Abstract
Anthracycline-based chemotherapy (ABC) is the most effective therapy for diffuse large B-cell lymphoma (DLBCL). The addition of rituximab to ABC in controlled trials has demonstrated superior survival, yet ABC is inconsistently utilized in elderly patients, and little is known about the penetrance or impact of rituximab with other treatments. We analyzed the treatment and survival patterns of 7559 patients with DLBCL over age 66 diagnosed from 1992 to 2002 using a linked Surveillance, Epidemiology and End Results (SEER)–Medicare database. Rituximab use was first detected in 1999 and by 2002 was incorporated in 79% of ABC-treated patients and 71% of patients treated with non-anthracycline chemotherapy, but only 12% of patients not receiving cytotoxic chemotherapy. ABC rates remained constant across time as did rates of no therapy, which were highest among the very old. Rituximab-associated survival improvements were seen among elderly treated with or without anthracyclines. Patients treated with rituximab but not anthracyclines had comparable survival to those treated with anthracycline but not rituximab.
Keywords: Non-Hodgkin lymphoma, immunotherapy, survival, elderly
Introduction
Diffuse large B-cell lymphoma (DLBCL) represents the most frequent histologic subtype of lymphoma. With a median age at presentation of 64 years, a substantial portion of newly diagnosed DLBCL occurs in elderly patients. Anthracycline-based chemotherapy (ABC) combinations offer the best opportunity for long-term disease-free survival in patients with DLBCL, and phase III studies demonstrate that the addition of rituximab improves survival of elderly patients, when added to ABC [1,2]. Nonetheless, results from phase III studies do not always translate into use or corresponding outcomes in the general population or elderly subgroups. As an example, an analysis of the Surveillance, Epidemiology and End Results (SEER)–Medicare linked database demonstrated that just over half of elderly patients with DLBCL throughout the 1990s received ABC, and over one-third received no chemotherapy at all within 6 months of diagnosis [3].
Rituximab (R) is a monoclonal antibody with broad anti-lymphoma activity across the spectrum of CD20+ B-cell lymphomas, and received Food and Drug Administration (FDA) approval in late 1997 as monotherapy following the demonstration of efficacy for palliative management of relapsed low-grade and follicular lymphomas [4]. Early studies demonstrated a response rate of 37% with rituximab as monotherapy in patients with DLBCL in 1998 [5]. The first phase II study combining rituximab with ABC (cyclophosphamide, doxorubicin, vincristine, and prednisone [CHOP]) in patients with DLBCL described a higher than anticipated complete response rate of 61%, at an international meeting in May 1998, followed by a published manuscript with 2-year follow-up in January 2001 [6,7]. The first phase III published report demonstrating a 13% improvement in overall survival (OS) in elderly (>60 years) patients with DLBCL treated with R-CHOP compared to CHOP was presented at an international meeting in December 2000 followed by a published report in January 2002 [8,9]. In March 2001, the British Columbia (BC) Cancer Agency recommended R-CHOP as preferred therapy for all newly diagnosed patients with advanced-stage DLBCL in the province of BC. A population-based retrospective analysis of this new treatment strategy demonstrated that among all adult patients with DLBCL who received ABC, the 2-year OS estimate increased from 52% to 78% in the post-rituximab era [10]. On 10 February 2006, the US FDA granted approval for rituximab use in the first-line treatment of patients with diffuse large B-cell, CD20-positive, non-Hodgkin lymphoma in combination with CHOP or other ABC regimens.
Little is known about patterns of rituximab use in elderly patients with DLBCL. We sought to understand whether rituximab use in these patients is influenced by specific clinical features, whether use is limited to concurrent ABC, and whether published results of the R-ABC combination resulted in increased utilization of ABC compared to the 1990s. Population registries are required to observe the treatment patterns for the oldest old, who are seldom included in randomized controlled trials. The linkage of the National Cancer Institute’s SEER cancer registry database to the Medicare database allows for the analysis of cancer treatment in relation to both demographic and clinical factors and cancer outcomes [11]. The objective of this study was to utilize a linkage of the SEER and Medicare databases to describe chemotherapy and rituximab practice patterns in a population-based study of elderly patients with DLBCL and assess whether survival changes correlate with these patterns.
Methods
Subject selection
We selected subjects from the SEER–Medicare linked database. The SEER registry data and Medicare claims have been linked for approximately 93% of persons with cancer aged 65 and older at the time of cancer diagnosis [11]. Participating registries collect data for all cancer patients diagnosed within their defined geographic area. Registry data include month and year of diagnosis, age at diagnosis, race, tumor stage, and histology. Medicare files from the Centers for Medicare and Medicaid Services (CMS) include demographic and enrollment information, date of death, and all bills submitted for inpatient hospital care, outpatient hospital care, and physician services.
The study population includes 34 963 cases of first primary non-Hodgkin lymphoma (NHL) diagnosed between 1992 and 2002 with a known month of diagnosis, aged 66 and over at the time of diagnosis. Cases were excluded if: (1) death date was recorded only in SEER and not Medicare or death date was before diagnosis date (n =71); (2) enrollment in parts A or B Medicare was not continuous for 12 months before or the earlier of 5 months after diagnosis or death, or there was health maintenance organization (HMO) enrollment any time during the 12 months before and 5 months after diagnosis (n =10 540); (3) a histology other than diffuse large cell or diffuse large B-cell lymphoma (ICD-O-3 [International Classification of Diseases for Oncology, 3rd edition] codes for DLBCL: 9679, 9680, 9684) (n =15 452); (4) the cancer site code was any part of the central nervous system (n =300); (5) the case was not microscopically confirmed (n =408); (6) chemotherapy type was unknown for all chemotherapy claims(n =497); or (7) census tract data were missing (n =136). After application of these criteria, a total of 7559 cases of DLBCL were available for study. Date of death information was complete through 2005 and 3-year survival follow-up was available for all patients.
Measures
First-course therapy was designated using the observed combinations of chemotherapy and rituximab received within 5 months of diagnosis. A therapy-related claim was any claim with one of the following codes [12] attached. The ICD-9 (International Classification of Diseases, 9th revision) diagnosis codes V58.1, V66.2, and V67.2, the ICD-9 procedure code 99.25, and the diagnosis-related group (DRG) code 410 were used to identify inpatient therapy administration. For outpatient and physician billing claims, Healthcare Common Procedures Classification System (HCPCS) codes for chemotherapy administration were used (Q0083, Q0084, Q0085, J9000–J9999, 964.xx, 965.xx) as well as relevant revenue center codes (0331, 0332, and 0335). Based on the HCPCS codes associated with treatment, indicator variables were used to classify patients by the treatments they received during their first course of treatment: ABC-only, ABC plus rituximab, non-ABC chemotherapy, non-ABC chemotherapy plus rituximab, rituximab only, and no therapy. Chemotherapy was categorized as ‘ABC’ if claims were found during this period for either doxorubicin or mitoxantrone. All other patients with chemotherapy claims were categorized as ‘non-ABC.’ Patients without chemotherapy or chemotherapy administration claim were categorized as ‘no therapy.’ Indicator variables for year of diagnosis were included to capture survival trends over the period. Other variables included age at diagnosis, race, gender, tumor stage, census tract, and socioeconomic variables from the Patient Entitlement and Diagnosis Summary File (PEDSF).
Statistical analysis
Using the two-sample median test and χ2 tests, patient survival was compared in the pre-rituximab versus rituximab periods for patients receiving ABC, non-ABC chemotherapy, and no chemotherapy. The pre-rituximab and rituximab eras were defined by the availability of rituximab (rituximab not available: January 1992–December 1998; rituximab available January 1999–December 2002). We then estimated two multivariate logistic models with 3-year survival after diagnosis as the dependent variable. The first model specified diagnosis year indicator variables (1 if patient was diagnosed in a given year, 0 otherwise) to capture survival trends along with patient baseline characteristics including age at diagnosis, race, gender, tumor stage, systemic symptoms, SEER registry, census tract variables, and comorbidities. Comorbidities were represented by two separate indicator variables measuring conditions in the year prior to NHL diagnosis. The indicators were based on the presence of at least one non-cancer Charlson condition [13] identified on inpatient claims and physician or outpatient claims, respectively, using the methodology developed by Klabunde et al. [14].
The second model included all of the variables specified in the first model and added five treatment indicator variables (ABC without rituximab was the reference group). We then compared the significance of the estimates for the diagnosis year indicator variables across model specifications to assess whether changes in treatment patterns were the source of survival trends.
Univariate and multivariate logistic regression models were run to assess factors related to rituximab use among those patients with known chemotherapy drugs who were diagnosed after rituximab approval (diagnosed after December 1998). In addition, we tested survival curve equality among treatment combinations (ABC only, R-ABC, R-other, other, R-only, and no treatment) using Wilcoxon and log-rank statistics.
Results
We identified 7559 patients aged 66 or over, diagnosed with diffuse large or diffuse large B-cell lymphoma (non-central nervous system primary) from 1992 to 2002 with continuous Medicare Part B coverage and no HMO coverage from 12 months prior to diagnosis and at least 5 months past diagnosis (or through death). Median age was 77 years with 30% over age 80. Nearly half had stage III/IV disease. Patients in the pre-rituximab and rituximab eras were well balanced with respect to baseline variables (Table I).
Table I.
Subject characteristics (n =7559).
| Characteristic | Therapy type and era
|
Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-rituximab
|
Post rituximab
|
|||||||||||
| ABC, n (%) | Non-ABC, n (%) | No therapy, n (%) | Era total, n (%) | ABC w/R, n (%) | ABC w/o R, n (%) | Non-ABC w/R, n (%) | Non-ABC w/o R, n (%) | No therapy, n (%) | R only, n (%) | Era total, n (%) | ||
| Total | 2193 | 307 | 1284 | 3784 | 968 | 1216 | 96 | 153 | 1228 | 114 | 3775 | 7559 |
| Age 66–70 | 577 (26) | 52 (17) | 169 (13) | 798 (21) | 244 (25) | 284 (23) | 14 (15) | 17 (11) | 128 (10) | 12 (11) | 699 (19) | 1497 (20) |
| Age 71–75 | 658 (30) | 78 (25) | 229 (18) | 965 (26) | 303 (31) | 334 (27) | 21 (22) | 35 (23) | 213 (17) | 17 (15) | 923 (24) | 1888 (25) |
| Age 76–80 | 568 (26) | 78 (25) | 288 (22) | 934 (25) | 239 (25) | 345 (28) | 22 (23) | 39 (25) | 282 (23) | 19 (17) | 946 (25) | 1880 (25) |
| Age 81–84 | 248 (11 | 59 (19) | 220 (17) | 527 (14) | 119 (12) | 150 (12) | 18 (19) | 30 (20) | 229 (19) | 24 (21) | 570 (15) | 1097 (15) |
| Age 85+ | 142 (6) | 40 (13) | 378 (29) | 560 (15) | 63 (7) | 103 (8) | 21 (22) | 32 (21) | 376 (31) | 42 (37) | 637 (17) | 1197 (16) |
| White | 2051 (94) | 279 (91) | 1159 (90) | 3489 (92) | 899 (93) | 1130 (93) | ★ | 136 (89) | 1106 (90) | ★ | ★ | 6961 (92) |
| Black | 55 (3) | ★ | 51 (4) | ★ | 24 (2) | 36 (3) | ★ | ★ | 49 (4) | ★ | ★ | 231 (3) |
| Asian | 63 (3) | 14 (5) | 62 (5) | 139 (4) | 28 (3) | 39 (3) | ★ | ★ | 43 (4) | ★ | 120 (3) | 259 (3) |
| Other | 24 (1) | ★ | 12 (1) | ★ | 17 (2) | 11 (1) | ★ | ★ | 30 (2) | ★ | ★ | 108 (1) |
| Stage I | 700 (32) | 89 (29) | 442 (34 | 1231 (33) | 252 (26) | 395 (32) | 21 (22) | 43 (28) | 386 (31 | 36 (32) | 1133 (30) | 2364 (31) |
| Stage II | 438 (20) | 63 (21) | 188 (15) | 689 (18) | 196 (20) | 252 (21) | 19 (20) | 36 (24) | 191 (16) | 21 (18) | 715 (19) | 1404 (19) |
| Stage III | 310 (14) | 36 (12) | 126 (10) | 472 (12) | 158 (16) | 185 (15) | ★ | ★ | 141 (11) | ★ | 532 (14) | 1004 (13) |
| Stage IV | 629 (29) | 102 (33) | 441 (34 | 1172 (31) | 314 (32) | 322 (26) | 25 (26) | 46 (30) | 403 (33 | 39 (34) | 1149 (30) | 2321 (31) |
| Unstaged | 116 (5) | 17 (6) | 87 (7) | 220 (6) | 48 (5) | 62 (5) | ★ | ★ | 107 (9) | ★ | 246 (7) | 466 (6) |
| Male | 998 (46) | 144 (47) | 543 (42 | 1685 (45) | 460 (48) | 541 (44) | 53 (55) | 79 (52) | 562 (46 | 47 (41) | 1742 (46) | 3427 (45) |
| Female | 1195 (54) | 163 (53) | 741 (58) | 2099 (55) | 508 (52) | 675 (56) | 43 (45) | 74 (48) | 666 (54) | 67 (59) | 2033 (54) | 4132 (55) |
| Inpatient claims comorbidity 0 | 1978 (90) | 266 (87) | 1013 (79) | 3257 (86) | 868 (90) | 1088 (89) | 71 (74) | 126 (82) | 938 (76) | 93 (82) | 3184 (84) | 6441 (85) |
| Inpatient claims comorbidity 1+ | 215 (10) | 41 (13) | 271 (21) | 527 (14) | 100 (10) | 128 (11) | 25 (26) | 27 (18) | 290 (24) | 21 (18) | 591 (16) | 1118 (15) |
| Physician claims comorbidity 0 | 1802 (82) | 241 (79) | 981 (76) | 3024 (80) | 732 (76) | 949 (78) | 71 (74) | 101 (66) | 918 (75) | 80 (70) | 2851 (76) | 5875 (78) |
| Physician claims comorbidity 1+ | 391 (18) | 66 (22) | 303 (24) | 760 (20) | 236 (24) | 267 (22) | 25 (26) | 52 (34) | 310 (25) | 34 (30) | 924 (24) | 1684 (22) |
Cell size suppressed to protect confidentiality.
ABC, anthracycline-based chemotherapy; R, rituximab; w/, with; w/o, without.
Patterns of therapy
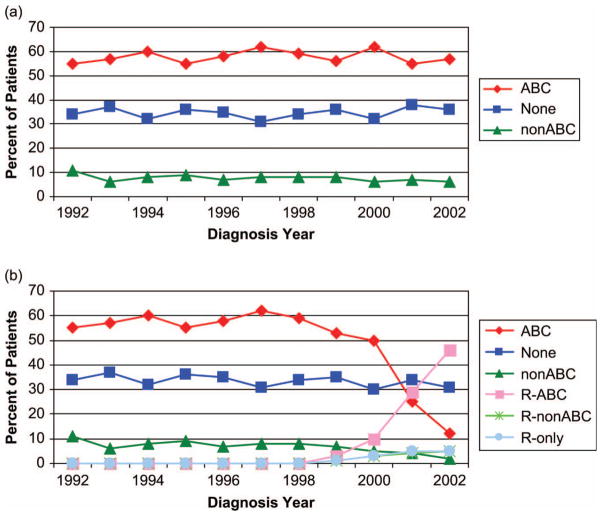
Sixty-seven percent of patients in the entire cohort received systemic therapy within 5 months of diagnosis and 58% received ABC chemotherapy. Among 1197 patients over age 85, 754 (63%) of these oldest patients received no therapy (Table I). Importantly, the rates of any chemotherapy use and of ABC chemotherapy use were stable over the observed time period, and did not increase in the rituximab era [Figure 1(A)]. Rituximab utilization was first detected in patients diagnosed in 1999, and by 2000 12% of patients were treated with ABC plus rituximab (R-ABC), representing 19% of patients receiving ABC [Figure 1(B)]. In 2000, rituximab was added among 10% of patients treated with non-ABC chemotherapy and among 5% of patients receiving no chemotherapy. By 2002, 45% of patients were treated with R-ABC, representing 79% of ABC-treated patients. Similarly, rituximab penetrance had increased to 71% of patients treated with non-ABC chemotherapy but only 12% of patients receiving no chemotherapy. Table II describes the factors related to rituximab use for the 2433 patients who received chemotherapy once rituximab was available (after December 1998). Multivariate analysis demonstrated that rituximab use correlated with advancing stage, inversely with increasing age, and marginally with anthracycline use, but not with comorbidity indices, reported B-symptoms, or gender.
Figure 1.
Distribution of initial management choices among elderly patients with diffuse large or diffuse large B-cell lymphoma over time from 1992 to 2002: (A) categorized by initial cytotoxic chemotherapy regimen (ABC, anthracycline-based combination); (B) categorized by initial (immuno)chemotherapy regimen (R, rituximab).
Table II.
Univariate and multivariate logistic regression analyses of rituximab use among those treated with known chemotherapy drugs who were diagnosed after rituximab became available (n =2433).
| Univariate odds ratio (95% CI) | p-Value | Adjusted odds ratio (95% CI) | p-Value | |
|---|---|---|---|---|
| Age at diagnosis (reference =66–70) | 0.0543 | 0.0163 | ||
| 71–75 | 1.024 (0.819–1.281) | 0.8326 | 1.055 (0.796–1.397) | 0.7094 |
| 76–80 | 0.793 (0.631–0.997) | 0.0470 | 0.764 (0.574–1.016) | 0.0637 |
| 81–84 | 0.888 (0.673–1.172) | 0.4014 | 0.698 (0.493–0.987) | 0.0422 |
| 85+ | 0.726 (0.528–0.999) | 0.0492 | 0.664 (0.444–0.992) | 0.0458 |
| Year of diagnosis (reference =2002) | <0.0001 | <0.0001 | ||
| 1999 | 0.014 (0.008–0.024) | <0.0001 | 0.014 (0.008–0.024) | <0.0001 |
| 2000 | 0.061 (0.047–0.079) | <0.0001 | 0.054 (0.041–0.072) | <0.0001 |
| 2001 | 0.327 (0.259–0.413) | <0.0001 | 0.307 (0.241–0.391) | <0.0001 |
| Stage (reference =stage 1) | 0.0046 | 0.0139 | ||
| Stage 2 | 1.198 (0.949–1.511) | 0.1282 | 1.220 (0.914–1.628) | 0.1766 |
| Stage 3 | 1.415 (1.100–1.819) | 0.0068 | 1.504 (1.099–2.060) | 0.0109 |
| Stage 4 | 1.478 (1.197–1.826) | 0.0003 | 1.559 (1.192–2.038) | 0.0012 |
| ABC use (reference =no) | 0.0827 | 0.0523 | ||
| Yes | 1.269 (0.970–1.660) | 0.0827 | 1.392 (0.997–1.994) | 0.0523 |
| Gender (reference = female) | 0.1513 | 0.1360 | ||
| Male | 1.125 (0.958–1.321) | 0.1513 | 1.165 (0.953–1.423) | 0.1360 |
| Physician claims comorbidity (reference =0) | 0.4797 | 0.7925 | ||
| ≥1 | 1.070 (0.887–1.291) | 0.4797 | 1.032 (0.817–1.303) | 0.7925 |
| Inpatient claims comorbidity (reference =0) | 0.7435 | 0.6009 | ||
| ≥1 | 1.043 (0.812–1.340) | 0.7435 | 0.920 (0.673–1.258) | 0.6009 |
| B-symptoms (reference =no) | 0.3936 | 0.8832 | ||
| Yes | 0.943 (0.751–1.185) | 0.6137 | 1.065 (0.800–1.419) | 0.6657 |
CI, confidence interval; ABC, anthracycline-based chemotherapy.
Survival patterns for entire cohort
Survival of patients with DLBCL aged 66 and older in our cohort improved over time. For survival analyses, the cohort was divided into two eras: era 1 =January 1992–December 1998; era 2 =January 1999–December 2002. Median survival for the two eras was 15 and 21 months, respectively (p <0.0001). Three-year OS for the two eras was 37% and 43%, respectively (p <0.0001). Among patients treated with ABC, the 3-year OS improved from 48% to 58% (p <0.0001).
Multivariate logistic regression analyses were performed to find plausible explanations for the observed improvements in survival. In the first model (Table III, columns 2–3), diagnosis year, specified using a series of indicator variables (reference = 1992–94), was highly associated with 3-year survival (p <0.0001), with survival increasing over time. In the second model (columns 4–5), with inclusion of the treatment category variables, the diagnosis year variables were less strongly associated, suggesting that the treatment pattern change over time was a dominant source of the positive survival trend. Adding rituximab to ABC was associated with increased 3-year survival relative to treatment with ABC-only (p <0.0001). Interestingly, there was no significant difference in 3-year survival between ABC-only patients (the reference category) and patients with other (non-ABC) chemotherapy plus rituximab. Patients with no chemotherapy or non-ABC chemotherapy without rituximab had lower 3-year survival probabilities (p <0.0001) relative to ABC-only. Patients with rituximab-only also had lower 3-year survival probability relative to ABC-only. Interestingly, the observed worse survival among the oldest old was less pronounced after the addition of the treatment variables (note that the 95% confidence interval for the 85+ age group effect for the model with treatments does not contain the 85+ estimate from the model without treatments), suggesting that the treatments received explain in part the strong age association with survival.
Table III.
Multivariate logistic regression models★ of 3-year overall survival.
| Model without treatments
|
Model with treatments
|
|||
|---|---|---|---|---|
| Adjusted odds ratio (95% CI) | p-Value | Adjusted odds ratio (95% CI) | p-Value | |
| Age at diagnosis (reference = 66–70) | <0.0001 | <0.0001 | ||
| 71–75 | 0.660 (0.570–0.764) | <0.0001 | 0.682 (0.586–0.793) | <0.0001 |
| 76–80 | 0.534 (0.461–0.619) | <0.0001 | 0.601 (0.515–0.700) | <0.0001 |
| 81–84 | 0.342 (0.287–0.407) | <0.0001 | 0.440 (0.367–0.529) | <0.0001 |
| 85+ | 0.148 (0.122–0.179) | <0.0001 | 0.246 (0.201–0.300) | <0.0001 |
| Year of diagnosis (reference = 1992–1994) | <0.0001† | 0.0122† | ||
| 1995–1996 | 0.916 (0.767–1.094) | 0.3334 | 0.907 (0.755–1.088) | 0.2928 |
| 1997–1998 | 1.196 (1.008–1.420) | 0.0403 | 1.128 (0.946–1.345) | 0.1788 |
| 1999–2000 | 1.330 (1.133–1.561) | 0.0005 | 1.203 (1.018–1.421) | 0.0298 |
| 2001–2002 | 1.609 (1.377–1.881) | <0.0001 | 1.231 (1.023–1.481) | 0.0278 |
| Treatment (reference = ABC) | <0.0001‡ | |||
| ABC + R | 1.730 (1.426–2.098) | <0.0001 | ||
| Non-ABC chemo | 0.563 (0.451–0.702) | <0.0001 | ||
| Non-ABC chemo + R | 1.258 (0.798–1.982) | 0.3226 | ||
| R only | 0.613 (0.395–0.952) | 0.0295 | ||
| No therapy | 0.289 (0.253–0.331) | <0.0001 | ||
The first model specification (columns 2–3) includes patient age (66–70) and diagnosis date (1992–1994) as reference. The second model specification (columns 4–5) adds treatment with ABC without rituximab (commonly the CHOP regimen) as reference group. In addition, control variables included race, stage, systemic symptoms, SEER registry, gender, comorbidity indicators, and census tract variables.
Wald χ2 testing the restriction that treatment does not affect survival.
Wald χ2 testing the restriction that treatment does not affect survival. CI, confidence interval; ABC, anthracycline-based chemotherapy; R, rituximab; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; SEER, Surveillance, Epidemiology and End Results.
Survival among treatment groups
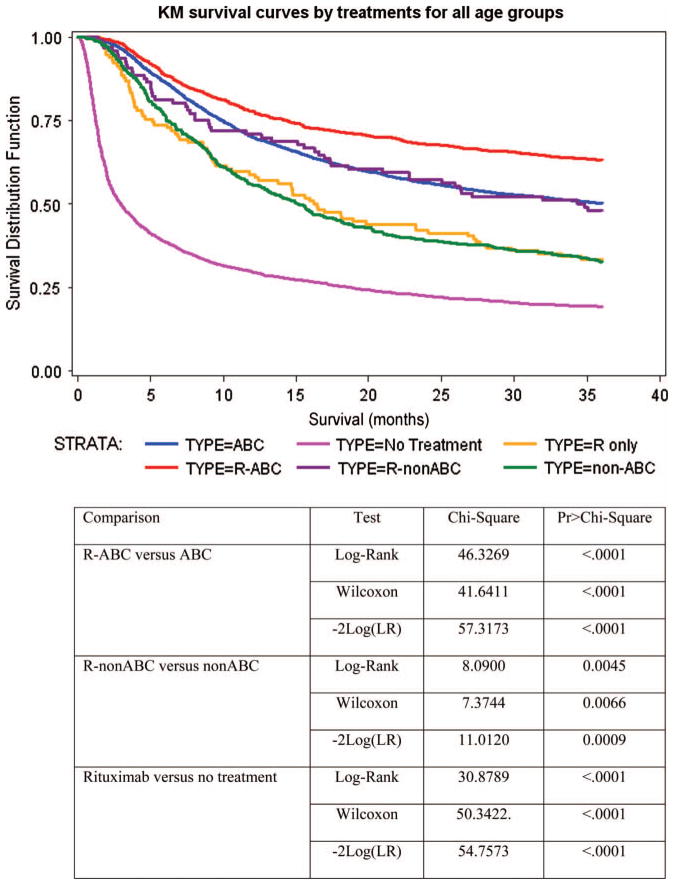
Kaplan–Meier survival curves among treatment groups are depicted in Figure 2. Survival improved for all subsets treated with rituximab when compared to their non-rituximab analog. As expected, individuals receiving R-ABC had the best overall survival. Notably, individuals treated with R-non-ABC had better overall survival than those treated with non-ABC without R (p <0.0001) and, consistent with the multivariate analyses, similar to those treated with ABC without rituximab. Those treated with rituximab alone had better survival than those who received no treatment (p <0.0001) and similar to those treated with non-ABC chemotherapy.
Figure 2.
Kaplan–Meier curves of overall survival estimates in 7559 patients aged 66 years or over diagnosed with diffuse large or diffuse large B-cell lymphoma from 1992 to 2002. All patients are censored at 36 months’ follow-up.
Discussion
This population-based study is the first to describe the diffusion of rituximab usage in Medicare patients with diffuse large cell lymphoma, and confirms the low rate of ABC use in older subjects with this disease even after high-profile studies of R-ABC in the elderly were published. The survival of all elderly patients with DLBCL has improved substantially since 1998, coincident with the diffusion of rituximab into the population. However, undertreatment of the oldest old is of potential concern. The oldest old were very unlikely to receive any therapy, and, if they received therapy, were least likely to receive rituximab. Survival was worse with advancing age, and the age relationship with survival was in part mediated by receipt of treatment. The improvement in survival associated with rituximab use would be expected among those treated with ABC, consistent with prospective clinical trials and the British Columbia Cancer Registry experience. However, the improved survival associated with rituximab addition to non-ABC regimens—equivalent to patients receiving ABC only—has not been previously described.
Rituximab appeared to be used largely as an addition to whatever intensity of chemotherapy a patient might have received, had they been diagnosed in the pre-1999 era. Because rituximab has a minimal frequency of cardiac toxicities, cytopenias, or fatigue, we had anticipated that many elderly or frail patients not deemed candidates for any cytotoxic therapy might have been treated with rituximab alone. However, the rate of ‘no therapy’ remained constant at over 30% after the availability of rituximab. Similarly, we had anticipated an increase in anthracycline use because of studies demonstrating R-CHOP as feasible and optimal for selected patients aged over 60 with DLBCL. However, rituximab introduction did not affect the rate of anthracycline use. Rates of systemic therapy use in general (67%) and anthracycline use specifically (58%) were stable throughout the study period. Thus, a large segment of the elderly population with DLBCL do not receive the generally accepted most effective treatment for DLBCL.
Individuals over age 60 years constitute over half of all patients diagnosed with DLBCL. Randomized trials have shown that patients over 60 respond well to ABC, and efforts to establish chemotherapy regimens for elderly patients that eliminate or utilize less anthracycline result in poorer outcome [15]. Anthracycline avoidance in this population is likely due to global concern for toxicity and tolerability in the elderly, with specific concerns for cardiac toxicity, cytopenias, fatigue, or generalized frailty. Cost–benefit studies utilizing phase III data have suggested that when combined with ABC, rituximab adds an acceptable $US20 000 or less per added year of life [16–18]. Given the relatively low penetration of ABC chemotherapy in the elderly, it remains critical to investigate the impact of new therapies in patients who are not deemed candidates for standard treatment regimens. This population-based study demonstrates that survival in the non-ABC group who received rituximab was comparable to that for the ABC group without rituximab. Based on these findings, rituximab alone or in combination with non-ABC chemotherapy should be investigated for such patients.
In 2002, the Groupe d’Etude des Lymphomes de l’Adulte (GELA) reported a randomized trial comparing R-CHOP to CHOP alone in patients over age 60 with DLBCL. The 2-year OS for CHOP was 57%, while for R-CHOP it was 70%. In that study, patient selection was restricted to subjects without serious concomitant disease, resulting in a median age of enrollment of 69 years, and any application to the general population of elderly patients has remained speculative. Similarly, the BC Cancer Agency reported that 2-year survival rates for all adults with DLBCL increased from 52% to 78%. In spite of a relatively advanced median age (75 years), we observed very similar 2-year OS rates: 59% and 68% in patients treated with ABC and R-ABC, respectively. Although the present study is observational, it is strongly suggestive that improved survival was attributable to rituximab.
Analyses of treatment outcomes for cancer patients utilizing SEER–Medicare data are limited by changes in pathologic classification and staging, uncertainties in the measurement of disease-free survival, and the time it takes to observe outcomes related to newly introduced therapies. This study has addressed these weaknesses in three ways. First, we limited our analysis to DLBCL, a diagnosis that has not substantially changed in classification during the study period. Second, we limited analysis to overall survival, which is easily defined, reproducibly measured, and clinically meaningful. Last, we had at least 3 years of survival data for all study subjects, a meaningful endpoint in DLBCL. Due to its observational design and reliance on registry and administrative claims data, this study was not able to overcome other weaknesses such as the use of billing data to ascertain treatment, and limited data on delivered dose intensity, functional status, or quality of life outcomes. Furthermore, this analysis does not account for treatment selection biases or the role of any therapy, including rituximab, in the second-line or subsequent management of DBCL in this cohort.
In conclusion, we describe for the first time the patterns of utilization of rituximab in the initial therapy of patients with DLBCL over 66 years of age. Utilization started shortly after the presentation of phase II data at a 1998 American Society of Clinical Oncology meeting, and increased steadily such that in 2002 rituximab was added to over three-quarters of anthracycline and non-anthracycline regimens, well before the 2006 FDA approval for this indication. It does not appear that rituximab changed the utilization of ABC or the frequency of total therapy avoidance, which remains very high in those aged over 85. Survival of all US patients with DLBCL over age 66 improved substantially, coincident with the diffusion of rituximab into treatment regimens, and subset analysis suggests that rituximab-associated survival improvement occurred with non-anthracycline containing regimens as well as anthracycline containing regimens. The expectation of greater adverse events should not preclude the treatment of older patients with optimal regimens; rather, the focus should be on finding optimal dosing for older patients, preventing toxicity with available supportive measures, and prospectively evaluating the comparative effectiveness of non-anthracycline and anthracycline-based immunochemotherapy regimens in the oldest patients.
Acknowledgments
This project received funding support from: (1) the Agency for Healthcare Research and Quality (AHRQ) Centers for Education and Research on Therapeutics cooperative agreement #5 U18 HSO16094; and (2) National Institutes of Health grant P50-CA97274 – Iowa/Mayo Lymphoma SPORE program.
This study used the linked SEER–Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology and End Results (SEER) Program tumor registries in the creation of the SEER–Medicare database.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- 1.Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2005;23:4117–4126. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 2.Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 3.Grann VR, Hershman D, Jacobson JS, et al. Outcomes and diffusion of doxorubicin-based chemotherapy among elderly patients with aggressive non-Hodgkin lymphoma. Cancer. 2006;107:1530–1541. doi: 10.1002/cncr.22188. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 5.Coiffier B, Haioun C, Ketterer N, et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood. 1998;92:1927–1932. [PubMed] [Google Scholar]
- 6.Link BK, Grossbard ML, Fisher RI, et al. Phase II pilot study of the safety and efficacy of rituximab in combination with CHOP chemotherapy in patients with previously untreated intermediate- or high-grade NHL. Proc Am Soc Clin Oncol. 1998;17:Abstract 7. [Google Scholar]
- 7.Vose JM, Link BK, Grossbard ML, et al. Phase II study of rituximab in combination with chop chemotherapy in patients with previously untreated, aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2001;19:389–397. doi: 10.1200/JCO.2001.19.2.389. [DOI] [PubMed] [Google Scholar]
- 8.Coiffier B, Lepage E, Herbrecht R, et al. Rituximab plus CHOP is superior to CHOP alone in elderly patients with diffuse large B-cell lymphoma: interim results of a randomized GELA study. Blood. 2000;96(Suppl 1):Abstract 950. [Google Scholar]
- 9.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 10.Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027–5033. doi: 10.1200/JCO.2005.09.137. [DOI] [PubMed] [Google Scholar]
- 11.Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 12.Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40(8 Suppl):IV55–IV61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 15.Sonneveld P, de Ridder M, van der Lelie H, et al. Comparison of doxorubicin and mitoxantrone in the treatment of elderly patients with advanced diffuse non-Hodgkin’s lymphoma using CHOP versus CNOP chemotherapy. J Clin Oncol. 1995;13:2530–2539. doi: 10.1200/JCO.1995.13.10.2530. [DOI] [PubMed] [Google Scholar]
- 16.Best JH, Hornberger J, Proctor SJ, Omnes LF, Jost F. Cost-effectiveness analysis of rituximab combined with chop for treatment of diffuse large B-cell lymphoma. Value Health. 2005;8:462–470. doi: 10.1111/j.1524-4733.2005.00037.x. [DOI] [PubMed] [Google Scholar]
- 17.Groot MT, Lugtenburg PJ, Hornberger J, Huijgens PC, Uylde Groot CA. Cost-effectiveness of rituximab (MabThera) in diffuse large B-cell lymphoma in The Netherlands. Eur J Haematol. 2005;74:194–202. doi: 10.1111/j.1600-0609.2004.00368.x. [DOI] [PubMed] [Google Scholar]
- 18.Hornberger JC, Best JH. Cost utility in the United States of rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone for the treatment of elderly patients with diffuse large B-cell lymphoma. Cancer. 2005;103:1644–1651. doi: 10.1002/cncr.20956. [DOI] [PubMed] [Google Scholar]