S, Ding Y, Song X, Fu Y, Luo Y. Overexpression of platelet-derived growth factor-bb increases tumor pericyte content via stromal-derived factor-1alpha/cxcr4 axis. Cancer Res. 2009;69:6057–6064. doi: 10.1158/0008-5472.CAN-08-2007. [DOI] [PubMed] [Google Scholar]
- 69.Pellet-Many C, Frankel P, Evans IM, Herzog B, Junemann-Ramirez M, Zachary IC. Neuropilin-1 mediates pdgf stimulation of vascular smooth muscle cell migration and signalling via p130cas. Biochem J. 2011;435:609–618. doi: 10.1042/BJ20100580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, Lindblom P, Shani M, Zicha D, Adams RH. Ephrin-b2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell. 2006;124:161–173. doi: 10.1016/j.cell.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 71.Salvucci O, Maric D, Economopoulou M, Sakakibara S, Merlin S, Follenzi A, Tosato G. Ephrinb reverse signaling contributes to endothelial and mural cell assembly into vascular structures. Blood. 2009;114:1707–1716. doi: 10.1182/blood-2008-12-192294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Erber R, Eichelsbacher U, Powajbo V, Korn T, Djonov V, Lin J, Hammes HP, Grobholz R, Ullrich A, Vajkoczy P. Ephb4 controls blood vascular morphogenesis during postnatal angiogenesis. EMBO J. 2006;25:628–641. doi: 10.1038/sj.emboj.7600949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Semela D, Das A, Langer D, Kang N, Leof E, Shah V. Platelet-derived growth factor signaling through ephrin-b2 regulates hepatic vascular structure and function. Gastroenterology. 2008;135:671–679. doi: 10.1053/j.gastro.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Okazaki T, Ni A, Baluk P, Ayeni OA, Kearley J, Coyle AJ, Humbles A, McDonald DM. Capillary defects and exaggerated inflammatory response in the airways of epha2-deficient mice. Am J Pathol. 2009;174:2388–2399. doi: 10.2353/ajpath.2009.080949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sanchez T, Hla T. Structural and functional characteristics of s1p receptors. J Cell Biochem. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 76.Tamama K, Kon J, Sato K, Tomura H, Kuwabara A, Kimura T, Kanda T, Ohta H, Ui M, Kobayashi I, Okajima F. Extracellular mechanism through the edg family of receptors might be responsible for sphingosine-1-phosphate-induced regulation of DNA synthesis and migration of rat aortic smooth-muscle cells. Biochem J. 2001;353:139–146. [PMC free article] [PubMed] [Google Scholar]
- 77.Kluk MJ, Hla T. Role of the sphingosine 1-phosphate receptor edg-1 in vascular smooth muscle cell proliferation and migration. Circ Res. 2001;89:496–502. doi: 10.1161/hh1801.096338. [DOI] [PubMed] [Google Scholar]
- 78.Osada M, Yatomi Y, Ohmori T, Ikeda H, Ozaki Y. Enhancement of sphingosine 1-phosphate-induced migration of vascular endothelial cells and smooth muscle cells by an edg-5 antagonist. Biochem Biophys Res Commun. 2002;299:483–487. doi: 10.1016/s0006-291x(02)02671-2. [DOI] [PubMed] [Google Scholar]
- 79.Boguslawski G, Grogg JR, Welch Z, Ciechanowicz S, Sliva D, Kovala AT, McGlynn P, Brindley DN, Rhoades RA, English D. Migration of vascular smooth muscle cells induced by sphingosine 1-phosphate and related lipids: potential role in the angiogenic response. Exp Cell Res. 2002;274:264–274. doi: 10.1006/excr.2002.5472. [DOI] [PubMed] [Google Scholar]
- 80.Tanimoto T, Lungu AO, Berk BC. Sphingosine 1-phosphate transactivates the platelet-derived growth factor beta receptor and epidermal growth factor receptor in vascular smooth muscle cells. Circ Res. 2004;94:1050–1058. doi: 10.1161/01.RES.0000126404.41421.BE. [DOI] [PubMed] [Google Scholar]
- 81.Bornfeldt KE, Graves LM, Raines EW, Igarashi Y, Wayman G, Yamamura S, Yatomi Y, Sidhu JS, Krebs EG, Hakomori S, et al. Sphingosine-1-phosphate inhibits pdgf-induced chemotaxis of human arterial smooth muscle cells: spatial and temporal modulation of pdgf chemotactic signal transduction. J Cell Biol. 1995;130:193–206. doi: 10.1083/jcb.130.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lockman K, Hinson JS, Medlin MD, Morris D, Taylor JM, Mack CP (2004) Sphingosine-1-phosphate stimulates smooth muscle cell differentiation and proliferation by activating separate serum response factor co-factors. J Biol Chem [DOI] [PubMed]
- 83.Hobson JP, Rosenfeldt HM, Barak LS, Olivera A, Poulton S, Caron MG, Milstien S, Spiegel S. Role of the sphingosine-1-phosphate receptor edg-1 in pdgf-induced cell motility. Science. 2001;291:1800–1803. doi: 10.1126/science.1057559. [DOI] [PubMed] [Google Scholar]
- 84.Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL. Edg-1, the g protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paik JH, Skoura A, Chae SS, Cowan AE, Han DK, Proia RL, Hla T (2004) Sphingosine 1-phosphate receptor regulation of n-cadherin mediates vascular stabilization. Genes Dev [DOI] [PMC free article] [PubMed]
- 87.McVerry BJ, Garcia JG. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cell Signal. 2005;17:131–139. doi: 10.1016/j.cellsig.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 88.Allende ML, Yamashita T, Proia RL. G-protein-coupled receptor s1p1 acts within endothelial cells to regulate vascular maturation. Blood. 2003;102:3665–3667. doi: 10.1182/blood-2003-02-0460. [DOI] [PubMed] [Google Scholar]
- 89.Du W, Takuwa N, Yoshioka K, Okamoto Y, Gonda K, Sugihara K, Fukamizu A, Asano M, Takuwa Y. S1p(2), the g protein-coupled receptor for sphingosine-1-phosphate, negatively regulates tumor angiogenesis and tumor growth in vivo in mice. Cancer Res. 2010;70:772–781. doi: 10.1158/0008-5472.CAN-09-2722. [DOI] [PubMed] [Google Scholar]
- 90.Ryu Y, Takuwa N, Sugimoto N, Sakurada S, Usui S, Okamoto H, Matsui O, Takuwa Y. Sphingosine-1-phosphate, a platelet-derived lysophospholipid mediator, negatively regulates cellular rac activity and cell migration in vascular smooth muscle cells. Circ Res. 2002;90:325–332. doi: 10.1161/hh0302.104455. [DOI] [PubMed] [Google Scholar]
- 91.Sugimoto N, Takuwa N, Okamoto H, Sakurada S, Takuwa Y. Inhibitory and stimulatory regulation of rac and cell motility by the g12/13-rho and gi pathways integrated downstream of a single g protein-coupled sphingosine-1-phosphate receptor isoform. Mol Cell Biol. 2003;23:1534–1545. doi: 10.1128/MCB.23.5.1534-1545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Allsopp G, Gamble HJ. Light and electron microscopic observations on the development of the blood vascular system of the human brain. J Anat. 1979;128:461–477. [PMC free article] [PubMed] [Google Scholar]
- 93.Caruso RA, Fedele F, Finocchiaro G, Pizzi G, Nunnari M, Gitto G, Fabiano V, Parisi A, Venuti A. Ultrastructural descriptions of pericyte/endothelium peg-socket interdigitations in the microvasculature of human gastric carcinomas. Anticancer Res. 2009;29:449–453. [PubMed] [Google Scholar]
- 94.Diaz-Flores L, Jr, Gutierrez R, Madrid JF, Saez FJ, Valladares F, Villar J, Diaz-Flores L. Peg-and-socket junctions between smooth muscle cells and endothelial cells in femoral veins are stimulated to angiogenesis by prostaglandin e and glycerols. Histol Histopathol. 2011;26:623–630. doi: 10.14670/HH-26.623. [DOI] [PubMed] [Google Scholar]
- 95.Wakui S, Yokoo K, Muto T, Suzuki Y, Takahashi H, Furusato M, Hano H, Endou H, Kanai Y. Localization of ang-1, -2, tie-2, and vegf expression at endothelial-pericyte interdigitation in rat angiogenesis. Lab Invest. 2006;86:1172–1184. doi: 10.1038/labinvest.3700476. [DOI] [PubMed] [Google Scholar]
- 96.Wakui S, Furusato M, Ohshige H, Ushigome S. Endothelial-pericyte interdigitations in rat subcutaneous disc implanted angiogenesis. Microvasc Res. 1993;46:19–27. doi: 10.1006/mvre.1993.1032. [DOI] [PubMed] [Google Scholar]
- 97.McGuire PG, Rangasamy S, Maestas J, Das A. Pericyte-derived sphinogosine 1-phosphate induces the expression of adhesion proteins and modulates the retinal endothelial cell barrier. Arterioscler Thromb Vasc Biol. 2011;31:e107–e115. doi: 10.1161/ATVBAHA.111.235408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gerhardt H, Wolburg H, Redies C. N-cadherin mediates pericytic-endothelial interaction during brain angiogenesis in the chicken. Dev Dyn. 2000;218:472–479. doi: 10.1002/1097-0177(200007)218:3<472::AID-DVDY1008>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 99.Sweet E, Abraham EH, D'Amore PA. Functional evidence of gap junctions between capillary endothelial cells and pericytes in vitro. Invest Ophthalmol Vis Sci. 1988;29:109a. [Google Scholar]
- 100.Hayashi K, Nakao S, Nakaoke R, Nakagawa S, Kitagawa N, Niwa M. Effects of hypoxia on endothelial/pericytic co-culture model of the blood–brain barrier. Regul Pept. 2004;123:77–83. doi: 10.1016/j.regpep.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 101.Hu J, Cotgreave IA. Differential regulation of gap junctions by proinflammatory mediators in vitro. J Clin Invest. 1997;99:2312–2316. doi: 10.1172/JCI119410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Larson DM, Carson MP, Haudenschild CC. Junctional transfer of small molecules in cultured bovine brain microvascular endothelial cells and pericytes. Microvasc Res. 1987;34:184–199. doi: 10.1016/0026-2862(87)90052-5. [DOI] [PubMed] [Google Scholar]
- 103.Little T, Xia J, Duling BR. Dye tracers define differential endothelial and smooth muscle coupling patterns within the arteriolar wall. Circ Res. 1995;76:498–504. doi: 10.1161/01.res.76.3.498. [DOI] [PubMed] [Google Scholar]
- 104.Spitznas M, Reale E. Fracture faces of fenestrations and junctions of endothelial cells in human choroidal vessels. Invest Ophthalmol. 1975;14:98–107. [PubMed] [Google Scholar]
- 105.de Wit C, Wolfle SE, Hopfl B. Connexin-dependent communication within the vascular wall: contribution to the control of arteriolar diameter. Adv Cardiol. 2006;42:268–283. doi: 10.1159/000092575. [DOI] [PubMed] [Google Scholar]
- 106.Figueroa XF, Duling BR. Gap junctions in the control of vascular function. Antioxid Redox Signal. 2009;11:251–266. doi: 10.1089/ars.2008.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hirschi KK, Burt JM, Hirschi KD, Dai C. Gap junction communication mediates transforming growth factor-beta activation and endothelial-induced mural cell differentiation. Circ Res. 2003;93:429–437. doi: 10.1161/01.RES.0000091259.84556.D5. [DOI] [PubMed] [Google Scholar]
- 108.Pries AR, Reglin B, Secomb TW. Remodeling of blood vessels: responses of diameter and wall thickness to hemodynamic and metabolic stimuli. Hypertension. 2005;46:725–731. doi: 10.1161/01.HYP.0000184428.16429.be. [DOI] [PubMed] [Google Scholar]
- 109.Dzau VJ, Gibbons GH. Vascular remodeling: mechanisms and implications. J Cardiovasc Pharmacol. 1993;21(Suppl 1):S1–S5. [PubMed] [Google Scholar]
- 110.Pries AR, Hopfner M, le Noble F, Dewhirst MW, Secomb TW. The shunt problem: control of functional shunting in normal and tumour vasculature. Nat Rev Cancer. 2010;10:587–593. doi: 10.1038/nrc2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gould VE, Mosquera JM, Leykauf K, Gattuso P, Durst M, Alonso A. The phosphorylated form of connexin43 is up-regulated in breast hyperplasias and carcinomas and in their neoformed capillaries. Hum Pathol. 2005;36:536–545. doi: 10.1016/j.humpath.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 112.Suarez S, Ballmer-Hofer K. Vegf transiently disrupts gap junctional communication in endothelial cells. J Cell Sci. 2001;114:1229–1235. doi: 10.1242/jcs.114.6.1229. [DOI] [PubMed] [Google Scholar]
- 113.van Hinsbergh VW, Koolwijk P. Endothelial sprouting and angiogenesis: matrix metalloproteinases in the lead. Cardiovasc Res. 2008;78:203–212. doi: 10.1093/cvr/cvm102. [DOI] [PubMed] [Google Scholar]
- 114.Hansen-Smith FM, Hudlicka O, Egginton S. In vivo angiogenesis in adult rat skeletal muscle: early changes in capillary network architecture and ultrastructure. Cell Tissue Res. 1996;286:123–136. doi: 10.1007/s004410050681. [DOI] [PubMed] [Google Scholar]
- 115.Gonul E, Duz B, Kahraman S, Kayali H, Kubar A, Timurkaynak E. Early pericyte response to brain hypoxia in cats: an ultrastructural study. Microvasc Res. 2002;64:116–119. doi: 10.1006/mvre.2002.2413. [DOI] [PubMed] [Google Scholar]
- 116.Dore-Duffy P, Owen C, Balabanov R, Murphy S, Beaumont T, Rafols JA. Pericyte migration from the vascular wall in response to traumatic brain injury. Microvasc Res. 2000;60:55–69. doi: 10.1006/mvre.2000.2244. [DOI] [PubMed] [Google Scholar]
- 117.Virgintino D, Girolamo F, Errede M, Capobianco C, Robertson D, Stallcup WB, Perris R, Roncali L. An intimate interplay between precocious, migrating pericytes and endothelial cells governs human fetal brain angiogenesis. Angiogenesis. 2007;10:35–45. doi: 10.1007/s10456-006-9061-x. [DOI] [PubMed] [Google Scholar]
- 118.Behrens P, Rothe M, Wellmann A, Krischler J, Wernert N. The ets-1 transcription factor is up-regulated together with mmp 1 and mmp 9 in the stroma of pre-invasive breast cancer. J Pathol. 2001;194:43–50. doi: 10.1002/path.844. [DOI] [PubMed] [Google Scholar]
- 119.Chantrain CF, Shimada H, Jodele S, Groshen S, Ye W, Shalinsky DR, Werb Z, Coussens LM, DeClerck YA. Stromal matrix metalloproteinase-9 regulates the vascular architecture in neuroblastoma by promoting pericyte recruitment. Cancer Res. 2004;64:1675–1686. doi: 10.1158/0008-5472.can-03-0160. [DOI] [PubMed] [Google Scholar]
- 120.Forsyth PA, Wong H, Laing TD, Rewcastle NB, Morris DG, Muzik H, Leco KJ, Johnston RN, Brasher PM, Sutherland G, Edwards DR. Gelatinase-a (mmp-2), gelatinase-b (mmp-9) and membrane type matrix metalloproteinase-1 (mt1-mmp) are involved in different aspects of the pathophysiology of malignant gliomas. Br J Cancer. 1999;79:1828–1835. doi: 10.1038/sj.bjc.6990291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nielsen BS, Sehested M, Kjeldsen L, Borregaard N, Rygaard J, Dano K. Expression of matrix metalloprotease-9 in vascular pericytes in human breast cancer. Lab Invest. 1997;77:345–355. [PubMed] [Google Scholar]
- 122.Yao JS, Chen Y, Zhai W, Xu K, Young WL, Yang GY. Minocycline exerts multiple inhibitory effects on vascular endothelial growth factor-induced smooth muscle cell migration: the role of erk1/2, pi3k, and matrix metalloproteinases. Circ Res. 2004;95:364–371. doi: 10.1161/01.RES.0000138581.04174.2f. [DOI] [PubMed] [Google Scholar]
- 123.Wang H, Keiser JA. Vascular endothelial growth factor upregulates the expression of matrix metalloproteinases in vascular smooth muscle cells: role of flt-1. Circ Res. 1998;83:832–840. doi: 10.1161/01.res.83.8.832. [DOI] [PubMed] [Google Scholar]
- 124.Wen J, Han M, Zheng B, Yang S. Comparison of gene expression patterns and migration capability at quiescent and proliferating vascular smooth muscle cells stimulated by cytokines. Life Sci. 2002;70:799–807. doi: 10.1016/s0024-3205(01)01446-1. [DOI] [PubMed] [Google Scholar]
- 125.Fabunmi RP, Baker AH, Murray EJ, Booth RF, Newby AC. Divergent regulation by growth factors and cytokines of 95 kda and 72 kda gelatinases and tissue inhibitors or metalloproteinases-1, -2, and −3 in rabbit aortic smooth muscle cells. Biochem J. 1996;315(Pt 1):335–342. doi: 10.1042/bj3150335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chang SH, Kanasaki K, Gocheva V, Blum G, Harper J, Moses MA, Shih SC, Nagy JA, Joyce J, Bogyo M, Kalluri R, Dvorak HF. Vegf-a induces angiogenesis by perturbing the cathepsin-cysteine protease inhibitor balance in venules, causing basement membrane degradation and mother vessel formation. Cancer Res. 2009;69:4537–4544. doi: 10.1158/0008-5472.CAN-08-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nagy JA, Feng D, Vasile E, Wong WH, Shih SC, Dvorak AM, Dvorak HF. Permeability properties of tumor surrogate blood vessels induced by vegf-a. Lab Invest. 2006;86:767–780. doi: 10.1038/labinvest.3700436. [DOI] [PubMed] [Google Scholar]
- 128.Banerjee S, Sengupta K, Dhar K, Mehta S, D'Amore PA, Dhar G, Banerjee SK. Breast cancer cells secreted platelet-derived growth factor-induced motility of vascular smooth muscle cells is mediated through neuropilin-1. Mol Carcinog. 2006;45:871–880. doi: 10.1002/mc.20248. [DOI] [PubMed] [Google Scholar]
- 129.Nehls V, Denzer K, Drenckhahn D. Pericyte involvement in capillary sprouting during angiogenesis in situ. Cell Tissue Res. 1992;270:469–474. doi: 10.1007/BF00645048. [DOI] [PubMed] [Google Scholar]
- 130.Schlingemann RO, Rietveld FJ, Kwaspen F, van de Kerkhof PC, de Waal RM, Ruiter DJ. Differential expression of markers for endothelial cells, pericytes, and basal lamina in the microvasculature of tumors and granulation tissue. Am J Pathol. 1991;138:1335–1347. [PMC free article] [PubMed] [Google Scholar]
- 131.Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2003;163:1801–1815. doi: 10.1016/S0002-9440(10)63540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang B, Dietrich UM, Geng JG, Bicknell R, Esko JD, Wang L. Repulsive axon guidance molecule slit3 is a novel angiogenic factor. Blood. 2009;114:4300–4309. doi: 10.1182/blood-2008-12-193326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jones CA, Nishiya N, London NR, Zhu W, Sorensen LK, Chan AC, Lim CJ, Chen H, Zhang Q, Schultz PG, Hayallah AM, Thomas KR, Famulok M, Zhang K, Ginsberg MH, Li DY. Slit2-robo4 signalling promotes vascular stability by blocking arf6 activity. Nat Cell Biol. 2009;11:1325–1331. doi: 10.1038/ncb1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Amselgruber WM, Schafer M, Sinowatz F. Angiogenesis in the bovine corpus luteum: an immunocytochemical and ultrastructural study. Anat Histol Embryol. 1999;28:157–166. doi: 10.1046/j.1439-0264.1999.00195.x. [DOI] [PubMed] [Google Scholar]
- 135.Redmer DA, Doraiswamy V, Bortnem BJ, Fisher K, Jablonka-Shariff A, Grazul-Bilska AT, Reynolds LP. Evidence for a role of capillary pericytes in vascular growth of the developing ovine corpus luteum. Biol Reprod. 2001;65:879–889. doi: 10.1095/biolreprod65.3.879. [DOI] [PubMed] [Google Scholar]
- 136.Reynolds LP, Grazul-Bilska AT, Redmer DA. Angiogenesis in the corpus luteum. Endocrine. 2000;12:1–9. doi: 10.1385/ENDO:12:1:1. [DOI] [PubMed] [Google Scholar]
- 137.Robinson RS, Woad KJ, Hammond AJ, Laird M, Hunter MG, Mann GE. Angiogenesis and vascular function in the ovary. Reproduction. 2009;138:869–881. doi: 10.1530/REP-09-0283. [DOI] [PubMed] [Google Scholar]
- 138.Ozerdem U, Alitalo K, Salven P, Li A. Contribution of bone marrow-derived pericyte precursor cells to corneal vasculogenesis. Invest Ophthalmol Vis Sci. 2005;46:3502–3506. doi: 10.1167/iovs.05-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ozerdem U, Stallcup WB. Early contribution of pericytes to angiogenic sprouting and tube formation. Angiogenesis. 2003;6:241–249. doi: 10.1023/B:AGEN.0000021401.58039.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ponce AM, Price RJ. Angiogenic stimulus determines the positioning of pericytes within capillary sprouts in vivo. Microvasc Res. 2003;65:45–48. doi: 10.1016/s0026286202000146. [DOI] [PubMed] [Google Scholar]
- 141.Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103:159–165. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wesseling P, Schlingemann RO, Rietveld FJ, Link M, Burger PC, Ruiter DJ. Early and extensive contribution of pericytes/vascular smooth muscle cells to microvascular proliferation in glioblastoma multiforme: an immuno-light and immuno-electron microscopic study. J Neuropathol Exp Neurol. 1995;54:304–310. doi: 10.1097/00005072-199505000-00003. [DOI] [PubMed] [Google Scholar]
- 143.Jung YD, Liu W, Reinmuth N, Ahmad SA, Fan F, Gallick GE, Ellis LM. Vascular endothelial growth factor is upregulated by interleukin-1 beta in human vascular smooth muscle cells via the p38 mitogen-activated protein kinase pathway. Angiogenesis. 2001;4:155–162. doi: 10.1023/a:1012291524723. [DOI] [PubMed] [Google Scholar]
- 144.Reinmuth N, Liu W, Jung YD, Ahmad SA, Shaheen RM, Fan F, Bucana CD, McMahon G, Gallick GE, Ellis LM. Induction of vegf in perivascular cells defines a potential paracrine mechanism for endothelial cell survival. FASEB J. 2001;15:1239–1241. doi: 10.1096/fj.00-0693fje. [DOI] [PubMed] [Google Scholar]
- 145.Darland DC, Massingham LJ, Smith SR, Piek E, Saint-Geniez M, D'Amore PA. Pericyte production of cell-associated vegf is differentiation-dependent and is associated with endothelial survival. Dev Biol. 2003;264:275–288. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 146.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by pdgf- b and vegf. Development. 1998;125:1591–1598. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 147.Gee MS, Procopio WN, Makonnen S, Feldman MD, Yeilding NM, Lee WM. Tumor vessel development and maturation impose limits on the effectiveness of anti-vascular therapy. Am J Pathol. 2003;162:183–193. doi: 10.1016/S0002-9440(10)63809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Helfrich I, Scheffrahn I, Bartling S, Weis J, von Felbert V, Middleton M, Kato M, Ergun S, Schadendorf D. Resistance to antiangiogenic therapy is directed by vascular phenotype, vessel stabilization, and maturation in malignant melanoma. J Exp Med. 2010;207:491–503. doi: 10.1084/jem.20091846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Erber R, Thurnher A, Katsen AD, Groth G, Kerger H, Hammes HP, Menger MD, Ullrich A, Vajkoczy P. Combined inhibition of vegf and pdgf signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004;18:338–340. doi: 10.1096/fj.03-0271fje. [DOI] [PubMed] [Google Scholar]
- 150.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 151.Shaheen RM, Tseng WW, Davis DW, Liu W, Reinmuth N, Vellagas R, Wieczorek AA, Ogura Y, McConkey DJ, Drazan KE, Bucana CD, McMahon G, Ellis LM. Tyrosine kinase inhibition of multiple angiogenic growth factor receptors improves survival in mice bearing colon cancer liver metastases by inhibition of endothelial cell survival mechanisms. Cancer Res. 2001;61:1464–1468. [PubMed] [Google Scholar]
- 152.Kuwabara T, Cogan DG. Mural cells of the retinal capillaries. Arch Ophthalmol. 1963;69:492–502. doi: 10.1001/archopht.1963.00960040498013. [DOI] [PubMed] [Google Scholar]
- 153.Feldman PS, Shneidman D, Kaplan C. Ultrastructure of infantile hemangioendothelioma of the liver. Cancer. 1978;42:521–527. doi: 10.1002/1097-0142(197808)42:2<521::aid-cncr2820420221>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 154.Egginton S, Hudlicka O, Brown MD, Graciotti L, Granata AL. In vivo pericyte-endothelial cell interaction during angiogenesis in adult cardiac and skeletal muscle. Microvasc Res. 1996;51:213–228. doi: 10.1006/mvre.1996.0022. [DOI] [PubMed] [Google Scholar]
- 155.Crocker DJ, Murad TM, Geer JC. Role of the pericyte in wound healing. An ultrastructural study. Exp Mol Pathol. 1970;13:51–65. doi: 10.1016/0014-4800(70)90084-5. [DOI] [PubMed] [Google Scholar]
- 156.Hobson B, Denekamp J. Endothelial proliferation in tumours and normal tissues: continuous labelling studies. Br J Cancer. 1984;49:405–413. doi: 10.1038/bjc.1984.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vartanian RK, Weidner N. Correlation of intratumoral endothelial cell proliferation with microvessel density (tumor angiogenesis) and tumor cell proliferation in breast carcinoma. Am J Pathol. 1994;144:1188–1194. [PMC free article] [PubMed] [Google Scholar]
- 158.Wang Y, Li Y, Zhu G, Wang X, Wu S, Zhang L, Gao X. ultrastructural and immunohistochemical characteristics of pericytes during neovascularization in breast carcinoma. Zhonghua Bing Li Xue Za Zhi. 2000;29:176–179. [PubMed] [Google Scholar]
- 159.Orlidge A, D'Amore PA. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol. 1987;105:1455–1462. doi: 10.1083/jcb.105.3.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Antonelli-Orlidge A, Saunders KB, Smith SR, D'Amore PA. An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proc Natl Acad Sci U S A. 1989;86:4544–4548. doi: 10.1073/pnas.86.12.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sato Y, Rifkin DB. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989;109:309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lafleur MA, Forsyth PA, Atkinson SJ, Murphy G, Edwards DR. Perivascular cells regulate endothelial membrane type-1 matrix metalloproteinase activity. Biochem Biophys Res Commun. 2001;282:463–473. doi: 10.1006/bbrc.2001.4596. [DOI] [PubMed] [Google Scholar]
- 163.Davis GE, Camarillo CW. An alpha 2 beta 1 integrin-dependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Exp Cell Res. 1996;224:39–51. doi: 10.1006/excr.1996.0109. [DOI] [PubMed] [Google Scholar]
- 164.Salazar R, Bell SE, Davis GE. Coordinate induction of the actin cytoskeletal regulatory proteins gelsolin, vasodilator-stimulated phosphoprotein, and profilin during capillary morphogenesis in vitro. Exp Cell Res. 1999;249:22–32. doi: 10.1006/excr.1999.4460. [DOI] [PubMed] [Google Scholar]
- 165.Davis GE, Pintar Allen KA, Salazar R, Maxwell SA. Matrix metalloproteinase-1 and −9 activation by plasmin regulates a novel endothelial cell-mediated mechanism of collagen gel contraction and capillary tube regression in three-dimensional collagen matrices. J Cell Sci. 2001;114:917–930. doi: 10.1242/jcs.114.5.917. [DOI] [PubMed] [Google Scholar]
- 166.Saunders WB, Bayless KJ, Davis GE. Mmp-1 activation by serine proteases and mmp-10 induces human capillary tubular network collapse and regression in 3d collagen matrices. J Cell Sci. 2005;118:2325–2340. doi: 10.1242/jcs.02360. [DOI] [PubMed] [Google Scholar]
- 167.Saunders WB, Bohnsack BL, Faske JB, Anthis NJ, Bayless KJ, Hirschi KK, Davis GE. Coregulation of vascular tube stabilization by endothelial cell timp-2 and pericyte timp-3. J Cell Biol. 2006;175:179–191. doi: 10.1083/jcb.200603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hayes AJ, Huang WQ, Yu J, Maisonpierre PC, Liu A, Kern FG, Lippman ME, McLeskey SW, Li LY. Expression and function of angiopoietin-1 in breast cancer. Br J Cancer. 2000;83:1154–1160. doi: 10.1054/bjoc.2000.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ahmad SA, Liu W, Jung YD, Fan F, Wilson M, Reinmuth N, Shaheen RM, Bucana CD, Ellis LM. The effects of angiopoietin-1 and −2 on tumor growth and angiogenesis in human colon cancer. Cancer Res. 2001;61:1255–1259. [PubMed] [Google Scholar]
- 170.Hawighorst T, Skobe M, Streit M, Hong YK, Velasco P, Brown LF, Riccardi L, Lange-Asschenfeldt B, Detmar M. Activation of the tie2 receptor by angiopoietin-1 enhances tumor vessel maturation and impairs squamous cell carcinoma growth. Am J Pathol. 2002;160:1381–1392. doi: 10.1016/S0002-9440(10)62565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Satoh N, Yamada Y, Kinugasa Y, Takakura N. Angiopoietin-1 alters tumor growth by stabilizing blood vessels or by promoting angiogenesis. Cancer Sci. 2008;99:2373–2379. doi: 10.1111/j.1349-7006.2008.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Metheny-Barlow LJ, Li LY. The enigmatic role of angiopoietin-1 in tumor angiogenesis. Cell Res. 2003;13:309–317. doi: 10.1038/sj.cr.7290176. [DOI] [PubMed] [Google Scholar]
- 173.Stoeltzing O, Ahmad SA, Liu W, McCarty MF, Parikh AA, Fan F, Reinmuth N, Bucana CD, Ellis LM. Angiopoietin-1 inhibits tumour growth and ascites formation in a murine model of peritoneal carcinomatosis. Br J Cancer. 2002;87:1182–1187. doi: 10.1038/sj.bjc.6600598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nakanishi H, Okayama M, Oguri K, Hayashi K, Tateno H, Hosoda S. Close association between tumour cells and vascular basement membrane in gastric cancers with liver metastasis. An immunohistochemical and electron microscopic study with special attention to extracellular matrices. Virchows Arch A Pathol Anat Histopathol. 1991;418:531–538. doi: 10.1007/BF01606504. [DOI] [PubMed] [Google Scholar]
- 175.Paulus W, Roggendorf W, Schuppan D. Immunohistochemical investigation of collagen subtypes in human glioblastomas. Virchows Arch A Pathol Anat Histopathol. 1988;413:325–332. doi: 10.1007/BF00783025. [DOI] [PubMed] [Google Scholar]
- 176.Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E, Munn LL, Jain RK. Kinetics of vascular normalization by vegfr2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 177.Jeon H, Ono M, Kumagai C, Miki K, Morita A, Kitagawa Y. Pericytes from microvessel fragment produce type iv collagen and multiple laminin isoforms. Biosci Biotechnol Biochem. 1996;60:856–861. doi: 10.1271/bbb.60.856. [DOI] [PubMed] [Google Scholar]
- 178.Mandarino LJ, Sundarraj N, Finlayson J, Hassell HR. Regulation of fibronectin and laminin synthesis by retinal capillary endothelial cells and pericytes in vitro. Exp Eye Res. 1993;57:609–621. doi: 10.1006/exer.1993.1166. [DOI] [PubMed] [Google Scholar]
- 179.Evensen L, Micklem DR, Blois A, Berge SV, Aarsaether N, Littlewood-Evans A, Wood J, Lorens JB. Mural cell associated vegf is required for organotypic vessel formation. PLoS One. 2009;4:e5798. doi: 10.1371/journal.pone.0005798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 181.Ergun S, Kilic N, Wurmbach JH, Ebrahimnejad A, Fernando M, Sevinc S, Kilic E, Chalajour F, Fiedler W, Lauke H, Lamszus K, Hammerer P, Weil J, Herbst H, Folkman J. Endostatin inhibits angiogenesis by stabilization of newly formed endothelial tubes. Angiogenesis. 2001;4:193–206. doi: 10.1023/a:1014027218980. [DOI] [PubMed] [Google Scholar]
- 182.Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114:5091–5101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nagy JA, Dvorak AM, Dvorak HF. Vascular hyperpermeability, angiogenesis, and stroma generation. Cold Spring Harb Perspect Med. 2012;2:a006544. doi: 10.1101/cshperspect.a006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dewever J, Frerart F, Bouzin C, Baudelet C, Ansiaux R, Sonveaux P, Gallez B, Dessy C, Feron O. Caveolin-1 is critical for the maturation of tumor blood vessels through the regulation of both endothelial tube formation and mural cell recruitment. Am J Pathol. 2007;171:1619–1628. doi: 10.2353/ajpath.2007.060968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dente CJ, Steffes CP, Speyer C, Tyburski JG. Pericytes augment the capillary barrier in in vitro cocultures. J Surg Res. 2001;97:85–91. doi: 10.1006/jsre.2001.6117. [DOI] [PubMed] [Google Scholar]
- 187.Kurzen H, Manns S, Dandekar G, Schmidt T, Pratzel S, Kraling BM. Tightening of endothelial cell contacts: a physiologic response to cocultures with smooth-muscle-like 10t1/2 cells. J Invest Dermatol. 2002;119:143–153. doi: 10.1046/j.1523-1747.2002.01792.x. [DOI] [PubMed] [Google Scholar]
- 188.Dohgu S, Takata F, Yamauchi A, Nakagawa S, Egawa T, Naito M, Tsuruo T, Sawada Y, Niwa M, Kataoka Y. Brain pericytes contribute to the induction and up-regulation of blood–brain barrier functions through transforming growth factor-beta production. Brain Res. 2005;1038:208–215. doi: 10.1016/j.brainres.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 189.Wang YL, Hui YN, Guo B, Ma JX. Strengthening tight junctions of retinal microvascular endothelial cells by pericytes under normoxia and hypoxia involving angiopoietin-1 signal way. Eye. 2007;21:1501–1510. doi: 10.1038/sj.eye.6702716. [DOI] [PubMed] [Google Scholar]
- 190.Hori S, Ohtsuki S, Hosoya K, Nakashima E, Terasaki T. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through tie-2 activation in vitro. J Neurochem. 2004;89:503–513. doi: 10.1111/j.1471-4159.2004.02343.x. [DOI] [PubMed] [Google Scholar]
- 191.Hunter AW, Barker RJ, Zhu C, Gourdie RG. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell. 2005;16:5686–5698. doi: 10.1091/mbc.E05-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rhett JM, Jourdan J, Gourdie RG. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol Biol Cell. 2011;22:1516–1528. doi: 10.1091/mbc.E10-06-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yonenaga Y, Mori A, Onodera H, Yasuda S, Oe H, Fujimoto A, Tachibana T, Imamura M. Absence of smooth muscle actin-positive pericyte coverage of tumor vessels correlates with hematogenous metastasis and prognosis of colorectal cancer patients. Oncology. 2005;69:159–166. doi: 10.1159/000087840. [DOI] [PubMed] [Google Scholar]
- 194.Welen K, Jennbacken K, Tesan T, Damber JE. Pericyte coverage decreases invasion of tumour cells into blood vessels in prostate cancer xenografts. Prostate Cancer Prostatic Dis. 2009;12:41–46. doi: 10.1038/pcan.2008.33. [DOI] [PubMed] [Google Scholar]
- 195.Taniguchi S, Takeoka M, Ehara T, Hashimoto S, Shibuki H, Yoshimura N, Shigematsu H, Takahashi K, Katsuki M. Structural fragility of blood vessels and peritoneum in calponin h1-deficient mice, resulting in an increase in hematogenous metastasis and peritoneal dissemination of malignant tumor cells. Cancer Res. 2001;61:7627–7634. [PubMed] [Google Scholar]
- 196.Xian X, Hakansson J, Stahlberg A, Lindblom P, Betsholtz C, Gerhardt H, Semb H. Pericytes limit tumor cell metastasis. J Clin Invest. 2006;116:642–651. doi: 10.1172/JCI25705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Nissen LJ, Cao R, Hedlund EM, Wang Z, Zhao X, Wetterskog D, Funa K, Brakenhielm E, Cao Y. Angiogenic factors fgf2 and pdgf-bb synergistically promote murine tumor neovascularization and metastasis. J Clin Invest. 2007;117:2766–2777. doi: 10.1172/JCI32479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Melnyk O, Zimmerman M, Kim KJ, Shuman M. Neutralizing anti-vascular endothelial growth factor antibody inhibits further growth of established prostate cancer and metastases in a pre-clinical model. J Urol. 1999;161:960–963. [PubMed] [Google Scholar]
- 199.Wang S, Voisin MB, Larbi KY, Dangerfield J, Scheiermann C, Tran M, Maxwell PH, Sorokin L, Nourshargh S. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Exp Med. 2006;203:1519–1532. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Voisin MB, Probstl D, Nourshargh S. Venular basement membranes ubiquitously express matrix protein low-expression regions: characterization in multiple tissues and remodeling during inflammation. Am J Pathol. 2010;176:482–495. doi: 10.2353/ajpath.2010.090510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Weisshardt P, Trarbach T, Durig J, Paul A, Reis H, Tilki D, Miroschnik I, Ergun S, Klein D. Tumor vessel stabilization and remodeling by anti-angiogenic therapy with bevacizumab. Histochem Cell Biol. 2012;137:391–401. doi: 10.1007/s00418-011-0898-8. [DOI] [PubMed] [Google Scholar]
- 202.Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M, Ancukiewicz M, Mrugala MM, Plotkin S, Drappatz J, Louis DN, Ivy P, Scadden DT, Benner T, Loeffler JS, Wen PY, Jain RK. Azd2171, a pan-vegf receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hwang JA, Lee EH, Kim HW, Park JB, Jeon BH, Cho CH. Comp-ang1 potentiates the antitumor activity of 5-fluorouracil by improving tissue perfusion in murine lewis lung carcinoma. Mol Cancer Res. 2009;7:1920–1927. doi: 10.1158/1541-7786.MCR-09-0041. [DOI] [PubMed] [Google Scholar]
- 205.Dirkx AE, oude Egbrink MG, Castermans K, van der Schaft DW, Thijssen VL, Dings RP, Kwee L, Mayo KH, Wagstaff J, Bouma-ter Steege JC, Griffioen AW. Anti-angiogenesis therapy can overcome endothelial cell anergy and promote leukocyte-endothelium interactions and infiltration in tumors. FASEB J. 2006;20:621–630. doi: 10.1096/fj.05-4493com. [DOI] [PubMed] [Google Scholar]
- 206.Hamzah J, Jugold M, Kiessling F, Rigby P, Manzur M, Marti HH, Rabie T, Kaden S, Grone HJ, Hammerling GJ, Arnold B, Ganss R. Vascular normalization in rgs5-deficient tumours promotes immune destruction. Nature. 2008;453:410–414. doi: 10.1038/nature06868. [DOI] [PubMed] [Google Scholar]