Abstract
Adult male rats gestationally exposed to di(n-butyl)phthalate (DBP) have dysgenetic testes characterized by seminiferous epithelial degeneration, clustering of Leydig cells, and decreased spermatogenesis. Cell proliferation and apoptosis are key processes regulating development of the testis, and alterations in these processes may underlie testicular dysgenesis.
Objective
to determine whether gestational exposure to DBP affects cell proliferation and apoptosis in the developing rat testis. Design: pregnant dams were exposed to different dose levels of DBP in mid-gestation and cellular outcomes in fetal and early postnatal testes were assayed by histological and morphometric approaches.
Results
gestational exposure to high dose DBP inhibited proliferation of fetal testicular somatic cells but did not affect apoptosis. Exposed fetal testes had a smaller volume and decreased cell numbers, with decreases in both the tubular and interstitial cell populations. A reduction was observed in the testis volume and altered seminiferous tubule morphometry at ≥50 mg/kg/d, and a decreased testicular cell number at ≥30 mg/kg/d DBP. The number of multinucleated gonocytes in DBP-exposed fetal testes increased after exposure to ≥100 mg/kg/d. The number of proliferating cells in the DBP-exposed testis rapidly rose after birth (when exposure stopped), and the testis volume and the total cell number was comparable to control by postnatal day 2. Conclusion: DBP reversibly inhibits proliferation of somatic cells in the fetal rat testis. Decreased proliferation, rather than increased apoptosis, is the underlying mechanism of altered fetal development of DBP-exposed seminiferous tubules contributing to testicular dysgenesis.
Keywords: Reproduction, antiandrogen, testicular dysgenesis, animal model, phthalate, fetus, somatic, Sertoli, Leydig, gonocytes
INTRODUCTION
Phthalates are high-production-volume chemicals used to soften and add flexibility to plastic consumer products including intravenous fluid bags and infusion sets, blood bags, children’s toys, and plastic curtains (Bradbury, 1996). These chemicals are also present in solvents, lubricating oils, fixatives, and detergents used in residential and commercial construction and in the automotive industry. Di(n-butyl) phthalate (DBP) is found in personal care products such as hair spray, nail polish, perfumes, and skin emollients (Koo et al., 2002). Ingestion is the major route of human exposure to this phthalate. In humans and rats, DBP is metabolized to mono(n-butyl) phthalate (MBP) in the stomach and small intestine (Rowland et al., 1977). MBP is the main active metabolite of DBP, and exposure to either DBP or MBP results in similar alterations in rat testes histopathology (Ema, 2002). Detectable levels of metabolites of at least four phthalates, including DBP, were found in urine samples collected from the general population (Blount et al., 2000; Silva et al., 2004). Infants and young children had the highest urine MBP concentrations followed by women (including women of reproductive age) and teenagers (Silva et al., 2004). Daily exposure to DBP is generally estimated to be below 10 μg/kg of body weight, but in some cases approaches 100 μg/kg.
Several studies have found associations between phthalate exposure and defects related to human reproductive health. Premature thelarche in girls with higher than control serum levels of di-ethyl phthalate (DEP), di-n-butyl phthalate (DBP) and di-2-ethylhexyl phthalate (DEHP) metabolites was found in a single case-control study (Colon et al., 2000). Exposure to DEHP was linked to shorter gestation resulting in preterm birth (Latini et al., 2003). Duty and colleagues showed a correlation between urinary levels of DBP and di-butyl-benzyl phthalate (DBzP) metabolites and reduced sperm concentration and motility (Duty et al., 2003a). These investigators also demonstrated an association between urinary concentration of a DEHP metabolite and increased DNA damage in sperm (Duty et al., 2003b). Swan and colleagues reported a correlation between decreased anogenital distance in male infants and concentration of DEHP, DBP, DBzP and mono-isobutyl phthalate metabolites in the mother’s prenatal urine (Swan et al., 2005). Although it is unknown whether an altered anogenital distance is related to male reproductive health problems in humans, decreased anogenital distance in laboratory animals is a sensitive and permanent biomarker of in utero phthalate exposure, and is associated with a host of male reproductive abnormalities (Barlow and Foster, 2003; Foster et al., 2001; Mylchreest et al., 1999).
In rats, male reproductive tract development is disrupted by DBP exposure (for review see (Kavlock et al., 2002)). Exposure to DBP on gestation days (gd) 12–18 significantly lowered levels of fetal testicular testosterone (Lehmann et al., 2004; Mylchreest et al., 2002) and altered testosterone - and dihydrotestosterone-dependent tissue development. Rats exposed to DBP in utero had decreased anogenital distance, retained nipples, delayed preputial separation, underdeveloped or absent epididymides, hypospadias, and cryptorchidism (Barlow et al., 2004; Fisher et al., 2003; Mylchreest et al., 1999). Some scrotal testes in these rats had focal areas of dysgenetic tubules characterized by degenerative seminiferous epithelium and reduced spermatogenesis (Barlow and Foster, 2003; Barlow et al., 2004; Fisher et al., 2003; Mylchreest et al., 1998). In general, the doses that caused adverse outcomes in rats were several orders of magnitude higher than maximal human exposures.
Testicular dysgenesis may result from altered development of the fetal testis or degeneration of the seminiferous epithelium later in life. Cellular and molecular events underlying development of dysgenetic seminiferous tubules in scrotal testes of adult male rats exposed to DBP in utero are not well understood. Development of seminiferous tubules begins in utero when fetal Sertoli and peritubular myoid cells surround primordial male germ cells. In humans, formation of primitive sex cords and migration of primordial germ cells into these cords starts in the sixth week of embryonic development, corresponding to gd 12 in the rat (Sharpe et al., 2003). During the fetal period, all somatic testicular cells (tunica albugenia, tunica vaginalis, Sertoli, peritubular, Leydig and other interstitial cells) proliferate and male germ cells (gonocytes) divide by mitosis and undergo apoptosis following enclosure in cords. Several lines of evidence suggest that DBP interferes with cell proliferation and apoptosis in the fetal testis. Fetal rat testes exposed to DBP in utero have large disorganized clusters of Leydig cells (Barlow et al., 2004; Fisher et al., 2003; Kleymenova et al., 2005; Shultz et al., 2001) and altered seminiferous tubule morphology suggestive of a decreased number, length or coiling of tubules (Kleymenova et al., 2005). Following exposure to DBP, the occurrence of fetal male germ cells containing multiple nuclei increases and these multinucleated gonocytes (MNGs) seem unable to complete postnatal mitosis (Barlow and Foster, 2003; Kleymenova et al., 2005). Expression of several genes known to regulate cell proliferation and apoptosis is changed in the fetal testis following exposure to DBP (Liu et al., 2005).
The objective of this study was to determine whether gestational exposure of male rats to high doses of DBP alters cell proliferation or apoptosis in the developing testis. Consequently, testicular morphometry was examined following a dose range of gestational exposure to DBP, including the highest levels of estimated human exposure. These studies provide direct evidence that DBP can inhibit cell proliferation in the fetal testis and also shed light on dose-dependent cellular consequences of gestational exposure to this phthalate.
MATERIALS AND METHODS
Animals and Exposure
Timed-mated Sprague-Dawley rats were obtained from Charles River Laboratories, Inc. (Raleigh, NC), with gd 0 defined as the day that sperm were identified in the vagina. The animals were housed and treated according to institutional and Federal guidelines for the care and use of laboratory animals. The dams were maintained on a standard 12-h light-dark cycle at 18–25° C and a relative humidity of 30–70%. Water and NIH-07 rodent chow (Zeigler Brothers, Gardners, PA) were provided ad libitum. Dams received 1ml/kg corn oil (MP Biochemicals, Irvine, CA) or DBP (98 % purity, Sigma, St Louis, MO) in corn oil by oral gavage. In cell kinetics studies, the DBP dose level was 500 mg/kg/d, and in dose-response studies the DBP dose levels were 0.1, 1, 10, 30, 50, 100, and 500 mg/kg/d. Verification of the DBP concentration in the dosing solutions was carried out prior to, and post dosing, by HPLC. In developmental kinetics studies, gavage was performed from gd 12 to gd 16–20, and dams were euthanized by CO2 on gd 17–21. Pups from dams treated from gd 12 to 21 were euthanized by CO2 on postnatal days (pnd) 1 and 2. One testis (right) from each fetus or pup from three corn oil- and 3 DBP- exposed litters was used to assess cellular responses at each gestational and postnatal time point. 5-Bromo-2-deoxyuridine (BrdU; 50 mg/kg in sterile PBS) was given to the dams by intraperitoneal injection 1 hr before euthanasia, or to post-natal pups subcutaneously 2 hours before euthanasia. In the dose response study, dams were treated from gd 12 to 20 and euthanized by CO2 on gd 21. The control group had 10 litters and DBP-exposed groups had 5 litters, except the 100 mg/kg/d group, which had 4 litters (one dam was not pregnant). From this study, two fetuses per litter and one testis (right) per fetus were analyzed for each end point described above.
Fetuses were obtained by Caesarian section, and males were identified by examining internal sex organs under a dissecting microscope. In gd 17–19 fetuses, both testes were left in situ. In gd 20–21 fetuses and all pups, the left testis with epididymis was removed and the right testis left in situ. Fetuses were fixed in modified Davidson fixative by immersion for 24 h, which provides superior preservation of morphological detail as compared to 10 % neutral buffered formalin and Bouin’s solution (Howroyd P. et al., 2005). Fixed in situ right testes were dissected, processed through graded alcohols, cleared in xylene, and embedded in paraffin.
Histopathology
Sections of 5 μm were cut and processed for hematoxylin and eosin staining (H&E), BrdU staining, and for terminal deoxynucleotide transferase-mediated deoxy-UTP nick labeling (TUNEL). H&E staining of fetal and postnatal rat testes fixed in Davidson’s was performed as previously described (Howroyd P. et al., 2005). Anti-BrdU staining was performed using the Dako EnVision+ horseradish peroxidase with DAB chromogen system (Dako, Carpinteria, CA). Antigen retrieval was conducted using Dako target retrieval solution in a de-cloaking chamber (Biocare Medical, Walnut Creek, CA) for 20 min at 95°C. Slides were allowed to cool to 85°C in the chamber. After a rinse in distilled water, slides were placed in Tris-buffered saline containing 0.05% Tween (TBST) and loaded on the Dako Autostainer for the remainder of the procedure. Endogenous peroxidases were blocked by 3 % hydrogen peroxide (Sigma). Dako protein block buffer was applied for 5 min. Anti-BrdU antibody (Caltage Labs, Burlingame, CA) at 1:12000 dilution was applied for 30 min. After rinsing with TBST, labeled polymer (Dako) was applied for 15 min. Following rinsing in TBST, DAB was applied for 5 min. Slides were rinsed in distilled water, counterstained with hematoxylin for 1 min, and rinsed in TBST. After dehydration in ascending grades of alcohol and clearing in xylene, slides were covered using Permount mounting media (Fisher, Pittsburg, PA).
TUNEL was performed using an ApopTag Red apoptosis detection kit (Intergen, Purchase, NY) according to the manufacturer’s protocol for indirect detection using a fluorescent- or DAB-labeled antibody. Hematoxylin was used as counterstain for DAB-labeled slides. Fluorescence-labeled slides were cover-slipped using “Hard Set” Vectashield mounting media (Vector Laboratories, Burlingame, CA) with DAPI as a counterstain.
Morphometry
Testis sections stained with H&E, BrdU, and TUNEL were used for morphometry. To minimize variability associated with the cellular heterogeneity of testicular sections, cell counts were performed on whole sections. Digital images were obtained at 40x magnification using a MagnaFire 2.0 digital camera mounted on BX61 Olympus microscope (Olympus, Melville, NY). Individual nuclei were used as counting objects. For each fetus, systematic random sampling was used to estimate the total number of cells per testis, the testis volume, and the number of tubular cross-sections per section. Three 5 μm H&E-stained transverse sections of each testis were assessed. Cell counts and measurements of the equatorial and polar radii of the sections were measured using Image Pro Plus 4.5 (IPP) software (Media Cybernetics, Silver Spring, MD). The total number of cells per testis was estimated as cell density multiplied by the testis volume. The cell density was estimated as the number of nuclei per section divided by volume of the section (section area multiplied by thickness of the section). Since we were examining the effects of DBP exposure and not the absolute numbers of cells per testis, the adjustment for cells present on two slides using the Abercrombie’s formula (Abercrombie, 1946) was not conducted. The volume of the testis was calculated using the volume equation of a spheroid. Tubular cross-sections were manually outlined on H&E sections using IPP tools and counted by IPP. The number of tubular cross-sections per section was calculated by dividing raw counts by the area of the section.
Calculation of the number of BrdU- and TUNEL-positive cells per testis was performed as described (Tarka-Leeds et al., 2003). This approach assumed that the volume of BrdU and TUNEL -positive cells was the same as the average volume of all cells on a section. The volume equation for a sphere was used to define the volume of a BrdU- positive nucleus. Diameters of 1000 individual BrdU-positive nuclei in control and DBP-exposed testes at all time points were measured using IPP and the mean of these measurements was used in calculating the BrdU-positive nuclear volume. The density of BrdU-positive cells was estimated as the number of BrdU-positive nuclei per section multiplied by the volume of BrdU-positive nuclei and divided by the volume of a section (as defined in the previous paragraph). The number of BrdU-positive cells per testis was estimated by multiplying the density of these cells by the testis volume. The same approach was used to estimate the number of TUNEL-positive cells per testis.
Previously we have demonstrated the occurrence of MNG in fetal rat testis exposed to 500 mg/kg/d DBP, with the maximal number of MNG observed on gd 21 (Kleymenova et al., 2005). MNGs are defined as gonocytes containing 2 or more nuclei within a single cytoplasm. In dose response studies, MNG were counted on three consecutive H&E-stained gd 21 sections by two investigators. One investigator had knowledge of the exposure and a second investigator was counting blindly. If the same multinucleated cell was present on two or three consecutive sections, this cell was counted only once.
Statistical Analyses
In all experiments, the dam was the experimental unit. The analysis was performed in SAS 8.2 (SAS institute, Cary, NC) using proc glm. A two-way analysis of variance (ANOVA) was performed on each variable to assess the significance of the treatment effect for each gestational day or for each dose level. After the variance of the linear model was estimated, an ANOVA-based t-test was conducted to compare the value of the control and DBP for each day or for each dose level. The p-values were adjusted for multiplicity for each experiment using the Bonferroni correction. For the total number of cells and the numbers of tubular and interstitial cells, a log-transformation of data was performed before the modeling. The log transformation made the residuals of the linear models more normally distributed (data not shown). Statistical significance was accepted at p < 0.05 unless otherwise noted.
RESULTS
Overview
The exposure paradigm involved gavage dosing of pregnant dams with DBP beginning on gd 12 with the last dose delivered 24 hrs before sacrifice, and assessments daily on gd 17–21. For the postnatal evaluation of pups on pnd 1–2, the last dose was delivered to the pregnant dams on gd 21, so this represents a time of post-exposure recovery. Throughout this developmental time course, morphometry was used to compare the effects of high dose DBP exposure (500 mg/kg/d) to control, evaluating testis volume, cells per testis (including total, tubular, and interstsitial cells), cellular proliferation as measured by BrdU incorporation, and apoptosis as measured by TUNEL positivity. This evaluation identified gd 21 as the time of greatest DBP-induced alteration in testicular parameters. A subsequent DBP dose-response evaluation was conducted at gd 21 over a wide dose range to identify the lowest observed adverse effect level for testis volume, total testicular cells, tubular cross-sections, and number of MNGs.
Developmental Time Course
The total number of cells and the volume of the testis were estimated in control and DBP-exposed (500 mg/kg/d) rat testes on gd 17–21 and pnd 1 and 2 using H&E stained sections and morphometry. DBP exposure produced a progressive qualitative decrease in the size of the fetal testis (Figure 1, compare panel CON [upper left] to panel 500 [lower right]). By gd 20 and 21, the volume of the DBP-exposed testes was significantly reduced compared with control (Figure 2A). The difference between control and DBP-exposed fetal testes was 51 % on gd 20 and 21. Postnatally, after the exposure had stopped, the decreased volume of DBP-exposed testis rapidly normalized. On Pnd 1, DBP-exposed testes were smaller (31%) than control testes, though this difference was insignificant after the Bonferroni correction for multiple comparisons (p=0.103). There was no difference in the testis volume between control and DBP-exposed testes by pnd 2.
Figure 1.
Representative whole mount images of rat fetal testis cross sections at gd 21 following DBP exposure. Pregnant dams were exposed beginning on gd 12 to corn oil (CON), or DBP at 0.1, 1, 10, 30, 50, 100, or 500 mg/kg/d (labeled in the upper right hand corner for each dose). There is a decrease in testis size that becomes apparent at 30 mg/kg/d. There is also a decrease in the number of seminiferous tubule cross sections and an increase in the size of the seminiferous tubules seen at 100 mg/kg/d that is most obvious at the highest dose (500 mg/kg/d). Bar, 200 μm; magnification is the same in all panels.
Figure 2.
Growth of the fetal and early postnatal rat testis exposed to 500 mg/kg/d DBP in utero. Testis volume (A), the total cell number (B), the number of tubular cells (C), and the number of interstitial cells (D) are shown. Data for corn oil-exposed rats are shown in open triangles and data for DBP-exposed rats are in black squares. *, statistical significance of the difference between corn oil (control) and DBP-exposed testes, #, marginal significance of the difference after the adjustment for multiple comparisons.
A similar pattern of response was observed for the total cell number per testis (Figure 2B). On gd 17, 19, 20, and 21 DBP-exposed fetal testes had significantly fewer cells compared with control testes, and the difference was 38, 48, 52, and 54 %, respectively. The effect of DBP treatment on the total cell number was insignificant by pnd 1 (p=0.055), and total cell counts were normalized by pnd 2. The mean cell density was the same in control and DBP-exposed testes at all time points (data not shown) indicating a lack of DBP effect on the mean cell volume. Consequences of DBP exposure on the testis volume and the cell number were most pronounced on gd 20–21.
Seminiferous tubules were identified on H&E-stained slides and cells associated with, and outside, the tubules were counted. The cells associated with the seminiferous tubules included proliferating peritubular and Sertoli cells, and mitotically quiescent gonocytes. The number of tubular cells in DBP-exposed testes was significantly decreased on gd 17,19–21 but not on gd 18 or pnd 1,2 (Figure 2C). The number of testicular cells outside the seminiferous tubules, including interstitial and tunica cells, followed the same pattern (Figure 2D).
To determine whether changes in cell proliferation or cell death were responsible for the DBP-induced alterations in testis volume and cell number, the incorporation of BrdU into dividing cells and the extent of TUNEL staining of apoptotic cells was evaluated during the developmental time course. BrdU was injected into the pregnant dams 1 hour prior to sacrifice, and into postnatal pups 2 hours before sacrifice. As expected for control testes, BrdU was detected in fetal Sertoli, peritubular, tunica, and interstitial cells, but not in gonocytes (Figure 3 CON). Qualitatively, the cellular distribution pattern and intensity of BrdU staining was the same in treated and control testes at all of the developmental time points (Figure 3, compare CON to 500). Using morphometry, the number of BrdU-positive cells per testis was determined with significant decreases in the number of stained cells in DBP-exposed (500 mg/kg/d) testes on gd 17, 20, and 21 (Figure 4A).). The decrease in the numbers of proliferating cells in control and DBP-exposed testes was 55±17, 70±33 and 64±29 % on gd 17, 20, and 21, respectively. There was a decrease in the number of these cells on 19 but statistical significance was not achieved. A sharp increase in the number of BrdU-positive cells in DBP-exposed testes occurred on pnd 1 and the 34 % difference in the number of BrdU-positive cells between DBP-exposed and control testes was not significant (p=0.23 after the Bonferroni correction). By pnd 2, the number of BrdU-positive cells was similar in control and treated testes.
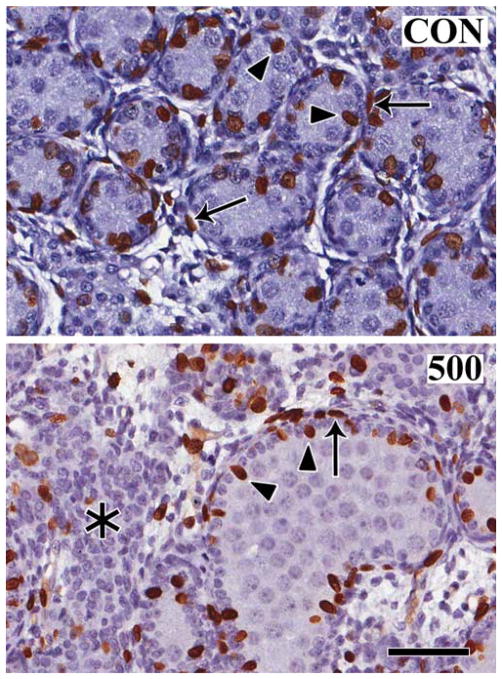
Figure 3.
Patterns of cell proliferation in the gd 21 fetal rat testis. BrdU was injected intraperitoneally into dams one hour before sacrifice, and fetal testis incorporation of the label was compared in corn oil control (CON) and following daily in utero exposure to 500 mg/kg/d DBP beginning on gd 12 (500). Note that the most abundant BrdU-positive (brown) proliferating cells are Sertoli cells (arrowheads) and peritubular cells (arrows), with some labeling of interstitial cells. Gonocytes, the cells occupying the center of the seminiferous tubules, are not labeled. Interestingly, the aggregates of Leydig cells (asterisk) in the DBP-exposed fetal testis have few proliferating cells. Bar, 100 μm; magnification is the same in both panels.
Figure 4.
Cell proliferation (A) and apoptosis (B) in the fetal rat testis following gestational exposure to 500 mg/kg/d DBP. Data for corn oil-exposed rats are shown in open triangles and data for DBP-exposed rats are in black squares. *, statistical significance of the difference between corn oil (control) and DBP-exposed testes (p<0.05).
Qualitatively, TUNEL staining detected few apoptotic cells per section in prenatal control and DBP-exposed (500 mg/kg/d) testes throughout the prenatal developmental time course. As expected (Boulogne et al., 1999), in the normal rat testis the number of apoptotic cells per section increased postnatally. However, at all time points examined in this study there was no difference in the pattern of TUNEL staining between control and DBP-exposed testes (data not shown). No significant difference in the number of apoptotic cells per testis between control and DBP-exposed testes was found at any of the developmental time points examined in this study (Figure 4B).
Dose-Response Relationships
Previous studies suggested that exposure to ≥500 mg/kg/d DBP in utero permanently affects the development of seminiferous tubules (Barlow et al., 2004; Fisher et al., 2003; Kleymenova et al., 2005). The developmental time course studies identified gd 20–21 as the time of greatest effect of DBP exposure (500 mg/kg/d) on testis volume and cell proliferation, as detected by both BrdU- or H&E-stained sections. Therefore, gd 21 was chosen as the developmental time for a detailed DBP dose-response study. For each sample, morphometric measurements were conducted on three consecutive H&E-stained sections of gd 21 testes. To represent each litter (the statistical unit), data were averaged from those two fetal testes with the best transverse sections (undistorted sections with the maximal polar to equatorial diameter ratio), as determined by a blinded observer.
By light microscopy, at low power, there was a clear DBP-dependent decrease in testis size at gd 21 with increasing dose (Figure 1). By light microscopy, at higher power, there were differences in the organization of the seminiferous tubules and the Leydig cells, and in the appearance of the gonocytes, that became increasingly exaggerated with increasing DBP. The apparent size of the seminiferous tubules increased with DBP dose, presumably reflecting a change in their length and degree of coiling (Figure 5). In addition, at the higher doses, there was a clustering of Leydig cells in the interstitial spaces, and the induction of numerous MNGs (Figure 5, lower right panel, 500 mg/kg/d).
Figure 5.
Dose-dependent changes in histopathology at gd 21 following DBP exposure. Shown are testis cross sections from fetal rats following exposure to corn oil (CON), or DBP at 0.1, 1, 10, 30, 50, 100, or 500 mg/kg/d (labeled in the upper right hand corner for each dose). In the control and at low doses of DBP, the seminiferous tubules are similar in size with little intervening interstitial space. At higher doses, beginning at 50 mg/kg/d, the seminiferous tubules show an increased size and abnormalities in shape. At doses of 100 mg/kg/d and above, increased numbers of MNGs (arrowheads) are seen. An obvious Leydig cells aggregate (asterisk) is seen within the interstitium at the highest dose examined (500 mg/kg/d). Bar, 50 μm; magnification is the same in all panels.
The dose-response relationships were quantitated for various endpoints, including testis volume, the total cell number per testis, the number of seminiferous tubule cross sections per testis cross section, and the number of MNGs per testis cross section. A significant DBP-induced decrease in testis volume was observed at ≥50 mg/kg/d (Figure 6A). The total cell number per testis was the most sensitive parameter; with statistical significance (p=0.050 after the Bonferroni adjustment) of the difference between control and treated groups achieved at ≥ 30 mg/kg/d DBP (Figure 6B). The change in the size and organization of the seminiferous tubules was quantitated by counting the number of tubular cross-section per section, with a significant decrease observed at ≥50 mg/kg/d DBP (Figure 6C). A statistically significant increase in the number of MNG was observed at ≥100 mg/kg/d DBP (Figure 6D). Taking these endpoints together, the fetal testis response to DBP occurs abruptly over a relatively narrow dose range (between ~10 and ~100 mg/kg/d).
Figure 6.
Dose-response relationships at gd 21. The testis volume (A), the total cell number (B), the number of tubular cross-sections (C), and the number of MNG (D). *, statistical significance of the difference between control and DBP-exposed testes.
DISCUSSION
Using morphometry, we established that gestational exposure to 500 mg/kg/d DBP starting on gd 12 significantly decreased the number of BrdU-positive cells, indicating a DBP-induced inhibition of somatic cell proliferation in the fetal testis. This decrease was first detected on gd 17, the earliest time point examined in our study, and persisted through gd 20–21. Consistent with the altered cell proliferation, testis cell number on gd 17 and 19–21 was significantly lower in the exposed testes as compared to control. The rates of cell proliferation and the total cell numbers reported here are consistent with previous data on fetal and early postnatal Sertoli and Leydig cell numbers in the rat (Sharpe et al., 2003; Zirkin and Ewing, 1987).
Interestingly, DBP exposure did not change the incidence of apoptosis, indicating that the smaller testis in DBP-exposed rat fetuses resulted from decreased somatic cell proliferation rather than increased cell death. Additionally, the similar rate of apoptosis in control and DBP-exposed testes prenatally indicates that the observed sharp postnatal increase in the number of proliferating cells was not a compensatory response for apoptosis in somatic cells during the fetal period of development. The staining pattern of BrdU-positive cells was similar in control and treated testes at all the developmental time points examined in this study. After birth, all cellular parameters examined in our study returned to control values, suggesting a release from a DBP-induced reduction of cell proliferation as the underlying mechanism of recovery.
The observed DBP-induced decrease in interstitial and tubular cell proliferation affected multiple cell types in the fetal testis. The calculation of cell proliferation rates assumed that DBP exposure did not change cell volume. This assumption is reasonable since the overall mean cell density in DBP-exposed fetal or postnatal rat testes did not change in our study. However, Manhood et al. reported a decreased volume of fetal Leydig cells exposed to DBP (Mahood et al., 2005). A decreased volume of DBP-exposed fetal Leydig cells would not negate our conclusion that DBP inhibits overall cell proliferation in the fetal testis, but should be taken into consideration in estimates of cell type-specific effects of DBP.
Exposure to DBP in utero has been reported to cause large aggregates of interstitial cells in the fetal rat testis (Fisher et al., 2003; Mahood et al., 2005; Mylchreest et al., 1998; Mylchreest et al., 1999; Mylchreest et al., 2002). This finding has led to the hypothesis that DBP exposure induces fetal Leydig cell proliferation as a compensatory response to reduced testosterone (Mylchreest et al., 2002). Research showing immunostaining patterns for proliferating cellular nuclear antigen (PCNA) and phospho-H3 indicated no increase in cell proliferation within interstitial cell aggregates (Barlow et al., 2003; Mahood et al., 2005). The BrdU cell proliferation studies presented here support these observations, showing a paucity of labeling within the aggregates. Moreover, stereological examination of fetal Leydig cell clusters in DBP-exposed rat testes demonstrated that aggregation of these cells was not due to an increase in the cell number but rather because of cell redistribution (Mahood et al., 2005).
A decrease in fetal testosterone is generally accepted as the mechanism by which in utero DBP exposure causes malformations in male reproductive organs in the rat. However, the role of altered androgen mediated signaling in DBP-induced inhibition of cell proliferation inhibition remains under investigation. Intratesticular testosterone concentration correlated with Sertoli cell numbers following DBP exposure in fetal rats, suggesting that testosterone is a regulator of Sertoli cell proliferation (Sharpe et al., 2003). Expression of the androgen receptor in actively proliferating fetal Leydig and peritubular cells suggests that testosterone may be involved in the regulation of proliferation of these cell types. Further, in androgen-dependent prostate cancer, cell cycle kinases CDK 2 and 4 are androgen-regulated genes, while expression of cyclin D inhibitor p16 was repressed in response to androgen (Lu et al., 1997). Chen et al. demonstrated activation of another cyclin D inhibitor p27 in the rat prostate following treatment with testosterone (Chen et al., 1996). When considered together, these data suggest the possibility that testosterone may regulate the cell cycle in these androgen-sensitive cell types, although effects on Leydig cell aggregation appear unrelated to intratesticular testosterone levels (Scott et al., 2007).
Adverse responses in adult rat testes that can affect fertility (such as reduced spermatogenesis, Sertoli cell-only tubules, and focal dysgenic tubules) have been reported following in utero exposure to ≥500 mg/kg/d DBP (Barlow and Foster, 2003; Barlow et al., 2004). When traditional endpoints such as weight and histopathology were assessed, DBP-induced alterations in the fetal rat testes were detected following exposure to ≥250 mg/kg/d (Kavlock et al., 2002). Recently, developmental exposure to DBP (100 mg/kg/d) was shown to induce Leydig cell aggregation, and an increased number of MNGs (Mahood et al., 2007). A significant reduction in fetal testicular testosterone that likely disrupts nipple differentiation was observed at 50 mg/kg/d DBP (Lehmann et al., 2004). We found a decreased total cell number in the fetal rat testis following exposure to 30 mg/kg/d, although this effect was transient and normalized postnatally. However, structural consequences of the decreased cell proliferation during fetal life may be permanent, since previous studies have demonstrated altered seminiferous tubules morphology postnatally following in utero DBP exposure (Barlow and Foster, 2003; Kleymenova et al., 2005). The current data suggests that altered post-natal development of seminiferous tubules may be due to decreased proliferation of tubular cells during fetal development. It is unclear whether the observed decrease in cell proliferation leads to fewer tubules or tubule malformation in DBP-exposed fetal testes. These dose response studies show that the effect of DBP on fetal seminiferous tubules can be detected at ≥50 mg/kg/d DBP, a dose level that significantly reduces fetal testicular testosterone (Lehmann et al., 2004). To what extent reduced testicular testosterone affects the development of seminiferous tubules in the fetal testis has yet to be determined.
In conclusion, these studies provide direct evidence that in utero exposure to DBP inhibits proliferation of somatic cells in the fetal testis resulting in a dose-dependent reversible decrease in the size of the fetal testes. These data confirm previous work that demonstrates that certain testicular responses to DBP exposure in utero are reversible. To date, the reported reversible effects include reduced fetal testicular testosterone, the collapsed fetal Sertoli cells cytoskeleton, and altered expression of smooth muscle actin in fetal peritubular myoid cells (Fisher et al., 2003; Kleymenova et al., 2005; Thompson et al., 2004). Further, a morphometric approach to quantifying cellular outcomes provides a sensitive measure of DBP-induced effects, with detection of significant abnormalities following as little as 30 mg/kg/d DBP exposure to pregnant dams. Data obtained by this approach will be useful for developing a mechanistic model that incorporates cellular responses in the fetal testis following exposure to various DBP dose levels.
Acknowledgments
These studies were funded by The American Chemistry Council’s Long-Range Research Initiative and EPA STAR grant R830766 (K.G.). This article does not necessarily reflect the views or policies of these organizations.
The authors thank Drs. S. Borghoff, D. Dorman, and M. Andersen for a thorough scientific review of this manuscript. Assistance of The Hamner Institute animal care and necropsy personnel is greatly appreciated. The authors thank Otis Lyght for help with BrdU staining.
Abbreviations
- BrdU
5-bromo-2-deoxyuridine
- DBP
di (n-butyl) phthalate
- DBzP
di (butyl-benzyl) phthalate
- DEHP
di (2-ethylhexyl) phthalate
- Gd
gestation days
- H & E
hematoxylin and eosin
- MBP
mono (n-butyl) phthalate
- MEHP
mono (ethyl-hexyl) phthalate
- MNG
multinucleated gonocytes
- PND
postnatal days
- TBST
tris-buffered saline with Tween
- TUNEL
terminal deoxynucleotide transferase-mediated deoxy-UTP nick labeling
References
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Barlow NJ, Foster PM. Pathogenesis of male reproductive tract lesions from gestation through adulthood following in utero exposure to Di(n-butyl) phthalate. Toxicol Pathol. 2003;31(4):397–410. doi: 10.1080/01926230390202335. [DOI] [PubMed] [Google Scholar]
- Barlow NJ, McIntyre BS, Foster PM. Male reproductive tract lesions at 6, 12, and 18 months of age following in utero exposure to di(n-butyl) phthalate. Toxicol Pathol. 2004;32(1):79–90. doi: 10.1080/01926230490265894. [DOI] [PubMed] [Google Scholar]
- Barlow NJ, Phillips SL, Wallace DG, Sar M, Gaido KW, Foster PM. Quantitative changes in gene expression in fetal rat testes following exposure to di(n-butyl) phthalate. Toxicol Sci. 2003;73(2):431–41. doi: 10.1093/toxsci/kfg087. [DOI] [PubMed] [Google Scholar]
- Blount BC, Silva MJ, Caudill SP, Needham LL, Pirkle JL, Sampson EJ, Lucier GW, Jackson RJ, Brock JW. Levels of seven urinary phthalate metabolites in a human reference population. Environ Health Perspect. 2000;108(10):979–82. doi: 10.1289/ehp.00108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulogne B, Olaso R, Levacher C, Durand P, Habert R. Apoptosis and mitosis in gonocytes of the rat testis during foetal and neonatal development. Int J Androl. 1999;22(6):356–65. doi: 10.1046/j.1365-2605.1999.00191.x. [DOI] [PubMed] [Google Scholar]
- Bradbury J. UK panics over phthalates in babymilk formulae. Lancet. 1996;347(9014):1541. doi: 10.1016/s0140-6736(96)90681-9. [DOI] [PubMed] [Google Scholar]
- Chen Y, Robles AI, Martinez LA, Liu F, Gimenez-Conti IB, Conti CJ. Expression of G1 cyclins, cyclin-dependent kinases, and cyclin-dependent kinase inhibitors in androgen-induced prostate proliferation in castrated rats. Cell Growth Differ. 1996;7(11):1571–8. [PubMed] [Google Scholar]
- Colon I, Caro D, Bourdony CJ, Rosario O. Identification of phthalate esters in the serum of young Puerto Rican girls with premature breast development. Environ Health Perspect. 2000;108(9):895–900. doi: 10.1289/ehp.108-2556932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duty SM, Silva MJ, Barr DB, Brock JW, Ryan L, Chen Z, Herrick RF, Christiani DC, Hauser R. Phthalate exposure and human semen parameters. Epidemiology. 2003a;14(3):269–77. [PubMed] [Google Scholar]
- Duty SM, Singh NP, Silva MJ, Barr DB, Brock JW, Ryan L, Herrick RF, Christiani DC, Hauser R. The relationship between environmental exposures to phthalates and DNA damage in human sperm using the neutral comet assay. Environ Health Perspect. 2003b;111(9):1164–9. doi: 10.1289/ehp.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema M. Antiandrogenic effects of dibutyl phthalate and its metabolite, monobutyl phthalate, in rats. Congenit Anom Kyoto. 2002;42(4):297–308. doi: 10.1111/j.1741-4520.2002.tb00896.x. [DOI] [PubMed] [Google Scholar]
- Fisher JS, Macpherson S, Marchetti N, Sharpe RM. Human ‘testicular dysgenesis syndrome’: a possible model using in-utero exposure of the rat to dibutyl phthalate. Hum Reprod. 2003;18(7):1383–94. doi: 10.1093/humrep/deg273. [DOI] [PubMed] [Google Scholar]
- Foster PM, Mylchreest E, Gaido KW, Sar M. Effects of phthalate esters on the developing reproductive tract of male rats. Hum Reprod Update. 2001;7(3):231–5. doi: 10.1093/humupd/7.3.231. [DOI] [PubMed] [Google Scholar]
- Howroyd P, Hoyle-Thacker R, Lyght O, Williams DEK. Morphology of the fetal rat testis preserved in different fixatives. Toxicol Pathology. 2005;33:300–304. doi: 10.1080/01926230590896145. [DOI] [PubMed] [Google Scholar]
- Kavlock R, Boekelheide K, Chapin R, Cunningham M, Faustman E, Foster P, Golub M, Henderson R, Hinberg I, Little R, Seed J, Shea K, Tabacova S, Tyl R, Williams P, Zacharewski T. NTP Center for the Evaluation of Risks to Human Reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di-n-butyl phthalate. Reprod Toxicol. 2002;16(5):489–527. doi: 10.1016/s0890-6238(02)00033-3. [DOI] [PubMed] [Google Scholar]
- Kleymenova E, Swanson C, Boekelheide K, Gaido KW. Biol Reprod:biolreprod.104.037184. 2005. Exposure In Utero to Di-(n-Butyl) Phthalate Alters the Vimentin Cytoskeleton of Fetal Rat Sertoli Cells and Disrupts Sertoli Cell-Gonocyte Contact. [DOI] [PubMed] [Google Scholar]
- Koo JW, Parham F, Kohn MC, Masten SA, Brock JW, Needham LL, Portier CJ. The association between biomarker-based exposure estimates for phthalates and demographic factors in a human reference population. Environ Health Perspect. 2002;110(4):405–10. doi: 10.1289/ehp.02110405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, Mazzeo P. In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environ Health Perspect. 2003;111(14):1783–5. doi: 10.1289/ehp.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann KP, Phillips S, Sar M, Foster PM, Gaido KW. Dose-dependent alterations in gene expression and testosterone synthesis in the fetal testes of male rats exposed to di (n-butyl) phthalate. Toxicol Sci. 2004;81(1):60–8. doi: 10.1093/toxsci/kfh169. [DOI] [PubMed] [Google Scholar]
- Liu K, Lehmann KP, Sar M, Young SS, Gaido KW. Biol Reprod:biolreprod.104.039404. 2005. Gene Expression Profiling Following In Utero Exposure to Phthalate Esters Reveals New Gene Targets in the Etiology of Testicular Dysgenesis. [DOI] [PubMed] [Google Scholar]
- Lu S, Tsai SY, Tsai MJ. Regulation of androgen-dependent prostatic cancer cell growth: androgen regulation of CDK2, CDK4, and CKI p16 genes. Cancer Res. 1997;57(20):4511–6. [PubMed] [Google Scholar]
- Mahood IK, Hallmark N, McKinnell C, Walker M, Fisher JS, Sharpe RM. Abnormal Leydig Cell Aggregation in the Fetal Testis of Rats Exposed to Di (n-Butyl) Phthalate and Its Possible Role in Testicular Dysgenesis. Endocrinology. 2005;146(2):613–623. doi: 10.1210/en.2004-0671. [DOI] [PubMed] [Google Scholar]
- Mahood IK, Scott HM, Brown R, Hallmark N, Walker M, Sharpe RM. In utero exposure to di(n-butyl) phthalate and testicular dysgenesis: comparison of fetal and adult end points and their dose sensitivity. Environ Health Perspect. 2007;115(Suppl 1):55–61. doi: 10.1289/ehp.9366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylchreest E, Cattley RC, Foster PM. Male reproductive tract malformations in rats following gestational and lactational exposure to Di(n-butyl) phthalate: an antiandrogenic mechanism? Toxicol Sci. 1998;43(1):47–60. doi: 10.1006/toxs.1998.2436. [DOI] [PubMed] [Google Scholar]
- Mylchreest E, Sar M, Cattley RC, Foster PM. Disruption of androgen-regulated male reproductive development by di(n-butyl) phthalate during late gestation in rats is different from flutamide. Toxicol Appl Pharmacol. 1999;156(2):81–95. doi: 10.1006/taap.1999.8643. [DOI] [PubMed] [Google Scholar]
- Mylchreest E, Sar M, Wallace DG, Foster PM. Fetal testosterone insufficiency and abnormal proliferation of Leydig cells and gonocytes in rats exposed to di(n-butyl) phthalate. Reprod Toxicol. 2002;16(1):19–28. doi: 10.1016/s0890-6238(01)00201-5. [DOI] [PubMed] [Google Scholar]
- Rowland IR, Cottrell RC, Phillips JC. Hydrolysis of phthalate esters by the gastrointestinal contents of the rat. Food Cosmet Toxicol. 1977;15(1):17–21. doi: 10.1016/s0015-6264(77)80257-5. [DOI] [PubMed] [Google Scholar]
- Scott HM, Hutchison GR, Mahood IK, Hallmark N, Welsh M, De Gendt K, Verhoeven G, O’Shaughnessy P, Sharpe RM. Role of androgens in fetal testis development and dysgenesis. Endocrinology. 2007;148(5):2027–36. doi: 10.1210/en.2006-1622. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125(6):769–84. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- Shultz VD, Phillips S, Sar M, Foster PM, Gaido KW. Altered gene profiles in fetal rat testes after in utero exposure to di(n-butyl) phthalate. Toxicol Sci. 2001;64(2):233–42. doi: 10.1093/toxsci/64.2.233. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL, Calafat AM. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect. 2004;112(3):331–8. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S, Teague JL Team, S.f.FF.R. Decrease in Anogenital Distance Among Male Infants with Prenatal Phthalate Exposure. Environ Health Perspect. 2005 doi: 10.1289/ehp.8100. available via http://dx.doi.org/ [Online 27 May 2005] [DOI] [PMC free article] [PubMed]
- Tarka-Leeds DK, Suarez JD, Roberts NL, Rogers JM, Hardy MP, Klinefelter GR. Gestational exposure to ethane dimethanesulfonate permanently alters reproductive competence in the CD-1 mouse. Biol Reprod. 2003;69(3):959–67. doi: 10.1095/biolreprod.103.017343. [DOI] [PubMed] [Google Scholar]
- Thompson CJ, Ross SM, Gaido KW. Di(n-butyl) phthalate impairs cholesterol transport and steroidogenesis in the fetal rat testis through a rapid and reversible mechanism. Endocrinology. 2004;145(3):1227–37. doi: 10.1210/en.2003-1475. [DOI] [PubMed] [Google Scholar]
- Zirkin BR, Ewing LL. Leydig cell differentiation during maturation of the rat testis: a stereological study of cell number and ultrastructure. Anat Rec. 1987;219(2):157–63. doi: 10.1002/ar.1092190208. [DOI] [PubMed] [Google Scholar]