Highlights
► Chicken follicle cells degrade corticosterone to mostly 20β-dihydrocorticosterone in vitro. ► Corticosterone did not affect enzymatic conversion of progesterone and DHEA by ovarian tissue. ► Metabolites such as 17α-hydroxyprogesterone, androstenedione and testosterone were formed.
Abbreviations: DHEA, dehydroeipandrosterone; FSH, follicle stimulating hormone; GnRH, gonadotropin-releasing hormone; HSD, hydroxysteroid dehydrogenase; LH, luteinizing hormone; P4, progesterone
Keywords: Stress, Reproduction, HPA-axis, HPG-axis, Poultry, β-Imaging
Abstract
Glucocorticoids affect reproductive hormone production in many species. In chickens, elevated plasma corticosterone down-regulates testosterone and progesterone concentrations in plasma, but also in egg yolk. This suppression could be mediated via the hypothalamic-pituitary system but also via local inhibition of gonadal activity by glucocorticoids. As the latter has not been tested in birds yet, we tested if corticosterone directly inhibits ovarian steroid synthesis under in vitro conditions. We hypothesized that degradation of corticosterone by follicular cells impairs their ability to synthesize reproductive hormones due to either inhibition of enzymes or competition for common co-factors. Therefore, we first established whether follicles degrade corticosterone. Follicular tissue was harvested from freshly euthanized laying hens and incubated with radiolabelled corticosterone. Radioactive metabolites were visualized and quantified by autoradiography. Follicles converted corticosterone in a time-dependent manner into metabolites with a higher polarity than corticosterone. The predominant metabolite co-eluted with 20β-dihydrocorticosterone. Other chicken tissues mostly formed the same metabolite when incubated with corticosterone. In a second experiment, follicles were incubated with either progesterone or dehydroepiandrosterone. Corticosterone was added in increasing dosages up to 1000 ng per ml medium. Corticosterone did not inhibit the conversion of progesterone and dehydroepiandrosterone into a number of different metabolites, including 17α-hydroxyprogesterone, androstenedione and testosterone. In conclusion, avian tissues degrade corticosterone mostly to 20β-dihydrocorticosterone and even high corticosterone dosages do not affect follicular hormone production under in vitro conditions.
1. Introduction
Ample research on hormone content in birds’ eggs has shown that environmental conditions experienced by the avian mother affect reproductive hormone concentrations in her eggs, which in turn influence the phenotype of the developing chick (Gil, 2003; von Engelhardt and Groothuis, 2011). These hormone- mediated maternal effects have been suggested to be an epigenetic mechanism to maximize reproductive success (Groothuis et al., 2005). However, the physiological mechanism allowing the female to modulate hormone content of her eggs is still elusive (Groothuis and Schwabl, 2008). In mammals, glucocorticoids exert suppressive effects on reproductive steroid hormone production of the gonads (Hardy et al., 2005; Moberg, 1991; Rivier and Rivest, 1991; Tilbrook et al., 2000). Accordingly, stressful conditions experienced by a female bird seem to change the hormone content of her eggs (Bertin et al., 2008; Henriksen et al., 2011b; Janczak et al., 2009; Okuliarova et al., 2010). In a recent experiment, Henriksen et al. Henriksen et al. (2011a) showed that elevated concentrations of circulating corticosterone lead to a decrease of reproductive hormones both in plasma and egg: Laying hens with corticosterone-releasing implants not only had lower plasma testosterone and progesterone levels than placebo implanted control females, they also produced eggs that contained less yolk testosterone and progesterone. It was thus concluded that corticosterone suppresses ovarian steroid hormone synthesis in chickens. However, the exact mode of action of glucocorticoids on the ovary’s hormone production was not addressed to date in avian species.
Based on mammalian research, glucocorticoids can affect gonadal function at multiple levels (Whirledge and Cidlowski, 2010). Glucocorticoids decrease synthesis and release of gonadotropin-releasing hormone (GnRH) from the hypothalamus by disrupting the GnRH pulse frequency (Bambino and Hsueh, 1981; Oakley et al., 2009), but can also modulate circulating levels of luteinizing hormone (LH) and follicle stimulating hormone (FSH) by inhibiting pituitary responsiveness to GnRH (Breen and Karsch, 2006; Matsuwaki et al., 2006; Saketos et al., 1993). Glucocorticoids can also exert direct action on the gonads themselves (Michael and Cooke, 1994; Tetsuka, 2007). This local effect of glucocorticoids on gonadal steroidogenesis is most likely receptor-mediated as effects can partly be prevented by blocking the glucocorticoid receptor with an antagonist (Dong et al., 2004; Orr and Mann, 1992). Corticosterone inhibits the enzymes 3β- and 17β-hydroxysteroid dehydrogenase (HSD) in Leydig cells (Orr et al., 1994; Sankar et al., 2000b). On the molecular level it was found that excess corticosterone suppresses mRNA expression of 3β-HSD1 and 17β-HSD3 enzymes (Badrinarayanan et al., 2006). This local suppressive effect of glucocorticoids on enzyme activity or availability greatly depends on the ability of the cells to modulate or regulate the amounts of glucocorticoids present. Most tissues convert glucocorticoids into their inactive 11-oxo-forms (Seckl and Walker, 2004; Tetsuka et al., 1999). For this oxidation, the presence of a co-factor that can be reduced is necessary. High amounts of glucocorticoids might thus lead to a shortage of co-factors which are also necessary for sex steroid synthesis, thereby decreasing enzymatic activity (Whirledge and Cidlowski, 2010). Latif et al. Latif et al. (2011) suggested that 11β-HSD1 is enzymatically coupled to 17β-HSD3, utilizing NADPH and NADP in intermeshed regeneration systems. Kavitha et al. Kavitha et al. (2006) showed that the inhibitory effect of corticosterone on Leydig cell steroidogenesis is mediated through defective co-factor generation, resulting in NADPH shortage caused by the involvement of corticosterone on glucose oxidation.
As the majority of the performed investigations used mammalian species, in birds knowledge about physiological mechanisms is much more fragmentary. It is however likely that glucocorticoids inhibit reproductive hormone production in birds also via at least two of the pathways described above: Circulating LH concentrations decreased due to glucocorticoid elevation (Etches et al., 1984; Goutte et al., 2010) indicating suppressive effects on the hypothalamic-pituitary level. It was also found that stress down-regulates reproductive hormones’ concentrations in chickens without affecting plasma levels of LH and FSH (Rozenboim et al., 2007), indicating a direct modulation of ovarian function. To our knowledge, the local action of glucocorticoids on the gonads has not been addressed to date in birds. We therefore investigated if corticosterone exerts an inhibitory effect on ovarian steroid hormone synthesis in the chicken ovary under in vitro conditions. We hypothesize that the degradation of corticosterone by enzymes present in the follicular cells inhibits the production of reproductive hormones by either competition for common co-factors or by inhibition of specific enzymes. We performed two experiments: In the first experiment we tested if corticosterone is metabolized by chicken follicle cells, thereby confirming the presence of enzymes and co-factors necessary for degradation. We incubated follicular tissue with radiolabelled corticosterone. The formed metabolites were separated via thin-layer chromatography and visualized and quantified by autoradiography. Tissues from other organs were also incubated with radiolabelled corticosterone to assess whether the formed metabolites are tissue-specific for the ovary. In the second experiment we investigated the enzymatic conversion of radiolabelled progesterone (P4) and dehydroepiandrosterone (DHEA) by follicular tissue in the presence of corticosterone. Unlabelled corticosterone was added in increasing concentrations to the cell culture medium. We chose the steroid hormones P4 and DHEA as precursor hormones to be able to differentiate if corticosterone selectively inhibits specific enzymes (17α-hydroxylase and 17,20-lyase for conversion of P4 into androstenedione and 3β-HSD for conversion of DHEA into androstenedione). Investigations were carried out using ovarian follicles of different sizes in both experiments, because the enzymatic capacities of birds’ follicles change during the course of maturation (Tilly et al., 1991).
2. Materials and methods
2.1. Animals
We used 16 female and 3 male adult laying hybrids that were offspring from chickens bred for another experiment (Henriksen et al. in prep.). Due to the set-up of the previous experiment all birds were cross-breeds between white Leghorn, ISA brown and Rhode Island Red laying hybrids. They were aged 37 weeks, housed in same-sex groups in open aviaries and received commercial layer mash and water ad libitum. All females regularly laid eggs. Average weight of the females was 2.2 ± 0.2 kg (mean ± sd). All birds were euthanized by intravenous injection of a barbiturate overdose followed by decapitation.
2.2. Experiment 1- metabolism of radiolabelled corticosterone by chicken tissues
Four females were used for experiment one. Immediately after euthanasia, the females’ ovaries were harvested. Three different sized follicles with mean (±sd) diameters of 34 ± 1 mm, 26 ± 2 mm and 10 ± 2 mm (referred to as F1, F2 and F3) respectively, were collected from each female. The follicles were cleaned from connecting tissue, opened and everted. By rinsing the tissue with pre-warmed physiological saline solution, yolk basal lamina and granulosa cells were flushed out (Gilbert et al., 1977). The remaining theca layers were washed with saline solution to remove any left over yolk and divided into tissue fragments of 0.05 g. Each tissue fragment was incubated in 1 ml medium (Eagles MEM) which contained 42,000 Bq per ml of radiolabelled corticosterone (NET-399; [1267-3H(N)]- corticosterone; 2830.5 GBq/mmol; obtained from Perkin Elmer, MA, USA). Samples were incubated in a water bath at 39 °C. Of every vial 0.2 ml were taken out after 1 h, 2 h, 4 h and 8 h of incubation respectively, and frozen immediately at −20 °C. Samples were transferred to the lab on dry ice. We also incubated radiolabelled corticosterone without any tissue material in medium only as control incubations, to assess a possible spontaneous degradation of corticosterone. Tissues from three adult males were incubated as described above for the follicle tissue. From each male, 0.05 g of tissue from testes, liver, lung, kidney, muscle, brain, adrenals and fat were collected and incubated in 1 ml medium, each together with the radiolabelled corticosterone. Again, 0.2 ml were taken out of each incubating vial after 1 h, 2 h and 4 h and frozen immediately.
2.3. Experiment 2- inhibitory effects of corticosterone on ovarian hormone synthesis
In total, twelve females were used for the second experiment. Again, three different sizes of follicles with mean (±sd) diameters of 33 ± 2 mm 24 ± 2 mm and 8 ± 1 mm (referred to as F1, F2 and F3) respectively, were prepared as described above. From all follicles, three tissue fragments of 0.05 g each were incubated in 1 ml cell culture medium. Unlabelled corticosterone was added to the media in three different concentrations (0 ng/ml, 100 ng/ml and 1,000 ng/ml). Subsamples of each six females were incubated with either 8,000 Bq per ml radio-labelled 4-pregnene-3,20-dione (P4) or 3β-hydroxyandrostene-17-one (DHEA), all radiolabelled hormones from Perkin Elmer, MA, USA). All samples were incubated for four hours at 39° C and frozen afterwards.
2.4. Extraction
We extracted the radioactive hormones and their metabolites by adding 2 times 5 ml of diethyl-ether to 0.2 ml of sample and shaking for 30 min. After centrifugation, samples were frozen at −20° C for a minimum of 3 h and the ether was then decanted into new vials. After evaporation of the solvent under N2 flow at 40° C, hormones were reconstituted in 0.3 ml of toluene. Afterwards, 0.1 ml were used for thin-layer chromatography and 10 μl in duplicates were mixed with 12 ml scintillation fluid (Quicksafe A No. 100800; Zinsser Analytic, Maidenhead, UK) to measure radioactivity in a liquid scintillation counter (Tri-Cab 2100 TR; Packard Instruments, Meridien, CT, USA) for 5 min while running a quench compensation program to calculate recoveries. We also measured 0.2 ml of the cell culture medium after the extraction to determine the amount of radioactivity that remained in the medium.
2.5. Thin layer chromatography
Thin layer chromatography was performed on TLC silica gel 60 aluminum sheets 20 × 20 cm (Merck JSC, Darmstadt, Germany) that were prewashed with pure methanol. After 0.1 ml of the sample were applied on the TLC plate via linomat (CAMAG Linomat III), plates were put into a TLC chamber which was fully saturated with the respective solvent.
In the corticosterone metabolism experiment, two different mobile phases were used after each other. Plates were first developed using toluene:acetone (1:1), until the solvent front reached 7 cm on the plate. Plates were dried under room temperature until the solvent had fully evaporated. In a second run, cyclohexane:ethylacetat (1:2) was used until the solvent front reached 14 cm. In the P4 and DHEA incubations, we used a mixture of toluene:ethylacetate:chloroform (1:3:5) as these solvents allowed P4, androstenedione, DHEA, 17α-hydroxyprogesterone, testosterone and estrogen standards to be reliably distinguished from each other. Plates remained in the TLC chamber until the solvent front reached 17 cm.
2.6. Visualization and quantification of radioactivity via ß-Imager
The TLC plates were dried with a hair drier for several minutes before they were inserted into the beta-imager measurement chamber. Tritium quantification was done in a beta-imager (Biospace Lab, Paris, France) for two hours. Quantification of the radioactivity was performed with the Beta Vision +2.0 program (Beta Imager 2000 Biospace Mesures, Paris, France). For each sample, the radioactivity measured over the whole length of the band was taken as 100%, to balance variations between the individual plates and runs. Measurements of the control incubations ranged from 219 to 316 cpm, resulting on a coefficient of variation of 18%. This number also includes variations caused by the extraction procedure itself.
2.7. Identification of metabolites
The ratio to front (Rf) values of the radioactive metabolites were compared to steroid standards that were run at the outermost left and right lane. Unlabelled steroid standards were visualized via sulfuric acid and heating, as described by Bamberg et al. Bamberg et al. (2004). The spots were marked by applying radioactivity on the established elution positions afterwards, to enable detection via the beta-imager. We tested the following standards in experiment one: corticosterone, 11-dehydro-corticosterone, aldosterone, 20α-dihydrocorticosterone, 20β-dihydrocorticosterone, 5α-3α,11β, 21-triol-20-one (allotetrahydrocorticosterone), 5α-3β,11β,21-triol-20-one, 5β-3α,11β,21-triol-20-one (tetrahydrocorticosterone), 5α-dihydrocorticosterone, 5β-dihydrocorticosterone.
2.8. Statistical analysis
Analyses were performed using a statistical software package (SIGMASTAT® Version 3.0 for Windows Jandel GmbH, Erkrath, Germany). In the first experiment, irrespective of their maturation stage, all follicles produced the same metabolites. The amounts of radioactivity found at the elution positions of the formed metabolites did not differ significantly between the three follicle sizes (RM ANOVAs on ranks: timepoint 1 h: x2 = 0.7; df = 2; p = 0.7; 2 h: x2 = 0.3; df = 2; p = 0.9; 4 h: x2 = 2.1; df = 2; p = 0.4; 8 h: x2 = 2.1; df = 2; p = 0.4). We therefore decided to combine the data from the three different follicle sizes. For each hen, we calculated mean concentrations of the three follicles to assess differences between time stages. Radioactivities found at the elution positions of corticosterone and at each formed metabolite were then compared between different incubation time points (1 h, 2 h, 4 h and 8 h of incubation) via RM ANOVA and pairwise multiple comparison procedure (Holm-Sidak). Differences between the amounts of formed metabolites at each time point were also assessed via ANOVA and post hoc tests (Holm-Sidak).
3. Results
3.1. Recoveries
In the first experiment, 65 ± 9% (mean ± sd) of the added radioactivity was recovered in the follicle samples resuspended in toluene, whereas about 12 ± 2% of radioactivity remained in the cell culture medium of the incubated follicles after twofold extraction with diethyl-ether. Tissue samples of males still contained about 10 ± 3% of the added radioactivity after the ether extraction and 67 ± 13% of the radioactivity was found in the toluene suspensions. In the second experiment, 11 ± 2% of the radiolabelled P4 metabolites remained in the cell culture medium after twofold extraction with diethyl-ether, whereas 5 ± 2% of the metabolites formed from DHEA were not extractable. After extraction, 59 ± 9% and 66 ± 12% of the added radioactivity were resuspended in toluene in the progesterone and DHEA incubation, respectively.
3.2. Experiment 1- metabolism of radiolabelled corticosterone by chicken tissues
No degradation of radiolabelled corticosterone was observed in the control incubations. When incubated in the presence of follicular tissue, radiolabelled corticosterone was metabolized in a time-dependent manner and converted into several radioactive metabolites. They all had a higher polarity than the corticosterone standard. Four major metabolites could be distinguished and were labelled M1 to M4 according to their elution profiles (M1=closest to the corticosterone standard; M4=closest to start line). In the course of the eight hours of incubation time, corticosterone concentrations decreased significantly (Table 1; RM ANOVA: F1,3 = 270; p < 0.001) whereas concentrations of the four formed metabolites increased significantly over time (RM ANOVAs, M1: F1,3 = 12.9; p = 0.001; M2: F1,3 = 105.1; p < 0.001; M3: F1,3 = 87.9; p < 0.001; M4: F1,3 = 66.3; p < 0.001). Metabolite concentrations also differed significantly within each time stage and one metabolite (M3) that showed the same chromatographic mobility as 20β-dihydrocorticosterone was predominant. At all time points, highest amounts of radioactivity were found at the elution position of M3 (ANOVAs, time point 1 h: F1,3 = 77.2; p < 0.001; 2 h: F1,3 = 56.4; p < 0.001; 4 h: F1,3 = 69.3; p < 0.001; 8 h: F1,3 = 42.6; p < 0.001). None of the three other metabolites that were formed co-eluted with any of the tested steroid standards. Two metabolites eluted between corticosterone and 20β-dihydrocorticosterone and one metabolite was more polar than 20β-dihydrocorticosterone (see Fig. 1). The other tissues metabolized the radiolabelled corticosterone similar to the females’ follicles. Again, only metabolites with a higher polarity than corticosterone were detected. Incubating radiolabelled corticosterone with tissue fragments from kidneys, adrenals and testes yielded radioactive metabolites with the same chromatographic mobilities as in the follicle incubation experiment. Only in testes however, the metabolite co-eluting with 20β-dihydrocorticosterone was predominant (see Fig. 2). Tissue samples from muscle, brain and lung showed low enzymatic activity after one and two hours of incubation. After four hours however, one or two additional metabolites appeared that did not co-elute with the radiolabelled metabolites found in the follicle incubations. Incubation of radiolabelled corticosterone with liver tissue produced a great number of different metabolites that could not be separated completely by the TLC. Samples from fat tissue could not be analysed as there were too many lipids interfering with the separation of the metabolites via TLC. Except for 20β-dihydrocorticosterone, none of the other steroid standards could be assigned to any of the metabolites formed by males’ tissues.
Table 1.
Percentages (means ± s.d.) of recovered radioactivity after incubating radiolabelled corticosterone for one, two, four and eight hours, respectively with follicle cells of three different sized follicles collected from each four laying hens.
| 1 h | 2 h | 3 h | 4 h | |
|---|---|---|---|---|
| Cort | 59.3 ± 1.2a | 39.0 ± 5.6b | 17.3 ± 4.7c | 5.9 ± 1.8d |
| M1 | 3.8 ± 0.8a | 7.8 ± 2.5a,b | 12.0 ± 5.7b,c | 16.5 ± 7.1c |
| M2 | 2.0 ± 0.4a | 4.1 ± 01.2b | 7.0 ± 1.6c | 11.5 ± 1.4d |
| M3 | 11.9 ± 2a | 24.3 ± 4.5b | 34.6 ± 2.9c | 42.0 ± 6.5d |
| M4 | 1.4 ± 0.3a | 2.4 ± 0.7b | 4.2 ± 0.9c | 6.3 ± 0.2d |
Cort = radioactivity found at established elution position of corticosterone; M1 – M4 = radioactivity found at elution positions of corticosterone metabolites; different superscripts in the same line indicate significant differences (p < 0.05) between time points.
Fig. 1.
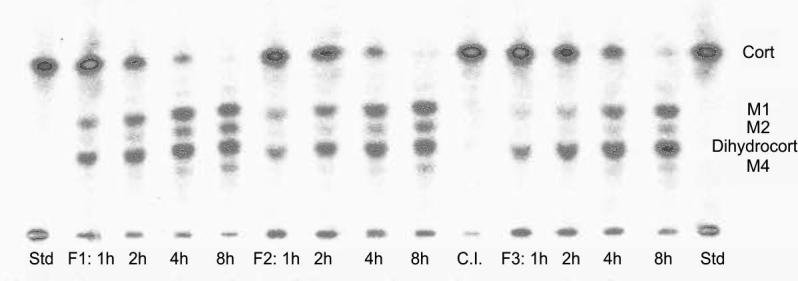
Thin layer chromatographic separations of recovered radioactivity after one, two, four and eight hours of incubation of radiolabelled corticosterone with tissue from different sized follicles (F1, F2, F3; see text) of one female; Std=corticosterone standard, C.I.=control incubation without tissue, Cort=elution position of corticosterone standard, Dihydrocort=elution position of 20β-dihydrocorticosterone standard, M1, M2, M4=formed metabolites.
Fig. 2.
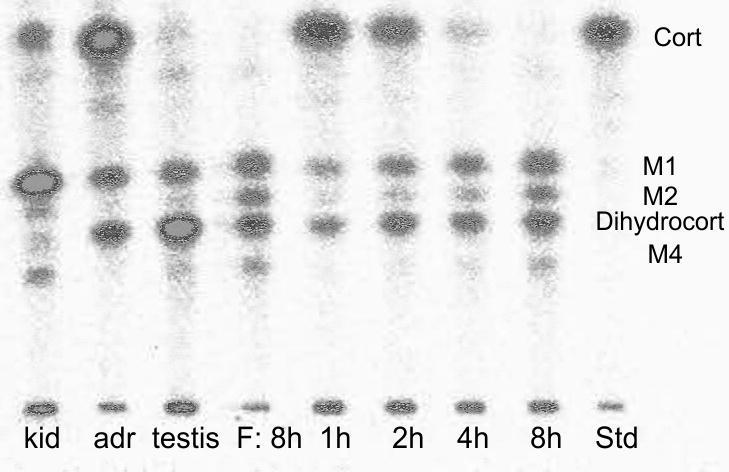
Thin layer chromatographic separations of radioactivity after four hours of incubation of radiolabelled corticosterone with tissue from kidneys (kid), adrenals (adr) and testis of one male, radioactivity and after one, two, four and eight hours of incubation of radiolabelled corticosterone with follicular tissue (F) of one female; Std=corticosterone standard; Cort=elution position of corticosterone, Dihydrocort=elution position of 20β-dihydrocorticosterone, M1, M2, M4=formed metabolites; note that the eight hour sample was applied twice for better comparison.
3.3. Experiment 2- inhibitory effects of corticosterone on ovarian hormone synthesis
In general, follicular tissues metabolized the added radiolabelled precursors independent of the absence or presence of corticosterone (see Figs. 3 and 4). All incubations yielded similar metabolite patterns and differences between the three corticosterone dosages were not detected. Several different metabolites were formed of which some could be assigned to the applied standards: In both the P4 and the DHEA incubations, one metabolite co-eluted with the androstenedione standard. In the P4 incubations, the two larger follicles (F1 and F2) formed a metabolite that co-eluted with the 17α-hydroxyprogesterone standard. This metabolite was not visible in the smallest follicles’ incubations. In the DHEA incubations, a major share of the formed metabolites showed the same chromatographic mobilities as androstenedione and testosterone, and there was more radioactivity found at these positions than at the DHEA spot, except in the smallest follicles’ incubations. In those follicles (F3), after four hours of incubation most of the radioactivity was still present at the position of DHEA, and compared to the larger follicles, less radioactivity eluted at the positions of androstenedione and testosterone. In both incubations, none of the formed metabolites could be assigned to the estrogen standards (estrone, 17α- and 17β-estradiol) because separation of radioactive metabolites with lower Rf values than testosterone was insufficient and did not allow a precise assignment to individual standards.
Fig. 3.
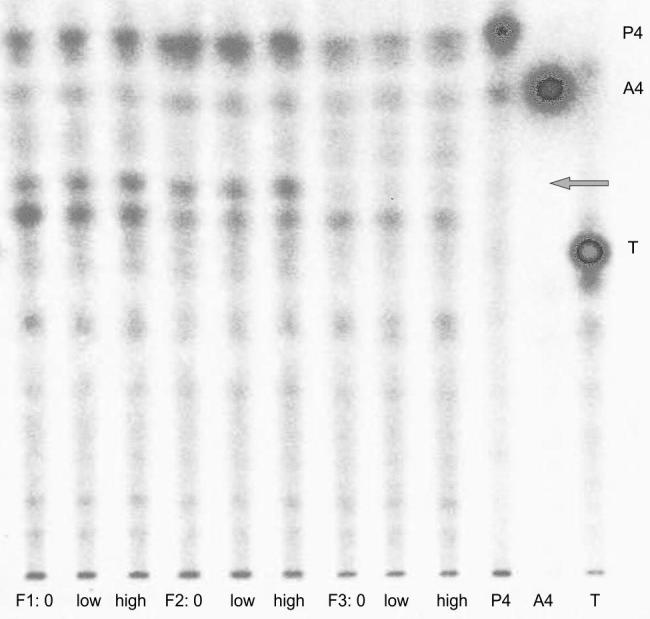
Thin layer chromatographic separations of radioactivity after four hours of incubating radiolabelled progesterone with tissue from different sized follicles (F1, F2, F3; see text) of one female together with unlabelled corticosterone added in three different concentrations to the cell culture medium (0 = 0 ng/ml, low = 100 ng/ml, high = 1000 ng/ml); P4 = progesterone standard, A4 = androstenedione standard, T = testosterone standard; elution position of 17α-hydroxyprogesterone indicated by arrow.
Fig. 4.
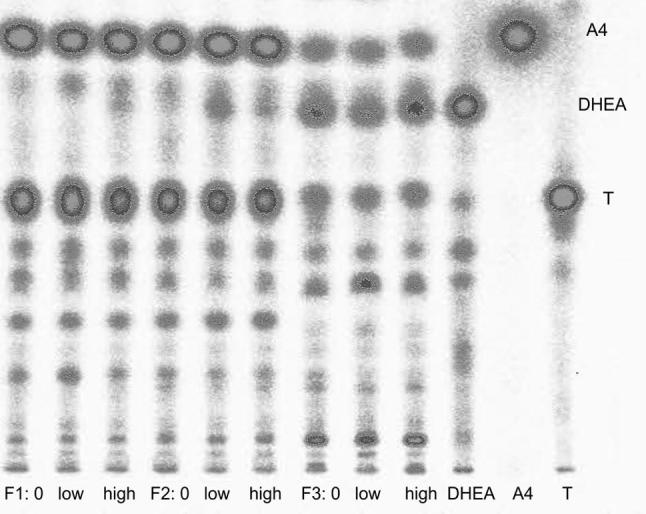
Thin layer chromatographic separations of radioactivity after four hours of incubating radiolabelled dehydroepiandrosterone with tissue from different sized follicles (F1, F2, F3 see text) of one female together with unlabelled corticosterone added in three different concentrations to the cell culture medium (0 = 0 ng/ml, low = 100 ng/ml, high = 1000 ng/ml); DHEA = dehydroepiandosterone standard, A4 = androstenedione standard; T = testosterone standard.
4. Discussion
The present study investigated a local inhibitory effect of glucocorticoids on ovarian hormone synthesis in chickens. We first tested if avian follicles have the enzymatic capacity to metabolize corticosterone and if the formed metabolites are tissue-specific. In a second experiment, we assessed a possible inhibitory effect of corticosterone on the conversion of P4 and DHEA by ovarian follicles. We expected that degradation of corticosterone by the follicular cells would impair their ability to synthesize reproductive hormones due to inhibition of certain enzymes or competition for common co-factors. To answer these questions we chose to incubate follicle cells with radiolabelled hormones and analysed the formed end products qualitatively and quantitatively via thin layer chromatography. Although this method is less sensitive or specific than other techniques such as measuring enzyme activity or enzyme gene expression it still seemed most appropriate to answer our questions. Measuring enzyme activity would have comprised several individual experiments, as corticosterone might inhibit different enzymes, furthermore a potential shortage of common co-factors would remain undetected. Assessing enzyme gene expression gives no indication about actual enzyme availability and activity and would thus give only inadequate information. As this is the first study ever on that particular topic, we think that results obtained by this rather classical methodology represent a profound and solid basis for further investigations. Incubation of radiolabelled corticosterone with theca cell tissue revealed that corticosterone was degraded in a time-dependent manner into more polar metabolites. The formed metabolites result from enzymatic activity of the ovarian tissue, as in the control incubations spontaneous degradation of radiolabelled corticosterone did not occur.
Investigations were carried out using ovarian follicles of three different sizes in both experiments, because the enzymatic capacities of birds’ follicles change during the course of maturation (Etches and Duke, 1984; Lee et al., 1998). In contrast to our expectations however, comparison between different sized follicles revealed no influence of maturation stage on metabolite patterns. This indicates the presence of similar enzymes throughout follicular growth, as all follicles converted corticosterone into the same metabolites. Another explanation might be that some of the produced metabolites were not extracted with the same efficiency as others. Furthermore in our experiment, the granulosa cell layer was removed during follicle preparation, which might have masked some differences between small and larger follicles. Granulosa cells are more active in large, preovulatory follicles (Tilly et al., 1991), but whether this also affects corticosterone metabolism at all cannot be answered in the present study. We found that all follicles produced similar amounts of the same metabolites. In all incubations, one metabolite co-eluted with 20β-dihydrocorticosterone was found in highest concentrations. This indicates that ovarian theca cells degrade corticosterone mainly via 20β-reduction. However, this pathway does not seem special for chicken follicular tissue: Incubation of corticosterone with other tissues yielded the same metabolite. In accordance with our observations, McNätt et al. McNätt et al. (1992) describe the exclusive conversion of cortisol to 20β-dihydrocortisol when incubated with chickens’ chorioallantoic membrane. These findings suggest the presence of the enzyme 20-HSD in several chicken tissues. Accordingly, Kucka et al. Kucka et al. (2006) showed 20-HSD activity in ovary, testis, kidney, intestines, liver, brain and oviduct. Also, mRNA expression of 20-HSD was confirmed by Bryndova et al. Bryndova et al. (2006) in several chicken tissues. In addition to 20β-dihydrocorticosterone, we found that the ovarian tissue also synthesized three other metabolites with higher polarity than corticosterone, which could not be assigned to any of the used standards. The same metabolites were also formed by testis, adrenals and kidney tissues, whereas liver, muscle, brain and lung produced small amounts of additional metabolites. The further biochemical characterisation of these metabolites was however beyond the scope of the present study. Overall, in both males’ and females’ tissues, none of the formed metabolites co-eluted with the tested 11-dihydrocorticosterone standard, suggesting no evidence for conversion of corticosterone into 11-dihydrocorticosterone. In general, birds seem to prefer reduction at position 20 for glucocorticoid metabolism (Vylitova et al., 1998), whereas in mammals glucocorticoid activity is regulated mostly via reduction or oxidation of position 11. In chicken ovaries 11β-HSD activity was not found, but the enzyme is present in the avian kidney (Klusonova et al., 2008; Kucka et al., 2006). However, in our kidney incubations we did not detect any radioactivity at the elution position of 11-dehydrocorticosterone. This could be due to differences in co-factor abundance, as for example the avian intestine converted corticosterone into 11-dehydrocorticosterone only in the presence of NAD+ or NADP+, whereas in the presence of NADPH, 11-dehydro-20-dihydrocorticosterone and 20-dihydrocorticosterone were formed (Vylitova et al., 1998). Similar conclusions were drawn by Katz et al. Katz et al. (2010), who found that ovaries formed more 11-oxo-metabolites whereas testes produced 20-dihydrocorticosterone. In our own experiment, metabolites produced by male and female gonads seemed highly similar. The other tissues investigated in the present study were all collected from male birds only. Besides the differences between male and female gonads described by Katz et al. Katz et al. (2010), none of the above cited authors observed sex differences in corticosterone metabolism, neither in peripheral tissues nor in the brain. However, in future studies it might still be of interest to assess potential sex differences in corticosterone metabolism in more detail, as type and amount of glucocorticoid metabolites in the excreta can largely differ between male and female individuals (Touma and Palme, 2005).
In the second experiment, we incubated follicular tissue with radiolabelled P4 and DHEA in the presence of different corticosterone dosages. Several metabolites were formed, unaffected by the presence of corticosterone. In the P4 incubations, two of the formed metabolites could be assigned to the androstenedione and the 17α-hydroxyprogesterone standards, respectively. Incubations with DHEA yielded two main metabolites that co-eluted with the androstenedione and testosterone standards, respectively. These findings clearly show that a certain share of the added radiolabelled precursors were converted by the ovarian tissue along the classical pathways described in literature, indicating activity of the enzymes 17α-hydroxylase and 17,20-lyase for conversion of P4 into 17α-hydroxyprogesterone and androstenedione as well as 3β-HSD for conversion of DHEA into androstenedione and 17-oxidoreductase for conversion androstenedione into testosterone (Payne and Hales, 2004). In general, the smallest follicles (F3) seemed to metabolize the precursors differently than the two larger follicles. In the P4 incubations, F3 cells did not form a metabolite that co-eluted with 17α-hydroxyprogesterone, which could be partly due to different enzymatic capacities: According to literature, small follicles convert pregnenolone to DHEA to further synthesize androstenedione and estrogens via the Δ5-pathway (Lee et al., 1998). During the course of maturation steroid production shifts towards the Δ4-pathway, with larger follicles now using P4 and 17α-hydroxyprogesterone as substrates for androstenedione production (Robinson and Etches, 1986). In the DHEA incubation however, small follicles seemed to metabolize the precursor to a lesser degree than larger follicles. This might simply be explained by the fact that, compared to larger follicles incubating tissue from smaller follicles resulted in fewer active follicle cells or reduced amounts of available co-factors. We refrained from quantifying exact amounts of the produced reproductive steroids, as the enzymatic activities of follicles and the different steroidogenic pathways during the course of maturation have already been well-investigated elsewhere (Etches and Duke, 1984; Lee et al., 1998; Porter et al., 1989). Furthermore, the main goal of the present study was to determine a possible inhibitory effect of corticosterone on follicular enzyme activity. We deduct from the present experiment that the added corticosterone had no influence on the metabolism of P4 and DHEA. The radiolabelled precursors were converted independently of the presence of corticosterone. This suggests that corticosterone did not inhibit enzymatic activity of ovarian cells, even at a concentration of 1,000 ng/ml cell culture medium. However, we do not think that the dosages we chose in our experiment were too low to produce any effect. In rats, Sankar et al. Sankar et al. (2000a) found that corticosterone concentrations of 200–400 ng/ml decreased basal and LH-stimulated testosterone production. As we did not add LH or FSH to our in vitro incubations, one explanation for our findings could be the fact that the presence of gonadotropins is still necessary for a local inhibition of corticosterone on ovarian activity in the chicken. To our knowledge, previous studies in birds on this topic do not exist and findings in mammals are ambiguous. Some in vitro studies assessed the suppressive effect of glucocorticoids on reproductive hormone synthesis in the presence of LH or FSH (Sankar et al., 2000a; Yang et al., 2001), whereas other authors found effects that were not gonadotropin-mediated (Badrinarayanan et al., 2006; Orr et al., 1994). Further studies in birds could explore a potential role of gonadotropins in mediating local inhibitory effects of glucocorticoids on reproductive hormone synthesis.
Furthermore, in vitro conditions certainly differ from the natural situation in the bird where the ovary is constantly synthesizing the whole cascade of reproductive hormones, thereby regenerating co-factors which might at the same time be necessary for corticosterone degradation. Although Rozenboim et al. Rozenboim et al. (2007) did not observe a decrease in chickens’ LH and FSH plasma levels after a stressor, it is still possible that the suppressive effect of glucocorticoids on reproductive hormone synthesis is mediated exclusively via the hypothalamic-pituitary-axis. Gonadotropins are secreted in pulsatile or episodic patterns (Gledhill and Follett, 1976; Vizcarra et al., 2004) which might complicate the detection of a decrease in plasma concentrations. In addition, other factors might be involved in the stress-related inhibition of the reproductive axis in avian species: Recent mammalian studies highlighted the relevance of substances such as IGF-1 or gonadotropin-inhibitory hormone in HPA-HPG interactions (Kirby et al., 2009; Viveiros and Liptrap, 1999).
Stressful stimuli have been shown to inhibit the cholesterol side-chain cleavage enzyme responsible for the conversion of cholesterol to pregnenolone (Stojkov et al., 2012). Future studies could address a possible effect of corticosterone on this early stage in reproductive steroid hormone synthesis. If glucocorticoids exert their inhibitory effects during such an early stage of the reproductive hormone production cascade, this might also imply that they affect the enzymatic activity of granulosa cells more than that of theca cells. Granulosa cells synthesise mainly progesterone, which is used subsequently by the theca layers to produce testosterone and estrogens (Porter et al., 1989). The granulosa itself has only very limited enzymatic capacity to further metabolize progesterone or DHEA, which we used as substrates in our second experiment. Therefore, the observed degradation of the added radiolabelled precursors is not influenced by the removal of the granulosa in the present study. Future experiments however should assess whether glucocorticoids inhibit earlier stages of reproductive hormone synthesis in the granulosa cell layer. In conclusion, our experiments show that corticosterone is enzymatically degraded by the chicken ovary. The reduction at position C20 seems to be a common feature of avian tissues, probably to prevent corticosterone from binding to mineralocorticoid receptors (DiBattista et al., 1989). In contrast to other tissues however, conversion of corticosterone by the ovary might serve an additional purpose: Overexposure to glucocorticoids can have detrimental effects on embryonic development (Drake et al., 2007). In mammals, the placenta inactivates glucocorticoids from the maternal circulation, thereby protecting the fetus from maternal stress (Murphy et al., 1974). In oviparous species, the follicular wall with its enzymatic abundance might represent a similar protective mechanism. Previous studies revealed that egg yolk contains only minor amounts of corticosterone (Rettenbacher et al., 2009) and showed that maternal stress can be mediated to the bird embryo via other hormones instead (Henriksen et al., 2011a). From our present results, a function of C20-reduced metabolites as mediators of maternal stress could be inferred. When a female bird is stressed and has elevated levels of plasma corticosterone during egg formation, 20β-dihydrocorticosterone might be transferred into the yolk and albumin. Further research should therefore confirm the presence of 20β-dihydrocorticosterone in avian eggs and whether it is a biologically active compound which can act as a hormonal signal for the bird embryo.
Acknowledgments
Rie Henriksen and Michael Lepschy were funded by grants of the Austrian Science Foundation (FWF Project No. 19169 and FWF Project No. 21607). Animals, materials and consumables used in the study were funded by the Austrian Science Foundation (FWF Project No. 19169 to Sophie Rettenbacher). The Beta Imager 2000 (Biospace Mesures, Paris, France) was purchased by means of the Vienna Science and Technology Fund (WWTF – UIP 2007). We are indebted to Amanda Aichinger whose assistance in developing the thin-layer protocols was indispensable. Katharina Mahr helped with the labwork. The first experiment was based on the work of baccalaureus student Nicole Schmitner who was supervised by Erich Möstl. We thank Rupert Palme and Claudia Ouschan for valuable discussions.
Contributor Information
Sophie Rettenbacher, Email: sophie.rettenbacher@vetmeduni.ac.at.
Rie Henriksen, Email: riehe@ifm.liu.se.
Ton G. Groothuids, Email: a.g.g.groothuis@rug.nl.
Michael Lepschy, Email: Michael.Lepschy@vetmeduni.ac.at.
References
- Badrinarayanan R., Rengarajan S., Nithya P., Balasubramanian K. Corticosterone impairs the mRNA expression and activity of 3beta- and 17beta-hydroxysteroid dehydrogenases in adult rat Leydig cells. Biochem. Cell Biol. 2006;84:745–754. doi: 10.1139/o06-074. [DOI] [PubMed] [Google Scholar]
- Bamberg E., Aichinger A., Mitteregger G. In vitro metabolism of dehydroepiandrosterone and testosterone by canine hair follicle cells. Vet. Dermatol. 2004;15:19–24. doi: 10.1111/j.1365-3164.2004.00366.x. [DOI] [PubMed] [Google Scholar]
- Bambino T.H., Hsueh A.J. Direct inhibitory effect of glucocorticoids upon testicular luteinizing hormone receptor and steroidogenesis in vivo and in vitro. Endocrinology. 1981;108:2142–2148. doi: 10.1210/endo-108-6-2142. [DOI] [PubMed] [Google Scholar]
- Bertin, Richard-Yris M.A., Houdelier C., Lumineau S., Möstl E., Kuchar A., Hirschenhauser K., Kotrschal K. Habituation to humans affects yolk steroid levels and offspring phenotype in quail. Horm. Behav. 2008;54:396–402. doi: 10.1016/j.yhbeh.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Breen K.M., Karsch F.J. Does season alter responsiveness of the reproductive neuroendocrine axis to the suppressive actions of cortisol in ovariectomized ewes? Biol. Reprod. 2006;74:41–45. doi: 10.1095/biolreprod.105.045898. [DOI] [PubMed] [Google Scholar]
- Bryndova J., Klusonova P., Kucka M., Mazancova-Vagnerova K., Miksik I., Pacha J. Cloning and expression of chicken 20-hydroxysteroid dehydrogenase. J. Mol. Endocrinol. 2006;37:453–462. doi: 10.1677/jme.1.02025. [DOI] [PubMed] [Google Scholar]
- DiBattista J.A., Mehdi A.Z., Sandor T. Steroid C-20 oxidoreductase activity of duck intestinal mucosa: the interrelations of the enzymatic activity with steroid binding. Gen. Comp. Endocrinol. 1989;74:136–147. doi: 10.1016/0016-6480(89)90122-6. [DOI] [PubMed] [Google Scholar]
- Dong Q., Salva A., Sottas C.M., Niu E., Holmes M., Hardy M.P. Rapid glucocorticoid mediation of suppressed testosterone biosynthesis in male mice subjected to immobilization stress. J. Androl. 2004;25:973–981. doi: 10.1002/j.1939-4640.2004.tb03170.x. [DOI] [PubMed] [Google Scholar]
- Drake A.J., Tang J.I., Nyirenda M.J. Mechanisms underlying the role of glucocorticoids in the early life programming of adult disease. Clin. Sci. (Lond.) 2007;113:219–232. doi: 10.1042/CS20070107. [DOI] [PubMed] [Google Scholar]
- Etches R.J., Williams J.B., Rzasa J. Effects of corticosterone and dietary changes in the hen on ovarian function, plasma LH and steroids and the response to exogenous LH-RH. J. Reprod. Fertil. 1984;70:121–130. doi: 10.1530/jrf.0.0700121. [DOI] [PubMed] [Google Scholar]
- Etches R.J., Duke C.E. Progesterone, androstenedione and oestradiol content of theca and granulosa tissues of the four largest ovarian follicles during the ovulatory cycle of the hen (Gallus domesticus) J. Endocrinol. 1984;103:71–76. doi: 10.1677/joe.0.1030071. [DOI] [PubMed] [Google Scholar]
- Gil D. Golden eggs: maternal manipulation of offspring phenotype by egg androgen in birds. Ardeola. 2003;50:281–294. [Google Scholar]
- Gilbert A.B., Evans A.J., Perry M.M., Davidson M.H. Method for separating granulosa-cells, basal lamina and theca of preovulatory ovarian follicle of domestic-fowl (Gallus-Domesticus) J. Reprod. Fertil. 1977;50:179–181. doi: 10.1530/jrf.0.0500179. [DOI] [PubMed] [Google Scholar]
- Gledhill B., Follett B.K. Diurnal variation and the episodic release of plasma gonadotrophins in Japanese quail during a photoperiodically induced gonadal cycle. J. Endocrinol. 1976;71:245–257. doi: 10.1677/joe.0.0710245. [DOI] [PubMed] [Google Scholar]
- Goutte, Angelier F., Chastel C.C., Trouve C., Moe B., Bech C., Gabrielsen G.W., Chastel O. Stress and the timing of breeding: glucocorticoid-luteinizing hormones relationships in an arctic seabird. Gen. Comp. Endocrinol. 2010;169:108–116. doi: 10.1016/j.ygcen.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Groothuis T.G., Muller W., von Engelhardt N., Carere C., Eising C. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 2005;29:329–352. doi: 10.1016/j.neubiorev.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Groothuis T.G., Schwabl H. Hormone-mediated maternal effects in birds: mechanisms matter but what do we know of them? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008;363:1647–1661. doi: 10.1098/rstb.2007.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy M.P., Gao H.B., Dong Q., Ge R., Wang Q., Chai W.R., Feng X., Sottas C. Stress hormone and male reproductive function. Cell Tissue Res. 2005;322:147–153. doi: 10.1007/s00441-005-0006-2. [DOI] [PubMed] [Google Scholar]
- Henriksen R., Groothuis T.G., Rettenbacher S. Elevated plasma corticosterone decreases yolk testosterone and progesterone in chickens: linking maternal stress and hormone-mediated maternal effects. PLoS One. 2011;6:e23824. doi: 10.1371/journal.pone.0023824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen R., Rettenbacher S., Groothuis T.G. Prenatal stress in birds: pathways, effects, function and perspectives. Neurosci. Biobehav. Rev. 2011;35:1484–1501. doi: 10.1016/j.neubiorev.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Janczak A.M., Torjesen P., Rettenbacher S. Environmental effects on steroid hormone concentrations in laying hens’ eggs. Acta Agric. Scand. Sect. A Anim. Sci. 2009;59:80–84. [Google Scholar]
- Katz, Oyama R.K., Feng N., Chen X., Schlinger B.A. 11beta-hydroxysteroid dehydrogenase type 2 in zebra finch brain and peripheral tissues. Gen. Comp. Endocrinol. 2010;166:600–605. doi: 10.1016/j.ygcen.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Kavitha T.S., Parthasarathy C., Sivakumar R., Badrinarayanan R., Balasubramanian K. Effects of excess corticosterone on NADPH generating enzymes and glucose oxidation in Leydig cells of adult rats. Hum. Exp. Toxicol. 2006;25:119–125. doi: 10.1191/0960327106ht591oa. [DOI] [PubMed] [Google Scholar]
- Kirby E.D., Geraghty A.C., Ubuka T., Bentley G.E., Kaufer D. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc. Natl. Acad. Sci. USA. 2009;106:11324–11329. doi: 10.1073/pnas.0901176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusonova P., Kucka M., Miksik I., Bryndova J., Pacha J. Chicken 11beta-hydroxysteroid dehydrogenase type 2: partial cloning and tissue distribution. Steroids. 2008;73:348–355. doi: 10.1016/j.steroids.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Kucka M., Vagnerova K., Klusonova P., Miksik I., Pacha J. Corticosterone metabolism in chicken tissues: evidence for tissue-specific distribution of steroid dehydrogenases. Gen. Comp. Endocrinol. 2006;147:377–383. doi: 10.1016/j.ygcen.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Latif S.A., Shen M., Ge R.S., Sottas C.M., Hardy M.P., Morris D.J. Role of 11beta-OH-C(19) and C(21) steroids in the coupling of 11beta-HSD1 and 17beta-HSD3 in regulation of testosterone biosynthesis in rat Leydig cells. Steroids. 2011;76:682–689. doi: 10.1016/j.steroids.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Lee K.A., Volentine K.K., Bahr J.M. Two steroidogenic pathways present in the chicken ovary: theca layer prefers delta 5 pathway and granulosa layer prefers delta 4 pathway. Domest. Anim. Endocrinol. 1998;15:1–8. doi: 10.1016/s0739-7240(97)00057-x. [DOI] [PubMed] [Google Scholar]
- Matsuwaki T., Kayasuga Y., Yamanouchi K., Nishihara M. Maintenance of gonadotropin secretion by glucocorticoids under stress conditions through the inhibition of prostaglandin synthesis in the brain. Endocrinology. 2006;147:1087–1093. doi: 10.1210/en.2005-1056. [DOI] [PubMed] [Google Scholar]
- McNätt L.G., Lane D., Clark A.F. Angiostatic activity and metabolism of cortisol in the chorioallantoic membrane (CAM) of the chick embryo. J. Steroid Biochem. Mol. Biol. 1992;42:687–693. doi: 10.1016/0960-0760(92)90109-v. [DOI] [PubMed] [Google Scholar]
- Michael A.E., Cooke B.A. A working hypothesis for the regulation of steroidogenesis and germ-cell development in the gonads by glucocorticoids and 11-beta-hydroxysteroid dehydrogenase (11-beta-hsd) Mol. Cell. Endocrinol. 1994;100:55–63. doi: 10.1016/0303-7207(94)90279-8. [DOI] [PubMed] [Google Scholar]
- Moberg G.P. How behavioral stress disrupts the endocrine control of reproduction in domestic animals. J. Dairy Sci. 1991;74:304–311. doi: 10.3168/jds.S0022-0302(91)78174-5. [DOI] [PubMed] [Google Scholar]
- Murphy B.E., Clark S.J., Donald I.R., Pinsky M., Vedady D. Conversion of maternal cortisol to cortisone during placental transfer to the human fetus. Am. J. Obstet. Gynecol. 1974;118:538–541. doi: 10.1016/s0002-9378(16)33697-3. [DOI] [PubMed] [Google Scholar]
- Oakley A.E., Breen K.M., Clarke I.J., Karsch F.J., Wagenmaker E.R., Tilbrook A.J. Cortisol reduces gonadotropin-releasing hormone pulse frequency in follicular phase ewes: influence of ovarian steroids. Endocrinology. 2009;150:341–349. doi: 10.1210/en.2008-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuliarova M., Sarnikova B., Rettenbacher S., Skrobanek P., Zeman M. Yolk testosterone and corticosterone in hierarchical follicles and laid eggs of Japanese quail exposed to long-term restraint stress. Gen. Comp. Endocrinol. 2010;165:91–96. doi: 10.1016/j.ygcen.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Orr T.E., Mann D.R. Role of glucocorticoids in the stress-induced suppression of testicular steroidogenesis in adult male rats. Horm. Behav. 1992;26:350–363. doi: 10.1016/0018-506x(92)90005-g. [DOI] [PubMed] [Google Scholar]
- Orr T.E., Taylor M.F., Bhattacharyya A.K., Collins D.C., Mann D.R. Acute immobilization stress disrupts testicular steroidogenesis in adult male rats by inhibiting the activities of 17 alpha-hydroxylase and 17,20-lyase without affecting the binding of LH/hCG receptors. J. Androl. 1994;15:302–308. [PubMed] [Google Scholar]
- Payne A.H., Hales D.B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- Porter T.E., Hargis B.M., Silsby J.L., Elhalawani M.E. Differential steroid-production between theca interna and theca externa cells – a 3-cell model for follicular steroidogenesis in avian species. Endocrinology. 1989;125:109–116. doi: 10.1210/endo-125-1-109. [DOI] [PubMed] [Google Scholar]
- Rettenbacher S., Möstl E., Groothuis T.G.G. Gestagens and glucocorticoids in chicken eggs. Gen. Comp. Endocrinol. 2009;164:125–129. doi: 10.1016/j.ygcen.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Rivier C., Rivest S. Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: peripheral and central mechanisms. Biol. Reprod. 1991;45:523–532. doi: 10.1095/biolreprod45.4.523. [DOI] [PubMed] [Google Scholar]
- Robinson F.E., Etches R.J. Ovarian steroidogenesis during follicular maturation in the domestic fowl (Gallus domesticus) Biol. Reprod. 1986;35:1096–1105. doi: 10.1095/biolreprod35.5.1096. [DOI] [PubMed] [Google Scholar]
- Rozenboim, Tako E., Gal-Garber O., Proudman J.A., Uni Z. The effect of heat stress on ovarian function of laying hens. Poult. Sci. 2007;86:1760–1765. doi: 10.1093/ps/86.8.1760. [DOI] [PubMed] [Google Scholar]
- Saketos M., Sharma N., Santoro N.F. Suppression of the hypothalamic-pituitary-ovarian axis in normal women by glucocorticoids. Biol. Reprod. 1993;49:1270–1276. doi: 10.1095/biolreprod49.6.1270. [DOI] [PubMed] [Google Scholar]
- Sankar B.R., Maran R.R., Sudha S., Govindarajulu P., Balasubramanian K. Chronic corticosterone treatment impairs Leydig cell 11beta-hydroxysteroid dehydrogenase activity and LH-stimulated testosterone production. Horm. Metab. Res. 2000;32:142–146. doi: 10.1055/s-2007-978609. [DOI] [PubMed] [Google Scholar]
- Sankar B.R., Maran R.R., Sivakumar R., Govindarajulu P., Balasubramanian K. Chronic administration of corticosterone impairs LH signal transduction and steroidogenesis in rat Leydig cells. J. Steroid Biochem. Mol. Biol. 2000;72:155–162. doi: 10.1016/s0960-0760(00)00019-4. [DOI] [PubMed] [Google Scholar]
- Seckl J.R., Walker B.R. 11beta-hydroxysteroid dehydrogenase type 1 as a modulator of glucocorticoid action: from metabolism to memory. Trends Endocrinol. Metab. 2004;15:418–424. doi: 10.1016/j.tem.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Stojkov N.J., Janjic M.M., Bjelic M.M., Mihajlovic A.I., Kostic T.S., Andric S.A. Repeated immobilization stress disturbed steroidogenic machinery and stimulated the expression of cAMP signaling elements and adrenergic receptors in Leydig cells. Am. J. Physiol. Endocrinol. Metab. 2012;302:E1239–E1251. doi: 10.1152/ajpendo.00554.2011. [DOI] [PubMed] [Google Scholar]
- Tetsuka M., Milne M., Simpson G.E., Hillier S.G. Expression of 11beta-hydroxysteroid dehydrogenase, glucocorticoid receptor, and mineralocorticoid receptor genes in rat ovary. Biol. Reprod. 1999;60:330–335. doi: 10.1095/biolreprod60.2.330. [DOI] [PubMed] [Google Scholar]
- Tetsuka M. Actions of glucocorticoid and their regulatory mechanisms in the ovary. Anim. Sci. J. 2007;78:112–120. [Google Scholar]
- Tilbrook A.J., Turner A.I., Clarke I.J. Effects of stress on reproduction in non-rodent mammals: the role of glucocorticoids and sex differences. Rev. Reprod. 2000;5:105–113. doi: 10.1530/ror.0.0050105. [DOI] [PubMed] [Google Scholar]
- Tilly J.L., Kowalski K.I., Johnson A.L. Stage of ovarian follicular development associated with the initiation of steroidogenic competence in avian granulosa cells. Biol. Reprod. 1991;44:305–314. doi: 10.1095/biolreprod44.2.305. [DOI] [PubMed] [Google Scholar]
- Touma C., Palme R. Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of validation. Ann. N. Y. Acad. Sci. 2005;1046:54–74. doi: 10.1196/annals.1343.006. [DOI] [PubMed] [Google Scholar]
- Viveiros M.M., Liptrap R.M. Glucocorticoid influence on porcine granulosa cell IGF-I and steroid hormone production in vitro. Theriogenology. 1999;51:1027–1043. doi: 10.1016/s0093-691x(99)80009-0. [DOI] [PubMed] [Google Scholar]
- Vizcarra J.A., Kreider D.L., Kirby J.D. Episodic gonadotropin secretion in the mature fowl: serial blood sampling from unrestrained male broiler breeders (Gallus domesticus) Biol. Reprod. 2004;70:1798–1805. doi: 10.1095/biolreprod.103.023143. [DOI] [PubMed] [Google Scholar]
- von Engelhardt N., Groothuis T.G.G. Maternal hormones in avian eggs. In: D.O. Norris, K.H. Lopez., editors. Birds. vol 4. Academic Press; New York: 2011. pp. 91–127. (Hormones and Reproduction of Vertebrates). [Google Scholar]
- Vylitova M., Miksik I., Pacha J. Metabolism of corticosterone in mammalian and avian intestine. Gen. Comp. Endocrinol. 1998;109:315–324. doi: 10.1006/gcen.1997.7035. [DOI] [PubMed] [Google Scholar]
- Whirledge S., Cidlowski J.A. Glucocorticoids, stress, and fertility. Minerva Endocrinol. 2010;35:109–125. [PMC free article] [PubMed] [Google Scholar]
- Yang J.G., Yu C.C., Li P.S. Dexamethasone enhances follicle stimulating hormone-induced P450scc mRNA expression and progesterone production in pig granulosa cells Chin. J. Physiol. 2001;44:111–119. [PubMed] [Google Scholar]


