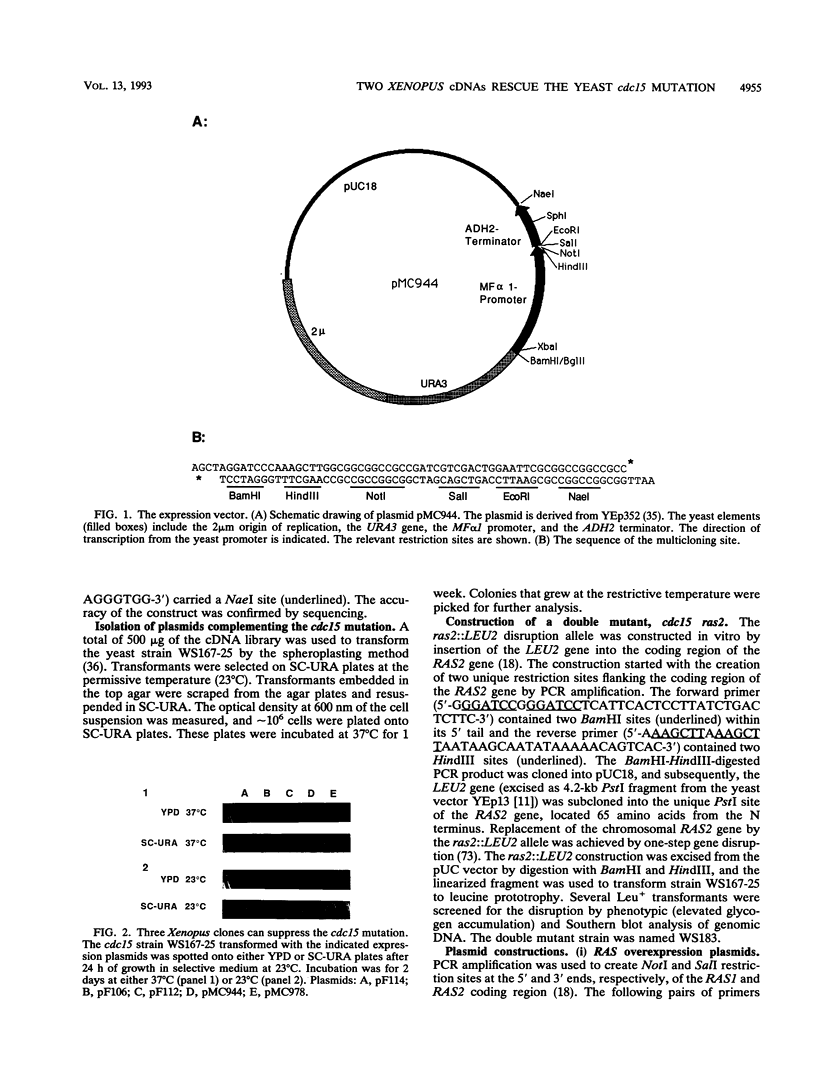
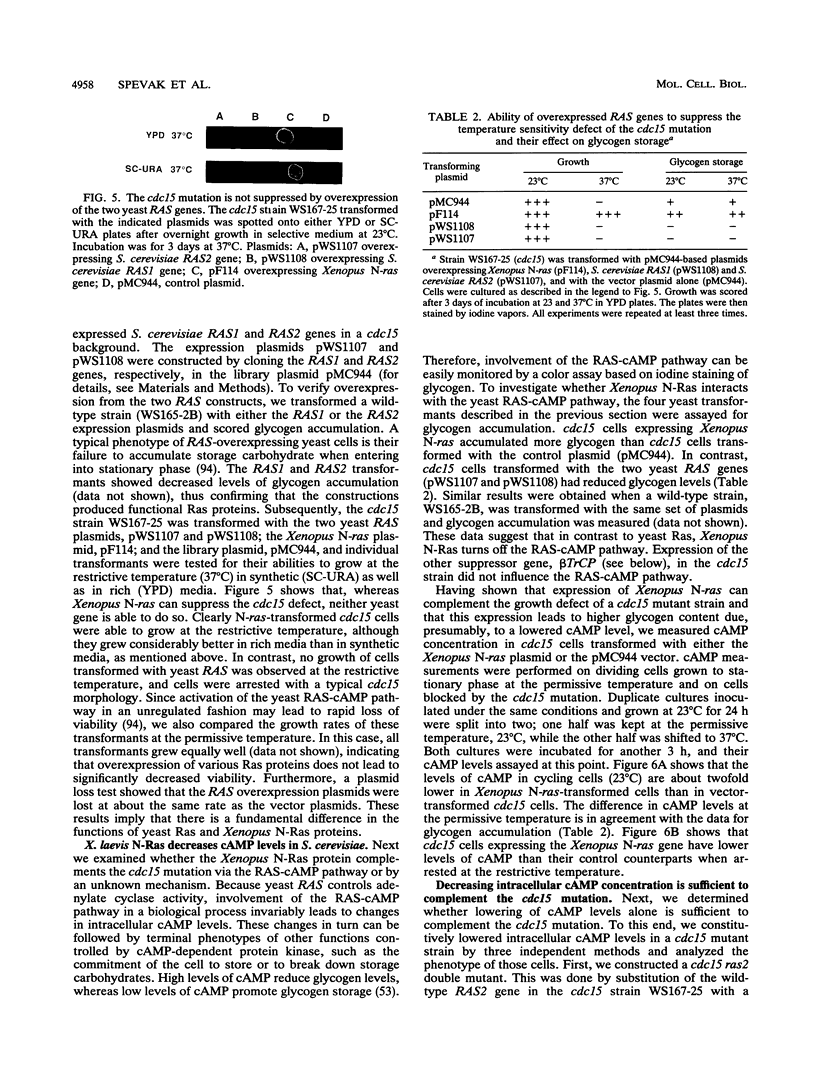
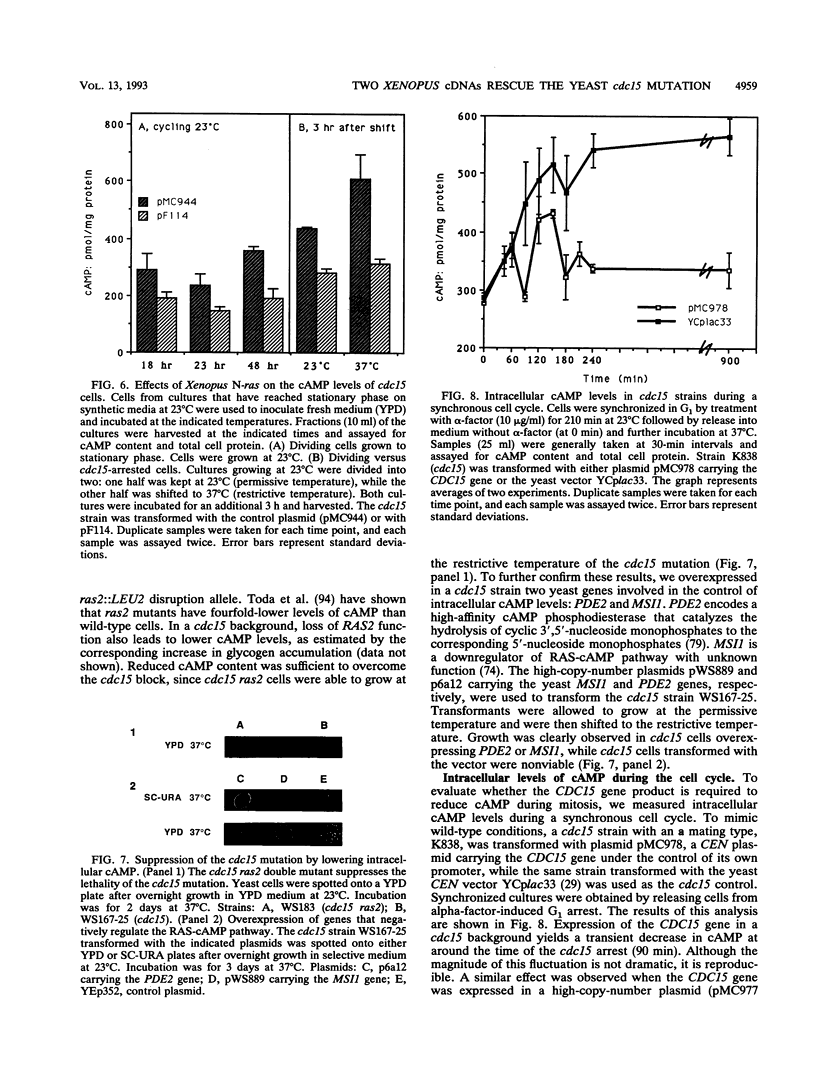
Abstract
We have constructed a Xenopus oocyte cDNA library in a Saccharomyces cerevisiae expression vector and used this library to isolate genes that can function in yeast cells to suppress the temperature sensitive [corrected] defect of the cdc15 mutation. Two maternally expressed Xenopus cDNAs which fulfill these conditions have been isolated. One of these clones encodes Xenopus N-ras. In contrast to the yeast RAS genes, Xenopus N-ras rescues the cdc15 mutation. Moreover, overexpression of Xenopus N-ras in S. cerevisiae does not activate the RAS-cyclic AMP (cAMP) pathway; rather, it results in decreased levels of intracellular cAMP in both mutant cdc15 and wild-type cells. Furthermore, we show that lowering cAMP levels is sufficient to allow cells with a nonfunctional Cdc15 protein to complete the mitotic cycle. These results suggest that a key step of the cell cycle is dependent upon a phosphorylation event catalyzed by cAMP-dependent protein kinase. The second clone, beta TrCP (beta-transducin repeat-containing protein), encodes a protein of 518 amino acids that shows significant homology to the beta subunits of G proteins in its C-terminal half. In this region, beta Trcp is composed of seven beta-transducin repeats. beta TrCP is not a functional homolog of S. cerevisiae CDC20, a cell cycle gene that also contains beta-transducin repeats and suppresses the cdc15 mutation.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andéol Y., Gusse M., Méchali M. Characterization and expression of a Xenopus ras during oogenesis and development. Dev Biol. 1990 May;139(1):24–34. doi: 10.1016/0012-1606(90)90275-n. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Baum E. Z., Bebernitz G. A. K-ras oncogene expression in Xenopus laevis. Oncogene. 1990 May;5(5):763–767. [PubMed] [Google Scholar]
- Beckner S. K., Hattori S., Shih T. Y. The ras oncogene product p21 is not a regulatory component of adenylate cyclase. Nature. 1985 Sep 5;317(6032):71–72. doi: 10.1038/317071a0. [DOI] [PubMed] [Google Scholar]
- Birchmeier C., Broek D., Wigler M. ras proteins can induce meiosis in Xenopus oocytes. Cell. 1985 Dec;43(3 Pt 2):615–621. doi: 10.1016/0092-8674(85)90233-8. [DOI] [PubMed] [Google Scholar]
- Bjørn S. P., Soltyk A., Beggs J. D., Friesen J. D. PRP4 (RNA4) from Saccharomyces cerevisiae: its gene product is associated with the U4/U6 small nuclear ribonucleoprotein particle. Mol Cell Biol. 1989 Sep;9(9):3698–3709. doi: 10.1128/mcb.9.9.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Deschenes R. J. The function of ras genes in Saccharomyces cerevisiae. Adv Cancer Res. 1990;54:79–139. doi: 10.1016/s0065-230x(08)60809-x. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Strathern J. N., Hicks J. B. Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene. 1979 Dec;8(1):121–133. doi: 10.1016/0378-1119(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Broek D., Toda T., Michaeli T., Levin L., Birchmeier C., Zoller M., Powers S., Wigler M. The S. cerevisiae CDC25 gene product regulates the RAS/adenylate cyclase pathway. Cell. 1987 Mar 13;48(5):789–799. doi: 10.1016/0092-8674(87)90076-6. [DOI] [PubMed] [Google Scholar]
- Bürglin T. R., Mattaj I. W., Newmeyer D. D., Zeller R., De Robertis E. M. Cloning of nucleoplasmin from Xenopus laevis oocytes and analysis of its developmental expression. Genes Dev. 1987 Mar;1(1):97–107. doi: 10.1101/gad.1.1.97. [DOI] [PubMed] [Google Scholar]
- Capon D. J., Chen E. Y., Levinson A. D., Seeburg P. H., Goeddel D. V. Complete nucleotide sequences of the T24 human bladder carcinoma oncogene and its normal homologue. Nature. 1983 Mar 3;302(5903):33–37. doi: 10.1038/302033a0. [DOI] [PubMed] [Google Scholar]
- Chester V. E. Heritable glycogen-storage deficiency in yeast and its induction by ultra-violet light. J Gen Microbiol. 1968 Apr;51(1):49–56. doi: 10.1099/00221287-51-1-49. [DOI] [PubMed] [Google Scholar]
- Culotti J., Hartwell L. H. Genetic control of the cell division cycle in yeast. 3. Seven genes controlling nuclear division. Exp Cell Res. 1971 Aug;67(2):389–401. doi: 10.1016/0014-4827(71)90424-1. [DOI] [PubMed] [Google Scholar]
- Dalrymple M. A., Petersen-Bjorn S., Friesen J. D., Beggs J. D. The product of the PRP4 gene of S. cerevisiae shows homology to beta subunits of G proteins. Cell. 1989 Sep 8;58(5):811–812. doi: 10.1016/0092-8674(89)90930-6. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Hough B. R. Genetic information in oocyte RNA. J Mol Biol. 1971 Mar 28;56(3):491–506. doi: 10.1016/0022-2836(71)90396-2. [DOI] [PubMed] [Google Scholar]
- DeFeo-Jones D., Tatchell K., Robinson L. C., Sigal I. S., Vass W. C., Lowy D. R., Scolnick E. M. Mammalian and yeast ras gene products: biological function in their heterologous systems. Science. 1985 Apr 12;228(4696):179–184. doi: 10.1126/science.3883495. [DOI] [PubMed] [Google Scholar]
- Dhar R., Nieto A., Koller R., DeFeo-Jones D., Scolnick E. M. Nucleotide sequence of two rasH related-genes isolated from the yeast Saccharomyces cerevisiae. Nucleic Acids Res. 1984 Apr 25;12(8):3611–3618. doi: 10.1093/nar/12.8.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynlacht B. D., Weinzierl R. O., Admon A., Tjian R. The dTAFII80 subunit of Drosophila TFIID contains beta-transducin repeats. Nature. 1993 May 13;363(6425):176–179. doi: 10.1038/363176a0. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Spottswood M. R. A new human p34 protein kinase, CDK2, identified by complementation of a cdc28 mutation in Saccharomyces cerevisiae, is a homolog of Xenopus Eg1. EMBO J. 1991 Sep;10(9):2653–2659. doi: 10.1002/j.1460-2075.1991.tb07808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F., Newport J. W. Evidence that the G1-S and G2-M transitions are controlled by different cdc2 proteins in higher eukaryotes. Cell. 1991 Aug 23;66(4):731–742. doi: 10.1016/0092-8674(91)90117-h. [DOI] [PubMed] [Google Scholar]
- Flessel M. C., Brake A. J., Thorner J. The MF alpha 1 gene of Saccharomyces cerevisiae: genetic mapping and mutational analysis of promoter elements. Genetics. 1989 Feb;121(2):223–236. doi: 10.1093/genetics/121.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong H. K., Hurley J. B., Hopkins R. S., Miake-Lye R., Johnson M. S., Doolittle R. F., Simon M. I. Repetitive segmental structure of the transducin beta subunit: homology with the CDC4 gene and identification of related mRNAs. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2162–2166. doi: 10.1073/pnas.83.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François J. M., Thompson-Jaeger S., Skroch J., Zellenka U., Spevak W., Tatchell K. GAC1 may encode a regulatory subunit for protein phosphatase type 1 in Saccharomyces cerevisiae. EMBO J. 1992 Jan;11(1):87–96. doi: 10.1002/j.1460-2075.1992.tb05031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiara J. B., Richardson H. E., Sugimoto K., Henze M., Lew D. J., Wittenberg C., Reed S. I. A cyclin B homolog in S. cerevisiae: chronic activation of the Cdc28 protein kinase by cyclin prevents exit from mitosis. Cell. 1991 Apr 5;65(1):163–174. doi: 10.1016/0092-8674(91)90417-w. [DOI] [PubMed] [Google Scholar]
- Gibbs J. B., Marshall M. S. The ras oncogene--an important regulatory element in lower eucaryotic organisms. Microbiol Rev. 1989 Jun;53(2):171–185. doi: 10.1128/mr.53.2.171-185.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz R. D., Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988 Dec 30;74(2):527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- Glotzer M., Murray A. W., Kirschner M. W. Cyclin is degraded by the ubiquitin pathway. Nature. 1991 Jan 10;349(6305):132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Gross E., Marbach I., Engelberg D., Segal M., Simchen G., Levitzki A. Anti-Cdc25 antibodies inhibit guanyl nucleotide-dependent adenylyl cyclase of Saccharomyces cerevisiae and cross-react with a 150-kilodalton mammalian protein. Mol Cell Biol. 1992 Jun;12(6):2653–2661. doi: 10.1128/mcb.12.6.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J. H., Stratowa C., Rutter W. J. Isolation of full-length putative rat lysophospholipase cDNA using improved methods for mRNA isolation and cDNA cloning. Biochemistry. 1987 Mar 24;26(6):1617–1625. doi: 10.1021/bi00380a020. [DOI] [PubMed] [Google Scholar]
- Hartley D. A., Preiss A., Artavanis-Tsakonas S. A deduced gene product from the Drosophila neurogenic locus, enhancer of split, shows homology to mammalian G-protein beta subunit. Cell. 1988 Dec 2;55(5):785–795. doi: 10.1016/0092-8674(88)90134-1. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Culotti J., Pringle J. R., Reid B. J. Genetic control of the cell division cycle in yeast. Science. 1974 Jan 11;183(4120):46–51. doi: 10.1126/science.183.4120.46. [DOI] [PubMed] [Google Scholar]
- Hill J. E., Myers A. M., Koerner T. J., Tzagoloff A. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast. 1986 Sep;2(3):163–167. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman C. S., Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57(2-3):267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Icho T., Wickner R. B. The MAK11 protein is essential for cell growth and replication of M double-stranded RNA and is apparently a membrane-associated protein. J Biol Chem. 1988 Jan 25;263(3):1467–1475. [PubMed] [Google Scholar]
- Kataoka T., Powers S., Cameron S., Fasano O., Goldfarb M., Broach J., Wigler M. Functional homology of mammalian and yeast RAS genes. Cell. 1985 Jan;40(1):19–26. doi: 10.1016/0092-8674(85)90304-6. [DOI] [PubMed] [Google Scholar]
- Keiper B. D., Wormington W. M. Nucleotide sequence and 40 S subunit assembly of Xenopus laevis ribosomal protein S22. J Biol Chem. 1990 Nov 15;265(32):19397–19400. [PubMed] [Google Scholar]
- Keleher C. A., Redd M. J., Schultz J., Carlson M., Johnson A. D. Ssn6-Tup1 is a general repressor of transcription in yeast. Cell. 1992 Feb 21;68(4):709–719. doi: 10.1016/0092-8674(92)90146-4. [DOI] [PubMed] [Google Scholar]
- Koff A., Cross F., Fisher A., Schumacher J., Leguellec K., Philippe M., Roberts J. M. Human cyclin E, a new cyclin that interacts with two members of the CDC2 gene family. Cell. 1991 Sep 20;66(6):1217–1228. doi: 10.1016/0092-8674(91)90044-y. [DOI] [PubMed] [Google Scholar]
- Langan T. A., Gautier J., Lohka M., Hollingsworth R., Moreno S., Nurse P., Maller J., Sclafani R. A. Mammalian growth-associated H1 histone kinase: a homolog of cdc2+/CDC28 protein kinases controlling mitotic entry in yeast and frog cells. Mol Cell Biol. 1989 Sep;9(9):3860–3868. doi: 10.1128/mcb.9.9.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. G., Nurse P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 1987 May 7;327(6117):31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- Lew D. J., Dulić V., Reed S. I. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell. 1991 Sep 20;66(6):1197–1206. doi: 10.1016/0092-8674(91)90042-w. [DOI] [PubMed] [Google Scholar]
- Lewin B. Driving the cell cycle: M phase kinase, its partners, and substrates. Cell. 1990 Jun 1;61(5):743–752. doi: 10.1016/0092-8674(90)90181-d. [DOI] [PubMed] [Google Scholar]
- Lorca T., Labbé J. C., Devault A., Fesquet D., Capony J. P., Cavadore J. C., Le Bouffant F., Dorée M. Dephosphorylation of cdc2 on threonine 161 is required for cdc2 kinase inactivation and normal anaphase. EMBO J. 1992 Jul;11(7):2381–2390. doi: 10.1002/j.1460-2075.1992.tb05302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léopold P., O'Farrell P. H. An evolutionarily conserved cyclin homolog from Drosophila rescues yeast deficient in G1 cyclins. Cell. 1991 Sep 20;66(6):1207–1216. doi: 10.1016/0092-8674(91)90043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller J. L., Butcher F. R., Krebs E. G. Early effect of progesterone on levels of cyclic adenosine 3':5'-monophosphate in Xenopus oocytes. J Biol Chem. 1979 Feb 10;254(3):579–582. [PubMed] [Google Scholar]
- Maller J. L., Krebs E. G. Progesterone-stimulated meiotic cell division in Xenopus oocytes. Induction by regulatory subunit and inhibition by catalytic subunit of adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1977 Mar 10;252(5):1712–1718. [PubMed] [Google Scholar]
- Marshall M. S., Gibbs J. B., Scolnick E. M., Sigal I. S. Regulatory function of the Saccharomyces cerevisiae RAS C-terminus. Mol Cell Biol. 1987 Jul;7(7):2309–2315. doi: 10.1128/mcb.7.7.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Ishikawa T. Genetic analysis of the role of cAMP in yeast. Yeast. 1985 Sep;1(1):15–24. doi: 10.1002/yea.320010103. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W., Solomon M. J., Kirschner M. W. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989 May 25;339(6222):280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- Nasmyth K., Adolf G., Lydall D., Seddon A. The identification of a second cell cycle control on the HO promoter in yeast: cell cycle regulation of SW15 nuclear entry. Cell. 1990 Aug 24;62(4):631–647. doi: 10.1016/0092-8674(90)90110-z. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Clapham D. E. Roles of G protein subunits in transmembrane signalling. Nature. 1988 May 12;333(6169):129–134. doi: 10.1038/333129a0. [DOI] [PubMed] [Google Scholar]
- Newport J. W., Kirschner M. W. Regulation of the cell cycle during early Xenopus development. Cell. 1984 Jul;37(3):731–742. doi: 10.1016/0092-8674(84)90409-4. [DOI] [PubMed] [Google Scholar]
- Ninomiya-Tsuji J., Nomoto S., Yasuda H., Reed S. I., Matsumoto K. Cloning of a human cDNA encoding a CDC2-related kinase by complementation of a budding yeast cdc28 mutation. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9006–9010. doi: 10.1073/pnas.88.20.9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P., Bissett Y. Gene required in G1 for commitment to cell cycle and in G2 for control of mitosis in fission yeast. Nature. 1981 Aug 6;292(5823):558–560. doi: 10.1038/292558a0. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Paris J., Le Guellec R., Couturier A., Le Guellec K., Omilli F., Camonis J., MacNeill S., Philippe M. Cloning by differential screening of a Xenopus cDNA coding for a protein highly homologous to cdc2. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1039–1043. doi: 10.1073/pnas.88.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J., Hunter T. p34cdc2: the S and M kinase? New Biol. 1990 May;2(5):389–401. [PubMed] [Google Scholar]
- Powers S., O'Neill K., Wigler M. Dominant yeast and mammalian RAS mutants that interfere with the CDC25-dependent activation of wild-type RAS in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Feb;9(2):390–395. doi: 10.1128/mcb.9.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst E., Kressmann A., Birnstiel M. L. Expression of sea urchin histone genes in the oocyte of Xenopus laevis. J Mol Biol. 1979 Dec 15;135(3):709–732. doi: 10.1016/0022-2836(79)90173-6. [DOI] [PubMed] [Google Scholar]
- Rebagliati M. R., Weeks D. L., Harvey R. P., Melton D. A. Identification and cloning of localized maternal RNAs from Xenopus eggs. Cell. 1985 Oct;42(3):769–777. doi: 10.1016/0092-8674(85)90273-9. [DOI] [PubMed] [Google Scholar]
- Reed S. I. G1-specific cyclins: in search of an S-phase-promoting factor. Trends Genet. 1991 Mar;7(3):95–99. doi: 10.1016/0168-9525(91)90279-Y. [DOI] [PubMed] [Google Scholar]
- Reed S. I., Wittenberg C. Mitotic role for the Cdc28 protein kinase of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5697–5701. doi: 10.1073/pnas.87.15.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Ruggieri R., Tanaka K., Nakafuku M., Kaziro Y., Toh-e A., Matsumoto K. MSI1, a negative regulator of the RAS-cAMP pathway in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8778–8782. doi: 10.1073/pnas.86.22.8778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. W., Smith M., Williamson V. M., Young E. T. Nucleotide sequence of the yeast alcohol dehydrogenase II gene. J Biol Chem. 1983 Feb 25;258(4):2674–2682. [PubMed] [Google Scholar]
- Sadler S. E., Maller J. L. A similar pool of cyclic AMP phosphodiesterase in Xenopus oocytes is stimulated by insulin, insulin-like growth factor 1, and [Val12,Thr59]Ha-ras protein. J Biol Chem. 1989 Jan 15;264(2):856–861. [PubMed] [Google Scholar]
- Sadler S. E., Maller J. L., Gibbs J. B. Transforming ras proteins accelerate hormone-induced maturation and stimulate cyclic AMP phosphodiesterase in Xenopus oocytes. Mol Cell Biol. 1990 Apr;10(4):1689–1696. doi: 10.1128/mcb.10.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sass P., Field J., Nikawa J., Toda T., Wigler M. Cloning and characterization of the high-affinity cAMP phosphodiesterase of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9303–9307. doi: 10.1073/pnas.83.24.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl R. H., Gietz R. D. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989 Dec;16(5-6):339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- Schweitzer B., Philippsen P. CDC15, an essential cell cycle gene in Saccharomyces cerevisiae, encodes a protein kinase domain. Yeast. 1991 Apr;7(3):265–273. doi: 10.1002/yea.320070308. [DOI] [PubMed] [Google Scholar]
- Segil N., Shrutkowski A., Dworkin M. B., Dworkin-Rastl E. Enolase isoenzymes in adult and developing Xenopus laevis and characterization of a cloned enolase sequence. Biochem J. 1988 Apr 1;251(1):31–39. doi: 10.1042/bj2510031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi N., Monteagudo M. C., Koshland D., Hogan E., Burke D. J. The CDC20 gene product of Saccharomyces cerevisiae, a beta-transducin homolog, is required for a subset of microtubule-dependent cellular processes. Mol Cell Biol. 1991 Nov;11(11):5592–5602. doi: 10.1128/mcb.11.11.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K., Birnbaum D., Ruley M. A., Fasano O., Suard Y., Edlund L., Taparowsky E., Goldfarb M., Wigler M. Structure of the Ki-ras gene of the human lung carcinoma cell line Calu-1. Nature. 1983 Aug 11;304(5926):497–500. doi: 10.1038/304497a0. [DOI] [PubMed] [Google Scholar]
- Simon M. I., Strathmann M. P., Gautam N. Diversity of G proteins in signal transduction. Science. 1991 May 10;252(5007):802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Smith M. E., Dickinson J. R., Wheals A. E. Intracellular and extracellular levels of cyclic AMP during the cell cycle of Saccharomyces cerevisiae. Yeast. 1990 Jan-Feb;6(1):53–60. doi: 10.1002/yea.320060106. [DOI] [PubMed] [Google Scholar]
- Smith R. C., Dworkin-Rastl E., Dworkin M. B. Expression of a histone H1-like protein is restricted to early Xenopus development. Genes Dev. 1988 Oct;2(10):1284–1295. doi: 10.1101/gad.2.10.1284. [DOI] [PubMed] [Google Scholar]
- Smith R. C., Dworkin M. B., Dworkin-Rastl E. Destruction of a translationally controlled mRNA in Xenopus oocytes delays progesterone-induced maturation. Genes Dev. 1988 Oct;2(10):1296–1306. doi: 10.1101/gad.2.10.1296. [DOI] [PubMed] [Google Scholar]
- Surana U., Robitsch H., Price C., Schuster T., Fitch I., Futcher A. B., Nasmyth K. The role of CDC28 and cyclins during mitosis in the budding yeast S. cerevisiae. Cell. 1991 Apr 5;65(1):145–161. doi: 10.1016/0092-8674(91)90416-v. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Nakafuku M., Satoh T., Marshall M. S., Gibbs J. B., Matsumoto K., Kaziro Y., Toh-e A. S. cerevisiae genes IRA1 and IRA2 encode proteins that may be functionally equivalent to mammalian ras GTPase activating protein. Cell. 1990 Mar 9;60(5):803–807. doi: 10.1016/0092-8674(90)90094-u. [DOI] [PubMed] [Google Scholar]
- Taparowsky E., Shimizu K., Goldfarb M., Wigler M. Structure and activation of the human N-ras gene. Cell. 1983 Sep;34(2):581–586. doi: 10.1016/0092-8674(83)90390-2. [DOI] [PubMed] [Google Scholar]
- Tatchell K. RAS genes and growth control in Saccharomyces cerevisiae. J Bacteriol. 1986 May;166(2):364–367. doi: 10.1128/jb.166.2.364-367.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Uno I., Ishikawa T., Powers S., Kataoka T., Broek D., Cameron S., Broach J., Matsumoto K., Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985 Jan;40(1):27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- Trahey M., McCormick F. A cytoplasmic protein stimulates normal N-ras p21 GTPase, but does not affect oncogenic mutants. Science. 1987 Oct 23;238(4826):542–545. doi: 10.1126/science.2821624. [DOI] [PubMed] [Google Scholar]
- Weeks D. L., Melton D. A. A maternal mRNA localized to the vegetal hemisphere in Xenopus eggs codes for a growth factor related to TGF-beta. Cell. 1987 Dec 4;51(5):861–867. doi: 10.1016/0092-8674(87)90109-7. [DOI] [PubMed] [Google Scholar]
- Whiteway M., Hougan L., Dignard D., Thomas D. Y., Bell L., Saari G. C., Grant F. J., O'Hara P., MacKay V. L. The STE4 and STE18 genes of yeast encode potential beta and gamma subunits of the mating factor receptor-coupled G protein. Cell. 1989 Feb 10;56(3):467–477. doi: 10.1016/0092-8674(89)90249-3. [DOI] [PubMed] [Google Scholar]
- Williams F. E., Trumbly R. J. Characterization of TUP1, a mediator of glucose repression in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Dec;10(12):6500–6511. doi: 10.1128/mcb.10.12.6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg C., Sugimoto K., Reed S. I. G1-specific cyclins of S. cerevisiae: cell cycle periodicity, regulation by mating pheromone, and association with the p34CDC28 protein kinase. Cell. 1990 Jul 27;62(2):225–237. doi: 10.1016/0092-8674(90)90361-h. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Connolly T., Futcher B., Beach D. Human D-type cyclin. Cell. 1991 May 17;65(4):691–699. doi: 10.1016/0092-8674(91)90100-d. [DOI] [PubMed] [Google Scholar]
- Yochem J., Byers B. Structural comparison of the yeast cell division cycle gene CDC4 and a related pseudogene. J Mol Biol. 1987 May 20;195(2):233–245. doi: 10.1016/0022-2836(87)90646-2. [DOI] [PubMed] [Google Scholar]
- de Barros Lopes M., Ho J. Y., Reed S. I. Mutations in cell division cycle genes CDC36 and CDC39 activate the Saccharomyces cerevisiae mating pheromone response pathway. Mol Cell Biol. 1990 Jun;10(6):2966–2972. doi: 10.1128/mcb.10.6.2966. [DOI] [PMC free article] [PubMed] [Google Scholar]