Abstract
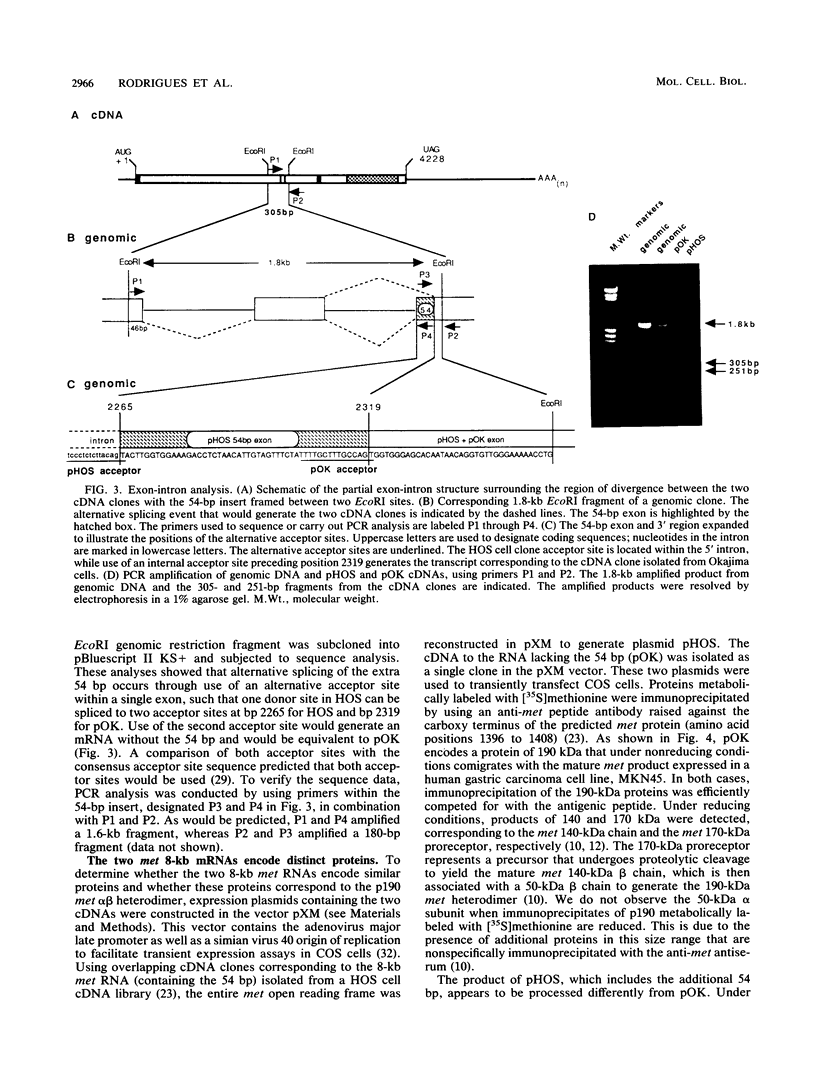
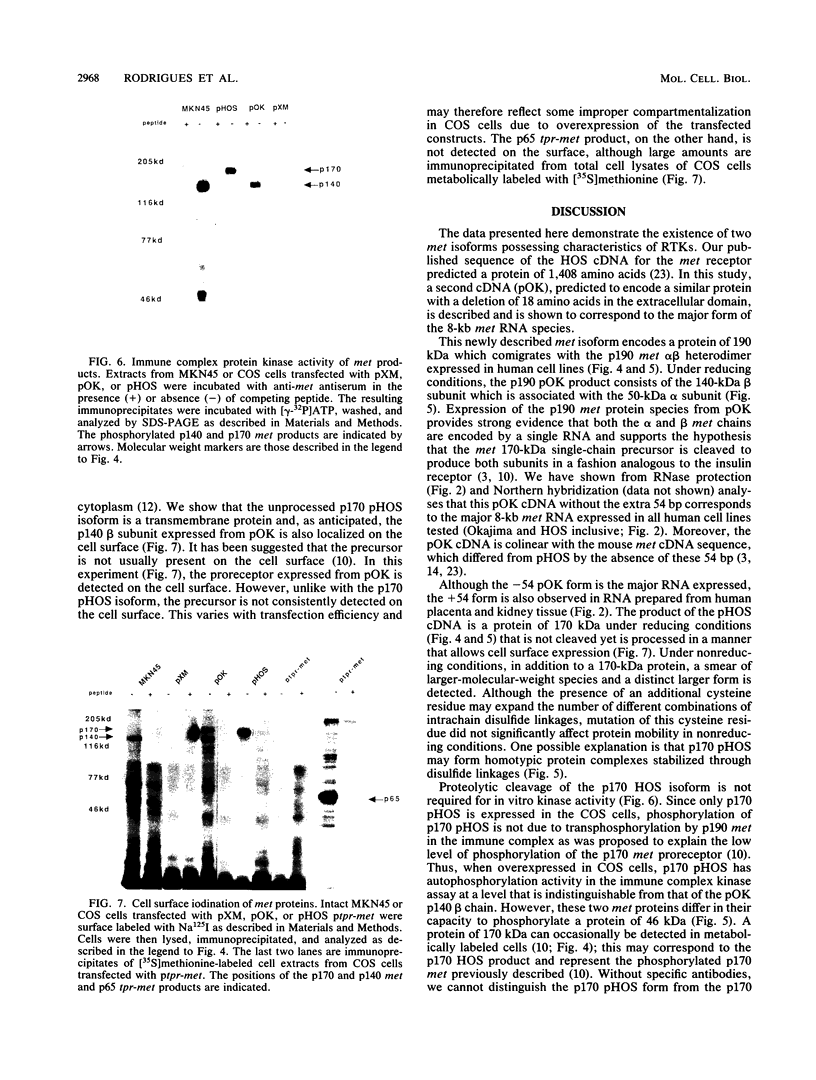
The met proto-oncogene is a member of the family of tyrosine kinase growth factor receptors. We describe the isolation and characterization of a cDNA clone (pOK) for the met receptor from a gastric carcinoma cell line. This clone differs from the published cDNA clone by the absence of 54 bp predicted to encode 18 amino acids in the extracellular domain. The pOK cDNA corresponds to the most abundant met RNA species of 8 kb expressed in human cell lines and tissue, and we show that there are in fact two 8-kb met receptor tyrosine kinase (RTK) isoforms that are generated by alternative splicing. This newly described met isoform when transiently expressed in COS cells encodes a protein of 190 kDa which corresponds in size to the p190 met alpha beta heterodimer expressed in human cell lines. Furthermore, we show that the 190-kDa product of pOK consists of the 140-kDa met beta subunit associated with the 50-kDa met alpha subunit. This finding suggests that both the alpha and beta met chains are encoded by this construct and confirms the hypothesis that a single chain precursor is cleaved to produce both subunits of met. In contrast, the previously characterized met isoform corresponds to a minor met RNA species and encodes a protein of 170 kDa that is not cleaved yet is processed in a manner that allows cell surface expression. Both met RTK isoforms are autophosphorylated in the in vitro kinase assay. These results suggest that different isoforms of the met RTK may have distinct biological activities.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreadis A., Gallego M. E., Nadal-Ginard B. Generation of protein isoform diversity by alternative splicing: mechanistic and biological implications. Annu Rev Cell Biol. 1987;3:207–242. doi: 10.1146/annurev.cb.03.110187.001231. [DOI] [PubMed] [Google Scholar]
- Breitbart R. E., Andreadis A., Nadal-Ginard B. Alternative splicing: a ubiquitous mechanism for the generation of multiple protein isoforms from single genes. Annu Rev Biochem. 1987;56:467–495. doi: 10.1146/annurev.bi.56.070187.002343. [DOI] [PubMed] [Google Scholar]
- Chan A. M., King H. W., Deakin E. A., Tempest P. R., Hilkens J., Kroezen V., Edwards D. R., Wills A. J., Brookes P., Cooper C. S. Characterization of the mouse met proto-oncogene. Oncogene. 1988 Jun;2(6):593–599. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cooper C. S., Park M., Blair D. G., Tainsky M. A., Huebner K., Croce C. M., Vande Woude G. F. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature. 1984 Sep 6;311(5981):29–33. doi: 10.1038/311029a0. [DOI] [PubMed] [Google Scholar]
- Cooper C. S., Tempest P. R., Beckman M. P., Heldin C. H., Brookes P. Amplification and overexpression of the met gene in spontaneously transformed NIH3T3 mouse fibroblasts. EMBO J. 1986 Oct;5(10):2623–2628. doi: 10.1002/j.1460-2075.1986.tb04543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulier F., Kumar R., Ernst M., Klein R., Martin-Zanca D., Barbacid M. Human trk oncogenes activated by point mutation, in-frame deletion, and duplication of the tyrosine kinase domain. Mol Cell Biol. 1990 Aug;10(8):4202–4210. doi: 10.1128/mcb.10.8.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M., Park M., Le Beau M. M., Robins T. S., Diaz M. O., Rowley J. D., Blair D. G., Vande Woude G. F. The human met oncogene is related to the tyrosine kinase oncogenes. 1985 Nov 28-Dec 4Nature. 318(6044):385–388. doi: 10.1038/318385a0. [DOI] [PubMed] [Google Scholar]
- Giordano S., Di Renzo M. F., Ferracini R., Chiadò-Piat L., Comoglio P. M. p145, a protein with associated tyrosine kinase activity in a human gastric carcinoma cell line. Mol Cell Biol. 1988 Aug;8(8):3510–3517. doi: 10.1128/mcb.8.8.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano S., Di Renzo M. F., Narsimhan R. P., Cooper C. S., Rosa C., Comoglio P. M. Biosynthesis of the protein encoded by the c-met proto-oncogene. Oncogene. 1989 Nov;4(11):1383–1388. [PubMed] [Google Scholar]
- Giordano S., Ponzetto C., Di Renzo M. F., Cooper C. S., Comoglio P. M. Tyrosine kinase receptor indistinguishable from the c-met protein. Nature. 1989 May 11;339(6220):155–156. doi: 10.1038/339155a0. [DOI] [PubMed] [Google Scholar]
- Gonzatti-Haces M., Seth A., Park M., Copeland T., Oroszlan S., Vande Woude G. F. Characterization of the TPR-MET oncogene p65 and the MET protooncogene p140 protein-tyrosine kinases. Proc Natl Acad Sci U S A. 1988 Jan;85(1):21–25. doi: 10.1073/pnas.85.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Iyer A., Kmiecik T. E., Park M., Daar I., Blair D., Dunn K. J., Sutrave P., Ihle J. N., Bodescot M., Vande Woude G. F. Structure, tissue-specific expression, and transforming activity of the mouse met protooncogene. Cell Growth Differ. 1990 Feb;1(2):87–95. [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Morrison M., Bayse G. S. Catalysis of iodination by lactoperoxidase. Biochemistry. 1970 Jul 21;9(15):2995–3000. doi: 10.1021/bi00817a010. [DOI] [PubMed] [Google Scholar]
- Mosthaf L., Grako K., Dull T. J., Coussens L., Ullrich A., McClain D. A. Functionally distinct insulin receptors generated by tissue-specific alternative splicing. EMBO J. 1990 Aug;9(8):2409–2413. doi: 10.1002/j.1460-2075.1990.tb07416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M., Dean M., Cooper C. S., Schmidt M., O'Brien S. J., Blair D. G., Vande Woude G. F. Mechanism of met oncogene activation. Cell. 1986 Jun 20;45(6):895–904. doi: 10.1016/0092-8674(86)90564-7. [DOI] [PubMed] [Google Scholar]
- Park M., Dean M., Kaul K., Braun M. J., Gonda M. A., Vande Woude G. Sequence of MET protooncogene cDNA has features characteristic of the tyrosine kinase family of growth-factor receptors. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6379–6383. doi: 10.1073/pnas.84.18.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petch L. A., Harris J., Raymond V. W., Blasband A., Lee D. C., Earp H. S. A truncated, secreted form of the epidermal growth factor receptor is encoded by an alternatively spliced transcript in normal rat tissue. Mol Cell Biol. 1990 Jun;10(6):2973–2982. doi: 10.1128/mcb.10.6.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino S., Bell G. I. Alternative splicing of human insulin receptor messenger RNA. Biochem Biophys Res Commun. 1989 Feb 28;159(1):312–316. doi: 10.1016/0006-291x(89)92439-x. [DOI] [PubMed] [Google Scholar]
- Seino S., Seino M., Nishi S., Bell G. I. Structure of the human insulin receptor gene and characterization of its promoter. Proc Natl Acad Sci U S A. 1989 Jan;86(1):114–118. doi: 10.1073/pnas.86.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987 Sep 11;15(17):7155–7174. doi: 10.1093/nar/15.17.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempest P. R., Cooper C. S., Major G. N. The activated human met gene encodes a protein tyrosine kinase. FEBS Lett. 1986 Dec 15;209(2):357–361. doi: 10.1016/0014-5793(86)81142-5. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Yang Y. C., Ciarletta A. B., Temple P. A., Chung M. P., Kovacic S., Witek-Giannotti J. S., Leary A. C., Kriz R., Donahue R. E., Wong G. G. Human IL-3 (multi-CSF): identification by expression cloning of a novel hematopoietic growth factor related to murine IL-3. Cell. 1986 Oct 10;47(1):3–10. doi: 10.1016/0092-8674(86)90360-0. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]