Abstract
Background
A mass of visceral adipose tissue is one of the most important determinants of progressive liver injury in nonalcoholic fatty liver disease (NAFLD). In accordance, nonalcoholic steatohepatitis (NASH) and fibrosis are believed to occur more commonly in morbidly obese patients compared with nonobese NAFLD patients.
Aim of the study
Comparative analysis of NAFLD histopathologic features and angiogenesis activity in morbidly obese and nonobese subjects.
Materials and methods
Biopsy samples from 40 severely obese (BMI ≥40 kg m−2) and 30 nonobese (BMI ≤30 kg m−2) NAFLD patients were examined. Kleiner’s classification was used to diagnose NASH by grading steatosis, cytoplasmatic ballooning of hepatocytes, and lobular inflammation. The severity of fibrosis was evaluated according to the liver fibrosis staging system. Qualitative and quantitative immunohistochemical analyses of VEGF A, Flk-1, and CD34 were performed to study angiogenesis and the terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) method was used to study hepatocyte apoptosis.
Results
Severely obese patients did not differ from nonobese patients with respect to age and sex distribution. NASH was diagnosed in nine (22.5%) severely obese patients and in seven (23.3%) nonobese patients. Fibrosis was more common in morbidly obese patients (82.5 vs. 43.5%, χ² = 11.71, p = 0.003) and was not associated with NASH. Moreover, the severity of fibrosis was greater in obese patients, as advanced fibrosis (bridging fibrosis and cirrhosis) occurred in six (15%) severely obese patients and in two (6.7%) nonobese patients. In morbidly obese individuals, angiogenesis was independent of NASH and was activated at the stage of simple steatosis. In severe obesity, there was a positive relationship between the stage of fibrosis and angiogenic activity.
Conclusion
In severely obese patients, fibrosis is probably promoted by mechanisms independent of NASH. In these patients, angiogenesis is activated early in the natural history of NAFLD and correlates with the severity of fibrosis.
Keywords: NAFLD, Morbid obesity, Fibrosis, Angiogenesis
Introduction
Nonalcoholic fatty liver disease (NAFLD) includes a wide spectrum of liver pathologies, ranging from pure steatosis, usually a benign and nonprogressive condition, to nonalcoholic steatohepatitis (NASH), which may progress to liver cirrhosis [1]. Studies on the natural history of NAFLD show that histopathologic progression occurs in 32–37% of patients over a period of 3–6 years and up to 12% of patients may progress to cirrhosis over a period of 8–10 years [2].
NAFLD is a growing problem due to the epidemic of obesity. Data from the USA suggest that 20–30% of the general population develop NAFLD and 3–5% develop NASH. It has been shown that the prevalence of NASH among morbidly obese patients is higher than in the general population; however, this prevalence was assessed as a broad range between 25 and 75% [3, 4].
Central obesity is the most important constituent of the metabolic syndrome and waist circumference is a key diagnostic feature of this syndrome. A high prevalence of NAFLD/NASH in patients with morbid obesity seems to be a consequence of a large amount of visceral tissue [large waist circumference and body mass index (BMI)] and advanced insulin resistance in this population. Visceral fat is active endocrine tissue that releases many peptides and hormones to the portal venous system, which reach the liver and promote insulin resistance and steatoinflammatory reactions. Moreover, activated macrophages located in visceral fat liberate proinflammatory cytokines that may interfere with liver resident macrophages and sinusoidal endothelial cells.
Angiogenesis is a critical step in tissue damage, healing, and vascular remodeling. Angiogenesis and sinusoidal remodeling occur concurrently with, or may even precede, fibrosis formation in most types of chronic liver disease [5, 6]. A number of experimental studies support a causative role for these vascular changes in the pathogenesis of fibrosis and portal hypertension. Recent data suggest that hepatic angiogenesis may be activated by certain adipose-related cytokines and hormones [7].
The aim of this study was to compare NAFLD histopathologic features and angiogenic activity in morbidly obese and nonobese subjects.
Materials and methods
Patient selection and clinical characteristics
The study was based on liver biopsy specimens from patients diagnosed with NAFLD from 2002 to 2007. Biopsy specimens embedded in paraffin blocks were retrieved from the files of two pathomorphology units. The liver samples were divided into two groups depending on the BMI of the sample donor. The first group included liver samples from consecutive patients with morbid obesity (BMI ≥40 kg m−2). These patients were referred to the bariatric surgical center where percutaneous liver biopsy was a routine procedure before surgery. The second group included randomly selected biopsy samples from nonobese NAFLD patients (BMI ≤30 kg m−2).
The biopsy samples were provided by the Department of Gastroenterology and Hepatology of the Medical University of Silesia. Liver biopsies were performed on patients with metabolic syndrome, echo-bright liver on ultrasound and chronic hypertransaminasemia. The diagnosis of metabolic syndrome was established according to International Diabetic Federation (IDF) criteria, where a required feature was waist circumference >94 cm in males and >80 cm in females (set points accepted for a European Caucasian population). Moreover, at least two of the following features had to be present: (1) fasting glucose >100 mg dl−1 or a previous diagnosis of diabetes; (2) arterial blood pressure >130/85 or pharmacologically treated hypertension; (3) triglyceride-levels >150 mg dl−1 or current use of fibrates; and (4) HDL-cholesterol <40 mg dl−1 (males) or 50 mg dl−1 (females).
The clinical data that excluded patients from the study were: regular consumption of alcohol, ≥120 or ≥70 g weekly in males and females, respectively; BMI <18.5 kg m−2; chronic or acute inflammatory bowel disease; past intestinal surgery; infection with hepatitis C virus (HCV) or hepatitis B virus (HBV); a saturation transferrin index >50%; a low serum level of ceruloplasmin; and administration of drugs known to induce liver steatosis, such as methotrexate, amiodarone, corticosteroids, or antidepressants. The histopathologic exclusion criteria were less than seven portal triads in the specimen, <5% of hepatocytes showing fatty degeneration, and the presence of features contradicting NAFLD as a main liver disorder. The number of patients/biopsies that were excluded from the study were 4 and 5, respectively. Finally, 40 patients were included in the morbid obesity group and 30 patients in the nonobese NAFLD group. All patient charts were reviewed to obtain clinical characteristics. The laboratory tests reviewed included the prothrombin index, serum levels of bilirubin, alanine and aspartate aminotransferases, alkaline phosphatase, γ-glutamyl transpeptidase, creatinine, and serologies of HCV and HBV.
Histopathology
Liver biopsy samples embedded in paraffin were cut into tissue sections 4 μm thick, mounted on adhesive slides, incubated at 58°C, and then deparaffinized in xylene and re-hydrated in alcohol solutions of decreasing concentrations. Sections were counterstained with hematoxylin–eosin and Masson trichrome. Histopathologic examination was simultaneously performed by two experienced pathologists using a double-headed microscope. Kleiner’s classification was used to grade steatosis, cytoplasmatic ballooning of hepatocytes, and lobular inflammation (NAFLD Activity Score: NAS) [8]. The histopathologic NAS score is defined as the unweighted sum of the scores for steatosis (0–3), lobular inflammation (0–3), and ballooning (0–2); thus ranging from 0 to 8. Cases with NAS of 0–2 were considered not diagnostic of NASH; however, scores of ≥5 were diagnosed as NASH. Cases with activity scores of 3 and 4 were considered as borderline (indeterminate) NASH. In addition, portal inflammation was assessed semiquantitatively: none or minimal (grade 0) and higher (grade 1). Severity of fibrosis was evaluated according to the simplified liver fibrosis staging system [8].
The terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) method was used to disclose hepatocyte apoptosis and qualitative and quantitative immunohistochemical analyses of VEGF A, Flk-1, and CD34 were performed to study angiogenesis.
-
TUNEL
Sections were deparaffinized, hydratated, and pretreated by microwave irradiation for 5 min with 0.01 M sodium citrate buffer at pH 6.0 to enhance TUNEL. The endogenous peroxidase was blocked with 3% hydrogen peroxide. The in situ detection kit, POD (Roche, Basel, Switzerland) for TUNEL was used according to the manufacturer’s instructions. The samples were incubated with the TUNEL reaction mixture containing terminal deoxynucleotidyl transferase (TdT) for 60 min at 37°C and consecutively with Converter POD for 30 min at the same temperature. TUNEL was visualized using 3.3-diaminobenzidine (DAB) as the chromogen. Samples were examined under ×200 magnification (Labophot 2 microscope, Nikon). The number of hepatocytes with a positive TUNEL reaction was recorded in five visual areas.
-
VEGF A, Flk-1
To retrieve the antigens, preparations were placed in TRS (Dako, Glostrup, Dania) solution and submerged in a water bath at 97°C for 30 min. After cooling and rinsing, the endogenous peroxidase was blocked with 3% hydrogen peroxide for 5 min. Next, the sections were incubated with primary antibodies to VEGF (Santa Cruz, USA) and to Flk-1 (Santa Cruz, USA) in dilutions 1:100 for 30 min at room temperature. The remaining immunohistochemical reaction was done with DAKO LSAB + (Dako, Glostrup, Denmark). Labeling was visualized using the chromogen DAB (Dako, Glostrup, Denmark). Sections were rinsed between consecutive laboratory steps with TBS/Tween (BUF 028, Serotec), then dehydrated, cleared in xylene, and coversliped with DPX.
Intensity of the reaction was assessed with densitometry in bioptate microphotography under ×200 magnification using Image-Pro Plus v.3.0 software (Media Cybernetics, Silver Spring, USA) with an Olympus microscope supplied with a digital camera.
-
CD34
To retrieve the antigen, preparations were placed in TRS solution, submerged in a water bath at 97°C for 30 min. Subsequently, the sections were incubated with CD34 antibody (Dako, Glostrup, Denmark) for 30 min. The remaining immunohistochemical reactions were performed with LSAB 2 (Dako, Glostrup, Denmark). DAB was used as the chromogen and the positive reaction for antigen CD34 was primarily evaluated under ×40 and ×100 magnifications to find the highest number of newly formed blood vessels (only a cytoplasmatic reaction was considered as positive). Blood vessels positive for CD34 were assessed in three visual areas.
Statistical analysis
The data were presented as medians with interquartile ranges for continuous variables and frequency distributions for categorical variables. Intergroup differences of quantitative variables were tested with Mann–Whitney U and ANOVA rank Kruskal–Wallis tests for independent variables. If a p value was <0.05, the Mann–Whitney U test was subsequently used in post hoc analysis. A Fisher’s test was applied to compare categorical variables. A χ² test was used to compare categorical variables between three or more groups. If a p value was <0.05, the Fisher’s test was subsequently used in post hoc analysis. A p value of <0.05 was considered to be statistically significant (Statsoft Statistica 7.0).
Results
Clinical characteristics of patients are shown in Table 1. Morbidly obese patients did not differ from nonobese NAFLD patients with respect to age, sex ratio, height, arterial blood pressure, and serum cholesterol levels. Morbidly obese patients had higher serum levels of triglycerides, lower serum levels of aminotransferases, and higher AST to ALT ratios.
Table 1.
Characteristics of morbidly obese and nonobese NAFLD patients
| Parameters | NAFLD patients | p | |
|---|---|---|---|
| Morbidly obese | Nonobese | ||
| Males/females | 16/24 | 15/15 | 0.47 |
| Age (years) | 41.0 (34.0–51.0) | 51.0 (36.0–54.0) | 0.17 |
| Body weight (kg) | 138 (123–175) | 83.8 (76.8–95.5) | <0.0001 |
| Height (cm) | 164 (162–171) | 169 (159–176) | 0.79 |
| BMI (kg/m2) | 49.9 (45.1–56.6) | 27.8 (26.6–28.6) | <0.0001 |
| Waist circumference (cm) | 137 (117–144) | 101 (97.5–108) | 0.0001 |
| Hip circumference (cm) | 141 (134–170) | 107 (104–112) | <0.0001 |
| Fasting glucose (mg/dl) | 5.6 (5.1–7.1) | 5.4 (4.9–6.2) | 0.33 |
| Triglycerides (mg/dl) | 187 (144–266) | 143 (119–199) | 0.049 |
| HDL (mg/dl) | 47.0 (42.0–48.0) | 41.0 (34.5–51.5) | 0.18 |
| Cholesterol (mg/dl) | 227 (198–265) | 220 (189–266) | 0.49 |
| AST (IU/l) | 28 (22–37) | 45 (35–68) | 0.004 |
| ALT (IU/l) | 36 (27–54) | 76 (52–111) | 0.0003 |
| AST/ALT | 0.92 (0.66–1.00) | 0.59 (0.44–0.72) | 0.001 |
| Systolic BP (mmHg) | 140 (130–140) | 130 (120–140) | 0.1 |
| Diastolic BP (mmHg) | 90 (80–90) | 85 (80–90) | 0.32 |
| Hemoglobin (g/dl) | 14.3 (13.0–15.9) | 14.9 (13.9–16.0) | 0.52 |
| Platelets (G/mm3) | 225 (210–264) | 235 (156–240) | 0.71 |
HDL high density lipoprotein, AST aspartate transaminase, ALT alanine transaminase, BP blood pressure
Median values (interquartile ranges), Mann–Whitney U test
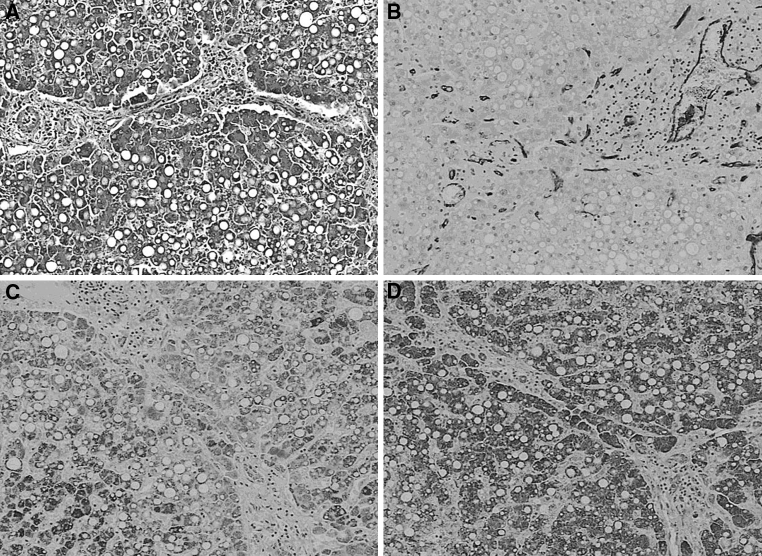
Histopathologic diagnosis for both groups is shown in Table 2. There was no difference in the prevalence of simple steatosis, borderline form, and NASH between morbidly obese and nonobese NAFLD patients (Table 2; χ² = 0.0084, p = 0.999). No difference was found with regard to grades of steatosis, cytoplasmatic ballooning, and activity of lobular inflammation (Table 3). In addition, the occurrence of moderate and severe portal inflammation (grade 1) was similar in obese and nonobese individuals (22.5 vs. 26.7%; p > 0.05). By contrast, fibrosis was more common in morbidly obese than in nonobese patients (Table 4; χ² = 11.71, p = 0.003). The fibrosis was found in 13 (43.5%) nonobese patients and in 33 (82.5%) severely obese patients. Moreover, advanced fibrosis (bridging fibrosis or cirrhosis) was diagnosed in two (6.7%) nonobese patients and in six (15%) morbidly obese patients (p < 0.005). The difference in fibrosis prevalence between obese and nonobese individuals was found only at the stage of simple steatosis or “borderline” lesions, according to Kleiner’s criteria, and was absent in patients with “definite” NASH (Table 5). The illustrative histopathologic images, all obtained from obese patients, are presented in Fig. 1.
Table 2.
Histopathological diagnosis according to NAFLD Activity Score in morbidly obese and nonobese patients
| NAFLD patients | Steatosis alone (%) | Borderline form (%) | NASH (%) |
|---|---|---|---|
| Obese | 16 (40) | 15 (37.5) | 9 (22.5) |
| Nonobese | 12 (40) | 11 (36.7) | 7 (23.3) |
Simple steatosis <3 points; indeterminate form 3–4 points, NASH ≥5 points
Table 3.
Gradings of different histopathological NAFLD features in morbidly obese and nonobese patients
| NAFLD patients | Steatosisa | Ballooningb | Lobular inflammationc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | 0 | 1 | 2 | 0 | 1 | 2 | 3 | |
| Obese | 15 | 12 | 13 | 25 | 14 | 1 | 21 | 12 | 5 | 2 |
| 37.5% | 30% | 32.5% | 62.5% | 35% | 2.5% | 52.5% | 30% | 12.5% | 5% | |
| Nonobese | 11 | 9 | 10 | 15 | 13 | 2 | 16 | 11 | 3 | 0 |
| 36.7% | 30% | 33.3% | 50% | 43.3% | 6.7% | 53.3% | 36.7% | 10% | 0% | |
aPercentage of hepatocytes involved with steatosis 5-33% (grade S1), 34-66% (grade S2); >66% (grade S3)
bBallooning: 0 = absence, 1 = few hepatocytes, 2 = numerous hepatocytes
cLobular inflammation: 0 = absence, 1 = <2 foci per single vision field, 2 = 2–4 foci per single vision field, 3 = 3- > 4 foci per single vision field
Table 4.
Severity of fibrosis in morbidly obese and nonobese NAFLD patients
| NAFLD patients | Fibrosis staging | ||
|---|---|---|---|
| F0 | F1 | F2 | |
| Obese | 7 | 27 | 6 |
| 17.5% | 67.5% | 15% | |
| Nonobese | 17 | 11 | 2 |
| 56.7% | 36.7% | 6.7% | |
F0, none; F1, moderate perisinusoidal or portal/periportal; F2, advanced fibrosis, i.e., bridging fibrosis or cirrhosis
Table 5.
Severity of fibrosis in morbidly obese and nonobese patients depending on diagnosis based on NAFLD Activity Score
| Group: | Obese | Nonobese | χ² | p | ||||
|---|---|---|---|---|---|---|---|---|
| Fibrosis: | F0 | F1 | F2 | F0 | F1 | F2 | ||
| Steatosis alone | 4 | 10 | 2 | 9 | 3 | 0 | 7.269 | 0.026 |
| 25% | 62.5% | 12.5% | 75% | 25% | ||||
| Indeterminate form | 2 | 11 | 2 | 7 | 3 | 1 | 7.095 | 0.027 |
| 13.3% | 73.3% | 13.3% | 63.6% | 27.3% | 9.1% | |||
| NASH | 1 | 6 | 2 | 1 | 5 | 1 | 0.177 | 0.915 |
| 11.1% | 66.7% | 22.2% | 14.3% | 71.4% | 14.3% | |||
Fig. 1.
Histopathologic images obtained from morbidly obese patients showing a stage-3 fibrosis (×100), b immune staining for CD34 in a patient with stage-3 fibrosis (×100), c immune staining for Flk-1, densitometry 0.631 (×100), and d immune staining for VEGF A, densitometry 0.649 (×100)
The number of hepatocytes with a positive TUNEL reaction was significantly higher in nonobese than obese patients (p = 0.011). Occurrence of apoptosis was not directly associated with histopathologic diagnosis but, in morbidly obese patients, was negatively related to the grade of steatosis (p = 0.019). This relationship was not found in nonobese patients.
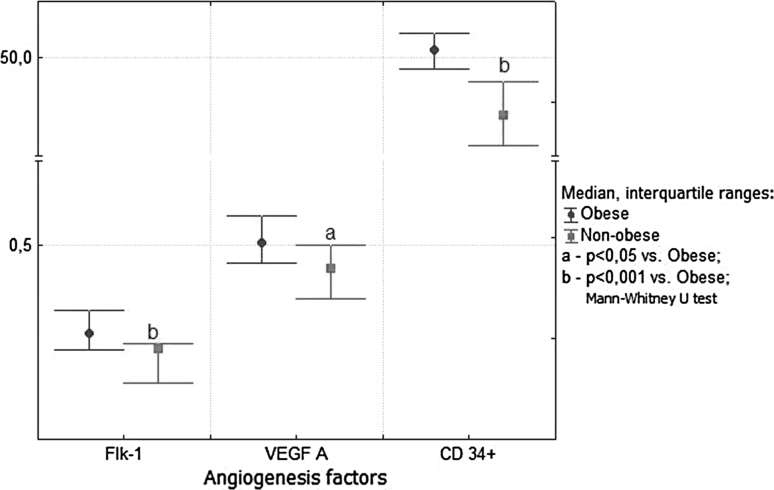
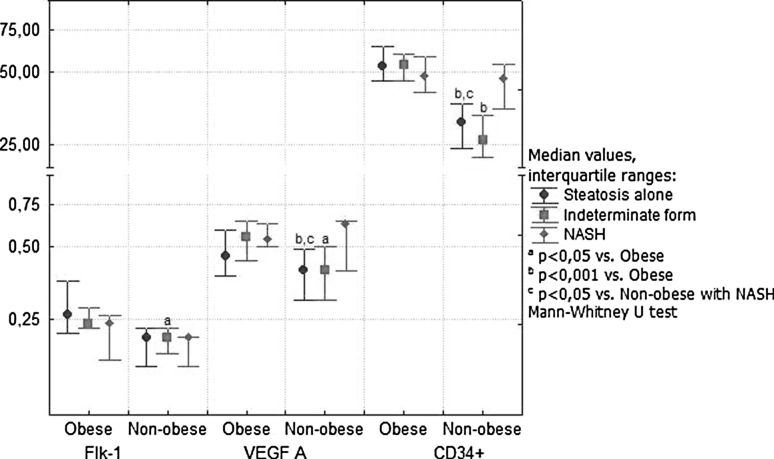
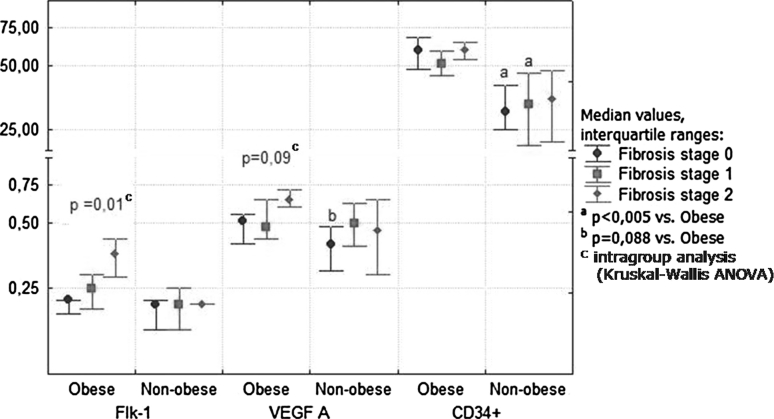
In severely obese patients, Flk-1 expression was positively correlated with VEFG-A expression (r = 0.36; p = 0.035). In these patients, the VEGF A and Flk-1 expressions and the density of newly formed CD34-positive blood vessels were significantly increased in comparison with nonobese NAFLD patients (Fig. 2). The increase in expression of angiogenic factors was observed in the stage of simple steatosis and “borderline” NASH (Fig. 3). The nonobese patients with NASH showed activation of VEGF-A and CD34 as compared with earlier stages of NAFLD. On the contrary, an advanced fibrosis was associated with further increase in expression of Flk-1 and VEGF only in obese patients (Fig. 4). There was a positive relationship in these patients between the stage of fibrosis and strength of Flk-1 cytoplasmatic expression (r = 0.56; p = 0.001). Such a relationship was not found in the nonobese group.
Fig. 2.
Strength of reactions with antibodies to Flk-1, VEGF A, and CD34 measured by densitometry in morbidly obese and nonobese NAFLD patients
Fig. 3.
Strength of reactions with antibodies to Flk-1, VEGF A, and CD34 measured by densitometry in morbidly obese and nonobese patients in relation to histopathologic diagnosis based on NAFLD Activity Scores
Fig. 4.
Liver expression of angiogenesis factors in relation to the severity of fibrosis in morbidly obese and nonobese NAFLD patients
Discussion
A mass of visceral adipose tissue is believed to be the most important determinant of progressive liver injury in NAFLD due to an inflammatory state and release of free fatty acids to the portal venous system. There have been many studies that characterized liver histopathology in patients with morbid obesity and nonobese NAFLD, but none focused on searching for histopathologic differences with regard to BMI using the same methodology. In randomly investigated morbidly obese and nonobese NAFLD patients who were age and sex matched, we found no difference in histopathologic severity of steatosis, hepatocyte ballooning, and inflammation. Instead, a higher prevalence of and more advanced fibrosis was associated with morbid obesity. These findings are in keeping with the current knowledge on liver status in patients with morbid obesity in whom severe fibrosis and cirrhosis were reported to be more common than in nonobese populations with NAFLD [9–12]. On the contrary, our study disagrees with a widespread opinion on more advanced steatosis and more common prevalence of NASH in morbidly obese people.
In different series of obese patients undergoing bariatric surgery, the prevalence of NASH was reported in the range of 24–36.4% [4, 12–15], being as high as 79.3% in a Taiwanese study [16]. This discrepancy may result from different histopathologic criteria for NASH and variable involvement of patients with type 2 diabetes. The NAFLD Activity Score system, which is now a standard approach to the diagnosis of NASH, specifies a group of diagnostically uncertain cases classified as borderline NASH. The presence of such patients whose liver disease may evolve in both directions explains the diversity of data on NASH prevalence in different cohorts. In the context of published data, the prevalence of NASH at 22.5% in morbidly obese subjects found in our study seems to be a realistic figure. However, the high prevalence of NASH in nonobese patients could be biased by selection criteria, as the decision on liver biopsy was based on the persistent elevation of aminotransferases. Knowledge on the occurrence of NASH in the general population with metabolic syndrome and BMI ranging from normal to excessive is rather limited. In one study, histopathology of liver donors disclosed steatosis in 38% and NASH in 16% of cases, and in another study the occurrence of NASH among liver donors did not exceed 2.5% [17, 18].
It is not clear if body weight itself has a direct influence on the degree of steatosis and risk for NASH. In a study by Gholam et al. [15], the histopathology of liver samples taken from 97 obese subjects clearly showed that the severity of metabolic abnormalities, such as hyperglycemia and insulin resistance, was more important for the development of NASH than an excess of adipose tissue. Similarly, Marchesini et al. [19] found that the number of metabolic syndrome features is more important for the development of NASH than body weight. In comparison with NASH, steatosis seems to be more dependent on BMI. Ryan et al. [20] found a more frequent occurrence of steatosis in patients with BMI between 26 and 37 kg m−² than ≤25 kg m−² (27 vs. 9%). A correlation between the degree of liver steatosis and BMI was also found by Marchesini et al. [19] and, Angelico et al. [21]. The causes of a similar degree of steatosis in morbidly obese and nonobese NAFLD patients in our study are unknown.
Hepatocyte apoptosis is a characteristic feature of progressive NASH [22, 23]. In our study, apoptosis was inversely related to the degree of steatosis in obese patients. This finding suggests that obese individuals with a high degree of hepatic steatosis may develop antiapoptotic mechanisms, contributing to the inhibition of pathways leading to NASH. Lower levels of ALT in obese patients confirm this hypothesis. In accordance, it has recently been found that obese patients with NAFLD demonstrate lower levels of aminotransferases than their nonobese counterparts [24].
Liver fibrosis is the most important step in the progression of NAFLD, demanding a different approach to the patient. In this study, morbid obesity was accompanied by more common and more significant liver fibrosis. A higher prevalence of liver fibrosis in obesity was reported in many studies [25–30]. Ratziu et al. [9] found bridging fibrosis in 30% and cirrhosis in 11% of patients with BMI >25 kg m−². Similarly, Angulo et al. [11] found moderate fibrosis in 55% and severe fibrosis in 12% of patients with BMI >35 kg m−². More importantly, our study clearly demonstrated that liver fibrosis predominated in patients, not accomplishing the criteria of NASH. There are two possible explanations for this relationship. First, fibrosis enforces the regression of inflammation and apoptosis/necrosis of hepatocytes. Second, NASH is not an indispensable intermediate step on the way to the development of significant fibrosis/cirrhosis in morbidly obese patients. Fibrosis without prior steatohepatitis is a known optional event in the natural history of alcoholic liver disease that pathomorphologically is very similar to NAFLD. It is conceivable that, in morbidly obese patients, high levels of adipokines and hyperinsulinemia stimulate directly or indirectly stellate cells without concurrent injury to hepatocytes.
Angiogenesis seems to play an important role in the progression of chronic liver disease as neovascularization has been shown to be closely associated with fibrosis and development of portosystemic microcollaterals [31]. The mechanisms of angiogenesis are executed by increased levels of VEGF-A and angiopoietin I and their receptors. Our study suggests that angiogenesis is triggered differently in obese and nonobese patients. In obesity, the angiogenesis was activated at an early stage of the natural history of NAFLD, namely simple steatosis, whereas in nonobese patients activated at the level of NASH. Development of NASH in severely obese patients had no influence on hepatic expression of angiogenic factors. By contrast, these factors were positively correlated with the severity of fibrosis. This observation clearly indicates that development of fibrosis is preceded by angiogenesis that is independent of NASH.
In conclusion, patients with morbid obesity do not differ in severity of steatosis, hepatocyte damage, and lobular inflammation compared with nonobese NAFLD subjects, however, they have more significant fibrosis. In severely obese patients, fibrosis and angiogenesis seem to be independent of NASH and are activated at the stage of simple steatosis.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Parekh S, Anania FA. Abnormal lipid and glucose metabolism in obesity: implications for nonalcoholic fatty liver disease. Gastroenterology. 2007;132:2191–2207. doi: 10.1053/j.gastro.2007.03.055. [DOI] [PubMed] [Google Scholar]
- 2.Angulo P. Obesity and nonalcoholic fatty liver disease. Nutr Rev. 2007;65:57–63. doi: 10.1301/nr.2007.jun.S57-S63. [DOI] [PubMed] [Google Scholar]
- 3.Tarantino G. Should nonalcoholic fatty liver disease be regarded as a hepatic illness only? World J Gastroenterol. 2007;13:4669–4672. doi: 10.3748/wjg.v13.i35.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boza C, Riquelme A, Ibañez L, et al. Predictors of nonalcoholic steatohepatitis (NASH) in obese patients undergoing gastric bypass. Obes Surg. 2005;15:1148–1153. doi: 10.1381/0960892055002347. [DOI] [PubMed] [Google Scholar]
- 5.Lee JS, Semela D, Iredale J, Shah VH. Sinusoidal remodeling and angiogenesis: a new function for the liver-specific pericyte? Hepatology. 2007;45:817–825. doi: 10.1002/hep.21564. [DOI] [PubMed] [Google Scholar]
- 6.Medina J, Sanz-Cameno P, Garcia-Buey L, Martin-Vilchez S, Lopez-Cabrera M, Moreno-Otero R. Evidence of angiogenesis in primary biliary cirrhosis: an immunohistochemical descriptive study. J Hepatol. 2005;42:124–131. doi: 10.1016/j.jhep.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Aleffi S, Petrai I, Bertolani C, et al. Upregulation of proinflammatory and proangiogenic cytokines by leptin in human hepatic stellate cells. Hepatology. 2005;42:1339–1348. doi: 10.1002/hep.20965. [DOI] [PubMed] [Google Scholar]
- 8.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 9.Ratziu V, Giral P, Charlotte F, et al. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117–1123. doi: 10.1016/S0016-5085(00)70364-7. [DOI] [PubMed] [Google Scholar]
- 10.Willner IR, Waters B, Patil SR, Reuben A, Morelli J, Riely CA. Ninety patients with nonalcoholic steatohepatitis: Insulin resistance, familial tendency, and severity of disease. Am J Gastroenterol. 2001;96:2957–2961. doi: 10.1111/j.1572-0241.2001.04667.x. [DOI] [PubMed] [Google Scholar]
- 11.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 12.Abrams GA, Kunde SS, Lazenby AJ, Clements RH. Portal fibrosis and hepatic steatosis in morbidly obese subjects: a spectrum of nonalcoholic fatty liver disease. Hepatology. 2004;40:475–483. doi: 10.1002/hep.20323. [DOI] [PubMed] [Google Scholar]
- 13.Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 14.Ong JP, Elariny H, Collantes R, et al. Predictors of nonalcoholic steatohepatitis and advanced fibrosis in morbidly obese patients. Obes Surg. 2005;15:310–315. doi: 10.1381/0960892053576820. [DOI] [PubMed] [Google Scholar]
- 15.Gholam PM, Flacbaum L, Machan JT, Charney DA, Kotler S. Nonalcoholic fatty liver disease in severely obese subjects. Am J Gastroenterol. 2007;102:399–408. doi: 10.1111/j.1572-0241.2006.01041.x. [DOI] [PubMed] [Google Scholar]
- 16.Huang HL, Lin WY, Lee LT, Wang HH, Lee WJ, Huang KC. Metabolic syndrome is related to nonalcoholic steatohepatitis in severely obese subjects. Obes Surg. 2007;17:1457–1463. doi: 10.1007/s11695-008-9423-0. [DOI] [PubMed] [Google Scholar]
- 17.Minervini MI, Ruppert K, Fontes P, et al. Liver biopsy findings from healthy potential liver donors: reasons for disqualification, silent diseases and correlation with liver injury tests. J Hepatol. 2009;50:501–510. doi: 10.1016/j.jhep.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 18.Lee JY, Kim KM, Lee SG, et al. Prevalence and risk factors of non-alcoholic fatty liver disease in potential living liver donors in Korea: a review of 589 consecutive liver biopsies in a single center. J Hepatol. 2007;47:239–244. doi: 10.1016/j.jhep.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 20.Ryan CK, Johnson LA, Germin BI, Marcos A. One hundred consecutive hepatic biopsies in the workup of living donors for right lobe liver transplantation. Liver Transpl. 2002;8:1114–1122. doi: 10.1053/jlts.2002.36740. [DOI] [PubMed] [Google Scholar]
- 21.Angelico F, Del Ben M, Conti R, et al. Non-alcoholic fatty liver syndrome: a hepatic consequence of common metabolic diseases. J Gastroenterol Hepatol. 2003;18:588–594. doi: 10.1046/j.1440-1746.2003.02958.x. [DOI] [PubMed] [Google Scholar]
- 22.Feldstein AE, Canbay A, Angulo P, et al. Hepatocyte apoptosis and Fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/S0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 23.Ribeiro PS, Cortez-Pinto H, Solá S, et al. Hepatocyte apoptosis, expression of death receptors, and activation of NF-kappaB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am J Gastroenterol. 2004;99:1708–1717. doi: 10.1111/j.1572-0241.2004.40009.x. [DOI] [PubMed] [Google Scholar]
- 24.Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT value. Hepatology. 2003;37:1286–1292. doi: 10.1053/jhep.2003.50229. [DOI] [PubMed] [Google Scholar]
- 25.Machado M, Marques-Vidal P, Corez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45:600–606. doi: 10.1016/j.jhep.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Schreuder TC, Verwer BJ, van Nieuwkerk CM, Mulder CJ. Nonalcoholic fatty liver disease: an overview of current insights in pathogenesis, diagnosis and treatment. World J Gastroenterol. 2008;14:2474–2486. doi: 10.3748/wjg.14.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 28.Sorrentino P, Tarantino G, Conca P, et al. Silent non-alcoholic fatty liver disease—a clinical-histological study. J Hepatol. 2004;41:751–757. doi: 10.1016/j.jhep.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Fassio E, Alvarez E, Dominguez N, Landeira G, Longo C. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology. 2004;40:820–826. doi: 10.1002/hep.20410. [DOI] [PubMed] [Google Scholar]
- 30.Park KS, Lee YS, Park HW, et al. Factors associated or related to with pathological severity of nonalcoholic fatty liver disease. Korean J Intern Med. 2004;19:19–26. doi: 10.3904/kjim.2004.19.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandez M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J. Angiogenesis in liver disease. J Hepatol. 2009;50:604–620. doi: 10.1016/j.jhep.2008.12.011. [DOI] [PubMed] [Google Scholar]