Abstract
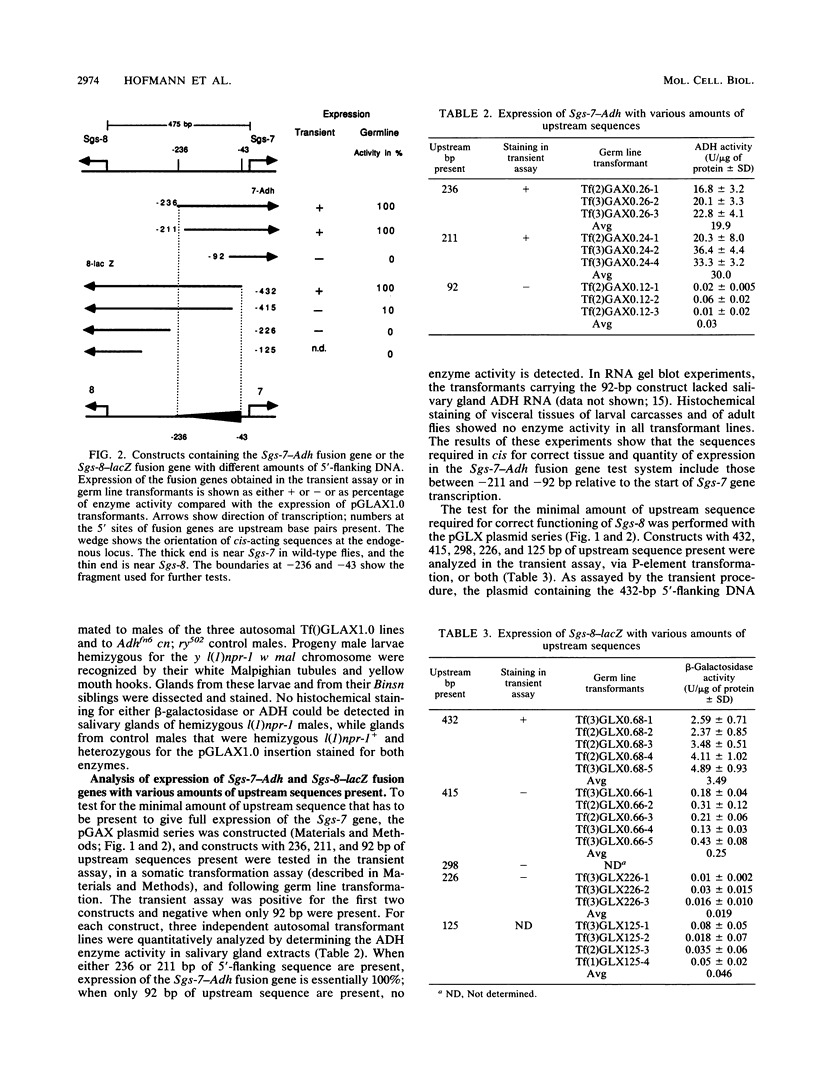
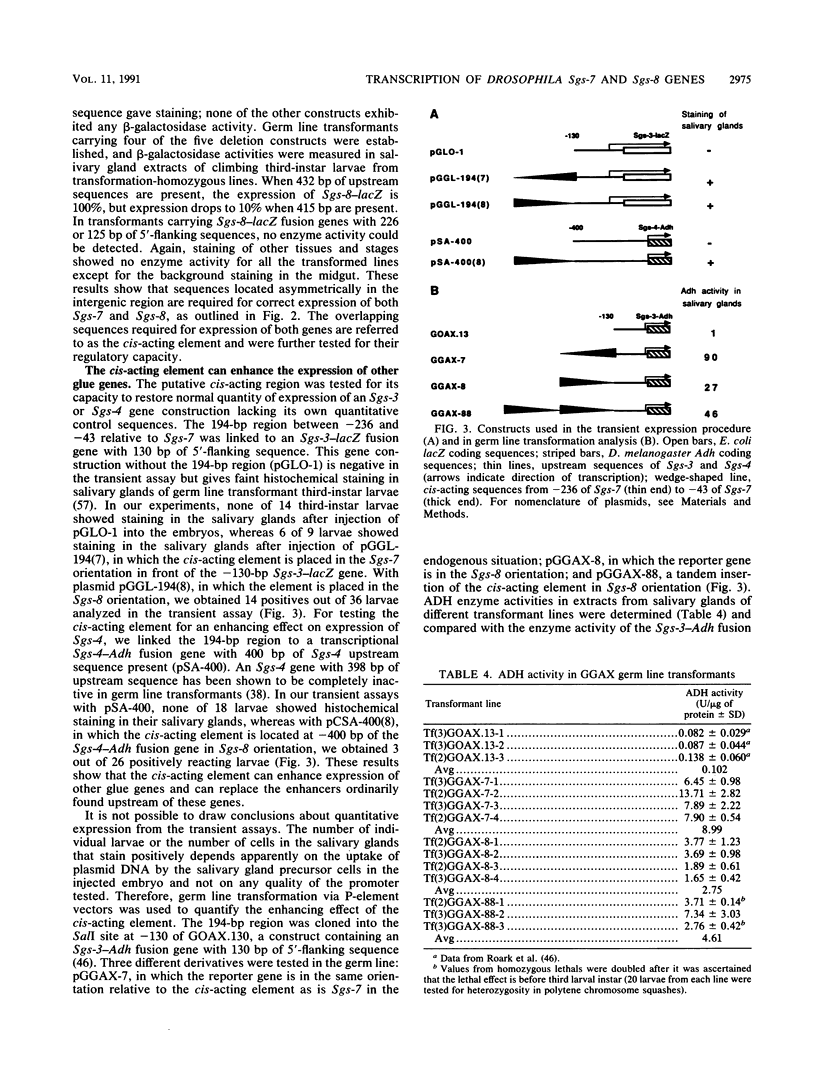
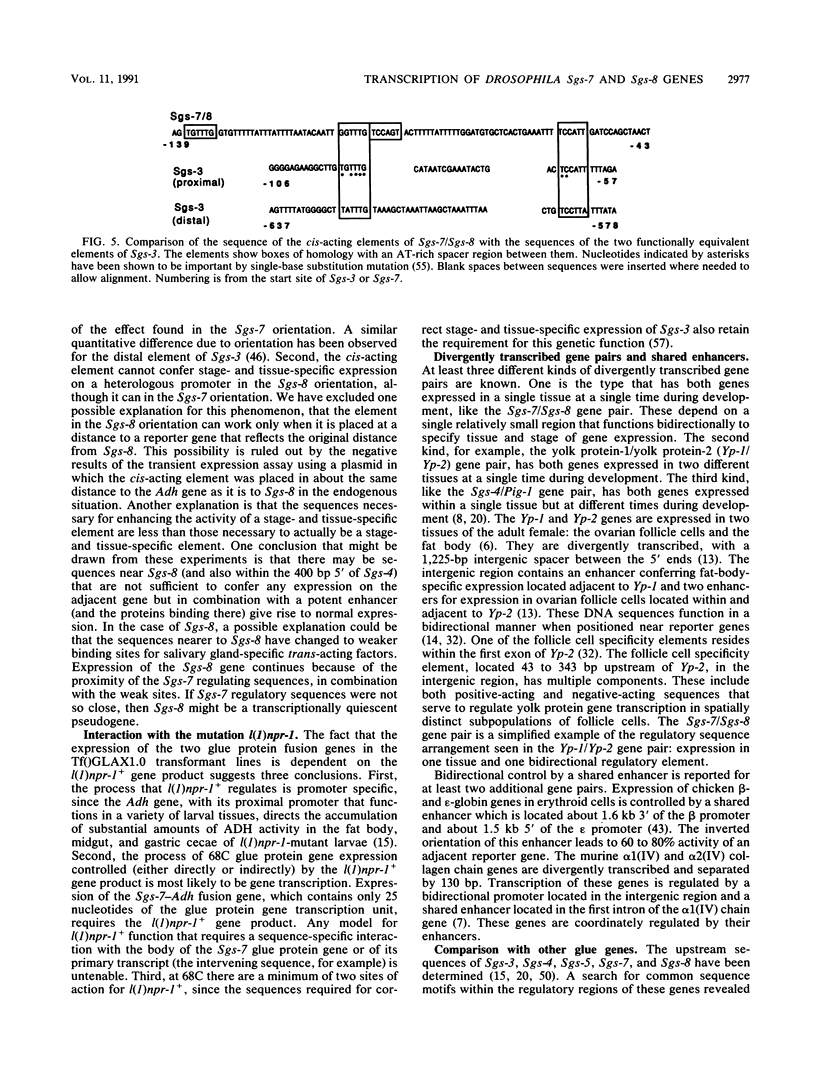
The Sgs-7 and Sgs-8 glue genes at 68C are divergently transcribed and are separated by 475 bp. Fusion genes with Adh or lacZ coding sequences were constructed, and the expression of these genes, with different amounts of upstream sequences present, was tested by a transient expression procedure and by germ line transformation. A cis-acting element for both genes is located asymmetrically in the intergenic region between -211 and -43 bp relative to Sgs-7. It is required for correct expression of both genes. This element can confer the stage- and tissue-specific expression pattern of glue genes on a heterologous promoter. An 86-bp portion of the element, from -133 to -48 bp relative to Sgs-7, is shown to be capable of enhancing the expression of a truncated and therefore weakly expressed Sgs-3 fusion gene. Recently described common sequence motifs of glue gene regulatory elements (T. Todo, M. Roark, K. Vijay Raghavan, C. A. Mayeda, and E.M. Meyerowitz, Mol. Cell. Biol. 10:5991-6002, 1990) are located within this 86-bp region.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckendorf S. K., Kafatos F. C. Differentiation in the salivary glands of Drosophila melanogaster: characterization of the glue proteins and their developmental appearance. Cell. 1976 Nov;9(3):365–373. doi: 10.1016/0092-8674(76)90081-7. [DOI] [PubMed] [Google Scholar]
- Belyaeva E. S., Vlassova I. E., Biyasheva Z. M., Kakpakov V. T., Richards G., Zhimulev I. F. Cytogenetic analysis of the 2B3-4-2B11 region of the X chromosome of Drosophila melanogaster. II. Changes in 20-OH ecdysone puffing caused by genetic defects of puff 2B5. Chromosoma. 1981;84(2):207–219. doi: 10.1007/BF00399132. [DOI] [PubMed] [Google Scholar]
- Benyajati C., Spoerel N., Haymerle H., Ashburner M. The messenger RNA for alcohol dehydrogenase in Drosophila melanogaster differs in its 5' end in different developmental stages. Cell. 1983 May;33(1):125–133. doi: 10.1016/0092-8674(83)90341-0. [DOI] [PubMed] [Google Scholar]
- Bonner J. J., Parks C., Parker-Thornburg J., Mortin M. A., Pelham H. R. The use of promoter fusions in Drosophila genetics: isolation of mutations affecting the heat shock response. Cell. 1984 Jul;37(3):979–991. doi: 10.1016/0092-8674(84)90432-x. [DOI] [PubMed] [Google Scholar]
- Bourouis M., Richards G. Remote regulatory sequences of the Drosophila glue gene sgs3 as revealed by P-element transformation. Cell. 1985 Feb;40(2):349–357. doi: 10.1016/0092-8674(85)90149-7. [DOI] [PubMed] [Google Scholar]
- Brennan M. D., Weiner A. J., Goralski T. J., Mahowald A. P. The follicle cells are a major site of vitellogenin synthesis in Drosophila melanogaster. Dev Biol. 1982 Jan;89(1):225–236. doi: 10.1016/0012-1606(82)90309-8. [DOI] [PubMed] [Google Scholar]
- Burbelo P. D., Martin G. R., Yamada Y. Alpha 1(IV) and alpha 2(IV) collagen genes are regulated by a bidirectional promoter and a shared enhancer. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9679–9682. doi: 10.1073/pnas.85.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. N., Malone T., Beckendorf S. K., Davis R. L. At least two genes reside within a large intron of the dunce gene of Drosophila. Nature. 1987 Oct 22;329(6141):721–724. doi: 10.1038/329721a0. [DOI] [PubMed] [Google Scholar]
- Crowley T. E., Bond M. W., Meyerowitz E. M. The structural genes for three Drosophila glue proteins reside at a single polytene chromosome puff locus. Mol Cell Biol. 1983 Apr;3(4):623–634. doi: 10.1128/mcb.3.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley T. E., Mathers P. H., Meyerowitz E. M. A trans-acting regulatory product necessary for expression of the Drosophila melanogaster 68C glue gene cluster. Cell. 1984 Nov;39(1):149–156. doi: 10.1016/0092-8674(84)90200-9. [DOI] [PubMed] [Google Scholar]
- Crowley T. E., Meyerowitz E. M. Steroid regulation of RNAs transcribed from the Drosophila 68c polytene chromosome puff. Dev Biol. 1984 Mar;102(1):110–121. doi: 10.1016/0012-1606(84)90179-9. [DOI] [PubMed] [Google Scholar]
- Garabedian M. J., Hung M. C., Wensink P. C. Independent control elements that determine yolk protein gene expression in alternative Drosophila tissues. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1396–1400. doi: 10.1073/pnas.82.5.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabedian M. J., Shepherd B. M., Wensink P. C. A tissue-specific transcription enhancer from the Drosophila yolk protein 1 gene. Cell. 1986 Jun 20;45(6):859–867. doi: 10.1016/0092-8674(86)90560-x. [DOI] [PubMed] [Google Scholar]
- Garfinkel M. D., Pruitt R. E., Meyerowitz E. M. DNA sequences, gene regulation and modular protein evolution in the Drosophila 68C glue gene cluster. J Mol Biol. 1983 Aug 25;168(4):765–789. doi: 10.1016/s0022-2836(83)80074-6. [DOI] [PubMed] [Google Scholar]
- Giangrande A., Mettling C., Richards G. Sps-3 transcript levels are determined by multiple remote sequence elements. EMBO J. 1987 Oct;6(10):3079–3084. doi: 10.1002/j.1460-2075.1987.tb02615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann A., Keinhorst A., Krumm A., Korge G. Regulatory sequences of the Sgs-4 gene of Drosophila melanogaster analysed by P element-mediated transformation. Chromosoma. 1987;96(1):8–17. doi: 10.1007/BF00285877. [DOI] [PubMed] [Google Scholar]
- Hofmann A., Korge G. Upstream sequences of dosage-compensated and non-compensated alleles of the larval secretion protein gene Sgs-4 in Drosophila. Chromosoma. 1987;96(1):1–7. doi: 10.1007/BF00285876. [DOI] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A., Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987 Dec 20;6(13):3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karess R. E., Rubin G. M. Analysis of P transposable element functions in Drosophila. Cell. 1984 Aug;38(1):135–146. doi: 10.1016/0092-8674(84)90534-8. [DOI] [PubMed] [Google Scholar]
- Korge G. Chromosome puff activity and protein synthesis in larval salivary glands of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4550–4554. doi: 10.1073/pnas.72.11.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korge G. Larval saliva in Drosophila melanogaster: production, composition, and relationship to chromosome puffs. Dev Biol. 1977 Jul 15;58(2):339–355. doi: 10.1016/0012-1606(77)90096-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Logan S. K., Wensink P. C. Ovarian follicle cell enhancers from the Drosophila yolk protein genes: different segments of one enhancer have different cell-type specificities that interact to give normal expression. Genes Dev. 1990 Apr;4(4):613–623. doi: 10.1101/gad.4.4.613. [DOI] [PubMed] [Google Scholar]
- Martin M., Giangrande A., Ruiz C., Richards G. Induction and repression of the Drosophila Sgs-3 glue gene are mediated by distinct sequences in the proximal promoter. EMBO J. 1989 Feb;8(2):561–568. doi: 10.1002/j.1460-2075.1989.tb03410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Martin A., Osmani A., Sofer W. A transient expression assay for tissue-specific gene expression of alcohol dehydrogenase in Drosophila. Dev Biol. 1986 Oct;117(2):574–580. doi: 10.1016/0012-1606(86)90326-x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McNabb S. L., Beckendorf S. K. Cis-acting sequences which regulate expression of the Sgs-4 glue protein gene of Drosophila. EMBO J. 1986 Sep;5(9):2331–2340. doi: 10.1002/j.1460-2075.1986.tb04501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestril R., Schiller P., Amin J., Klapper H., Ananthan J., Voellmy R. Heat shock and ecdysterone activation of the Drosophila melanogaster hsp23 gene; a sequence element implied in developmental regulation. EMBO J. 1986 Jul;5(7):1667–1673. doi: 10.1002/j.1460-2075.1986.tb04410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerowitz E. M., Hogness D. S. Molecular organization of a Drosophila puff site that responds to ecdysone. Cell. 1982 Jan;28(1):165–176. doi: 10.1016/0092-8674(82)90386-5. [DOI] [PubMed] [Google Scholar]
- Nickol J. M., Felsenfeld G. Bidirectional control of the chicken beta- and epsilon-globin genes by a shared enhancer. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2548–2552. doi: 10.1073/pnas.85.8.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue M. L., Gall J. G. Nucleic acid hybridization to the DNA of cytological preparations. Methods Cell Biol. 1975;10:1–16. doi: 10.1016/s0091-679x(08)60727-x. [DOI] [PubMed] [Google Scholar]
- Raghavan K. V., Crosby M. A., Mathers P. H., Meyerowitz E. M. Sequences sufficient for correct regulation of Sgs-3 lie close to or within the gene. EMBO J. 1986 Dec 1;5(12):3321–3326. doi: 10.1002/j.1460-2075.1986.tb04646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramain P., Giangrande A., Richards G., Bellard M. Analysis of a DNase I-hypersensitive site in transgenic Drosophila reveals a key regulatory element of Sgs3. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2718–2722. doi: 10.1073/pnas.85.8.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roark M., Raghavan K. V., Todo T., Mayeda C. A., Meyerowitz E. M. Cooperative enhancement at the Drosophila Sgs-3 locus. Dev Biol. 1990 May;139(1):121–133. doi: 10.1016/0012-1606(90)90283-o. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Vectors for P element-mediated gene transfer in Drosophila. Nucleic Acids Res. 1983 Sep 24;11(18):6341–6351. doi: 10.1093/nar/11.18.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore E. M., Guild G. M. Closely linked DNA elements control the expression of the Sgs-5 glue protein gene in Drosophila. Genes Dev. 1987 Oct;1(8):829–839. doi: 10.1101/gad.1.8.829. [DOI] [PubMed] [Google Scholar]
- Shore E. M., Guild G. M. Larval salivary gland secretion proteins in Drosophila structural analysis of the Sgs-5 gene. J Mol Biol. 1986 Jul 20;190(2):149–158. doi: 10.1016/0022-2836(86)90288-3. [DOI] [PubMed] [Google Scholar]
- Sofer W., Ursprung H. Drosophila alcohol dehydrogenase. Purification and partial characterization. J Biol Chem. 1968 Jun 10;243(11):3110–3115. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982 Oct 22;218(4570):341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Todo T., Roark M., Raghavan K. V., Mayeda C., Meyerowitz E. Fine-structure mutational analysis of a stage- and tissue-specific promoter element of the Drosophila glue gene Sgs-3. Mol Cell Biol. 1990 Nov;10(11):5991–6002. doi: 10.1128/mcb.10.11.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]



