Abstract
Study Objectives:
Although impairment of daytime functioning is a symptom of many sleep disorders, there are limited data on their nature for some patient groups. The role of the circadian system on impaired functioning, specifically the wake maintenance zone (WMZ)—a ∼3-h window of reduced sleep propensity that occurs shortly before the onset of melatonin synthesis—has received little attention. The study examined the influence of the WMZ on neurobehavioral performance under normal conditions and following sleep deprivation.
Methods:
Thirty-one adults (8 F; 18-29 y) completed an in-patient protocol including a baseline day (8-h sleep:16-h wake) and a ∼50-h constant routine (CR), including regular assessment of plasma melatonin and neurobehavioral performance (i.e., auditory and visual psychomotor vigilance tests [aPVT, vPVT], Digit Symbol Substitution Test [DSST], and subjective sleepiness).
Results:
Performance in the 3 hours before the onset of melatonin secretion (i.e., the expected WMZ) was significantly improved compared to performance during a 3-hour block earlier in the biological day, despite a longer time awake. The improvement during WMZ was most prominent after extended wakefulness (i.e., day 2 of the CR).
Conclusions:
These results suggest that alignment of circa-dian phase with respect to sleep-wake timing may affect cognitive performance, particularly when homeostatic sleep pressure is high, and especially when performance is assessed in the evening, near the predicted WMZ. The potential contribution of the WMZ to sleep-onset insomnia complaints should be assessed further, using objective neurobehavioral testing and simultaneous circadian phase measurement.
Citation:
Shekleton JA; Rajaratnam SMW; Gooley JJ; Van Reen E; Czeisler CA; Lockley SW. Improved neurobehavioral performance during the wake maintenance zone. J Clin Sleep Med 2013;9(4):353-362.
Keywords: Cognition, sleep regulation, circadian, performance, melatonin, two-process model, PVT, DLMO
The assessment of daytime neurobehavioral impairments in sleep disordered patients has produced inconsistent results, particularly in patients with insomnia.1 In other sleep disorder populations, such as circadian rhythm sleep disorders (CRSDs), there is a paucity of investigations into the daytime impairments associated with the disorders.2 Yet, reports of daytime impairment are a core symptom in the diagnosis of many sleep disorders. The circadian system is known to modulate alertness and performance patterns, and is known to be dysregulated in CRSD. One mechanism by which the circadian system may manifest in the daytime impairments in these patients is via altered timing or amplitude of the wake maintenance zone (WMZ).
The WMZ has previously been described as a 2- to 3-h window of reduced sleep propensity that occurs immediately prior to the evening onset of melatonin secretion and under normal conditions, occurs several hours prior to bedtime.3,4 This window is characterized by low sleep propensity and prolonged sleep latency and is usually only observed during conditions in which the circadian and homeostatic sleep-wake regulation process are desynchronized, such as during jet lag, shift work, and forced or spontaneous desynchronization studies.3–6
The two-process model of sleep-wake regulation accounts for the WMZ.7 The homeostatic process (Process S) predicts increasing sleep propensity with increasing time awake, and therefore peaks towards the end of the waking day.8,9 Process C is the endogenous circadian rhythm in sleep propensity, which reaches its peak at ∼3-6 am in normally entrained individuals. The maximal circadian drive for alertness occurs in the early evening around 6-10 pm, and opposes the rising homeostatic sleep pressure. In this manner, Process C and S work in opposition to maintain consolidated bouts of sleep and wakefulness; the homeostatic drive for sleepiness that increases during the daytime is opposed by the increasing circadian drive for wakefulness, permitting consolidated wakefulness across the daytime.7,10 The onset of melatonin secretion marks the start of the biological night and is closely associated with a decreasing circadian wake-promoting signal, ending the WMZ and opening the “sleep gate.”11
BRIEF SUMMARY
Current Knowledge/Study Rationale: While the role of the circadian system in the regulation of neurobehavioural performance is widely recognized, the impact of the wake maintenance zone (WMZ; a ∼3 hour window of reduced sleep propensity that occurs shortly before the onset of melatonin synthesis) on daytime performance has received little attention. This study investigated whether neurobehavioural performance improved during the WMZ under normal conditions and under increasing durations of sleep deprivation.
Study Impact: The wake maintenance zone is readily apparent, particularly during sleep deprivation. The findings suggest that misalignment of circadian phase with respect to sleep-wake timing may affect cognitive performance, and may impact the daytime functioning of those with abnormal sleep-wake timing, for example in patients with circadian rhythm sleep disorders or insomnia.
The daily pattern of neurobehavioral performance is also under circadian and homeostatic regulation, and both subjective and objective measures of alertness and cognition are explained by the two-process model (for overview see Dijk and von Schantz12). It is hypothesized that cognitive performance is improved during the WMZ, compared to earlier in the biological day, due to the circadian drive for alertness overriding the increase in homeo-static sleep pressure at this time. Consistent with this hypothesis, forced desynchrony protocols have demonstrated that alertness and neurobehavioral performance (e.g., digit symbol substitution task, psychomotor vigilance task) are highest at the equivalent to 9 pm under normally entrained conditions.5,13 Similarly, visual inspection of neurobehavioral performance data collected across 30 to 72 hours of extended wakefulness also suggests that performance can improve in the hours immediately prior to habitual bedtime, corresponding to the WMZ.14–16
The wake-promoting role of the circadian system appears to be crucial for optimal cognitive functioning, particularly in the latter part of the day when elevated homeostatic sleep pressure may otherwise compromise waking function.6,17 Given the close temporal relationship between the WMZ and circadian phase, particularly the onset of melatonin secretion, it is important to measure a robust marker of circadian phase when attempting to define the WMZ. Anchoring performance trials relative to habitual bedtime may not provide optimal accuracy to identify the WMZ, given that even with strict adherence to regular sleep-wake schedules there is often a 5- to 6-hour range in the phase relationship between dim light melatonin onset (DLMO) and bedtime.18,19
The aim of this study was to determine whether the WMZ can be observed from neurobehavioral performance measures taken under normal conditions and under conditions of increasing homeostatic sleep pressure but without circadian misalignment. This research was proposed to increase our understanding of the time course of neurobehavioral performance in healthy individuals relative to circadian phase and as a reference for future comparisons to those with abnormal sleep-wake timing, for example, night shift workers or patients with circadian rhythm sleep disorders or insomnia.
METHODS
Participants
Thirty-one healthy individuals (23 M, 8 F), 21.84 ± 2.56 (M ± SD) years of age were studied. The study was approved by the Partners Human Research Committee and Monash University Human Research Ethics Committee; all participants gave written informed consent. Participants were included if they met the following conditions: no night work in the previous 3 years, no transmeridian travel (across > 2 time zones) in the previous 3 months; and no history of psychiatric illness or sleep disorders, no other serious or chronic medical disorders or conditions. All participants were healthy upon psychological or psychiatric examination, physical examination, routine laboratory tests, and electrocardiogram (ECG). Participants also had scores within the normal range on validated questionnaires Symptom Checklist 90R, Beck Depression Inventory, Minnesota Multiphasic Personality Index, State Anxiety Index, Pittsburgh Sleep Quality Index, and Horne-Östberg Morningness Eveningness Questionnaire. All participants who successfully completed relevant inpatient protocols at the Intensive Physiological Monitoring (IPM) Unit of the Center for Clinical Investigation at Brigham and Women's Hospital from 2007 until mid-2010 were available for analysis. From this cohort of 34 participants, 31 were included in the current analysis; 3 participants were excluded for missing or incomplete melatonin data.
Pre-Laboratory Protocol
Participants were required to maintain a regular (8-h time in bed), self-determined sleep-wake schedule for ≥ 7 days prior to the inpatient study; adherence was verified by wrist activity (Actiwatch-L, Mini-Mitter Inc., Bend. OR), twice-daily calls to a time-stamped voicemail service, and sleep diaries. Participants were instructed to refrain from using any prescription or non-prescription medications, supplements, recreational drugs, caffeine, alcohol, and nicotine for ≥ 3 weeks before admission. Compliance with these instructions was verified by urine toxicology during screening and upon admission to the laboratory.
Study Protocol
Participants were studied for 9 days as part of a longer protocol, the details of which are published elsewhere.20 Only the data from Days 3-5 are included here, which consisted of a baseline day (Day 3) followed by a ∼50-h Constant Routine (CR) protocol (Day 4 to 5, see Figure 1). Participants remained in individual light- and sound-attenuated suites (with bathroom) throughout the study. A strict time-free environment was maintained. Days 1 to 3 were baseline days, during which participants followed an 8-h: 16-h sleep-wake cycle based on their average bed and wake times for the 7 days prior to admission. They were free to engage in sedentary activities when not occupied with study requirements. On Days 1 to 3, ambient light levels were < 190 lux (0.48 W/m2 measured in the horizontal plane at 183 cm), generated by ceiling mounted 4100K fluorescent lamps (Philips Lighting, The Netherlands). Midway through Day 3, 8 h prior to habitual bedtime, and during the CR on days 4 and 5, light levels were maintained at < 3 lux maximum when measured as above and at 0 lux during sleep episodes. From awakening on Day 4 and throughout Day 5, a ∼50-h CR procedure was imposed. Participants were required to remain awake (enforced by trained technicians) in semi-recumbent bed rest, with daily nutritional intake divided into hourly equicaloric portions.21 The first “day” of the CR procedure was, therefore, essentially a repeat of the Baseline Day, except that ambient light levels and posture remained constant. The second “day” of the Constant Routine imposed substantial sleep deprivation but was otherwise identical to the first Constant Routine day.
Figure 1. Study protocol.
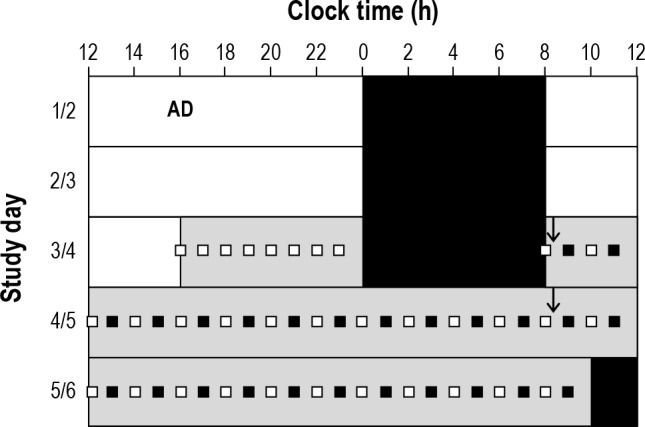
Admission on Day 1 (AD) is shown, followed by study events on Days 3 through 6. Study events were scheduled according to each person's habitual pre-study sleep-wake times: areas filled with white represent wake (< 190 lux); areas filled with black represent sleep (0 lux); areas filled with gray represent dim lighting (< 3 lux); the arrows show the start of constant routine day 1 and constant routine day 2, respectively; aPVT, KSS, and plasma melatonin sample; aPVT, vPVT, DSST, KSS, and plasma melatonin sample.
Melatonin Measurement
Plasma or salivary melatonin was sampled every 30-60 minutes. Blood samples were collected through an indwelling intravenous catheter placed in a forearm vein to allow for continuous collection of blood during sleep and wake episodes. Melatonin assays were carried out by the Core Laboratory of the Clinical Research Centre at the Brigham and Women's Hospital using established radioimmunoassay techniques.20 The plasma melatonin intraassay coefficient of variation (CV) was 10.0% at 1.9 pg/mL and 7.2% at 21.9 pg/mL, and the interassay CV was 12.65% at 3.06 pg/mL and 12.12% at 22.36 pg/mL. The saliva melatonin intraassay CV was 4.1% at 3.56 pg/mL and 4.8% at 24.2 pg/mL, and the interassay CV was 12.15% at 2.37 pg/mL and 10.20% at 19.58 pg/mL.
Neurobehavioral Performance, Sleepiness, and Alertness
Participants completed a comprehensive neurobehavioral test battery that included a visual psychomotor vigilance task (vPVT), an auditory PVT (aPVT), and a Digit Symbol Substitution Test (DSST). These tasks were chosen because they have been shown to be sensitive to sleep loss and circadian phase.13–15 The aPVT and vPVT are both 10-min sustained attention tasks and have an interstimulus interval randomly varying between 1 and 9 seconds. For the aPVT, participants were required to respond as quickly as possible to an auditory tone by pressing a button box. On the vPVT, participants responded as fast as possible to the appearance of a millisecond counter in the center of the computer screen. For both tasks, mean reaction time (RT) was averaged across the 10-min trial, and the number of lapses (RTs > 500 msec) occurring during the 10-min trial was recorded.
The DSST is test of processing speed (with an accuracy tradeoff) and working memory, in which 9 symbol-number pairs appear at the top of a computer screen and a symbol appears in the middle of the screen. Participants were required to respond as quickly as possible by entering the corresponding number. The symbol-number pairs were fixed and the target symbol changed with each trial. Performance was measured by the number of correct responses within the tasks' 2-min time limit.
To measure subjective sleepiness, participants completed the Karolinksa Sleepiness Scale, a 9-point scale requiring participants to rate how sleepy they have felt in the preceding 5 minutes. Higher scores indicate higher levels of sleepiness.
Every 120 min while awake, the participants completed a full battery (KSS, aPVT, vPVT, DSST), and every 60 min the participants completed a shortened battery (KSS, aPVT).
Data Analysis
Dim light melatonin onset (DLMO) times were calculated for each participant according to the 25% threshold method; the amplitude (mesor to peak or trough) of the melatonin rhythm during the constant routine was estimated by fitting a 3-harmonic model to the melatonin data,22 and DLMO was defined as the clock time at which the plasma melatonin rhythm exceeded a 50% threshold of the amplitude (25% of the peak-to-trough amplitude).23 The biological night was defined as the duration from DLMO to dim light melatonin offset (DLMOff), defined as the clock time at which the melatonin level dropped below the 50% threshold.24,32
For each individual participant, each trial of the aPVT, vPVT, DSST, and KSS was assigned to the Baseline Day (BD), Constant Routine day 1 (CRD1), or Constant Routine day 2 (CRD2) as follows: all performance trials occurring from 8 h prior to habitual bedtime (when the protocol on BD commenced) until DLMOff on BD were labelled as BD; all trials from the start of CRD1 until DLMOff on CRD1 were labelled as CRD1; and all performance trials occurring after DLMOff on CRD1 until DLMOff on CRD2 were labelled as CRD2 trials. In Figures 2–4, only time points where more than 50% of participants recorded a performance trial were plotted.
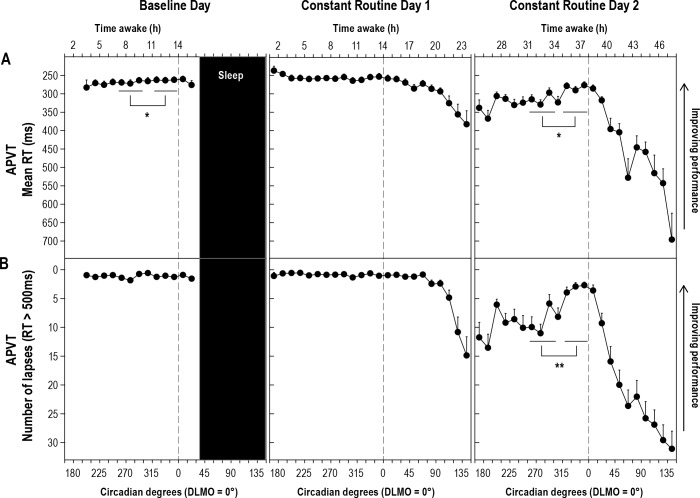
Figure 2. Performance on the APVT across three study days.
Performance on the auditory psychomotor vigilance task (aPVT) at each time point relative to circadian phase plotted across Baseline Day and Constant Routine Days 1 and 2. (A) mean reaction time (RT) on aPVT in milliseconds; (B) Number of lapses on aPVT. Testing was carried out every 15°/∼60 minutes. DAY or biological daytime period, corresponds to 255.00-299.99°, left most line. WMZ, the expected WMZ corresponds to 315.00 to 359.99°, right most line. Symbols are mean and error bars are SEMs. Vertical line corresponds to DLMO or 0°/360° circadian degrees. Sleep episode bar is indicative only, sleep time occurred at different circadian phases across individuals. *Post hoc analysis of Study day × Block interaction for aPVT mean RT. Baseline Day: p = 0.033, partial h2 = 0.15. Constant Routine Day 2: p = 0.001, Partial h2 = 0.32. **Post hoc analysis of Study day × Block interaction for aPVT # lapses. Constant Routine Day 2: p < 0.0001, Partial h2 = 0.39.
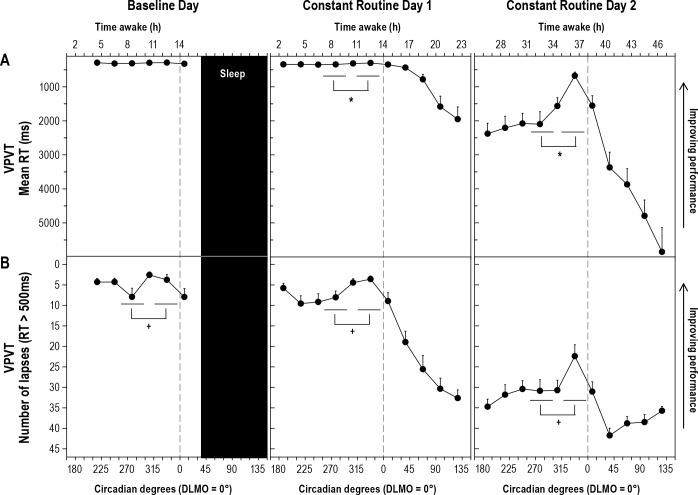
Figure 3. Visual Psychomotor Vigilance Task (VPVT) performance across three study days.
Performance on the visual psychomotor vigilance task (vPVT) at each time point relative to circadian phase plotted across baseline day and constant routine days 1 and 2. (A) vPVT mean reaction time (RT) in milliseconds; (B) Number of lapses on vPVT. Testing was carried out every 30°/∼120 minutes. For statistical analysis, data were assigned to 15° “bins” and the mean of all available trials occurring within each 45°/∼3-h WMZ or DAY block was then included. See legend of Figure 2 for additional information. *Post hoc analysis of Study day × Block interaction for vPVT mean RT. Constant Routine Day 1: p = 0.008, Partial h2 = 0.23. Constant Routine Day 2: p = 0.005, Partial h2 = 0.26. +Main effect of Block comparison, without interaction effect, p < 0.0001, Partial h2 = 0.49.
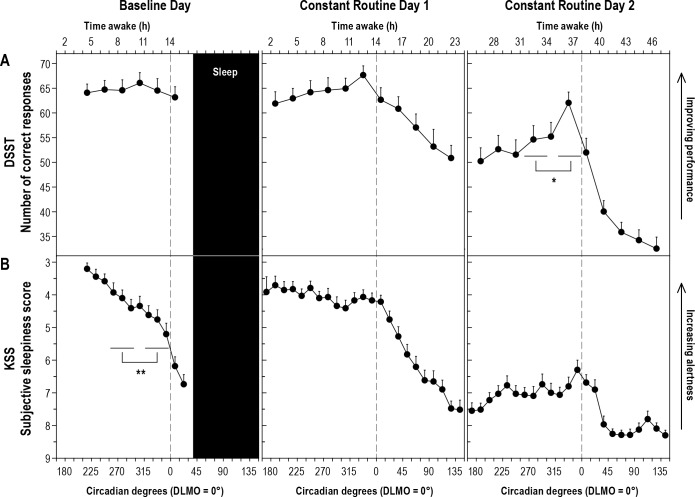
Figure 4. Digit Symbol Substitution Task (DSST) performance and subjective sleepiness scores (KSS) across three study days.
Performance on the Digit Symbol Substitution Task (DSST) (A) and scores on the Karolinska Sleepiness Scale (KSS) (B) at each time point relative to circadian phase plotted across baseline day and constant routine days 1 and 2. DSST/A: Testing was carried out every 30°/∼120 minutes. KSS/B: Testing was carried out every 15°/∼60 minutes. See legend of Figure 3 for additional information. *Post hoc analysis of Study day × Block interaction, p = 0.007, Partial h2 = 0.22. **Post hoc analysis of Study day × Block interaction, p = 0.006, Partial h2 = 0.24.
Each DLMO on each study day (BD, CRD1, and CRD2) was assigned a circadian phase of 0°/360°, and each performance trial was labelled relative to the number of degrees it occurred prior to DLMO on that day. For ease of analysis, each participant's trials occurring over a 45° range from 255.00 to 299.99° (≅3 h when 24 h = 360°) were averaged, and this mean value was labelled DAY, corresponding to the block during the biological day. This range was chosen to minimize the potential effects from sleep inertia or mid-afternoon dip.25 Based on previous research,3,4 the WMZ was expected to occur over the 3 h prior to DLMO; therefore, trials occurring from 315.00 to 359.99° were averaged together and this mean value was labelled WMZ. Trials occurring from 300.00 to 314.99° were not included in either block to provide a clear distinction between blocks. Performance trials of the aPVT and KSS were carried out approximately every 15° (60 min) and approximately every 30° (120 min) for the vPVT and DSST.
Neurobehavioral performance measures were analyzed using two-way repeated measures ANOVA, with factors study day (BD, CRD1, CRD2) and block (DAY, WMZ). Post hoc analysis was performed using one-way ANOVA. Multiple comparisons were not corrected for, but exact p values are reported. Huhyn-Feldt corrections were applied to counteract violations of sphericity, and adjusted df and F values are reported where appropriate. Unless otherwise specified, data are reported as mean ± SD (range). SPSS Statistics Version 19.0 (SPSS Inc.) was used for all data analysis.
RESULTS
The timing of melatonin onset, hours of wakefulness at DLMO, and phase angle (habitual bedtime – DLMO) were comparable across the 3 study days (see Table 1).
Table 1.
Dim light melatonin onset (DLMO), hours of wakefulness at DLMO, and phase angle between habitual bedtime and DLMO on each study day

Overall average performance was worse after 24 h of wakefulness (CRD2) for all indices of performance as compared to both CRD1 and BD (main effect of study day, p < 0.0001; post hoc testing p < 0.0001 for both days for all performance variables; data not shown). Differences in performance were less consistent between CRD1 and BD; aPVT mean reaction times were faster on CRD1 than on BD (p = 0.034), whereas vPVT mean reaction times were faster (p = 0.003) and subjective sleepiness was higher (p = 0.038) on BD than CRD1. There was no difference in overall performance between BD and CRD1 for aPVT lapses, vPVT lapses, or DSST correct responses (p > 0.05; see Table 2 and Figures 2–4).
Table 2.
Neurobehavioral performance and sleepiness measures
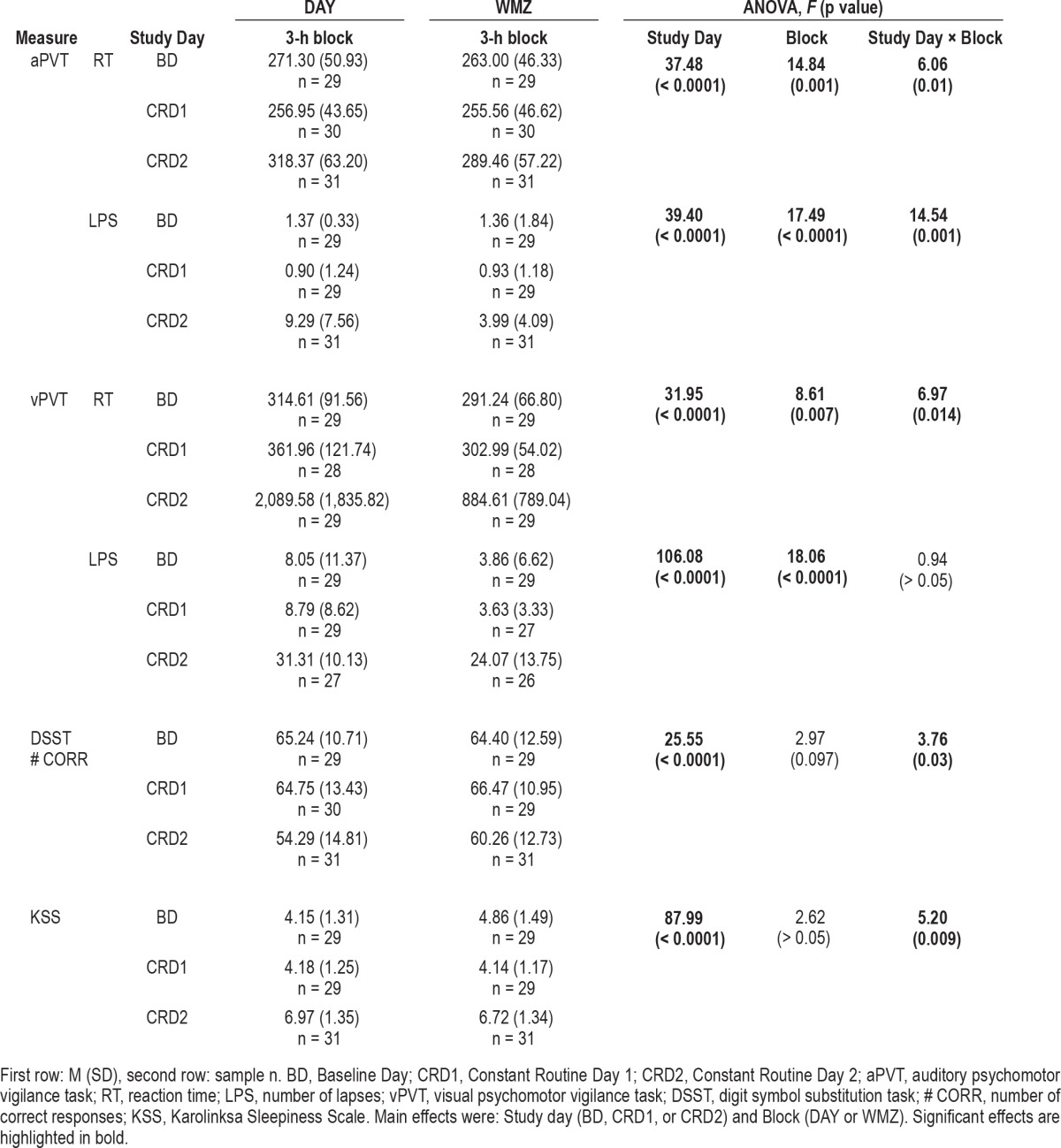
When performance during the 3-h WMZ was compared with performance during the 3-h block earlier in the day (DAY), mean reaction time and number of lapses significantly improved during the WMZ for the aPVT and vPVT (main effect of Block, p < 0.01; Table 2). There was a significant Study Day × Block interaction for all these variables, however (p < 0.05), except vPVT lapses (Table 2). Post hoc analysis showed that vPVT mean reaction times were significantly faster during the WMZ on CRD2 (p = 0.008) and on CRD1 (p = 0.005) than earlier in the day (Figure 3A). For aPVT reaction time and lapses, there was a significant improvement in performance during the WMZ on CRD2 (Figure 2). Only aPVT reaction time was better during the WMZ on BD than earlier in each of those days (p = 0.033; Figure 2A).
A significant Study Day × Block interaction on the DSST number of correct responses (p < 0.05; Table 2), followed by post hoc testing revealed a significant improvement in performance during the WMZ on CRD2 (p = 0.007; Figure 4A). In addition, a significant Study Day × Block interaction on the KSS (p < 0.05; Table 2), followed by post hoc testing revealed a significant increase in subjective sleepiness during the WMZ on BD (p = 0.006; Figure 4B).
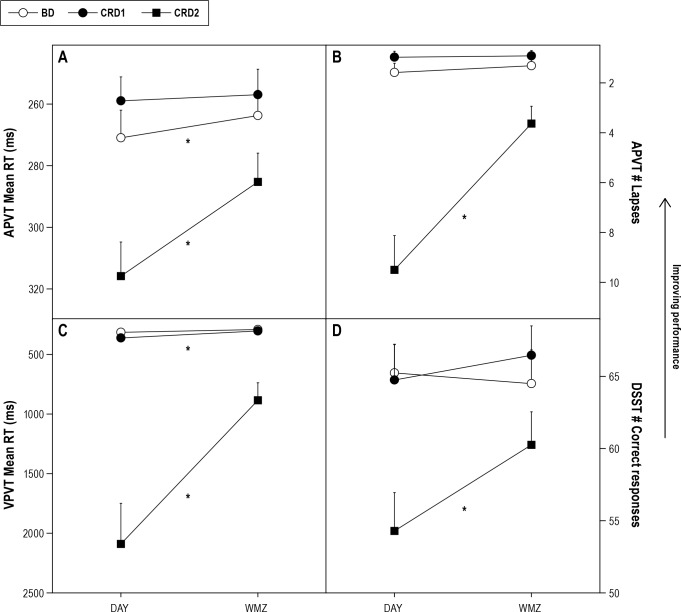
There was a greater relative improvement in reaction time (vPVT, aPVT), number of lapses (aPVT), and number of DSST correct responses during the WMZ on CRD2 when compared to either CRD1 or BD, although absolute performance during WMZ on CRD2 was never restored to that of the corresponding times on CRD1 or BD (Figure 5).
Figure 5. Improvement in performance during the wake maintenance zone (WMZ) on Constant Routine Day 2 (CRD2) compared to Constant Routine Day 1 (CRD1) and Baseline Day (BD).
Only significant Study day × Block interaction effects are represented. A: aPVT mean reaction time; B: aPVT number of lapses; C: vPVT mean reaction time; D: DSST, number of correct responses. *p < 0.05.
DISCUSSION
This study demonstrated a robust effect of the wake maintenance zone (WMZ) on neurobehavioral performance. While the WMZ has previously been observed in spontaneous internal desynchrony studies3 and using specialized forced desynchrony and ultrashort sleep-wake studies (a type of forced desynchrony),4,5 the current study shows that the WMZ can be observed for neurobehavioral performance during the usual waking day following either a full night of sleep or a sleepless night. Our study demonstrated that there was robust evidence of a WMZ on neurobehavioral performance following extended sleep deprivation, but that it was much more difficult to detect after only ∼10 hours of wakefulness. The findings of this study add to our understanding of why daytime performance patterns may be altered in patients who demonstrate a disrupted circadian rhythm, and suggest that the inter-individual variability in symptoms may be due, in part, to the circadian system.
Our protocol permitted examination of the WMZ across three days and under two different levels of sleep pressure. On all three days, circadian phase remained relatively stable with only minimal drifts due to intrinsic circadian period,20 and, because circadian phase was measured directly from the melatonin rhythm, the WMZ could be defined relative to onset of melatonin production. Following extended wakefulness, on Constant Routine Day 2, performance on all measures was relatively improved in the three hours prior to melatonin onset as compared to a 3-hour window earlier in the day, despite a longer duration awake, illustrating a robust alerting response during that time. Weaker and more inconsistent alerting responses were observed under the baseline day and the first “day” of the Constant Routine, when there was minimal prior sleep deprivation.
Following extended sleep deprivation, performance was not restored to baseline levels during the WMZ. Although the relative improvement in performance during the WMZ was greatest during Constant Routine Day 2, this effect was measurable because of the poor performance levels earlier in the day, following ∼30 hours of sleep deprivation. It appears that the WMZ is best observed when performance is substantially degraded by high homeostatic sleep pressure that builds during extended wakefulness. We could only reliably observe the WMZ when homeostatic sleep pressure was high enough to permit an “unmasking” of the circadian alerting signal. When assessed after only ∼10 hours of wakefulness on the baseline day of CR, homeostatic sleep pressure had not yet degraded performance to a low enough level for the circadian alerting signal to exert a robust improvement.
Self-ratings of sleepiness did not show the same response as the objective performance measures. Other studies have also reported a mismatch between subjective reports of sleepiness and objective performance measures.26,27 Self-reported sleepiness continued to rise prior to melatonin onset on the baseline day, and sleepiness ratings did not show a significant improvement during the WMZ on either of the Constant Routine days, although the shape of the KSS profiles during CR approaches that of other outcomes. The question of statistical power is therefore raised. With 31 participants, we had sufficient power to detect a robust response for performance measures after extended wakefulness (i.e., when effect sizes were large). We might have observed similar responses on performance measures on the other days with a much larger sample size; we estimated that we would need 146 individuals participants in order to detect a difference (medium effect size) on any given day.28
The present findings are generally in agreement with other studies that report that circadian modulation of neurobehavioral performance increases at times of increasing homeostatic sleep pressure.5,6,15,17,29,30 The pattern of increased circadian modulation of performance with increasing hours awake, however, is not consistently found across all neurobehavioral performance measures,5 which may be due to differences in protocol (e.g., short prior wake episodes and therefore low homeostatic sleep pressure), task characteristics (i.e., higher attentional load), or low statistical power.5
It is noted that the current study included only healthy, young adult participants. The extent to which these findings would generalize to other populations, specifically sleep disorder patients and older individuals, remains to be determined. The neurobehavioral tests used in this study assessed a limited range of cognitive functions, namely sustained attention and processing speed/working memory. Future studies should investigate the impact of the WMZ on higher-level cognitive functions, such as decision making and learning.
On any given day in the current study, the clock time corresponding to the start of the estimated WMZ (based on an individual's DLMO) ranged from 4 pm until almost 9:30 pm. Likewise, the phase angle between the start of the WMZ and habitual bedtime ranged from 2.67 to 6.42 hours, with an average of 4.87 hours. The range in phase angles indicates that if the WMZ had been estimated from habitual bedtime31 or without a biological marker,14,15 the WMZ may be missed completely in some participants or with a large error (> 1 h) on average. Some studies report that optimal performance occurs earlier than the expected WMZ when referenced to core body temperature minimum under entrained conditions (e.g., 180 to 210°, ∼5-8 pm),14,32 although data binning methods and infrequent performance trials may affect the ability to detect accurately the effect of the WMZ on neurobehavioral performance.14,32 It is therefore suggested that even if an individual has a regular sleep-wake schedule, a strongly endogenous phase marker, ideally DLMO, is required to define the WMZ accurately and performance changes in relation to it.
While the mechanisms by which the circadian system mediates the alerting signal during the WMZ is unknown, it has been proposed that the evening increase in hypocretin/orexin, identified as a key neurotransmitter underlying the circadian wake promoting signal,33 may contribute to the WMZ phenomenon. While there is some uncertainty as to whether humans show a wake promoting pattern of hypocretin release,34 and the role of orexin as a circadian wake promoting signal is not supported by studies of orexin knockout mice,35 others have proposed that the hypocretin/orexin system contributes to the circadian-dependent changes in waking EEG during the WMZ.29
The present data illustrate the pivotal role of the circadian system in the regulation of neurobehavioral performance and in particular of improved performance during the WMZ. This finding suggests that small misalignments of circadian phase may alter the time course of alertness and performance,36 particularly when performance is assessed during or immediately after the WMZ or under conditions of high homeostatic sleep pressure. Circadian rhythm sleep disorders (CRSDs), which are characterized by a mismatch between the internal biological clock and external clock time, are likely to be associated with significant neurobehavioral performance deficits that are not only a consequence of short duration or poor quality sleep but are a product of individuals attempting to undertake cognitive tasks at an adverse circadian phase.36
Those with extreme eveningness preference (delayed sleep phase disorder), who tend to sleep at an earlier circadian phase than normal,37 may be at particular risk of sleep disruption if attempting to sleep during the WMZ. Similarly, given that a subset of traditional insomnia diagnoses may also be due to altered circadian phase,3,38 the misalignment of the WMZ may not only contribute to the underlying sleep pathology but may also contribute to the variability seen in the daytime performance of such patients,1 particularly among individuals whose insomnia is associated with elevated homeostatic sleep pressure. The current results emphasize that the WMZ should be taken into account when investigating the potential neurobehavioral deficits associated with CRSDs and insomnia, particularly sleep onset insomnia, as a failure to detect symptoms of impaired cognitive functioning may reflect measurements taken during the WMZ.
It may also be possible to capitalize on the WMZ as a time of relatively better neurobehavioral performance for those working non-standard or extended work hours. For example, during evening or night shift work, it would be beneficial to align periods of the most demanding work or to schedule shift end time (and hence the commute home) during the WMZ prior to the onset of melatonin secretion. In order to optimize work schedules in such a way that they reflect the underlying circadian process, however, the development of simple methods for the detection of circadian phase or the onset or timing of melatonin secretion in a field setting is needed.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Rajaratnam has served as a consultant to Vanda Pharmaceuticals, Philips Respironics, and EdanSafe, and has received research grants and/or unrestricted educational grants from Vanda Pharmaceuticals, Takeda Pharmaceuticals North America, Philips Lighting, Philips Respironics, Cephalon, and ResMed Foundation, and reimbursements for conference travel expenses from Vanda Pharmaceuticals. He has received equipment donations or other support from Optalert, Compumedics, and Tyco Healthcare. He has also served as an expert witness, consultant or advisor to shift work organizations and regulatory agencies. Dr. Czeisler has received consulting fees from or served as a paid member of scientific advisory boards for: Actelion, Ltd., Bombardier, Inc., Boston Celtics, Cephalon, Inc., Delta Airlines, Eli Lilly and Co., Garda Siochana Inspectorate, Gerson Lehrman Group, Global Ground Support, Johnson & Johnson, Koninklijke Philips Electronics, N.Y., Minnesota Timberwolves, Norfolk Southern, Novartis, Portland Trail Blazers, Respironics, Inc., Sepracor, Inc., Sleep Multimedia, Inc., Somnus Therapeutics, Inc.; Yanda Pharmaceuticals, Inc. and Zeo Inc. Dr. Czeisler owns an equity interest in Lifetrac, Inc., Somnus Therapeutics, Inc., Yanda Pharmaceuticals, Inc., and Zeo Inc. and received royalties from the Massachusetts Medical Society/New England Journal of Medicine, McGraw Hill, the New York Times Penguin Press and Philips Respironics, Inc. Dr. Czeisler has received lecture fees from Accreditation Council of Graduate Medical Education, Alliance for Epilepsy Research, American Academy of Sleep Medicine, Cephalon, Inc., Duke University School of Medicine, Harvard School of Public Health, Mount Sinai School of Medicine, National Academy of Sciences, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDKINIH), National Sleep Foundation, New England College of Occupational and Environmental Medicine (NECOEM), North East Sleep Society, Office of Rare Diseases Research (NIH), Rockpointe, Sleep Research Society, Society for Obstetric Anesthesia and Perinatology (SOAP), St. Luke's Roosevelt Hospital, University of Chicago, University of Colorado, the University of Virginia Medical Center, the University of Washington Medical Center, and the University of Wisconsin Medical School. Dr. Czeisler has also received research prizes with monetary awards from the American Academy of Sleep Medicine, clinical trial research contracts from Cephalon, Inc., an investigator-initiated research grant from Cephalon, Inc., and his research laboratory at the Brigham and Women's Hospital has received unrestricted research and education funds and/or support for research expenses from Cephalon, Inc., Koninklijke Philips Electronics, NY, ResMed, ResMed Foundation; Committee for Interns and Residents, the CIR Policy and Education Initiative and the Brigham and Women's Hospital. The Harvard Medical School Division of Sleep Medicine (HMS/DSM) which Dr. Czeisler directs, has received unrestricted research and educational gifts and endowment funds from Boehringer Ingelheim Pharmaceuticals, Inc., Cephalon, Inc., George H. Kidder, Esq., Gerald McGinnis, GlaxoSmithKline, Herbert Lee, Hypnion, Jazz Pharmaceuticals, Jordan's Furniture, Merck & Co., Inc., Peter C. Farrell, Ph.D., Pfizer, ResMed, Respironics, Inc., Sanofi-Aventis, Inc., Sealy, Inc., Sepracor, Inc., Simmons, Sleep Health Centers LLC, Spring Aire, Takeda Pharmaceuticals and Tempur-Pedic. The HMS/ DSM has received gifts from many outside organizations and individuals including: Brigham and Women's Hospital (Development Office), Catalyst Group, Cephalon, Inc., Committee for Interns and Residents, Eisai, Inc., Farrell Family Foundation, Jordan's Furniture, Lilly USA, LLC, Neurocare Center for Sleep, Philips-Respironics, Inc., Praxair US Homecare, Sanofi-Aventis, Inc., Select Comfort Corporation, Sepracor, Inc., Sleep HealthCenters LLC, Somaxon Pharmaceuticals, Synchrony Healthcare Communications, Yanda Pharmaceuticals, Inc., Wake Up Narcolepsy, Inc., Water-mark Medical and Zeo, Inc. The HMS/DSM Sleep and Health Education Program has received Educational Grant funding from Cephalon, Inc., Takeda Pharmaceuticals, Sanofi-Aventis, Inc. and Sepracor, Inc. Dr. Czeisler is the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc. and holds a number of process patents in the field of sleep/circadian rhythms (e.g., photic resetting of the human circadian pacemaker). Since 1985, Dr. Czeisler has also served as an expert witness on various legal cases related to sleep and/or circadian rhythms. Dr. Lockley has received consulting fees from Apollo Lighting and Wyle Integrated Science and Engineering (NASA) and holds current consulting contracts with Naturebright, Sound Oasis and Wyle Integrated Science and Engineering (NASA). He is/was a consultant on federally-funded projects at Brigham and Women's Hospital, Thomas Jefferson University and Warwick Medical School. He has received lecture fees from Takeda Pharmaceuticals North America, I Slept Great/Euforma, LLC and Emergency Social Services Association Conference, UK and unrestricted equipment gifts from ResMed Inc, Philips Lighting and Bionetics Corporation. He received an unrestricted monetary gift to support research from Swinburne University of Technology, Australia, and a fellowship gift from Optalert, Pty Melbourne, Australia. Further he has received advance author payment and royalties from Oxford University Press, and honoraria from Servier Inc. for writing an article for Dialogues in Clinical Neuroscience and from AMO Inc., for writing an educational monograph, neither of which refer to the companies' products*. He has received honoraria and/or travel and accommodation support for invited seminars, conference presentations or teaching from 2nd International Symposium on the Design of Artificial Environments, 8th International Conference on Managing Fatigue, American Academy of Sleep Medicine, American Society for Photobiology, Apollo Lighting, Bar Harbor Chamber of Commerce, Bassett Research Institute, Canadian Sleep Society, Committee of Interns and Residents, Coney Island Hospital, FASEB, Harvard University, Illinois Coalition for Responsible Outdoor Lighting, International Graduate School of Neuroscience, Japan National Institute of Occupational Safety and Health, Lightfair, National Research Council Canada, New York Academy of Sciences, North East Sleep Society, Ontario Association of Fire Chiefs, Philips Lighting, Thomas Jefferson University, University of Montreal, University of Tsukuba, University of Vermont College of Medicine, Utica College, Vanda Pharmaceuticals, Velux, Warwick Medical School, Woolcock Institute of Medical Research, Wyle Integrated Science and Engineering (NASA), investigator-initiated research grants from Respironics Inc., Philips Lighting, Apollo Lighting and Alcon Inc., service agreement with Vanda Pharmaceuticals, sponsor-initiated research contract with Vanda Pharmaceuticals, two investigator-initiated research grants from the ResMed Foundation. Dr. Lockley holds a process patent for the use of short-wavelength light for resetting the human circadian pacemaker and improving alertness and performance which is assigned to the Brigham and Women's Hospital per Hospital policy. He has also received revenue from a patent on the use of short-wavelength light which is assigned to the University of Surrey. Dr. Lockley has also served as a paid expert witness on behalf of two public bodies on arbitration panels related to sleep, circadian rhythms and work hours. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Shekleton JA, Rogers NL, Rajaratnam SM. Searching for the daytime impairments of primary insomnia. Sleep Med Rev. 2010;14:47–60. doi: 10.1016/j.smrv.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Reid KJ, McGee-Koch LL, Zee PC. Cognition in circadian rhythm sleep disorders. Prog Brain Res. 2011;190:3–20. doi: 10.1016/B978-0-444-53817-8.00001-3. [DOI] [PubMed] [Google Scholar]
- 3.Strogatz SH, Kronauer RE, Czeisler CA. Circadian pacemaker interferes with sleep onset at specific times each day: role in insomnia. Am J Physiol. 1987;253(1 Pt 2):R172–8. doi: 10.1152/ajpregu.1987.253.1.R172. [DOI] [PubMed] [Google Scholar]
- 4.Lavie P. Ultrashort sleep-waking schedule. III. ‘Gates’ and ‘forbidden zones’ for sleep. Electroencephalogr Clin Neurophysiol. 1986;63:414–25. doi: 10.1016/0013-4694(86)90123-9. [DOI] [PubMed] [Google Scholar]
- 5.Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol. 1999;277:R1152–63. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- 6.Dijk DJ, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1992;1:112–7. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 7.Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166:63–8. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- 8.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 9.Daan S, Beersma DG, Borbely AA. Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol. 1984;246:R161–83. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- 10.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–38. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lavie P. Melatonin: role in gating nocturnal rise in sleep propensity. J Biol Rhythms. 1997;12:657–65. doi: 10.1177/074873049701200622. [DOI] [PubMed] [Google Scholar]
- 12.Dijk DJ, von Schantz M. Timing and consolidation of human sleep, wakefulness, and performance by a symphony of oscillators. J Biol Rhythms. 2005;20:279–90. doi: 10.1177/0748730405278292. [DOI] [PubMed] [Google Scholar]
- 13.Wright KP, Jr., Hull JT, Czeisler CA. Relationship between alertness, performance, and body temperature in humans. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1370–7. doi: 10.1152/ajpregu.00205.2002. [DOI] [PubMed] [Google Scholar]
- 14.Johnson MP, Duffy JF, Dijk DJ, Ronda JM, Dyal CM, Czeisler CA. Short-term memory, alertness and performance: a reappraisal of their relationship to body temperature. J Sleep Res. 1992;1:24–9. doi: 10.1111/j.1365-2869.1992.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 15.Cajochen C, Khalsa SB, Wyatt JK, Czeisler CA, Dijk DJ. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am J Physiol. 1999;277:R640–9. doi: 10.1152/ajpregu.1999.277.3.r640. [DOI] [PubMed] [Google Scholar]
- 16.Babkoff H, Caspy T, Mikulincer M. Subjective sleepiness ratings: the effects of sleep deprivation, circadian rhythmicity and cognitive performance. Sleep. 1991;14:534–9. doi: 10.1093/sleep/14.6.534. [DOI] [PubMed] [Google Scholar]
- 17.Czeisler CA, Dijk D, Duffy JF. Entrained phase of the circadian pacemaker serves to stabilize alertness and performance throughout the habitual waking day. In: Ogilvie RD, Harsh JR, editors. Sleep onset: Normal and abnormal processes. Washington, DC: American Psychological Association; 1994. pp. 89–120. [Google Scholar]
- 18.Gooley JJ, Chamberlain K, Smith KA, et al. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrinol Metab. 2011;96:E463–72. doi: 10.1210/jc.2010-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gronfier C, Wright KP, Jr., Kronauer RE, Czeisler CA. Entrainment of the human circadian pacemaker to longer-than-24-h days. Proc Nat Acad Sci U S A. 2007;104:9081–6. doi: 10.1073/pnas.0702835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gooley JJ, Rajaratnam SM, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010;2:31–3. doi: 10.1126/scitranslmed.3000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duffy JF, Dijk DJ. Getting through to circadian oscillators: why use constant routines? J Biol Rhythms. 2002;17:4–13. doi: 10.1177/074873002129002294. [DOI] [PubMed] [Google Scholar]
- 22.Shanahan TL, Kronauer RE, Duffy JF, Williams GH, Czeisler CA. Melatonin rhythm observed throughout a three-cycle bright-light stimulus designed to reset the human circadian pacemaker. J Biol Rhythms. 1999;14:237–53. doi: 10.1177/074873099129000560. [DOI] [PubMed] [Google Scholar]
- 23.Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17:181–93. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- 24.Hughes RJ, Sack RL, Lewy AJ. The role of melatonin and circadian phase in age-related sleep-maintenance insomnia: assessment in a clinical trial of melatonin replacement. Sleep. 1998;21:52–68. [PubMed] [Google Scholar]
- 25.Van Dongen HP, Dinges DF. Sleep, circadian rhythms, and psychomotor vigilance. Clin Sports Med. 2005;24:237–49. vii–viii. doi: 10.1016/j.csm.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Van Dongen HP, Dinges DF. Investigating the interaction between the homeo-static and circadian processes of sleep-wake regulation for the prediction of waking neurobehavioural performance. J Sleep Res. 2003;12:181–7. doi: 10.1046/j.1365-2869.2003.00357.x. [DOI] [PubMed] [Google Scholar]
- 27.Frey DJ, Badia P, Wright KP., Jr Inter- and intra-individual variability in performance near the circadian nadir during sleep deprivation. J Sleep Res. 2004;13:305–15. doi: 10.1111/j.1365-2869.2004.00429.x. [DOI] [PubMed] [Google Scholar]
- 28.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 29.Cajochen C, Wyatt JK, Czeisler CA, Dijk DJ. Separation of circadian and wake duration-dependent modulation of EEG activation during wakefulness. Neuro-science. 2002;114:1047–60. doi: 10.1016/s0306-4522(02)00209-9. [DOI] [PubMed] [Google Scholar]
- 30.Jewett ME, Wyatt JK, Ritz-De Cecco A, Khalsa SB, Dijk DJ, Czeisler CA. Time course of sleep inertia dissipation in human performance and alertness. J Sleep Res. 1999;8:1–8. doi: 10.1111/j.1365-2869.1999.00128.x. [DOI] [PubMed] [Google Scholar]
- 31.Graw P, Krauchi K, Knoblauch V, Wirz-Justice A, Cajochen C. Circadian and wake-dependent modulation of fastest and slowest reaction times during the psychomotor vigilance task. Physiol Behav. 2004;80:695–701. doi: 10.1016/j.physbeh.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Kline CE, Durstine JL, Davis JM, Moore TA, Devlin TM, Youngstedt SD. Circa-dian rhythms of psychomotor vigilance, mood, and sleepiness in the ultra-short sleep/wake protocol. Chronobiol Int. 2010;27:161–80. doi: 10.3109/07420521003648604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeitzer JM, Buckmaster CL, Parker KJ, Hauck CM, Lyons DM, Mignot E. Circa-dian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J Neurosci. 2003;23:3555–60. doi: 10.1523/JNEUROSCI.23-08-03555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grady SP, Nishino S, Czeisler CA, Hepner D, Scammell TE. Diurnal variation in CSF orexin-A in healthy male subjects. Sleep. 2006;29:295–7. doi: 10.1093/sleep/29.3.295. [DOI] [PubMed] [Google Scholar]
- 35.Mochizuki T, Crocker A, McCormack S, Yanagisawa M, Sakurai T, Scammell TE. Behavioral state instability in orexin knock-out mice. J Neurosci. 2004;24:6291–300. doi: 10.1523/JNEUROSCI.0586-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lockley SW, Dijk DJ, Kosti O, Skene DJ, Arendt J. Alertness, mood and performance rhythm disturbances associated with circadian sleep disorders in the blind. J Sleep Res. 2008;17:207–16. doi: 10.1111/j.1365-2869.2008.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dijk DJ, Lockley SW. Integration of human sleep-wake regulation and circadian rhythmicity. J Appl Physiol. 2002;92:852–62. doi: 10.1152/japplphysiol.00924.2001. [DOI] [PubMed] [Google Scholar]
- 38.Morris M, Lack L, Dawson D. Sleep-onset insomniacs have delayed temperature rhythms. Sleep. 1990;13:1–14. doi: 10.1093/sleep/13.1.1. [DOI] [PubMed] [Google Scholar]