Abstract
Study Objectives:
CPAP is used as the first-line treatment for patients with severe OSA, but this machine is not always feasible to use on the long term. We performed a clinical trial to determine whether patients with OSA could use a mandibular advancement splint (MAS) as a short-term treatment alternative to CPAP.
Methods:
Twenty-two patients adherent with CPAP therapy were recruited to the study. Each patient used the MAS for approximately 4 months. The transition between CPAP to MAS was gradual, and patients were asked to start using MAS together with CPAP during the MAS titration until subjective improvement or maximum mandibular advancement was achieved. Sleepiness (ESS), quality of life (SAQLI), and polysomnography were recorded prior to and after MAS titration. Patients recorded CPAP or MAS usage for the following 3 months.
Results:
Seven women and 12 men with a mean age of 53.8 (± 12.1) years and mean body mass index of 28.1 (± 4.8) kg/m2 completed the clinical trial. Prior to MAS, CPAP adherence was 5.8 h/night. AHI decreased significantly with MAS use compared to baseline (30.7 ± 23.1 vs 13.2 ± 11; p < 0.01). Fourteen patients (74%) had > 50% decrease in their AHI, while 2 patients had an increase in their AHI. There were no significant differences in SAQLI between MAS and CPAP treatment, while ESS decreased significantly on MAS. MAS self-reported usage was correlated with treatment efficacy (r = 0.52; p < 0.05). Seventy-five percent of the patients reported being sufficiently satisfied with MAS to continue to use it as an alternative short-term therapy.
Conclusions:
MAS partially or completely reduced sleep disordered breathing in the majority of selected, successfully CPAP-treated severe OSA patients. Many patients can probably effectively use MAS as a short-term treatment alternative to CPAP.
Clinical Trial Registration:
ClinicalTrials.gov registration number NCT00358605.
Commentary:
A commentary on this article appears in this issue on page 325.
Citation:
Almeida FR; Mulgrew A; Ayas N; Tsuda H; Lowe AA; Fox N; Harrison S; Fleetham JA. Mandibular advancement splint as short-term alternative treatment in patients with obstructive sleep apnea already effectively treated with continuous positive airway pressure. J Clin Sleep Med 2013;9(4):319-324.
Keywords: Obstructive sleep apnea, CPAP, oral appliance, mandibular advancement splint, alternative treatment
Obstructive sleep apnea (OSA) is a common syndrome that is characterized by recurrent episodes of partial or complete upper airway obstruction during sleep. OSA is associated with reduced quality of life, decreased cardiovascular health and increased healthcare utilization, car crashes, and mortality.1,2 There are a variety of treatment options currently available for OSA including lifestyle modifications, continuous positive airway pressure (CPAP), mandibular advancement splints (MAS), and corrective upper airway surgery. CPAP is the most efficient treatment for OSA and has been demonstrated to improve daytime symptoms and reduce cardiovascular disease and car crashes.3,4 Despite improvements in the portability of CPAP machines, many patients find this treatment not feasible when away from home. CPAP machines require access to a reliable electrical source while asleep. This can sometimes be provided by a battery, but is quite cumbersome and needs periodic recharging. Many patients faced with short-term difficulties complying with CPAP during periods of travel or vacation stop treatment in these circumstances. MAS are now widely used for the treatment of OSA both as a primary therapy and as an alternative for patients with severe OSA who are unwilling or unable to tolerate CPAP.5 MAS are a simple, reversible, quiet, and cost effective therapy for selected patients with OSA.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Many patients with OSA who are under CPAP treatment will experience temporary circumstances during which CPAP is not convenient, and therefore adherence is compromised. There is a lack of studies evaluating the combination of CPAP and OA where patients can alternate between the two therapies.
Study Impact: This study may impact clinical treatment recommendations with a patient-centered treatment approach. Patients can express treatment preferences; deliberate the options; and agree on a treatment plan implementing both devices (CPAP and OA). This combination of therapies allows greater flexibility of treatment and opportunity for ongoing adherence during those temporary circumstances in which CPAP cannot be used.
In randomized controlled trials comparing both modalities of treatment, CPAP therapy is consistently more effective in reducing sleep disordered breathing events.6–9 While CPAP and MAS have similar subjective and cardiovascular outcomes, patients tolerate MAS better.8–11 The superior patient satisfaction associated with the use of MAS reflects the relative simplicity and convenience of this form of treatment. Patients are offered one or the other treatment, but the combination of these two specific therapies warrants further investigation. To our knowledge, there has only been one previous study12 that has attempted the combination of these two therapies, with the disadvantage of using a non-titratable device without a follow-up by a dentist for adjustments. The objective of this study was to determine the feasibility of using MAS as an alternative treatment in patients previously established on CPAP. This combination of treatments would permit greater flexibility and improved treatment adherence in circumstances where CPAP cannot be tolerated.
METHODS
Participants
Patients with symptomatic OSA and an apnea-hypopnea index (AHI) > 10 events/h adherent with CPAP (> 4 h/night) for a minimum period of 3 months were recruited to the study. Patients were excluded if they had any comorbid diseases, had a safety-critical occupation, were looking for an alternative treatment to stop CPAP treatment, or had dental contraindications to MAS treatment, (< 8 teeth per arch or severe periodontal disease).13 This study was approved by the UBC Human Ethics Committee, and all patients signed a consent form prior to inclusion in the study.
Polysomnography
All patients had baseline diagnostic polysomnography (PSG) prior to initiation of CPAP treatment. The laboratory PSG was performed according to practice parameters published by the American Sleep Disorders Association Standards of Practice Committee.14 Standard measurements included electroencephalography (EEG), electrooculography (EOG), submental and tibial electromyography (EMG), electrocardiography (ECG), chest and abdominal respiratory impedance plethysmography (Respitrace; Ambulatory Monitoring Systems, Ardsley, NY), arterial oxygen saturation was recorded using a pulse oximeter (Model N-100; Nellcor Inc., Hayward, CA, USA), and nasal airflow was recorded using nasal cannulae connected to a pressure transducer (Ultima Airflow Pressure Sensor; Braebon Medical Corp., Carp, Ontario, Canada). PSG data were analyzed manually according to AASM criteria by a certified polysomnographic technologist. All patients were prescribed CPAP and had used it consistently for a minimum of 3 months. CPAP adherence was objectively measured prior to recruitment.
Questionnaires
Patients completed an Epworth Sleepiness Scale (ESS)15 and quality of life and side effects (SAQLI) questionnaires16 at recruitment while on CPAP. ESS and SAQLI were repeated after MAS titration and while on MAS for ≥ 2 weeks. Patients were then informed of the results of the PSG and were instructed to use either the MAS or CPAP at their discretion for the following 3 months. They were instructed to keep a 3-month diary where they tracked which treatment was used each night. After this 3-month period, patient and partner satisfaction were assessed using visual analog scales. At the end of the study, patients were asked whether they were sufficiently satisfied with MAS to continue with it as a short-term treatment alternative and/or purchase it at a similar cost to CPAP.
MAS Protocol
Patients were fitted with a titratable MAS (Klearway).17 To ensure safety and adherence, the transition between CPAP to MAS was gradual, and patients were asked to start using MAS together with CPAP. Patients were instructed to advance the MAS 0.5 mm per week. Patients were then advised to stop using the CPAP for 1 or 2 nights to evaluate the efficacy of the MAS once a month. CPAP pressure was not adjusted during treatment, since after titration patients would use only one treatment at a time. Patients were evaluated by the dentist once a month, and further titration was recommended based on the patient's subjective evaluation. Once patients felt that the MAS had either successfully reduced the snoring and sleepiness or if the maximum mandibular advancement had been achieved, patients underwent PSG with the MAS and also repeated the ESS and SAQLI questionnaires. Treatment success was defined as AHI ≤ 10; suboptimal treatment if 20 ≤ AHI > 10; or treatment failure if AHI > 20 or ESS > 9 or ESS > 4 CPAP value. Treatment efficacy was also described as a decrease in the AHI greater than 50%, a decrease of less than 50%, or increase of the AHI compared to the baseline AHI. The clinical trial was registered as ClinicalTrials.gov registration number NCT00358605.
Study Design
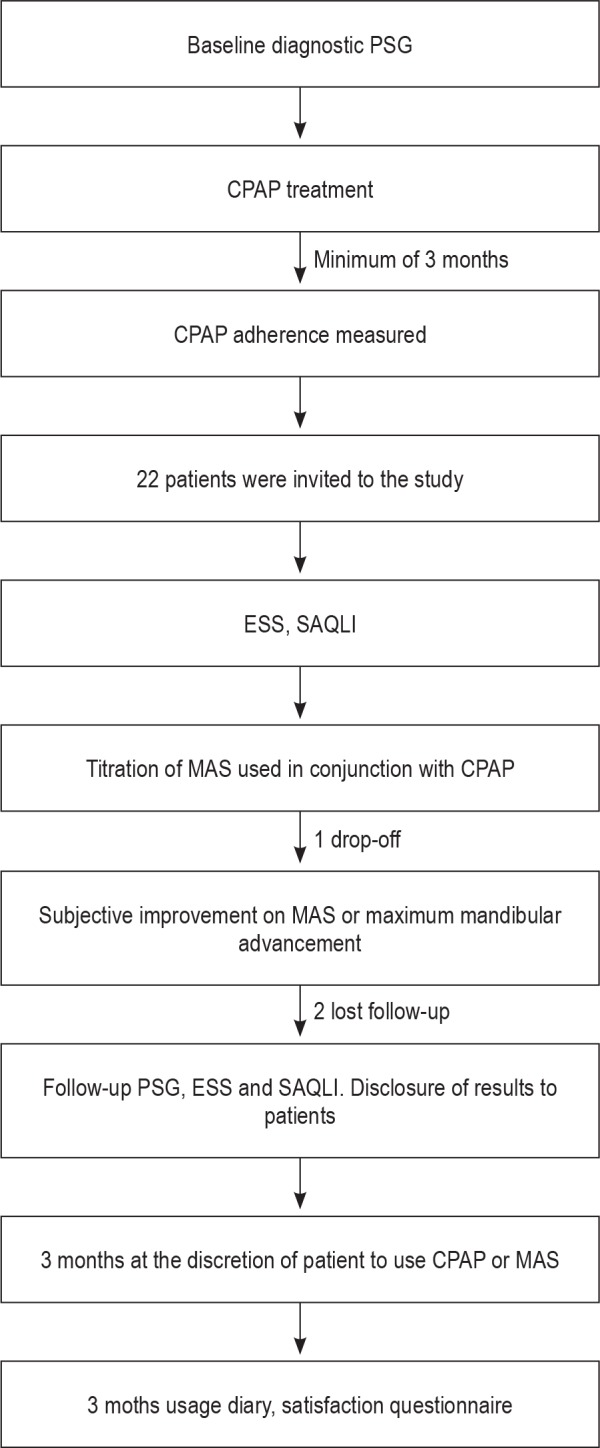
The study design included the patient's diagnostic PSG and the assurance that patients were adherent to CPAP for a minimum of a 3-month period. Once patients were included in the study, they answered the ESS and SAQLI while on CPAP. All patients received a custom-made adjustable MAS. After the adjustment period, all patients underwent a repeat PSG, ESS, and SAQLI while on MAS treatment alone. After MAS outcomes disclosure, patients were followed for 3 months with a treatment use diary where they could use CPAP or MAS at their will. A schematic flow chart of the study design is shown in Figure 1.
Figure 1. Schematic flow chart of the study design.
Statistical Analysis
Statistical analyses were performed using SPSS version 15.0 (SPSS Inc., Chicago, Illinois, USA). Descriptive data were presented by percentage, mean, and standard deviation. Comparisons between baseline and follow-up for AHI, ESS, SAQLI, and treatment usage were analyzed using paired Student t-tests, and Pearson correlation tests were applied to determine the correlation between MAS AHI and days per month of MAS usage. A p value of 0.5 was considered statistically significant.
RESULTS
Participants
Nineteen of 22 patients recruited for the study completed the trial. One patient could not tolerate the MAS due to excessive salivation, and 2 patients were unable to attend the follow-up PSG. Seven women and 12 men completed the clinical trial with a mean age of 53.8 (± 12.1) years and a mean body mass index (BMI) of 28.1 (± 4.8) kg/m2. CPAP adherence was 5.8 (± 0.8) h per night prior to the study. Baseline demographic data is provided in Table 1.
Table 1.
Demographic data for 19 sleep apnea patients who completed the study

Polysomnography
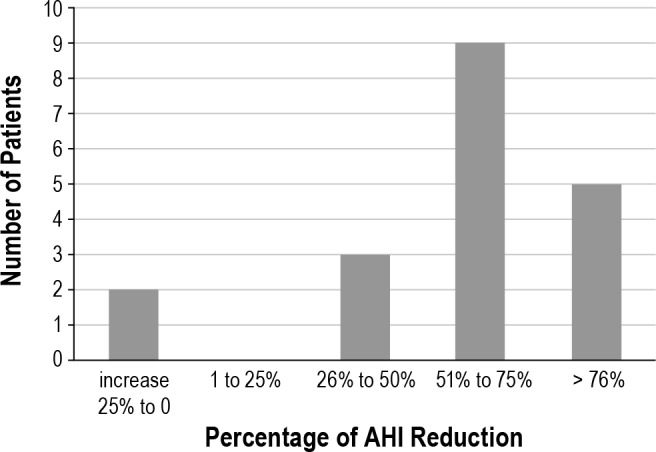
Baseline AHI compared to AHI while on MAS treatment decreased significantly from 30.7 ± 23.1 to 13.2 ± 11.0 events/h (p < 0.01). Patient baseline and follow-up (MAS) AHI data are shown in Figure 2. The MAS successfully treated 47% of the patients, while 21% reached suboptimal results and 32% were considered treatment failures. Fourteen of 19 patients (74%) showed a reduction ≥ 50% in the AHI. Further changes in the AHI expressed as percentage are shown in Figure 3. In the evaluation of sleep quality, MAS treatment did not change sleep architecture (as percentages of sleep stage N1, N2, N3, or REM) and did not significantly improve the mean oxygen saturation.
Figure 2. Significant reduction of the apnea and hypopnea index when comparing no treatment to MAS treatment (p < 0.01).
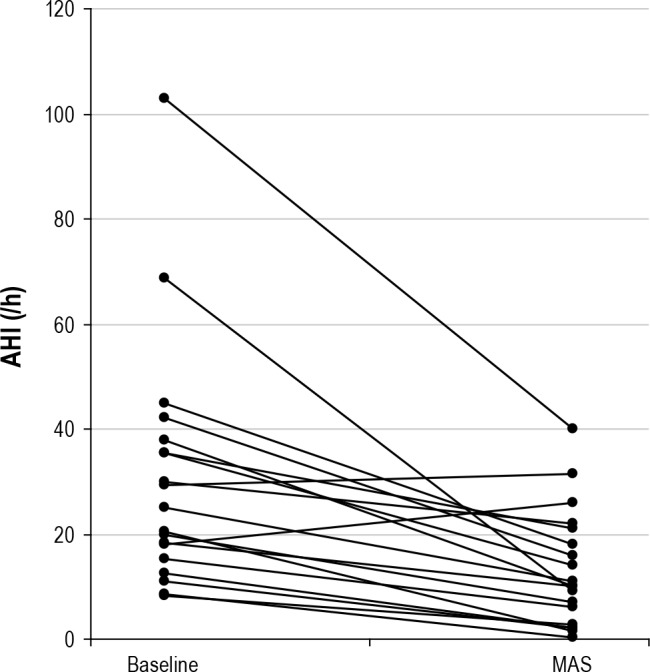
Figure 3. Distribution of the number of patients with their respective percentage of change in the AHI.
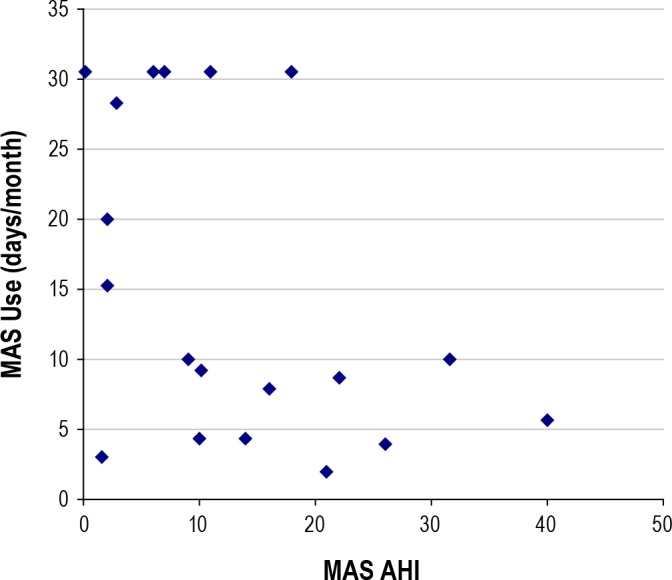
In the 3-month follow-up period, MAS usage reflected treatment efficacy, with a significant negative correlation between days per month of MAS use and MAS AHI (r = -0.45 p < 0.05; Figure 4). There were two exceptions, one patient in whom the MAS was highly effective but used it infrequently, and one patient used the MAS exclusively despite MAS treatment AHI > 20/h.
Figure 4. Significant correlation between AHI when wearing a MAS (MAS-AHI) and days per month of MAS usage (r = -0.454; p = 0.05).
Questionnaires
While on CPAP treatment, patients reported a mean ESS of 8.5 and a SAQLI score of 4.8. There was significant improvement in ESS while on MAS compared to CPAP (5.5 ± 3.0 vs 8.5 ± 4.3) and no difference in SAQLI while on MAS compared to CPAP (5.5 ± 0.9 vs 4.8 ± 0.7). There was no significant difference in reported side effects with MAS compared to CPAP, but MAS side effects (salivation and jaw discomfort) were more common in patients who were infrequent users of MAS. Six patients (31%) used MAS exclusively during the first month of follow-up. There was no significant difference between treatment nights per month (16.1 ± 12.1 nights on CPAP and 15.0 ± 11.4 nights on MAS). Sixteen patients (84%) reported wearing MAS while on vacation or traveling, and 11 (58%) reported using it at home. Sixteen patients completed a follow-up questionnaire. Patient preference between treatments did not show a statistical significant difference, as 56% of the patients preferred the MAS, 31% preferred CPAP, and 13% responded that they had no preference. In terms of patient perception of treatment efficacy, 19% of the patients felt that the MAS was more effective than CPAP, 44% felt both treatments were equally effective, and 37% felt that CPAP was more effective than MAS, which was not significantly different. When bed partners were asked about treatment preference, a significantly higher number of patients preferred MAS treatment (88%), while 6% preferred CPAP and 6% showed no preference (Figure 5). At the end of the trial, 85% of the patients reported using MAS, and 75% of them said they would purchase the MAS at a similar cost to the CPAP.
Figure 5. Subjective evaluation of treatment.
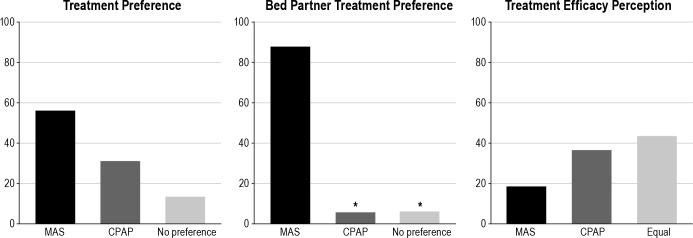
Data presented as percentage of patients. *CPAP and No preference significantly different than MAS preference, p < 0.05.
DISCUSSION
We have demonstrated that patients with OSA established on CPAP can use MAS as an alternative treatment. MAS was effective in reducing sleep disordered breathing in the majority of patients. With the exception of one patient, all subjects found MAS useful and have subsequently alternated between CPAP and MAS at home or used it solely for traveling. For patients adherent to CPAP treatment, this modality is well accepted and not inconvenient, although many patients may not use it while traveling. We have demonstrated that MAS is useful as an alternative therapy and patients will tend to use MAS while traveling instead of avoiding treatment in these circumstances. This is the first study to use titratable MAS on patients compliant to CPAP that investigates the applicability of having an alternate treatment for traveling.
Treatment preference outcomes, with a higher percentage of patients preferring MAS over CPAP, were similar to previous studies,9,10 although this is the first report to evaluate and describe a significantly higher preference in favor of MAS by the bed partner. The opportunity for an easier treatment approach was well received by most patients and bed partners, and we further hypothesize that these patients will have a tendency to spend fewer days without the use of any treatment modality. The treatment approach to OSA should always be individualized; a patient-tailored therapy requires patient involvement in planning and decision-making in their treatment. Patient lifestyle, including consistent access to a reliable electrical source while asleep, should be evaluated; patients may be frustrated if their needs are not taken into account.18
For the evaluation of the treatment effects on sleepiness and quality of life, previous studies have shown that patients with OSA present with a significant impairment in daytime sleepiness, quality of life, and neurocognitive function, which can be improved with CPAP and MAS therapy.6–9,19 Randomized control trials have shown no significant differences in subjective sleepiness and quality of life when comparing CPAP to MAS.8,20,21 Our study showed an interesting and important finding in terms of daytime sleepiness. When compared to sleepiness on CPAP, patients showed a significant decrease in their ESS score when using MAS. One could hypothesize that patients were less likely to occasionally drop treatment, and therefore the long-term effects on sleepiness were further consolidated.
The level of CPAP compliance effects on sleepiness has been widely studied. The assumption of good CPAP compliance use is commonly described as a 4 h per night on 70% of nights.22 Antic and colleagues,23 though, identified a treatment dose effect for subjective sleepiness, where a greater percentage of patients achieved normal functioning with longer use of CPAP. Based on our results, we hypothesize that combination therapy may be similar to higher hours to CPAP use, and combination therapy might be superior to CPAP alone in terms of the subjective sleepiness outcomes.
Quality of life is often measured in comparative studies. Similar to the present trial, previous studies found similar efficacy in MAS and CPAP when assessed by FOSQ, SF-36, anxiety and depression scales, or Nothingham health profiles.8,9,20 In contrast, there are two studies showing a greater impact of CPAP compared to MAS in quality of life.21,24 The appliance used in these studies was not titratable and thus likely did not achieve ideal mandibular protrusion with less efficacy than a titratable MAS. According to Lettieri and collaborators,25 adjustable devices are superior to single jaw position appliances and produce a greater reduction in the AHI, especially in moderate to severe OSA.
With regards to the type of MAS treatment, titratable MAS have higher efficacy rates,25 and a careful follow-up by the dentist is recommended by the American Academy of Sleep Medicine.26 We are aware of one previous study which addressed the feasibility of using MAS as an adjunct treatment in patients previously established on CPAP.12 The study used a non-adjustable MAS at 75% of mandibular advancement. Some patients had the appliance mailed to them, and there was minimal dental follow-up. The study measured CPAP and MAS efficacy with a portable monitor rather than detailed polysomnography. Without the refined adjustments and progressive titration of the MAS conducted by a dentist, of the 50 patients in that study who were fitted for MAS, only 9 were able to complete the study and 31 patients withdrew due to side effects from the MAS. The authors speculate that “insecurities” related to withdrawal from CPAP therapy as well as the use of a relatively simple MAS may have been contributory factors to the low acceptance rate. The current study also reinforces that a specialized dentist in the field should be responsible for insertion of the device and follow-up of the patients and that gradual titration of the mandible is extremely important to improve MAS efficacy. Despite the use of titration, according to our study protocol, patients were not asked to further adjust their appliances after the follow-up PSG. It is common in clinical practice to continue mandibular advancement if residual apneas are seen and further protrusion is possible. Therefore, an even higher success rate may have been achieved. Titration procedures are extremely important and are clearly related to MAS adherence and efficacy.27
The effect of treatment in the reduction of apneas is very important. Our study raises one issue in that some of our patients effectively treated with CPAP and provided with short-term MAS treatment subsequently discontinued their CPAP treatment in favor of MAS. It is imperative that the patients are aware of the efficacy of MAS. In the present study we found a positive correlation between the reduction in the AHI and MAS usage, showing that the allowance for patients to utilize both therapies is safe. In this regard, a follow-up sleep assessment should always be considered as part of a treatment protocol and in every patient with OSA treated with MAS. Despite the presence of residual apneas and the inferior efficacy of MAS compared to CPAP, it is interesting that some studies have shown that with a higher adherence to MAS, patients have similar health outcomes related to blood pressure8 and endothelial function.28 In a recent study, Lam and collaborators29 evaluated a group of severe OSA patients with recent diagnosed hypertension or on hypertensive drugs and found that despite the partial reduction in the AHI (67 to 25/h), there was significant improvement in the systolic and diastolic blood pressure measurements after one year of treatment.
This study has some clear limitations. Patients recruited to the study were biased to those who were willing to try another therapy because of lifestyle issues. It is possible that these patients, despite good initial reported CPAP adherence, were not as compliant to treatment on the long-term. Objective MAS adherence could not be obtained, as there was no readily available MAS adherence monitor in the market at the time of this study. Subjective treatment adherence reporting generally overestimates actual objective treatment adherence. This is a short-term study; the evaluation of usage of these treatments over a one-year period would have been ideal.
Despite the short-term evaluation, we believe this study reflects some patient behavior in daily life, such as changing treatment for relief of side effects or for convenience during travel. A different treatment option is likely useful when patients are experiencing specific side effects or discomfort, which could be relieved with the other form of therapy or while away from home.
The present study suggests that the mandibular advancement splint is a safe short-term alternative treatment approach for CPAP compliant patients. Since patient involvement in treatment choices may increase adherence with treatment; patient involvement would tend to increase overall adherence in the long term and decrease subjective sleepiness. Patient-tailored treatment is synonymous with good medicine, and life-long therapies are dependent on the patient's cooperation and adherence. We believe that it is important to include patients in decisions about their treatment and to offer more than one type of therapy for OSA patients who are potentially good candidates for MAS therapy.
CONCLUSIONS
A majority of patients with OSA already successfully treated with CPAP can effectively be treated with MAS as a short-term alternative treatment when travelling, when an electrical supply is not available, or on days that patients are not willing to use CPAP and tend to forgo treatment. A combination of therapies allows greater flexibility of treatment and opportunity for ongoing adherence in circumstances where CPAP cannot be used.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was performed at the University of British Columbia, approved by the UBC Human Ethics Committee and all patients signed a consent form prior to inclusion in the study. This study was supported by the BC Lung Foundation, a non-profit organization.
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Flemons WW. Clinical practice. Obstructive sleep apnea. N Engl J Med. 2002;347:498–504. doi: 10.1056/NEJMcp012849. [DOI] [PubMed] [Google Scholar]
- 3.George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax. 2001;56:508–12. doi: 10.1136/thorax.56.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy P, Pepin JL, McNicholas WT. Should all sleep apnoea patients be treated? Yes. Sleep Med Rev. 2002;6:17–26. doi: 10.1053/smrv.2002.0209. discussion 7. [DOI] [PubMed] [Google Scholar]
- 5.Kushida CA, Morgenthaler TI, Littner MR, et al. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep. 2006;29:240–3. doi: 10.1093/sleep/29.2.240. [DOI] [PubMed] [Google Scholar]
- 6.Aarab G, Lobbezoo F, Heymans MW, Hamburger HL, Naeije M. Long-term follow-up of a randomized controlled trial of oral appliance therapy in obstructive sleep apnea. Respiration. 2011;82:162–8. doi: 10.1159/000324580. [DOI] [PubMed] [Google Scholar]
- 7.Aarab G, Lobbezoo F, Hamburger HL, Naeije M. Oral appliance therapy versus nasal continuous positive airway pressure in obstructive sleep apnea: a randomized, placebo-controlled trial. Respiration. 2011;81:411–9. doi: 10.1159/000319595. [DOI] [PubMed] [Google Scholar]
- 8.Barnes M, McEvoy RD, Banks S, et al. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:656–64. doi: 10.1164/rccm.200311-1571OC. [DOI] [PubMed] [Google Scholar]
- 9.Gagnadoux F, Fleury B, Vielle B, et al. Titrated mandibular advancement versus positive airway pressure for sleep apnoea. Eur Respir J. 2009;34:914–20. doi: 10.1183/09031936.00148208. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson KA, Ono T, Lowe AA, Keenan SP, Fleetham JA. A randomized crossover study of an oral appliance vs nasal-continuous positive airway pressure in the treatment of mild-moderate obstructive sleep apnea. Chest. 1996;109:1269–75. doi: 10.1378/chest.109.5.1269. [DOI] [PubMed] [Google Scholar]
- 11.Trzepizur W, Gagnadoux F, Abraham P, et al. Microvascular endothelial function in obstructive sleep apnea: Impact of continuous positive airway pressure and mandibular advancement. Sleep Med. 2009;10:746–52. doi: 10.1016/j.sleep.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Smith DM, Stradling JR. Can mandibular advancement devices be a satisfactory substitute for short term use in patients on nasal continuous positive airway pressure? Thorax. 2002;57:305–8. doi: 10.1136/thorax.57.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almeida FR, Lowe AA. Principles of oral appliance therapy for the management of snoring and sleep disordered breathing. Oral Maxillofac Surg Clin North Am. 2009;21:413–20. doi: 10.1016/j.coms.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Chesson AL, Jr., Ferber RA, Fry JM, et al. The indications for polysomnography and related procedures. Sleep. 1997;20:423–87. doi: 10.1093/sleep/20.6.423. [DOI] [PubMed] [Google Scholar]
- 15.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 16.Flemons WW, Reimer MA. Development of a disease-specific health-related quality of life questionnaire for sleep apnea. Am J Respir Crit Care Med. 1998;158:494–503. doi: 10.1164/ajrccm.158.2.9712036. [DOI] [PubMed] [Google Scholar]
- 17.de Almeida FR, Lowe AA, Tsuiki S, et al. Long-term compliance and side effects of oral appliances used for the treatment of snoring and obstructive sleep apnea syndrome. J Clin Sleep Med. 2005;1:143–52. [PubMed] [Google Scholar]
- 18.Sabaté E WHO Adherence to Long Term Therapies Project., Global Adherence Interdisciplinary Network., World Health Organization. Dept. of Management of Noncommunicable Diseases. Adherence to long-term therapies: evidence for action. Geneva: World Health Organization; 2003. [Google Scholar]
- 19.Gotsopoulos H, Kelly JJ, Cistulli PA. Oral appliance therapy reduces blood pressure in obstructive sleep apnea: a randomized, controlled trial. Sleep. 2004;27:934–41. doi: 10.1093/sleep/27.5.934. [DOI] [PubMed] [Google Scholar]
- 20.Hoekema A, Voors AA, Wijkstra PJ, et al. Effects of oral appliances and CPAP on the left ventricle and natriuretic peptides. Int J Cardiol. 2008;128:232–9. doi: 10.1016/j.ijcard.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Lam B, Sam K, Mok WY, et al. Randomised study of three non-surgical treatments in mild to moderate obstructive sleep apnoea. Thorax. 2007;62:354–9. doi: 10.1136/thx.2006.063644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–9. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antic NA, Catcheside P, Buchan C, et al. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep. 2011;34:111–9. doi: 10.1093/sleep/34.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engleman HM, McDonald JP, Graham D, et al. Randomized crossover trial of two treatments for sleep apnea/hypopnea syndrome: continuous positive airway pressure and mandibular repositioning splint. Am J Respir Crit Care Med. 2002;166:855–9. doi: 10.1164/rccm.2109023. [DOI] [PubMed] [Google Scholar]
- 25.Lettieri CJ, Paolino N, Eliasson AH, Shah AA, Holley AB. Comparison of adjustable and fixed oral appliances for the treatment of obstructive sleep apnea. J Clin Sleep Med. 2011;7:439–45. doi: 10.5664/JCSM.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanderveken OM, Devolder A, Marklund M, et al. Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am J Respir Crit Care Med. 2008;178:197–202. doi: 10.1164/rccm.200701-114OC. [DOI] [PubMed] [Google Scholar]
- 27.de Almeida FR. Complexity and efficacy of mandibular advancement splints: understanding their mode of action. J Clin Sleep Med. 2011;7:447–8. doi: 10.5664/JCSM.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itzhaki S, Dorchin H, Clark G, Lavie L, Lavie P, Pillar G. The effects of 1-year treatment with a herbst mandibular advancement splint on obstructive sleep apnea, oxidative stress, and endothelial function. Chest. 2007;131:740–9. doi: 10.1378/chest.06-0965. [DOI] [PubMed] [Google Scholar]
- 29.Lam B, Sam K, Lam JC, Lai AY, Lam CL, Ip MS. The efficacy of oral appliances in the treatment of severe obstructive sleep apnea. Sleep Breath. 2011;15:195–201. doi: 10.1007/s11325-011-0496-y. [DOI] [PubMed] [Google Scholar]


