Abstract
In concert with the development of new materials in the last decade, the need for toxicological studies of these materials has been increasing. These new materials include a group of rare earths (RE). The use of RE nanotechnology is being considered in some green applications, to increase their efficiency by using nano-sized RE compounds, and therefore hazard evaluation and risk assessment are highly recommended. This review was conducted through an extensive contemplation of the literatures in toxicology with in vitro and in vivo studies. Major aspects reviewed were the toxicological evaluations of these elements and metallic compounds at the molecular and cellular level, animal and human epidemiological studies and environmental and occupational health impacts on workers. We also discuss the future prospect of industries with appliances using RE together with the significance of preventive efforts for workers' health. To establish a safe and healthy working environment for RE industries, the use of biomarkers is increasing to provide sustainable measure, due to demand for information about the health risks from unfavorable exposures. Given the recent toxicological results on the exposure of cells, animals and workers to RE compounds, it is important to review the toxicological studies to improve the current understanding of the RE compounds in the field of occupational health. This will help to establish a sustainable, safe and healthy working environment for RE industries.
Keywords: Rare earths, Toxicology, Environmental health, Occupational health
Introduction
The rare earth elements (RE) are a group of metals comprised of yttrium, fourteen lanthanide elements, and sometimes scandium. Their unique physical and chemical properties have rendered them indispensable for a growing number of critical technologies. For example, neodymium is vital to high-performance permanent magnets, and yttrium is a promising raw material for superconductors and laser technology [1].
RE exist in a wide range of mineral types, including halides, carbonates, oxides, phosphates and silicates. Cerium, the dominant RE, is used in catalytic converters in cars, enabling them to run at high temperatures, and plays a crucial role in the chemical reactions in the converter. Lanthanum is used in camera and telescope lenses. Compounds containing lanthanum are used extensively in carbon lighting applications, such as studio lighting and cinema projection. Neodymium is used to make the powerful magnets used in loudspeakers and computer hard drives, to enable them to be smaller and more efficient. Magnets containing neodymium are also used in green technologies, such as the manufacture of wind turbines and hybrid cars. Praseodymium is used to create strong metals for aircraft engines, and it is also a component of a special sort of glass, used to make visors to protect welders and glassmakers. Gadolinium is used in X-ray, magnetic resonance image scanning systems, and also in television screens. Yttrium, terbium and europium are important in making televisions, computer screens and other devices that have visual displays, as they are used in making materials that give off different colors. Europium is also used in making control rods in nuclear reactors [2]. The commercial applications of RE are summarized in Table 1.
Table 1.
Commercial applications of rare earths
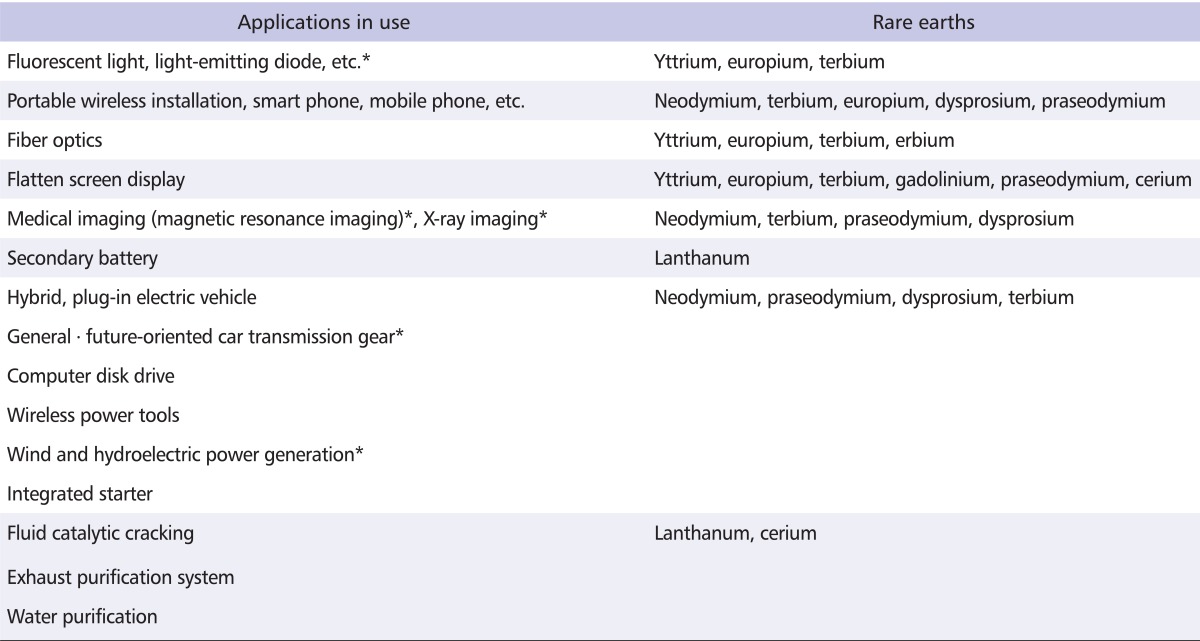
*Can apply to green technologies, including fluid catalytic cracking.
Nevertheless, there are many environmental issues associated with RE production. Reports indicate that the chemicals used in the refining process have been responsible for the disease and occupational poisoning of local residents, water pollution, and the destruction of farmland. Occupational and public safety and health risks related to the rare earths may be addressed at their mining, transportation, processing, and waste disposal, as well as decommissioning, stages [3]. The possible contaminants cause negative effects on aquatic and terrestrial organisms, as well as on humans. In some cases, they increase the mortality rates of aquatic and terrestrial organisms [4]; and some of the radionuclides and metals contaminants are even classified by international and federal health agencies as human carcinogens.
Given the recent toxicological results on the exposure of cells, animals and workers to rare earth compounds, it is important to review the toxicological studies, in order to improve the current understanding of the rare earth compounds. This will also help to establish a sustainable, safe and healthy working environment for the RE industries.
Methods
This study was conducted through an extensive review of the literature. Literature review techniques were used to find relevant articles in the toxicology, with in vitro and in vivo studies, industrial hygiene, and epidemiologic literature.
Extensive Internet searching was used as the primary tool for this review. Various websites, including Google Scholar (http://scholar.google.com), ScienceDirect (www.sciencedirect.com), and PubMed (http://www.ncbi.nlm.nih.gov/pubmed/), were also searched. Searches were conducted using keywords similar to the following: rare earth AND toxicology AND worker OR environment OR occupation AND health OR industry. These searches yielded more than 100 references. The references were reviewed further for information regarding occupational or environmental aspects. As a result of this further examination, 84 citations were deemed relevant to this study, and are included as references in this report.
The major aspects reviewed were the toxicological evaluations of these elements and metallic compounds at the molecular and cellular level, animal and human epidemiological studies, and environmental and occupational health impacts on workers. Part of the literature review also included gathering information on the chemical and toxicological properties of each compound of interest.
Specifically, material safety data sheets (MSDS) were collected from various publicly available Internet sites, such as KOSHANET, as shown in the tables, in which detailed information has been collected and summarized.
We also discuss the future prospects of industries with appliances using rare earths, and the significance of preventive efforts for workers' health.
Results
For the past three decades, most attention in heavy metal toxicology has been paid to cadmium, mercury, lead, chromium, nickel and tin, because these metals have widely polluted the environment. However, with the development in the last decade of new materials, the need for toxicological studies of these materials has been increasing. These new materials include a group of RE. Although some RE have been used for superconductors, plastic magnets, and ceramics, few toxicological data are available, in comparison with other heavy metals. In this review, we present an overview of the health hazards of RE and related compounds, including recent studies.
Historical background of occupational health research with RE
A case of RE pneumoconiosis is described of a man working in a lithographic laboratory as a photoengraver, exposed to the smoke of cored carbon arc lamps over a period of 46 years, who developed an interstitial pneumoconiosis. The findings strongly suggest that the pneumoconiosis diagnosis has to do with occupational exposure to RE dusts, and calls attention to proposals for maximum permissible concentration limits of occupational exposure to RE [5,6].
Little is known of the biological effects of occupational exposure to the lanthanides. A case of a photoengraver professionally exposed to cored arc light carbon fumes doped with Ce was studied, to establish whether the observed pulmonary alterations were related to the exposure to RE present in cored arc light carbon [7]. The first case of RE pneumoconiosis described had worked as a photoengraver for 13 years, and had not been exposed for 17 years. The diagnosis is derived from the finding in the bronchoalveolar lavage (BAL) fluid of abnormal levels of La, Ce, Nd, Sm, Tb, Yb and Lu. Abnormal levels of RE were demonstrated also in the nails, suggesting absorption of the RE from the lung [8]. Slowly progressive restriction in respiratory function was observed in five reproduction photographers, who had been exposed for more than a decade to the fumes of carbon arc lamps [9]. The case report of a movie projectionist describes his approximately 25 years of occupational exposure to carbon arc lamp fumes [10]. With the increasingly widespread use of RE, there is a likelihood that further occupational groups may have significant but unrecognized exposure.
A retrospective study was conducted to evaluate the lung retention of particles containing cerium in subjects with and without previous occupational exposure to mineral dusts [11]. Lanthanides were extracted from the lung tissue of a subject with a history of potential exposure to carbon-arc lamp emissions in printing shops. They showed the presence of elemental Ce, La and Nd, at concentrations higher than the average concentration measured in other workers who had died of cancer at various sites [12].
Experiments on animals and the culture of rats' peritoneal macrophages (MΦ) covered fluorides of rare earth metals (REM) assigned to the yttrium group. The authors recommend control of the level of yttrium, terbium and lutetium fluorides in the air of the workplace, through the maximum admissible concentrations (MACs) for the fluorides of 2.5 mg/m3 (maximal single concentration) and 0.5 mg/m3 (average shift concentration), and the level of ytterbium fluoride as moderate fibrogenic dust of 6 mg/m3 [13].
For diagnostic purposes, mineralogical analysis was performed in BAL fluid and lung tissue from a 58-year-old patient previously exposed to asbestos and RE dusts. These results suggest that RE is metabolized, and should be considered as biopersistent in the human respiratory tract, since occupational inquiries revealed that exposure to cerium oxide abrasive powder had ceased at least 15 years earlier [14]. A report describes a male patient with a 35-year history of optical lens grinding, associated with exposure to CeO2. Besides reinforcing the contention that REM are potentially harmful, it is suggested that such agents may be causally related to the development of pulmonary fibrosis [15]. A 60-year-old male subject who worked as a movie projectionist and who was exposed for 12 years to RE from cored arc light carbon electrodes was investigated. The research tended to exclude other occupational or non-occupational lung diseases. The relation between the observed interstitial lung fibrosis and occupational exposure to RE, however, is highly probable [16].
Environmental and occupational health problems with RE
The relationship between cerium content in human breast milk and blood plasma or serum was evaluated. The results contribute to setting reference values of cerium in human breast milk and blood plasma/serum, and indicate a varying cerium amount that depends on the cerium environmental pollution. The cerium content in plasma/serum could possibly be an indicator for environmental cerium, which is not valid for breast milk [17]. The research of hair content of RE in young children aged 0-3 years whose mothers live in a RE mining area of Jiangxi Province was conducted. It is concluded that the hair level of RE can be used as a biomarker to reflect body's level of exposure to RE. The hair level of RE in young children and their mothers decreased with the increase of the distance from their home to the RE mining area. Young children living in the area with RE mining may be the high-exposure population, and their hair level of RE was significantly higher than that in their mothers [18].
Based on the new information, there is ongoing exposure of a large population to new diesel emissions generated from using fuel additives containing CeO2 nanoparticles, for which the environmental and public health impacts of this new technology are unknown. Therefore, there is an absolutely critical need to investigate integrated exposure and to conduct toxicological studies, in order to accurately assess the environmental, ecological and health implications of nano-RE [19].
These environmental and occupational health problems commonly result from insufficient environmental regulations and controls in the areas where REs are mined and processed. One of the most significant reports related to the radioactivity of some ores, is that refining one ton of RE oxide can potentially produce 1.4 t of radioactive waste [20]. However, there are indications that China is becoming increasingly aware of the environmental impacts of RE production. It has closed 80 RE production facilities, in an attempt to improve efficiency and environmental performance, which is likely to impact on global supplies [21].
Poor hygiene and the unsafe act of not wearing breathing respirators among workers contributed to radiation risk, following exposures to radioactive emitters. A longer duration of employment and poorer occupational hygiene explained the high chromosomal aberrations frequency among workers [22]. In Malaysia, some plants were built very near to housing areas, some even as close as 20 m, and these residents could potentially be exposed to radiation in the suspended radioactive dust blown from such plants [23]. The impacts on nearby residents from other non-radioactive suspended particles, such as RE minerals and silica, needed further investigation, because such mineral dust has been shown to cause pneumoconiosis [24].
In the course of the extracting, separating and refining processes of RE, a large number of chemical materials are applied, leading to a huge amount of waste gas, waste water and solid waste. In China, after several decades of RE mining and processing, with little regard to health, safety and the environment, regulations have been tightened, and will oblige all RE smelting separation facilities to install health, safety and environmental protection systems [25].
A comprehensive review presents the accumulation and toxicity of RE in the human body, with stress on the potential hazard to environmental and occupational health of the RE fertilizers used in agricultural production. Long-term intake of low dose RE may lead to accumulation in the bone structure, leading to changes in the bone tissue and an increased bone marrow micronucleus (MN) rate, and further, to generation of genotoxicity in bone marrow cells [26]. Workers in the transportation of raw materials and mineral, such as port or local suppliers, and those who work in the processing plant, as well as in the transportation of minerals within the plant, are exposed to safety and health risk. Drivers of vehicles carrying such minerals are also at radiological risk [27].
Some of the occupational health and safety issues with RE are represented in Table 2.
Table 2.
Occupational health and safety issues with rare earths
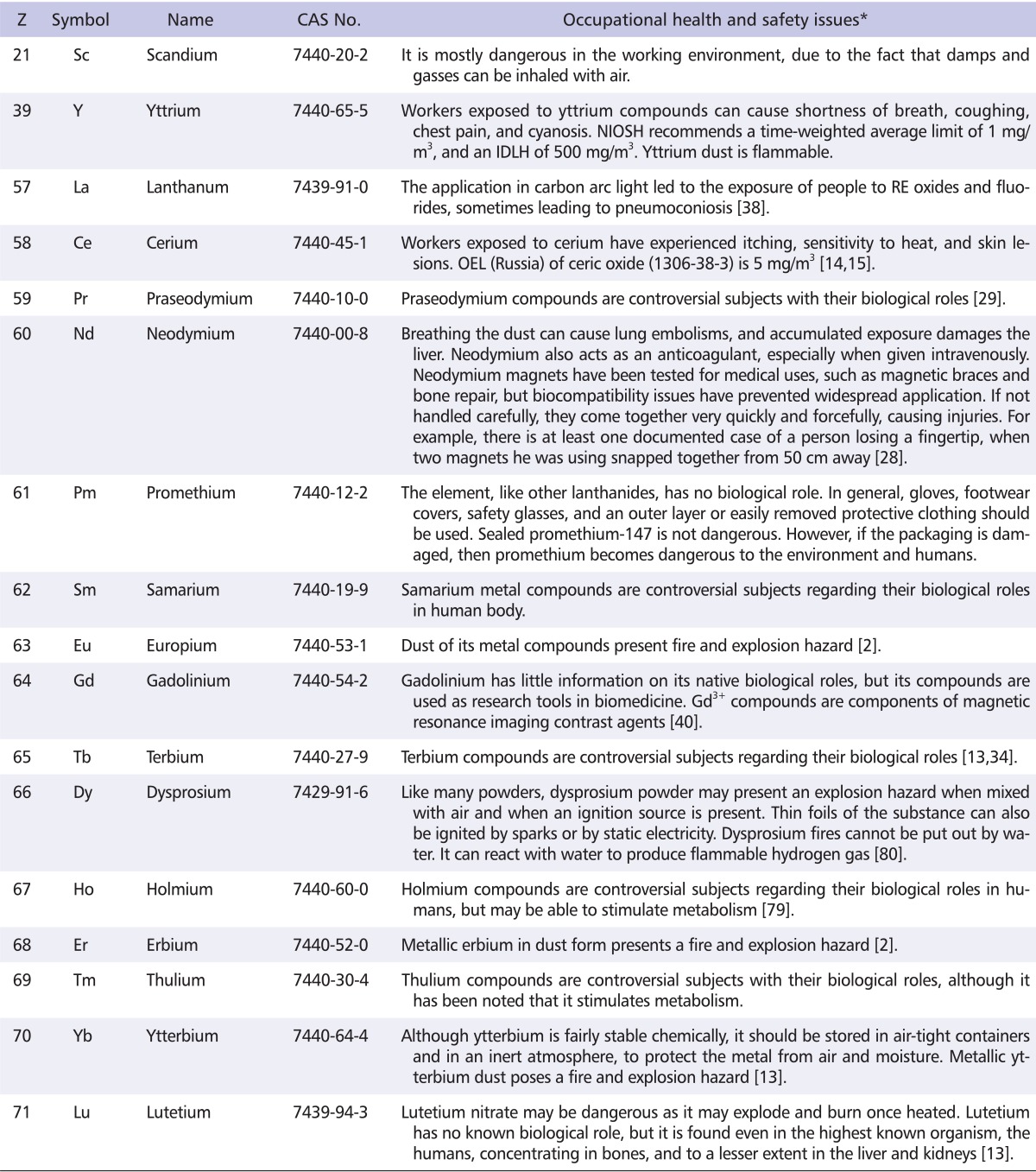
*Mostly referred from ChemIDplus Advanced (http://chem.sis.nlm.nih.gov/chemidplus/) and material safety data sheets information in KOSHANET (http://www.kosha.or.kr/bridge?menuId=69). Searches were conducted using keywords chemical name AND/OR CAS number.
RE: rare earths, Z: atomic number, NIOSH: National Institute for Occupational Safety and Health, IDLH: immediately dangerous to life or health concentrations, OEL: occupational exposure limits.
Toxicological evaluation with in vitro assay
The REM cerium, lanthanum, and neodymium were each evaluated in an in vitro cytotoxicity assay system using rat pulmonary alveolar MΦ; only lanthanum chloride (LC50 = 52 µM), cerium chloride (LC50 = 29 µM), and neodymium oxide (LC50 = 101 µM) displayed significant cytotoxicity. These findings suggest that REM fumes should be considered as cytotoxic to lung tissue, and therefore potentially fibrogenic [28].
In the liver, gadolinium selectively inhibits secretion by Kupffer cells, and decreases cytochrome P450 activity in hepatocytes, thereby protecting liver cells against the toxic products of xenobiotic biotransformation. Praseodymium ion (Pr3+) produces the same protective effect in liver tissue cultures. Trivalent lanthanide ions, especially La3+ and Gd3+, block different calcium channels in human and animal cells. Dy3+ and La3+ block Ca2+-ATPase and Mg2+-ATPase, while Eu3+ and Tb3+ inhibit calcineurin. It is likely that lanthanides significantly and uniquely affect biochemical pathways, thus altering physiological processes in the tissues of humans and animals [29]. The focus of alloy development for biomedical applications should include most defined alloy compositions, with well-known tissue-specific and systemic effects [30].
It was shown that nanoceria particles prevent increases in the intracellular concentrations of reactive oxygen intermediates (ROIs) in the primary cell cultures of rat retina, and prevent loss of vision, due to light-induced degeneration of photoreceptor cells. These indicate that the nanoceria particles may be effective in inhibiting the progression of ROI-induced cell death [31]. The cytotoxicity and oxidative stress caused by 20 nm CeO2 nanoparticles in cultured human lung cancer cells was investigated. Cell viability decreased significantly, due to the function of nanoparticle dose and exposure time. It is concluded that free radicals generated by exposure to CeO2 nanoparticles produce significant oxidative stress in the cells, as reflected by reduced glutathione (GSH) and alpha-tocopherol levels; the toxic effects of CeO2 nanoparticles are dose dependent and time dependent [32]. The ability of CeO2 nanoparticles to confer radioprotection against gastrointestinal epithelium suggests that they protect against radiation-induced damage, both by acting as free-radical scavengers, and by increasing the production of superoxide dismutase (SOD) 2 before radiation insult [33]. In studying the effects of lanthanum, cerium, yttrium and terbium ions on the respiratory burst of peritoneal MΦ, it was concluded that lanthanide ions can inhibit the production of active oxygen free radicals at low concentration, but it turned out contrary at high concentration [34]. The ability of nanoceria was investigated to scavenge free radicals, or reactive oxygen species (ROS), and inhibit inflammatory mediator production in J774A.1 murine MΦ. Cells internalize nanoceria, the treatment is nontoxic, and oxidative stress and pro-inflammatory iNOS protein expression are abated with stimulation. It is suggested that CeO2 nanoparticles are well tolerated in mice, and are incorporated into cellular tissues [35]. Long term effects of small dosage with LaCl3, SmCl3 and YbCl3 on cultured human breast cancer cells, when exposed to 0.1 mM/L of LaCl3, SmCl3 and YbCl3, respectively, for 16 months show increased mitotic activity and synthesis of DNA [36].
To study the hemolysis of human erythrocytes induced by RE ions Ln3+ and their complexes [Ln(Cit)2]3- (Cit=citric group), it was concluded that the RE ions Ln3+ have low critical hemolytic concentration, and indicates Ln3+ acts strongly on erythrocytes, and exhibits obvious cellular toxicity [37].
Lanthanum chloride, cerium chloride and mixed RE chloride at levels of 0.5 to 1.5 mM/L could inhibit the obvious growth of cancer cells, and the expression of tumor suppressor gene p53, p16 and p21 increased, and that of gene nm23 lowered [38]. In a study of the suppression effect of light RE on the proliferation of two cancer cell lines, Northern blot analysis revealed the marked up-regulation of p53, p16 (MTS1), p21 (WAF1) gene expressions in PAMC82 cells treated with lanthanum chloride and cerium chloride, as compared to control cells. The light RE studied has certain suppression effects on the proliferation of cancer cells. This effect might be related to the decrease of calmodulin and up-regulation of some gene expressions in cancer cells [39]. The genotoxicity of Lanthanum (III) and Gadolinium (III) in primary cultures of human peripheral lymphocytes (HPL) was measured in the induction of MN, single strand break, and unscheduled DNA synthesis. The results suggest possible DNA cleavage by RE in human primary peripheral blood lymphocytes. It has been reported that lanthanide metal ions and their complexes can catalyze the nonenzymic sequence-selective hydrolytic scission of isolated linear DNA and RNA, under mild physiological conditions [40]. To explore a new agent for inhibiting leukemic cells, the effects of RE compounds on the growth and apoptosis of HL-60 and NB4 cells were investigated. The results indicate that at certain concentrations, RE compounds may inhibit the growth of leukemic cells, induce them to apoptosis, and have no significant inhibitory effects on normal bone marrow hematopoietic progenitor cells (CFU-GM) [41]. The trivalent ion of a RE, lanthanum, was studied for the effects on the growth, transformation, and gene expression of Escherichia coli. The results showed that La3+ at concentrations from 50 to 150 µg/mL stimulated both endogenic and ectogenic metabolism, but had few effects on gene expression. La3+ at lower concentrations (0.5-30 µg/mL) intensively inhibits E. coli-absorbing external DNA, decreasing the transformation efficiency [42]. RE exposure in mononuclear cells from human peripheral blood (PBMNCs) increased the telomerase activity and the percentages of cells in the S-phase and the G2/M phase, but it had no effect on the apoptotic rate of PBMNCs. Under exposure to lower concentrations of RE, the telomerase activity of PBMNCs in the exposed group was higher than that of the control group, and there was no effect on the apoptotic rate of PBMNCs, but diploid DNA replication was promoted, and the percentages of G2/M- and S-phase cells increased [43].
Exploring the influence of LaCl3 on inducible nitric oxide synthase (iNOS) expression in RAW264.7 MΦ with lipopolysaccharide (LPS) induction, the RAW264.7 cells with overexpression of iNOS accounted for 44.4%, which was obviously higher than that in the LaCl3 + LPS group (11.8%, p < 0.05). LaCl3 can suppress LPS-induced iNOS overexpression at mRNA and protein level and reduce NO production, indicating that LaCl3 can antagonize the excessive activation of iNOS induced by LPS [44]. The effects of lanthanum on Jurkat cells and HPL were examined, and found that it was cytotoxic and genotoxic to both cell lines. Additionally, HPL were more sensitive to La treatment than Jurkat cells, and necrosis was the pathway by which La induced cytotoxicity [45].
Different concentrations (0.001-50 µg/mL) of CeO2 nanoparticles were treated, and measured mRNA levels of the three inflammatory markers intercellular adhesion molecule (ICAM)-1, interleukin (IL)-8, and monocyte chemotactic protein (MCP)-1. They caused very little inflammatory response in human aortic endothelial cells (HAECs), even at the highest dose [46].
The effects of LaCl3 on the production of nitric oxide (NO) and tumor necrosis factor (TNF)-α and the expression of iNOS and TNF-α in RAW 264.7 cells were examined. It was found that the LPS-elicited excessive production of nitric oxide and TNF-α in RAW 264.7 cells was inhibited significantly in the presence of LaCl3, and the attenuation of iNOS and TNF-α occurred at mRNA level. These results indicated the involvements of PKC/Ca2+ and NF-κB in the attenuation of NO and pro-inflammatory cytokine production by LaCl3. They suggest a possible therapeutic application of this agent for treating inflammatory diseases [47]. The effect of LaCl3 on the proliferation and migration activity of human cervical cancer cells in vitro was investigated. It was concluded that LaCl3 can inhibit the proliferation and migration of cervical cancer cells, and induce apoptosis by down-regulating cyclin D1, A20, and MMP-9 expressions in vitro [48].
Sister chromatid exchanges (SCEs) were measured and DNA damage investigated, using the alkaline Comet assay on cultured human lens epithelial cells, exposed to 5 and 10 µg/mL of nanoceria. Nanoceria at these dosages did not cause any DNA damage, or significant increases in the number of SCEs [49].
To understand the interactions of synthesized nanoparticles with bacterial systems, growth and viability of the Gram-negative species E. coli and Shewanella oneidensis, a metal-reducing bacterium, and the Gram-positive species Bacillus subtilis were examined, relative to CeO2 particle size, growth media, pH, and dosage. For E. coli and B. subtilis, clear strain- and size-dependent inhibition were observed, whereas S. oneidensis appeared to be unaffected by the particles [50].
Toxicological evaluation with in vivo study
40 BALB/C mice were randomly divided into two groups, and were treated with lethal dose of LPS and LaCl3 processed LPS, respectively. The TNF-α secretion and TNF-α mRNA expression level of the MΦ from mice treated by LaCl3 processed LPS were obviously lower than those by LPS only (p < 0.01). The mortality of the mice treated by lethal dose of the LPS that had been processed by LaCl3 was significantly lower than that by lethal dose of LPS only. LaCl3 possessed the capacity of lowering the toxicity of LPS, and inhibiting the TNF-α secretion and TNF-α mRNA expression in murine MΦ stimulated by LPS [51]. The effects of LaCl3 (Lads) on mouse liver were studied. The results showed that on the 2nd day after intravenous injection of LaCl3, dense bodies formed by La deposition were found in Kupffer's cells in the 1 mg/kg group; La deposition was also found in hepatocytes in the 5 mg/kg group; extensive degeneration and necrosis of hepatocytes were observed in the 20 mg/kg group, and all the mice in this group died on the 3rd day; but in the 80 mg/kg group, the liver structure changed little, except for the reduction of glycogen. On the 10th day, the liver structures recovered to normal in the 1 mg/kg and 5 mg/kg groups, while intralobular spot necrosis and local disappearance of glycogen were visible in the 80 mg/kg group [52].
The distribution and accumulation of Ce in the viscera and tissues of mice that were fed two kinds of fodder containing Ce in 200 and 800 mg/kg dose, respectively, were studied at different times, using a radioisotope tracer 141Ce. The results showed that Ce residues were distributed in all the animal's viscera and tissue, but were higher in the eye, bone, testis, brain, heart and adipose, and the accumulation of cerium increased with increasing dose and prolonging feed time. The accumulation of cerium was much higher in the eye, than in other viscera and tissue [53]. The influence of oral administration of Ce was studied in relation to metallothionein (MT) and GSH content in the organs of the imprinting control region (ICR) mice. It was concluded that orally administered Ce increases MT and GSH as an antioxidant in the mouse liver, and these reaction are probably caused by increases in the oxidative stress with Ce [54].
In an effort to investigate the effects of exposure to lanthanoids (Ln) on the immune response and liver function, mice were orally exposed to LaCl3, CeCl3, and NdCl3 at 2, 10, and 20 mg/kg doses for 30 days, respectively. The findings showed that exposure to Ln affected the cell and humoral immunity, and disturbed the liver function in mice. In addition, Ce3+ was found to exhibit higher toxicity than La3+ and Nd3+ [55]. In order to compare the toxicity of Ln on organs, mice were exposed to LaCl3, CeCl3, and NdCl3 at a dose of 20 mg/kg for 60 days. The results suggested that long-term exposure to Ln resulted in histopathological changes of liver, kidney, and heart, and their functional damages [56]. In order to study the mechanisms underlying the effects of Ln on the brain, ICR mice were injected with a single 20 mg/kg dose of LaCl3, CeCl3, and NdCl3 into the abdominal cavity, daily, for 14 days. The results showed that CeCl3 and NdCl3 could induce some neurons to turn into inflammatory cells and slight edema, but brain pathological changes from the LaCl3-treated group were not observed. Injury of the brain and oxidative stress occurred, as Ln appeared to trigger a cascade of reactions, such as lipid peroxidation, decreases of the total antioxidation capacity and activities of antioxidative enzymes, excessive release of nitric oxide, increase of glutamic acid, and downregulated level of acetylcholinesterase activities. Furthermore, both Ce3+ and Nd3+ exhibited higher oxidative stress and toxicity on the brain than La3+, and Ce3+ caused more severe brain injuries and oxidative stress than Nd3+, implying that the differences in brain injuries caused by Ln might be related to the number of 4f electrons of Ln [57]. To investigate whether Ce exposure causes neurotoxicological effects, ICR mice were exposed to CeCl3 through intragastric administration at 0, 2, 10, and 20 mg/kg for 60 days. The results indicated that CeCl3 exposure could impair the learning ability, which is attributed to the disturbance of the homeostasis of trace elements, enzymes, and neurotransmitter systems in the mouse brain [58]. The study investigated the signal pathway of hippocampal apoptosis induced by intragastric administration of CeCl3 for 60 days. It showed that cerium had been significantly accumulated in the mouse hippocampus, and CeCl3 caused hippocampal apoptosis and the impairment of spatial recognition memory of mice. CeCl3 effectively activated caspase-3 and -9, inhibited Bcl-2, increased the levels of Bax and cytochrome c, and promoted accumulation of ROS in the mouse hippocampus. This implied that CeCl3 induced apoptosis in the mouse hippocampus could be triggered via a mitochondrion mediated pathway [59]. To understand the liver injury induced by intragastric administration of CeCl3 for 60 days, the ultrastructure of hepatocyte, various oxidative stress parameters, and the stress-related gene expression levels were investigated for the mouse liver. The results demonstrated that CeCl3 had an obvious accumulation in the mouse liver, leading to a classical laddering cleavage of DNA and hepatocyte apoptosis. CeCl3 significantly promoted the accumulation of ROS and inhibited the stress related gene expression of SOD, catalase (CAT), GSH peroxidase, MT, heat-shock protein 70, GSH-S-transferase, P53, and transferring; and it effectively activated the cytochrome p450 1A. This implied that CeCl3 resulted in apoptosis and alteration of expression levels of the genes related to metal detoxification/metabolism regulation and the radical scavenging action in mice [60]. The result of the investigation of the molecular mechanism of inflammatory response in the mouse liver caused by exposure to CeCl3, showed that exposure to CeCl3 decreased body weight, and caused cerium accumulation in the mouse liver, and histopathological changes of the liver (such as inflammatory cell infiltration). Taken together with biochemical assays, the inflammation of mice liver caused by exposure to CeCl3 might be closely associated with the alteration of inflammatory cytokine expressions in the mouse liver, and the signal transducing events happening in CeCl3-induced MΦ of liver sequentially might occur via activation of TLRs→TNF-α→NIK→IκB kinase (including IKK1, IKK2)→NF-κB (including NF-κBP52, NF-κBP65)→inflammation [61]. Ablactated Wistar rats were used, and small doses (0.05, 0. 025 mg/kg) of SmCl3 and PrCl3 were given in different ways. The results showed that both SmCl3 and PrCl3 could decrease the activity of peroxidase and increase the activity of SOD, but the effects of PrCl3 were better than those of SmCl3 [62].
The metabolic accumulation and species of lanthanum in Wistar rat liver were investigated, after the rats were fed with low dose of lanthanum. It was found that the content of La in the liver increased regularly with increase of the dose and time of drug delivery. After the administration was stopped for a certain time, a part of lanthanum in the liver was metabolized, but the metabolic rate was very slow. The lanthanum in rat liver was distributed in soluble protein, mostly with a molecular weight of more than 60,000 [63]. To study the deposit of lanthanum in rat liver, young Wistar rats were divided into 6 groups, which were fed lanthanum nitrate (La(NO3)3) for six months, and the metabolic accumulation of La in rat liver was investigated. It was concluded the La(NO3)3 can enter cells, and the content of La in the liver increases regularly with increase of dose and time of drug delivery [64].
The gene expression between La(NO3)3 in exposed and control rats were compared in vivo. Rats were fed with La(NO3)3 once a day at a dose of 20 mg/kg for one month by gavage. With DNA microarray, 136 differentially expressed genes were identified, including 131 over-expressed genes, and 5 under-expressed genes. Most of these differentially expressed genes were cell signal and transmission genes, and genes associated with metabolism, protein translation and synthesis [65]. The rats were fed with water dissolved Y3+ at different levels (0, 53.4, 5,340 mg/L) for 7 months. The gene expression in brain tissue was detected with oligonucleotide microarray. The results show that, compared to the control, 789 genes express differentially, with 507 over-expressed genes and 282 under-expressed genes in the high-dose group (5,340 mg/L), of which most were related to cell receptor, cell signal and transmission, and ionic passage. Forty-four genes were found to express differentially, including 32 over-expressed genes and 12 under-expressed genes in the low-dose group (53.40 mg/L), of which most were related to cell skeleton and movement, immunity, and DNA binding protein. These results suggest that Y3+ can change the expression of some genes, which may be responsible for the toxicity of RE on learning and memory [66].
In order to examine the short-, medium- and long-term effects of CeO2 particles of different sizes on the lung, 10-week-old male Wistar rats were administered particles of CeO2 at 34 mg/kg, by a single intratracheal instillation. The instillation of Ce-F particles primarily induced inflammation, granulomas, mobilization and impairment of alveolar MΦ and pulmonary alveolar proteinosis. The pulmonary toxicity of Ce-F-instilled rats was found to be markedly enhanced, in sharp contrast to that of Ce-C-instilled rats, on the basis of equal mass concentration, suggesting clear dependence of the pulmonary toxicity on numbers and sizes of particles [67]. The rats were exposed to CeO2 nanoparticles (NPs), which caused cytotoxicity, and oxidative stress and inflammation in the lungs were evaluated. It was suggested that the acute exposure of CeO2 NPs through an inhalation route may induce cytotoxicity via oxidative stress, and lead to a chronic inflammatory response [68]. In order to obtain more insight, the tissue distribution, accumulation and elimination of CeO2 nanoparticles after inhalation exposure with micro and nano CeO2 particles (nominal primary particle size: < 5,000 nm, 40 nm and 5-10 nm) were investigated. It was concluded that no clear effect of the primary particle size or surface area on pulmonary deposition and extrapulmonary tissue distribution could be demonstrated. This is most likely explained by the similar aerodynamic diameter of the CeO2 particles in air, as a result of the formation of aggregates, and irrespective of possible differences in surface characteristics. The implications of the accumulation of CeO2 particles for systemic toxicological effects after repeated chronic exposure via ambient air are significant, and require further exploration [69]. To assess the potential risk of mixed RE Changle for human embryo, it was used for a transplacental MN test, and single cell gel electrophoresis (SCGE) technique was used to detect DNA damage of an embryo. It was concluded that mixed RE Changle is restricted by placenta membrane from entering the embryo body, and more than 2 mg/kg mixed RE Changle may cross the placenta barrier, and cause DNA damage of the hepatocyte and developing erythrocyte of the rat embryo [70].
The anti-inflammatory effects of LaCl3 on LPS challenged mice were examined. The results indicated that LaCl3 can greatly decrease the secretion of TNF-α and IL-1β, as well as TNF-α mRNA expression in mice challenged with LPS. To clarify the mechanism involved, the effects of LaCl3 on the activation of NF-κB were examined, both in liver and in peritoneal MΦ. The LPS-induced activation of NF-κB was significantly blocked by LaCl3. These findings demonstrate that the inhibition of LPS-induced inflammatory media, such as TNF-α and IL-1β, by LaCl3, is due to the inhibition of NF-κB activation [71].
Fluorophore (carboxyfluorescein) conjugated CeO2 NPs (CCNPs) was utilized to study the mechanism of uptake, and to elucidate the subcellular localization of CCNPs, using a keratinocyte model system. The rapid uptake (within 3 hours) of CCNPs that was observed was governed by energy-dependent, clathrin-mediated and caveolae-mediated endocytic pathways, and it was found that CCNPs co-localized with mitochondria, lysosomes and endoplasmic reticulum, as well as being abundant in the cytoplasm and the nucleus. Therefore, CCNPs likely act as cellular antioxidants in multiple compartments of the cell, imparting protection against a variety of oxidant injuries [72].
Fruit flies (Drosophila melanogaster) were fed on media with different dose of ceric sulfate (1, 4, 16, 64, 256, 1,024 mg/L, corresponding to cerium concentrations of 0.45, 1.65, 6.91, 26.3, 104 and 429 mg/g culture medium). There was a significant decrease in mean lifespan and maximum lifespan, with increasing doses of cerium. At some concentrations, there was a decrease in reproductive output, in particular for concentrations of 46.91 mg/g. Cerium caused a significant increase in malondialdehyde (MDA) content and decrease in SOD and CAT activities, at concentrations of 46.91 mg/g. It was suggested that cerium may result in oxidative toxicity to D. melanogaster [73].
This was to study the effects of RE exposure on human telomerase and apoptosis of PBMNCs, and it was concluded that the telomerase activity of PBMNCs in the RE exposed group was higher than that of the control group, and that there is no effect on the apoptotic rate of PBMNCs, but that it may promote diploid DNA replication, and increase the percentage of G2/M and S phase cells [74].
In an effort to combat the harmful effects of radiation exposure, it was proposed that CeO2 nanoparticles (free-radical scavengers) protect normal tissue from radiation-induced damage. It was shown that CeO2 nanoparticles are well tolerated by live animals, and they prevent the onset of radiation-induced pneumonitis, when delivered to live animals exposed to high doses of radiation. This provides a tremendous potential for radioprotection, and can lead to significant benefits for the preservation of human health and quality of life, for humans receiving radiation therapy [75].
To study the effects of environmental exposure to RE on health in children, the level of physical growth and development, function of immune system and intelligence were chosen as the health-response indexes of children. It was concluded that children aged 7-10 years in a RE ore area may have a higher RE burden in the body, and exposure to RE could have adverse influences in children [76]. Also, to demonstrate the validity of using scalp hair RE content as a biomarker of human RE exposure, data were collected on RE exposure levels from children aged 11-15 years old living in an ion-adsorptive type light RE mining area and surrounding areas in southern China. The children living in this mining area should be regarded as a high-risk group with RE (especially light RE) exposure, and their health status should be examined from a RE health risk assessment perspective [77].
RE can lead to morphological changes of the liver. After RE enter the liver, they will interact with many proteins and other molecules in the cells. RE have effects on the activities of enzymes, and interfere in physiological functions of the liver, via informational molecules [78]. The effect of holmium ions on DNA breakage of mice liver cells, and the effect of holmium element on apoptosis of zoic cells were studied in vivo. It is possible that the extension of DNA breakage caused by holmium ions to mice liver cells was associated with dose, treating duration and DNA repair, which had no specificity. It was suggested that holmium element could not induce apoptosis of mice liver cells in vivo [79]. A summary of toxicological information with RE is represented in Table 3.
Table 3.
Summary of toxicological information with rare earths
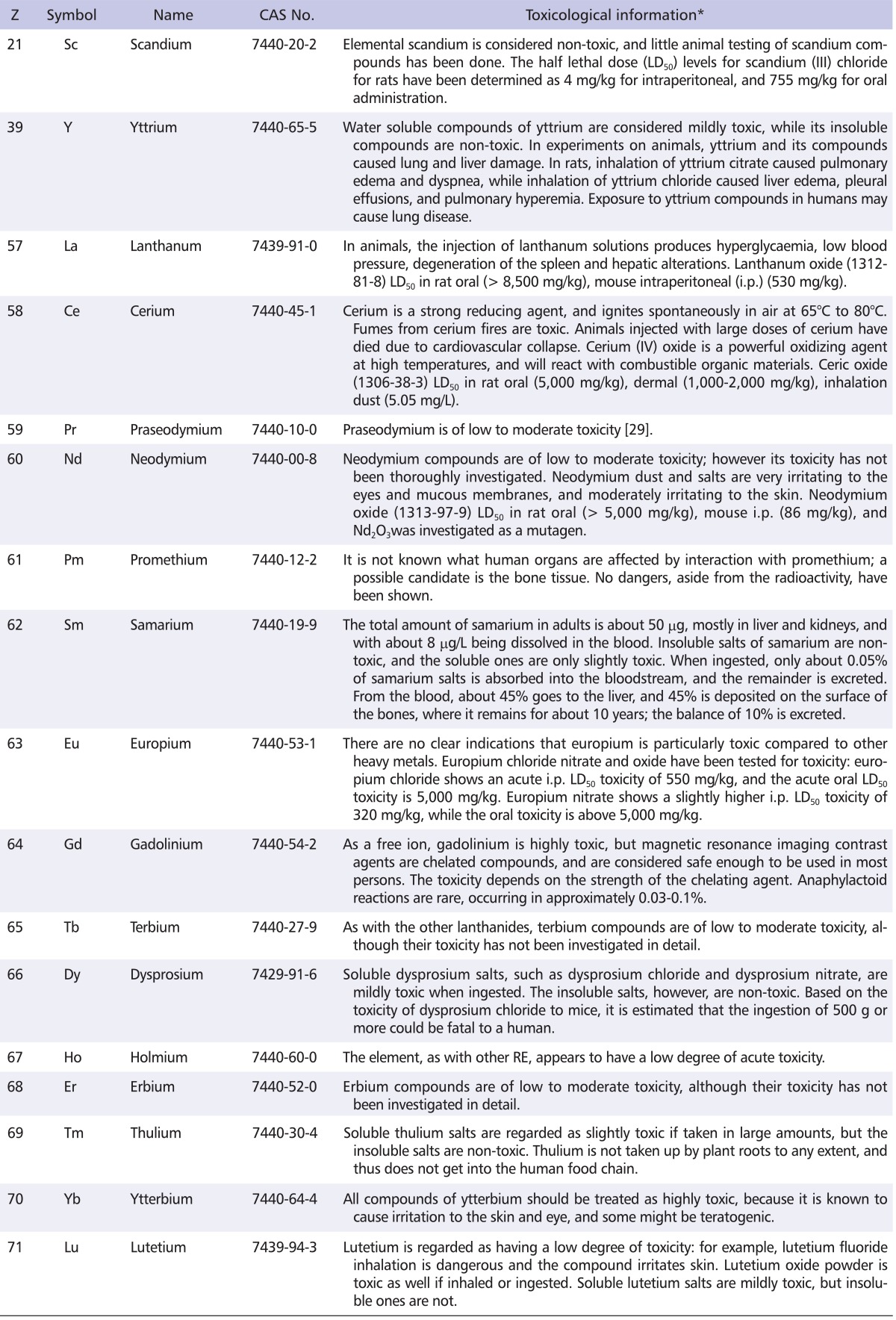
Z: atomic number.
*Mostly referred from National Toxicology Program database search application (http://tools.niehs.nih.gov/ntp_tox/index.cfm) and material safety data sheets information in KOSHANET (http://www.kosha.or.kr/bridge?menuId=69). Searches were conducted using keywords chemical name AND/OR CAS number.
Discussion
The mining, transportation, processing, and waste disposal stages of RE may have very serious environmental and occupational risks. The main risks are the tailings, which are a mixture of small-size particles, waste water and flotation chemicals, and arise at the concentration of the mined ore. Generally, most RE deposits contain radioactive materials, which impose the risk of radioactive dust and water emissions. During their processing, wet gravity separation, dry high magnetic and electrostatic physical separation methods are used. Poor ventilation of dusty working areas, poor hygiene on the part of the workers, and the absence of or improper use of personal protective equipment (such as respirators) increase the likelihood of exposures, and aggravate the risk from lung related diseases, such as pneumoconiosis [10]. Pneumoconiosis comprise a wide spectrum of conditions, ranging from diseases characterized by diffuse collagenous pulmonary reactions, to relatively small lung burdens of bioactive dusts (e.g., silicosis and asbestosis) to diseases characterized by largely non-collagenous reactions in the face of heavy lung dust burdens (e.g., coal workers' pneumoconiosis) [24,27].
There have also been recycling efforts in the RE industry. In the past, with the low prices accorded to REM, there was no incentive to undertake recycling of the elements. With the anticipated increase in price in the various REM, recycling is becoming more attractive. However, the recovery of RE from production waste is not yet practiced. There are various studies in China on the recovery of REM from neodymium magnet scrap and waste, and dysprosium oxide, or Dy2O3, could be recovered to an extent of over 99%. Lanthanum and cerium from used Ni-MH batteries used for hybrid electric vehicles is refined, and the recovered metals reused in new batteries. In general, recycling of RE and the availability of recycling plants and technologies is uncommon. Recycling for small amounts of magnetic scrap containing Nd, Pr and Dy, and small amounts of yttrium from laser and garnet applications, were undertaken. Furthermore, there is no current industrial recycling process for the recovery of RE from Ni-MH batteries containing La, Ce, Nd and Pr [80].
So far, increasing biological interest is emerging for nanotechnology that can use nanomaterials to improve pharmacological treatments. In particular, CeO2 nanoparticles, considered one of the most interesting nanomaterials for their catalytic properties, show a promise for application in therapy [35]. Due to the presence of oxygen vacancies on its surface and autoregenerative cycle of its two oxidation states, Ce3+ and Ce4+, nanoceria can be used as an antioxidant agent. Because many disorders are associated with oxidative stress and inflammation, CeO2 nanoparticles may be a tool for the treatment of these pathologies [61]. The opinions, sometimes conflicting, of the scientific community about nanoceria have been analyzed, together with its capability to protect from various damages that induce cells to death, and to reduce oxidative stress, associated with a consequent reduction of inflammation [81]. The use of nanotechnology in some green applications is being considered, in order to increase their efficiency by using nano-sized RE compounds. Hazard evaluation and risk assessment addressing social and environmental aspects are therefore highly recommended [82]. RE can be recycled from the scrap produced in the manufacturing of neodymium-iron-boron magnets. The manufacture of magnets increases annually, and generates a significant amount of scrap; so an effective means of recycling neodymium from this source would be highly beneficial [83].
The attention towards RE, including key and critical elements, together with RE production, could contaminate the environment if best management practices are not used, and if the operation is not closely monitored. Possible contaminants include, but are not limited to, radionuclides, RE, metals, and other potential contaminants, such as fluorine and asbestos minerals. Mining exposes these possible contaminants while refining isolates, and concentrates the possible contaminants. This implies that persistent applications of RE may lead to potential environmental and occupational risk in the future.
Inhalational or intratracheal exposure of animals to RE has been proven to cause acute pneumonitis with neutrophil infiltration in the lung; long-term exposure to RE dust seems to cause pneumoconiosis in humans. However, the mechanism of neutrophil recruitment or interaction of RE with lung cells has not been fully investigated.
There appeared to be a small probability of worker illness due to exposure to RE, although it is well known that some metals are industrial hazards, and that their inhalation plays an important role in determining pulmonary histopathologic alterations. However, long-term exposure of occupational workers to RE dusts can induce bronchiolar, alveolar, and interstitial histologic reactions in the lung, observed in the cases presented in this review. The toxicological significance of these findings may be that of RE as irritative agents, causing long-term morphologic changes in the lung, with fibrosis of the interstitial tissue and bronchitis of the respiratory tract.
For occupational health, further research is required to evaluate the hazards or risks for workers, and measuring the RE levels at which health problems are observed. Environmental and occupational toxicology testing of RE requires the development of agreed testing protocols and guidelines, to allow for the comparison and interpretation of data from the studies.
Occupational exposures to natural and man-made chemicals are a major cause of disease. One of the primary challenges of molecular occupational epidemiology, therefore, is to improve exposure assessment. Progress has been made with biomarkers, such as carcinogens and their metabolites, DNA, and protein adducts and mutations measured in various tissues and body fluids. This review considers some of the principles behind the application of exposure biomarkers in RE treated workers. The use of biomarkers is increasing for sustainable measure to establish a safe and healthy working environment for RE industries, due to growing demands for information about health risks from unfavorable exposures. Biomarkers provide information about individual loads; therefore biomarkers of intermediate endpoints benefit, in comparison with those of exposure, from the fact that they are closer to the adverse outcome in the pathway from exposure to health effects, and may provide powerful information for intervention. The potential of new technologies, such as transcriptomics, proteomics and metabonomics, to provide a step change in occupational exposure assessment is discussed [84]. It is suggested that a concerted effort is now required, with appropriate funding, to develop and validate the required exposure assessment tools.
Acknowledgments
This study was supported by the intramural research fund of the Occupational Safety and Health Research Institute (OSHRI).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Angerer G, Erdmann L, Marscheider-Weidemann F, Scharp M, Lüllmann A, Handke V, Marwede M. Rohstoffe für Zukunftstechnologien: Einfluss des branchenspezifischen Rohstoffbedarfs in rohstoffintensiven Zukunftstechnologien auf die zukünftige Rohstoffnachfrage. Stuttgart (Germany): Fraunhofer IRB Verlag; 2009. German. [Google Scholar]
- 2.British Geological Survey-Rare Earth Elements [Internet] Nottingham (UK): Natural environment research council; 2011. [cited 2012 Jun 14]. Available from: http://www.MineralsUK.com. [Google Scholar]
- 3.Hilsum L. The Sunday Times. London ed. 2009. Dec 06, Chinese pay toxic price for a green world. Post#27707605. [Google Scholar]
- 4.Paul J, Campbell G. Investigating rare earth element mine development in EPA region 8 and potential environmental impacts. Additional review by Region 8 Mining Team Members. Washington, DC: Environmental protection agency (US); 2011. Report No.: EPA Document-908R11003. [Google Scholar]
- 5.Sabbioni E, Pietra R, Gaglione P, Vocaturo G, Colombo F, Zanoni M, Rodi F. Long-term occupational risk of rare-earth pneumoconiosis. A case report as investigated by neutron activation analysis. Sci Total Environ. 1982;26:19–32. doi: 10.1016/0048-9697(82)90093-6. [DOI] [PubMed] [Google Scholar]
- 6.Vocaturo G, Colombo F, Zanoni M, Rodi F, Sabbioni E, Pietra R. Human exposure to heavy metals. Rare earth pneumoconiosis in occupational workers. Chest. 1983;83:780–783. doi: 10.1378/chest.83.5.780. [DOI] [PubMed] [Google Scholar]
- 7.Pietra R, Sabbioni E, Orvini E, Vocaturo G, Colombo F, Zanoni M, Rodi F. Occupational risk to rare earths. Inorganica Chim Acta. 1984;94:143–144. [Google Scholar]
- 8.Sulotto F, Romano C, Berra A, Botta GC, Rubino GF, Sabbioni E, Pietra R. Rare-earth pneumoconiosis: a new case. Am J Ind Med. 1986;9:567–575. doi: 10.1002/ajim.4700090609. [DOI] [PubMed] [Google Scholar]
- 9.Vogt P, Spycher MA, Rüttner JR. Pneumoconiosis caused by "rare earths" (cer-pneumoconiosis) Schweiz Med Wochenschr. 1986;116:1303–1308. German. [PubMed] [Google Scholar]
- 10.Waring PM, Watling RJ. Rare earth deposits in a deceased movie projectionist. A new case of rare earth pneumoconiosis? Med J Aust. 1990;153:726–730. doi: 10.5694/j.1326-5377.1990.tb126334.x. [DOI] [PubMed] [Google Scholar]
- 11.Pairon JC, Roos F, Iwatsubo Y, Janson X, Billon-Galland MA, Bignon J, Brochard P. Lung retention of cerium in humans. Occup Environ Med. 1994;51:195–199. doi: 10.1136/oem.51.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dufresne A, Krier G, Muller JF, Case B, Perrault G. Lanthanide particles in the lung of a printer. Sci Total Environ. 1994;151:249–252. doi: 10.1016/0048-9697(94)90474-x. [DOI] [PubMed] [Google Scholar]
- 13.Neizvestnova EM, Grekhova TD, Privalova LI, Babakova OM, Konovalova NE, Petelina EV. Hygienic regulation of yttrium, terbium, ytterbium and lutetium fluorides in the air of the workplace. Med Tr Prom Ekol. 1994;(7):32–35. [PubMed] [Google Scholar]
- 14.Pairon JC, Roos F, Sébastien P, Chamak B, Abd-Alsamad I, Bernaudin JF, Bignon J, Brochard P. Biopersistence of cerium in the human respiratory tract and ultrastructural findings. Am J Ind Med. 1995;27:349–358. doi: 10.1002/ajim.4700270304. [DOI] [PubMed] [Google Scholar]
- 15.McDonald JW, Ghio AJ, Sheehan CE, Bernhardt PF, Roggli VL. Rare earth (cerium oxide) pneumoconiosis: analytical scanning electron microscopy and literature review. Mod Pathol. 1995;8:859–865. [PubMed] [Google Scholar]
- 16.Porru S, Placidi D, Quarta C, Sabbioni E, Pietra R, Fortaner S. The potencial role of rare earths in the pathogenesis of interstitial lung disease: a case report of movie projectionist as investigated by neutron activation analysis. J Trace Elem Med Biol. 2001;14:232–236. doi: 10.1016/S0946-672X(01)80008-0. [DOI] [PubMed] [Google Scholar]
- 17.Höllriegl V, González-Estecha M, Trasobares EM, Giussani A, Oeh U, Herraiz MA, Michalke B. Measurement of cerium in human breast milk and blood samples. J Trace Elem Med Biol. 2010;24:193–199. doi: 10.1016/j.jtemb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Peng RL, Pan XC, Xie Q. Relationship of the hair content of rare earth elements in young children aged 0 to 3 years to that in their mothers living in a rare earth mining area of Jiangxi. Zhonghua Yu Fang Yi Xue Za Zhi. 2003;37:20–22. Chinese. [PubMed] [Google Scholar]
- 19.Cassee FR, van Balen EC, Singh C, Green D, Muijser H, Weinstein J, Dreher K. Exposure, health and ecological effects review of engineered nanoscale cerium and cerium oxide associated with its use as a fuel additive. Crit Rev Toxicol. 2011;41:213–229. doi: 10.3109/10408444.2010.529105. [DOI] [PubMed] [Google Scholar]
- 20.Jiabao L, Jie L. Rare earth industry adjusts to slow market. Beijing (China): China Daily; [updated 2010 Jan 12; cited 2012 Jun 14]. Available from: http://www.chinadaily.com.cn. [Google Scholar]
- 21.Williams SH. In-situ neutron diffraction analysis of deformation behavior of ductile rare earth intermetallic YCu. Paper 10863 [graduate thesis and dissertation] Ames (IA): Iowa State University; 2009. [cited 2012 Jun 14]. Available from: http://lib.dr.iastate.edu/etd/10863. [Google Scholar]
- 22.Kandar MZ, Bhari IB. Radiation-induced chromosomal aberrations among TENORM workers: amang- and ilmenite-processing workers of Malaysia. Mutat Res. 1996;351:157–161. doi: 10.1016/0027-5107(95)00174-3. [DOI] [PubMed] [Google Scholar]
- 23.Ismail B, Redzuwan Y, Chua RS, Shafiee W. Radiological impacts of the amang processing industry on neighbouring residents. Appl Radiat Isot. 2001;54:393–397. doi: 10.1016/s0969-8043(00)00106-8. [DOI] [PubMed] [Google Scholar]
- 24.Becklake MR. The mineral dust diseases. Tuber Lung Dis. 1992;73:13–20. doi: 10.1016/0962-8479(92)90074-T. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z. Outline on the development and policies of China rare earth industry [Internet] Beijing (China): Chinese Society of Rare Earths; 2010. [cited 2012 Jun 14]. Available from: http://www.cs-re.org.cn/en/modules.php?name=news&file=article&sid=35. [Google Scholar]
- 26.Chen Z, Zhu X. Accumulation of rare earth elements in bone and its toxicity and potential hazard to health. J Ecol Rural Environ [Internet] 2008. [cited 2012 Jun 14]. Available from: http://en.cnki.com.cn/Article_en/CJFDTotal-NCST200801019.htm. Chinese.
- 27.Yoon HK, Moon HS, Park SH, Song JS, Lim Y, Kohyama N. Dendriform pulmonary ossification in patient with rare earth pneumoconiosis. Thorax. 2005;60:701–703. doi: 10.1136/thx.2003.006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmer RJ, Butenhoff JL, Stevens JB. Cytotoxicity of the rare earth metals cerium, lanthanum, and neodymium in vitro: comparisons with cadmium in a pulmonary macrophage primary culture system. Environ Res. 1987;43:142–156. doi: 10.1016/s0013-9351(87)80066-x. [DOI] [PubMed] [Google Scholar]
- 29.Pałasz A, Czekaj P. Toxicological and cytophysiological aspects of lanthanides action. Acta Biochim Pol. 2000;47:1107–1114. [PubMed] [Google Scholar]
- 30.Feyerabend F, Fischer J, Holtz J, Witte F, Willumeit R, Drücker H, Vogt C, Hort N. Evaluation of short-term effects of rare earth and other elements used in magnesium alloys on primary cells and cell lines. Acta Biomater. 2010;6:1834–1842. doi: 10.1016/j.actbio.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Patil S, Seal S, McGinnis JF. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat Nanotechnol. 2006;1:142–150. doi: 10.1038/nnano.2006.91. [DOI] [PubMed] [Google Scholar]
- 32.Lin W, Huang YW, Zhou XD, Ma Y. Toxicity of cerium oxide nanoparticles in human lung cancer cells. Int J Toxicol. 2006;25:451–457. doi: 10.1080/10915810600959543. [DOI] [PubMed] [Google Scholar]
- 33.Colon J, Hsieh N, Ferguson A, Kupelian P, Seal S, Jenkins DW, Baker CH. Cerium oxide nanoparticles protect gastrointestinal epithelium from radiation-induced damage by reduction of reactive oxygen species and upregulation of superoxide dismutase 2. Nanomedicine. 2010;6:698–705. doi: 10.1016/j.nano.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 34.Liu HX, Liu XT, Lu JF, Li RC, Wang K. The effects of lanthanum, cerium, yttrium and terbium ions on respiratory burst of peritoneal macrophage (MΦ) J Beijing Med Univ [Internet] 2000. [cited 2012 Jun 14]. Available from: http://en.cnki.com.cn/Article_en/CJFDTOTAL-BYDB200003003.htm. Chinese.
- 35.Hirst SM, Karakoti AS, Tyler RD, Sriranganathan N, Seal S, Reilly CM. Anti-inflammatory properties of cerium oxide nanoparticles. Small. 2009;5:2848–2856. doi: 10.1002/smll.200901048. [DOI] [PubMed] [Google Scholar]
- 36.Nie Y. Long term effects of small dosage of chlorides of La, Sm and Yb on cultured cells. J Jilin Univ (Medicine Edition) [Internet] 1992. [cited 2012 Jun 14]. Available from: http://en.cnki.com.cn/Article_en/CJFDTOTAL-BQEB199206000.htm. Chinese.
- 37.Lao FY, Li RC, Wang K. Study on the hemolysis of human erythrocytes induced by rare earth ion Ln3+ and their complex ion [Ln(Cit)2]3- Beijing Da Xue Xue Bao. 2002;34:163–166. [Google Scholar]
- 38.Xiao B, Ji Y, Cui M. Effects of lanthanum and cerium on malignant proliferation and expression of tumor-related gene. Zhonghua Yu Fang Yi Xue Za Zhi. 1997;31:228–230. Chinese. [PubMed] [Google Scholar]
- 39.Ji YJ, Xiao B, Wang ZH, Cui MZ, Lu YY. The suppression effect of light rare earth elements on proliferation of two cancer cell lines. Biomed Environ Sci. 2000;13:287–292. [PubMed] [Google Scholar]
- 40.Yongxing W, Xiaorong W, Zichun H. Genotoxicity of lanthanum (III) and gadolinium (III) in human peripheral blood lymphocytes. Bull Environ Contam Toxicol. 2000;64:611–616. doi: 10.1007/s001280000047. [DOI] [PubMed] [Google Scholar]
- 41.Dai Y, Li J, Li J, Yu L, Dai G, Hu A, Yuan L, Wen Z. Effects of rare earth compounds on growth and apoptosis of leukemic cell lines. In Vitro Cell Dev Biol Anim. 2002;38:373–375. doi: 10.1290/1071-2690(2002)038<0373:EORECO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 42.Wenhua L, Ruming Z, Zhixiong X, Xiangdong C, Ping S. Effects of La3+ on growth, transformation, and gene expression of Escherichia coli. Biol Trace Elem Res. 2003;94:167–177. doi: 10.1385/BTER:94:2:167. [DOI] [PubMed] [Google Scholar]
- 43.Yu L, Dai Y, Yuan Z, Li J. Effects of rare earth elements on telomerase activity and apoptosis of human peripheral blood mononuclear cells. Biol Trace Elem Res. 2007;116:53–59. doi: 10.1007/BF02685918. [DOI] [PubMed] [Google Scholar]
- 44.Lou YL, Guo F, Wang Y, Xie A, Liu YX, Li GH. Inhibitory effect of lanthanum chloride on the expression of inducible nitric oxide synthase in RAW264.7 macrophages induced by lipopolysaccharide. Zhonghua Shao Shang Za Zhi. 2007;23:280–283. Chinese. [PubMed] [Google Scholar]
- 45.Paiva AV, de Oliveira MS, Yunes SN, de Oliveira LG, Cabral-Neto JB, de Almeida CE. Effects of lanthanum on human lymphocytes viability and DNA strand break. Bull Environ Contam Toxicol. 2009;82:423–427. doi: 10.1007/s00128-008-9596-1. [DOI] [PubMed] [Google Scholar]
- 46.Gojova A, Lee JT, Jung HS, Guo B, Barakat AI, Kennedy IM. Effect of cerium oxide nanoparticles on inflammation in vascular endothelial cells. Inhal Toxicol. 2009;21(Suppl 1):123–130. doi: 10.1080/08958370902942582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo F, Lou Y, Feng N, Li G, Xie A, Huang X, Wang Y. Exposure to lanthanum compound diminishes LPS-induced inflammation-associated gene expression: involvements of PKC and NF-kappaB signaling pathways. Biometals. 2010;23:669–680. doi: 10.1007/s10534-010-9327-z. [DOI] [PubMed] [Google Scholar]
- 48.Liu SS, Lu D, Miao LF, Xiong QY, Chen XP, Wang Y, Guo F. Effects of lanthanum chloride on proliferation and migration of human cervical cancer cell line HeLa cells. Zhonghua Fu Chan Ke Za Zhi. 2010;45:609–613. Chinese. [PubMed] [Google Scholar]
- 49.Pierscionek BK, Li Y, Yasseen AA, Colhoun LM, Schachar RA, Chen W. Nanoceria have no genotoxic effect on human lens epithelial cells. Nanotechnology. 2010;21:035102. doi: 10.1088/0957-4484/21/3/035102. [DOI] [PubMed] [Google Scholar]
- 50.Pelletier DA, Suresh AK, Holton GA, McKeown CK, Wang W, Gu B, Mortensen NP, Allison DP, Joy DC, Allison MR, Brown SD, Phelps TJ, Doktycz MJ. Effects of engineered cerium oxide nanoparticles on bacterial growth and viability. Appl Environ Microbiol. 2010;76:7981–7989. doi: 10.1128/AEM.00650-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Yuan K, Cao Y, Li G, Dai Y, Zhou S. The influence of lanthanum chloride on the TNFalpha expression of murine peritoneal macrophages stimulated by lipopolysaccharide. Zhonghua Shao Shang Za Zhi. 2002;18:102–104. Chinese. [PubMed] [Google Scholar]
- 52.Chen D, Zou Z, Zhu X, Chen A. Effects of lanthanum trichloride in different doses on the liver cells in mice. J Jilin Univ (Medicine Edition) [Internet] 1993. [cited 2012 Jun 14]. Available from: http://en.cnki.com.cn/Article_en/CJFDTOTAL-BQEB199305012.htm. Chinese.
- 53.Chen Z, Liu Y, Wang Y. Study on distributions and accumulations of rare earth element cerium (141Ce) in mice. J Nanjing Agric Univ [Internet] 2000. [cited 2012 Jun 14]. Available from: http://en.cnki.com.cn/Article_en/CJFDTOTAL-NJNY200003025.htm. Chinese.
- 54.Kawagoe M, Hirasawa F, Cun Wang S, Liu Y, Ueno Y, Sugiyama T. Orally administrated rare earth element cerium induces metallothionein synthesis and increases glutathione in the mouse liver. Life Sci. 2005;77:922–937. doi: 10.1016/j.lfs.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 55.Cheng J, Cheng Z, Hu R, Cui Y, Cai J, Li N, Gui S, Sang X, Sun Q, Wang L, Hong F. Immune dysfunction and liver damage of mice following exposure to lanthanoids. Environ Toxicol. 2011 doi: 10.1002/tox.20773. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 56.Cheng J, Li N, Cai J, Cheng Z, Hu R, Zhang Q, Wan F, Sun Q, Gui S, Sang X, Wang L, Hong F. Organ histopathological changes and its function damage in mice following long-term exposure to lanthanides chloride. Biol Trace Elem Res. 2012;145:361–368. doi: 10.1007/s12011-011-9193-8. [DOI] [PubMed] [Google Scholar]
- 57.Zhao H, Cheng Z, Hu R, Chen J, Hong M, Zhou M, Gong X, Wang L, Hong F. Oxidative injury in the brain of mice caused by lanthanid. Biol Trace Elem Res. 2011;142:174–189. doi: 10.1007/s12011-010-8759-1. [DOI] [PubMed] [Google Scholar]
- 58.Zhao H, Cheng Z, Cheng J, Hu R, Che Y, Cui Y, Wang L, Hong F. The toxicological effects in brain of mice following exposure to cerium chloride. Biol Trace Elem Res. 2011;144:872–884. doi: 10.1007/s12011-011-9045-6. [DOI] [PubMed] [Google Scholar]
- 59.Cheng Z, Li N, Cheng J, Hu R, Gao G, Cui Y, Gong X, Wang L, Hong F. Signal pathway of hippocampal apoptosis and cognitive impairment of mice caused by cerium chloride. Environ Toxicol. 2011;27:707–718. doi: 10.1002/tox.20696. [DOI] [PubMed] [Google Scholar]
- 60.Zhao H, Cheng J, Cai J, Cheng Z, Cui Y, Gao G, Hu R, Gong X, Wang L, Hong F. Liver injury and its molecular mechanisms in mice caused by exposure to cerium chloride. Arch Environ Contam Toxicol. 2012;62:154–164. doi: 10.1007/s00244-011-9672-0. [DOI] [PubMed] [Google Scholar]
- 61.Li N, Cheng J, Cheng Z, Hu R, Cai J, Gao G, Cui Y, Wang L, Hong F. Molecular mechanism of inflammatory response in mouse liver caused by exposure to CeCl(3) Environ Toxicol. 2011 doi: 10.1002/tox.20727. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 62.Cui L, Li Y, Liu XL, Nic Y. Effect of SmCl3 and PrCl3 on morphology of liver and activities of LPO and SOD in rats. J Norman Bethune Univ Med Sci [Internet] 1994. [cited 2012 Jun 14]. Available from: http://en.cnki.com.cn/Article_en/CJFDTOTAL-BQEB404.014.htm. Chinese.
- 63.Lu R, Zhu YM, Chen HT, Zhao DQ, Ni JZ, Chen D, Nie YX, Liu Y. Species of lanthanum in Wistar rat liver. Chinese J Inorg Chem. 2000;16:273–278. Chinese. [Google Scholar]
- 64.Liu Y, Chen D, Chen A. Study on lanthanum deposit in liver of rats chronically exposed to lanthanum nitrate at low dose. J Health Toxicol [Internet] cited 2012 Jun 14. 2003. Available from: http://en.cnki.com.cn/Article_en/CJFDTOTAL-WSDL200304002.htm. Chinese.
- 65.Zhao H, Hao WD, Xu HE, Shang LQ, Lu YY. Gene expression profiles of hepatocytes treated with La(NO3)3 of rare earth in rats. World J Gastroenterol. 2004;10:1625–1629. doi: 10.3748/wjg.v10.i11.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang W, Zhang P, Liu J, Xue Y. Effect of long-term intake of Y3+ in drinking water on gene expression in brains of rats. J Rare Earths. 2006;24:369–373. [Google Scholar]
- 67.Toya T, Takata A, Otaki N, Takaya M, Serita F, Yoshida K, Kohyama N. Pulmonary toxicity induced by intratracheal instillation of coarse and fine particles of cerium dioxide in male rats. Ind Health. 2010;48:3–11. doi: 10.2486/indhealth.48.3. [DOI] [PubMed] [Google Scholar]
- 68.Srinivas A, Rao PJ, Selvam G, Murthy PB, Reddy PN. Acute inhalation toxicity of cerium oxide nanoparticles in rats. Toxicol Lett. 2011;205:105–115. doi: 10.1016/j.toxlet.2011.05.1027. [DOI] [PubMed] [Google Scholar]
- 69.Geraets L, Oomen AG, Schroeter JD, Coleman VA, Cassee FR. Tissue distribution of inhaled micro- and nano-sized cerium oxide particles in rats: Results from a 28-day exposure study. Toxicol Sci. 2012;127:463–473. doi: 10.1093/toxsci/kfs113. [DOI] [PubMed] [Google Scholar]
- 70.Zhou L, Li S, Chem H, Huan K, Nie Y. DNA damage effect of mixed rare earth Changle crossing placenta barrier on rat embryo. J Rare Earths. 2003;21:176–179. [Google Scholar]
- 71.Guo F, Guo X, Xie A, Lou YL, Wang Y. The suppressive effects of lanthanum on the production of inflammatory mediators in mice challenged by LPS. Biol Trace Elem Res. 2011;142:693–703. doi: 10.1007/s12011-010-8792-0. [DOI] [PubMed] [Google Scholar]
- 72.Singh S, Kumar A, Karakoti A, Seal S, Self WT. Unveiling the mechanism of uptake and sub-cellular distribution of cerium oxide nanoparticles. Mol Biosyst. 2010;6:1813–1820. doi: 10.1039/c0mb00014k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang SF, Li ZY, Wang XQ, Wang QX, Hu FF. Cerium caused life span shortening and oxidative stress resistance in Drosophila melanogaster. Ecotoxicol Environ Saf. 2010;73:89–93. doi: 10.1016/j.ecoenv.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 74.Yu L, Dai YC, Yuan ZK, Li J. Effects of rare earth compounds on human peripheral mononuclear cell telomerase and apoptosis. Zhonghua Yu Fang Yi Xue Za Zhi. 2004;38:248–251. Chinese. [PubMed] [Google Scholar]
- 75.Colon J, Herrera L, Smith J, Patil S, Komanski C, Kupelian P, Seal S, Jenkins DW, Baker CH. Protection from radiation-induced pneumonitis using cerium oxide nanoparticles. Nanomedicine. 2009;5:225–231. doi: 10.1016/j.nano.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 76.Fan G, Yuan Z, Zheng H, Liu Z. Study onthe effects of exposure to rare earth elements and health-responses in children aged 7-10 years. Wei Sheng Yan Jiu. 2004;33:23–28. Chinese. [PubMed] [Google Scholar]
- 77.Tong SL, Zhu WZ, Gao ZH, Meng YX, Peng RL, Lu GC. Distribution characteristics of rare earth elements in children's scalp hair from a rare earths mining area in southern China. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2004;39:2517–2532. doi: 10.1081/ese-200026332. [DOI] [PubMed] [Google Scholar]
- 78.Lu R, Ni J. Mechanism of rare earth effect on liver. J Chinese Rare Earth Soc [Internet] 2002. [cited 2012 Jun 14]. Available from: http://en.cnki.com.cn/Article_en/CJFDTOTAL-XTXB200203000.htm. Chinese.
- 79.Wang C, Min L, Wu W. Effects of holmium element on DNA lesion of mice liver cells in vivo. Carcinog Teratog Mutagen [Internet] 2004. [cited 2012 Jun 14]. Available from: http://en.cnki.com.cn/Article_en/CJFDTOTAL-ABJB200404011.htm. Chinese.
- 80.Goonan TG. U.S. Geological Survey Scientific Investigations Report 2011-5094 [Internet] Reston (VA): U.S. Geological Survey; 2011. [cited 2012 Jun 14]. Rare earth elements-end use and recyclability. Available from: http://pubs.usgs.gov/sir/2011/5094/ [Google Scholar]
- 81.Celardo I, Traversa E, Ghibelli L. Cerium oxide nanoparticles: a promise for applications in therapy. J Exp Ther Oncol. 2011;9:47–51. [PubMed] [Google Scholar]
- 82.Study into the feasibility of protecting and recovering critical raw materials through infrastructure development in the south east of England final report. Project ref: LIFE08 ENV/UK/000208 [Internet] Reading (UK): European Pathway to Zero Waste, Environment Agency; 2011. [cited 2012 Jun 14]. Available from: http://www.environment-agency.gov.uk/static/documents/Business/EPOW-recovering-critical-raw-materials-T5v2.pdf. [Google Scholar]
- 83.Takeda O, Okabe TH, Umetsu Y. Recovery of neodymium from a mixture of scrap and other scrap. J Alloys Compd. 2006;408-12:387–390. [Google Scholar]
- 84.Wild CP. Environmental exposure measurement in cancer epidemiology. Mutagenesis. 2009;24:117–125. doi: 10.1093/mutage/gen061. [DOI] [PMC free article] [PubMed] [Google Scholar]


