Abstract
Background:
Studies have indicated that the prevalence of obstructive sleep apnea-hypopnea syndrome (OSAHS) is similar between white and African American patients, but it is unclear if there are differences in the severity of OSAHS. We hypothesized that in patients with diagnosed OSAHS, African Americans would have higher apnea-hypopnea index (AHI) and higher mortality than white individuals.
Methods:
We analyzed a prospectively collected database of 512 patients studied between July 1996 through February 1999. Inclusion criteria included age ≥ 18 y, AHI ≥ 5/h, and full-night PSG. Statistical analysis was performed to determine the association between race and AHI while controlling for the effect of confounders and effect modifiers, which included gender, age, body mass index, and comorbidities.
Results:
The database included 340 African American and 172 white patients. AHI was higher in African American patients (median 32.7/h IQR 3.3-69.2) than white patients (22.4/h IQR 12.8-40.6, p = 0.01). Age, sex, and BMI were found to be effect modifiers and were included in final models. In the final model, African American men younger than 39 years and between 50 and 59 years were found to have a higher AHI than white men in the same age ranges.
Conclusions:
African American men younger than 39 years and between 50 and 59 years have a higher AHI compared to white men of the same ages after correcting for confounders and effect modifiers. There was no difference in mortality between African Americans and whites with OSAHS in this cohort.
Citation:
Pranathiageswaran S; Badr MS; Severson R; Rowley JA. The influence of race on the severity of sleep disordered breathing. J Clin Sleep Med 2013;9(4):303-309.
Keywords: Mortality, race, sleep disordered breathing
Epidemiologic studies suggest that race may influence the prevalence and outcome of chronic illness. There is evidence that the prevalence of obstructive sleep apnea/hypopnea syndrome (OSAHS) is higher in younger (< 25 years) African Americans than younger white individuals, with no difference in prevalence in the middle-aged1 or in the elderly (> 55 years).2 In contrast, there is evidence that the prevalence of severe OSAHS (defined by an apnea-hypopnea index [AHI] > 30) is increased in African American elderly.2 Overall, it is unclear whether race is a determinant of sleep apnea severity and outcome.1–3 Moreover, a racial difference may be due to increased prevalence of obesity and hypertension in African Americans, both of which have been associated with increased OSAHS severity.4,5 The purpose of this study was to determine whether the severity and mortality of sleep apnea were higher in African American patients with OSAHS compared to white individuals. To study this hypothesis, we compared polysomnographic variables of OSAHS in a cohort of African American and white patients diagnosed with OSAHS in an urban clinical sleep center and determined survival of the members of the cohort after approximately 10 years.
METHODS
The Human Investigation Committee of Wayne State University approved this study. Consent was waived as the study was an analysis of a database.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Previous work has shown differences in the prevalence of obstructive sleep apnea (OSA) between whites and African-Americans but there is less data as to whether there is a difference in severity of OSA between the races. We aimed to compare apnea-hypopnea index and mortality between white and African-Americans diagnosed with OSA in an urban sleep center.
Study Impact: In addition to gender and age, AHI also varies by race being more predominant in African-American men. The interaction between gender and race suggests that both neurochemical and anatomic factors contribute to the severity of OSA.
Subjects and Data Generation
We analyzed a computerized database of patients with an AHI ≥ 5/h, studied in the sleep center between July 1996 through February 1999. Patients from this time period were selected as we had self-identified racial demographics during this period, as many of these patients were also included in a previously reported study from our center.6 We chose not to include patients with an AHI < 5/h because we were interested in the influence of race on the AHI in patients with OSA, not the predictors of OSAHS in patients presenting to a sleep center, which have been determined.1,7–9 We excluded patients who underwent a split-night polysomnography to compare sleep architecture between the groups. Because there were very small numbers of other racial and ethnic groups, only African American and white patients were included in the analysis.
The following variables were included in the analysis: gender, self-identified race, age, body-mass index (BMI), neck circumference measured in the sleep center at the time of the sleep study, and comorbidities. The following comorbidities were identified as they have been associated with OSAHS: hypertension,10 diabetes mellitus,11 and heart disease.12 Presence of lung disease was also included. Comorbidities were considered present if the patient provided the diagnosis as part of the medical evaluation or if the patient was on a medication consistent with one of the comorbidities. Zip codes were used to estimate the patient's median income using the website, http://homeadvisor.msn.com. In March 2009, the Social Security Death Database (Social Security Death Index search results using http://ssdi.rootsweb.ancestry.com/cgi-bin/ssdi.cgi) was used to determine if each patient was alive or had died since the initial PSG.
Sleep-study variables included: total sleep time (TST), time in bed (TIB), sleep efficiency, percentage of the TST for different stages of sleep, and multiple indices of sleep disordered breathing including the apnea index (AI), hypopnea index (HI), apnea-hypopnea index (AHI), and the percentage of sleep time with a saturation < 90%.
Polysomnography
Polysomnography was performed as previously described.6,12 Respiration was monitored throughout the night with thermo-couples at the nose and mouth and thoracic strain gauges. Oxy-hemoglobin saturation was obtained with an oximeter. Sleep stage scoring was performed using standard criteria in use at that time.13 Apnea was defined as complete cessation of airflow for ≥ 10 sec and labeled as obstructive if there was effort noted in the strain gauge channel and central if effort was absent. Hypopnea was defined as a reduction in the airflow ≥ 10 sec associated with either an arousal or a 3% drop in the oxyhemoglobin saturation. The AHI, apnea index (AI), and hypopnea index (HI) were calculated using standard definitions.
Analysis/Statistical Methods
Prior to the statistical analysis, AHI, race, and other variables were checked for missing values and their suitability (normality of the variable dataset) for multivariate analysis. One subject had a missing value for BMI which was replaced by the mean value for all subjects. There were no other missing values. As the AHI was not normally distributed, a natural log transformation was applied. Demographic and sleep study variables were compared between the 2 groups using the Mann-Whitney rank sum test for continuous variables or χ2 test for nominal variables.
The goal of the statistical analysis was to determine the association between race and AHI while controlling for effects of confounders and effect modifiers. To achieve this goal, multivariate linear regression was used to assess the effect of race on AHI, while controlling for confounding and effect modification. In the first model, logeAHI was the dependent factor and race the independent factor. In subsequent models, a potential effect modifier, such as BMI, was added both individually and as an interaction term computed by multiplying race and the potential modifier (race*BMI). The following effect modifiers were added to the basic model: age, BMI, sex, presence of hypertension, diabetes mellitus, heart disease, and lung disease. The interaction terms were statistically significant for age (p = 0.019), sex (p = 0.011), and lung disease (p = 0.011).
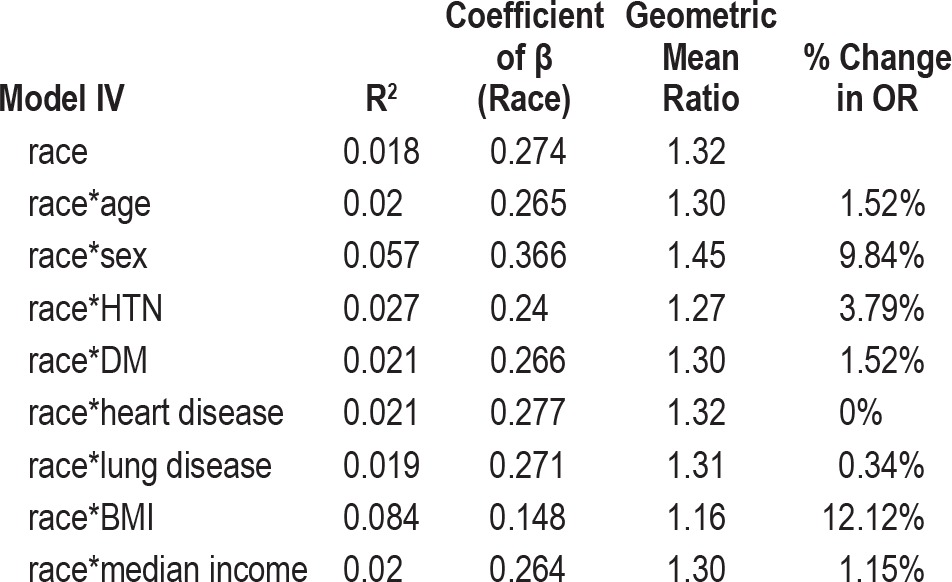
Linear regression was also used to assess potential confounders. The risk estimate (or geometric mean ratio, 1.32) associated with race was compared between a model including only race and a model including both race and the potential confounder. Sex, age, BMI, presence of hypertension, diabetes mellitus, heart disease, and lung disease were assessed as potential confounders. If the risk estimate for race differed by > 5% between the 2 models, that covariate was considered an important confounder and was included in the final model.
Variables found to be both a confounder and an effect modifier were treated as effect modifiers in the final model. As age and sex were found to be effect modifiers, age and sex stratified regression models were run with logeAHI as the independent variable and race and BMI as independent variables.
Cox regression analysis was run to identify the predictors of the mortality for the patients with the AHI > 5. Death was considered as the outcome of interest. The period of follow-up was calculated from the date of the patient having PSG to the date of death for the participants with the event, while for those without the event, the period of follow-up was calculated from the date of the PSG to date that they were checked for the mortality data. Sex, age, BMI, median income, presence of hypertension, heart disease, lung disease, diabetes mellitus, logeAHI, and logeAI were run as covariates.
RESULTS
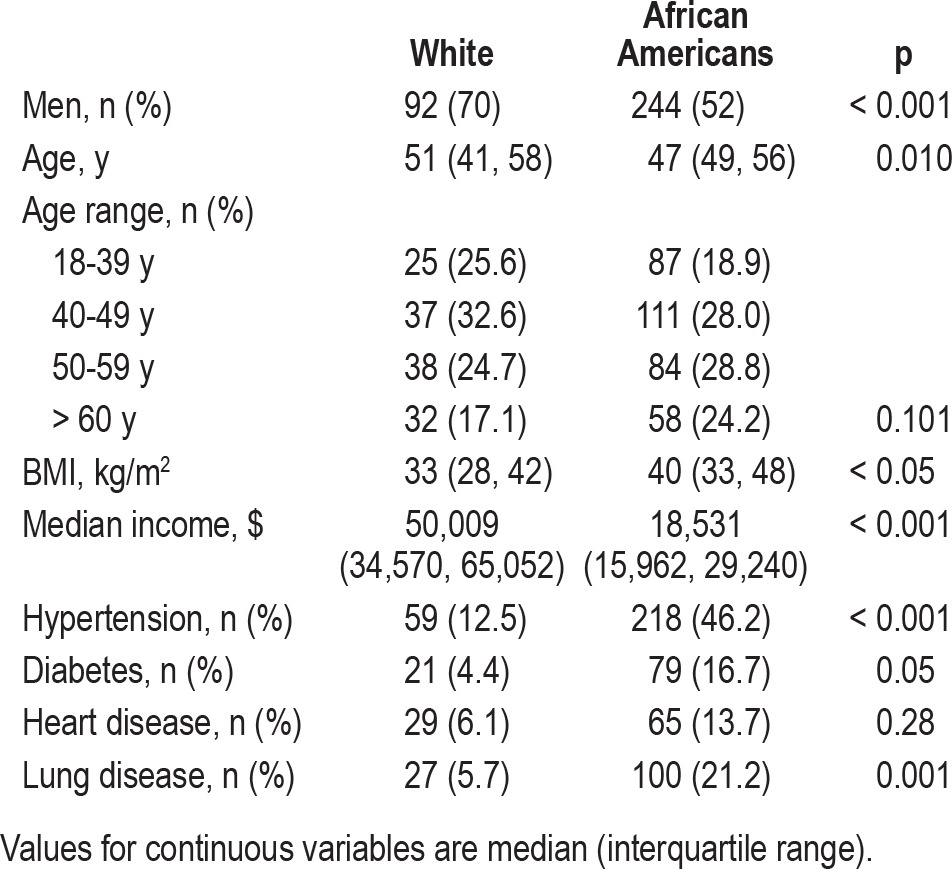
During the time period, 867 patients presented for evaluation. Of these, 272 were excluded for AHI < 5/h, and 123 were excluded for split-night studies. This resulted in 472 charts for analysis. There were 244 (52%) men and 228 (48%) women. Among the 340 African Americans, there were 152 (45%) men and 188 (55%) women. Among the 132 white patients, there were 92 (70%) men and 40 (30%) women (Table 1). The prevalence of hypertension was higher in the African Americans (46.2% v. 12.5%, p < 0.001), but there was no difference in the prevalence of diabetes, heart or lung disease between the 2 groups.
Table 1.
Patient demographics
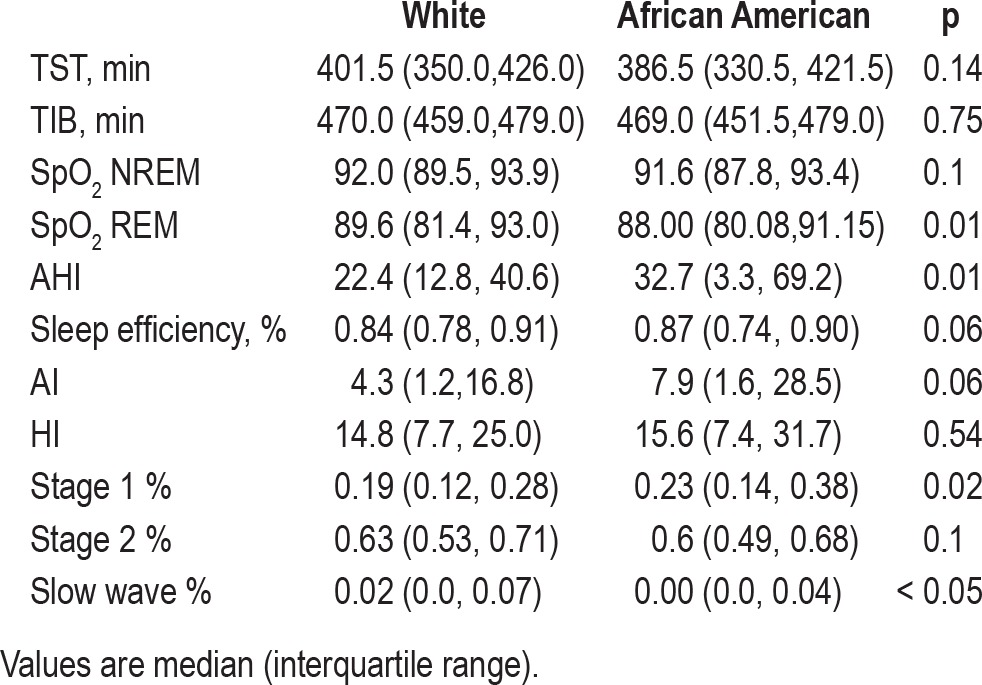
Sleep architecture and indices of sleep disordered breathing were compared by running a nonparametric test (Mann-Whitney rank sum; see Table 2). Whites and African Americans differed significantly in %Stage 1, %slow wave, AHI events/h, and SpO2 during REM; they did not differ in time in bed (TIB), total sleep time (TST), sleep efficiency, %Stage 2, %REM, AI (events/h), HI (events/h), and SpO2 in NREM.
Table 2.
Sleep study architecture and indices of sleep disordered breathing
To ascertain the independent effect of race on AHI, we built several multivariate linear regression models to assess the potential confounding and effect modification of the relationship between race and increasing AHI by age, gender, HTN, DM, heart disease, lung disease, BMI, and income (Table 3). In evaluating effect modification, we found that the relationship between race and AHI severity varied by age level (interaction p = 0.019), by gender (interaction p = 0.011), and by whether or not the patient had lung disease (interaction p = 0.011). In further modeling, the interaction term for race and lung disease was not significant, so this term was dropped. Thus, we retained interaction terms for race*age and race*gender in all future analyses. When we evaluated potential confounding (Table 4), there was a 12.1% decrease in the main effect term for race when BMI was included in the model. Based on these findings, we subsequently included BMI in all further models.
Table 3.
Measures of potential modifiers of the effect of race on sleep disordered breathing
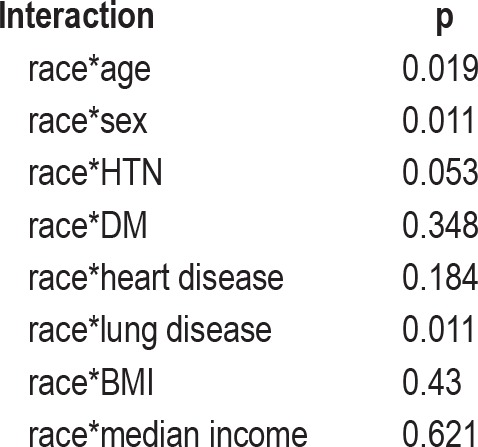
Table 4.
Measures of potential confounders of the effect of race on sleep disordered breathing, sleep disorders center
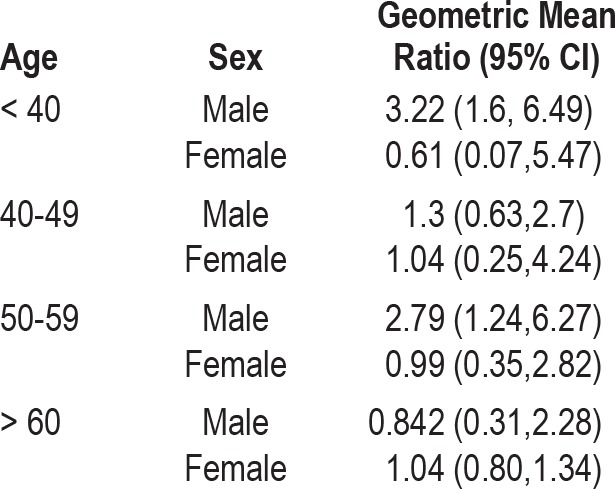
Age and gender specific regression models were run with race and BMI as the independent variable and logeAHI as the dependent variable (Table 5). In the age and gender stratified model, being an African American male ≤ 39 years increased the logeAHI by 1.17 or AHI by 3.21/h (p < 0.05) compared to a white male in the same age range with the same BMI. For the age group of 50-59 years, being an African American male increased logeAHI by 1.03 units or increased AHI by 2.79/h compared to a white male in the same age range with the same BMI.
Table 5.
Univariate estimates of the risk (risk estimate) of a one unit increase in the apnea hypopnea index in African Americans compared to whites, stratified by age and sex
The mean follow-up period was 10.5 ± 5.3 years. During the follow-up period, 20 white patients died (11.6% of group; 11 men, 9 women); in the African American group, 57 (17.1% of cohort; 34 men, 23 women) patients died. In the Cox regression analysis (Table 6 and Figure 1) for the whole cohort, only heart disease (p = 0.002), diabetes mellitus (p = 0.005), age (p < 0.001), and male gender (p = 0.027) were identified as significant predictors of the mortality for patients with AHI > 5. Baseline AHI and race were not identified as independent predictors.
Table 6.
Multivariate analysis of the risk (hazard ratio) of death associated with AHI, race, and other covariates
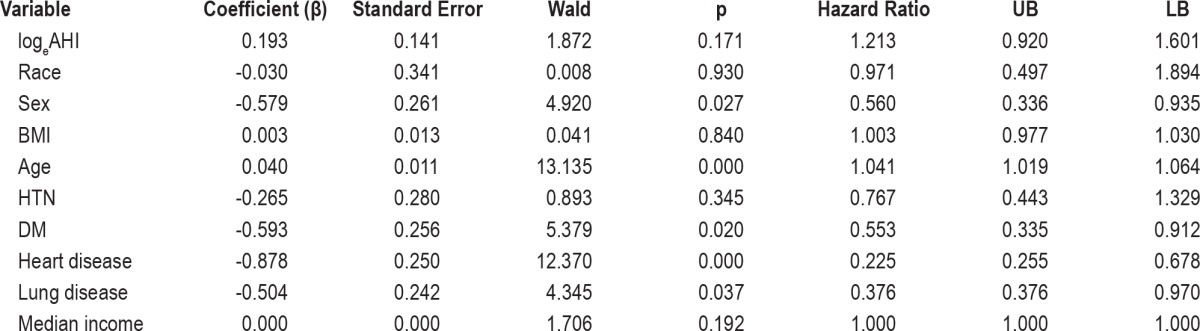
Figure 1. Kaplan-Meier survival curves for African American (dashed line) and white (solid line) patients.
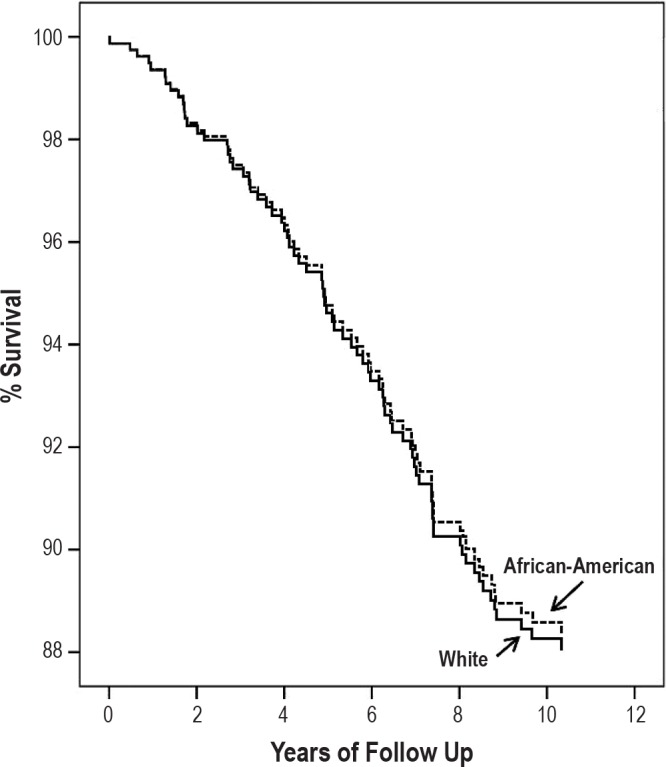
There was no difference in survival between the two groups.
In summary, AHI was higher in African American males younger than 39 years, and between 50-59 years of age. There was no difference in AHI between African American and white females. Longitudinal analysis of mortality found that race and severity of OSA were not predictors of increased mortality in patient with sleep apnea.
DISCUSSION
Our study demonstrated the following novel findings: (1) Sleep apnea severity, expressed as AHI, was higher in African American males (age ≤ 39 years and 50-59 years), even after controlling for BMI. (2) Race and AHI were not identified as independent predictors of mortality in this cohort of patients with OSAHS.
Our study demonstrated higher AHI in African American patients relative to white patients with OSAHS. Our study corroborates previous epidemiologic studies addressing the relationship between race and AHI. Data from the Cleveland Cohort Study indicated that African Americans had a higher AHI at younger ages but a similar AHI between the ages of 50 and 60 years.1 In a cohort of elderly patients, Ancoli-Israeli et al. found no difference in the overall AHI between African Americans and whites, though they did find that the AHI was higher in African Americans with severe OSAHS (AHI ≥ 30).2 Both of these studies were community-based cohorts including subjects with and without OSAHS. Our study extended the previous findings by demonstrating that the severity of OSAHS is influenced by race, age, and gender.
Epidemiologic studies have shown significant cardiovascular mortality disparities between African American and white populations.14–17 These disparities have been found to be associated with disparities in other chronic illnesses, such as diabetes and hypertension.17 There is also evidence that the degree of disparity is greater in younger than older individuals.16 OSAHS is an additional chronic illness that could contribute to mortality and, possibly, the racial disparities in cardiovascular mortality. Recent epidemiologic studies have shown that individuals with OSAHS have increased mortality compared to individuals without OSAHS.18–20 These studies have generally shown that the increased mortality was seen in individuals with severe OSAHS, generally defined as an AHI ≥ 30/h, with one of the studies showing that the effect of mortality was primarily observed in men under the age of 70 years.20 Thus, we were surprised that race and AHI did not predict mortality, even among younger African American men who had significantly higher AHI. Thus, while young and middle-aged African American men have a greater burden of disease, as measured by AHI, this burden does not translate into increased mortality when other demographic and comorbidities are considered.
We intended to study the influence of race on sleep apnea severity and mortality, we are cognizant that race is primarily a social construct. Therefore, considering racial differences in biological processes that influence OSAHS,3,21 race may represent a marker rather than a specific biologic risk factor for increased severity of AHI.22 In addition, other relevant factors, such as socioeconomic status,16,23 educational status,15 degree of urbanization,24 and access to care25 are significant determinants of morbidity and mortality and contribute to the apparent racial disparities. For this cohort, we used median income data (based upon home address zip codes) as a surrogate for socioeconomic status, but did not find that socioeconomic status was a confounder or effect modifier in this population despite the clear difference in median income between the two groups. However, income may not be sufficient to detect disparities in access to care.26 Finally, the use of race in this context could limit the generalizability to populations with different racial demographics or evolving and/or complex social settings.
Potential Mechanisms
Group differences in the prevalence and severity of OSAHS are generally attributed to group differences in either anatomic factors or neurochemical control of breathing during sleep.27 Previous work has shown that there are differences in craniofacial structure, as indicated by cephalometrics1 and anthropometric facial features,28 between African American and white men. In particular, the evidence suggests that hard tissue anatomic risk associated with shape and size of the skeletal components of the upper airway may be relatively more important in whites, while soft tissue structures may be more important in African Americans.28 We hypothesize that these anatomic differences may predispose to differences in upper airway mechanics and collapsibility between African Americans and white subjects. However, to our knowledge, there are no published reports to support this hypothesis.
Alternatively, the increased AHI in African Americans compared to whites could suggest that African Americans have an increased apneic threshold (are more susceptible to developing apnea) compared to white subjects; however, there is no experimental proof of such a difference, and preliminary data from our group does not indicate a difference in the apneic threshold.29 However, it has been shown that African American individuals have a greater ventilatory response to hypoxia21 and an increased peripheral response to hypercapnia compared to white individuals30 during NREM sleep, suggesting that differences in neurochemical control of breathing could contribute to the increased AHI in African Americans.
Our study found a significant interaction between gender and race, with the increased in AHI being found only in African American men. The above discussion on the differences in the pathogenesis of OSA suggests a potential explanation for this finding. We speculate that an increased susceptibility to hypocapnic apnea (men)31 in combination with differences in upper airway mechanics (African Americans) could result in a risk of OSA that is greater than either risk factor alone. This is, however, a hypothesis awaiting experimental proof.
In addition to pathophysiologic mechanisms, there could be cultural reasons for a difference in AHI. Specifically, reduced access to care or cultural differences in reporting symptoms of OSAHS could result in delayed presentation and higher AHI. However, in the Sleep Heart Health Study, African American women were more likely to report snoring than white women with no difference between African American and white men.32 However, there is evidence that African Americans answer and interpret elements of the Epworth Sleepiness Scale differently than whites,33 suggesting that cultural differences could exist in reporting of symptoms.
Epidemiologic studies have found an increased prevalence of OSA with increasing age.2,8,34 Ware and colleagues found that age was a significant predictor of AHI in a clinical sample.35 In this study, we found that the racial differences in AHI were most apparent in certain age groups, similar to other investigators who also found an increased AHI in younger African Americans.1 The reasons for age being an influence on AHI in only certain age ranges are unclear. Several studies of the influence of age on upper airway mechanics and control of breathing do not show an aging effect36–39 and of those that do, the decrement in function is present in an older age group, not younger.40,41
Limitations
We included comorbid conditions in the analysis because of known associations between several medical conditions and OSA, including heart disease12 and hypertension.10,11 While we did find associations for several of the comorbidities to AHI, the final regression models did not include any of these factors. There are several potential explanations. First, comorbidities other than hypertension were self-identified by the patients, and it is conceivable that patients did not disclose all relevant associated diagnoses. Second, the comorbidities could have been present but undiagnosed at the time of presentation to the sleep center. Third, the comorbidities analyzed could be either independent of or secondary to the OSA and therefore not predictive of OSA severity. For instance, comorbidities could be coexistent because of the common factor of obesity. Finally, it should be noted that we found that comorbidities such as heart disease and diabetes to be associated with a decreased risk of mortality; generally these comorbidities are associated with an increased risk of death. However, we note that those associations are generally from epidemiologic studies and ours is a clinical study. We speculate that the patients with these comorbidities were being actively treated, thus causing a potential protective effect.
Referral bias could result in our clinic population not being representative of patients with OSA. We note that in our clinic population the ratio of men to women is nearly 1:1, in contrast to the usual 3-5:1 reported in other clinic based population studies.35,42,43 Our patients are also extremely obese (mean BMI > 35 kg/m2), which also contrasts to previous clinic-based population studies. However, both of these demographic characteristics are consistent with the urban community our sleep center serves and previous studies from our center.6,44
We were unable to analyze the effect of neck circumference in this population because we only had this measurement in a subset of patients with disproportionate data collection between the gender and racial groups. Several previous studies have shown the importance of neck circumference in the prediction of sleep apnea,44–46 and some have suggested that it is a better predictor of OSA severity than BMI. It is conceivable that substitution of neck circumference into our regression analyses could have changed the results. However, in a recent study from our center, we found that both neck circumference and BMI are independent predictors of AHI in a patient population very similar to that in this study.44
O'Connor and colleagues found that men were more likely to have supine dominant OSA.42 Therefore, it is possible that the increased AHI in our study were secondary to the African American men spending more time in the supine position than white men. We do not have body position data for our patients so cannot disprove this hypothesis. However, we do not know of study that has shown that members of different races or cultures spend different amounts of time supine.
We limited our sample to patients with full-night sleep studies and those with an AHI ≥ 5/hour. We included only full-night studies so that we could compare sleep architecture between the two groups and so that all REM periods would be included in the analysis of sleep disordered breathing indices (important for patients with more mild REM-associated events). In theory, exclusion of patients with split-night studies removed patients with more severe OSAHS, thus minimizing differences between the two groups. However, split-night studies in our center can be performed for a wide range of AHI, thus minimizing this limitation. We excluded patients with AHI < 5/h, to better compare AHI in patients with sleep disordered breathing, not all patients presenting to a sleep center. However, limiting the population in this way could have introduced selection bias and favored the results showing a difference in AHI between the two races. In theory, the methodological design of a cross-sectional or longitudinal would be better suited to eliminate selection bias. In addition, that AHI was analyzed as a continuous variable, excluding patients with AHI < 5/h, could have contributed to the lack of significance in AHI predicting mortality. Finally, while the calculated power of the mortality data was 0.8, it should be noted that the sample size was determined not by a power calculation, as this was a convenience sample of patients with self-described race and sufficient follow-up.
In summary, we have extended previous findings by showing that African American men have a higher AHI than white men in certain age ranges, with no differences between African American and white women. The interaction between gender and race suggests that increased severity of sleep disordered breathing may require both an increased susceptibility to hypocapnic apnea (men) and a smaller upper airway (African Americans).
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. 1997;155:186–92. doi: 10.1164/ajrccm.155.1.9001310. [DOI] [PubMed] [Google Scholar]
- 2.Ancoli-Israel S, Klauber MR, Stepnowsky C, Estline E, Chinn A, Fell R. Sleep-disordered breathing in African-American elderly. Am J Respir Crit Care Med. 1995;152:1946–9. doi: 10.1164/ajrccm.152.6.8520760. [DOI] [PubMed] [Google Scholar]
- 3.Cakirer B, Hans MG, Graham G, Aylor J, Tishler PV, Redline S. The relationship between craniofacial morphology and obstructive sleep apnea in whites and in African-Americans. Am J Respir Crit Care Med. 2001;163:947–50. doi: 10.1164/ajrccm.163.4.2005136. [DOI] [PubMed] [Google Scholar]
- 4.Newman AB, Nieto FJ, Guidry U, et al. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. Am J Epidemiol. 2001;154:50–9. doi: 10.1093/aje/154.1.50. [DOI] [PubMed] [Google Scholar]
- 5.Dempsey JA, Skatrud JB, Jacques AJ, et al. Anatomic determinants of sleep-disordered breathing across the spectrum of clinical and nonclinical male subjects. Chest. 2002;122:840–51. doi: 10.1378/chest.122.3.840. [DOI] [PubMed] [Google Scholar]
- 6.Rowley JA, Aboussouan LS, Badr MS. The use of clinical prediction formulas in the evaluation of obstructive sleep apnea. Sleep. 2000;23:929–38. doi: 10.1093/sleep/23.7.929. [DOI] [PubMed] [Google Scholar]
- 7.Redline S, Kump K, Tishler PV, Browner I, Ferrette V. Gender differences in sleep-disordered breathing in a community-based sample. Am J Respir Crit Care Med. 1994;149:722–6. doi: 10.1164/ajrccm.149.3.8118642. [DOI] [PubMed] [Google Scholar]
- 8.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–8. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 9.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–13. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 10.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 11.Newman AB, Nieto FJ, Guidry U, et al. Relation of sleep-disordered breathing to cardiovascular disease risk factors. Am J Epidemiol. 2001;154:50–9. doi: 10.1093/aje/154.1.50. [DOI] [PubMed] [Google Scholar]
- 12.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 13.Rechtschaffen A, Kales AA. A manual of standardized terminology, techniques and scoring system for the sleep stages of human subjects. Washington, DC: National Institutes of Health; 1968. [Google Scholar]
- 14.Murray CJ, Kulkarni SC, Michaud C, et al. Eight Americas: investigating mortality disparities across races, counties, and race-counties in the United States. PLoS Med. 2006;3:e260. doi: 10.1371/journal.pmed.0030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–41. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 16.Jolly S, Vittinghoff E, Chattopadhyay A, Bibbins-Domingo K. Higher cardiovascular disease prevalence and mortality among younger blacks compared to whites. Am J Med. 2010;123:811–8. doi: 10.1016/j.amjmed.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Wong MD, Shapiro MF, Boscardin WJ, Ettner SL. Contribution of major diseases to disparities in mortality. N Engl J Med. 2002;347:1585–92. doi: 10.1056/NEJMsa012979. [DOI] [PubMed] [Google Scholar]
- 18.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 20.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crisostomo I, Zayyad A, Carley DW, et al. Chemo- and baroresponses differ in African-Americans and Caucasians in sleep. J Appl Physiol. 1998;85:1413–20. doi: 10.1152/jappl.1998.85.4.1413. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan JB, Bennett T. Use of race and ethnicity in biomedical publication. JAMA. 2003;289:2709–16. doi: 10.1001/jama.289.20.2709. [DOI] [PubMed] [Google Scholar]
- 23.Kanjilal S, Gregg EW, Cheng YJ, et al. Socioeconomic status and trends in disparities in 4 major risk factors for cardiovascular disease among US adults, 1971-2002. Arch Intern Med. 2006;166:2348–55. doi: 10.1001/archinte.166.21.2348. [DOI] [PubMed] [Google Scholar]
- 24.Erwin PC, Fitzhugh EC, Brown KC, Looney S, Forde T. Health disparities in rural areas: the interaction of race, socioeconomic status, and geography. J Health Care Poor Underserved. 2010;21:931–45. doi: 10.1353/hpu.0.0336. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Geiss LS, Cheng YJ, Beckles GL, Gregg EW, Kahn HS. The missed patient with diabetes: how access to health care affects the detection of diabetes. Diabetes Care. 2008;31:1748–53. doi: 10.2337/dc08-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moy E, Bartman BA, Weir MR. Access to hypertensive care. Effects of income, insurance, and source of care. Arch Intern Med. 1995;155:1497–502. [PubMed] [Google Scholar]
- 27.Badr MS. Pathophysiology of upper airway obstruction during sleep. Clin Chest Med. 1998;19:21–32. doi: 10.1016/s0272-5231(05)70429-9. [DOI] [PubMed] [Google Scholar]
- 28.Cakirer B, Hans MG, Graham G, Aylor J, Tishler PV, Redline S. The relationship between craniofacial morphology and obstructive sleep apnea in whites and in African-Americans. Am J Respir Crit Care Med. 2001;163:947–50. doi: 10.1164/ajrccm.163.4.2005136. [DOI] [PubMed] [Google Scholar]
- 29.Rowley JA, Badr MS. Determinants of the hypocapnic apneic threshold: influence of race. Am J Respir Crit Care Med. 2007;175:A995. [Google Scholar]
- 30.Bobat I, Mateika JH, Qureshi T, Badr MS. Peripheral not central chemoreflex responsiveness is enhanced in African-Amercians compared to Caucasians during wakefulness. Am J Respir Crit Care Med. 2004;169:A60. [Google Scholar]
- 31.Zhou XS, Shahabuddin S, Zahn BR, Babcock MA, Badr MS. Effect of gender on the development of hypocapnic apnea/hypopnea during NREM sleep. J Appl Physiol. 2000;89:192–9. doi: 10.1152/jappl.2000.89.1.192. [DOI] [PubMed] [Google Scholar]
- 32.O'Connor GT, Lind BK, Lee ET, et al. Variation in symptoms of sleep-disordered breathing with race and ethnicity: the Sleep Heart Health Study. Sleep. 2003;26:74–9. [PubMed] [Google Scholar]
- 33.Hayes AL, Spilsbury JC, Patel SR. The Epworth score in African American populations. J Clin Sleep Med. 2009;5:344–8. [PMC free article] [PubMed] [Google Scholar]
- 34.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14:486–95. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ware JC, McBrayer RH, Scott JA. Influence of sex and age on duration and frequency of sleep apnea events. Sleep. 2000;23:165–70. 15. [PubMed] [Google Scholar]
- 36.Browne HA, Adams L, Simonds AK, Morrell MJ. Ageing does not influence the sleep-related decrease in the hypercapnic ventilatory response. Eur Respir J. 2003;21:523–9. doi: 10.1183/09031936.03.00039002. [DOI] [PubMed] [Google Scholar]
- 37.Browne HA, Adams L, Simonds AK, Morrell MJ. Impact of age on breathing and resistive pressure in people with and without sleep apnea. J Appl Physiol. 2001;90:1074–82. doi: 10.1152/jappl.2001.90.3.1074. [DOI] [PubMed] [Google Scholar]
- 38.Rowley JA, Zhou X, Vergine I, Shkoukani MA, Badr MS. Influence of gender on upper airway mechanics: upper airway resistance and Pcrit. J Appl Physiol. 2001;91:2248–54. doi: 10.1152/jappl.2001.91.5.2248. [DOI] [PubMed] [Google Scholar]
- 39.Pokorski M, Marczak M. Ventilatory response to hypoxia in elderly women. Ann Hum Biol. 2003;30:53–64. doi: 10.1080/03014460210162000. [DOI] [PubMed] [Google Scholar]
- 40.Fogel RB, White DP, Pierce RJ, et al. Control of upper airway muscle activity in younger versus older men during sleep onset. J Physiol. 2003;553:533–44. doi: 10.1113/jphysiol.2003.045708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Worsnop C, Kay A, Kim Y, Trinder J, Pierce R. Effect of age on sleep onset-related changes in respiratory pump and upper airway muscle function. J Appl Physiol. 2000;88:1831–9. doi: 10.1152/jappl.2000.88.5.1831. [DOI] [PubMed] [Google Scholar]
- 42.O'Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:1465–72. doi: 10.1164/ajrccm.161.5.9904121. [DOI] [PubMed] [Google Scholar]
- 43.Kushida CA, Efron B, Guilleminault C. A predictive morphometric model for the obstructive sleep apnea syndrome. Ann Intern Med. 1997;127:581–7. doi: 10.7326/0003-4819-127-8_part_1-199710150-00001. [DOI] [PubMed] [Google Scholar]
- 44.Tashkandi Y, Badr MS, Rowley JA. Determinants of the apnea index in a sleep center population. Sleep Breath. 2005;9:181–6. doi: 10.1007/s11325-005-0034-x. [DOI] [PubMed] [Google Scholar]
- 45.Young T, Palta M, Badr MS. Sleep-disordered breathing (letter) N Engl J Med. 1993;329:1429–30. [Google Scholar]
- 46.Stradling JR, Crosby JH. Predictors and prevalence of obstructive sleep apnea and snoring in 1001 middle aged men. Thorax. 1991;46:85–90. doi: 10.1136/thx.46.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]


