Abstract
Background
It is estimated that up to 24% of the population in Germany suffers from allergic rhinoconjunctivitis and 5% from allergic asthma. Allergic rhinoconjunctivitis is closely related to other atopic diseases.
Methods
This review is based on pertinent publications retrieved by a selective search of the Medline database, guidelines from Germany and abroad, and Cochrane meta-analyses.
Results
Specific immunotherapy (SIT) is the only diseases-modifying treatment option for allergies. Meta-analysis reveals standardized mean differences in allergic rhinitis symptom scores of -0.73 for subcutaneous immunotherapy (SCIT) and -0.49 for sublingual immunotherapy (SLIT); the corresponding mean differences in medication scores are -0.57 and -0.32, respectively. The treatment should be carried out for at least three years. It is indicated when the symptoms are severe and allergen avoidance is not a realistic option. The efficacy of treatment depends on the allergen dose; thus, every allergen preparation should be evaluated individually, independent of route of administration. SCIT can cause systemic adverse effects, including anaphylaxis. SLIT is safer but often causes allergic symptoms of the oral mucosa at the beginning of treatment.
Conclusion
Even though the efficacy of SIT is well documented, it is still underused. SIT should be offered as standard treatment to patients suffering from allergic rhinitis.
An increase in allergies to ubiquitous aeroallergens over the last few decades has been well documented (1). Among 13- and 14-year olds around the world, the prevalence of self-reported rhinitis symptoms now ranges from 3.2% to 66% (2). It is estimated that up to 24% of the German population suffers from allergic rhinoconjunctivitis, and 5% from allergic asthma (3). The symptoms are classified as either intermittent (present for less than four weeks per year) or persistent (present for more than four weeks per year). They are called moderate to severe if rinorrhea, nasal obstruction, and eye symptoms interfere with the untreated patient’s daily activities and/or sleep. An inhalation allergy should always be considered as a diagnostic possibility when the symptoms tend to arise at certain times of year or in particular situations. The allergic march often starts in childhood with atopic dermatitis and food allergies, followed by allergic asthma and allergic rhinoconjunctivitis.
Allergies are often trivialized and held to be more of a nuisance than a disease (4). Allergy is often not even considered as a possible cause of the patient’s symptoms. On the other hand, many people know they have allergies but still do not consult a doctor (3). In a recent Forsa survey (5), 58% of the persons with allergies who responded said that they treated their allergy themselves with non-prescription medications or allergen avoidance. 28% received specific immunotherapy (SIT), and 70% of those receiving SIT were satisfied with the outcome.
The purpose of treating allergies is to lessen the burden of symptoms and to prevent disease progression. SIT is currently the only disease-modifying treatment capable of inducing tolerance to individual allergens. When indicated, it can be performed successfully even in childhood (6, 7).
Prevalence.
Among 13- and 14-year olds around the world, the prevalence of self-reported rhinitis symptoms now ranges from 3.2% to 66%.
The socioeconomic cost of respiratory allergies in Europe has been estimated to lie between 36.7 and 385.1 billion euros per year, depending on the assumptions on which the calculation is based (8). The more severe the allergy, the more difficult and expensive the treatment. The overall cost per patient is about € 1670/year for mild asthma and about €6000/year for severe asthma (9).
Learning objectives
This article is intended to inform readers of the current state of knowledge regarding specific immunotherapy:
its indications, contraindications, and adverse effects,
its efficacy and the principles of allergen extract selection,
and the manner in which the treatment is administered.
Methods
This article is based on information from the following sources:
an S2 guideline on specific immunotherapy (SIT) (13),
-
the position papers of
the recommendations of the British Society for Allergy and Clinical Immunology (19),
the guidelines of the Committee for Medicinal Products for Human Use (CHMP) (20, 21), and
pertinent publications retrieved by selective searches in the Medline database (main search term: “allergen-specific immunotherapy”), with special attention to recent large-scale, double-blind placebo-controlled (DBPC) clinical trials.
The treatment of allergic diseases of the respiratory tract
Allergen avoidance is the first recommendation for patients with inhalative allergies. It may be successful, for example, for patients with allergies to pets. For patients with mite allergies, however, adequate allergen avoidance is often impossible, while the attempt to avoid pollen would most probably interfere excessively with a “normal” lifestyle.
The cost of respiratory allergies.
The socioeconomic cost of respiratory allergies in Europe has been estimated to lie between 37 and 385 billion euros per year, depending on the assumptions on which the calculation is based.
Antihistamines can be used for the symptomatic treatment of allergic rhinoconjunctivitis. Topical and systemic corticosteroids and leukotriene receptor antagonists can also be used to treat allergic asthma. These medications relieve symptoms, but they are not curative.
The efficacy of SIT has been documented in a large number of clinical trials. The results of many trials employing diverse allergen preparations in various dosages have been summarized in meta-analyses (10, 11) whose findings are presented in Table 1.
Table 1. Meta-analyses of SCIT and SLIT:Calculation of effect strengths.
| SCIT (10) | SLIT (11) | |
| SMD (95% CI) | SMD (95% CI) | |
| Symptoms | -0.73 | -0.49 |
| (-0.97 to -0.5) | (-0.64 to -0.34) | |
| Medications | -0.57 | –0.32 |
| (-0.82 to -0.33) | (-0.43 to -0.21) |
SMD, standardized mean difference; SCIT, subcutaneous immunotherapy; SLIT, sublingual immunotherapy; CI, confidence interval
A meta-analysis of the efficacy of subcutaneous immunotherapy (SCIT) for allergic asthma yielded standardized mean differences (SMDs) of -0.59 (-0.83, -0.35) for the symptom score and -0.53 (-0.80, -0.27) for the medication score (12).
Despite differences in the study design of trials analyzing the efficacy of symptomatic treatments and of SIT there is ecidence that the therapeutic effect of SCIT is superior to that of symptomatic medication even during the first year of treatment (Table 2) (22).
Table 2. The efficacy of SCIT compared to symptomatic medications*.
| Total nasal symptom score, | Total symptom score, | |||
| TNSS | TSS | |||
| RCI | SMD | RCI | SMD | |
| SCIT | -34.7% +/- 6.8% | –0.94; | –32.9% +/- 12.7% | –0.86; |
| 95% CI, –1.45 to –0.43 | 95% CI, –1.17 to –0.55 | |||
| Mometasone fuorate | –31.8% +/- 16.7% | –0.47; | ||
| 95% CI, –0.63 to –0.32 | ||||
| Montelukast | –6.3% +/- 3.0% | –0.24; | ||
| 95% CI, –0.33 to –0.16 | ||||
| Desloratadine | -12% +/-5.1% | -1.0; | ||
| 95% CI, -1.68 to -0.32 | ||||
SCIT, subcutaneous immunotherapy; RCI, relative clinical improvement; SMD, standardized mean difference.
*Total nasal symptom score (TNSS) and total symptom score (TSS) according to (22);CI, confidence interval
The mechanism of action of specific immunotherapy
Until a few years ago, specific immunotherapy was thought to exert its beneficial effect mainly by inducing “blocking” IgG antibodies. This concept, with its functional implications for antigen presentation and effector cell function, has now been supplemented by that of regulation of the allergen-specific immune response. When the immune system is confronted with a high enough dose of allergen, tolerance is induced. The precise mechanism of action of specific immunotherapy has not yet been definitively characterized (23, 24).
Allergen avoidance.
Allergen avoidance is the first treatment option that should be considered. It may be successful for patients with allergies to pets.
Dose-dependence of the immunological effect—the definition of an optimal therapeutic dose
The dose-effect relationship of SIT has been shown in clinical trials (14). The clinical benefit of a mite extract containing 7 µg of Der p 1 per injection in the maintenance phase (Der p 1 is a major allergen of the house-dust mite Dermatophagoides pteronyssinus) cannot be significantly augmented by raising the dose to 21 µg of Der p 1, although this does lead to an increase in adverse effects. The efficacy of 0.7 µg of Der p 1 was in the range of placebo (25). Circa 8 µg of group 5 allergen in a grass-pollen tablet is about as effective as placebo, and three times this dose (25 µg per day) yields the maximum effect, without any further benefit from still higher doses (26). These findings imply that it is possible to determine optimal dosages for SCIT and SLIT.
Allergen quantification for immunotherapy
The standardization and characterization of allergen extracts was once left entirely up to the manufacturers. The characterization of extract strength is based on measurement of protein concentrations, electrochemical techniques such as immunoelectrophoresis, wheal diameter in prick tests, and in vitro inhibition tests (RAST inhibition). The development of obligatory, validated methods for allergen quantification has now become a matter of central importance.
Allergen extracts.
Extract strength is determined by measurement of protein concentrations, biochemical methods, wheal diameter in prick tests, and in vitro inhibition tests.
Allergen extracts contain proteins, glycoproteins, lectins, DNA, pigments, and other substances. The immune system interacts with only a few allergens, which are called major allergens if more than 50% of patients sensitized against the allergen extract react to this particular protein. It should be a requirement that the concentration of a major allergen is declared. This is principally unproblematic when an allergen source contains only a single major allergen (e.g., Bet v 1 in birch-pollen extract), but extracts containing multiple major allergen proteins (e.g., grass-pollen extracts) are more difficult to characterize.
The quantification of individual proteins is further complicated by the fact that allergens are found in nature in different isoforms, with potentially varying biological activity. This may affect the results of protein quantification by immunological techniques.
A comparison of major allergen concentrations in different extracts can only be valid if obligatory and validated analytic methods have been used, with comparison to standardized reference allergens. In a continuation of the European CREATE project (27), a round-robin testing program (BSP90) is now being carried out with the support of the European Directorate for Quality Assurance in Medicine (EDQM) for the testing of biological reagents and standardized analytic methods for allergens in extracts. Once these reagents and methods have been published in the European Pharmacopoeia, they will become obligatory.
The allergen concentrations that are currently declared by manufacturers are an inadequate basis for the evaluation of allergen extracts. There is no substitute for clinical trials to demonstrate the efficacy of each and every allergen extract.
Clinical trials to determine efficacy.
Clinical trials are needed to determine the efficacy of allergen extracts. Efficacy can not be assessed on the basis of the allergen concentrations specified by manufacturers.
Requirements for allergen extracts that are to be used for SIT
A European technical guideline currently delineates the qualitative requirements for allergen extracts and their classification and composition. The most important new feature of this guideline is the definition of homologous groups of allergens, which is based on structural similarity, rather than on botanical relatedness (20). Another guideline of the European Medicines Agency (EMA) contains recommendations for the performance of clinical trials with allergen preparations; from now on, such preparations are to be developed in a standardized, stepwise algorithm with dose-finding studies and double-blind, placebo-controlled trials of efficacy (21). In Germany, more than 6000 different allergen mixtures were commercially available until the Regulation on Therapeutic Allergens (Therapie-Allergene-Verordnung, TAV) went into effect in November 2008. Most of these mixtures were then withdrawn from the market (data from the Paul Ehrlich Institute).
At present, single-substance preparations are preferred, as defined according to the concept of homologous allergen groups. The major allergens of grass and grain pollens are so highly homologous that “mixtures” of pollens from these sources are considered to be monopreparations. The same holds for allergens from birch, hazel, and alder pollen.
The evaluation of clinical effects in SIT trials
The clinical effect of SIT is evaluated by a comparison of the symptom and medication scores (SMS) during the allergy season of patients receiving either the active substance or placebo. The results obtained in different trials cannot be direcly compared for a number of reasons, but mainly because no standardized SMS has yet been defined.
Evaluating the clinical effect of SIT.
The clinical effect of SIT is evaluated by a comparison of the symptom and medication scores (SMS) during the allergy season of patients receiving either the active substance or placebo.
A 30% difference in SMS between the active-substance group and the placebo group used to be required as the criterion for a clinically significant effect. This requirement was based on evaluation of the per-protocol (PP) population, measuring the real effect of SIT itself. At present, however, intention-to-treat (ITT) analysis is considered standard. In this method, the data from all patients enrolled in the study, including those with low compliance, are included in the analysis so that the findings will more closely correspond to the effect that can be expected under real-life conditions. The two methods of analysis can yield very disparate results: In a trial of tree-pollen SIT, for example, the SMS improvement was found to be 38.9% by PP analysis, but only 11.5% by ITT analysis (28).
The selection of allergen products
One allergen preparation cannot be recommended over another, because the findings relating to products tested in different clinical trials cannot be compared. In general, the allergen to be used should be selected by a physician who has undergone further training in allergology within his or her specialty (e.g., internal medicine), or who has specialty certification in allergology itself. The recommendations of the current guideline (13) are as follows:
The efficacy of allergen preparations should be evaluated individually and independently of route of administration (e.g., subcutaneous or sublingual).
High-quality allergen extracts with demonstrated efficacy should be used.
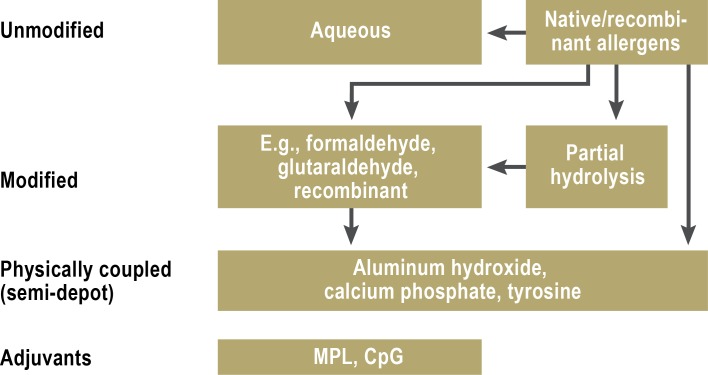
Allergen extracts are categorized as either unmodified (native) or modified (allergoids). The latter are proteins that have been treated with formaldehyde or glutaraldehyde to lower their binding affinity for IgE.
The selection of an allergen product.
The efficacy of allergen preparations should be evaluated individually and independently of route of administration, and high-quality allergen extracts of documented efficacy should be used.
In the extracts that are commonly used today, aluminum hydroxide is the most common adsorbent for prolonging release of the allergen. Some preparations additionally contain adjuvants to enhance the immunological effect (Figure).
Figure.
Allergen preparations for specific immunotherapy (SIT); modified from (21) MPL, monophosphoryl lipid A; CpG, cytosine phosphatidylguanine
Evidence.
The best evidence for the efficacy of SCIT and SLIT is for the treatment of allergic rhinoconjunctivitis with grass-pollen extracts. Patients should be informed of both options and choose between them in collaboration with the physician.
The best currently available evidence for the efficacy of SCIT and SLIT concerns the treatment of allergic rhinoconjunctivitis with grass-pollen extracts. Grass-pollen tablets (SLIT) have been officially approved for the treatment of adults and children on the basis of results from clinical trials. The German pediatric specialty society, however, currently hesitates to recommend SLIT because of concern about local adverse effects that are common at the start of treatment and that may interfere with long-term compliance. Long-term trials have been published that document the sustained effect of SLIT in adults after the end of treatment (29, 30). The efficacy of SLIT against allergic asthma, its potential preventive effect against asthma, and its potential preventive effect against new sensitization have not yet been adequately studied; large-scale prospective studies of these questions are currently in progress, and their findings should be published in the next few years (31). The state of the evidence for SLIT with other antigens is held to be inadequate by most specialists in Germany (13), although individual extracts from other allergen sources have achieved the status of an approved product. Information about the approval status of allergen preparations in Germany is available on the Internet (32, in German).
It is currently up to the allergist to evaluate the efficacy of allergen preparations. In the future, however, all newly approved allergen extracts will be subject to the requirements of the TAV, and their efficacy will have to be demonstrated in clinical trials whose validity will be assessed by the Paul Ehrlich Institute. Some allergen extracts are still available today whose efficacy cannot be evaluated at all because they have not been clinically tested.
The available evidence for the efficacy of SCIT and SLIT against grass-pollen allergy currently supports a positive recommendation for the preparations that have been shown to be effective in clinical trials. Patients should be informed about both treatment options so that they can make an informed choice between them in collaboration with the treating physician. (Under the new Patients’ Rights Law in Germany [Patientenrechtegesetz], patients have the right to participate in decision-making). For allergens of other sources, the evidence for SCIT is somewhat stronger and for this reason it is preferred by many allergists in Germany (Table 2). It bears pointing out once again that current evidence permits neither a comparison of SCIT against SLIT nor a comparison of different SCIT preparations with one another.
The indications for SIT
It is stated in the German guideline (13) that SIT is indicated when the causative allergen cannot be avoided, or when avoidance is not adequately effective; for further requirements, see Box 1.
Box 1. Preconditions for SIT*.
Sensitization to an aeroallergen and demonstration of clinical relevance; for perennial allergens, specific provocation is generally necessary (nasal, conjunctival, sometimes bronchial [in adults]).
SIT must have a documented effect against the disease to be treated; according to the current state of the evidence, this is now the case for allergic rhinoconjunctivitis and allergic asthma.
A suitable allergen extract must be available. The extract’s efficacy and adverse effects must be analyzed in clinical trials according to the specifications of the Paul Ehrlich Institute (double-blind, placebo-controlled trial of adequate size).
The patient must be willing and able to keep up with treatment regularly for three years.
*modified from (13); SIT, specific immunotherapy
Immunotherapy.
Immunotherapy can be recommended if the symptoms are moderate or severe and have been present for more than two years, and if allergen avoidance is impossible or insufficiently effective.
As a rule of thumb, immunotherapy can be recommended if the symptoms have been present for more than two years, if allergen avoidance is impossible or insufficiently effective, and if the patient suffers from moderate or severe symptoms that would call for long-term symptomatic treatment. There is no longer any reason to set age limits (in the past, immunotherapy was said to be indicated only for patients between the ages of 5 and 50). The treatment of children requires special consideration of the individual compliance factor; school-age children are generally capable of taking their medication as instructed. In elderly patients, the contraindications to SIT play an increasingly important role. These contraindications are derived from the potential dangers of treatment (Box 2).
Box 2. Contraindications of SIT*.
Partly controlled or uncontrolled asthma with FEV1 less than 70% of normal despite adequate treatment
Severe autoimmune diseases and immunodeficiency states
Current cancer
Beta-blockers (as eye drops as well) are, according to the guideline (13), a contraindication for SCIT, but not for SLIT. Nonetheless, the package inserts of certain high-dose preparations for SLIT name beta-blockers as a contraindication.
Cardiovascular diseases that elevate the risk of complications after the administration of epinephrine.
SIT should not be initiated during pregnancy, but an uncomplicated SIT may be continued if the patient becomes pregnant while SIT is ongoing.
*modified from (13); SIT, specific immunotherapy; SLIT, sublingual immunotherapy; SCIT, subcutaneous immunotherapy
The benefits and risks of the treatment should be weighed against each other for each patient. For medicolegal reasons, special attention should also be paid to all information contained in the package insert.
Predictors of the success of SIT
SIT is especially likely to succeed in patients who are sensitized against a single allergen and are in an early stage of disease. Thus, early treatment with SIT is recommended, particularly for children. The treatment is also effective, however, for patients sensitized against multiple allergens (33). For them, too, SIT is the only treatment than can have a lasting effect on the disease.
According to the guideline, patients may be treated with up to three allergen extracts at once (13). The selection is based on the severity and duration of symptoms, the degree to which they impair the patient’s quality of life, and the avoidability of the individual allergens.
Individual prediction of the response to SIT is currently not possible. A potential approach that is currently under discussion is to analyze IgE antibodies against allergen components and then treat patients who are found to be sensitized to major allergens (34, 35).
The duration of the therapeutic benefit of SIT is also hard to predict in individual cases. The longest published follow-up to date is for SCIT with a grass-pollen allergoid: The effect was still demonstrable 12 years after the end of treatment (36).
The recommendation to repeat the treatment if allergic rhinoconjunctivitis recurs after initially successful SIT is based on clinical experience, rather than on data from clinical trials, as adequate evidence of this type is not yet available.
The performance of SIT
The recommendations contained in published guidelines from Germany and other countries are based on evidence from clinical trials and meta-analyses (10– 19).
The success of SIT.
SIT is likely to succeed in patients who are sensitized against a single antigen in an early stage of disease, but it is also effective in those sensitized against multiple allergens. SIT is the only treatment with a lasting effect on the disease.
The treatment begins at a low dose, which is gradually increased until the maintenance dose is reached. This process may take up to 20 weeks with native allergens; with allergoids, a single day may suffice. Clinical trials have been conducted to test the efficacy of treatment with allergoid extracts of grass pollen (37– 39), tree pollen (40), and mites (e1, e2).
Depending on the preparation, the treatment with with pollen extract can be given either before the allergy season or year-round; some allergoids have been approved for both treatment schedules. No adequate comparison of these two treatment schedules has yet been published.
SCIT.
The adverse effects of SCIT include allergic reactions ranging all the way to severe anaphylaxis. The physician’s office must be properly equipped for the treatment of anaphylaxis, and the injection must be carried out by the physician.
The adverse effects of SCIT include allergic reactions that can range all the way to severe anaphylaxis. No official statistics on severe reactions have been published, but cases of fatal anaphylaxis are known to have occurred in recent years. The physician must inform the patient thoroughly of all possible adverse effects (including generalized skin reactions, systemic reactions, and anaphylaxis) and document in writing that this information has been provided. The injection must be performed by the physician, who must have experience in the treatment of systemic allergic reactions. The physician’s office should be properly equipped for the treatment of anaphylaxis, with the appropriate medications and equipment close at hand. As a minimum, the following must be available:
a defibrillator
an IV infusion set
epinephrine
an antihistamine
an inhalable beta-2-sympathomimetic drug
a corticosteroid for intravenous administration.
The patient should be asked about current contraindications before every injection (Box 3). 30 minutes of observation after each injection are obligatory. Intense physical activity shortly before or after the injection may induce an anaphylactic reaction and should therefore be strictly avoided.
Box 3. Obligatory questions before each injection.
Did you have any adverse effects from the last injection?
Do you have any allergy symptoms now?
Are you now suffering from any new medical condition?
Are you now taking any other medication?
SLIT is administered either year-round (pre-saisonal) or before and during the allergy season (pre/co-saisonal). In patients who are to receive high-dose preparations, the initial administration must be performed under medical supervision. The patient must have an intact oral mucosa, as mucosal injuries may promote adverse effects. After dental extractions or gingival treatments, SLIT should be temporarily interrupted. SLIT should not be administered immediately after tooth-brushing.
Pretreatment with an antihistamine is a useful means of diminishing the local reactions that often arise at the beginning of treatment. SLIT causes mild adverse effects more commonly, but severe ones less commonly, than SCIT. To our knowledge, fatal adverse reactions have never been reported for SLIT.
Injuries of the oral mucosa and SLIT.
Patients taking SLIT must have an intact oral mucosa, as mucosal injuries promote adverse effects.
Whatever the mode of administration, SIT should be continued for at least three years. It has been shown for SCIT that longer treatment confers no statistically significant additional benefit (e3).
It is recommended that the treatment be continued until no further therapeutic benefit is observable, or for one further year after complete relief of symptoms has been achieved.
If allergic symptoms recur after initially successful SIT, repetition of the treatment is recommended.
The ideal time to start SIT
There was an earlier recommendation to start SIT before the allergy season. Updosing during the pollen season should be avoided, however, due to a potentially increased risk of adverse effects.
No studies have yet been published addressing the question of when the effect of SCIT sets in. For SLIT with a grass-pollen tablet, statistically significant efficacy has been documented 30 days after the start of treatment (e4).
The effect of concurrent medication on the efficacy of SIT
An effect of concurrent treatment on the efficacy of SIT has not been systematically studied in clinical trials. Immune suppressants would presumably diminish the treatment effect, and, as a rule, immune-suppressed or immunocompromised patients should not undergo SIT. In a small-scale trial in which children with asthma and house-dust mite allergy were treated with SIT with a house-dust mite extract, simultaneous treatment with montelukast apparently lowered the efficacy of SIT (e5).
There is insufficient evidence for further recommendations to avoid or specifically add any particular medication.
Adjuvants
The purpose of adding adjuvants is to enhance the immunological effect of SIT by modulating the allergen-specific T-cell response in the direction of tolerance. For this purpose an allergen extract containing monophosphoryl lipid A is available in Germany. The postulated enhancement of the immunological effect by the bacterial molecule contained in this preparation cannot be judged at present, as there has been no comparative clinical study of the efficacy of allergen treatment with and without added adjuvants.
Adverse effects of SLIT.
SLIT causes mild adverse effects more commonly, but severe ones less commonly, than SCIT.
In animals, aluminum hydroxide has been found to to have strong adjuvant effects (e6). No tests have yet been performed to determine whether it might act as one in man as well.
Other molecules that might be useful as adjuvants are now being clinically tested.
Recombinant allergens
The major allergens of the main inhaled allergen sources have been characterized and can be produced with recombinant techniques. The problem of standardization can thus be solved for SIT, as large amounts of these proteins can be produced with consistent quality. Clinical trials of the use of recombinant grass pollen, birch pollen, and cat allergens have been published.
In a proof-of-concept study, SCIT with a mixture of five recombinant grass-pollen proteins led to a 38.5% reduction in SMS compared to placebo (e7).
15 µg of natural or recombinant Bet v 1 were as effective as treatment with a native birch-pollen extract containing 15 µg of Bet v 1 (e8).
Experimental alternatives.
Recently, intralymphatic immunotherapy (ILIT) and epicutaneous allergen application have been described. In a proof-of-concept study, three applications of a very small amount of allergen in an inguinal lymph node sufficed for effective treatment.
By analogy with the production of allergoids, the three-dimensional structure of recombinant proteins can be modified. Testing of a modified Bet v 1 molecule (folding variant) has shown it to be clinically effective and well tolerated at a high dose (80 µg) (e9).
Peptides containing the necessary T-cell epitopes are promising substances for treatment with relatively few adverse effects once the IgE-binding epitopes that cause adverse effects have been eliminated. This approach is used in the treatment of cat allergy (e10).
Modes of administration
Both the subcutaneous and the sublingual administration of allergen extracts are now well established. Recently, intralymphatic immunotherapy (ILIT) and epicutaneous allergen application have been described as potential alternatives. In a proof-of-concept study, three injections of very small allergen amounts in an inguinal lymph node sufficed for effective treatment (e11). Epicutaneous allergen application has also been found to have an effect (e12). It is not yet clear whether allergen preparations for intralymphatic or epicutaneous application will become commercially available.
SIT.
If the patient’s symptoms are clearly connected to allergen exposure, SIT should be offered as the standard treatment. To be successful, SIT must be given for at least three years.
Concluding remarks
SCIT and SLIT are now the only treatments for allergic diseases that induce long-lasting disease modification with sustained improvement of symptoms. The therapeutic potential of SIT is not reflected by its current use in Germany. Patients with allergies are undertreated here. All allergic patients should undergo an appropriate diagnostic evaluation followed by appropriate allergy treatment, and SIT should be offered as the standard treatment for suitable patients.
Allergen preparations with documented safety and efficacy are to be preferred. The new German Regulation on Therapeutic Allergens (TAV) ought to simplify the choice of suitable preparations.
To be successful, SIT should be given for an adequate period of time (at least three years). Recent surveys indicate, however, that less than half of all patients who start SIT continue to receive it for the full three years (e25). The main reasons for premature termination of treatment are apparently adverse effects, insufficient efficacy, and the expenditure of time. Means of improving patient compliance need to be developed.
Table 3. SCIT versus SLIT: the available evidence.
| Studies on: | SCIT | SLIT |
| Dose-effect relationship | studied for various allergens (14) | studied for various allergens (14) |
| Definition of the optimal dose | documented in one DBPC trial of a mite allergen (23) | documented in one DBPC trial of a grass-pollen tablet (24) |
| Efficacy after 1 year of treatment | determined for multiple allergens in DBPC trials, some of which were on a large scale (10, 37, 39, 40, e1, e2) | determined in large-scale DBPC trials for grass-pollen extracts (11, 26, 30, e13– e19) |
| Efficacy after 2 and 3 years of treatment | shown in trials of various allergens (e3) | shown in large-scale DBPC trials of grass-pollen extracts (29, 30, e13) |
| Sustained therapeutic benefit | shown in multiple trials, most of which were controlled (6, 24, 36, e20) | shown in trials of adequate size for grass-pollen extracts for adults (27, 28) |
| Efficacy for allergic asthma | shown for various allergens (39) | small effect in meta-analysis (e21) |
| Asthma prevention | no DBPC trials; positive findings in controlled trials (e22) | no DBPC trials; positive findings (e23) |
| Prevention of new sensitization | shown in controlled trials of individual allergens (e22) | no DBPC trials; positive findings (e24) |
SCIT, subcutaneous immunotherapy; SLIT, sublingual immunotherapy; DBPC, double-blind, placebo-controlled
Further Information on CME.
This article has been certified by the North Rhine Academy for Postgraduate and Continuing Medical Education. Deutsches Ärzteblatt provides certified continuing medical education (CME) in accordance with the requirements of the Medical Associations of the German federal states (Länder). CME points of the Medical Associations can be acquired only through the Internet, not by mail or fax, by the use of the German version of the CME questionnaire within 6 weeks of publication of the article. See the following website: cme.aerzteblatt.de
Participants in the CME program can manage their CME points with their 15-digit “uniform CME number” (einheitliche Fortbildungsnummer, EFN). The EFN must be entered in the appropriate field in the cme.aerzteblatt.de website under “meine Daten” (“my data”), or upon registration. The EFN appears on each participant’s CME certificate.
The CME unit “Postoperative Care and Follow-up After Coronary Stenting“ (Issue 5/2013) can be accessed until 15 March 2013. For issue 13/2013, we plan to offer the topic “The Prevention, Diagnosis, and Treatment of Premature Labor.”
Solutions to the CME questions in Issue 1–2/2013:
Scharhag J, Löllgen H, Kindermann W: Competitive Sports and the Heart: Benefit or Risk?
Solutions: 1c, 2a, 3c, 4c, 5e, 6c, 7e, 8c, 9b, 10d\
Please answer the following questions to participate in our certified Continuing Medical Education program. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
Which of these situations is the best indication for SIT?
When symptomatic treatment is no longer adequate
When symptoms of asthma are present
When the patient is allergic to multiple allergens
When marked symptoms are present in the early stage of the disease
After the first season of symptoms
Question 2
Which of the following is a requirement for the approval of future drug products for use as treatment allergens?
Their efficacy must be tested in large-scale clinical trials.
They must consist of a mixture of multiple allergen extracts.
There must be a standardized means of determining the quantity of major allergen.
They must be characterized with electrochemical testing.
The manufacturer must provide information about extract strength.
Question 3
How can the efficacy of allergen preparations be evaluated?
By the allergen concentration declared by the manufacturer
By the concentration of a major allergen
By clinical trials
By observations from clinical practice
By in vitro testing
Question 4
Which of the following is a contraindication for SCIT with pollen extracts?
The use of beta-blocker eye drops
Sensitization to multiple allergens
Longstanding allergy
Mild allergic asthma
A history of breast cancer, without recurrence
Question 5
For what type of extract is there the best evidence for efficacy against allergy, with either SCIT or SLIT?
Birch
Rye
Wheat
Grass pollen
Hazelnut
Question 6
For what condition is SIT clearly indicated?
Atopic dermatitis
Mild, intermittent rhinoconjunctivitis
Severe asthma
Persistent allergic rhinoconjunctivitis
Pollen-associated food allergy
Question 7
What is the main advantage of recombinant allergens?
They can be precisely standardized.
They are more effective.
They are better tolerated.
They are easier to produce.
They have a longer shelf-life.
Question 8
For what condition(s) has sublingual immunotherapy been shown to be effective?
Allergic asthma in children
Mainly for rhinoconjunctivitis due to grass-pollen allergy
For the prevention of new sensitization
For allergic asthma in adults
For all allergens
Question 9
Which of the following is particularly important for the evaluation of a potential causal link between a perennial allergen and rhinoconjunctivitis, as opposed to seasonal allergens?
A nasal provocation test
A prick test
The demonstration of specific IgE antibodies
An intracutaneous test
An epicutaneous test
Question 10
After how many days of treatment has SLIT with a grass-pollen tablet been shown to have a statistically significant benefit?
25 days
30 days
35 days
40 days
45 days
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Prof. Brehler has received consultant’s fees and reimbursement of participation fees for scientific meetings from Allergopharma, Novartis, and Stallergenes. He has received reimbursement of travel and accommodation expenses from Allergopharma, Novartis, Stallergenes, and ALK. He has also received honoraria for the preparation of continuing medical education presentations from Allergopharma, Novartis, Stallergenes, Bencard, and HAL. He has received payment for the performance of clinical trials on behalf of Allergopharma, Novartis, Leti, HAL, Stallergenes and ALK (funds deposited in an account of his hospital department for external research support).
Prof. Klimek has received consultant’s fees from ALK, Allergopharma, Bencard, Boehringer, Lofarma, and Novartis. He has also received reimbursement of participation fees in scientific meetings and continuing medical education events, and of travel expenses, from ALK, Allergopharma, Bencard, HAL, Novartis, Lofarma, Roxall, and Stallergenes. He has received payment for the preparation of continuing medical education presentations from ALK, Allergopharma, Bencard, GSK, HAL, Lofarma, Novartis, Roxall, and Stallergenes. He has received honoraria for carrying out clinical trials on behalf of ALK, Allergopharma, ArtuBiologicals, Bencard, Biomay, Bionorica, BMBF, Cytos, EAACI, HAL, GSK, Leti, Lofarma, Novartis, PierreFabre, Roxall, Ursapharm, Teva, Stallergenes, and Zambon. He has received payment for a research project from Bencard, ALK, Allergopharma, and HAL.
Prof. Kopp has received consultant’s fees from Bencard, ALK, Abelló, and Novartis. He has received honoraria for preparing continuing medical education events from Bencard and Novartis and for performing clinical trials on behalf of Novartis.
Prof. Virchow has received consultant’s fees from Allergopharma, ALK, and Stallergenes.
References
- 1.Asher MI, Montefort S, Björkstén B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 2.Björkstén B, Clayton T, Ellwood P, Stewart A, Strachan D. ISAAC Phase III Study Group Worldwide time trends for symptoms of rhinitis and conjunctivitis: Phase III of the International Study of Asthma and Allergies in Childhood. Pediatr Allergy Immunol. 2008;19:110–124. doi: 10.1111/j.1399-3038.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- 3.Ring J, Bachert C, Bauer C P, Czech W, editors. München: Urban & Vogel; 2009. Weißbuch Allergie in Deutschland. [Google Scholar]
- 4.Maurer M, Zuberbier T. Undertreatment of rhinitis symptoms in Europe: findings from a cross-sectional questionnaire survey. Allergy. 2007;62:1057–1063. doi: 10.1111/j.1398-9995.2007.01367.x. [DOI] [PubMed] [Google Scholar]
- 5.FORSA. Umfrage. www.derma.de/fileadmin/eingang/FORSA_Allergien.de. 2012.
- 6.Jacobsen L, Niggemann B, Dreborg S, et al. (The PAT investigator group). Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–948. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 7.Calderon MA, Gerth van Wijk R, Eichler I, et al. Perspectives on allergen-specific immunotherapy in childhood: an EAACI position statement. Pediatr Allergy Immunol. 2012;23:300–306. doi: 10.1111/j.1399-3038.2012.01313.x. [DOI] [PubMed] [Google Scholar]
- 8.Zuberbier T, Lötvall J. Allergies have a socioeconomic impact: a model calculation. Allergy. 2008;63(Suppl. 88):612–621. [Google Scholar]
- 9.Graf von der Schulenburg JM, Greiner W, Molitor S, Kielhorn A. Medizinische Ökonomie - Kosten der Asthmatherapie nach Schweregrad, Eine empirische Untersuchung. Medizinische Klinik. 1996;91:670–676. [PubMed] [Google Scholar]
- 10.Calderon MA, Alves B, Jacobson M, Hurwitz B, Sheikh A, Durham S. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database of Systematic Reviews. 2007;(Issue 1) doi: 10.1002/14651858.CD001936.pub2. Art. No.: CD001936. DOI: 10.1002/14651858.CD001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radulovic S, Calderon MA, Wilson D, Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2010;12 doi: 10.1002/14651858.CD002893.pub2. CD002893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database of Systematic Reviews. 2010;(Issue 8) doi: 10.1002/14651858.CD001186.pub2. Art. No.: CD001186. DOI: 10.1002/14651858.CD001186.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Kleine-Tebbe J, Bufe A, Ebner C, et al. Leitlinien der Deutschen Gesellschaft Allergologie und klinische Immunologie (DGAKI), des Ärzteverbandes Deutscher Allergologen (ÄDA), der Gesellschaft für pädiatrische Allergologie und Umweltmedizin (GPA), der Österreichischen Gesellschaft für Allergologie und Immunologie (ÖGAI) und der Schweizerischen Gesellschaft für Allergologie und Immunologie (SGAI). Die spezifische Immuntherapie (Hyposensibilisierung) bei IgE vermittelten allergischen Erkrankungen. Allergo J. 2009;18:508–537. [Google Scholar]
- 14.Calderon MA, Larenas D, Kleine-Tebbe J, et al. European Academy of Allergy and Clinical Immunology task force report on ’dose-response relationship in allergen-specific immunotherapy’. Allergy. 2011;66:1345–1359. doi: 10.1111/j.1398-9995.2011.02669.x. [DOI] [PubMed] [Google Scholar]
- 15.Malling HJ, Abreu-Nogueira J, Alvarez-Cuesta E, et al. Local immunotherapy. Allergy. 1998;53:933–944. doi: 10.1111/j.1398-9995.1998.tb03793.x. [DOI] [PubMed] [Google Scholar]
- 16.Zuberbier T, Bachert C, Bousquet PJ, et al. GA2 LEN/EAACI pocket guide for allergen-specific immunotherapy for allergic rhinitis and asthma. Allergy. 2010;65:1525–1530. doi: 10.1111/j.1398-9995.2010.02474.x. [DOI] [PubMed] [Google Scholar]
- 17.Bousquet J, Lockey R, Malling HJ. WHO position paper. Allergen immunotherapy: therapeutic vaccines for allergic diseases. Allergy. 1998;53(Suppl 54):1–42. doi: 10.1016/s0091-6749(98)70271-4. [DOI] [PubMed] [Google Scholar]
- 18.Canonica GW, Bousquet J, Casale T, et al. Sublingual immunotherapy: World Allergy Organization Position Paper 2009. Allergy. 2009 64;(Suppl 91):1–59. doi: 10.1111/j.1398-9995.2009.02309.x. [DOI] [PubMed] [Google Scholar]
- 19.Walker SM, Durham SR, Till SJ, et al. British Society for Allergy and Clinical Immunology; Immunotherapy for allergic rhinitis. Clin Exp Allergy. 2011;41:1177–1200. doi: 10.1111/j.1365-2222.2011.03794.x. [DOI] [PubMed] [Google Scholar]
- 20.Guidline on allergen products. Production and quality issues des comittee for medical products for human use (CHMP) www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003333.pdf.
- 21.Guidline on the clinical development of products for specific immunotherapy for the treatment of allergic diseases des comittee for medical products for human use (CHMP) www.emea.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003605.pdf.
- 22.Matricardi PM, Kuna P, Panetta V, Wahn U, Narkus A. Subcutaneous immunotherapy and pharmacotherapy in seasonal allergic rhinitis: a comparison based on meta-analyses. J Allergy Clin Immunol. 2011;128:791–799. doi: 10.1016/j.jaci.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 23.Fujita H, Soyka MB, Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. Clin Transl Allergy. 2012;2 doi: 10.1186/2045-7022-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.James LK, Shamji MH, Walker SM, et al. Long-term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J Allergy Clin Immunol. 2011;127:509–516. doi: 10.1016/j.jaci.2010.12.1080. [DOI] [PubMed] [Google Scholar]
- 25.Haugaard L, Dahl R, Jacobsen L. A controlled dose-response study of immunotherapy with standardized, partially purified extract of house dust mite: Clinical efficacy and side effects. J Allergy Clin Immunol. 1993;91:709–722. doi: 10.1016/0091-6749(93)90190-q. [DOI] [PubMed] [Google Scholar]
- 26.Didier A, Malling HJ, Worm M, Horak F, et al. Optimal dose, efficacy, and safety of once-daily sublingual immunotherapy with a 5-grass pollen tablet for seasonal allergic rhinitis. J Allergy Clin Immunol. 2007;120:1338–1345. doi: 10.1016/j.jaci.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 27.Chapman MD, Ferreira F, Villalba M, et al. CREATE consortium. The European Union CREATE project: a model for international standardization of allergy diagnostics and vaccines. J Allergy Clin Immunol. 2008;122:882–889. doi: 10.1016/j.jaci.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 28.Pfaar O, Robinson DS, Sager A, Emuzyte R. Immunotherapy with depigmented-polymerized mixed tree pollen extract: a clinical trial and responder analysis. Allergy. 2010;65:1614–1621. doi: 10.1111/j.1398-9995.2010.02413.x. [DOI] [PubMed] [Google Scholar]
- 29.Durham SR, Emminger W, Kapp A, et al. SQ-standardized sublingual grass immunotherapy: Confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol. 2012;129:717–725. doi: 10.1016/j.jaci.2011.12.973. [DOI] [PubMed] [Google Scholar]
- 30.Ott H, Sieber J, Brehler R, Fölster-Holst R, Kapp A, Klimek L, Pfaar O, Merk H. Efficacy of grass pollen sublingual immunotherapy for three consecutive seasons and after cessation of treatment: the ECRIT study. Allergy. 2009;64:1394–1401. doi: 10.1111/j.1398-9995.2009.02194.x. [DOI] [PubMed] [Google Scholar]
- 31.Valovirta E, Berstad AK, de Blic J, et al. GAP investigators. Design and recruitment for the GAP trial, investigating the preventive effect on asthma development of an SQ-standardized grass allergy immunotherapy tablet in children with grass pollen-induced allergic rhinoconjunctivitis. Clin Ther. 2011;33:1537–1546. doi: 10.1016/j.clinthera.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 32.Paul-Ehrlich Institut. Therapieallergene. www.pei.de/DE/arzneimittel/allergene/therapieallergene/therapieallergene-inhalt.html. 2013. Last accessed on 12 February.
- 33.Calderón MA, Cox L, Casale TB, Moingeon P, Demoly P. Multiple-allergen and single-allergen immunotherapy strategies in polysensitized patients: Looking at the published evidence. J Allergy Clin Immunol. 2012;129:924–934. doi: 10.1016/j.jaci.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 34.Valenta R, Laffer S, Vrtala S, et al. Recombinant allergens. Steps on the way to diagnosis and therapy of type I allergy. Adv Exp Med Biol. 1996;409:185–196. [PubMed] [Google Scholar]
- 35.Schmid-Grendelmeier P. Recombinant allergens. For routine use or still only science? Hautarzt. 2010;61:946–953. doi: 10.1007/s00105-010-1967-y. [DOI] [PubMed] [Google Scholar]
- 36.Eng PA, Borer-Reinhold M, Heijnen IA, Gnehm HP. Twelve-year follow-up after discontinuation of preseasonal grass pollen immunotherapy in childhood. Allergy. 2006;61:198–201. doi: 10.1111/j.1398-9995.2006.01011.x. [DOI] [PubMed] [Google Scholar]
- 37.Corrigan CJ, Kettner J, Doemer C, Cromwell O, Narkus A, Study Group. Efficacy and safety of preseasonal-specific immunotherapy with an aluminium-adsorbed six-grass pollen allergoid. Allergy. 2005;60:801–807. doi: 10.1111/j.1398-9995.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 38.DuBuske LM, Frew AJ, Horak F, et al. Ultrashort-specific immunotherapy successfully treats seasonal allergic rhinoconjunctivitis to grass pollen. Allergy Asthma Proc. 2011;32:239–247. doi: 10.2500/aap.2011.32.3453. [DOI] [PubMed] [Google Scholar]
- 39.Pfaar O, Urry Z, Robinson DS, et al. A randomized placebo-controlled trial of rush preseasonal depigmented polymerized grass pollen immunotherapy. Allergy. 2012;67:272–279. doi: 10.1111/j.1398-9995.2011.02736.x. [DOI] [PubMed] [Google Scholar]
- 40.Pfaar O, Robinson DS, Sager A, Emuzyte R. Immunotherapy with depigmented-polymerized mixed tree pollen extract: a clinical trial and responder analysis. Allergy. 2010;65:1614–1621. doi: 10.1111/j.1398-9995.2010.02413.x. [DOI] [PubMed] [Google Scholar]
- e1.Zielen S, Kardos P, Madonini E. Steroid-sparing effects with allergen-specific immunotherapy in children with asthma: a randomized controlled trial. J Allergy Clin Immunol. 2010;126:942–949. doi: 10.1016/j.jaci.2010.06.002. [DOI] [PubMed] [Google Scholar]
- e2.Riechelmann H, Schmutzhard J, van der Werf JF, Distler A, Kleinjans HA. Efficacy and safety of a glutaraldehyde-modified house dust mite extract in allergic rhinitis. Am J Rhinol Allergy. 2010;24:104–109. doi: 10.2500/ajra.2010.24.3508. [DOI] [PubMed] [Google Scholar]
- e3.Durham SR, Walker SM, Varga EM, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–475. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- e4.Horak F, Zieglmayer P, Zieglmayer R, et al. Early onset of action of a 5-grass-pollen 300-IR sublingual immunotherapy tablet evaluated in an allergen challenge chamber. J Allergy Clin Immunol. 2009;124:471–477. doi: 10.1016/j.jaci.2009.06.006. [DOI] [PubMed] [Google Scholar]
- e5.Majak P, Rychlik B, Pulaski L, et al. Montelukast treatment may alter the early efficacy of immunotherapy in children with asthma. J Allergy Clin Immunol. 2010;125:1220–1227. doi: 10.1016/j.jaci.2010.02.034. [DOI] [PubMed] [Google Scholar]
- e6.Rask C, Lund L, Lund G. An alternative allergen: adjuvant formulation potentiates the immunogenicity and reduces allergenicity of a novel subcutaneous immunotherapy product for treatment of grass-pollen allergy. Clin Exp Allergy. 2012;42:1356–1356. doi: 10.1111/j.1365-2222.2012.04026.x. [DOI] [PubMed] [Google Scholar]
- e7.Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005;116:608–613. doi: 10.1016/j.jaci.2005.06.004. [DOI] [PubMed] [Google Scholar]
- e8.Pauli G, Larsen TH, Rak S, et al. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2008;122:951–960. doi: 10.1016/j.jaci.2008.09.017. [DOI] [PubMed] [Google Scholar]
- e9.Kettner J, Meyer H, Cromwell O, Narkus A, Jost K. Specific immunotherapy with recombinant birch pollen allergen rBet v 1-FV results of 2 years treatment (Phase II trial) Allergy. 2007;62 [Google Scholar]
- e10.Worm M, Lee HH, Kleine-Tebbe J, et al. Development and preliminary clinical evaluation of a peptide immunotherapy vaccine for cat allergy. J Allergy Clin Immunol. 2011;127:89–97. doi: 10.1016/j.jaci.2010.11.029. [DOI] [PubMed] [Google Scholar]
- e11.Senti G, Prinz Vavricka BM, Erdmann I , et al. Intralymphatic allergen administration renders specific immunotherapy faster and safer: a randomized controlled trial. Proc Natl Acad Sci U S A. 2008;105:17908–17912. doi: 10.1073/pnas.0803725105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e12.Senti G, von Moos S, Tay F, et al. Epicutaneous allergen-specific immunotherapy ameliorates grass pollen-induced rhinoconjunctivitis: A double-blind, placebo-controlled dose escalation study. J Allergy Clin Immunol. 2012;129:128–135. doi: 10.1016/j.jaci.2011.08.036. [DOI] [PubMed] [Google Scholar]
- e13.Nelson HS, Nolte H, Creticos P, Maloney J, Wu J, Bernstein DI. Efficacy and safety of timothy grass allergy immunotherapy tablet treatment in North American adults. J Allergy Clin Immunol. 2011;127:72–80. doi: 10.1016/j.jaci.2010.11.035. [DOI] [PubMed] [Google Scholar]
- e14.Blaiss M, Maloney J, Nolte H, Gawchik S, Yao R, Skoner DP. Efficacy and safety of timothy grass allergy immunotherapy tablets in North American children and adolescents. J Allergy Clin Immunol. 2011;127:64–71. doi: 10.1016/j.jaci.2010.11.034. [DOI] [PubMed] [Google Scholar]
- e15.Pfaar O, Klimek L. Efficacy and safety of specific immunotherapy with a high-dose sublingual grass pollen preparation: a double-blind, placebo-controlled trial. Ann Allergy Asthma Immunol. 2008;100:256–263. doi: 10.1016/s1081-1206(10)60451-6. [DOI] [PubMed] [Google Scholar]
- e16.Mösges R, Brüning H, Hessler HJ, Götz G, Knaussmann HG. Sublingual immunotherapy in pollen-induced seasonal rhinitis and conjunctivitis: a randomized controlled trial. Acta Dermatovenerol Alp Panonica Adriat. 2007;16:143–148. [PubMed] [Google Scholar]
- e17.Wahn U, Tabar A, Kuna P, et al. Efficacy and safety of 5-grass-pollen sublingual immunotherapy tablets in pediatric allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2009;123:160–166. doi: 10.1016/j.jaci.2008.10.009. [DOI] [PubMed] [Google Scholar]
- e18.Wahn U, Klimek L, Ploszczuk A, et al. High-dose sublingual immunotherapy with single-dose aqueous grass pollen extract in children is effective and safe: A double-blind, placebo-controlled study. J Allergy Clin Immunol. 2012;130:886–893. doi: 10.1016/j.jaci.2012.06.047. [DOI] [PubMed] [Google Scholar]
- e19.Worm M. Efficacy and tolerability of high dose sublingual immunotherapy in patients with rhinoconjunctivitis. Eur Ann Allergy Clin Immunol. 2006;38:55–60. [PubMed] [Google Scholar]
- e20.Tabar AI, Arroabarren E, Echechipía S, García BE, Martin S, Alvarez-Puebla MJ. Three years of specific immunotherapy may be sufficient in house dust mite respiratory allergy. J Allergy Clin Immunol. 2011;127:57–63. doi: 10.1016/j.jaci.2010.10.025. [DOI] [PubMed] [Google Scholar]
- e21.Calamita Z, Saconato H, Pelá AB, Atallah AN. Efficacy of sublingual immunotherapy in asthma: systematic review of randomized-clinical trials using the Cochrane Collaboration method. Allergy. 2006;61:1162–1172. doi: 10.1111/j.1398-9995.2006.01205.x. [DOI] [PubMed] [Google Scholar]
- e22.Jacobsen L, Wahn U, Bilo MB. Allergen-specific immunotherapy provides immediate, long-term and preventive clinical effects in children and adults: the effects of immunotherapy can be categorised by level of benefit—the centenary of allergen specific subcutaneous immunotherapy. Clinical and Translational Allergy. 2012;2 doi: 10.1186/2045-7022-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e23.Novembre E, Galli E, Landi F, et al. Coseasonal sublingual immunotherapy reduces the development of asthma in children with allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2004;114:851–857. doi: 10.1016/j.jaci.2004.07.012. [DOI] [PubMed] [Google Scholar]
- e24.Des Roches A, Paradis L, Knani J, et al. Immunotherapy with a standardized Dermatophagoides pteronyssinus extract. VI. Specific immunotherapy prevents the onset of new sensitizations in children. J Allergy Clin Immunol. 1997;99:450–453. doi: 10.1016/s0091-6749(97)70069-1. [DOI] [PubMed] [Google Scholar]
- e25.Sieber J, De Geest S, Shah-Hosseini K, Mösges R. Medication persistence with long-term, specific grass pollen immunotherapy measured by prescription renewal rates. Curr Med Res Opin. 2011;27:855–861. doi: 10.1185/03007995.2011.559538. [DOI] [PubMed] [Google Scholar]