Abstract
Leukocyte antigen CD38 expression is an early marker of all-trans retinoic acid (ATRA) stimulated differentiation in the leukemic cell line HL-60. It promotes induced myeloid maturation when overexpressed, whereas knocking it down is inhibitory. It is a type II membrane protein with an extracellular C-terminal enzymatic domain with NADase/NADPase and ADPR cyclase activity and a short cytoplasmic N-terminal tail. Here we determined whether CD38 enzymatic activity or the cytoplasmic tail is required for ATRA-induced differentiation. Neither a specific CD38 ectoenzyme inhibitor nor a point mutation that cripples enzymatic activity (CD38 E226Q) diminishes ATRA-induced differentiation or G1/0 arrest. In contrast a cytosolic deletion mutation (CD38 Δ11–20) prevents membrane expression and inhibits differentiation and G1/0 arrest. These results may be consistent with disrupting the function of critical molecules necessary for membrane-expressed CD38 signal transduction. One candidate molecule is the Src family kinase Fgr, which failed to undergo ATRA-induced upregulation in CD38 Δ11–20 expressing cells. Another is Vav1, which also showed only basal expression after ATRA treatment in CD38 Δ11–20 expressing cells. Therefore, the ability of CD38 to propel ATRA-induced myeloid differentiation and G1/0 arrest is unimpaired by loss of its ectoenzyme activity. However a cytosolic tail deletion mutation disrupted membrane localization and inhibited differentiation. ATRA-induced differentiation thus does not require the CD38 ectoenzyme function, but is dependent on a membrane receptor function.
Keywords: CD38 signaling, HL-60 differentiation, ATRA
Introduction
All-trans retinoic acid (ATRA) leads to the myeloid differentiation and G1/0 arrest of HL-60 human myeloblastic leukemia cells. The process may depend on the early ATRA-induced expression of the leukocyte antigen CD38, a 45 kDa type II transmembrane glycoprotein that has both enzymatic and receptor functions. It is an early biomarker of ATRA-induced differentiation in the HL-60 cell line that is detectable after 6 h of treatment and reaches maximum expression within 16 h [1]. CD38 may play a causal role in HL-60 myeloid differentiation, since RNAi directed toward CD38 crippled ATRA induction [2]. Transfectants that overexpress wild-type CD38 show an enhanced rate of differentiation indicated by increased inducible oxidative metabolism by 48 h and G1/0 arrest by 72 h [1].
CD38 is an ectoenzyme that catalyzes the formation of adenosine diphosphate ribose (ADPR), cyclic ADPR (cADPR), and nicotinamide from NAD+ under neutral pH; or NAADP+ from NADP under acidic conditions [3]. Both cADPR and NAADP+ facilitate calcium signaling. ATRA-treated HL-60 cells release nuclear calcium in response to cADPR production that correlates with the presence of nuclear CD38 protein, suggesting a role in differentiation [4]. However, ATRA-induced differentiation causes a decrease in total cellular calcium levels, and studies of calcium flux inhibition during ATRA treatment also suggested independence [5,6]. Thus the precise role of calcium flux and its stimulation is not fully understood.
In addition to its enzymatic activity, CD38 has receptor functions that participate in diverse signaling mechanisms that vary with cell type and differentiation status [7]. Membrane-expressed CD38 forms lateral associations with CD3 on T lymphocytes; with surface Ig, CD19, and CD21 on B cells; and with CD16 on NK cells to produce signaling events [8–10]. In human B cell precursors, ligation results in tyrosine phosphorylation of proteins such as Syk, phospholipase C-γ, and the p85 subunit of PI3K [11]. In myeloid cells, CD38 mo (Ab)-induced tyrosine phosphorylation can be mediated through FcγII receptors [12]. In HL-60 cells CD38-agonist interaction also results in phosphorylation of c-Cbl, a cytosolic adapter molecule known to promote MAPK signaling and ATRA induced differentiation [13,14]. Fluorescence resonance energy transfer (FRET) data and immunoprecipitation experiments show that these proteins exist in a complex [15].
CD38 also drives MAPK activation after agonist ligation, which is orchestrated by Raf, MEK, and ERK [16,17]. Transient or protracted signaling from this cascade can lead to either cell proliferation or differentiation respectively [18], and sustained MAPK signaling is required for ATRA-induced differentiation [19,20].
In myeloid cells, CD38 signaling may promote either cell proliferation or growth inhibitory signals [21,22]. The apparently divergent functions, particularly within myeloid cell lines, make the role of CD38 somewhat enigmatic. It may reflect the function of different domains and their relative activities in different contexts. Given that the enzymatic activity, receptor signaling, and downstream effectors of CD38 might produce divergent outcomes, and that CD38 likely participates directly in differentiation, we investigated which domains of CD38 are required for ATRA-induced HL-60 myeloid differentiation. Our results showed that the enzymatic activity of CD38 is expendable, while the transmembrane proximal cytosolic region needed for membrane expression is required.
Materials and methods
Cell culture
HL-60 human myeloblastic leukemia cells and stable transfectant cell lines (CD38 E226Q, WT38, and CD38 Δ11–20) were grown in RPMI 1640 supplemented with 5% heat-inactivated fetal bovine serum purchased from Invitrogen (Carlsbad, CA) in a 5% CO2 humidified atmosphere at 37 °C. All-trans-retinoic acid (ATRA) was purchased from Sigma (St. Louis, MO) and solubilized in ethanol. Cells were cultured in a final concentration of 1 μM. Arabinosyl 2′-fluoro-2′-deoxy NAD (F-araNAD) small molecules were suspended in water and cells were cultured in a final concentration of 5 μM. For some experiments additional 5 μM doses were added every 24 h to compensate for possible inhibitor degradation.
Antibodies and reagents
F-araNAD was synthesized following published procedures [23]. Antibodies for cytometric analysis of CD38 and CD11b, and CD38 Western blot antibody were purchased from Invitrogen and BD Pharmingen (San Jose, CA), respectively. Fgr antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA); HRP anti-mouse, HRP anti-rabbit, Vav1, GAPDH, and p-ERK were purchased from Cell Signaling (Danvers, MA); and anti-total ERK from Zymed (San Francisco, CA). M-PER Mammalian Protein Extraction Reagent lysis buffer was purchased from Pierce (Rockford, IL). Protease and phosphatase inhibitors, nicotinamide guanine dinucleotide (NGD+), propidium iodide, dimethyl sulfoxide (DMSO), and 12-O-Tetradecanoylphorbol 13-acetate (TPA) were purchased from Sigma, and 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA) or DCFH was purchased from Invitrogen.
Generation of stable transfectants
WT38 cell lines were created as previously described [1]. Stable transfectants were sorted to enrich the population for cells with transfected expression by flow cytometry using anti-CD38. CD38 E226Qwas created by transfectingHL-60 cells with a pIRES-hr GFP II vector purchased from Stratagene (La Jolla, CA) containing a CD38 PCR product flanked by 5′ EcoRV (GATATCATGGCCAACTGCGAGTTCAGCCCGGTG) and 3′ Not1 restriction sites (GCGGCCGCTCAGATGTGCAAGATGAATCCTCAGGATTTTTCAC). Site-directed mutagenesis was used to create the CD38 E226Q point mutation using the (GCACTTTTGGGAGTGTGCAAGTCCATAATTTGCAACCAG) sequence. Stable transfectants were sorted by flow cytometry based on CD38 immunostaining.
The CD38 Δ11–20 cell line was created using an identical pIRES-hr GFP II vector with CD38 cDNA containing a deletion of amino acids 11–20 with a HindIII site bridging nucleotides 30–61. The cDNA fragment was cloned into the vector with 5′ EcoRV and 3′ Not1 restriction sites. Segment 1: 5′ EcoRV forward (GATATCAGTGAAACAGAAGGGGAGGTGCAGTTTCAG) and 3′ HindIII reverse (AAGCTTCCCGGACACCGGGCTGAACTCGCAGTT); Segment 2: 3′ HindIII forward (AAGCTTGCCCAACTCTGTCTTGGCGTCAGTATCCTG) and 5′ Not1 reverse (GCGGCCGCTCAGATGTGCAAGATGAATCCTCAGGATTT). Stable transfectants were created by flow cytometric sorting for GFP-positive cells. Stably transfected cell lines containing CD38 E226Q mutations and the CD38 Δ11–20 truncation were further verified by mRNA isolation followed by reverse transcriptase PCR amplification and gel analysis and sequencing.
mRNA isolation and sequencing
Total RNA was extracted from the CD38 E226Q and CD38 Δ11–20 cell lines with Invitrogen Trizol reagent per the manufacturer's protocol. Total RNA was measured by a spectrofluorometer (Beckman Coulter, CA), and reverse transcriptase polymerase chain reaction (RT-PCR) was performed using Invitrogen SuperScript One-Step RT-PCR with Platinum Taq. The appropriate DNA sequences were then verified by sequencing at Cornell University's Life Sciences Core Laboratory.
Flow cytometric phenotypic analysis
Immunostaining for CD11b and CD38 was performed as previously described using a Becton Dickinson LSR II flow cytometer (San Jose, CA) [15]. Gating was set to exclude 95% of the negative control. Respiratory burst capability was measured as previously described [6]. Propidium iodide (PI) cell cycle analysis was performed as previously described [15].
Western blot analysis
For Western blot analysis, cells were pelleted, washed, and lysed with ice cold Pierce M-PER Mammalian Protein Extraction Reagent (Rockford, IL) with a protease inhibitor cocktail containing 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF), pepstatinA, E-64, bestatin, leupeptin, and aprotinin (Sigma); and a phosphatase inhibitor cocktail containing sodium vanadate, sodium molybdate, sodium tartrate, and imidazoleprotease (Sigma). Samples were incubated on ice and debris was pelleted. Protein content was determined by Pierce BCA protein reagent assay. Lysate was subjected to standard SDS-PAGE. Membranes were then probed with antibodies described above. All blot films shown were transferred to the same membrane and are the same exposure.
Detection of CD38 enzymatic activity
ADP-ribosyl cyclase activity was detected by fluorometric analysis of the NGD+ metabolic product cyclic GDP-ribose (cGDPR) using a spectrofluorometer (Horiba Jobin Yvon Fluoro Max3, NJ) as previously described [24,25]. For the CD38 F-araNAD inhibitor assay, cells were cultured for 48 h untreated or in the presence of 1 μM ATRA to induce CD38 expression. Appropriate samples were treated with 5 μM F-araNAD or left untreated for 30 min with constant rotation at 37 °C with protease and phosphatase inhibitors. All samples evaluated for CD38 enzymatic activity were treated with 100 μM NGD+ for 1 h with constant rotation at 37 °C. Cells were then pelleted, and supernatant was subjected to fluorometric analysis for cGDPR.
Statistics
Statistics were analyzed by JMP Statistical Discovery Software and Microsoft Excel statistical software.
Results
CD38 enzymatic inhibitors do not compromise ATRA-induced differentiation or G1/0 arrest
CD38 enzymatic ADPR cyclase activity is important for calcium mobilization and therefore potentially relevant to differentiation. The enzymatic activity and the receptor signaling of CD38 are reportedly uncoupled, and ADPR or cADPR generation is thought to be independent of signaling mechanisms [26,27]. Therefore, we investigated whether crippling the enzymatic activity of CD38 influenced ATRA-induced differentiation.
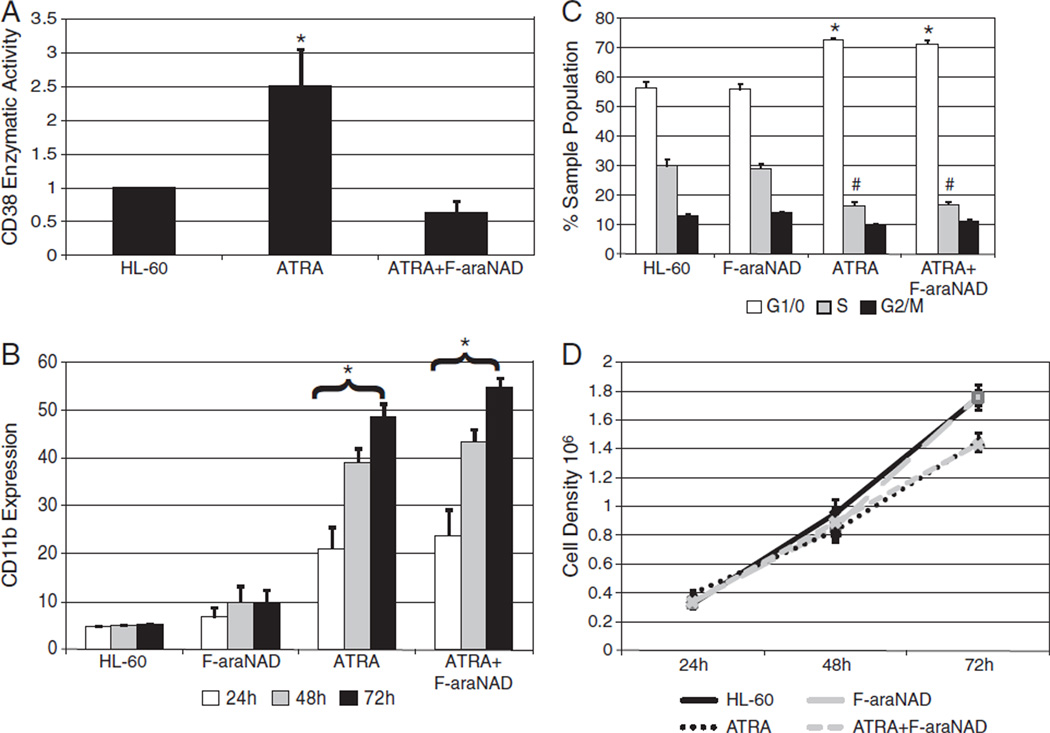
We first tested whether a small molecule inhibitor of CD38 can interfere with ATRA-induced HL-60 cell differentiation. Arabinosyl 2′-fluoro-2′-deoxy NAD (F-araNAD)was added with ATRA. F-araNAD is a cell impermeable known suicide substrate that inhibits CD38 ectoenzyme activity by forming a covalent adduct with E226 in the active pocket [23,28]. CD38 has the potential to be internalized [29–32], including to the nucleus. Since the binding of F-araNAD to CD38 is covalent, then the internalized CD38 would be expected to retain the inhibitor. To verify the ability of F-araNAD to block CD38 enzymatic activity, we used the fluorometric NGD+ substrate assay. NGD+ is a NAD+ analog that can be cyclized by CD38 to cGDPR, a stable fluorescent product. We compared the fluorescence of untreated HL-60 cells with and without NGD+ substrate and found no significant differences, indicating that untreated control cells had negligible ADPR cyclase activity (data not shown). We then compared the relative amount of cGDPR fluorescence generated by untreated cells, ATRA-treated HL-60 cells expressing CD38, or cells co-treated with ATRA plus 5 μM F-araNAD (Fig. 1A). Fluorescence is reported in arbitrary units where 1 is the null background of control cells without CD38. While there was a significant difference influorescence between untreated cells and cells treated with ATRA, there were no apparent differences between control cells and co-treated cells. The inhibitor was therefore effective in crippling CD38 catalytic activity.
Fig. 1.
CD38 inhibitors do not affect ATRA-induced differentiation markers. A: Cells were cultured for 48 h with or without 1 μM ATRA and evaluated for cGDPR production by NGD+ assay. The fold increase of cGDPR production was calculated by normalizing each sample to an arbitrary null control value of 1 (*p = < 0.05). B: Membrane CD11b expression was measured by flow cytometry at indicated time points. (*p = < 0.05 significantly higher than HL-60 cells that were not treated with ATRA.) C: HL-60 cells were treated or not with 1 μM ATRA for 72 h. Appropriate samples were incubated with 5 μM F-araNAD. Cell cycle phase distribution was determined by flow cytometry with propidium iodide staining. (*p = < 0.05 significantly higher than untreated G1/0 sample groups; #p = < 0.05 significantly lower than untreated S sample groups.) D: Cell density was measured using a hemocytometer and 0.2% Trypan Blue exclusion staining.
Next we determined the effects of CD38 inhibitors on ATRA-induced differentiation. ATRA treatment causes HL-60 cells to exhibit differentiation markers such as the α-integrin receptor subunit CD11b. F-araNAD had no apparent effect on ATRA-induced CD11b expression, with or without ATRA treatment (Fig. 1B).
ATRA-treated HL-60 cells also undergo G1/0 enrichment, indicating cell cycle arrest. Cells were cultured with or without retinoic acid, and with or without 5 μM F-araNAD for 72 h. The proportion of G1/0 cells in neither ATRA-treated samples nor untreated samples was affected by inhibitor treatment (Fig. 1C). Cell density was also measured during 72 h to detect growth arrest (Fig. 1D). ATRA-treated samples, regardless of F-araNAD treatment, show similar growth retardation at 72 h. Untreated HL-60 cells and cells treated only with the inhibitor show continuous exponential growth. Therefore, inhibition of CD38 enzymatic activity by F-araNAD did not affect several markers of ATRA-induced myeloid differentiation. A second inhibitor, Arabinosyl-2′-fluoro-2′-deoxy nicotinamide mononucleotide (F-araNMN), was also tested and similarly failed to affect ATRA-induced differentiation and corroborated these results (data not shown).
An E226Q mutation does not alter CD38 effects on ATRA-induced differentiation or G1/0 arrest
The experiments using pharmacological inhibition indicated that inhibiting the ectoenzyme activity of the endogenous CD38 did not affect induced differentiation, suggesting that the ectoenzyme activity is not critical. This gives rise to an anticipation that was tested. Since ectopic expression of wild-type CD38 promotes induced differentiation, then the enzymatically inactive [33] CD38 E226Q catalytic mutant would be expected to do the same if the ectoenzyme activity was not needed for this. Therefore, we ectopically expressed CD38 E226Q and investigated whether or not overexpression of CD38 E226Q was able to similarly drive ATRA induction.
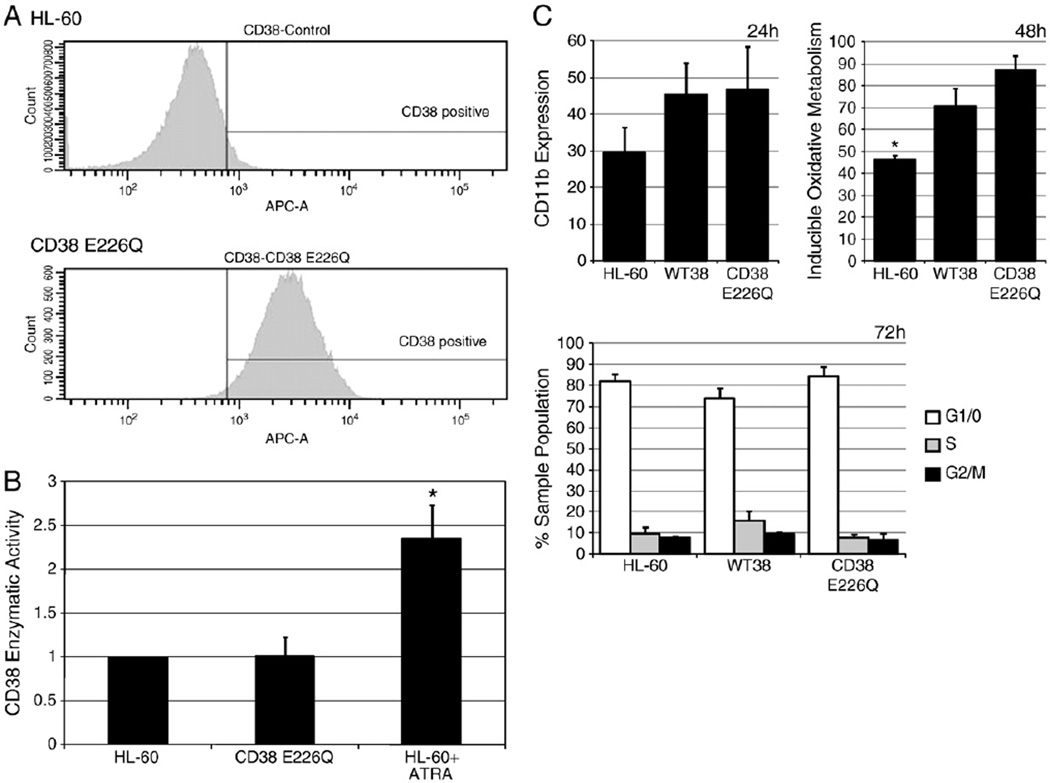
We created cells stably transfected with the CD38 E226Q catalytic mutant and verified the mutation by sequencing. Expression of protein was confirmed by flow cytometry of immunostained cells (Fig. 2A). Cell viability was unaffected by E226Q expression since cells showed normal growth, no increase in Trypan Blue exclusion staining, and no detectable sub-G1 population using cytometric propidium iodide staining (data not shown). Expression of CD38 after ATRA treatment was comparable in both WT38 and CD38 E226Q transfectants, allowing comparison without caveats due to achieving different expression levels (data not shown). The enzymatic activity for each cell line was tested using the NGD+ assay. cGDPR levels in E226Q cells were comparable to that of untreatedHL-60 cells, showing that the point mutation was effective in crippling catalytic activity (Fig. 2B). ATRA-treated HL-60 cells expressing CD38 were used as a positive control.
Fig. 2.
CD38 E226Q is able to promote ATRA induction similar to wild-type CD38. A: Flow cytometry immunostaining for CD38 in untreated HL-60 cells and CD38 E226Q stable transfectants. B: Cells were assayed for cGDPR production by NGD+ assay. The fold increase of cGDPR production was calculated by normalizing each sample to an arbitrary null control value of 1 (*p = < 0.05). C: Cells were treated for 24 h with ATRA, and induced CD11b expression was measured by flow cytometry (top left); cells were treated for 48 h with ATRA, and inducible oxidative metabolism was measured by DCF assay (top right; *p = < 0.5 significantly lower than WT38 and CD38 E226Q); cell lines were cultured with ATRA for 72 h and cell cycle phase distribution was determined by nuclear propidium iodide staining (bottom). No significant differences (p = < 0.05) were detected between WT38 and CD38 E226Q samples in any of the above experiments.
Cells stably transfected with wild-type CD38 (WT38) showed enhanced ATRA-induced differentiation [1]. To determine if CD38 E226Q and WT38 cells propelled differentiation similarly, we analyzed several markers to investigate whether or not the catalytic mutation had any affect. We first compared CD11b expression in response to ATRA in HL-60, WT38, and CD38 E226Q cells. After 24 h, both WT38 and CD38 E226Q showed increased CD11b expression compared to ATRA-treated HL-60 cells (Fig. 2C, top left). We then compared oxidative metabolism capability. After 48 h ATRA-treated WT38 and CD38 E226Q showed a comparable increase in inducible oxidative metabolism, which was significantly higher than ATRA-treated HL-60 cells (Fig. 2C, top right). These results suggested that both WT38 and CD38 E226Q expressing cells are similarly capable of enhancing functional differentiation. Finally, to determine if there were effects on cell cycle inhibition, we analyzed ATRA-induced G1/0 arrest. ATRA-treated HL-60, CD38 E226Q, and WT38 cells all showed similar G1/0 arrest by 72 h (Fig. 2C, bottom). CD38 E226Q transfected cells thus underwent differentiation indistinguishably from WT38 cells in response to ATRA. Like wild-type CD38, CD38 E226Q was also able to enhance CD11b expression and inducible oxidative metabolism compared to HL-60 cells. This confirms the anticipation of the earlier F-araNAD results.
A cytosolic deletion prevents membrane expression in CD38 transfectants
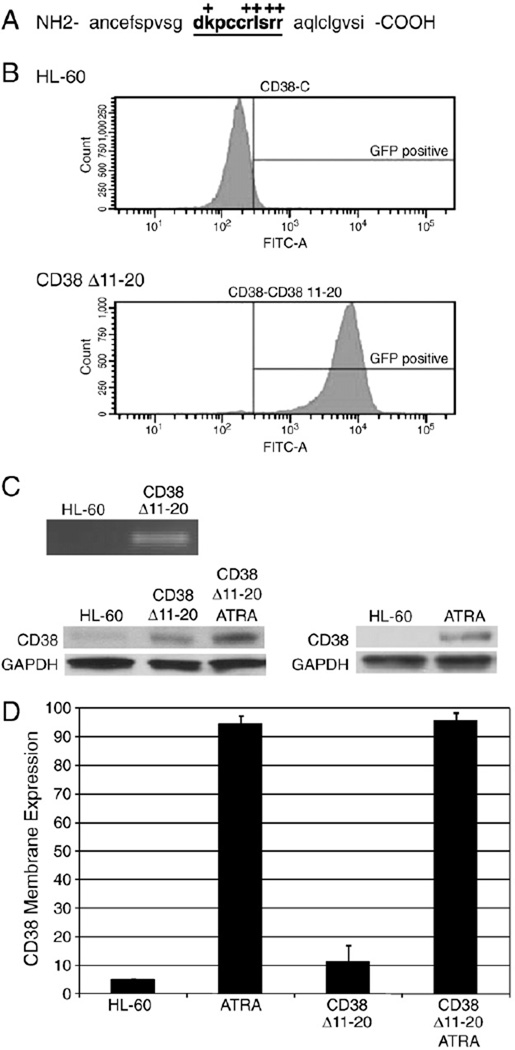
The CD38 cytosolic N-terminal tail is dispensable for signaling in pro-B cells [34,35]. However, Moreno-Garcia et al. show that the cytosolic tail is important for CD38 homodimer stabilization and a truncation mutant decreases the half-life of membrane expression in murine B lymphocytes [36], which may be important for signaling. To assess the contribution of the short CD38 cytosolic tail to ATRA induction, we determined whether a deletion affected membrane expression of CD38 or ATRA-induced HL-60 differentiation. Ten amino acids were truncated in the cytosolic N terminus proximal to the transmembrane region (Fig. 3A). These 10 residues were reported to be necessary for interaction with Lck, a Src family kinase, in T cell signaling events [9,37] and were considered good candidates for interactions with other proteins in different cell lines.
Fig. 3.
A CD38 cytosolic deletion mutant does not express on the membrane. A: CD38 N-terminal cytosolic and transmembrane amino acids are shown. Deleted amino acids are in bold and underlined. Positively charged amino acids are indicated with a +. B: GFP-positive population in HL-60 and CD38 Δ11–20 cells. C: mRNA from HL-60 cells and CD38 Δ11–20 was subjected to reverse-transcriptase PCR for CD38 expression and visualized on an agarose gel (top). Western blot for total CD38 protein in CD38 Δ11–20 and HL-60 cells, with and without 48 h of ATRA treatment (bottom). Untreated HL-60 cells were used as a negative control for showing CD38 Δ11–20 expression in transfectants. D: Membrane expression of CD38 was measured after 24 h of culture by immunostaining and flow cytometry.
Stable transfectants expressing the CD38 Δ11–20 deletion were isolated by FACS sorting using a GFP marker co-expressed from a CD38 Δ11–20/GFP bicistronic transcript with an IRES element (Fig. 3B). The deletion was confirmed by mRNA isolation followed by reverse transcriptase PCR and gel analysis, and protein expression was confirmed by Western blot (Fig. 3C). Cell viability was unaffected by CD38Δ11–20 overexpression since cells showed normal growth, no increase in Trypan Blue exclusion staining, and no detectable sub-G1 population using cytometric propidium iodide staining (data not shown). Interestingly, although ATRA-treated HL-60 cells expressing wild-type CD38 were able to be membrane-labeled with antibody, cells expressing CD38 Δ11–20 showed minimal labeling and no significant difference in membrane expression compared to the untreated control (Fig. 3D). The lack of CD38 Δ11–20 membrane expression could reflect the loss of the positively charged residues in the transmembrane-proximal cytosolic region, which may be essential for proper membrane insertion and orientation. This is consistent with studies that report CD38 mutant proteins with truncated cytosolic regions show significantly reduced membrane expression and half-lives compared to wild-type protein [36]. After CD38 Δ11–20 transfectants were treated with ATRA, membrane expression of CD38 was comparable to that of ATRA-treated HL-60 cells consistent with endogenous CD38 expression.
CD38 Δ11–20 cripples ATRA-induced differentiation induction
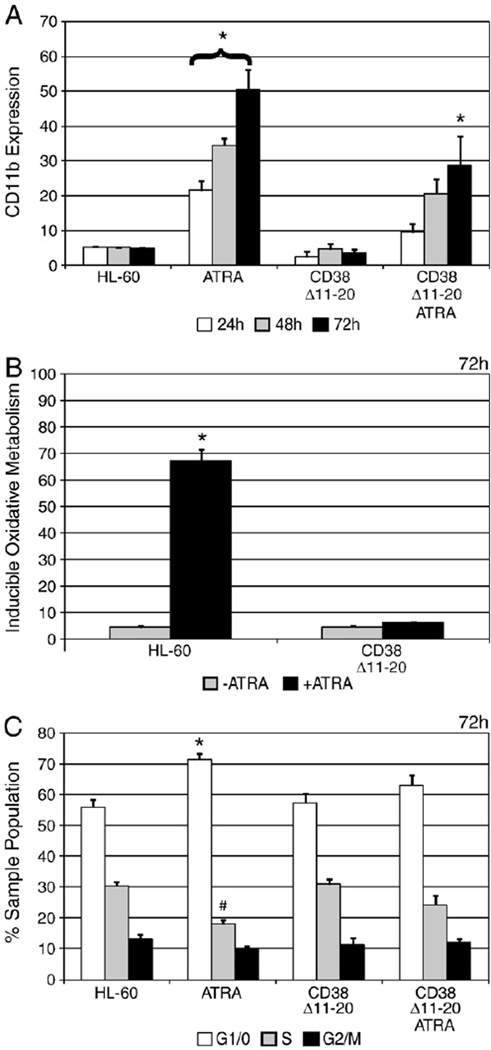
To determine the effect of the Δ11–20 deletion on ATRA induction, we conducted flow cytometric analyses of HL-60 cells and CD38 Δ11–20 transfectants to assess their ability to undergo differentiation. Compared to ATRA-treated HL-60 cells, treated CD38Δ11–20 transfectants showed significantly less CD11b expression at 24 and 48 h, and decreased expression at 72 h (Fig. 4A). This indicated that expression of the Δ11–20 deletion decreased ATRA-induced expression of CD11b.
Fig. 4.
CD38 Δ11–20 expression interrupts differentiation markers. A: Membrane CD11b expression was measured by immunostaining and flow cytometry at indicated time points. (*p = < 0.05 significantly higher than untreated HL-60 cells at the same time point.) B: After 72 h, inducible oxidative metabolism was measured by flow cytometry using the DCF assay. (*p = < 0.05 significantly higher than untreated HL-60 cells.) C: Cells were cultured for 72 h with or without ATRA and cell cycle phase distribution was determined by nuclear propidium iodide staining. (*p = < 0.05 G1/0 phase was significantly enriched compared to untreated HL-60 cells; #p = < 0.05 S phase significantly lower than untreated HL-60 cells.)
Next we analyzed inducible oxidative metabolism after ATRA treatment. For HL-60 cells, the percentage capable of producing reactive oxygen species (ROS) increased to approximately 70% at 72 h compared to the untreated control (Fig. 4B). However, CD38 Δ11–20 transfectants showed a lack of oxidative metabolism capability, indicating CD38 Δ11–20 transfectants are unable to undergo the inducible respiratory burst characteristic of mature myeloid cells.
After 72 h of ATRA treatment, HL-60 cells showed significant G1/0 DNA enrichment compared to the untreated counterparts (Fig. 4C). However, after ATRA treatment the percentage of CD38 Δ11–20 cells in G1/0 was not significantly different than untreated HL-60 cells, indicative of crippled ATRA-induced G1/0 arrest. Together, these results indicate that CD38 Δ11–20 is able to inhibit terminal differentiation as evidenced by crippled G1/0 enrichment and oxidative metabolism, and decreased expression of CD11b.
CD38 Δ11–20 expression affects signaling proteins regulated by ATRA
MAPK signaling can result in cell proliferation or arrest and differentiation, depending on receptor type and signaling longevity [18,38]. During ATRA-induced HL-60 differentiation, a protracted Raf/MEK/ERK signal is required, and inhibitors of Raf and MEK block HL-60 leukemic cell maturation [19,20]. Overexpression of wild-type CD38 drives MAPK signaling on its own and results in sustained, upregulated ERK signaling without ATRA treatment [1]. This motivated interest in whether the CD38 Δ11–20 mutant is capable of driving MAPK signaling.
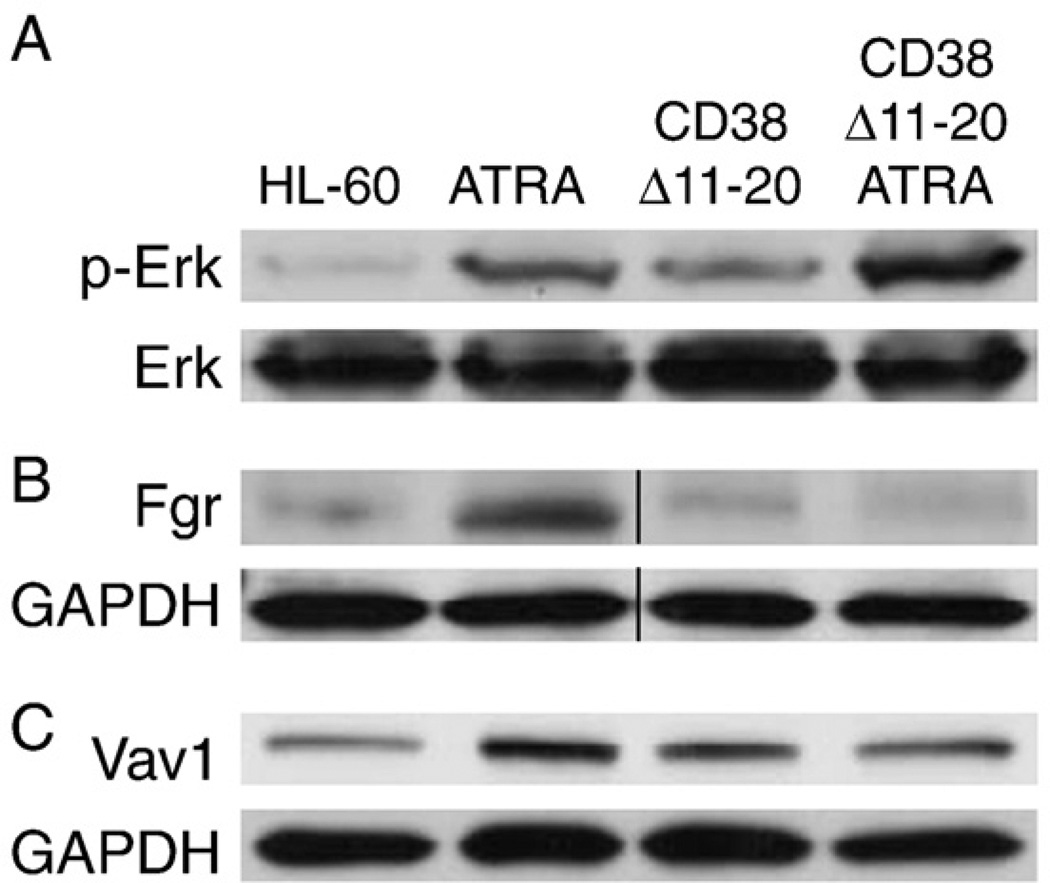
HL-60 cells and CD38 Δ11–20 transfectants were ATRA-treated or left untreated for 48 h, and phosphorylated ERK1/2 was assessed (Fig. 5A). The CD38 Δ11–20 transfectants showed enhanced ERK activation compared to HL-60 cells. ATRA increased activated ERK in both cases with CD38 Δ11–20 cells still exceeding HL-60. Hence the CD38 Δ11–20 cells were still capable of producing a persistent cellular MAPK signal, but it did not propel ATRA-induced differentiation.
Fig. 5.
CD38 Δ11–20 expression modulates some ATRA-regulated signaling molecules. A: HL-60 cells or CD38 Δ11–20 cells were treated or not with ATRA for 48 h and Western blot analysis for ERK phosphorylation was performed with total ERK used as a loading control. B: Western blot for Fgr expression after 48 h of ATRA treatment or untreated culture. GAPDH was used as a loading control. C: Western blot of Vav1 expression after 48 h of ATRA treatment or untreated culture. GAPDH was used as a loading control.
While CD38 Δ11–20 appears to be capable of generating a MAPK signal, its inhibitory effect on ATRA-induced differentiation suggests that this signaling differs from that of wild-type CD38. One such possibility is disruption of MAPK regulators such as Src family kinases and the guanine nucleotide exchange factor Vav1 [39–42]. ATRA upregulates Fgr and Vav1 [43,44]; and Vav1 interacts with CD38 through a c-Cbl containing complex [14]. This motivated interest in the effect of CD38 Δ11–20 on Fgr and Vav1.
Fgr upregulation is proposed to prevent apoptosis and promote granulocytic differentiation [43,45]. Therefore, Fgr may be important for ATRA-induced signaling mechanisms and cell survival through maturation. Treated HL-60 cells showed upregulated expression of Fgr while CD38 Δ11–20 cells did not (Fig. 5B). Premature cell death was not observed with Trypan Blue exclusion staining nor was the presence of sub-G1 populations detectable by flow cytometry (data not shown), indicating the cells were not experiencing elevated apoptosis. These results implied that the expression of CD38 Δ11–20 interfered with the expected ATRA-induced Fgr upregulation.
Vav1, a guanine nucleotide exchange factor (GEF) for the Rho family of Ras-related GTPases, becomes upregulated [44] after ATRA treatment in HL-60 cells and can be phosphorylated by Fgr [46]. After 48 h of ATRA, HL-60 cells showed a modest increase in total Vav1 expression (Fig. 5C). However, there was no change in Vav1 expression in CD38 Δ11–20 cells. These results indicated that ATRA-induced Vav1 upregulation was also defective when CD38 Δ11–20 was expressed.
Discussion
CD38 is a membrane-expressed protein that is used as a prognostic indicator in leukemia. While it is often considered a negative marker in chronic lymphocytic leukemia (CLL), the consequences of CD38 expression are somewhat enigmatic in acute myelogenous leukemia (AML). Because CD38 is associated with signaling mechanisms that may induce proliferation, differentiation, or apoptosis [22,27,47] the role of CD38 in cell survival and terminal arrest remains ambiguous. This could reflect the diversity of its functions and capabilities.
CD38 is known to have both enzymatic and receptor functions. The extracellular domain is able to catalyze the formation of cADPR and NAADP+, which are both calcium-mobilizing second messengers. CD38 also participates in a variety of signaling events by serving as a ligand-activated receptor and by forming lateral associations with other proteins at the cell membrane. Here we attempt to segregate several of these functions and determine which are important for ATRA-induced myeloid differentiation in HL-60 human leukemia cells. We find that a cytosolic deletion mutant (CD38 Δ11–20) which does not membrane express interrupted differentiation, as evidenced by a lack oxidative metabolism, insignificant growth arrest, and decreased CD11b expression. Expression of CD38 Δ11–20 also caused failure to upregulate markers including Fgr and Vav1 which are induced by ATRA and may be MAPK modulators. In contrast, crippling enzymatic activity by inhibitors had no effect on ATRA-induced myeloid differentiation. Also, cells expressing catalytically inactive CD38 showed similar enhanced differentiation as cells expressing wild-type CD38.
CD38 Δ11–20 transfectants failed to show ATRA-induced G1/0 enrichment and oxidative metabolism, suggesting that they are unable to undergo respiratory burst and growth arrest characteristic of terminal differentiation. Src family tyrosine kinases such as Fgr, which is expressed in differentiated myeloid cells after ATRA treatment, mediate respiratory burst [46,48]. Fgr failed to up-regulate in ATRA-treated CD38 Δ11–20 cells. The transfectants also showed decreased ATRA-induced expression of CD11b, an α-integrin receptor subunit. Treating HL-60 cells with molecules that upregulate CD11b increases inducible oxidative metabolism [6,49]. Membrane-localized Fgr plays a role in integrin receptor signaling [50], and integrin signaling may be important for hematopoietic myelo-monocytic differentiation [51,52]. This suggests that the compromised induced expression of both Fgr and CD11b may decrease the oxidative metabolism capability in CD38 Δ11–20 cells that is normally characteristic of differentiated myelocytes.
CD38 Δ11–20 expression also disrupted ATRA-induced expression of Vav1, which is tyrosine phosphorylated by Fgr [46]. Vav1 is a Rho/Rac guanine nucleotide exchange factor that also regulates PI3K activity during myeloid differentiation [45,53] and MAPK signaling in lymphocytes [39,40]. Overexpression of Vav1 enhances myeloid differentiation and coincides with nuclear translocation of its tyrosine-phosphorylated form and nucleoskeleton rearrangement [44,54]. ATRA-treated HL-60 cells showed an increase in Vav1 expression compared to untreated cells, while CD38 Δ11–20 transfectants did not.
The MAPK signaling cascade is also activated in HL-60 cells after ATRA treatment and is required for differentiation progression [19,55]. In CD38 Δ11–20 transfectants ERK phosphorylation was enhanced after 48 h of ATRA treatment but cell differentiation was inhibited. These results indicate that while MAPK signaling is necessary, it is not sufficient in itself for ATRA-induced differentiation. It is possible that CD38 Δ11–20 is changing the character of the MAPK cascade and affecting cellular outcomes by disrupting MAPK signaling regulators, which may include Vav1, Fgr, and other molecules. The MAPK network employs numerous adaptor, scaffolding, and inhibitory accessory proteins that regulate signaling; and direct MAPK signaling effectors such as Raf, MEK, and ERK may also serve as scaffolds, adaptors, and inhibitors themselves to finely tune the outcome of MAPK signaling (reviewed in [56]). Therefore CD38 Δ11–20, or ATRA-modulated signaling molecules that are not upregulated in CD38 Δ11–20 cells, could be aberrantly interacting with or affecting the regulation of signaling networks, such as MAPK, that are important for ATRA myeloid differentiation induction. It is also possible that CD38 Δ11–20 itself may disrupt protein–protein interactions with ATRA-induced, endogenous membrane CD38 and its downstream effectors, thus crippling wild-type signaling. Further research will be needed to elucidate how the CD38 Δ11–20 deletion mutant inhibited differentiation, but the discovery that it appears to act as a dominant negative is noteworthy. While the results in this study support the notion that CD38 plays a direct role in differentiation, we cannot rule out the possibility that the proteins affected by CD38 Δ11–20 expression, such as CD11b, Fgr, and Vav1, either act independently or as accessory signaling proteins, and their disruption interferes with differentiation.
In contrast, crippling enzymatic cADPR catalytic activity, either by treatment with a specific small molecule inhibitor or by site-directed mutagenesis, does not impair ATRA-induced differentiation progression. WT38 and CD38 E226Q transfectants that overexpress wild-type or catalytically inactive protein, respectively, show similar enhanced differentiation compared to parental HL-60 cells. Regulation of ATRA-induced differentiation by CD38 thus does not appear to depend on its ecto-enzyme activity. The results of other studies are consistent with this premise. CD38 enzymatic activity and receptor functions are uncoupled in a murine pro-B leukemic cell line. Receptor signaling cascades show dependence on downstream tyrosine kinase activity but are independent of ADP-ribosyl cyclase and NAD-glycohydrolase mechanisms [27,57].
In conclusion, these results suggest that membrane-expressed CD38 may be required for the regulation and activation of downstream signaling mechanisms induced by ATRA treatment, which a non-membrane, cytosolic deletion mutant blocks. CD38 apparently promotes ATRA-induced myeloid differentiation through its receptor but not ecto-enzymatic functions.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH) R01 GM086703 (HL), CA033505 (AY), and 1U54 CA143876 (Craighead), New York State Stem Cell Science (NYSTEM) (AY), the National Institute of Environmental Health Sciences (NIEHS) (AY), the Progetti Ricerca Interesse Nazionale (PRIN) (FM), the University of Torino (Italy) (FM), the Regione Piemonte (FM), the Italian Association for Cancer Research (AIRC, Milan) (FM), and the Fondazione Internazionale Ricerche Medicina Sperimentale (FIRMS) (FM).
Footnotes
The authors report no competing financial conflicts of interest.
REFERENCES
- 1.Lamkin TJ, Chin V, Varvayanis S, Smith JL, Sramkoski RM, Jacobberger JW, Yen A. Retinoic acid-induced CD38 expression in HL-60 myeloblastic leukemia cells regulates cell differentiation or viability depending on expression levels. J. Cell. Biochem. 2006;97:1328–1338. doi: 10.1002/jcb.20745. [DOI] [PubMed] [Google Scholar]
- 2.Munshi CB, Graeff R, Lee HC. Evidence for a causal role of CD38 expression in granulocytic differentiation of human HL-60 cells. J. Biol. Chem. 2002;277:49453–49458. doi: 10.1074/jbc.M209313200. [DOI] [PubMed] [Google Scholar]
- 3.Lee HC. A unified mechanism of enzymatic synthesis of two calcium messengers: cyclic ADP-ribose and NAADP. Biol. Chem. 1999;380:785–793. doi: 10.1515/BC.1999.098. [DOI] [PubMed] [Google Scholar]
- 4.Yalcintepe L, Albeniz I, Adin-Cinar S, Tiryaki D, Bermek E, Graeff RM, Lee HC. Nuclear CD38 in retinoic acid-induced HL-60 cells. Exp. Cell Res. 2005;303:14–21. doi: 10.1016/j.yexcr.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Yen A, Freeman L, Powers V, Van Sant R, Fishbaugh J. Cell cycle dependence of calmodulin levels during HL-60 proliferation and myeloid differentiation. No changes during pre-commitment. Exp. Cell Res. 1986;165:139–151. doi: 10.1016/0014-4827(86)90539-2. [DOI] [PubMed] [Google Scholar]
- 6.Reiterer G, Yen A. Platelet-derived growth factor receptor regulates myeloid and monocytic differentiation of HL-60 cells. Cancer Res. 2007;67:7765–7772. doi: 10.1158/0008-5472.CAN-07-0014. [DOI] [PubMed] [Google Scholar]
- 7.Funaro A, Spagnoli GC, Ausiello CM, Alessio M, Roggero S, Delia D, Zaccolo M, Malavasi F. Involvement of the multilineage CD38 molecule in a unique pathway of cell activation and proliferation. J. Immunol. 1990;145:2390–2396. [PubMed] [Google Scholar]
- 8.Mallone R, Funaro A, Zubiaur M, Baj G, Ausiello CM, Tacchetti C, Sancho J, Grossi C, Malavasi F. Signaling through CD38 induces NK cell activation. Int. Immunol. 2001;13:397–409. doi: 10.1093/intimm/13.4.397. [DOI] [PubMed] [Google Scholar]
- 9.Munoz P, Navarro MD, Pavon EJ, Salmeron J, Malavasi F, Sancho J, Zubiaur M. CD38 signaling in T cells is initiated within a subset of membrane rafts containing Lck and the CD3-zeta subunit of the T cell antigen receptor. J. Biol. Chem. 2003;278:50791–50802. doi: 10.1074/jbc.M308034200. [DOI] [PubMed] [Google Scholar]
- 10.Funaro A, De Monte LB, Dianzani U, Forni M, Malavasi F. Human CD38 is associated to distinct molecules which mediate transmembrane signaling in different lineages. Eur. J. Immunol. 1993;23:2407–2411. doi: 10.1002/eji.1830231005. [DOI] [PubMed] [Google Scholar]
- 11.Silvennoinen O, Nishigaki H, Kitanaka A, Kumagai M, Ito C, Malavasi F, Lin Q, Conley ME, Campana D. CD38 signal transduction in human B cell precursors. Rapid induction of tyrosine phosphorylation, activation of syk tyrosine kinase, and phosphorylation of phospholipase C-gamma and phosphatidylinositol 3-kinase. J. Immunol. 1996;156:100–107. [PubMed] [Google Scholar]
- 12.Inoue S, Kontani K, Tsujimoto N, Kanda Y, Hosoda N, Hoshino S, Hazeki O, Katada T. Protein-tyrosine phosphorylation by IgG1 subclass CD38 monoclonal antibodies is mediated through stimulation of the FcgammaII receptors in human myeloid cell lines. J. Immunol. 1997;159:5226–5232. [PubMed] [Google Scholar]
- 13.Kontani K, Kukimoto I, Nishina H, Hoshino S, Hazeki O, Kanaho Y, Katada T. Tyrosine phosphorylation of the c-cbl proto-oncogene product mediated by cell surface antigen CD38 in HL-60 cells. J. Biol. Chem. 1996;271:1534–1537. doi: 10.1074/jbc.271.3.1534. [DOI] [PubMed] [Google Scholar]
- 14.Shen M, Yen A. c-Cbl tyrosine kinase-binding domain mutant G306E abolishes the interaction of c-Cbl with CD38 and fails to promote retinoic acid-induced cell differentiation and G0 arrest. J. Biol. Chem. 2009;284:25664–25677. doi: 10.1074/jbc.M109.014241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen M, Yen A. c-Cbl interacts with CD38 and promotes retinoic acid-induced differentiation and G0 arrest of human myeloblastic leukemia cells. Cancer Res. 2008;68:8761. doi: 10.1158/0008-5472.CAN-08-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zubiaur M, Izquierdo M, Terhorst C, Malavasi F, Sancho J. CD38 ligation results in activation of the Raf-1/mitogen-activated protein kinase and the CD3-zeta/zeta-associated protein-70 signaling pathways in Jurkat T lymphocytes. J. Immunol. 1997;159:193–205. [PubMed] [Google Scholar]
- 17.Zubiaur M, Guirado M, Terhorst C, Malavasi F, Sancho J. The CD3-gamma delta epsilon transducing module mediates CD38-induced protein-tyrosine kinase and mitogen-activated protein kinase activation in Jurkat T cells. J. Biol. Chem. 1999;274:20633–20642. doi: 10.1074/jbc.274.29.20633. [DOI] [PubMed] [Google Scholar]
- 18.Traverse S, Gomez N, Paterson H, Marshall C, Cohen P. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem. J. 1992;288(Pt 2):351–355. doi: 10.1042/bj2880351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yen A, Roberson MS, Varvayanis S, Lee AT. Retinoic acid induced mitogen-activated protein (MAP)/extracellular signal-regulated kinase (ERK) kinase-dependent MAP kinase activation needed to elicit HL-60 cell differentiation and growth arrest. Cancer Res. 1998;58:3163–3172. [PubMed] [Google Scholar]
- 20.Wang J, Yen A. A MAPK-positive feedback mechanism for BLR1 signaling propels retinoic acid-triggered differentiation and cell cycle arrest. J. Biol. Chem. 2008;283:4375–4386. doi: 10.1074/jbc.M708471200. [DOI] [PubMed] [Google Scholar]
- 21.Todisco E, Suzuki T, Srivannaboon K, Coustan-Smith E, Raimondi SC, Behm FG, Kitanaka A, Campana D. CD38 ligation inhibits normal and leukemic myelopoiesis. Blood. 2000;95:535–542. [PubMed] [Google Scholar]
- 22.Konopleva M, Estrov Z, Zhao S, Andreeff M, Mehta K. Ligation of cell surface CD38 protein with agonistic monoclonal antibody induces a cell growth signal in myeloid leukemia cells. J. Immunol. 1998;161:4702–4708. [PubMed] [Google Scholar]
- 23.Jiang H, Congleton J, Liu Q, Merchant P, Malavasi F, Lee HC, Hao Q, Yen A, Lin H. Mechanism-based small molecule probes for labeling CD38 on live cells. J. Am. Chem. Soc. 2009;131:1658–1659. doi: 10.1021/ja808387g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graeff RM, Mehta K, Lee HC. GDP-ribosyl cyclase activity as a measure of CD38 induction by retinoic acid in HL-60 cells. Biochem. Biophys. Res. Commun. 1994;205:722–727. doi: 10.1006/bbrc.1994.2725. [DOI] [PubMed] [Google Scholar]
- 25.Graeff RM, Walseth TF, Fryxell K, Branton WD, Lee HC. Enzymatic synthesis and characterizations of cyclic GDP-ribose. A procedure for distinguishing enzymes with ADP-ribosyl cyclase activity. J. Biol. Chem. 1994;269:30260–30267. [PubMed] [Google Scholar]
- 26.Lund FE, Muller-Steffner HM, Yu N, Stout CD, Schuber F, Howard MC. CD38 signaling in B lymphocytes is controlled by its ectodomain but occurs independently of enzymatically generated ADP-ribose or cyclic ADP-ribose. J. Immunol. 1999;162:2693–2702. [PubMed] [Google Scholar]
- 27.Lund FE, Muller-Steffner H, Romero-Ramirez H, Moreno-Garcia ME, Partida-Sanchez S, Makris M, Oppenheimer NJ, Santos-Argumedo L, Schuber F. CD38 induces apoptosis of a murine pro-B leukemic cell line by a tyrosine kinase-dependent but ADP-ribosyl cyclase- and NAD glycohydrolase-independent mechanism. Int. Immunol. 2006;18:1029–1042. doi: 10.1093/intimm/dxl037. [DOI] [PubMed] [Google Scholar]
- 28.Sauve AA, Deng H, Angeletti RH, Schramm VL. A covalent intermediate in CD38 is responsible for ADP-ribosylation and cyclization reactions. J. Am. Chem. Soc. 2000;122:7856–7859. [Google Scholar]
- 29.Munoz P, Mittelbrunn M, de la Fuente H, Perez-Martinez M, Garcia-Perez A, Ariza-Veguillas A, Malavasi F, Zubiaur M, Sanchez-Madrid F, Sancho J. Antigen-induced clustering of surface CD38 and recruitment of intracellular CD38 to the immunologic synapse. Blood. 2008;111:3653–3664. doi: 10.1182/blood-2007-07-101600. [DOI] [PubMed] [Google Scholar]
- 30.Trubiani O, Guarnieri S, Orciani M, Salvolini E, Di Primio R. Sphingolipid microdomains mediate CD38 internalization: topography of the endocytosis. Int. J. Immunopathol. Pharmacol. 2004;17:293–300. doi: 10.1177/039463200401700309. [DOI] [PubMed] [Google Scholar]
- 31.Funaro A, Reinis M, Trubiani O, Santi S, Di Primio R, Malavasi F. CD38 functions are regulated through an internalization step. J. Immunol. 1998;160:2238–2247. [PubMed] [Google Scholar]
- 32.Zocchi E, Franco L, Guida L, Piccini D, Tacchetti C, De Flora A. NAD+-dependent internalization of the transmembrane glycoprotein CD38 in human Namalwa B cells. FEBS Lett. 1996;396:327–332. doi: 10.1016/0014-5793(96)01125-8. [DOI] [PubMed] [Google Scholar]
- 33.Munshi C, Aarhus R, Graeff R, Walseth TF, Levitt D, Lee HC. Identification of the enzymatic active site of CD38 by site-directed mutagenesis. J. Biol. Chem. 2000;275:21566–21571. doi: 10.1074/jbc.M909365199. [DOI] [PubMed] [Google Scholar]
- 34.Kitanaka A, Suzuki T, Ito C, Nishigaki H, Coustan-Smith E, Tanaka T, Kubota Y, Campana D. CD38-mediated signaling events in murine pro-B cells expressing human CD38 with or without its cytoplasmic domain. J. Immunol. 1999;162:1952–1958. [PubMed] [Google Scholar]
- 35.Lund FE. Signaling properties of CD38 in the mouse immune system: enzyme-dependent and -independent roles in immunity. Mol. Med. 2006;12:328–333. doi: 10.2119/2006-00099.Lund. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moreno-Garcia ME, Partida-Sanchez S, Primack J, Sumoza-Toledo A, Muller-Steffner H, Schuber F, Oppenheimer N, Lund FE, Santos-Argumedo L. CD38 is expressed as noncovalently associated homodimers on the surface of murine B lymphocytes. Eur. J. Biochem. 2004;271:1025–1034. doi: 10.1111/j.1432-1033.2004.04006.x. [DOI] [PubMed] [Google Scholar]
- 37.Cho YS, Han MK, Choi YB, Yun Y, Shin J, Kim UH. Direct interaction of the CD38 cytoplasmic tail and the Lck SH2 domain. Cd38 transduces T cell activation signals through associated Lck. J. Biol. Chem. 2000;275:1685–1690. doi: 10.1074/jbc.275.3.1685. [DOI] [PubMed] [Google Scholar]
- 38.Santos SD, Verveer PJ, Bastiaens PI. Growth factor-induced MAPK network topology shapes Erk response determining PC-12 cell fate. Nat. Cell Biol. 2007;9:324–330. doi: 10.1038/ncb1543. [DOI] [PubMed] [Google Scholar]
- 39.Reynolds LF, de Bettignies C, Norton T, Beeser A, Chernoff J, Tybulewicz VL. Vav1 transduces T cell receptor signals to the activation of the Ras/ERK pathway via LAT, Sos, and RasGRP1. J. Biol. Chem. 2004;279:18239–18246. doi: 10.1074/jbc.M400257200. [DOI] [PubMed] [Google Scholar]
- 40.Fujikawa K, Miletic AV, Alt FW, Faccio R, Brown T, Hoog J, Fredericks J, Nishi S, Mildiner S, Moores SL, Brugge J, Rosen FS, Swat W. Vav1/2/3-null mice define an essential role for Vav family proteins in lymphocyte development and activation but a differential requirement in MAPK signaling in T and B cells. J. Exp. Med. 2003;198:1595–1608. doi: 10.1084/jem.20030874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan GR, Xiao CL, He GW, Yin XF, Chen NP, Cao Y, He QY. Global phosphoproteomic effects of natural tyrosine kinase inhibitor, genistein, on signaling pathways. Proteomics. 2010;10:976–986. doi: 10.1002/pmic.200900662. [DOI] [PubMed] [Google Scholar]
- 42.Kim LC, Song L, Haura EB. Src kinases as therapeutic targets for cancer. Nat. Rev. Clin. Oncol. 2009;6:587–595. doi: 10.1038/nrclinonc.2009.129. [DOI] [PubMed] [Google Scholar]
- 43.Katagiri K, Yokoyama KK, Yamamoto T, Omura S, Irie S, Katagiri T. Lyn and Fgr protein-tyrosine kinases prevent apoptosis during retinoic acid-induced granulocytic differentiation of HL-60 cells. J. Biol. Chem. 1996;271:11557–11562. doi: 10.1074/jbc.271.19.11557. [DOI] [PubMed] [Google Scholar]
- 44.Bertagnolo V, Brugnoli F, Mischiati C, Sereni A, Bavelloni A, Carini C, Capitani S. Vav promotes differentiation of human tumoral myeloid precursors. Exp. Cell Res. 2005;306:56–63. doi: 10.1016/j.yexcr.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 45.Notario V, Gutkind JS, Imaizumi M, Katamine S, Robbins KC. Expression of the fgr protooncogene product as a function of myelomonocytic cell maturation. J. Cell Biol. 1989;109:3129–3136. doi: 10.1083/jcb.109.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fumagalli L, Zhang H, Baruzzi A, Lowell CA, Berton G. The Src family kinases Hck and Fgr regulate neutrophil responses to N-formyl-methionyl-leucyl-phenylalanine. J. Immunol. 2007;178:3874–3885. doi: 10.4049/jimmunol.178.6.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yue J, Wei W, Lam CM, Zhao YJ, Dong M, Zhang LR, Zhang LH, Lee HC. CD38/cADPR/Ca2+ pathway promotes cell proliferation and delays nerve growth factor-induced differentiation in PC12 cells. J. Biol. Chem. 2009;284:29335–29342. doi: 10.1074/jbc.M109.049767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paul R, Obermaier B, Van Ziffle J, Angele B, Pfister HW, Lowell CA, Koedel U. Myeloid Src kinases regulate phagocytosis and oxidative burst in pneumococcal meningitis by activating NADPH oxidase. J. Leukoc. Biol. 2008;84:1141–1150. doi: 10.1189/jlb.0208118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reiterer G, Bunaciu RP, Smith JL, Yen A. Inhibiting the platelet derived growth factor receptor increases signs of retinoic acid syndrome in myeloid differentiated HL-60 cells. FEBS Lett. 2008;582:2508–2514. doi: 10.1016/j.febslet.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suen PW, Ilic D, Caveggion E, Berton G, Damsky CH, Lowell CA. Impaired integrin-mediated signal transduction, altered cytoskeletal structure and reduced motility in Hck/Fgr deficient macrophages. J. Cell Sci. 1999;112(Pt 22):4067–4078. doi: 10.1242/jcs.112.22.4067. [DOI] [PubMed] [Google Scholar]
- 51.Komano Y, Nanki T, Hayashida K, Taniguchi K, Miyasaka N. Identification of a human peripheral blood monocyte subset that differentiates into osteoclasts. Arthritis Res. Ther. 2006;8:R152. doi: 10.1186/ar2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sudhakaran PR, Radhika A, Jacob SS. Monocyte macrophage differentiation in vitro: fibronectin-dependent upregulation of certain macrophage-specific activities. Glycoconj. J. 2007;24:49–55. doi: 10.1007/s10719-006-9011-2. [DOI] [PubMed] [Google Scholar]
- 53.Bertagnolo V, Brugnoli F, Marchisio M, Celeghini C, Carini C, Capitani S. Association of PI 3-K with tyrosine phosphorylated Vav is essential for its activity in neutrophil-like maturation of myeloid cells. Cell. Signal. 2004;16:423–433. doi: 10.1016/j.cellsig.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 54.Bertagnolo V, Marchisio M, Brugnoli F, Bavelloni A, Boccafogli L, Colamussi ML, Capitani S. Requirement of tyrosine-phosphorylated Vav for morphological differentiation of all-trans-retinoic acid-treated HL-60 cells. Cell Growth Differ. 2001;12:193–200. [PubMed] [Google Scholar]
- 55.Wang J, Yen A. A MAPK-positive feedback mechanism for BLR1 signaling propels retinoic acid-triggered differentiation and cell cycle arrest. J. Biol. Chem. 2008;283:4375. doi: 10.1074/jbc.M708471200. [DOI] [PubMed] [Google Scholar]
- 56.Kolch W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat. Rev. Mol. Cell Biol. 2005;6:827–837. doi: 10.1038/nrm1743. [DOI] [PubMed] [Google Scholar]
- 57.Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, Vaisitti T, Aydin S. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol. Rev. 2008;88:841–886. doi: 10.1152/physrev.00035.2007. [DOI] [PubMed] [Google Scholar]