Abstract
Background
Depression in the context of bipolar disorder (BDd) is often misdiagnosed as unipolar disorder depression (UDd) leading to poor clinical outcomes for many bipolar sufferers. We examined neural circuitry supporting emotion regulation in females with either BDd or UDd as a first stage toward identifying biomarkers that may differentiate BDd from UDd.
Method
Fifty-seven females aged 18–45 years participated in this study: 23 with UDd, 18 with bipolar disorder type I depression (BDId) and 16 healthy females. During 3-T functional magnetic resonance imaging (fMRI), the participants performed an emotional face n-back (EFNBACK) task, that is an n-back task with high (2-back) and low (0-back) memory load conditions flanked by two positive, negative or neutral face distracters. This paradigm examines executive control with emotional distracters–emotion regulation.
Results
High memory load with neutral face distracters elicited greater bilateral and left dorsal anterior midcingulate cortex (dAMCC) activity in UDd than in healthy and BDId females respectively, and greater bilateral putamen activity in both depressed groups versus healthy females. High memory load with happy face distracters elicited greater left putamen activity in UDd than in healthy females. Psychotropic medication was associated with greater putamen activity to these contrasts in UDd females.
Conclusions
During high memory load with neutral face distracters, elevated dAMCC activity in UDd suggests abnormal recruitment of attentional control circuitry to maintain task performance, whereas elevated putamen activity unrelated to psychotropic medication in BDId females may suggest an attentional bias toward ambiguous neutral face distracters. Differential patterns of functional abnormalities in neural circuitry supporting attentional control during emotion regulation, especially in the dAMCC, is a promising neuroimaging measure to distinguish UDd from BDId in females.
Keywords: Bipolar disorder, depression, emotion regulation, emotional n-back, unipolar disorder
Introduction
The absence of biologically relevant diagnostic markers of bipolar disorder (BD) results in misdiagnosis of the illness as recurrent unipolar disorder (UD) depression in 60% of BD individuals seeking treatment for depression, leading to poor clinical outcomes (Hirschfeld et al. 2003; Langenecker et al. 2010). It is therefore crucial that objective markers of BD are identified to help distinguish BD from UD as early as possible in depressed individuals. A first stage toward this ultimate goal is the identification of biological markers reflecting pathophysiologic processes that may differ between BD and UD depression (Strakowski et al. 2002; Drevets, 2003; Almeida et al. 2010).
Emotional over-reactivity and emotion dysregulation characterize BD. A better understanding of functional abnormalities in neural circuitry supporting emotion regulation may help to provide biological measures reflecting pathophysiologic processes distinguishing BD from recurrent UD depression. To date, the majority of neuroimaging studies in BD depressed (BDd) and recurrent UD depressed (UDd) individuals have focused on examination of neural circuitry supporting emotion processing. These studies reported abnormally elevated amygdala and striatal activity to negative and positive emotional stimuli in BDd (and subsyndromally depressed) adults (Lawrence et al. 2004; Malhi et al. 2004; Chen et al. 2006). In UDd adults, studies reported abnormally elevated amygdala and striatal activity to negative emotional stimuli, but less consistently to positive emotional stimuli (Fu et al. 2004; Surguladze et al. 2005; Anand et al. 2007; Dannlowski et al. 2007; Fales et al. 2008), and abnormally reduced activity in the striatum to positive emotional and rewarding stimuli (Surguladze et al. 2005; Epstein et al. 2006; McRae et al. 2008).
Emotion regulation neural circuitry comprises regions implicated in early appraisal of emotional information during ‘automatic’ or implicit subprocesses of emotion regulation. These areas include rostral and subgenual regions of the anterior cingulate cortex (ACC; Brodmann areas BA 24/25 respectively), orbitofrontal cortex (OFC: BA 11), mediodorsal prefrontal cortex (mdPFC: medial BA 9/10), and medial components of the ventrolateral prefrontal cortex (VLPFC: medial BA 47). Regions implicated in more demanding executive and attentional control processes, which support more effortful, emotion regulation processes, include the dorsal ACC (dorsal BA 24/32) and, in particular, the dorsal anterior midcingulate cortex (dAMCC: the most dorsal parts of BA 24/32), lateral VLPFC (BA 47) and dorsolateral prefrontal cortex (DLPFC: BA 44/46 and lateral BA 9) (Ochsner & Gross, 2005; Langenecker et al. 2007; Phillips et al. 2008).
To date, only one study has examined emotion regulation neural circuitry in BDd adults (Deckersbach et al. 2008). A combination n-back working memory (WM) task and sad or neutral mood induction was used. Participants either maintained or suppressed a sad or neutral mood induced during the first 30 s of the WM task. This study reported greater left rostral/dorsal ACC (BA 32) activity during the sad condition and greater right rostral/dorsal ACC (BA 32) during the neutral mood induction condition for BDd relative to healthy females. In addition, BDd females had more activity than healthy females in DLPFC (BA 9/46) to the sad condition. Greater activity in these regions suggested greater demand for recruitment of neural regions supporting more effortful regulation of emotion in BDd than in healthy females.
In UDd adults, numerous studies have examined activity in emotion regulation neural circuitry during paradigms involving either effortful direction of attention away from emotionally distracting information or more automatic emotion regulation subprocesses. Reduced DLPFC activity in UDd versus healthy adults was shown during a WM task following negative emotional word stimuli (Siegle et al. 2007). Other studies reported reduced DLPFC activity in UDd adults attempting to ignore negative stimuli (Fales et al. 2008), and during both unattended and attended emotional judgment (Grimm et al. 2008). Studies examining effortful emotion regulation showed greater left dorsal ACC (BA 32) activity during an emotional Stroop task in UDd versus healthy adults (Mitterschiffthaler et al. 2008), and greater activity in rostral/dorsal ACC during an affective go/no-go task in UDd versus healthy adults to sad targets and in healthy versus UDd adults to happy targets (Elliott et al. 2002). The latter study also showed greater DLPFC activity in healthy versus UDd adults to neutral targets and in UDd versus healthy adults to sad targets. Additionally, one study reported abnormal recruitment of bilateral DLPFC, together with abnormal positive subgenual ACC–amygdala functional connectivity, in UDd versus healthy adults during cognitive appraisal of emotion, an effortful emotion regulation subprocess (Johnstone et al. 2007).
The above studies suggest overlapping, but distinct, functional abnormalities in neural circuitry supporting emotion regulation subprocesses in UDd and BDd adults. No neuroimaging studies, however, have compared neural circuitry directly during emotion regulation paradigms in BDd and UDd adults. Furthermore, no neuroimaging studies have evaluated the extent to which neutral stimuli (neutral faces) may serve as distracters during the performance of executive and attentional control tasks.
This is the first study to our knowledge to compare neural activity during effortful emotion regulation in BDd, UDd and healthy females. The overall aim of this study was to examine activity in neural circuitry supporting emotion regulation in BDd and UDd adults, using an emotional WM paradigm, as a first stage toward identification of biomarkers reflecting pathophysiological processes that differentiate BDd from UDd. We focused on females, given the higher prevalence of depression in females (Weissman et al. 1996), and gender differences in neural activity during emotion processing and regulation (Schienle et al. 2005; Koch et al. 2007; McRae et al. 2008). We also focused on BD type I depression (BDId) rather than BD type II (BDII) depression, given the few studies and limited understanding of the pathophysiology of BDII. We chose to examine neural circuitry supporting effortful emotion regulation specifically, maintaining executive control throughout emotional and emotionally ambiguous distraction using an emotional n-back paradigm. Similar paradigms have been used to separately examine emotion regulation neural circuitry in BDd or UDd. The task requires direction of attention away from negative (fear), positive (happy) or neutral face distracters in order to perform a 2-back WM task. The task has the advantage of being able to be used to examine neural activity during WM task performance in the presence of emotional and neutral face distracters. The latter is important because findings suggest that neutral faces may be perceived as ambiguous and potentially more salient or threatening in mood-disordered relative to healthy individuals (Langenecker et al. 2005; Rich et al. 2006).
In our primary hypotheses, we focused on an examination of activity within key a priori neural regions implicated in executive and attentional control (DLPFC, rostral/dorsal ACC, dAMCC). In our secondary hypotheses, we focused on key regions implicated in emotion processing (amygdala, striatum).
Our two primary hypotheses were guided by findings in UDd adults of abnormally reduced DLPFC activity during a WM task in the context of negative emotional distraction (Siegle et al. 2002) and also abnormal recruitment of dorsal ACC during an affective go/no-go task (Elliott et al. 2002). We hypothesized that, relative to healthy females:
-
(1)
UDd females would show significantly reduced DLPFC activity but abnormally elevated rostral/dorsal ACC and dAMCC activity during the 2-back memory load condition with negative emotional (fearful face-versus-no face) distracters.
Findings from the one existing study in BDd adults examining neural circuitry during a WM task in the context of negative mood induction (Deckersbach et al. 2008) allowed us to hypothesize that, relative to healthy females:
-
(2)
BDId females would show significantly elevated DLPFC rostral/dorsal ACC, and dAMCC activity during the 2-back memory load condition with both negative and positive emotional (fearful face-versus-no face and happy face-versus-no face) distracters.
With no extant studies directly comparing neural activity in BDId and UDd adults during emotion regulation tasks, we were unable to make specific hypotheses regarding the comparison of BDId and UDd females.
Our two secondary hypotheses were guided by findings in UDd adults of abnormally elevated amygdala and striatal activity to negative emotional stimuli, but abnormally reduced activity in the striatum to positive emotional and rewarding stimuli (Fu et al. 2004; Surguladze et al. 2005; Anand et al. 2007; Dannlowski et al. 2007; Fales et al. 2008; McRae et al. 2008) and, in BDId (and subsyndromally depressed) adults, abnormally elevated amygdala and striatal activity to both negative and positive emotional stimuli (Lawrence et al. 2004; Malhi et al. 2004; Chen et al. 2006). We hypothesized that, relative to healthy females:
-
(3)
UDd females would show significantly greater activity in amygdala and striatum during the 2-back memory load condition with negative emotional (fearful) distracters, but significantly reduced activity in striatum during the 2-back memory load condition with positive emotional (happy) distracters.
-
(4)
BDId females would show elevated activity in amygdala and striatum during the 2-back memory load condition with negative emotional (fearful) or positive emotional (happy) distracters.
Method
Participants
Fifty-seven females aged 18–45 years participated in the study. Twenty-three UDd and 18 BDId in a depressed episode during scanning [diagnosed by a trained clinician using the Structured Clinical interview of DSM-IV, Research Version (SCID-P; First et al. 1995); a 25-item Hamilton Depression Rating Scale (HAMD-25; Hamilton, 1960) score >10; and a Young Mania Rating Scale (YMRS; Young et al. 1978) score <10]. Sixteen healthy female participants had no personal or family history (first-degree relative) of psychiatric disorder. All participants were native English speakers and right-handed (Annett, 1970). The groups did not differ on age. Depressed female groups did not differ on depression severity, mania rating, state anxiety severity, age of onset of illness, duration of illness or smoking status (Table 1 and online Supplementary Tables S1A and S1B).
Table 1.
Demographic characteristics of the sample
| BDId (n=18) | UDd (n=23) | Healthy (n=16) | Test | Significance | |
|---|---|---|---|---|---|
| Age (years) | 31.94 (8.54) | 29.74 (8.22) | 32.76 (6.50) | F(2, 54)=0.87 | 0.463 |
| NART | 112.93 (8.41) | 111.67 (8.84) | 111.09 (7.13) | F(2, 49)=0.20 | 0.819 |
| HAMD-25 | 24.17 (8.44) | 26.26 (6.30) | N.A. | t(39)=−0.911 | 0.368 |
| YMRS | 3.94 (2.90) | 4.17 (2.50) | N.A. | t(39)=0.272 | 0.787 |
| STAI | 51.78 (11.02) | 54.43 (8.94) | N.A. | t(39)=0.853 | 0.399 |
| Age of illness onset (years) | 17.94 (6.38) | 17.30 (7.23) | N.A. | t(39)=−0.296 | 0.769 |
| Illness duration (years) | 14.00 (6.63) | 12.44 (7.38) | N.A. | t(39)=−0.703 | 0.487 |
| Current (proportion) | |||||
| Antidepressant use | 6/18 | 20/23 | N.A. | ||
| Antipsychotic use | 9/18 | 2/23 | N.A. | ||
| Mood stabilizer use | 11/18 | 1/23 | N.A. | ||
| Benzodiazepine use | 3/18 | 5/23 | N.A. | ||
| Smoking use | 9/19 | 5/23 | 3/16 | F(2, 54)=2.69 | 0.077 |
| Lifetime (proportion) | |||||
| Drug/alcohol abuse/dependence | 7/18 | 7/23 | |||
| Anxiety disorder | 11/18 | 15/23 | |||
BDId, Bipolar I depression; UDd, unipolar disorder depression; NART, National Adult Reading Test; HAMD-25, 25-item Hamilton Rating Scale for Depression; YMRS, Young Mania Rating Scale; STAI, Speilberger State Trait Anxiety Inventory; N.A., not applicable.
Values given are mean (standard deviation) or proportion.
Exclusion criteria for all participants included a history of head trauma/neurological disease, serious medical illness, cognitive impairment (IQ <85/Mini Mental State Examination score <24; Folstein et al. 1975; Blair & Spreen, 1989), severe visual disturbance, and borderline personality disorder. Exclusion criteria also included a lifetime history of alcohol or substance abuse/dependence for healthy females, and alcohol or substance abuse/dependence in the past 3 months for depressed females (determined by SCID-P, saliva, and urine screen). The presence of psychotic symptoms in UDd and BDId females was also an exclusion criterion. In addition, magnetic resonance imaging (MRI) exclusion criteria included a history of metal in the body, a positive pregnancy test, and claustrophobia.
Participants were recruited using local advertising and clinician referral from the Western Psychiatric Institute and Clinic at the University of Pittsburgh Medical Center (UPMC). The study was approved by the Institutional Review Board at the University of Pittsburgh, and all participants provided written informed consent prior to participation.
Medication
Psychotropic medication was used by 87% of UDd females and 77.8% of BDId females. Individual medication subclasses (antidepressants, antipsychotics, anxiolytics, and mood stabilizers) were documented (Table 1 and Supplementary Table S1).
Paradigm
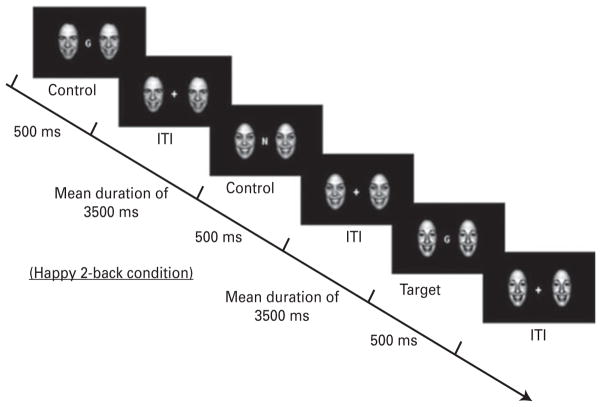
An emotional face n-back (EFNBACK) task was used to examine the ability to recruit prefrontal ‘executive control’ systems in the context of simultaneously presented emotionally salient distracting stimuli while performing a WM task (Ochsner & Gross, 2005; Ladouceur et al. 2009). The EFNBACK task is a modified version of the n-back WM task (Ladouceur et al. 2009), which consists of visually presenting a pseudorandom sequence of letters while participants respond to a prespecified letter appearing on the computer screen. The n-back task included two memory load conditions: a no-memory load (0-back; e.g. press the button to ‘M’) and a high memory load [2-back; e.g. press the button whenever the current letter is identical to the letter present two trials back (L–X–L)] (Fig. 1). The emotional n-back task comprised the original n-back task flanked by two emotional or neutral face distracters (Tottenhan et al. 2009). A no-face condition controlled for the interference related to a face distracter on either side of the letter in the 2- and 0-back task conditions and allowed us to examine all three emotional faces (neutral, fear and happy) as distracters. In addition, there were eight different stimulus blocks: two memory-load conditions (0-back and 2-back), each with one of four emotional face distracter conditions (none, neutral, fearful or happy). The task comprised three 7-min 4-s runs, for a total of 24 blocks, presented in a pseudo-randomized order. Each block included 12 trials. Each trial comprised a letter flanked with either no pictures or identical pictures of an actor’s facial expression (neutral, fearful or happy). The trial duration was 500 ms. The inter-trial interval was a jittered fixation cross (mean duration= 3500 ms). Participants responded as quickly as possible with their index finger to the target letter. Brief instructions were presented on the screen for 4000 ms at the beginning of each block. Detailed instructions were provided during task practice prior to the scanning session.
Fig. 1.
Illustration of the Emotional Face n-Back (EFNBACK) task. This is an example of the 2-back (high attentional demand) happy-face distracter condition. During the 0-back (low attentional demand) condition, participants must respond to the letter M. ITI, intertribal stimulus interval. (Copyright © 2009 by the American Psychological Association. Reproduced with permission. The official citation that should be used in referencing this material is Ladouceur et al. 2009. The use of APA information does not imply endorsement by the APA.)
Neuroimaging data acquisition and analysis
All neuroimaging datawere collected on a 3.0-T Siemens Trio MRI scanner at the Magnetic Resonance Imaging Center, UPMC. Structural T1-weighted volumetric scans were obtained using a magnetization-prepared rapid gradient-echo (MP-RAGE) sequence; 192 1.0-mm axial slices; flip angle 9°; field of view (FOV)=256 mm; repetition time (TR)=2200 ms; echo time (TE)=3.29 ms; matrix=256×256. Blood oxygen level-dependent (BOLD) images were acquired with a gradient-echo echo planar imaging (EPI) sequence with 39 3.2 mm axial slices; flip angle 90°; FOV= 205 mm; TR=2000 ms; TE=28 ms; matrix=64×64.
Data were preprocessed and analyzed using Statistical Parametric Mapping software (SPM5; www.fil.ion.ucl.ac.uk/spm). Preprocessing to correct for differences in acquisition comprised slice time correction, realignment and unwarping, co-registration, normalization into a standard stereotactic space (Montreal Neurological Institute, MNI; www.bic.mni.mcgill.ca), resampled to 2×2×2 mm3 voxels, and spatially smoothed using a 6-mm full-width at half-maximum (FWHM) Gaussian kernel. Trials with incorrect behavioral responses were excluded from functional MRI (fMRI) data analysis.
A two-level random-effects procedure was used to analyze fMRI data. At the first level, individual whole-brain statistical maps were constructed to evaluate each of the three main condition contrasts of interest: (1) 2-back:fear face-no face; (2) 2-back:happy face-no face; (3) 2-back:neutral face-no face. Movement parameters from the realignment stage of preprocessing were included as covariates of no interest.
To test our specific hypotheses, at the second level, three 3×1 factorial designs were used to examine the main effect of group upon neural activity during executive control in the presence of negative emotional (fear), positive emotional (happy) or neutral face emotional distracters within a priori regions of interest (ROIs, described below). The three models were: (1) group (BDId, UDd, healthy)×2-back (memory load condition): fear face (emotional distracter) versus no face distracter, (2) group×2-back:happy face versus no face distracter, and (3) group × 2-back:neutral face versus no face distracter. The no face distracter was used to fully evaluate the salience of all three emotional faces (neutral, fearful and happy).
The WFU PickAtlas (Wake Forest University, USA) was used in the second-level processing to create anatomical masks for bilateral ROIs (Maldjian et al. 2003). ROIs for WM load–executive control analysis included the bilateral DLPFC (BA 9/46) and dorsal ACC–dAMCC (BA 24/32). Subcortical regions supporting emotion processing included the bilateral amygdala and striatum [caudate head and body, putamen, and ventral striatum (bilateral spheres −9, 9, −8 and 9, 9, −8; radius=8)]. The entire ROI was used, followed by specific identification of neural structures corresponding to the MNI coordinates of the peak voxels. To examine the main effect of group, we used a voxelwise threshold of p≤0.05. In addition, to correct for multiple voxelwise comparisons within each ROI, the AlphaSim program was used (http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim), with 1000 Monte Carlo simulations. AlphaSim limits the overall type 1 error to 0.05 by computing minimum cluster size thresholds for each ROI (Table 2). The AlphaSim program is a validated technique for correction for multiple voxelwise tests in ROI analyses (Ward, 2002).
Table 2.
Between-group differences during working memory and emotion conditionsa
| Contrast | Region Comparison | Area | BA | Cluster k | MNI | p | d |
|---|---|---|---|---|---|---|---|
| 2-back:neutral-2 back:no face | |||||||
| dAMCC | |||||||
| AlphaSim threshold: k≥44 | |||||||
| Main effect of group | dAMCC | 24 | 65 | −4, −4, 36 | 0.021 | ||
| UDd>H | dAMCC | 24 | 316 | −4, −10, 38 | 0.001 | 0.75 | |
| dAMCC | 24 | 264 | 4, −10, 40 | 0.002 | 0.69 | ||
| UDd>BPId | dAMCC | 24 | 53 | −4, −10, 28 | 0.011 | 0.90 | |
| Striatum | |||||||
| AlphaSim threshold: k≥ 79 | |||||||
| Main effect of group | Putamen | 125 | 26, 10, −2 | 0.010 | |||
| UDd>H | Putamen | 151 | −22, 0, 6 | 0.005 | 0.59 | ||
| Putamen | 255 | 14, 8, 2 | 0.008 | 0.72 | |||
| BPId>H | Putamen | 85 | −26, 12, 2 | 0.003 | 0.73 | ||
| Putamen | 316 | 24, 10, −4 | 0.005 | 0.83 | |||
| 2-back:happy-2-back:no face | |||||||
| Striatum | |||||||
| AlphaSim threshold: k≥ 79 | |||||||
| Main effect of group | Putamen | 94 | −24, −4, 8 | 0.006 | |||
| UDd>H | Putamen | 358 | −24, −4, 8 | 0.001 | 0.73 | ||
| BDId>H | Putamen | 128 | 22, 4, −2 | 0.020b | 0.69 | ||
dAMCC, Dorsal anterior midcingulate cortex; BA, Brodmann area; MNI, Montreal Neurological Institute coordinates; BDId, bipolar disorder type I depressed; UDd, recurrent unipolar disorder depressed; H, healthy; d, effect size of observed differences calculated using Cohen’s d. Striatum=bilateral caudate head and body, putamen, ventral striatum. dAMCC=bilateral BA24 and 32.
Region of interest (ROI) analyses, with a voxelwise threshold of p<0.05. To control for multiple voxelwise comparisons, contiguous voxel thresholds were constructed individually at p<0.05 using AlphaSim for each ROI. Each line in the table represents the voxel of peak activity difference within the specified region. No regions exceeded AlphaSim thresholds of 0.05.
This region just failed to meet our conservative voxelwise threshold of p<0.016 (to control for the three tests of group in each a priori ROI).
Significant main effects of group were examined using post-hoc, pairwise between-group comparisons on activity in bilateral ROIs showing a significant main effect of group. To control for the three post-hoc pairwise comparisons in each ROI for each condition showing a main effect of group, we used a voxelwise threshold of p≤0.016 (0.05/3), followed by the AlphaSim program to compute clusterwise thresholds at p<0.05, as above.
In supplementary analyses, a similar series of three 3×1 factorial models were conducted for the less cognitively demanding 0-back condition, and therefore not expected to engage executive control neural circuitry.
Relationships between task performance, characteristic variables and neural activity
For each of the 2-back stimulus conditions, a mean BOLD signal was extracted from clusters in all ROIs showing significant between-group differences in activity. Two-tailed bivariate correlations were computed between activity in each of these clusters and reaction time differences for the corresponding stimulus conditions (e.g. 2-back:happy-no face neural activity and 2-back:happy-no face reaction time). Further correlational analyses were conducted for BDId and UDd females between activity in clusters in ROIs showing significant abnormal activity relative to healthy females and demographic and clinical variables: age, HAMD-25 total score, YMRS total score, state anxiety, age of illness onset, and illness duration. Independent t tests were used to compare depressed females in each diagnostic group who were taking versus those who were not taking psychotropic medication classes (antidepressants, anxiolytics, antipsychotics, mood stabilizers); those with versus those without previous history of substance abuse/dependence; co-morbid anxiety disorders, and smoking status.
Results
Task performance
Performance on the 2-back task was good (mean accuracy=92.82%) with no main effect of group. Accuracy did not differ significantly among emotional face distracter and the no-distracter conditions (Supplementary Table S2).
Repeated-measures analysis showed no significant between-group differences in reaction time [F(2, 54)= 0.183, p = 0.834]. A trend for a main effect of group was observed for accuracy [F(2, 54)=2.62, p = 0.08] but there were no post-hoc significant pairwise between-group differences in accuracy using either Bonferroni or Tukey HSD correction. Reaction times were slower for face distracter trials compared with no-face distracter trials (all p<0.001; Supplementary Table S2).
Neuroimaging findings
Findings for the main effect of group and post-hoc pairwise tests are shown in Table 2. Within-group findings for mean percentage BOLD responses on the 2-back and 0-back conditions show the extent of activation differences (see Supplementary Tables S3A and S3B).
Primary hypotheses findings in main a priori ROIs: DLPFC, rostral/dorsal and dAMCC
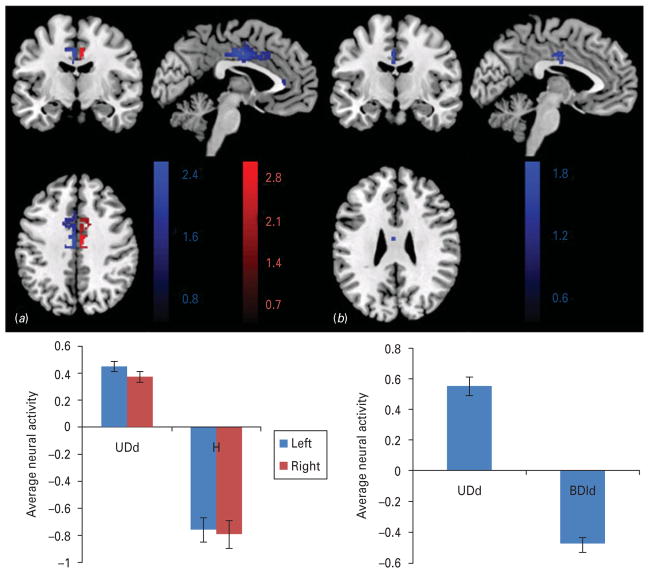
There was no significant effect of group on activity in the DLPFC or in the rostral/dorsal ACC. However, there was a significant effect of group in the left dAMCC (BA 32) on the 2-back:neutral-2-back:no face condition (F=5.37, p<0.05, corrected; Table 2).
Post-hoc comparisons in dAMCC to the 2-back:neutral-2-back:no face condition
UDd females showed significantly greater left and right dAMCC (BA 24) activity than healthy females (left: t=3.06, p<0.05, corrected, Cohen’s d=0.75; right: t=2.95, p<0.05, corrected, Cohen’s d=0.69). UDd females also showed significantly greater activity in the left dAMCC (BA 24) than BDId females (t=2.32, p<0.05, corrected, Cohen’s d=0.90; Table 2 and Fig. 2). UDd females showed activation whereas healthy females showed deactivation in the bilateral dAMCC. BDId females showed deactivation in the left dAMCC and activation in the right dAMCC (Supplementary Table S3A).
Fig. 2.
Group differences in the dorsal anterior midcingulate cortex (dAMCC) blood oxygen level-dependent (BOLD) signal during the attentional control of emotion on the 2back: neutral-2back: no face contrast. Females with unipolar disorder depression (UDd) exhibited (a) significantly greater activity in the dAMCC (peak voxel left: −4, −10, 38; voxels=316, right: 4, −10, 40; voxels=264) versus healthy controls during the 2-back neutral-no face distracter condition; and (b) significantly greater activity in the dAMCC (peak voxel left: −4, −10, 28; voxels=53) versus bipolar I depression (BDId) during the 2-back neutral-no face distracter condition. UDd females showed activation whereas healthy females showed deactivation in the bilateral dAMCC and BDId females deactivation in the left dAMCC. Color bars indicate t values of the statistical parametric mapping (SPM).
Secondary hypotheses findings in main a priori ROIs: amygdala and striatum
There was no significant effect of group on activity in the amygdala. However, there was a significant effect of group in the left putamen on the 2-back:happy-2-back:no face condition (F=7.68, p<0.05, corrected; Table 2) and in the right putamen on the 2-back:neutral-2-back:no face condition (F=6.8, p<0.05, corrected; Table 2).
Post-hoc comparisons in the striatum to the 2-back:happy-2-back:no face and 2-back:neutral-2-back:no face conditions
2-back:happy-2-back:no face
UDd females showed significantly greater left putamen activity than healthy females (t=3.02, p<0.05, corrected, Cohen’s d=0.73). BDId females showed greater right putamen activity than healthy females, although this just missed our conservative voxelwise threshold of p≤0.016 (Cohen’s d=0.69) for pairwise between-group comparisons. Healthy females showed deactivation whereas UDd and BDId females showed activation in these regions (Table 2; Supplementary Table S3A).
2-back:neutral-2-back:no face
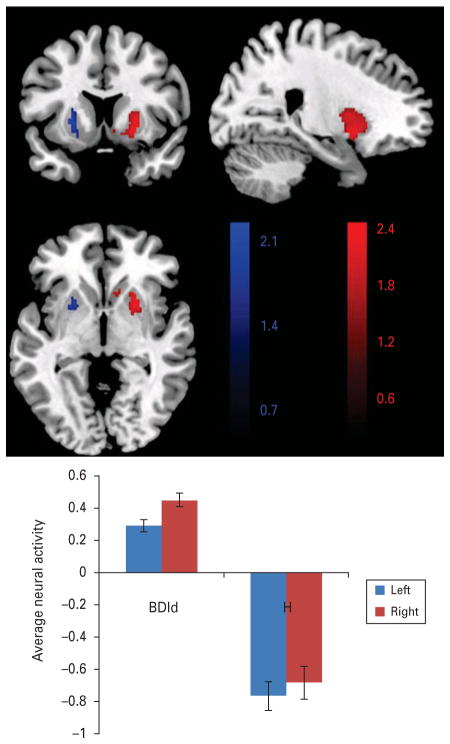
UDd females showed significantly greater activity in the right (and left) putamen (right: t=2.39, p<0.05, corrected, Cohen’s d=0.72; left: t=2.56, p<0.05, corrected, Cohen’s d=0.59) relative to healthy females. BDId females showed significantly greater right (and left) putamen activity (right: t=2.60, p<0.05, corrected, Cohen’s d=0.83; left: t=2.77, p<0.05, corrected, Cohen’s d=0.73) relative to healthy females (Table 2 and Fig. 3). Healthy females showed deactivation whereas UDd and BDId females showed activation in these regions (Supplementary Table 3A).
Fig. 3.
Group differences in the putamen BOLD signal during the attentional control of emotion on the 2back:neutral-2back:no face contrast. BDId individuals exhibited significantly greater activity in the putamen (peak voxel left: −26, 12, 2; voxels=85, right: 24, 10, −4; voxels=316) versus healthy controls during the 2-back neutral-no face distracter condition; BDId females showed activation, while healthy females showed deactivation in bilateral putamen. Color bar indicates t values of the statistical parametric mapping (SPM). BDId=Bipolar disorder type I depressed; H=healthy dAMCC=bilateral BA 24 and 32.
Exploratory analyses in regions showing a main effect of group
With regard to the 2-back:neutral-no face condition, UDd females taking antidepressant medication showed greater right putamen activity than UDd females not taking antidepressant medication [t(21)= −2.19, p = 0.04]. For the 2-back:happy-no face condition, UDd females taking benzodiazepines showed greater left putamen activity than UDd females not taking benzodiazepines [t(21)=−2.11, p=0.045]. There were no other significant exploratory relationships (Supplementary Tables S4A–S4C).
Discussion
The aim of this study was to identify neuroimaging measures that could differentiate BDId from UDd females during emotion regulation. Neural activity during an executive control task with emotional distracters (emotional n-back) was examined. Our findings indicate differential patterns of abnormal activity in the dAMCC and striatal attentional control neural circuitry, in BDId and UDd groups during the demanding 2-back condition of the task with neutral face distracters, with moderate to large effect sizes, while maintaining an equivalent task performance with healthy groups. The most interesting finding was that UDd females showed abnormally elevated dAMCC activity during this condition relative to both BDId and healthy females.
In partial support of hypotheses 1 and 2, UDd females showed abnormally elevated activity in the dAMCC, a key neural region supporting effortful cognitive/attentional control (Bush & Shin, 2006), during the cognitively demanding 2-back task with face distracters. This was, however, to the neutral condition and not to the negative emotion (fear) condition. Contrary to our hypothesis, BDId females did not show abnormally elevated dorsal ACC/dAMCC activity during the 2-back:fear-no face condition (or to any of the three 2-back:face distracter conditions). UDd females also recruited the left dAMCC to a greater extent than BDId females to the 2-back:neutral-no face condition. Here, UDd females showed activation, whereas BDId and healthy females showed deactivation, in the dAMCC to the 2-back:neutral-no face condition. Neutral faces have been shown to be perceived as more ambiguous and potentially threatening in psychiatric groups relative to healthy groups (Langenecker et al. 2005; Rich et al. 2006). Given that all three groups performed equally well on the task, our findings suggest that, during the demanding 2-back condition with neutral face distracters, only the UDd females needed to recruit attentional control neural circuitry to successfully direct attention away from the ambiguous, neutral face distracters. These findings may therefore suggest that neutral face distracters may have been perceived as more distracting by UDd than by BDId females. Given the literature indicating an abnormal negative emotional attentional bias in UDd, but less consistently in BDId (Phillips, 2003; Phillips et al. 2003; Pavuluri et al. 2007), and the frequent misclassification of neutral faces as negative emotional faces even in healthy individuals (Young et al. 1997), these findings may reflect the fact that neutral faces may have been perceived as depicting more negative emotion, different from the fearful face, in UDd than the other groups. Less pronounced levels of abnormal activation in UDd, and deactivation in healthy, females in dAMCC to the 2-back:fear-no face and 2-back:happy-no face conditions probably accounted for the absence of significant group effects in this region to these conditions.
In partial support of hypothesis 4, BDId females showed significantly greater bilateral putamen activity than healthy females during the 2-back:neutral-no face condition, and greater right putamen activity than healthy females during the 2-back:happy-no face condition, although the latter just missed our conservative voxelwise threshold. BDId females activated, whereas healthy females deactivated, these regions. The putamen is implicated in motor control but has also been shown to be involved in orienting responses to emotional stimuli (Surguladze et al. 2003, 2005; Gruber et al. 2004; Haber et al. 2006). Given that the main findings were to the neutral condition, BDId females may have shown a greater orienting response, supported by putamen activity, to the more ambiguous neutral face distracters than healthy females, but, unlike UDd females, performed the task as well as healthy females without additional recruitment of dAMCC. In UDd females, abnormally elevated right putamen activity during the 2-back:neutral-no face condition, and left putamen activity during the 2-back:happy-no face condition, may have been confounded by antidepressants and benzodiazepines respectively. The absence of a significant group effect on striatal activity during the 2-back:fear-no face condition most probably resulted from the fact that both BDId and healthy females showed deactivation, and UDd females small levels of activation, in the striatum to this condition.
The absence of significant group effects in the DLPFC and amygdala to any of the three main 2-back conditions resulted from similar levels of activation/deactivation in these regions to these conditions in all groups. Our main findings therefore suggest that between-group differences in neural activity in UDd and BDId females to the 2-back conditions were present in neural regions supporting attentional control (dAMCC) and orientation to salient stimuli (putamen) rather than in neural regions supporting executive control in general (DLPFC) or emotion processing (amygdala).
The pattern of deactivations observed in a priori regions merits further discussion. Our three main stimulus condition contrasts were designed to Distinguishing unipolar and bipolar depression in females 9 examine neural activity during WM with, versus without, the presence of face distracters. Greater activity in the dAMCC and DLPFC to these condition contrasts would thus suggest a need to recruit more executive and attentional control neural circuitry to help direct attention away from face distracters to perform the WM task. The pattern of predominant deactivation observed in healthy females in the majority of a priori neural regions to most condition contrasts suggests that healthy females were not distracted by face stimuli, and in fact needed to recruit the DLPFC and dAMCC less during the demanding 2-back task in the presence of face distracters than during performance of the task without face distracters. These findings suggest that performance of the task may have been less effortful for healthy than for depressed females. By contrast, both depressed groups, and especially UDd females, showed patterns of activation in many a priori regions to most condition contrasts. UDd females, in particular, may have been more distracted by face stimuli than the other two groups during the 2-back task, and may therefore have needed to recruit neural regions, especially the dAMCC supporting attentional control, to a significantly greater extent than other groups to enable accurate task performance.
There are limitations to this study. We examined females only, thereby avoiding the potential confound of gender differences in neural activity during task performance, but also limiting generalizability of our findings to BDId and UDd males. Future studies should compare neural activity during the task in males and females in each of the groups. Most depressed females were medicated, as was necessary given their depression severity. Our findings, however, suggest few effects of psychotropic medication upon neural activity in UDd females, and no significant effects of such medication in BDId females. In addition, we did not examine the potential impact of the menstrual/hormonal cycle upon neural activity because menstrual history data were not collected. Given the literature indicating gray matter abnormalities, particularly in subcortical regions, in mood-disordered individuals (Lorenzetti et al. 2009), future studies should also aim to examine the extent to which potential gray matter abnormalities in subcortical (and other) ROIs may impact functional abnormalities in these regions in BDId and UDd individuals.
This is the first study to directly compare neural circuitry supporting executive and attentional control processes in the context of emotional distracters using an emotional WM task in BDId and UDd females with similar levels of severity and duration of depressive illness. Our findings suggest, in the two depressed groups, differential patterns of functional abnormalities in the dAMCC and putamen during successful maintenance of attention to task during emotional distraction and in the dAMCC in particular. Specifically, UDd females recruited the dAMCC to a significantly greater extent than healthy and BDId females when directing attention away from neutral face distracters during the 2-back condition. By contrast, BDId females showed abnormally elevated putamen activity to neutral, and to a lesser extent happy, face distracters during the 2-back condition, which may suggest an orienting bias toward these distracters in this group. Our findings help to further understand functional abnormalities in the dAMCC and striatum, supporting executive control during emotion regulation, which may differentiate BDI from UD depression in females, and are a step toward identifying neuroimaging measures reflecting pathophysiologic processes that may accurately distinguish the two types of depression.
Acknowledgments
This work was supported by National Institute of Mental Health (NIMH) grants 5R01MH041712-19, R01MH07697, R01MH073953 and U01MH092221 (to M.L.P.), K-23MH074459 (to S.L.), and NIMH K01MH083001 and the National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Award (to C.L.). We thank the staff of the Phillips Functional Neuroimaging Studies and the participants.
Footnotes
These data were presented as a poster at the Society of Biological Psychiatry Conference, San Francisco, 2011.
Supplementary material accompanies this paper on the Journal’s website (http://journals.cambridge.org/psm).
Declaration of Interest
None.
References
- Almeida JR, Versace A, Hassel S, Kupfer DJ, Phillips ML. Elevated amygdala activity to sad facial expressions: a state marker of bipolar but not unipolar depression. Biological Psychiatry. 2010;67:414–421. doi: 10.1016/j.biopsych.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Gardner K, Lowe M. Reciprocal effects of antidepressant treatment on activity and connectivity of the mood regulating circuit: an fMRI study. Journal of Neuropsychiatry Clinical Neuroscience. 2007;19:274–282. doi: 10.1176/appi.neuropsych.19.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annett M. A classification of hand preference by association analysis. British Journal of Psychology. 1970;61:303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Blair K, Spreen O. Predicting premorbid IQ: a revision of the National Adult Reading Test. Clinical Neuropsychologist. 1989;3:129–136. [Google Scholar]
- Bush G, Shin LM. The Multi-Source Interference Task: an fMRI task that reliably activates the cingulofrontal-parietal cognitive/attention network. Nature Protocols. 2006;1:308–313. doi: 10.1038/nprot.2006.48. [DOI] [PubMed] [Google Scholar]
- Chen C, Lenox B, Jacob R, Calder A, Lupson V, Bisbrown-Chippendale R, Suckling J, Bullmore E. Explicit and implicit facial affect recognition in manic and depressed states of bipolar disorder: a functional magnetic resonance imaging study. Biological Psychiatry. 2006;59:31–39. doi: 10.1016/j.biopsych.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Kugel H, Arolt V, heindel W, Kersting A, Baune B, Suslow T. Amygdala reactivity to masked negative faces is associated with automatic judgmental bias in major depression: a 3T fMRI study. Journal of Psychiatry and Neuroscience. 2007;32:423–429. [PMC free article] [PubMed] [Google Scholar]
- Deckersbach T, Rauch S, Buhlman U, Ostacher M, Beucke J, Nierenberg A, Sachs G, Dougherty DD. An fMRI investigation of working memory and sadness in females with bipolar disorder: a brief report. Bipolar Disorders. 2008;10:928–942. doi: 10.1111/j.1399-5618.2008.00633.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Annals of the New York Academy of Sciences. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. The neural basis of mood-congruent processing biases in depression. Archives of General Psychiatry. 2002;59:597–604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, Hochberg H, Murrough J, Strohmayer E, Stern E, Silbersweig DA. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. American Journal of Psychiatry. 2006;163:1784–1790. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- Fales C, Barch D, Rundle M, Mintun M, Snyder A, Cohen J, Mathews J, Sheline Y. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biological Psychiatry. 2008;63:377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders, Version 2.0. Biometric Research Department, New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Folstein M, Folstein S, McHugh P. ‘Mini-Mental State’. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fu CHY, Williams SCR, Cleare AJ, Brammer MJ, Walsh ND, Kim J, Andrew CM, Pich EM, Williams PM, Reed LJ, Mitterschiffthaler MT, Suckling J, Bullmore ET. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Archives of General Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Grimm S, Beck J, Schuepbach D, Hell D, Boesinger P, Permpohl F, Niehaus l, Boeker H, Northoff G. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorder. Biological Psychiatry. 2008;63:369–376. doi: 10.1016/j.biopsych.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Rogowska J, Yurgelun-Todd DA. Decreased activation of the anterior cingulate in bipolar patients: an fMRI study. Journal of Affective Disorders. 2004;82:191–201. doi: 10.1016/j.jad.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Haber SN, Kim K-S, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. Journal of Neuroscience. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld RM, Lewis L, Vornik LA. Perceptions and impact of bipolar disorder: how far have we really come? Results of the National Depressive and Manic-Depressive Association 2000 survey of individuals with bipolar disorder. Journal of Clinical Psychiatry. 2003;64:161–174. [PubMed] [Google Scholar]
- Johnstone T, van Reekum C, Urry H, Kalin N, Davidson R. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. Journal of Neuroscience. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K, Pauly K, Kellermann T, Seiferth NY, Reske M, Backes V, Stöcker T, Shah NJ, Amunts K, Kircher T, Schneider F, Habel U. Gender differences in the cognitive control of emotion: an fMRI study. Neuropsychologia. 2007;45:2744–2754. doi: 10.1016/j.neuropsychologia.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Silk JS, Dahl RE, Ostapenko L, Kronhaus DM, Phillips ML. Fearful faces influence attentional control processes in anxious youth and adults. Emotion. 2009;9:855–864. doi: 10.1037/a0017747. [DOI] [PubMed] [Google Scholar]
- Langenecker S, Bieliauskas L, Rapport L, Zubieta J, Wilde E, Berent S. Face emotion perception and executive functioning deficitis in depression. Journal of Clinical and Experimental Neuropsychology. 2005;27:320–333. doi: 10.1080/13803390490490515720. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Kennedy S, Guidotti L, Briceno E, Own L, Hooven T, Young E, Akil H, Noll DC, Zubieta J. Frontal and limbic activation during inhibitory control predicts treatment response in major depressive disorder. Biological Psychiatry. 2007;62:1272–1280. doi: 10.1016/j.biopsych.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker SA, Saunders E, Kade A, Ransom M, McInnis M. Intermediate cognitive phenotypes in bipolar disorder. Journal of Affective Disorders. 2010;122:285–293. doi: 10.1016/j.jad.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips ML. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biological Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Lorenzetti V, Allen NB, Fornito A, Yücel M. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. Journal of Affective Disorders. 2009;117:1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Malhi G, Lagopoulos J, Ward P, Kumari V, Mitchell P, Parker GB, Ivanovski B, Schdev P. Cognitive generation of affect in bipolar depression: an fMRI study. European Journal of Neuroscience. 2004;19:741–754. doi: 10.1111/j.0953-816x.2003.03159.x. [DOI] [PubMed] [Google Scholar]
- McRae K, Ochsner KN, Mauss IB, Gabrieli JJD, Gross JJ. Gender differences in emotion regulation: an fMRI study of cognitive reappraisal. Group Processes and Intergroup Relations. 2008;11:143–162. doi: 10.1177/1368430207088035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitterschiffthaler MT, Williams SCR, Walsh ND, Cleare AJ, Donaldson C, Scott J, Fu CHY. Neural basis of the emotional Stroop interference effect in major depression. Psychological Medicine. 2008;38:247–256. doi: 10.1017/S0033291707001523. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Oconnor MM, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biological Psychiatry. 2007;62:158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Phillips M, Ladouceur C, Drevets W. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML. Understanding the neurobiology of emotion perception: implications for psychiatry. British Journal of Psychiatry. 2003;182:190–192. doi: 10.1192/bjp.182.3.190. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biological Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, Fromm SJ, Pine DS, Leibenluft E. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proceedings of the National Academy of Sciences USA. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienle A, Schäfer A, Stark R, Walter B, Vaitl D. Gender differences in the processing of disgust- and fear-inducing pictures: an fMRI study. Neuroreport. 2005;16:277–280. doi: 10.1097/00001756-200502280-00015. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger AV, Carter CS. Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biological Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Adler CM, DelBello MP. Volumetric MRI studies of mood disorders: do they distinguish unipolar and bipolar disorder ? Bipolar Disorders. 2002;4:80–88. doi: 10.1034/j.1399-5618.2002.01160.x. [DOI] [PubMed] [Google Scholar]
- Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SCR, Phillips ML. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biological Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Surguladze SA, Brammer MJ, Young AW, Andrew C, Travis MJ, Williams SCR, Phillips ML. A preferential increase in the extrastriate response to signals of danger. NeuroImage. 2003;19:1317–1328. doi: 10.1016/s1053-8119(03)00085-5. [DOI] [PubMed] [Google Scholar]
- Tottenhan N, Tanaka J, Leon A, McCarry T, Nurse M, Hare T. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B. AlphaSim. National Institute of Mental Health; Bethesda, MD: 2002. [Google Scholar]
- Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu H-G, Joyce PR, Karam EG, Lee C-K, Lellouch J, Lépine J-P, Newman SC, Rubio-Stipec M, Wells JE, Wickramaratne PJ, Wittchen H-U, Yeh E-K. Cross-national epidemiology of major depression and bipolar disorder. Journal of the American Medical Association. 1996;276:293–299. [PubMed] [Google Scholar]
- Young AW, Rowland D, Calder AJ, Etcoff NL, Seth A, Perrett DI. Facial expression megamix: tests of dimensional and category accounts of emotion recognition. Cognition. 1997;63:271–313. doi: 10.1016/s0010-0277(97)00003-6. [DOI] [PubMed] [Google Scholar]
- Young R, Biggs J, Ziegler V, Meyer D. A rating scale for mania: reliability, validity, and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]