Abstract
Clinical studies of T cell profiles from cancer patients have shown a skewing toward a type-2 T cell response with decreased cytotoxic T cell function. However, the primary cause of this shift remains unknown. Here we show that tumor-released Survivin, an inhibitor of apoptosis (IAP) protein and tumor-specific antigen, is taken up by T cells and alters their response. The addition of Survivin to T cell cultures resulted in decreased T cell proliferation and reduced cytotoxic CD8+ T cell function. Additionally, type 1 cell numbers and IFN-γ and IL-2 production were significantly reduced, while IL-4 release and type 2 T cell numbers increased. In contrast, the function and numbers of Th17 and T regulatory cells were not affected. These studies show that tumor-released Survivin modulates T cells resulting in a phenotype similar to that observed in cancer patients with a polarity shift from a type 1 to a type 2 response.
Electronic supplementary material
The online version of this article (doi:10.1007/s12307-012-0096-9) contains supplementary material, which is available to authorized users.
Keywords: Survivin, T cell, Microenvironment, Th1, Th2
Introduction
The tumor microenvironment is characterized by the infiltration of T cells. Investigation into the role of T cells in tumor progression has demonstrated the importance of the inflammatory T cell response in elimination of neoplastic cells. The importance of T cell subsets in the anti-tumor response is of interest as imbalances in these cells and the cytokines they produce have been shown to alter the ability of the immune system to eradicate the tumor. Of particular interest are the type 1 and type 2 T cells, which are characterized by production of IFN-γ and IL-2 or IL-4 and IL-10, respectively. These cytokines play a significant role in the progression of tumor growth or eradication. The type 1 T cell cytokine IFN-γ is vital to the anti-tumor response as it recruits and activates macrophages, as well as increasing the cytotoxic response by CD8+ T cells [1]. Mice deficient in IFN-γ have been shown to spontaneously develop tumors and have poor tumor clearance, illustrating the importance of IFN-γ in anti-tumor immunity [1]. Production of IL-2 by type 1 cells increases the proliferation of nearby T cells, aiding the immune response by inducing clonal expansion of tumor-reactive cells. The antagonists of type 1 cells are the type 2 Tc2 and Th2 cells, which release IL-4, IL-5, IL-6 and IL-10 [2, 3]. Type 2 cytokines prevent the differentiation of naïve T cells into type 1 cells and oppose the functions of type 1 cytokines [3]. IL-10 decreases the cytotoxic response by decreasing antigen presentation by macrophages to Th1 cells [4, 5], while IL-4 leads to decreased type 1 cell differentiation and IFN-γ production [5]. In cancer, IL-4 has been shown to prevent apoptosis of malignant cells, as well as increase the metastatic potential of breast cancer cells through polarization of infiltrating macrophages [6].
In addition to Th1 and Th2 cells, the CD4+ T cell population also includes Th17 and T regulatory (Treg) cells [3]. Pro-inflammatory Th17 cells produce IL-17 to recruit neutrophils and induce the production of pro-inflammatory factors in immune cells [7, 8]. In contrast to their Th17 counterpart, Treg cells suppress inflammatory responses and regulate the function of other T cell populations [3, 9–11]. Characterized as CD4+CD25+CD127lowFoxp3+, Tregs are important in preventing over-activation of the immune system as well as responses to autoantigens [10, 12].
Peripheral blood and intratumoral T cells from cancer patients frequently exhibit altered T cell populations. Type 1 responses are typically decreased with a concomitant increase in type 2 cell numbers and cytokine levels [13–22]. The potential for metastasis and angiogenesis is increased by the presence of IL-4 and IL-6, respectively, and the type 2 polarization increases both of these cytokines [6, 23]. This shift is correlated to poor disease progression, as the anti-tumor response is inhibited and a pro-tumor environment is created [13, 14, 24]. Additionally, Treg cells have been implicated in promoting tumor growth by suppressing activation of tumor-specific cytotoxic T cells due to their expression of auto antigens [11, 25–27]. As with type 2 T cells, increased numbers of Tregs within the tumor correlates with poor prognosis and decreased responsiveness to immunotherapies [25–27]. The role of Th17 cells in cancer is still unclear. The inflammatory Th17 response correlates with poor disease outcome in prostate, head and neck and colorectal cancer [28, 29]. However, IL-17 knockout mice exhibited increased susceptibility to metastasis of colon cancer MC38 cells, demonstrating an possible anti-tumor function of Th17 cells [30, 31].
While altered T cell populations in the tumor have been well documented from a clinical aspect, what leads to these changes has not been fully elucidated. Much evidence points to tumor-released factors such as tumor proteins and cytokines [9, 32, 33]. One tumor antigen recently shown to be released from cancer cells into the extracellular space is the inhibitor of apoptosis (IAP) protein Survivin [34, 35]. Within the cancer cell, Survivin functions to prevent cell death in response to apoptotic signals [36, 37]. In its extracellular context, Survivin is released and taken up by surrounding malignant cells, which increases their proliferative rate, resistance to therapy and their invasive potential [34]. Here we show a novel role for tumor-released Survivin as a modulator of the T cell response, capable of inhibiting their proliferation and inducing a shift to a type 2 response, similar to that observed in cancer patients.
Results
T Cells Take Up Tumor-Released Survivin
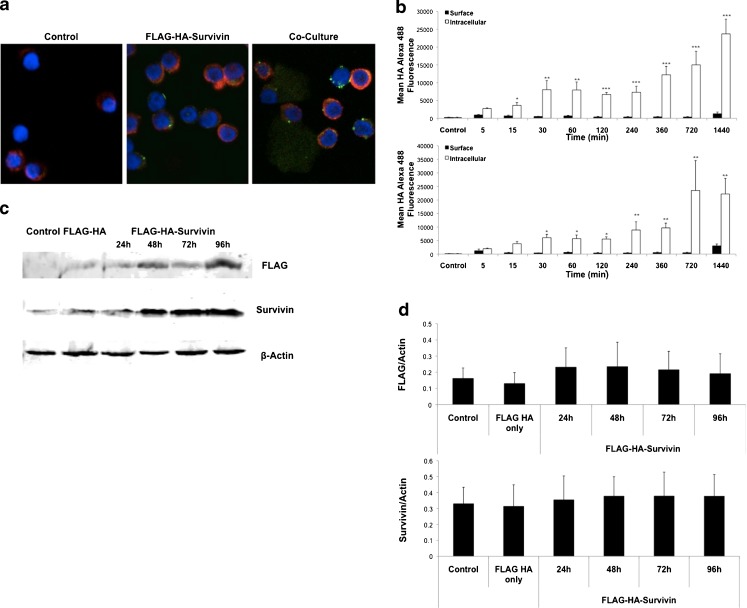
Recent studies have demonstrated that uptake of Survivin by cancer cells leads to a more aggressive phenotype [34]. To determine if tumor-infiltrating T cells would also be able to take up tumor-released Survivin, FLAG-HA-tagged Survivin was collected from cancer cell culture medium using dual-affinity purification and added to T cell cultures. Peripheral blood mononuclear cells (PBMCs) were isolated from normal, cancer-free donors and CD3+ T cells isolated by magnetic separation. Using FLAG-HA-Survivin released by POZn-WT-Survivin HeLa S cells as previously described [34] and at a non-toxic concentration (Supplementary Figure 1), we analyzed for both surface binding and intracellular uptake of Survivin in T cells. Cells were cultured for 24 h in control R10 or FLAG-HA-Survivin medium and stained for confocal microscopy with anti-HA and anti-actin. HA staining was detected in T cells cultured with FLAG-HA-Survivin (Fig. 1a), with some colocalization with actin as well as areas of pyknotic surface staining, demonstrating both surface binding and intracellular uptake of FLAG-HA-Survivin. Similar results were seen in T cells that were directly cultured with POZn-WT-Survivin cells (Fig. 1a). Surface binding and intracellular uptake were further evaluated by flow cytometry from 5 min to 24 h in culture. Cells were stained for FLAG-HA-Survivin using anti-HA and anti-Survivin antibodies. T cells were gated on CD3+CD4+ or CD3+CD8+ and the gating for Survivin and HA determined using appropriate isotype controls (Supplementary Figure 2a). Figure 1b shows the presence of surface-bound Survivin-HA on both CD4+ and CD8+ cells at 5 min, with significant uptake over the next 24 h (1440 min). At 30 min and later, surface Survivin levels significantly decrease (Fig. 1b) which is also representative of uptake. To determine the stability of FLAG-HA-Survivin, as endogenous Survivin is targeted by the ubiquitin-proteasome pathway, we evaluated the presence of FLAG-HA-Survivin in T cells at 24, 48, 72 and 96 h by Western blot to determine if FLAG-HA-Survivin levels are maintained or degraded following uptake (Fig. 1c, d). Endogenous Survivin, which is normally expressed in T cells, was detected in Control and FLAG-HA only samples, as well as FLAG-HA-Survivin samples at all timepoints (Fig. 1c, d). FLAG-tagged Survivin was detected only in FLAG-HA-Survivin samples and was retained in all timepoints and did not appear to be degraded (Fig. 1c). Additionally, densitometry of replicate Western blots showed no significant difference in endogenous Survivin levels in the presence of FLAG-HA-Survivin (Fig. 1d).
Fig. 1.
Normal T cells take up tumor-released Survivin. a Normal T cells were isolated by magnetic separation and cultured with FLAG-HA-Survivin or co-cultured with POZn-WT-Survivin cells for 24 h. Confocal images show uptake and surface binding of Survivin using a 60x oil immersion objective lens (Red – Actin, Green – FLAG-HA-Survivin, blue – DAPI). Representative of 2 independent experiments. b Surface and intracellular mean fluorescence intensity of HA in CD4+ (top) and CD8+ (bottom) T cells over time. Representative of 3 independent experiments. (Mean ± SD for 3 independent experiments; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 Intracellular vs. Surface). c Western blotting of T cells shows the presence of intracellular Survivin and FLAG tagged Survivin over time. Blots are representative of 3 independent experiments. d Densitometry shows Mean ± SD for 3 independent experiments, 2 blots each, n = 6
Survivin Uptake Results in Diminished T Cell Proliferation
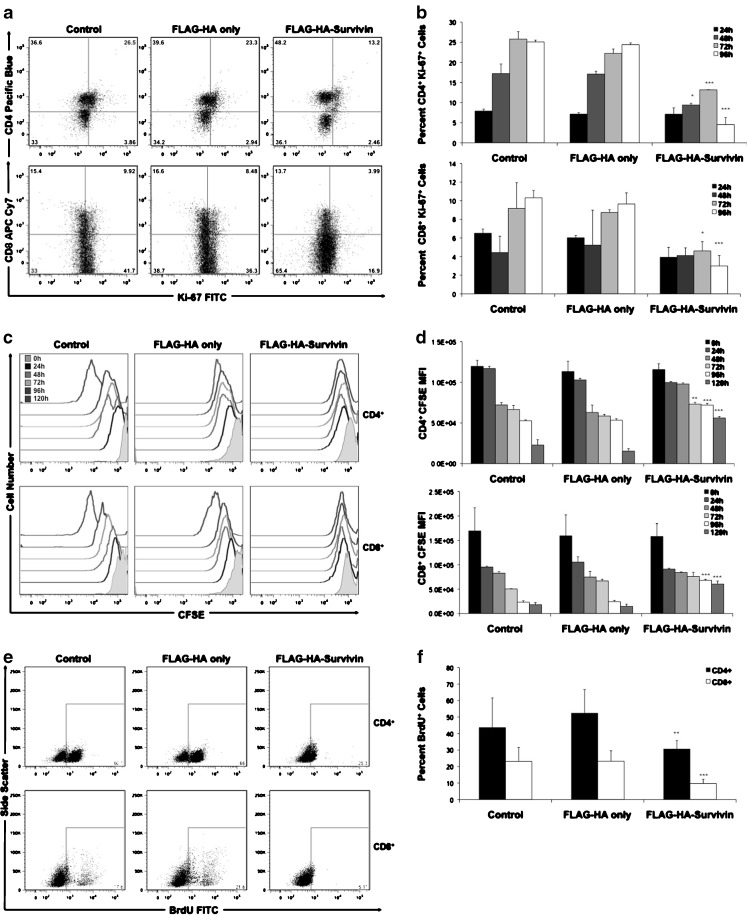
Uptake of Survivin by malignant cells has been shown to increase their proliferative rate [34]. To determine if Survivin uptake has a similar effect on T cells, we analyzed their proliferation using the cell-cycle-associated nuclear antigen Ki-67 [38], CFSE staining and BrdU incorporation. T cells were isolated from healthy, cancer-free donors, stimulated with anti-CD3/CD28 T cell expansion beads and cultured in Control, FLAG-HA peptide or FLAG-HA-Survivin medium. Expression of Ki-67 was analyzed at 24, 48, 72 and 96 h. Figure 2a shows representative staining of Ki-67 at 72 h post-stimulation. At 72 and 96 h, Ki-67 expression was significantly decreased in both CD8+ and CD4+ T cells when cultured with FLAG-HA-Survivin compared to Control and FLAG-HA only medium (Fig. 2b). CFSE is a cytosolic fluorescent dye used to detect proliferation, with a dilution of the dye observed with each cell division [39]. CFSE staining showed dilution in response to stimulation in Control and FLAG-HA only cells, which was prevented by the addition of FLAG-HA-Survivin (Fig. 2c, d). These results were confirmed by a BrdU incorporation assay. T cells were cultured as before and BrdU added after 72 h in culture. Cells were harvested 24 h later and analyzed for nuclear BrdU by flow cytometry. T cells cultured with FLAG-HA-Survivin showed significantly reduced percentage of cells with BrdU incorporation (Fig. 2e, f). Additionally, total T cell numbers after stimulation were significantly decreased in FLAG-HA-Survivin cultures compared to Controls (Supplementary Figure 3a). To ensure this difference in cell number was not due to cell death, we analyzed viability using 7-AAD staining. No significant difference in viability was seen in FLAG-HA-Survivin compared to controls (Supplementary Figure 3b). This data shows an anti-proliferative function for Survivin uptake in T cells.
Fig. 2.
T cell proliferation is inhibited by culture with tumor-released Survivin. a Representative Ki-67 staining at 72 h post-stimulation with anti-CD3/anti-CD28 microbeads. b Ki-67 expression was evaluated over time in T cells cultured with FLAG-HA-Survivin. CD4+Ki-67+ and CD8+Ki-67+ cell numbers are decreased after culture with FLAG-HA-Survivin compared to control. c and d CFSE dilution post-activation is significantly reduced by Survivin in CD8+ and CD4+ T cells compared to controls at 72, 96 and 120 h. e and f BrdU incorporation is diminished in CD8+ and CD4+ T cell cultures containing Survivin. Results in a, c and e are representative of 3 independent experiments. Data in b, d, and f are Means ± SD of 3 independent experiments, n = 9. *p < 0.05, **p < 0.01, ***p < 0.001
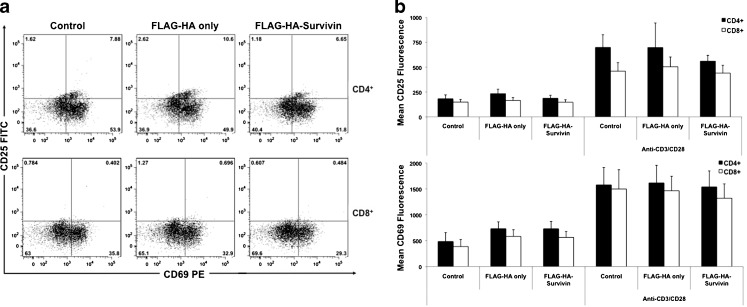
Survivin’s ability to prohibit proliferation in T cells is in stark contrast to the increased proliferative rate seen in malignant cells after Survivin uptake [34]. One key difference between T cell and tumor cell proliferation is the requirement of activation signalling for T cell clonal expansion [40, 41]. Survivin’s anti-proliferative effect on T cells could be due to an interference with T cell activation. To examine this possibility, T cells were stimulated in the presence or absence of extracellular FLAG-HA-Survivin and analyzed for the expression of activation markers CD25 and CD69. We found that the addition of FLAG-HA-Survivin during stimulation had no effect on the expression of activation antigens on T cell cultures (Fig. 3a, b). Additionally, upregulation of Survivin is required for proper T cell activation and proliferation [40] and the presence of exogenous FLAG-HA-Survivin does not significantly alter Survivin protein expression levels after activation (Supplementary Figure 4). From these results we conclude the inhibition of proliferation by Survivin likely occurs after cells have been activated and is not due to a reduction in activation-induced Survivin expression.
Fig. 3.
Survivin does not affect T cell activation. a CD25 and CD69 staining of CD4+ and CD8+ T cells cultured with anti-CD3/anti-CD28 microbeads for 24 h show no change in CD25+CD69+ cell numbers. Results representative of 3 independent experiments. b CD25 and CD69 MFI are and unchanged in CD8+ and CD4+ Survivin-cultured cells. Results show Mean ± SD, n = 9
Survivin Decreases the Cytotoxic Function of CTLs
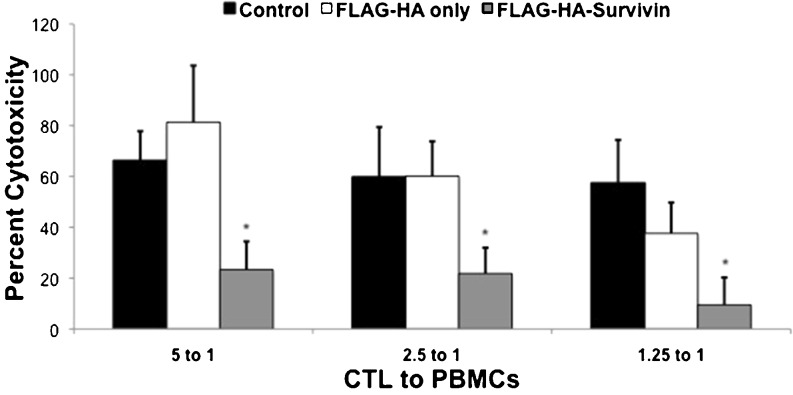
Tumor-specific CTLs are important in the destruction of cancer cells [10]. We have observed decreased proliferation of CTLs when stimulated in the presence of Survivin (Fig. 2), which would likely diminish the effectiveness of a T cell response. It is not known if Survivin also alters the cytotoxic function of CTLs, so we evaluated this using a cytotoxicity assay. CTLs were isolated from a single donor and cultured with Control, FLAG-HA or FLAG-HA-Survivin medium and stimulated with PMA and ionomycin for 24 h. The cells were then added in culture with pooled PBMCs from other healthy donors at 5:1, 2.5:1 and 1.25:1 CTL:PBMC ratios. The ability of the CTLs to kill foreign PBMCs was then evaluated using by lactate dehydrogenase release. The cytotoxic function of CTLs was diminished at all CTL:PBMC ratios after culture with FLAG-HA-Survivin compared to control conditions (Fig. 4). This may demonstrate a possible immune evasion function for tumor-released Survivin by preventing CTL-mediated killing.
Fig. 4.
Survivin decreases cytotoxicity of CD8+ T cells. CD8+ cells from a single donor were cultured with Survivin for 24 h and their cytotoxic function assessed by the CytoTox 96 Non-Radioactive Cytotoxicity Assay. Results show Mean ± SD of 3 independent experiments, n = 3. *p < 0.05
Survivin Does Not Affect T Regulatory Cells
Decreased activity of cytotoxic CD8+ T cells in cancer patients has been correlated to increased numbers of tumor-infiltrating T regulatory cells (Tregs) [10, 42]. To determine if the decreased CTL function observed in the presence of Survivin could be due in part to Tregs, we analyzed both the number and function of Tregs after culture with Survivin. CD3+ cells were cultured with Survivin or Control conditions with stimulation for 48 h and analyzed for the number of CD4+CD25+CD127low Treg cells. No differences were seen in the number of Tregs in the presence of Survivin compared to Controls (Supplementary Figure 5). To analyze the function of Tregs after culture with Survivin, CD4+CD127low Tregs were isolated by magnetic separation. After 24 h culture with Control, FLAG-HA, or FLAG-HA-Survivin medium, the cells were washed and plated with PBMCs and anti-CD3/anti-CD28 microbeads for 4 h. Analysis of CD154 and CD69 levels on non-Treg cells was used to determine the ability of Tregs to suppress activation. We observed that Treg function was not altered by culture with tumor-released Survivin (Supplementary Figure 5). Due to these results, the decreased cytotoxic function of CD8+ cells is likely a direct effect of Survivin, and not through increased Treg numbers or function.
Survivin Skews the Balance of Type 1 and Type 2 T Cells
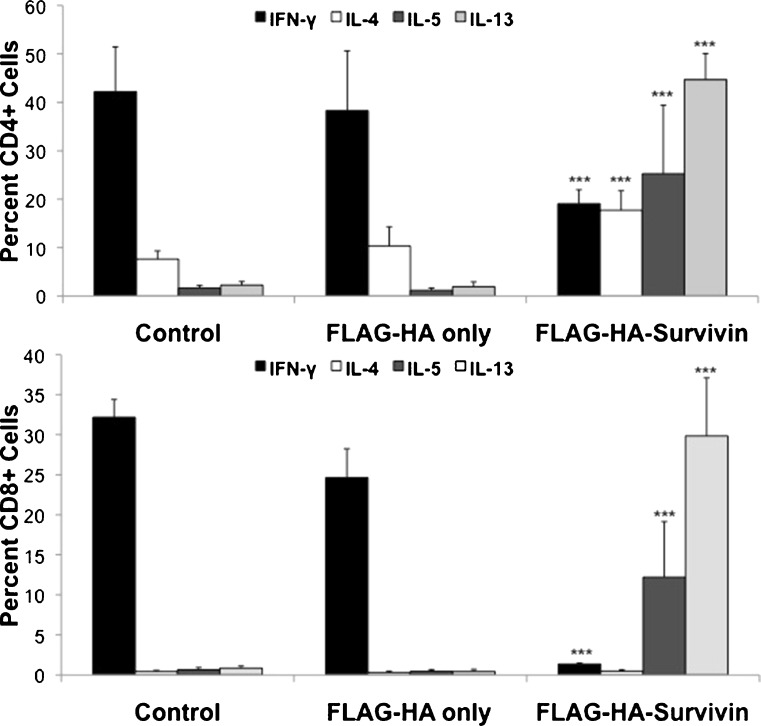
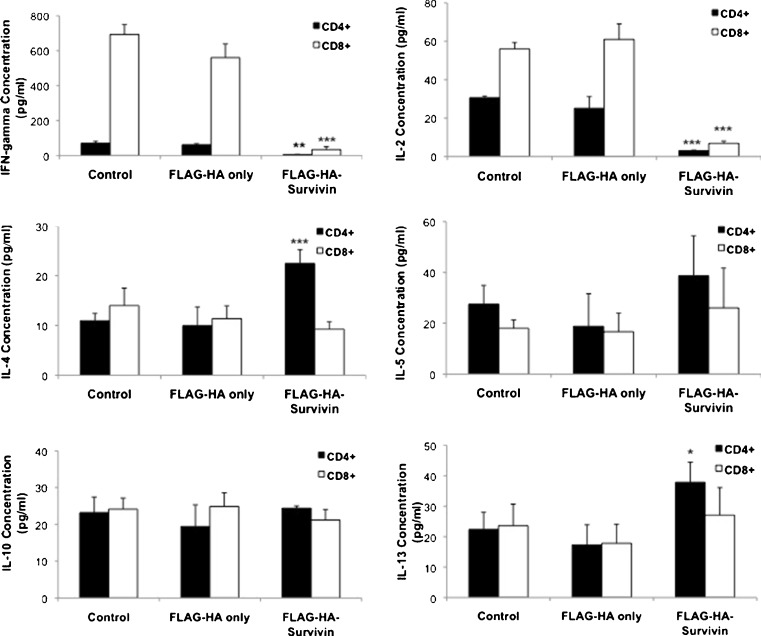
Cancer patients frequently exhibit skewing of helper T cell populations with an increase in Type 2 cells, a concomitant decrease in the Type 1 response [13–15, 17, 18, 43], and cancer-type specific alteration of Th17 cells [28, 29]. To evaluate the effects of tumor-released Survivin in this population skewing, intracellular cytokine staining was used to quantify the number of Tc1, Tc2, Th1, Th2, and Th17 cells (Fig. 5). Supplementary Figure 6 shows representative gating of intracellular cytokine analysis for CD4+ and CD8+ cells. After culture with FLAG-HA-Survivin for 48 h, the numbers of Th1 (CD4+IFN-γ+) and Tc1 (CD8+ IFN-γ+) cells were significantly decreased (Fig. 5). Additionally, CD4+ and CD8+ cells expressing the type 2 cytokines IL-4, IL-5 and IL-13 increased substantially in FLAG-HA-Survivin cultures (Fig. 5), though the change in IL-4 expressing CD8+ cells was not significant. No significant change was seen in CD4+IL-17+ or CD8+IL-17+ cell numbers (Supplementary Figure 7). To quantify the effects on cytokine release, isolated CD4+ and CD8+ cells were plated in Control, FLAG-HA only or FLAG-HA-Survivin medium for 48 h, and the culture supernatants evaluated by ELISA (Fig. 6). Production of the type 1 cytokines IFN-γ and IL-2 was significantly reduced in both CD4+ and CD8+ cultures (Fig. 6). Additionally, IL-4 and IL-13 production by CD4+ cells was significantly increased when cultured with FLAG-HA-Survivin, but a significant change was not seen in IL-5 or IL-10 levels (Fig. 6). These findings demonstrate a shift from a type 1 to a type 2 T cell response in the presence of tumor-released Survivin.
Fig. 5.
Polarization to a type 2 phenotype by Survivin. Survivin significantly decreases CD4+IFN-γ+ and CD8+IFN-γ+ and increases CD4+Il-5+, CD8+Il-5+, CD4+IL-13+, CD8+IL-13+, and CD4+IL-4+ cell numbers. No change was seen in IL-17+ cell numbers. Results show Mean ± SD from 3 independent experiments, n = 9. *p < 0.05, **p < 0.01 and ***p < 0.001
Fig. 6.
Type 1 cytokines are decreased and Type 2 cytokines increased by Survivin. Cytokine ELISA shows decreased production of inflammatory cytokines IFN-γ and IL-2 by CD4+ and CD8+ T cells and increased IL-4 and IL-13 production by CD4+ cells in the presence of Survivin. No change was observed in IL-5 or IL-10 release. Results show Mean ± SD from 2 independent experiments, n = 6. *p < 0.05, **p < 0.01 and ***p < 0.001
Discussion
The ability of the immune system to detect and destroy tumor cells plays an important role in decreasing tumor growth and improving patient prognosis, as patients with fewer tumor-specific T cells often have increased tumor growth and metastasis [13–17]. Here we have shown that the tumor-released protein Survivin [35] is taken up by T cells and is capable of altering their function. Like that described by Mera et al. using recombinant protein [44], tumor-released Survivin is able to bind to the surface of T cells, and as described here, is also taken up into T cells (Fig. 1). Similar results were also observed in T cells after culture with POZn-WT-Survivin HeLa-S cells (Fig. 1a), leading us to believe that Survivin uptake could occur within the tumor microenvironment. Whether similar uptake and cellular modulation of granulocytes and monocytes would be recorded is yet to be investigated. Also the mode of uptake, whether receptor-mediated or not has yet to be fully evaluated. It is also important to note that, due to the allotypic differences between the donor T cells and the POZn-WT-Survivin cells, tumor cell death was observed in our confocal microscopy (Data Not Shown). The inhibition of T cell division by Survivin (Fig. 2) is in contrast to its effects on malignant cells [34]. Based on the evaluation of activation markers CD25 and CD69 (Fig. 3), Survivin is not inhibiting T cell stimulation, but is possibly acting downstream of activation signaling. Additionally, no changes in activation-induced endogenous Survivin in the presence of FLAG-HA-Survivin were observed (Supplementary Figure 4). Due to the significantly diminished proliferation observed in both CD4+ and CD8+ populations from cancer-free donors (Fig. 2), we believe a similar effect would be seen in tumor-specific T cells and overall T cell populations from cancer patients, though these studies have yet to be accomplished. In addition to their decreased proliferative capacity, Survivin-exposed CD8+ T cells showed decreased cytotoxic function compared to controls (Fig. 4). Many previous studies have demonstrated the inhibition of tumor-specific CTL function by T regulatory cells [45, 46]. In our studies, Survivin did not significantly alter Treg numbers or function (Supplementary Figure 5). Because of these observations, we hypothesize that Survivin’s inhibition of cytotoxic function is independent of Treg-mediated CTL suppression. The suppression of cytotoxic T cell function is an important mechanism of immune escape for many cancers [33, 47, 48]. The release of Survivin may be one way tumors evade T cell-mediated cytotoxicity.
Although Treg populations were not affected by extracellular Survivin (Supplementary Figure 5), type 1 and type 2 T cell populations were significantly altered. The decrease in IFN-γ and IL-2 production by T cells, combined with the significant drop in IFN-γ+ cells demonstrates an inhibition of the anti-tumor response (Fig. 5). The importance of IFN-γ to immune surveillance has been shown previously in IFN-γ deficient mice and it is frequently decreased in cancer patients [14, 15, 49, 50]. The overall shift from a type 1 to a type 2 response was shown both by the decrease in IFN-γ+ T cells and the increase in IL-4+, IL-5+ and IL-13+ T cells in response to tumor-released Survivin (Fig. 5). The importance of CD4+IL-4+cells in malignancy was recently demonstrated in a mouse mammary carcinoma model, in which production of IL-4 by helper T cells was able to polarize macrophages, resulting in increased lung metastasis [6]. Similarly, increased plasma levels of IL-13 in patients with pancreatic, gastric and esophageal cancers was positively correlated to increased numbers of myeloid derived suppressor cells [51]. Both these studies showed important downstream effects of the shift to a type 2 response, but did not examine the preliminary cause of the shift. Importantly, when combined with this study and our previous work showing Survivin-associated invasiveness [34], a more complete picture emerges with Survivin inducing the type 2 response, which can then lead to increased metastasis and complete polarization of the immune response. Alteration of the immune response toward the type 2 phenotype has been demonstrated in many types of cancer, including lymphoma, breast, prostate and lung cancer, though the reason for this shift was undetermined [13–17]. Similar studies have been performed looking at Th17 cells in cancer patients [29, 30, 52, 53]. Unlike the Th1/Th2 shift, which is common across patients with a variety of cancer types, the role of Th17 cells appear to be cancer-type specific. In prostate and ovarian cancers, increased numbers of Th17 cells in the tumor correlated with better patient outcome, demonstrating an anti-tumor role [28, 29]. Conversely, in colorectal carcinoma, higher levels of serum IL-17 correlated to poor patient prognosis [54]. In our studies, no change in IL-17-expressing cells was observed after exposure to Survivin (Supplementary Figure 7), unlike our observed type 1 and type 2 T cell shift Fig. 6.
In this study we show that the polarization of the T cell response leading to a pro-tumor response could be mediated in part by tumor-released Survivin. The ability of Survivin to decrease the proliferation and cytotoxicity of T cells and alter the T cell response to malignant cells, as well as positively affect tumor growth and aggressiveness, makes it an important therapeutic target for the treatment of cancer.
Materials and Methods
Preparation of FLAG-HA-Tagged Survivin
The development of POZn-WT-Survivin HeLa-S cells stably expressing FLAG-HA-Survivin has been described previously [34]. Cells were grown in a 5 L bioreactor at 37°C in Joklik medium supplemented with 10% FCS, L-glutamine and penicillin/streptomycin. After 1 week of growth the conditioned medium was collected and centrifuged to remove cells and debris. Anti-FLAG beads (20 μl/ml; Sigma, St. Louis, MO) were added to the medium and rotated overnight at 4°C. The beads were pelleted by centrifugation and Survivin eluted using FLAG peptides. This process was repeated using anti-HA beads (20μl/ml). Free FLAG and HA peptides were removed from the preparation by dialysis against a 10 mM HEPES buffer (pH 7.5) overnight at 4°C. The concentration of Survivin was 1.25 ng/ml as determined by ELISA (R&D Systems, Minneapolis, MN) as previously described [34]. Synthesized FLAG and HA peptides were added to 10 mM HEPES buffer at a concentration of 1.25 pg/ml for use as a control.
T Cell Isolation and Culture
Peripheral blood from normal, healthy donors was collected into potassium EDTA vacuettes as approved by the Institutional Review Board at Loma Linda University. PBMCs were isolated by Ficoll-Hypaque (GE Healthcare, Piscataway, NJ) centrifugation as described previously [10]. T cell populations were isolated using the MACS Pan T Cell Isolation Kit II (Miltenyi Biotec, Auburn, CA). Cells were cultured in R10 medium (RPMI supplemented with 10% FBS, L-glutamine, penicillin/streptomycin, and 0.1% 2-mercaptoethanol), FLAG-HA medium (R10 with 1.25 pg/ml FLAG and HA peptides) or FLAG-HA-Survivin medium (R10 with 1.25 pg/ml FLAG-HA-Survivin; Supplementary Figure 1). For co-culture experiments, T cells were cultured 100:1 with POZn-WT-Survivin cells in R10 medium.
Flow Cytometric Analysis of Survivin Uptake
T cells were cultured in 96-well plates in R10 or FLAG-HA-Survivin medium at a concentration of 1 × 106 cells/ml. Cells were harvested at 5, 15, 30, 60, 120, 240, 360, 720, and 1440 min and stained for surface-bound or intracellular Survivin using rabbit anti-HA, goat anti-rabbit Alexa Fluor 488 secondary (Invitrogen, Carlsbad, CA), and anti-Survivin Alexa Fluor 647 antibodies (Cell Signalling, Danvers, MA). For intracellular staining, cells were fixed and permeabilized using the eBioscience Fixation/Permeabilization Kit (San Diego, CA). Cells were stained for T cell surface antigens with monoclonal antibodies against CD3, CD4, and CD8 (BioLegend, San Diego, CA). Data was collected on the MACSQuant flow cytometer (Miltenyi Biotec, Auburn, CA) and analyzed with FlowJo analysis software. Intracellular MFI was calculated as Total MFI of permeabilized cells minus the Surface Stained MFI.
Confocal Microscopy
T cells were cultured in 12-well plates in R10 or FLAG-HA-Survivin medium at 1 × 106 cells/ml. Co-cultures were performed with 1 × 106 T cells/ml and 1 × 104 POZn-WT-Survivin cells/ml. Cells were harvested after 24 h in culture, fixed with 3.7% paraformaldehyde and permeabilized with 0.1% PBS-saponin. Cells were stained with anti-FLAG and anti-Actin primary antibodies and goat anti-mouse Alexa Fluor 488 and goat anti-rabbit Alexa Fluor 555 secondary antibodies (Invitrogen, Carlsbad, CA). Slides were imaged using the Zeiss LSM 710 NLO laser scanning confocal imaging system, equipped with an Axio Observer Z1 inverted microscope.
Western Blots
For Western blots of Survivin uptake, T cells were cultured with Survivin, harvested at 24, 48, 72, and 96 h, and washed with PBS. To analyze Survivin expression after activation, T cells were cultured with R10, FLAG-HA or FLAG-HA-Survivin medium and activated using anti-CD3/anti-CD28 T cell expansion beads (25 μl/ml; Invitrogen, Carlsbad, CA). Cells were harvested at 0, 24, 48, 72, and 96 h. Whole cell lysates were prepared and separated using 12% Bis-Tris polyacrylamide gels as described previously [34]. Proteins were transferred onto nitrocellulose membranes and probed using mouse monoclonal anti-FLAG (Invitrogen, Carlsbad, CA), rabbit polyclonal anti-Survivin (Novus Biologicals, Littleton, CO), and rabbit polyclonal anti-Actin (Cell Signalling, Danvers, MA). Secondary IR-Dye conjugated goat anti-rabbit and goat anti-mouse antibodies (LICOR, Lincoln, NE) were utilized and bands detected using the Odyssey imaging system (LICOR, Lincoln, NE).
T Cell Proliferation Analysis by CFSE and Ki-67
T cells were stained with 0.5 μM CellTrace carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes, Carlsbad, CA) for 15 min at 37°C and the reaction quenched with 5 volumes of cold R10 medium (RPMI supplemented with 10% FBS, L-glutamine, penicillin/streptomycin, and 0.1% 2-mercaptoethanol). Cells were plated in a 96-well plate at 2.5 × 105 cells/well in R10, FLAG-HA, or FLAG-HA-Survivin medium, stimulated with anti-CD3/anti-CD28 T cell expansion beads (6.25 μl/well; Invitrogen, Carlsbad, CA) and harvested at 0, 24, 48, 72, 96 and 120 h. Cells were stained for surface antigens CD3, CD4, CD8 and for viability using 7-AAD (BioLegend, San Diego, CA). For Ki-67 analysis cells were cultured as above and harvested at 24, 48, 72, and 96 h. Cells were stained for surface markers, fixed and permeabilized using a Fixation/Permeabilization Kit (eBioscience, San Diego, CA) and stained for intracellular Ki-67 (BD Biosciences, San Jose, CA).
Analysis of T Cell Proliferation by BrdU Incorporation
T cells were plated in a 96-well plate at 2.5 × 105 cells/well in R10, FLAG-HA, or FLAG-HA-Survivin medium and stimulated with anti-CD3/anti-CD28 T cell expansion beads (6.25 μl/well; Invitrogen, Carlsbad, CA). At 72 h in culture, 10 μM BrdU was added to each well and the cells harvested 24 h after BrdU addition. Cells were stained for surface CD3, CD4 and CD8, fixed and permeabilized using BD Cytofix/Cytoperm Buffer (BD Biosciences, San Jose, CA), and frozen at -80°C overnight in freezing medium (10% DMSO, 90% heat-inactivated FBS). Cells were thawed and then washed using BD Cytofix/Cytoperm and then washed with BD Perm/Wash Buffer, after which they were re-fixed as before. Cells were treated with DNase (300μg/ml) and stained with anti-BrdU FITC (BD Biosciences, San Jose, CA).
Analysis of T Cell Activation
Isolated T cells were cultured and stimulated as described above. After 24 h, cells were harvested and stained with monoclonal antibodies against CD3, CD4, CD8 (BioLegend, San Diego, CA), CD25 and CD69 (BD Biosciences, San Jose, CA) and analyzed by flow cytometry.
Analysis of CTL Cytotoxic Function
CD8+ CTLs were isolated from a single healthy donor using the MACS anti-PE magnetic separation kit (Miltenyi Biotec, Auburn, CA) and anti-CD8 PE antibodies (BD Biosciences, San Jose, CA). CTLs were cultured in 12-well plates as described above and stimulated with PMA (10 ng/ml) and ionomycin (0.1 μg/ml). After 24 h in culture the cells were washed and harvested. Pooled PBMCs from various healthy donors were put into a 96-well plate with CTLs at 5:1, 2.5:1, and 1.25:1 (CTL:PBMC) ratios. The cytotoxic function of the CTLs was analyzed using the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega, Fitchburg, WI).
T Regulatory (Treg) Cell Analysis
CD3+ T cells were isolated and plated in 96-well plates as described above and stimulated for 48 h with anti-CD3/anti-CD28 T cell expansion beads (6.25 μl/well; Invitrogen, Carlsbad, CA). Cells were stained for CD3, CD4, CD8 (BioLegend, San Diego, CA), CD25 and CD127 (BD Biosciences, San Jose, CA) and CD4+CD25+CD127low Treg populations analyzed by flow cytometry.
T Regulatory Cell Activity Assay
T regulatory cells were isolated from PBMCs using the EasySep Human CD4+CD127low T Cell Enrichment Kit (Stem Cell Technologies, Vancouver, BC, Canada) and cultured in 12-well plates at 1 × 106 cells/ml in R10, FLAG-HA, or FLAG-HA-Survivin medium. After 24 h, cells were washed, harvested and added to PBMCs at a 0:1, 0.25:1 or 0.5:1 (Treg:PBMC) concentration. Inhibition of cell activation by Tregs was evaluated using the BD FastImmune Human Regulatory T Cell Function Kit (BD Biosciences, San Jose, CA) as per the manufacturer’s instructions.
Intracellular Cytokine Analysis
CD3+ T cells were isolated, plated in 96-well plates as described above and stimulated with PMA (10 ng/ml) and ionomycin (0.1 μg/ml) in the presence of 3 μM monensin to inhibit cytokine release. After 6 h of activation, the cells were harvested and stained for T cell surface markers. Cells were fixed and permeabilized and intracellularly stained with biotinylated antibodies against IFN-γ, IL-4, IL-5, IL-13 or IL-17 followed by streptavidin-APC (eBioscience, San Diego, CA).
Cytokine ELISA
Isolated CD4+ and CD8+ cells were stimulated with anti-CD3/anti-CD28 microbeads for 48 h and PMA and ionomycin added for the final 6 h as previously described [55]. Cell supernatants were isolated and analyzed using Th1/Th2, IL-5, and IL-13 Ready-Set-Go! ELISA kits (eBioscience) as per the manufacturer’s protocol. Detection limits for these ELISAs are IFN- γ – 4 pg/ml; IL-2 – 4 pg/ml; IL-4 – 2 pg/ml; IL-5 – 4 pg/ml; IL-10 – 2 pg/ml; IL-13 – 4 pg/ml; as described by the manufacturer’s Technical Data Sheet.
Statistical Analysis
Results are shown as mean ± standard deviation. Statistical significance was determined by Student’s t-test for independent samples or ANOVA with Tukey’s post-hoc analysis, as appropriate. Results with p ≤ 0.05 were considered significant.
Electronic Supplementary Material
ESM 1 (PPT 2.52 mb)
Acknowledgements
We thank M. Rubalcava, S. Wilson and the Center for Perinatal Biology for assistance with confocal microscopy, I. Baez for assistance with flow cytometry techniques and J.A. Jutzy for assistance in procuring necessary reagents.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
Footnotes
Grant Support
NCMHD Project EXPORT Program 5P20MD001631/Project 3 (NRW). Funding was also obtained as part of a start-up package from Loma Linda University’s Center for Molecular Biology and Gene Therapy, now the Center for Health Disparities and Molecular Medicine (NRW) and a National Merit Test Bed (NMTB) award sponsored by the Department of the Army under Cooperative Agreement Number DAMD17-97-2-7016 (NRW). Imaging was performed in the LLUSM Advanced Imaging and Microscopy Core that is supported by NSF Grant No. MRI-DBI 0923559 (SMW) and the Loma Linda University School of Medicine.
References
- 1.Riemensberger J, Bohle A, Brandau S. IFN-gamma and IL-12 but not IL-10 are required for local tumour surveillance in a syngeneic model of orthotopic bladder cancer. Clin Exp Immunol. 2002;127:20–26. doi: 10.1046/j.1365-2249.2002.01734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodland DL, Dutton RW. Heterogeneity of CD4+ and CD8+ T cells. Curr Opin Immunol. 2003;15:336–342. doi: 10.1016/S0952-7915(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 3.Zhu J, Paul WE. CD4 T cells: fates, functions and faults. Blood. 2008;112(5):1557–1563. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 5.Wurtz O, Bajenoff M, Guerder S. IL-4-mediated inhibition of IFN-gamma production by CD4+ T cells proceeds by several developmentally regulated mechanisms. Int Immunol. 2004;16(3):501–508. doi: 10.1093/intimm/dxh050. [DOI] [PubMed] [Google Scholar]
- 6.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16(2):91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang XO, Pappu B, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns K, Watowich SS, Tian Q, Jetten AM, Dong C. TH17 lineage differentiation is programmed by orphan nuclear receptors RORalpha and RORgamma. Immunity. 2008;28(1):29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203(10):2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao Y, Schaue D, McBride WH. Modification of the tumor microenvironment to enhance immunity. Front Biosci. 2007;12:3576–3600. doi: 10.2741/2336. [DOI] [PubMed] [Google Scholar]
- 10.Chikamatsu K, Sakakura K, Whiteside TL, Furuya N. Relationships between regulatory T cells and CD8+ effector populations in patients with squamous cell carcinoma of the head and neck. Head & Neck. 2007;29:120–127. doi: 10.1002/hed.20490. [DOI] [PubMed] [Google Scholar]
- 11.Clarke SL, Betts GJ, Plant A, Wright KL, El-Shanawany TM, Harrop R, Torkington J, Rees BI, Williams GT, Gallimore AM, Godkin AJ. CD4+CD25+FOXP3+ regulatory T cells suppress anti-tumor immune responses in patients with colorectal cancer. PLoS One. 2006;1(1):e129. doi: 10.1371/journal.pone.0000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, de St F, Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ Treg cells. J Exp Med. 2006;203(7):1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehi K, Ishigami S, Masamoto I, Uenosono Y, Natsugoe S, Arigami T, Arima H, Kijima Y, Yoshinaka H, Yanagita S, Kozono T, Funasako Y, Maruyama I, Aikou T. Analysis of T-helper type1 and 2 cells and T-cytotoxic type 1 and 2 cells of sentinel lymph nodes in breast cancer. Oncol Rep. 2008;19:601–607. [PubMed] [Google Scholar]
- 14.Filella X, Alcover J, Zarco MA, Beardo P, Molina R, Ballesta AM. Analysis of type T1 and T2 cyokines in patients with prostate cancer. Prostate. 2000;44:271–275. doi: 10.1002/1097-0045(20000901)44:4<271::AID-PROS2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 15.Ito N, Nakamura H, Netsugi H, Ohgi Dissociation between T helper type 1 and type 2 differentiation and cytokine produciton in tumor-infiltrating lymphocytes in pateints with lung cancer. Surgery Today. 2001;31:390–394. doi: 10.1007/s005950170127. [DOI] [PubMed] [Google Scholar]
- 16.Mainou-Fowler T, Taylor PRA, Miller S, Dickinson AM, Proctor SJ. Intracellular cytokine profiles by peripheral blood CD3+ T-cells in patients with classical Hodgkin lymphoma. Leukemia & Lymphoma. 2003;44(8):1331. doi: 10.1080/1042819031000090246. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama H, Kitayama J, Muto T, Nagawa H. Characterization of intracellular cytokine profile of CD4+ T cells in peripheral blood and tumor-draining lymph nodes of patients with gastrointestinal cancer. Jpn J Clin Oncol. 2000;30(7):301–305. doi: 10.1093/jjco/hyd078. [DOI] [PubMed] [Google Scholar]
- 18.Schreck S, Briebel D, Buettner M, Distel L, Grabenbauer G, Young LS, Niedobitek G. Prognostic impact of tumour-infiltrating Th2 and regulatory T cells in classical Hodgkin lymphoma. Hematol Oncol. 2009;27:31–39. doi: 10.1002/hon.878. [DOI] [PubMed] [Google Scholar]
- 19.Ubukata H, Motohashi G, Tabuchi T, Nagata H, Konishi S, Tabuchi T. Evaluations of inteferon-gamma/interleukin-4 ratio and neutrophil/lymphocyte ratio as prognostic indicators in gastric cancer patients. J Surg Oncol. 2010;102:742–747. doi: 10.1002/jso.21725. [DOI] [PubMed] [Google Scholar]
- 20.De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C, Protti MP. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208(3):469–478. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma A, Khan R, Joshi S, Kumar L, Sharma M. Dysregulation in T helper 1/T helper 2 cytokine ratios in patients with multiple myeloma. Leukemia & Lymphoma. 2010;51(5):920–927. doi: 10.3109/10428191003699563. [DOI] [PubMed] [Google Scholar]
- 22.Kristensen VN, Vaske CJ, Ursini-Siegel J, Van Loo P, Nordgard SH, Sachidanandam R, Sorlie T, Warnberg F, Haakensen VD, Helland A, Naume B, Perou CM, Haussler D, Troyanskaya OG, Borresen-Dale AL (2011) Integrated molecular profiles of invasive breast tumors and ductal carcinoma in situ (DCIS) reveal differential vascular and interleukin signaling. PNAS 10 [DOI] [PMC free article] [PubMed]
- 23.Naldini A, Pucci A, Bernini C, Carraro F. Regulation of angiogenesis by Th1 and Th2 type cytokines. Curr Pharm Des. 2003;9:511–519. doi: 10.2174/1381612033391423. [DOI] [PubMed] [Google Scholar]
- 24.Murakami H, Ogawara H, Hiroshi H. Th1/Th2 cells in patients with multiple myeloma. Hematology. 2004;9(1):41–46. doi: 10.1080/10245330310001652437. [DOI] [PubMed] [Google Scholar]
- 25.Sellitto A, Galizia G, De Fanis U, Lieto E, Zamboli A, Orditura M, De Vita F, Giunta R, Lucivero G, Romano C. Behavior of circulating CD4+CD25+Foxp3+ regulatory T cells in colon cancer patients undergoing surgery. J Clin Immunol. 2011;31:1095–1104. doi: 10.1007/s10875-011-9585-8. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Yang B, Liu H, Hu Y, Yang J, Wu L, Zhou Z, Jiao S (2011) Regulatory T cells increase in breast cancer and in stage IV breast cancer. Cancer Immunology, Immunotherapy [DOI] [PMC free article] [PubMed]
- 27.Yamagami W, Susumu N, Tanaka H, HIrasawa A, Banno K, Suzuki N, Tsuda H, Tsukazaki K, Aoki D. Immunofluorescence-detected infiltration of CD4+FOXP3+ regulatory T cells is relevant to the prognosis of patients with endometrial cancer. Int J Gynecol Canc. 2011;21:1628–1634. doi: 10.1097/IGC.0b013e31822c271f. [DOI] [PubMed] [Google Scholar]
- 28.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, Chang A, Coukos G, Liu R, Zou W. Phenotype, distribution, generation and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sfanos KS, Burno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, Meeker AK, Isaacs WB, Drake CG. Phenotypic analysis of prostate-infiltrating lymphocytes reveals Th17 and Treg skewing. Clin Cancer Res. 2008;14(11):3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114:357–359. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li C, Zhao Y, Zhang W, Zhang W. Increased prevalence of TH17 cells in the peripheral blood of patients with head and neck squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(1):80–89. doi: 10.1016/j.tripleo.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 32.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:2904–2912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H, Grizzle WE. Exosomes and cancer: a newly described pathway of immune suppression. Clin Cancer Res. 2011;17:959–964. doi: 10.1158/1078-0432.CCR-10-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan S, Aspe JR, Asumen MG, Almaguel F, Odumosu O, Acevedo-Martinez S, De Leon M, Langridge WH, Wall NR. Extracellular, cell-permeable survivin inhibits apoptosis while promoting proliferative and metastatic potential. Br J Cancer. 2009;100(7):1073–1086. doi: 10.1038/sj.bjc.6604978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan S, Jutzy JMS, Aspe JR, McGregor DW, Neidigh JW, Wall NR. Survivin is released from cancer cells via exosomes. Apoptosis. 2011;16(1):1–12. doi: 10.1007/s10495-010-0534-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adida C, Crotty PL, McGrath J, Berrebi D, Diebold J, Altieri DC. Developmentally regulated expression of the novel cancer anti-apoptosis gene survivin in human and mouse differentiation. Am J Pathol. 1998;152:43–49. [PMC free article] [PubMed] [Google Scholar]
- 37.Dohi T, Beltrami E, Wall NR, Plescia J, Altieri DC. Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J Clin Invest. 2004;114:1117–1127. doi: 10.1172/JCI22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182(3):311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 39.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171(1):131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 40.Song J, So T, Cheng M, Tang X, Croft M. Sustained survivin expression from OX40 costimulatory signals drives T cell clonal expansion. Immuunity. 2005;22:621–631. doi: 10.1016/j.immuni.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Morris J, Waldmann T (2000) Advances in interleukin 2 receptor targeted treatment. Annals of the Rheumatic Diseases Suppl 1:I109–114 [DOI] [PMC free article] [PubMed]
- 42.Ke X, Wang J, Li L, Chen IH, Wang H, Yang XF. Roles of CD4+CD25high Foxp3+ Tregs in lymphomas and tumors are complex. Front Biosci. 2008;13:2986–3001. doi: 10.2741/2986. [DOI] [PubMed] [Google Scholar]
- 43.Manel N, Unutmaz D, Littman DR. The differentiation of human Th17 cells requires transforming growth factor beta and induction of the nuclear receptor RORγt. Nat Immunol. 2008;9(6):641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mera S, Magnusson M, Tarkowski A, Bokarewa M. Extracellular survivin up-regulates adhesion molecules on the surface of leukocytes changing their reactivity pattern. J Leukoc Biol. 2008;83:149–154. doi: 10.1189/jlb.0507287. [DOI] [PubMed] [Google Scholar]
- 45.Chattopadhyay S, Mehrortra S, Chhabra A, Hegde U, Mukherji B, Chakraborty NG (2006) Effect of CD4+CD25+ and CD4+CD25- T regulatory cells on the generation of cytolytic T cell response to a self but human tumor-associated epitope in vitro. J Immunol 176(2) [DOI] [PubMed]
- 46.James E, Yeh A, King C, Korangy F, Bailey I, Boulanger DS, Van den Eynde BJ, Murray N, Elliott TJ. Differential suppression of tumor-specific CD8+ T cells by regulatory T cells. J Immunol. 2010;185(9):5048–5055. doi: 10.4049/jimmunol.1000134. [DOI] [PubMed] [Google Scholar]
- 47.Koyama S, Koike N, Adachi S. Expression of TNF-related apoptosis-inducing ligand (TRAIL) and its receptors in gastric carcinoma and tumor-infiltrating lymphocytes: a possible mechanism of immune evasion of the tumor. J Cancer Res Clin Oncol. 2002;128:73–80. doi: 10.1007/s004320100292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koyama S, Koike N, Adachi S. Fas receptor counterattack against tumor-infiltrating lymphocytes in vivo as a mechanism of immune escape in gastric carcinoma. J Cancer Res Clin Oncol. 2001;127:20–26. doi: 10.1007/s004320000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ellyard JI, Simson L, Parish CR. Th2-mediated anti-tumour immunity: friend or foe? Tissue Antigens. 2007;70:1–11. doi: 10.1111/j.1399-0039.2007.00869.x. [DOI] [PubMed] [Google Scholar]
- 50.Hamai A, Benlalam H, Meslin F, Hasmim M, Carre T, Akalay I, Janji B, Berchem G, Noman MZ, Chouaib S. Immune surveillance of human cancer: if the cytotoxic T-lymphocytes play the music, does the tumor system call the tune? Tissue Antigens. 2010;75:1–8. doi: 10.1111/j.1399-0039.2009.01401.x. [DOI] [PubMed] [Google Scholar]
- 51.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW (2011) Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunology, Immunotherapy Epub (Epub):e1–e6 [DOI] [PMC free article] [PubMed]
- 52.Maniati E, Soper R, Hagemann T. Up for Mischief? IL-17/Th17 in the tumour microenviroment. Oncogene. 2010;29(42):5653–5662. doi: 10.1038/onc.2010.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. Malignant B cells skew the balance of regulatory T cells and Th17 cells in B-cell non-Hodgkin's lymphoma. Cancer Res. 2009;69(13):5522–5530. doi: 10.1158/0008-5472.CAN-09-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu J, Duan Y, Cheng X, Chen X, Xie W, Long H, Lin Z, Zhu B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem Biophys Res Commun. 2011;407(2):348–354. doi: 10.1016/j.bbrc.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 55.Jung T, Schauer U, Heusser C, Neumann C, Rieger C. Detection of intracellular cytokines by flow cytometry. J Immunol Methods. 1993;159(1–2):197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ESM 1 (PPT 2.52 mb)