Abstract
Background
Drug-coated balloons (DCBs) have been developed for the percutaneous treatment of coronary artery disease. An initial focus has been the management of in-stent restenosis (ISR) but randomised controlled trials (RCTs) have been small and powered only for angiographic endpoints.
Objective
The aim of the work was to assess the clinical and angiographic outcomes of patients treated for ISR with DCB versus control (balloon angioplasty or drug-eluting stents) by a meta-analysis of RCTs.
Methods
A comprehensive search was performed of RCTs where patients with ISR were randomly assigned to either DCB or alternative coronary intervention. Outcome measurements were death, myocardial infarction (MI), target lesion revascularisation (TLR), binary definition of restenosis and in-lesion late luminal loss (LLL).
Results
Four studies were identified that fulfilled the inclusion criteria. Pooled odds ratios (ORs) were calculated for patients treated for ISR (n = 399). Mean follow-up duration was 14.5 months. DCBs were associated with lower rates of TLR [8.8 vs. 29.7 % OR (95 % confidence interval, CI) 0.20 (0.11–0.36), p < 0.0001], binary restenosis [10.3 vs. 41.3 % OR (95 % CI) 0.13 (0.07–0.24), p < 0.00001] and MI [0.5 vs. 3.8 %, OR (95 % CI) 0.21 (0.04–1.00), p = 0.05]. No significant heterogeneity was identified.
Conclusion
Drug-coated balloons appear to be effective versus control in reducing TLR and possibly MI versus balloon angioplasty or drug-eluting stents in the management of ISR.
Keywords: Coronary artery disease, Angioplasty, Drug-coated balloons, Drug-eluting stents, Meta-analysis, Randomized controlled trials
Introduction
Several drug-coated balloons (DCBs) have been developed for the percutaneous treatment of coronary artery disease. They are all coated with the anti-proliferative drug paclitaxel with or without a carrier that influence not only drug solubility but also drug transfer and biological efficacy. In practise, the DCB is inflated within the coronary artery, with direct drug delivery to the coronary endothelium [1]. The aim of local drug delivery is to inhibit neo-intimal hyperplasia and promote rapid healing of the treated vessel. In comparison with drug eluting stents (DES), the current gold standard strategy for percutaneous coronary intervention (PCI), DCBs have theoretical benefits that include more uniform drug distribution at higher doses, no permanent vascular scaffold left in situ and no need for a polymer. These features allow for shorter durations of dual anti-platelet therapy and may eliminate some of the stimuli that predispose to stent thrombosis [1, 2].
With the widespread successful application of DES [3], it is not currently clear where DCBs may provide additional benefits. Restenosis within previously implanted bare-metal stents (BMS) or DES is considered a possible indication for DCB therapy [4]. Several randomised controlled trials (RCTs) have been performed, but they have been small and powered for angiographic endpoints.
The goal of this meta-analysis was to determine the role of DCB in the management of in-stent restenosis (ISR).
Methods
The present meta-analysis was performed according to the established methods of Cochrane Guidelines [5] and in compliance with the PRISMA statement [6] for reporting systematic reviews and meta-analyses in health care interventions.
Study eligibility and search strategy
Studies were eligible for inclusion if patients were randomly assigned to either DCB or an alternative coronary intervention, with appropriate reporting of methodologies, baseline patient and procedural data and clinical events at least 6 months following the index procedure. Published manuscripts and adequately reported oral abstracts were considered for inclusion to minimise the risk of publication bias. No language barrier was applied. Medline, Embase, and Cochrane databases were searched, as well as the web-based resources, ‘clinicaltrials.gov’ and Google Scholar. Search terms were “drug eluting balloon”, “drug coated balloon”, “paclitaxel eluting balloon”, “randomised controlled trial”, “controlled clinical trial”, “double-blind”, “placebo”, and “random”. Where unpublished RCTs were identified, Scientific Sessions of the American College of Cardiology [http://www.acc.org], American Heart Association [http://www.aha.org], European Society of Cardiology [http://www.escardio.org], Transcatheter Cardiovascular Therapeutics [http://www.tctmd.com] and EuroPCR [http://www.europcr.com] websites were searched. The literature searches were performed independently by two authors. Titles and abstracts were reviewed to determine appropriateness for further review. Full texts of studies of potential interest were then retrieved. Only studies of restenosis were included in this meta-analysis.
Clinical outcomes and definitions
Data were extracted on baseline variables and on clinical and angiographic outcomes. Outcomes of interest defined a priori were all-cause mortality, myocardial infarction (MI, defined according to each study protocol), target lesion revascularisation (TLR, defined as re-intervention on the index treated lesion), binary restenosis (defined as ≥50 % luminal diameter stenosis by quantitative coronary angiography), and in-lesion late luminal loss (LLL). LLL was the difference between the in-segment minimal lumen diameter after the procedure and at angiographic follow-up, as evaluated by quantitative coronary angiography.
Statistical analysis
Odds ratio (OR) and 95 % confidence interval (95 % CI) were used as summary statistics for binary data, whereas mean difference (MD) was used for continuous data. Heterogeneity was assessed by Cochran’s Q test, with a 2-tailed p = 0.1. The statistical inconsistency test (I2) {[(Q−df)/Q] × 100 %, where Q is the chi-squared statistic and df its degrees of freedom} was also employed to overcome the low statistical power of Cochran’s Q test. Pooled ORs were calculated using a Fixed Effect Model with the Mantel–Haenszel method. The DerSimonian and Laird Random Effects Model was used in case of significant heterogeneity and/or moderate or significant inconsistency (>50 %) across studies. The potential publication bias was examined by constructing a ‘funnel plot’, in which the standard error (SE) of the ln OR was plotted against the OR of the chosen outcome. Finally, we addressed the influence of each study by testing whether, deleting each in turn, would have changed significantly the pooled results of the meta-analysis (sensitivity analysis). Review Manager 5.1 (The Nordic Cochrane Center, Købehvn, Denmark) and SPSS for Windows version 15 (SPSS, Chicago, Illinois) were used for statistical computations.
Results
Literature search
The initial search identified 897 studies of potential relevance. After assessment of title and abstract, 17 studies were reviewed in full text for eligibility (Fig. 1). Two further studies were identified through review of full text articles. Eight studies were reviewed in full text but not included because they were non-randomised registry studies or case series [7–14]. Further RCTs that focused on different aspects of de novo disease (e.g., bifurcation, MI, small vessels) were also excluded [15–19]. The RCT PACCOCATH I was initially reported at 1-year follow-up [20], but was excluded in favour of the combined PACCOCATH I and II study [21] which had identical protocols and were jointly reported at 2-year follow-up. Thus, four studies were considered eligible for inclusion in the meta-analysis [21–24]. The internal validity of the included studies was appraised by two unblinded reviewers.
Fig. 1.
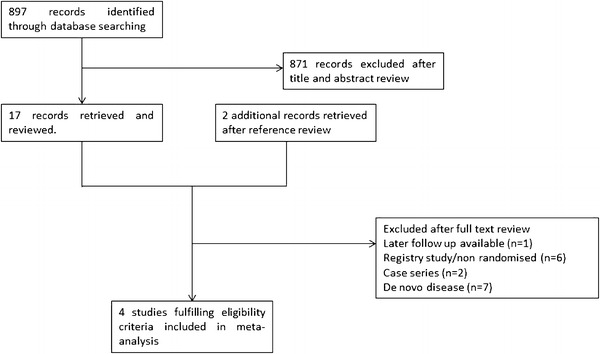
Study flow chart
Baseline study characteristics
The studies included in the meta-analysis are summarised in Table 1. Four studies compared DCBs with either conventional balloon angioplasty (POBA) or DES in the treatment of restenosis [21–24]. Three studies used Sequent Please (B. Braun, Melsungen, Germany) [22–24] and one used Paccocath (Bayer Schering Pharma, Berlin, Germany) [21]; both balloons have the same carrier matrix and are coated with 3 μg paclitaxel. Studies were multi-centre in three cases and single centre in one. One study was double-blind, two were single-blind, and one was unblinded. In total, clinical follow-up was available for all 399 patients enroled in the studies of ISR, with angiographic follow-up available for 355 patients (89.0 %). The mean duration of follow-up was 14.5 months.
Table 1.
Summary of characteristics of randomised control trials included in the meta-analysis
| Author/acronym | Years | Setting | Blinding | DCB | Comparator | Lesion characteristic | Total, n | Clinical follow-up, n | Angiographic follow-up, n | Follow-up(months) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DCB | Control | DCB | Control | |||||||||
| Habara et al. [19] | 2011 | Single centre | Single | Sequent please | POBA | ISR of SES | 50 | 25 | 25 | 23 | 24 | 6 |
| PACCOCATH ISR I AND II [18] | 2008 | Multicentre, Germany | Double | Paccocath | POBA | ISR of BMS or DES | 108 | 54 | 54 | 49 | 48 | 24 |
| PEPCAD II ISR [20] | 2009 | Multicentre, Germany | Unblinded | Sequent please | DESa | ISR of BMS | 131 | 66 | 65 | 57 | 59 | 12 |
| PEPCAD-DES [23] | 2011 | Multicentre, Germany | Single | Sequent please | POBA | ISR of DES | 110 | 72 | 38 | 64 | 31 | 12 |
DCB drug-coated balloon, POBA balloon angioplasty, DES drug-eluting stent, SES sirolimus-eluting stent, BMS bare-metal stent
aTaxus Libertè
Mortality
The odds ratios (ORs) for mortality in the RCTs of ISR are shown in Fig. 2. There were a total of 16 deaths (Table 2). Mortality rates were numerically lower in DCB-treated patients than in controls with a trend towards statistical significance: the incidence of death was 5/217 (2.3 %) in the DCB group and 11/182 (7.6 %) in the control group [OR (95 % CI) 0.36 (0.12–1.02), p = 0.06], as shown in Fig. 2.
Fig. 2.
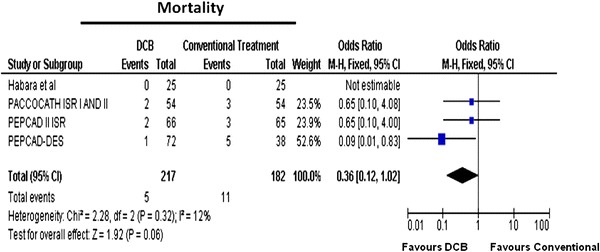
Meta-analysis for mortality in in-stent restenosis for DCB versus control; individual and overall odds ratios of mortality after treatment with DCB or control PCI are reported
Table 2.
Summary of clinical outcomes in drug-eluting balloon randomised controlled trials
| Study | Follow-up (months) | Patients at follow-up | Death (n) | MI (n) | TLR (n) | ||||
|---|---|---|---|---|---|---|---|---|---|
| DCB | Control | DCB | Control | DCB | Control | DCB | Control | ||
| Habara et al. [19] | 6 | 25 | 25 | 0 | 0 | 0 | 0 | 1 | 10 |
| PACCOCATH ISR I AND II [18] | 24 | 54 | 54 | 2 | 3 | 1 | 5 | 3 | 20 |
| PEPCAD II ISR [20] | 12 | 66 | 65 | 2 | 3 | 0 | 1 | 4 | 10 |
| PEPCAD-DES [23] | 12 | 72 | 38 | 1 | 5 | 0 | 1 | 11 | 14 |
| Overall | 217 | 182 | 5 | 11 | 1 | 7 | 19 | 54 | |
Myocardial infarction
Among the studies of ISR, fewer MIs occurred in the DCB-treated group (Table 2). This finding was of borderline statistical significance: 1/217 (0.5 %) patients in the DCB group and 7/182 (3.8 %) in the control group sustained an MI [OR (95 % CI) 0.21 (0.04–1.00), p = 0.05). Figure 3 shows the individual and overall ORs for MI.
Fig. 3.
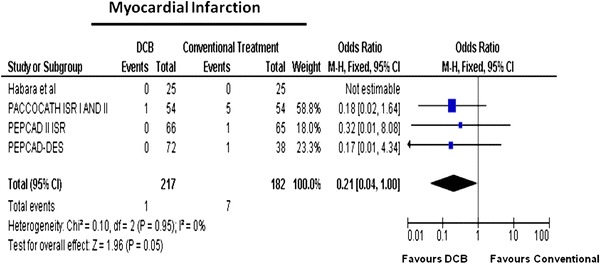
Meta-analysis for myocardial infarction in in-stent restenosis for DCB versus control; individual and overall odds ratios of incident myocardial infarction after treatment with DCB or control PCI are reported
Target lesion revascularisation
When compared with controls, DCB use was associated with significantly reduced TLR rates in patients treated for ISR (Table 2): 19/217 (8.8 %) versus 54/182 (29.7 %) [OR (95 % CI)] 0.20 (0.11–0.36), p < 0.0001, Fig. 4).
Fig. 4.
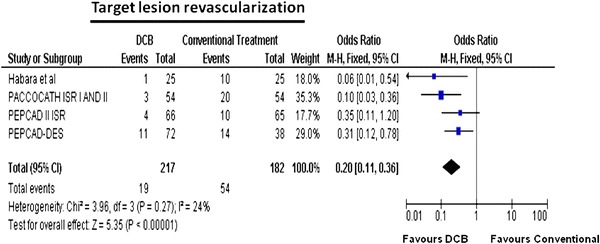
Meta-analysis for target lesion revascularisation in-stent restenosis for DCB versus control: individual and overall odds ratios of target lesion revascularisation after treatment with DCB or control PCI are reported
Binary restenosis and late lumen loss
As shown in Table 3, the rate of binary restenosis was reduced with the DCB strategy as compared to controls: 20/193 (10.3 %) versus 18/162 (41.3 %). Figure 5 demonstrates that binary restenosis was significantly reduced in DCB-treated patients [OR (95 % CI) 0.13 (0.07–0.24), p < 0.00001]. Mean in-stent LLL in the DCB group was 0.23 versus 0.75 mm in the control group (Table 3). Patients treated with DCB had significantly less in-stent LLL than patients receiving control [MD (95 % CI) −0.50 (−0.71; −0.30) mm, p < 0.00001, Fig. 6).
Table 3.
Summary of angiographic outcomes in drug-eluting balloon randomised controlled trials
| Study | Angiographic follow-up (n) | Binary restenosis | Late luminal loss | |||
|---|---|---|---|---|---|---|
| DCB | Control | DCB | Control | DCB | Control | |
| Habara et al. [19] | 23 | 24 | 2 | 15 | 0.17 ± 0.45 | 0.72 ± 0.56 |
| PACCOCATH ISR I AND II [18] | 49 | 48 | 3 | 24 | 0.14 ± 0.46 | 0.81 ± 0.79 |
| PEPCAD II ISR [20] | 57 | 59 | 4 | 10 | 0.19 ± 0.39 | 0.45 ± 0.68 |
| PEPCAD-DES [23] | 64 | 31 | 11 | 18 | 0.43 ± 0.61 | 1.03 ± 0.77 |
| Overall | 193 | 162 | 20 | 67 | 0.23 | 0.75 |
Fig. 5.
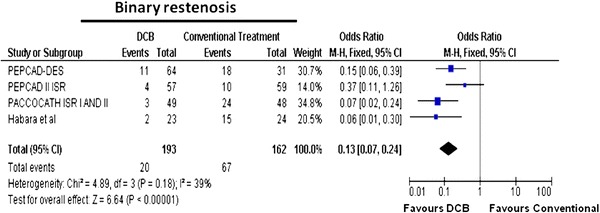
Meta-analysis for binary restenosis in in-stent restenosis for DCB versus control; individual and overall odds ratios of binary restenosis after treatment with DCB or control PCI are reported
Fig. 6.
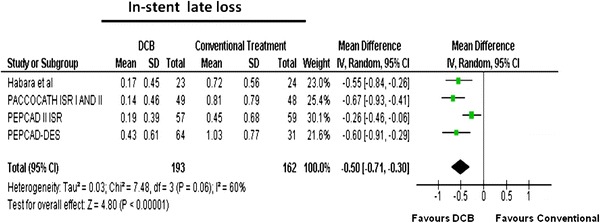
Meta-analysis for in-stent/lesion late luminal loss in in-stent restenosis for DCB versus control; individual and overall means and standard deviations of in-stent/lesion late luminal loss after treatment with DCB or control PCI are reported
Sensitivity analysis
Sensitivity analysis, performed by removing each of the studies one at a time, demonstrated that no single study influenced the overall results. Sensitivity analysis, performed by including each of the studies one at a time according to different length of follow-up, from the lowest to the highest, showed that different follow-up times did not influence the overall results.
Test for interaction
The interaction test showed no significant difference in TLR results when DCB treatment was compared to control patients treated with either POBA [21, 22, 24] or DES [23] (χ 2 = 0.54, df = 1, p = 0.46).
Publication bias
The funnel plot for mortality did not show asymmetry by visual inspection suggesting no publication bias (Fig. 7); similarly, Egger’s test was not significant, thus excluding the presence of publication bias; the same results were observed for all the chosen outcomes.
Fig. 7.
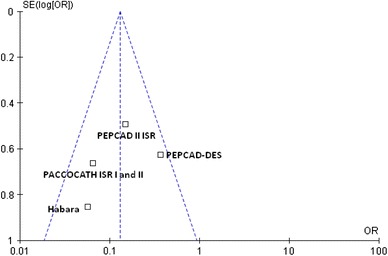
Funnel plot for the mortality outcome. The sample size of each study (measured as standard error of the treatment effect) was plotted against the odds ratio for overall mortality
Number needed to treat
The absolute difference in event rates results in five patients needed to treat to prevent one TLR, and three patients to prevent one binary restenosis (Fig. 8).
Fig. 8.
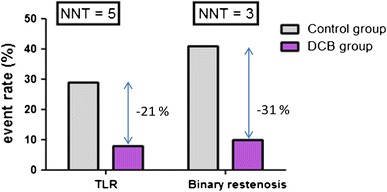
Absolute differences in rates of target lesion revascularisation (TLR) and binary restenosis after treatment with DCB or control PCI for in-stent restenosis
Discussion
The present meta-analysis demonstrates that the use of DCB in the management of ISR is associated with reduction in the incidence of binary restenosis, in-stent LLL, TLR and possibly MI compared with controls (balloon angioplasty or drug eluting stent).
In the United States, DCBs are not currently approved by the regulatory authorities. However, DCBs have a class IIa, level of evidence B indication in the ESC revascularisation guidelines for the management of ISR in bare-metal stent. Recruiting large patient numbers to RCTs for ISR is difficult; as such, individual studies included were relatively small. Meta-analyses in these circumstances are particularly useful. By pooling existing data, this study has provided more robust clinical evidence for a broader group of patients including restenosis of previously implanted DES.
Management of in-stent restenosis
In-stent restenosis has been reduced but not eliminated by the use of DES. Indeed, given the numbers of more complex interventions with DES, ISR will remain a prevalent clinical presentation and on-going challenge for interventional cardiologists. Current percutaneous treatment options for ISR include treatment with balloon angioplasty (with or without cutting balloons) and the placement of a second (drug-eluting) stent. Vascular brachytherapy has previously been advocated, but is not currently in widespread use. In studies using implantation of a second DES, further re-intervention rates remain high [25]. Thus, there is scope for improved and expanded treatment options in this patient group.
Drug-coated balloons have been studied versus POBA or DES in RCTs of stable patients presenting with restenosis of either a BMS or DES [21–24]. DCB included in the analysis were similar technologies; both balloons have the same carrier matrix and are coated with 3 μg paclitaxel. Overall, less LLL and lower rates of binary restenosis were observed with DCB versus control. The pooled estimates also demonstrated a significant reduction in re-intervention on the target lesion when patients were treated with DCB. Studies included reported clinical follow-up between 6 and 24 months. More recently the PACCOCATH studies showed a durable benefit to 5 years in DCB-treated patients [26].
In the three studies where DCBs were compared with POBA [21, 22, 24], the MD in LLL was remarkably consistent. A single study, PEPCAD II ISR, that compared DCB with a DES (Taxus libertē), showed a trend in favour of DCB but did not achieve statistical significance for a reduction in clinical TLR [23]. Further comparisons with newer generation DES would give additional useful information. PEPCAD II ISR, however, did contribute to the overall impression of superiority for DCB over alternative treatments, with a significant reduction in LLL and numerically fewer binary restenosis and TLR [23]. Furthermore, no interaction with type of control with respect to TLR was demonstrated in the present analysis.
In the management of restenosis, DCBs have the practical advantage of not placing a further layer of metal within a coronary artery and requiring shorter durations of dual anti-platelet therapy. The results of the analysis were consistent, with no evidence of statistical heterogeneity. Therefore, DCBs appear to be an effective choice in the management of ISR.
Perhaps more surprisingly, borderline significant (p = 0.05) lower rates of MI were also observed for DCB over controls (1/217 vs. 7/182). The absolute MI rate in this meta-analysis was small, with 8/399 patients reporting an MI. This finding was mainly powered by the PACCOCATH ISR I and II that reported follow-up data at 2 years [21]. A potential explanation for this small but significant absolute difference is that restenosis in itself can manifest as MI. This proportion was estimated at 3.5 % (death or MI) in the TAXUS clinical trials of de novo coronary artery disease, although in unselected practise this has been estimated up to 9.5 % [27, 28]. Based on DES versus BMS RCTs, it has been hypothesised that reducing restenosis can “offset” the impact of late stent thrombosis on the end points of MI and death [27]. Given that no stent thrombosis was recorded, it is possible that DCBs provide the benefit of a reduction in MI by attenuating binary restenosis and LLL, without “trade-off” in this subgroup. More detailed information on the timing of MI in relation to TLR, and a greater number of studied patients and clinical events would be required to confirm this finding and explanation.
Limitations
In general, the RCTs were well reported and the data were easily abstracted from the published manuscripts. It is recognised as a limitation that the three of the included RCTs were either unblinded [23], or single blind [22, 24]. In addition, the meta-analysis would have been strengthened by the existence of more studies employing DES control.
Many of the included RCTs allowed angiographic rather than purely clinical driven revascularisation of target lesions. RCTs are known in other fields to increase the frequency of TLR clinical end points by the “oculo-stenotic” reflex. It is possible, therefore, that some cases of TLR would not have occurred had protocol angiography not been performed. This limitation in directly applying these research findings to real world clinical practise is mitigated by the important information gained by quantitative angiography in these experimental circumstances.
We opted in this meta-analysis to focus on studies of ISR. This approach has the advantage of comparing treatment in a defined lesion pathology, with studies of similar DCB. It was therefore considered valid to calculate pooled estimates for these studies. However, it could be seen as a limitation that we excluded studies of de novo disease from the current analysis. DCB devices used in some de novo lesion studies have varied significantly in their design to current devices (e.g., no carrier molecule, “DCB-facilitated” BMS), which in some cases have not been made available for use due to lack of efficacy [16, 29]. Furthermore, for those remaining DCB tested in RCTs, both the patient sub-groups (e.g. AMI, stable angina), and comparator stents (e.g. DES, EPC capture stents) were incongruent, rendering a comparison limited at best [17]. Thus, it was concluded that a fair comparison is not currently possible. DCBs remain in a developmental stage in de novo disease, and further RCTs are awaited.
Finally, a limitation of this meta-analysis, common to all the meta-analyses based on study-level data, is the lack of individual patient data that would have further improved the results. However, an in-depth robust statistical analysis in the present study revealed no heterogeneity or publication bias. Further, given our study does not contain patient level data, it is not possible to determine if there were differences in the degree of angina pectoris and dyspnoea before and following DCB treatment.
Conclusion
Drug-coated balloons appear effective versus controls (balloon angioplasty or DES) in improving angiographic outcomes, reducing TLR and possibly MI when used in the management of restenosis of previously implanted stents.
Acknowledgments
The present contribution is a project of Systematic Investigation and Research on Interventions and Outcomes (SIRIO)-MEDICINE, a group of senior scientists and fellows collaborating worldwide to pursue research and innovation in medicine.
Abbreviations
- BMS
Bare-metal stent
- CI
Confidence interval
- DCB
Drug-coated balloon
- DES
Drug-eluting stent
- EPC
Endothelial progenitor cell
- ISR
In-stent restenosis
- LLL
Late luminal loss
- MD
Mean difference
- MI
Myocardial infarction
- OR
Odds ratio
- POBA
Balloon angioplasty
- PACCOCATH ISR
Treatment of coronary in-stent restenosis with a catheter
- PCI
Percutaneous coronary intervention
- PEPCAD II
Paclitaxel-coated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis
- PEPCAD DES
Prospective randomized trial of a paclitaxel-coated balloon vs. uncoated balloon angioplasty in patients with drug-eluting stent restenosis
- PEPPER
Paclitaxel releasing balloon in patients presenting with in-stent restenosis
- RCT
Randomised controlled trial
- TLR
Target lesion revascularisation
References
- 1.Cortese B, Bertoletti A. Paclitaxel coated balloons for coronary artery interventions: A comprehensive review of preclinical and clinical data. Int J Cardiol. 2011;161(1):4–12. doi: 10.1016/j.ijcard.2011.08.855. [DOI] [PubMed] [Google Scholar]
- 2.Waksman R, Pakala R. Drug-eluting balloon: the comeback kid? Circ Cardiovasc Interv. 2009;2(4):352–358. doi: 10.1161/CIRCINTERVENTIONS.109.873703. [DOI] [PubMed] [Google Scholar]
- 3.Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schomig A, Pfisterer ME, Stone GW, Leon MB, de Lezo JS, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370(9591):937–948. doi: 10.1016/S0140-6736(07)61444-5. [DOI] [PubMed] [Google Scholar]
- 4.Scheller B, Speck U, Bohm M. Prevention of restenosis: is angioplasty the answer? Heart. 2007;93(5):539–541. doi: 10.1136/hrt.2007.118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higgins J, Green S, editors. Cochrane handbook for systematic reviews of intervention. London: Wiley; 2011. [Google Scholar]
- 6.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Cremers B, Clever Y, Schaffner S, Speck U, Bohm M, Scheller B. Treatment of coronary in-stent restenosis with a novel paclitaxel urea coated balloon. Minerva Cardioangiol. 2010;58(5):583–588. [PubMed] [Google Scholar]
- 8.Fanggiday JC, Stella PR, Guyomi SH, Doevendans PA. Safety and efficacy of drug-eluting balloons in percutaneous treatment of bifurcation lesions: the DEBIUT (drug-eluting balloon in bifurcation Utrecht) registry. Catheter Cardiovasc Interv. 2008;71(5):629–635. doi: 10.1002/ccd.21452. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed W, Shah MA, Thaver AM, Mirza J. Drug-eluting balloon (DEB) for de novo coronary artery disease and in-stent restenosis: immediate and intermediate term results from a prospective registry. J Pak Med Assoc. 2011;61(2):157–160. [PubMed] [Google Scholar]
- 10.Nakamura S, Ogawa H, Bae J-H, Cahyadi YH, Udayachalerm W, Tresukosol D. Comparison of efficacy and safety between sirolimus paclitaxel everolimus-eluting stent and sequent please a drug-eluting balloon on the outcome of patients with diffuse in-stent restenosis after bare metal stent implantation. J Am Coll Cardiol. 2011;57(14 Suppl S):E1638. doi: 10.1016/S0735-1097(11)61638-0. [DOI] [Google Scholar]
- 11.Sgueglia GA, Todaro D, Bisciglia A, Conte M, Stipo A, Pucci E. Kissing inflation is feasible with all second-generation drug-eluting balloons. Cardiovasc Revasc Med. 2011;12(5):280–285. doi: 10.1016/j.carrev.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Unverdorben M, Kleber FX, Heuer H, Figulla HR, Vallbracht C, Leschke M, Cremers B, Hardt S, Buerke M, Ackermann H, et al. Treatment of small coronary arteries with a paclitaxel-coated balloon catheter. Clin Res Cardiol. 2010;99(3):165–174. doi: 10.1007/s00392-009-0101-6. [DOI] [PubMed] [Google Scholar]
- 13.Vaquerizo B, Serra A, Miranda-Guardiola F, Martinez V, Antoni Gomez-Hospital J, Iniguez A, Fernandez E, Ramon Rumoroso J, Bosa F, Otaegui I. One-year outcomes with angiographic follow-up of paclitaxel-eluting balloon for the treatment of in-stent restenosis: insights from Spanish multicenter registry. J Interv Cardiol. 2011;24(6):518–528. doi: 10.1111/j.1540-8183.2011.00667.x. [DOI] [PubMed] [Google Scholar]
- 14.Stella PR, Belkacemi A, Waksman R, Stahnke S, Torguson R, von Strandmann RP, Agostoni P, Sangiorgi G, Silber S. The valentines trial: results of the first one week worldwide multicentre enrolment trial, evaluating the real world usage of the second generation DIOR paclitaxel drug-eluting balloon for in-stent restenosis treatment. EuroIntervention. 2011;7(6):705–710. doi: 10.4244/EIJV7I6A113. [DOI] [PubMed] [Google Scholar]
- 15.Wohrle J, Birkemeyer R, Markovic S, Nguyen TV, Sinha A, Miljak T, Spiess J, Rottbauer W, Rittger H. Prospective randomised trial evaluating a paclitaxel-coated balloon in patients treated with endothelial progenitor cell capturing stents for de novo coronary artery disease. Heart. 2011;97(16):1338–1342. doi: 10.1136/hrt.2011.226563. [DOI] [PubMed] [Google Scholar]
- 16.Cortese B, Micheli A, Picchi A, Coppolaro A, Bandinelli L, Severi S, Limbruno U. Paclitaxel-coated balloon versus drug-eluting stent during PCI of small coronary vessels, a prospective randomised clinical trial. The PICCOLETO study. Heart. 2010;96(16):1291–1296. doi: 10.1136/hrt.2010.195057. [DOI] [PubMed] [Google Scholar]
- 17.Stella P (2011) Drug eluting balloon in acute myocardial infarction. In: TCT, San Francisco, 2011
- 18.Belkacemi A. A multicenter randomized comparison of drug-eluting balloon plus bare metal stent versus bare metal stent versus drug-eluting stent in bifurcation lesions: 6-month angiographic and 12-month clinical results of DEBUIT. In: EuroPCR; 2011. [DOI] [PubMed] [Google Scholar]
- 19.Mathey DG, Wendig I, Boxberger M, Bonaventura K, Kleber FX. Treatment of bifurcation lesions with a drug-eluting balloon: the PEPCAD V (paclitaxel eluting PTCA balloon in coronary artery disease) trial. EuroIntervention. 2011;7(Suppl K):K61–K65. doi: 10.4244/EIJV7SKA11. [DOI] [PubMed] [Google Scholar]
- 20.Scheller B, Hehrlein C, Bocksch W, Rutsch W, Haghi D, Dietz U, Bohm M, Speck U. Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. N Engl J Med. 2006;355(20):2113–2124. doi: 10.1056/NEJMoa061254. [DOI] [PubMed] [Google Scholar]
- 21.Scheller B, Hehrlein C, Bocksch W, Rutsch W, Haghi D, Dietz U, Bohm M, Speck U. Two year follow-up after treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. Clin Res Cardiol. 2008;97(10):773–781. doi: 10.1007/s00392-008-0682-5. [DOI] [PubMed] [Google Scholar]
- 22.Habara S, Mitsudo K, Kadota K, Goto T, Fujii S, Yamamoto H, Katoh H, Oka N, Fuku Y, Hosogi S, et al. Effectiveness of paclitaxel-eluting balloon catheter in patients with sirolimus-eluting stent restenosis. JACC Cardiovasc Interv. 2011;4(2):149–154. doi: 10.1016/j.jcin.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Unverdorben M, Vallbracht C, Cremers B, Heuer H, Hengstenberg C, Maikowski C, Werner GS, Antoni D, Kleber FX, Bocksch W, et al. Paclitaxel-coated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis. Circulation. 2009;119(23):2986–2994. doi: 10.1161/CIRCULATIONAHA.108.839282. [DOI] [PubMed] [Google Scholar]
- 24.Rittger H, Brachmann J, Sinha AM, Waliszewski M, Ohlow M, Brugger A, Thiele H, Birkemeyer R, Kurowski V, Breithardt OA, et al. A randomized, multicenter, single-blinded trial comparing paclitaxel-coated balloon angioplasty with plain balloon angioplasty in drug-eluting stent restenosis: the PEPCAD-DES study. J Am Coll Cardiol. 2012;59(15):1377–1382. doi: 10.1016/j.jacc.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 25.Mehilli J, Byrne RA, Tiroch K, Pinieck S, Schulz S, Kufner S, Massberg S, Laugwitz KL, Schomig A, Kastrati A. Randomized trial of paclitaxel- versus sirolimus-eluting stents for treatment of coronary restenosis in sirolimus-eluting stents: the ISAR-DESIRE 2 (intracoronary stenting and angiographic results: drug eluting stents for in-stent restenosis 2) study. J Am Coll Cardiol. 2010;55(24):2710–2716. doi: 10.1016/j.jacc.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Scheller B, Clever YP, Kelsch B, Hehrlein C, Bocksch W, Rutsch W, Haghi D, Dietz U, Speck U, Bohm M, et al. Long-term follow-up after treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. JACC Cardiovasc Interv. 2012;5(3):323–330. doi: 10.1016/j.jcin.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Stone GW, Ellis SG, Colombo A, Dawkins KD, Grube E, Cutlip DE, Friedman M, Baim DS, Koglin J. Offsetting impact of thrombosis and restenosis on the occurrence of death and myocardial infarction after paclitaxel-eluting and bare metal stent implantation. Circulation. 2007;115(22):2842–2847. doi: 10.1161/CIRCULATIONAHA.106.687186. [DOI] [PubMed] [Google Scholar]
- 28.Chen MS, John JM, Chew DP, Lee DS, Ellis SG, Bhatt DL. Bare metal stent restenosis is not a benign clinical entity. Am Heart J. 2006;151(6):1260–1264. doi: 10.1016/j.ahj.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Hamm C (2009) PEPCAD III: A randomized trial comparing a paclitaxel-eluting PTCA-balloon in combination with the coroflex blue stent versus the sirolimus coated Cypher stent in the treatment of coronary artery disease. In: American Heart Association, Scientific Sessions, 2009


