Abstract
The core of human language, which differentiates it from the communicative abilities of other species, is the set of combinatorial operations called syntax. For over a century researchers have attempted to understand how this essential function is organised in the brain. Here we combine behavioural and neuroimaging methods, with left hemisphere-damaged patients and healthy controls, to identify the pathways connecting the brain regions involved in syntactic processing. In a previous fMRI study (Tyler et al. 2010b) we established that regions of left inferior frontal cortex and left posterior middle temporal cortex were activated during syntactic processing. These clusters were used here as seeds for probabilistic tractography analyses in patients and controls, allowing us to delineate, and measure the integrity of, the white matter tracts connecting the frontal and temporal regions active for syntax. We found that structural disconnection in either of two fibre bundles - the arcuate fasciculus or the extreme capsule fibre system - was associated with syntactic impairment in patients. The results demonstrate the causal role in syntactic analysis of these two major left hemisphere fibre bundles - challenging existing views about their role in language functions, and providing a new basis for future research in this key area of human cognition.
Keywords: diffusion tensor imaging, connectivity, tractography, stroke, grammar
Syntax lies at the heart of the human language faculty, providing the structural principles that make it possible for us to combine words into meaningful sentences. Neuropsychological and neuroimaging studies show that this core function, unique to humans, involves a primarily left-lateralized fronto-temporal network (Dapretto and Bookheimer 1999; Tyler and Marslen-Wilson 2008; Snijders et al. 2009). Mainly focused on cortical grey matter, this research shows how frontal and temporal regions are modulated by the demands of syntactic processing in language comprehension and production.
The structural connections between anterior and posterior language regions via white matter fibre tracts, although known for more than 100 years (Wernicke 1874; Dejerine 1895), were largely neglected until Geschwind (1965) revived Wernicke’s ‘disconnection’ framework, in which language deficits could arise from disconnections between distant cortical regions. Within Geschwind’s model the main emphasis was on the left arcuate fasciculus (AF), a large fibre bundle running dorsally along the frontal, inferior parietal, and perisylvian temporal cortices, connecting the classical language regions of Broca (left inferior frontal gyrus; LIFG) and Wernicke (left posterior superior and middle temporal gyrus; LpSTG/LpMTG). The AF was thought to be the primary tract supporting speech production, linking phonological representations in posterior temporal cortex to articulatory-motor representations in inferior frontal and motor cortex, a view which has carried through into recent models of the neural language system (Hickok and Poeppel 2000; Hickok and Poeppel 2004; Hickok and Poeppel 2007).
Advances in in vivo tractography using diffusion-weighted MR imaging (Schmahmann et al. 2007; Frey et al. 2008), building on earlier work with radioisotope tract tracing methods in the macaque (e.g. Petrides and Pandya 1988), have recently highlighted the potential importance of a second major fronto-temporal pathway, termed the extreme capsule fibre system (EmC). The EmC, like the AF, provides a direct structural link between anterior and posterior language cortices, running ventrally rather than dorsally along the superior temporal gyrus to connect inferior frontal, temporal and inferior parietal cortices (Makris and Pandya 2009). This ventral pathway is argued to play a key role in language comprehension (Saur et al. 2008), complementing the role of the dorsal AF pathway in language production. The research we present here challenges this emerging orthodoxy. It uses a unique combination of behavioural, functional, and structural neuroimaging techniques, applied to both healthy and brain-damaged groups, to show that an intact AF and EmC are both required to support the syntactic processes underpinning language comprehension, and that the role of the AF is by no means limited to language production.
As a direct test of the functional significance of the AF and the EmC in syntactic analysis (Caplan et al. 1996; Hagoort 2003; Hagoort 2005; Grodzinsky and Friederici 2006; Tyler and Marslen-Wilson 2008; Tyler et al. 2010a), given their likely roles as the primary mediators of information transfer between anterior and posterior language areas, we ask whether disruption to these tracts, either together or singly, is associated with deficits in syntactic comprehension. Seeds for a probabilistic tractography analysis were taken from a previous fMRI study (Tyler et al. 2010b) investigating the neural regions involved in the semantic and syntactic analyses of spoken sentences. Both patients and healthy age-matched controls participated in this study, providing both activation and behavioural data associated with syntactic analysis. Consistent with previous studies (Tyler et al. 2010a; Tyler et al. 2011) the controls showed significant clusters of activation in LpMTG and LIFG for stimuli that loaded on syntactic analysis, with the LIFG activity consisting of one cluster primarily located in BA45 and another primarily in BA44. Tracking between these clusters served to identify the major tracts which are likely to support syntactic performance in the normal system. Following classical neuropsychological inference, evidence from patients with damaged tracts tests the validity of this model. To do this requires behavioural evidence about levels of impairment in patients’ syntactic performance. We obtained these data from two sources: a word monitoring task and a sentence-picture matching task, both of which have been used extensively to assess syntactic deficits in aphasic patients (Saffran et al. 1980; Ostrin and Tyler 1995; Berndt et al. 1996; Berndt et al. 2004b; Mitchum et al. 2004; Tyler et al. 2010b). We related behavioural performance on these two tasks to tract integrity across patients and controls in order to assess the role of fronto-temporal structural connectivity in syntactic comprehension. This constitutes a strong and direct test of whether the dorsal and ventral fronto-temporal white matter pathways are functionally critical for this key cognitive function.
Methods
Participants
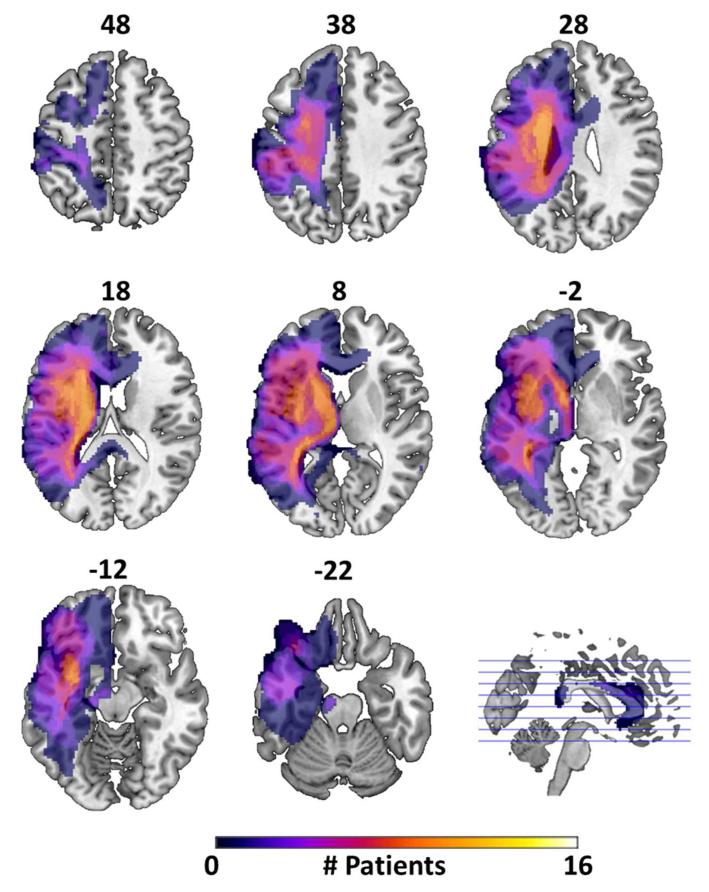
Sixteen patients (aged 33-76 years; 5 female) and fourteen healthy controls (aged 58-66 years; 8 female) participated. All controls were neurologically normal, right-handed British English speakers, and were free of psychoactive medication for at least 1 year prior to the time of testing. Patients were recruited from our panel of volunteers and from local stroke groups. All were stable at time of testing and had been discharged from hospital a minimum of 1.3 (mean 7.5) years prior to participation. They all gave informed consent (Addenbrookes NHS Trust Local Research Ethics Committee) and understood task instructions, were right handed prior to stroke, had lesions restricted to the left hemisphere, and showed no MR contraindications or artefacts on functional images. 14 patients’ lesions were caused by stroke (in each case the patient’s first) and 2 were due to excised tumours. Figure 1 shows the distribution of lesion locations for the patients, which cover large portions of the left insula, basal ganglia, inferior and middle frontal gyri, superior and inferior parietal lobule, and superior and middle temporal gyri (see also ‘lesion detection’ section below). Functional imaging data from the patients used in this study, and details of their language abilities across a range of language tests, has been reported previously (Tyler et al. 2010b).
Figure 1. Lesion Frequency Map.
Axial slices showing extent and variability of lesion location within the patient group, as defined by the automated lesion detection method of Stamatakis & Tyler (2005). Numbers above each slice give its z coordinate in MNI space. Colour bar indicates number of patients whose lesions fell within that region.
Behavioural stimuli and tasks
We used two tasks to measure syntactic and semantic processing: (a) Word monitoring study: The word monitoring task (Marslen-Wilson and Tyler 1975; Marslen-Wilson and Tyler 1980) provides a behavioural measure of the ability to construct on-line syntactic and semantic representations of spoken language, and has been used extensively to test language performance in a variety of special populations (Hodges et al. 1994; Tyler et al. 2010b). Many studies have shown that patients with brain damage can reliably perform the task; it elicits fast RTs and few errors (Tyler 1992; Hodges et al. 1994). In the experiment, participants heard 3 different types of spoken sequences in which the availability of syntactic and semantic sentential information was manipulated: Normal Prose (NP), which has both grammatical structure and sentential meaning (e.g. “He was trying to find the name of the TREE he planted last year”), Anomalous Prose (AP), which has grammatical structure but lacks sentential meaning (e.g. “She was writing to use the college of a FISH she opened last week”), and Random Word Order (RWO), with no grammatical structure or sentential meaning (e.g. “Use was college a to writing she of ROAD last opened she week”). There were 30 items in each condition, presented in a blocked design to avoid frequent task-switching, which may introduce confounding task-related cognitive demands in patients.
Listeners were instructed to press a response key when they heard a pre-specified target word (in capitals in the examples above) in a sequence. Target words (and pictures denoting the same concept) were presented visually and remained on the screen throughout the trial to reduce working memory demands. Target words could occur either early or late in the sentence. Reaction times were recorded from the onset of each target word. The difference in reaction times to targets occurring late and early in the sentence, which we refer to as the word position effect (WPE; (Marslen-Wilson and Tyler 1975; Tyler 1992), was used as a behavioural measure of sentence comprehension. Previous studies have established that in NP, faster reaction times for later word positions (resulting in a significant WPE) reflects the on-line construction of a meaningful representation spanning the sentence, whereas significant WPEs for AP reflect the on-line development of syntactic representations without the contribution of sentential or pragmatic meaning. In RWO, RTs are not facilitated consistently (and may even be slowed down) by later-occurring target words, due to the absence of a coherent syntactic or semantic analysis of the sentence (Marslen-Wilson and Tyler 1980; Tyler 1992). This contrast between the lack of a WPE in RWO and its presence in NP and AP indicates normal comprehension performance (Marslen-Wilson and Tyler 1975; Marslen-Wilson and Tyler 1980; Tyler 1992).
(b) Sentence-picture matching task: As a second behavioural measure of sentence comprehension, we used a sentence-picture matching test (Tyler et al. 2002; Tyler et al. 2004). In this test, which is routinely used to assess syntactic deficits in aphasic patients (Caramazza and Zurif 1976; Saffran et al. 1980; van der Lely and Harris 1990; Berndt et al. 1996; Berndt et al. 2004; Mitchum et al. 2004), subjects hear a sentence, either in the active or the passive voice, which describes two participants engaged in an action (e.g., “The man hugs the woman” or “The woman is hugged by the man”), which they are asked to compare against a set of three line drawings. The 34 sentences are all semantically ‘reversible’, meaning that either person mentioned can potentially perform the action. Only one picture is correct. The two ‘foil’ pictures either contain a lexical distracter involving a change of meaning (e.g. a picture of a man painting a woman), or a reverse role distracter in which the agent of the action becomes its recipient (e.g. a picture of a woman hugging a man). When a patient makes reverse role errors and few lexical distracter errors, this indicates difficulties with syntax in the presence of intact semantics (Tyler et al. 2002; Tyler et al. 2004). In keeping with previous neuropsychological research, we expect passive sentences to generate more reverse role errors since they cannot be interpreted using a canonical word order strategy.
Imaging acquisition and analyses
Participants were scanned at the MRC Cognition and Brain Sciences Unit, Cambridge, in a Siemens 3T Tim Trio MRI scanner (Siemens Medical Solutions, Camberley, UK). T1-weighted structural images were acquired at 1mm isotropic resolution in the sagittal plane, using an MPRAGE sequence with TR=2250ms, TI=900ms, TE=2.99ms and flip angle=9°. Diffusion-weighted images were acquired in 64 directions with two averages, with TR=6.5s, TE=93ms, b=1000 s/mm3 and GRAPPA parallel reconstruction (acceleration factor=2). Each volume consisted of 48 slices in the intercommissural plane, 2.5 mm thick with 0.5mm gap, with an in-plane resolution of 1.8mm and FOV = 230mm × 230mm.
T1-weighted images were processed in SPM5 (Wellcome Trust Centre for Neuroimaging, London, UK). Unified, nonlinear normalisation was used with increased regularization, which has given reliable normalisation in previous studies on lesioned brains (Tyler et al. 2005; Crinion et al. 2007). Assessment was carried out by visually inspecting the spatially normalized images. In two patients, the algorithm did not fit the image to the template, probably because of misclassification of the dura as grey matter during the segmentation stage. The images of these two patients were re-normalised using standard regularization and cost function masking (Brett et al. 2001).
Lesion detection
We used an automated procedure (Stamatakis and Tyler 2005) to delineate the sites of damaged tissue. For each patient, the spatially normalized, skull-stripped T1-weighted structural image was smoothed using a Gaussian kernel (10mm FWHM) and entered into a two-sample t-test with a set of images from twenty age-matched controls. Voxels were classified as damaged if their T1 signal intensity was significantly lower in the patients than controls, having accounted for global signal differences. To avoid classifying enlarged sulci near intact tissue as lesion, voxel-level and cluster size thresholds were adjusted on an individual basis. For one patient, whose lesion was caused by subcortical haemorrhage (which shows up poorly on T1-weighted images, but prominently on the mean T2-weighted EPI images as a region of reduced signal), we used the same lesion detection procedure but on the mean functional image. With this modified method we were able to automatically delineate this patient’s lesion. Lesions detected in this way were binarized and summed to produce the lesion overlap image shown in Fig. 1, as well as the lesion surface renderings in Fig. 4.
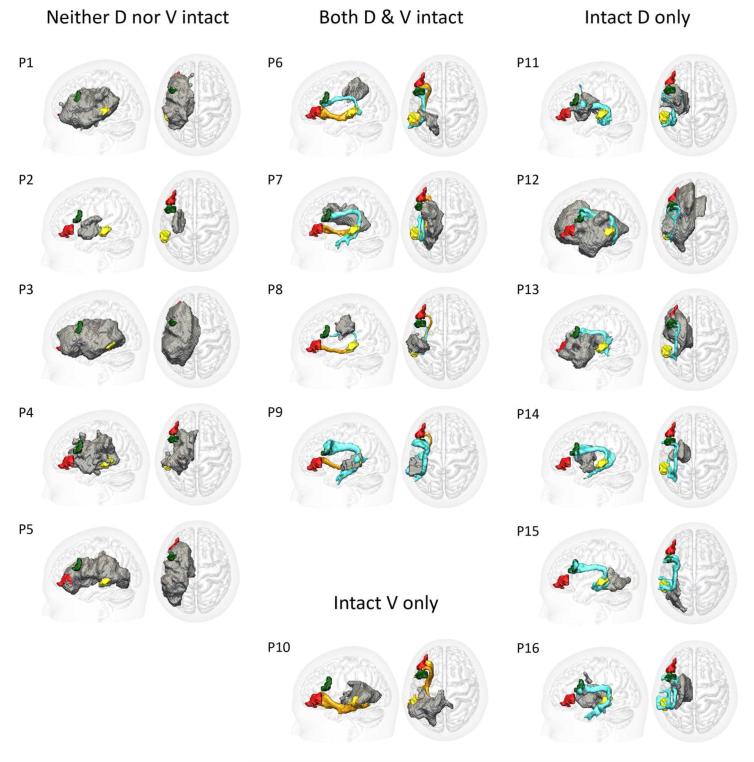
Figure 4. Patients’ Tracts.
Surface renderings of individual tractography reconstructions in the left hemisphere for each of the 16 patients. Grey regions indicate the outer surface of the lesioned tissue, as defined by the automated lesion detection method of Stamatakis & Tyler (2005).
Tractography
Diffusion data were analysed using a combination of tools from the FSL (Smith et al. 2004; Behrens et al. 2007) and SPM software packages. Eddy current correction, skull stripping, tensor fitting, and probabilistic tractography were all carried out using programs from FSL’s diffusion toolkit (FDT). Mappings between diffusion space and MNI space were generated by concatenating the transform generated from affine registration of the least diffusion weighted image (b0) to the T1 image with the transform generated from unified segmentation and normalization of the T1 image in SPM5. These mappings were used to transform the fMRI clusters to each subject’s native diffusion space, where they were used as seeds in two 2-region-of-interest (ROI) probabilistic tractography analyses. Algorithmically, this type of analysis proceeds by propagating streamlines (representing potential fibre bundle trajectories) through the 3D image volume by sampling from the local probability distribution of (confidence in estimates of) fibre orientations at an initial seed voxel, then following the sampled direction on to the next voxel and repeating the process until a stopping criterion is reached. Repeating this process multiple (5000, in our case) times for each voxel in the seed ROI results in a global connectivity or ‘path’ distribution, in which intensities represent the number of streamline visitations at each voxel, and the confidence bounds on the spatial location of the most probable single connection (Behrens et al. 2003). When a 2-ROI approach is used, separate path distributions are generated from the first and then from the second seed ROI, both with the added constraint that only those streamlines initiated from the first ROI that reach a voxel in the second ROI (or vice versa) and do not enter any exclusion masks are retained; the output is then the sum of these two distributions.
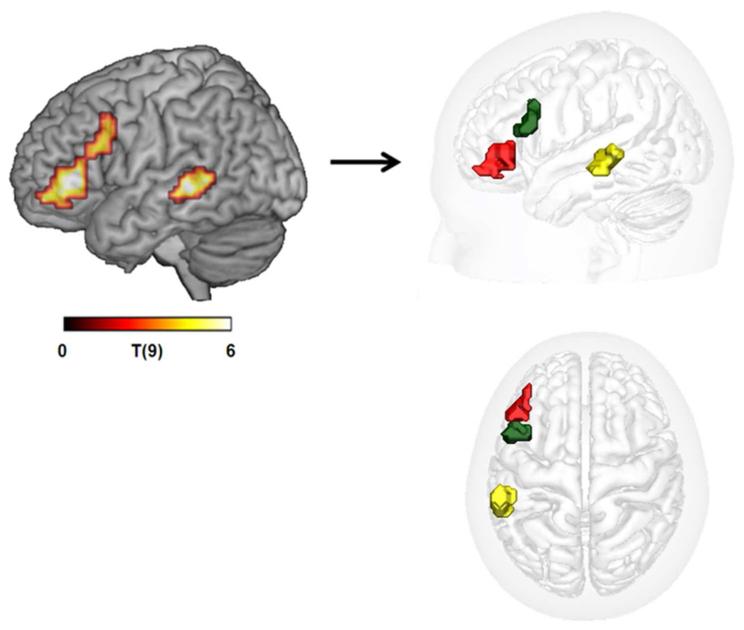
The seeds used were defined from clusters of activation obtained for (a different group of) control participants in a previous fMRI study (Tyler et al. 2010b), where the contrast of AP-RWO revealed 3 regions of activation (voxel level p>.001, cluster level corrected at .05) that were maximally sensitive to syntactic processing over and above activity accruing from individual words (for task details see ‘Word Monitoring Study’ above). These 3 regions, all in the LH, included IFG BA44, IFG BA45, and pMTG 21/222.
Because the focus here is on within-hemisphere left fronto-temporal connections, we used a single sagittal slice at the midline as an exclusion mask, which has the effect of rejecting any streamlines entering the opposite hemisphere from the final output path distributions. Additional exclusion masks were also used in a second set of tractography analyses, which allowed us to separate the AF and EmC components of the fibre tracts connecting the LIFG and LpMTG seeds (see Results for details). For each tractography reconstruction, the path distribution images were thresholded at 0.1×10−4 times the total number of streamlines used to generate the distribution (# voxels in LpMTG seed × 5000 + # voxels in LIFG seed × 5000). This threshold was selected empirically on the basis of the control group data, it being the highest (most stringent) value that still showed maximal agreement in the reconstructed pathways across all control subjects.
This general fMRI+DWI procedure, whereby fMRI-derived regions of interest are used to define seeds for DWI fibre tracking, has been used in a number of recent studies investigate the white matter architecture of the neural language system (e.g. Hagmann et al. 2006; Powell et al. 2006; Saur et al. 2008). The present study is, however, the first to our knowledge to employ this technique in conjunction with neuropsychological, structural, and functional imaging data to assess the relationship between fronto-temporal connectivity and syntactic structure.
As a complementary measure of potential anatomical disconnection in the patients, we examined white matter microstructural integrity, as indexed by the standard four tensor-derived scalar metrics: fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD). These quantities were measured at the stereotaxic locations of the AF and EmC using a recently developed method for constructing probabilistic templates from group tractography data (Hua et al. 2008; see also Thiebaut de Schotten et al., 2008). Spatially normalized tractography images from the control group for each of the reconstructed tracts were thresholded as described above, binarized, and averaged. The resulting group ‘tract probability map’ had voxel intensities ranging from 0 (tract passed through that location in zero subjects) to 1 (tract passed through that location in all subjects). These maps were then used as templates for extracting values from each subject’s spatially normalised FA, MD, AD, and RD images. Following Hua et al. (2008), we scaled the contribution of each voxel using the equation
where Pi is the intensity of the tract probability map at voxel i and FAi is the intensity of the FA (and similarly for MD, AD, and RD) image at the corresponding voxel. The effect of applying this formula is to weight more heavily those core white matter areas that are consistent across subjects, and thus most likely to belong to the tract of interest. This template approach allows us to obtain a measure of the white matter integrity within each respective pathway, and therefore to ascertain whether the lack of tracts seen in some patients’ tractography results are due simply to damage to the seeds, or to the core white matter structures themselves.
Results
(a) Tractography
We obtained functionally relevant seeds for probabilistic tractography from the fMRI analyses described in Tyler et al. (2010b), where the contrast of AP-RWO, which highlights syntactic processing functions as distinct from the processing of the phonology and meaning of individual words, identified significant clusters of activation in left inferior frontal cortex (BA44 and BA45) and left posterior temporal cortex (BA 21/22). The tractography results for the healthy controls (Fig. 3), based on these functionally determined seeds, demonstrate the involvement of two major dorsal and ventral pathways, as hypothesized earlier. The first of these exits the LpMTG cluster medially to join the AF as it arches around the caudal end of the sylvian fissure, then course through the white matter beneath the inferior parietal lobe and motor cortices before entering the LBA44 cluster at its most posterior and medial aspect. The second pathway exits the LpMTG cluster at its most anterior and medial aspect to join the EmC fibre bundle as it passes across the deep white matter of the temporal lobe and behind the posterior bank of the medial insula, then heads anteriorly along the extreme/external capsule conduit before arching around the anterior bank of the medial insula and extending laterally through orbito- and inferior-frontal (pars triangularis) areas to reach the BA45 cluster. The ventral pathway was never observed for the LpMTG←→LBA44 seed pair. However, in a small number of subjects, initial tractography results for the LpMTG←→LBA45 seed pair picked up part of the same dorsal pathway invariably seen in the LpMTG←→LBA44 tractography. Similarly, these subjects also showed dorsal tracts for the LpMTG←→LBA44 pair that continued past the LBA44 cluster along the white matter beneath the lateral frontal cortex to reach the LBA45 cluster. Such inter-subject variability in the frontal extent of the AF is consistent with recent reports (Frey et al. 2008; Glasser and Rilling 2008; Rilling et al. 2008). However, because this variability was not the focus of our investigation, and because we wanted to obtain quantitative measurements from the EmC without contamination from the AF, we opted to separate the two pathways at the tractography stage. To do this, we re-ran the LpMTG←→LBA45 tractography twice, with two additional waypoint and exclusion masks. The first was placed in the inferior parietal white matter, following the AF tractography reconstruction protocol described by Catani et al. (2008). The second was placed in the medial insula white matter, following the EmC reconstruction protocol described by Makris & Pandya (2009). The two new ROIs were defined in standard space, and transformed to native diffusion space in the same manner as the fMRI-derived seeds. They were both designed to cover the intended territories generously, so as to allow for imprecision when moving from standard to native space.
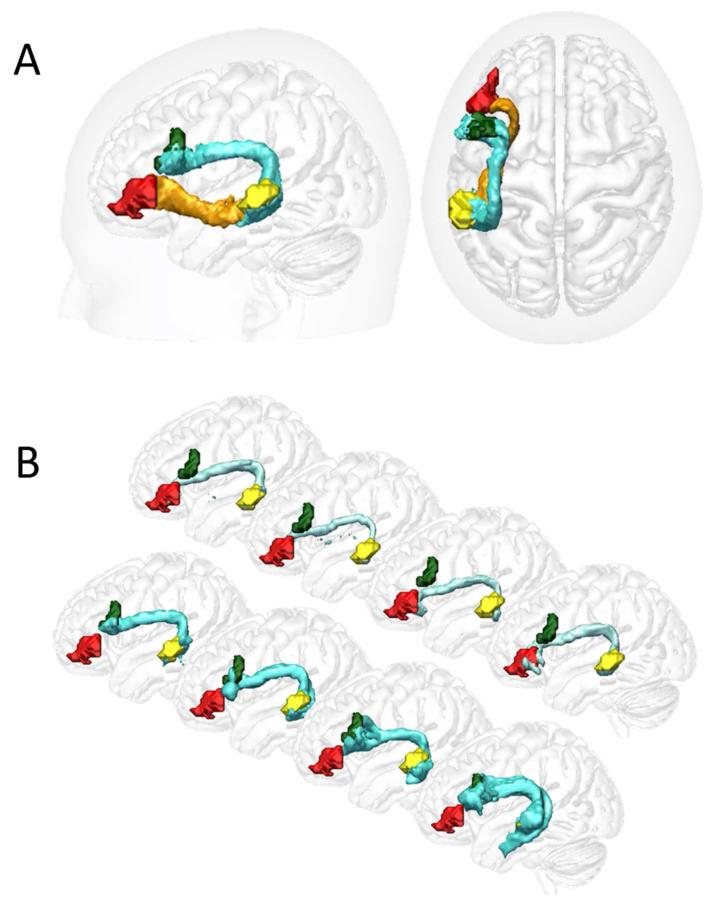
Figure 3. Control group tracts.
Surface renderings of dorsal (AF; light blue) and ventral (EmC; orange) pathways. A) Group average pMTG←→BA44 dorsal and pMTG←→BA45 ventral pathways. B) Renderings of the two dorsal pathways (pMTG←→BA44; A, and pMTG←→BA45) in four example subjects.
The first tractography re-run aimed to isolate the AF component of the original LpMTG←→LBA45 path distribution by adding the inferior parietal ROI as a waypoint mask and the medial insula ROI as an exclusion mask. Conversely, the second re-run isolated the EmC component by adding the inferior parietal ROI as an exclusion mask and the medial insula ROI as a waypoint mask. In those individuals where both dorsal-going and ventral-going streamlines were seen in the original LpMTG←→LBA45 path distribution, this procedure cleanly separated the two, whilst otherwise showing exactly the same structures as in the original tractography analysis. Examples of subjects where the dorsal LpMTG←→LBA45 pathway was observed are shown in Fig. 3. Our focus for the remainder of this paper will however be on the two robustly observed tracts: the dorsal AF pathway subtending the LpMTG←→LBA44 connection, and the LpMTG←→LBA45 connection subtended by the ventral EmC pathway. Every control subject showed this two-tract pattern. The patients, in contrast, presented a variable pattern (Fig. 4), falling into four groups on the basis of their tractography reconstructions: (i) both pathways were intact (4 out of 16 patients), (ii) intact dorsal but no ventral pathway (6 patients); (iii) intact ventral but no dorsal pathway (1 patient), and (iv) neither pathway intact (5 patients).
We interpreted cases where tractography in patients failed to identify either the dorsal LpMTG←→LBA44 or the ventral LpMTG←→LBA45 pathways that were robustly observed in all controls as reflecting a structural disconnection between the frontal and temporal regions in question, resulting from damage to some part of the subtending pathway. Such results, however, could potentially be due to an overlap of the lesions and seed ROIs - in which case it would be damage to the seed regions, rather than to their anatomical connections per se, that determines whether or not a given connection is successfully identified. Inspection of individual patients’ structural images confirmed that this possibility was not sufficient to explain the majority of the tractography results. Not only were there patients with high % seed/lesion overlap but who nevertheless showed intact connecting pathways (e.g. Patients 10, 12, and 13 in Fig. 4); there were also patients displaying disconnections who showed zero seed/lesion overlap (e.g. Patients 2 and 14 in Fig. 4).
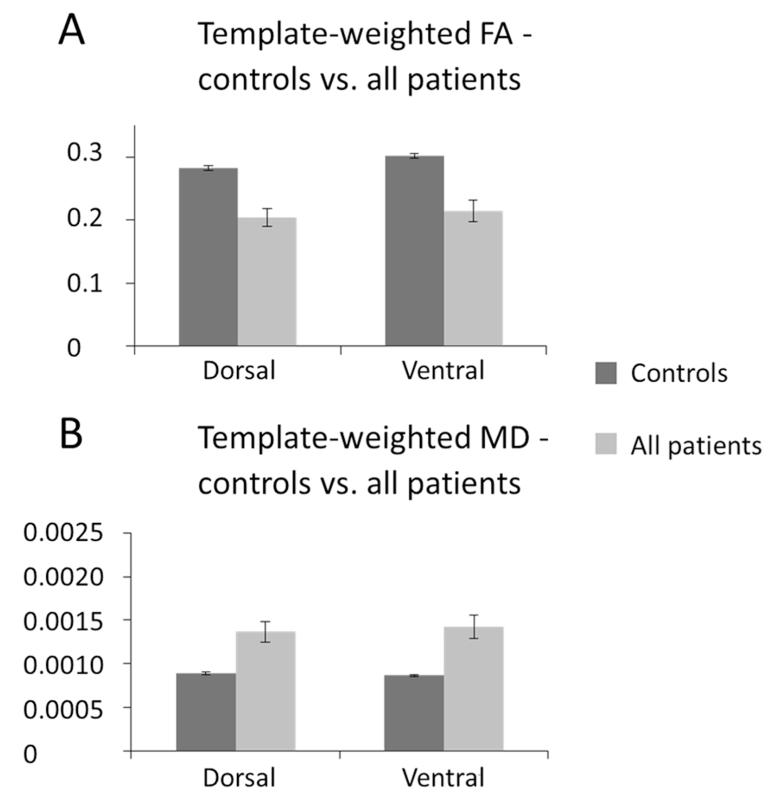
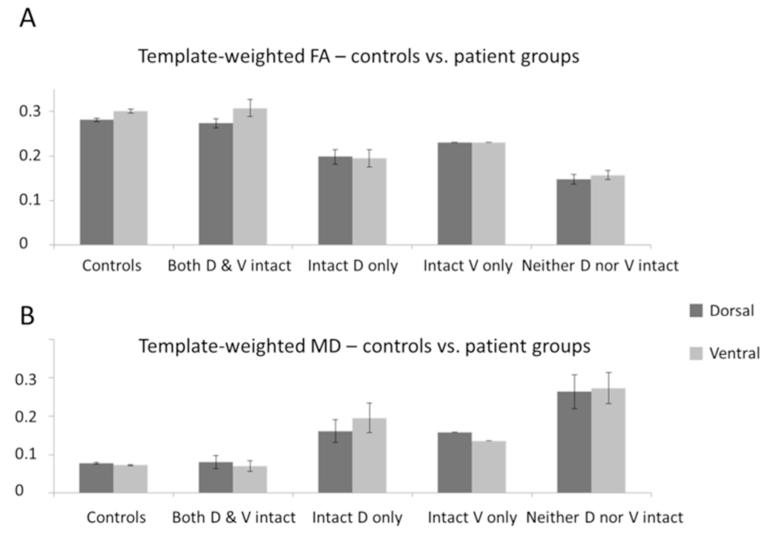
We further investigated the possibility that the disconnections observed in tractography might not be due to AF/EmC damage using the tract template method of Hua et al. (2008). This analysis provided voxelwise measures of white matter integrity at the anatomical locations normally occupied by the two tracts. Results (Fig. 5) showed clearly that white matter integrity in both the dorsal and ventral pathways was significantly reduced in patients compared to controls, as indexed by a lower FA values (dorsal t=5.3, p<0.001; ventral t=4.89, p<0.005) and higher MD values (dorsal t = -3.88,p<0.005; ventral t =−4.15, p<0.001). This was accompanied by significant increases in both AD (dorsal t =−3.7, p<0.005; ventral t =−3.96, p<0.005) and RD (dorsal t=−3.99, p<0.005; ventral t=−4.38, p<0.005), indicating a general reduction in the prevalence of structures such as axonal membranes, neurofilaments, or glial cells that normally impede water diffusion in neural tissue (Beaulieu 2002). Furthermore, when the data were broken up into the four sub-groups defined from fibre tracking results in the patients (Fig. 6), the tract MD values followed the pattern that would be predicted from the tractography results - namely lower MD in the dorsal than the ventral pathway for the ‘intact dorsal but no ventral’ group, and vice versa for the ‘intact ventral but no dorsal’ group. This is consistent with the claim that the fronto-temporal ‘disconnections’ observed in the fibre tracking data of individual patients are associated with (deep) white matter damage, rather than simply lesions in the seed regions.
Figure 5. Tract Template Analyses – controls vs. all patients.
Mean FA (A) and MD (B) values weighted by tract probability, extracted from dorsal pMTG←→BA44 (AF) and ventral pMTG←→BA45 (EmC) pathway templates following the method of Hua et al. (2008). Controls show significantly higher FA and lower MD than patients within both the AF and EmC template.
Figure 6. Tract Template Analyses – controls vs. patient groups.
Mean FA and MD values weighted by tract probability, extracted from dorsal pMTG←→BA44 (AF) and ventral pMTG←→BA45 (EmC) pathway templates, for the control and each of the four patient groups. As predicted by the individual tractography results, patients with an intact dorsal but no ventral connection showed higher MD in the EmC than the AF, whereas patients with intact ventral but no dorsal connections showed higher MD in the AF than the EmC.
b) Behavioural measures of syntactic impairment and relation to tract status
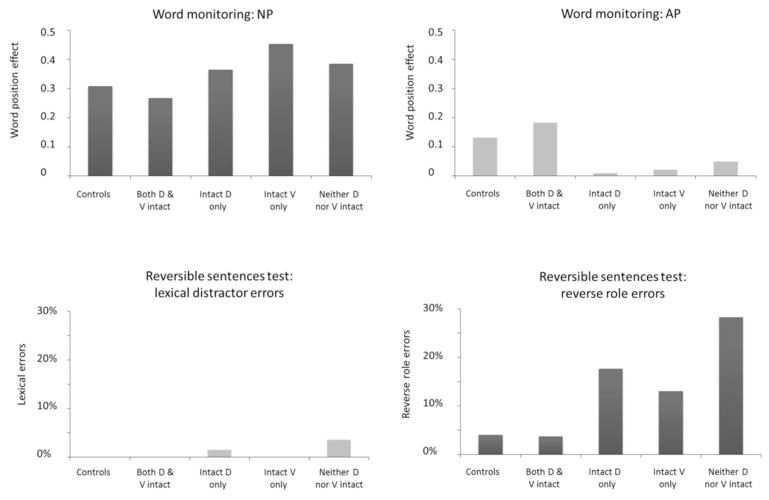
The results of the two sets of behavioural measures (word-monitoring and sentence-picture matching) are presented in Fig. 7, broken down into the control scores and the patient scores in each of the four tract status groups (as listed above). The two graphs on the right hand side of the figure (AP word position effect in word monitoring; upper right, and reverse role errors in sentence-picture mapping; lower right) show performance on the subsets of each task that tap specifically into syntactic processing of spoken sentences. Each Patients’ performance was highly similar across the two tasks, with WPE scores for AP being significantly (negatively) correlated with increased number of reverse role errors in the sentence-picture matching task (r=−0.63, p<.01). Similarly, the control participants and the patients with both tracts intact showed comparable WPE scores for AP, as well as making very few (<10%) reverse role errors in the sentence-picture mapping task.
Figure 7. Behavioural Data.
Top row: Averaged word position effect (WPE) on normal prose (top left) and anomalous prose (top right) conditions in the word monitoring study, shown for the controls and each of the tract status-based patient groups. Bottom row: Average percent semantic (lexical distracter; bottom left) and syntactic (reverse role; bottom right) errors in the sentence-picture matching task. The controls and patients with both tracts show a large AP WPE in the word monitoring study and make few syntactic errors in the sentence-picture matching task, both indicating good syntactic comprehension. D=dorsal (pMTG←→BA44) tract, V = ventral (pMTG←→BA45) tract.
In contrast, patients with either one or both connections disrupted showed reduced or absent WPEs in AP, and made significantly more syntactic errors on the sentence-picture matching task (range of reverse role errors: 13-28%). These parallels between the two types of behavioural assessment establish the generality of the patients’ syntactic deficits. Patients with disrupted tracts differed from normals on both tests, with smaller WPE in AP and increased syntactic errors on the sentence-picture matching task. This held true irrespective of which tract was damaged. For both behavioural measures, disruption of either one or both connections gave a 75% probability of having a syntactic deficit. This difference in degree of syntactic deficit in patients with intact as opposed to damaged tracts was significant (χ2(1) = 6.86, p<.05). No significant deficit was seen however for the other portions of the tasks (Fig. 7, left hand side) where performance did not critically depend on intact capacities for syntactic analysis. Patients with damaged tracts made fewer semantic (lexical distracter) than syntactic errors on the sentence-picture matching task, indicating that syntax suffers more from damage to these connections than does semantic processing. Similarly, processing of NP sentences seemed unaffected by tract damage, with patients and controls all producing comparable WPEs in NP, where they can use individual word meanings and their pragmatic implications to construct a representation of the sentence.
Discussion
This study examined the role in syntactic comprehension of the two major white matter tracts – the arcuate fasciculus and the extreme capsule fibre system - that provide direct connections between the posterior and anterior cortical regions that are critical for language function. We obtained functional seeds for tractography analyses from an fMRI study in which both patients and controls had participated (Tyler et al. 2010b), and related the presence/absence of white matter tracts connecting these regions and their structural integrity to performance on two tests of syntactic processing. Behavioural data from both tests showed similar patterns of syntactic deficits and demonstrated that the degree of syntactic impairment in each patient was closely related to variations in their tract status. Strikingly, the patients with both tracts intact did not differ from normal controls, either in terms of size of anomalous prose WPE or in the percentage of syntactic errors on the sentence-picture matching task, even in the presence of substantial LH lesions. Additional analyses established that the disconnections observed in tractography were indeed associated with white matter damage, and were not artefacts caused by damage in the seed regions.
Confirmation that disruptions to these fronto-temporal connections had a disproportionately larger effect on syntax than other aspects of spoken language comprehension came from additional data provided by the word monitoring and sentence-picture matching studies. Patients in each tract group showed a normal WPE in normal prose in the word monitoring study and made few semantic errors in the sentence-picture matching task, suggesting that their ability to process the semantics of sentences was significantly less disrupted than their ability to process syntax. The combination of data from brain-damaged patients, linking variation in tract integrity with the preservation of syntax, together with the parallel data from healthy participants, provides the critical conditions for determining which specific pathways play a necessary role in syntactic analysis during language comprehension (Chatterjee 2005). These results challenge several recent claims that assign disjoint roles to AF and EmC pathways in language function. The results are inconsistent with the view that the AF is involved only in language production (Hickok and Poeppel 2007), that the EmC takes the primary role in language comprehension (Saur et al. 2008), or that only the AF is involved in complex syntactic analysis, with the EmC supporting semantic processes (Friederici 2009). Instead, we see that both dorsal and ventral left hemisphere pathways are necessary for successful syntactic comprehension, a finding which emphasizes the critical role of left fronto-temporal connectivity in syntactic analysis. As we have recently argued (Rolheiser et al. 2011), this synergistic relationship between the two pathways in syntactic analysis is likely to extend to all levels of linguistic analysis, in both comprehension and production. Patients’ impairments and language activations are thus better explained by differential relative loading on the dorsal and ventral tracts, rather than a binary delegation of function to one or the other.
Previous studies have shown that lesions in either LIFG or LMTG are associated with syntactic deficits (Caplan et al. 1996; Tyler and Marslen-Wilson 2008). The present results document the anatomical underpinnings of the functional relationship between these two regions, with communication between them being necessary for syntactic processing. In contrast to their key role in syntactic analysis, damage to either AF or EmC pathways did not significantly disrupt the patients’ ability to construct a semantic representation of the sentences, with all patients showing a normal word position effect in NP, and preserved semantic function in other tasks (see Fig. 7). This is consistent with claims we have made elsewhere (Bozic et al. 2010) for the separability of specifically linguistic processes, requiring the intact LH perisylvian system, from more distributed, bihemispheric systems for interpreting sensory inputs (including speech) in their semantic and pragmatic contexts. Word monitoring in NP sentences reflects listeners’ ability to rely primarily on the combined meanings of individual words and their pragmatic implications, with a lesser contribution of syntax. We suggest that syntactic analysis requires both information flow between LpMTG and frontal cortex and integration within sub-regions of LIFG, in order to link the different kinds of combinatorial processes involved in syntactic analysis. This is inconsistent with a focus on syntax-specific regions acting within the IFG complex, and more in keeping with network approaches to cognitive functions (Tyler and Marslen-Wilson 2008; Tyler et al. 2010a), providing a framework for merging the many fMRI studies which show activity in BA44, BA45 and/or BA47 during syntactic comprehension (e.g. Friederici et al. 2003; Musso et al. 2003; Tyler et al. 2010b).
Figure 2. Left posterior middle temporal and two left inferior frontal clusters activated in the AP-RWO contrast in the fMRI study.
These three regions were used as seeds for tractography. Left: colourmap showing T-score activation values for AP-RWO contrast. Right: Binary masks of the three separated clusters: BA45 (red), BA44 (green), pMTG (yellow).
Acknowledgements
This research was supported by a Medical Research Council (UK) programme grant (grant number G0500842 to LKT) and Medical Research Council Cognition and Brain Sciences Unit funding (U.1055.04.002.00001.01 to WMW). EAS is supported by the Stephen Erskine Fellowship, Queens’ College, Cambridge. We thank Paul Wright for his help with the fMRI study.
References
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. Nmr in Biomedicine. 2002;15(7-8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Johansen-Berg H, Jbabdi S, Rushworth MFS, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34(1):144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn. Reson. Med. 2003;50(5):1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Berndt RS, Mitchum C, Burton M, Haendiges A. Comprehension of reversible sentences in aphasia: The effects of verb meaning. Cognitive Neuropsychology. 2004;21(2-4):229–245. doi: 10.1080/02643290342000456. [DOI] [PubMed] [Google Scholar]
- Berndt RS, Mitchum CC, Haendiges AN. Comprehension of reversible sentences in ‘agrammatism’: a meta-analysis. Cognition. 1996;58(3):289–308. doi: 10.1016/0010-0277(95)00682-6. [DOI] [PubMed] [Google Scholar]
- Bozic M, Tyler LK, Ives DT, Randall B, Marslen-Wilson WD. Bihemispheric foundations for human speech comprehension. Proc. Natl. Acad. Sci. U. S. A. 2010;107(40):17439–17444. doi: 10.1073/pnas.1000531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage. 2001;14(2):486–500. doi: 10.1006/nimg.2001.0845. [DOI] [PubMed] [Google Scholar]
- Caplan D, Hildebrandt N, Makris N. Location of lesions in stroke patients with deficits in syntactic processing in sentence comprehension. Brain. 1996;119(3):933–949. doi: 10.1093/brain/119.3.933. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Zurif EB. Dissociation of Algorithmic and Heuristic Processes in Language Comprehension - Evidence from Aphasia. Brain Lang. 1976;3(4):572–582. doi: 10.1016/0093-934x(76)90048-1. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. A madness to the methods in cognitive neuroscience? J. Cogn. Neurosci. 2005;17(6):847–849. doi: 10.1162/0898929054021085. [DOI] [PubMed] [Google Scholar]
- Crinion J, Ashburner J, Leff A, Brett M, Price C, Friston K. Spatial normalization of lesioned brains: performance evaluation and impact on fMRI analyses. Neuroimage. 2007;37(3):866–875. doi: 10.1016/j.neuroimage.2007.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapretto M, Bookheimer SY. Form and content: Dissociating syntax and semantics in sentence comprehension. Neuron. 1999;24(2):427–432. doi: 10.1016/s0896-6273(00)80855-7. [DOI] [PubMed] [Google Scholar]
- Dejerine J. Anatomie des centres nerveux. Rueff et Cie; Paris: 1895. [Google Scholar]
- Frey S, Campbell JS, Pike GB, Petrides M. Dissociating the human language pathways with high angular resolution diffusion fiber tractography. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28(45):11435–11444. doi: 10.1523/JNEUROSCI.2388-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, Campbell JSW, Pike GB, Petrides M. Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J. Neurosci. 2008;28(45):11435–11444. doi: 10.1523/JNEUROSCI.2388-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD. Pathways to language: fiber tracts in the human brain. Trends in Cognitive Sciences. 2009;13(4):175–181. doi: 10.1016/j.tics.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Ruschemeyer S-A, Hahne A, Fiebach CJ. The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb. Cortex. 2003;13(2):170–177. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. Brain. 1965;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Rilling JK. DTI Tractography of the Human Brain’s Language Pathways. Cereb. Cortex. 2008;18(11):2471–2482. doi: 10.1093/cercor/bhn011. [DOI] [PubMed] [Google Scholar]
- Grodzinsky Y, Friederici AD. Neuroimaging of syntax and syntactic processing. Curr. Opin. Neurobiol. 2006;16:240–246. doi: 10.1016/j.conb.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Martuzzi R, Maeder P, Clarke S, Thiran JP, Meuli R. Hand preference and sex shape the architecture of language networks. Human Brain Mapping. 2006;27(10):828–835. doi: 10.1002/hbm.20224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagoort P. How the brain solves the binding problem for language: a neurocomputational model of syntactic processing. Neuroimage. 2003;20(Supplement 1):S18–S29. doi: 10.1016/j.neuroimage.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Hagoort P. On Broca, brain, and binding: a new framework. Trends in Cognitive Sciences. 2005;9(9):416–423. doi: 10.1016/j.tics.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Towards a functional neuroanatomy of speech perception. Trends in Cognitive Sciences. 2000;4(4):131–138. doi: 10.1016/s1364-6613(00)01463-7. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92(1-2):67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nature Reviews: Neuroscience. 2007;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Tyler LK. Loss of semantic memory: Implications for the modularity of mind. Cognitive Neuropsychology. 1994;11:505–542. [Google Scholar]
- Hua K, Zhang JY, Wakana S, Jiang HY, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PCM, Mori S. Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39(1):336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Pandya DN. The extreme capsule in humans and rethinking of the language circuitry. Brain Structure & Function. 2009;213(3):343–358. doi: 10.1007/s00429-008-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marslen-Wilson WD, Tyler LK. Processing structure of sentence perception. Nature. 1975;257(5529):784–786. doi: 10.1038/257784a0. [DOI] [PubMed] [Google Scholar]
- Marslen-Wilson WD, Tyler LK. The temporal structure of spoken language understanding. Cognition. 1980;8(1):1–71. doi: 10.1016/0010-0277(80)90015-3. [DOI] [PubMed] [Google Scholar]
- Mitchum CC, Haendiges AN, Berndt RS. Response strategies in aphasic sentence comprehension: An analysis of two cases. Aphasiology. 2004;18(8):675–692. [Google Scholar]
- Musso M, Moro A, Glauche V, Rijntjes M, Reichenbach J, Buchel C, Weiller C. Broca’s area and the language instinct. Nature Neuroscience. 2003;6(7):774–781. doi: 10.1038/nn1077. [DOI] [PubMed] [Google Scholar]
- Ostrin RK, Tyler LK. Dissociations of lexical function: Semantics, syntax and morphology. Cognitive Neuropsychology. 1995;12(4):345–389. [Google Scholar]
- Petrides M, Pandya DN. Association fiber pathways to the frontal cortex from the superior temporal region in the rhesus monkey. J. Comp. Neurol. 1988;273:52–66. doi: 10.1002/cne.902730106. [DOI] [PubMed] [Google Scholar]
- Powell HM, Parker GJM, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CAM, Barker GJ, Noppeney U, Koepp J, Duncan JS. Hemispheric asymmetries in language-related pathways: a combined functional MRI and tractography study. Neuroimage. 2006;32(1):388–399. doi: 10.1016/j.neuroimage.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Glasser MF, Preuss TM, Ma X, Zhao T, Hu X, Behrens TEJ. The evolution of the arcuate fasciculus revealed with comparative DTI. Nat. Neurosci. 2008;11(4):426–428. doi: 10.1038/nn2072. [DOI] [PubMed] [Google Scholar]
- Rolheiser T, Stamatakis EA, Tyler LK. Dynamic Processing in the Human Language System: Synergy between the Arcuate Fascicle and Extreme Capsule. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31(47):16949–16957. doi: 10.1523/JNEUROSCI.2725-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffran E, Schwartz E, Marin O. The word order problem in agrammatism: 11. Production. Brain Lang. 1980;10:263–280. doi: 10.1016/0093-934x(80)90056-5. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kummerer D, Kellmeyer P, Vry M-S, Umarova R, Musso M, Glauche V, Abel S, et al. Ventral and dorsal pathways for language. Proc. Natl. Acad. Sci. U. S. A. 2008;105(46):18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D’Arceuil HE, de Crespigny AJ, and Wedeen, V. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130(3):630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Snijders TM, Vosse T, Kempen G, Van Berkum JJA, Petersson KM, Hagoort P. Retrieval and Unification of Syntactic Structure in Sentence Comprehension: an fMRI Study Using Word-Category Ambiguity. Cerebral Cortex. 2009;19(7):1493–1503. doi: 10.1093/cercor/bhn187. [DOI] [PubMed] [Google Scholar]
- Stamatakis EA, Tyler LK. Identifying lesions on structural brain images - validation of the method and application to neuropsychological patients. Brain Lang. 2005;94:167–177. doi: 10.1016/j.bandl.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Kinkingnehun S, Delmaire C, Lehericy S, Duffau H, Thivard L, Volle E, Levy R, Dubois B, Bartolomeo P. Visualization of disconnection syndromes in humans. Cortex. 2008;44(8):1097–1103. doi: 10.1016/j.cortex.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Tyler LK. Spoken language comprehension: An experimental approach to disordered and normal processing. MIT Press; Cambridge, MA: 1992. [Google Scholar]
- Tyler LK, de Mornay-Davies P, Anokhina R, Longworth C, Randall B, Marslen-Wilson WD. Dissociations in processing past tense morphology: Neuropathology and behavioural studies. J. Cogn. Neurosci. 2002;14(1):79–94. doi: 10.1162/089892902317205348. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Marslen-Wilson W, Stamatakis EA. Dissociating neuro-cognitive component processes: voxel-based correlational methodology. Neuropsychologia. 2005;43(5):771–778. doi: 10.1016/j.neuropsychologia.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Marslen-Wilson WD. Fronto-temporal brain systems supporting spoken language comprehension. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2008;363(1493):1037–1054. doi: 10.1098/rstb.2007.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Marslen-Wilson WD, Randall B, Wright P, Devereux BJ, Zhuang J, Papoutsi M, Stamatakis EA. Left inferior frontal cortex and syntax: function, structure and behaviour in patients with left hemisphere damage. Brain. 2011;134(Pt 2):415–431. doi: 10.1093/brain/awq369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Shafto MA, Randall B, Wright P, Marslen-Wilson WD, Stamatakis EA. Preserving Syntactic Processing across the Adult Life Span: The Modulation of the Frontotemporal Language System in the Context of Age-Related Atrophy. Cerebral Cortex. 2010;20(2):352–364. doi: 10.1093/cercor/bhp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Stamatakis EA, Jones RW, Bright P, Acres K, Marslen-Wilson WD. Deficits for semantics and the irregular past tense: a causal relationship? J. Cogn. Neurosci. 2004;16(7):1159–1172. doi: 10.1162/0898929041920559. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Wright P, Randall B, Marslen-Wilson WD, Stamatakis EA. Reorganization of syntactic processing following left-hemisphere brain damage: does right-hemisphere activity preserve function? Brain. 2010;133(11):3396–3408. doi: 10.1093/brain/awq262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Wright P, Randall B, Marslen-Wilson WD, Stamatakis EA. Reorganization of syntactic processing following LH brain damage: does right-hemisphere activity preserve function? Brain. 2010;133(11):3396–3408. doi: 10.1093/brain/awq262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lely HKJ, Harris M. Comprehension of reversible sentences in specifically language impaired children. J. Speech Hear. Disord. 1990;55:101–117. doi: 10.1044/jshd.5501.101. [DOI] [PubMed] [Google Scholar]
- Wernicke C. Der aphasische Symptomencomplex. Cohen and Weigert; Breslau: 1874. [Google Scholar]