Abstract
Recombinant human erythropoietin (rhEPO), a glycohormone, is one of the leading biopharmaceutical products. The production of rhEPO is currently restricted to mammalian cell expression systems because of rhEPO's highly complex glycosylation pattern, which is a major determinant for drug-efficacy. Here we evaluate the ability of plants to produce different glycoforms of rhEPO. cDNA constructs were delivered to Nicotiana benthamiana (N. benthamiana) and transiently expressed by a viral based expression system. Expression levels up to 85 mg rhEPO/kg fresh leaf material were achieved. Moreover, co-expression of rhEPO with six mammalian genes required for in planta protein sialylation resulted in the synthesis of rhEPO decorated mainly with bisialylated N-glycans (NaNa), the most abundant glycoform of circulating hEPO in patients with anemia. A newly established peptide tag (ELDKWA) fused to hEPO was particularly well-suited for purification of the recombinant hormone based on immunoaffinity. Subsequent lectin chromatography allowed enrichment of exclusively sialylated rhEPO. All plant-derived glycoforms exhibited high biological activity as determined by a cell-based receptor-binding assay. The generation of rhEPO carrying largely homogeneous glycosylation profiles (GnGnXF, GnGn, and NaNa) will facilitate further investigation of functionalities with potential implications for medical applications.
Keywords: Erythropoietin, Glycoengineering, Plants, Purification, Sialylation
1 Introduction
Erythropoietin (EPO) is a glycoprotein mainly produced in the adult kidney, and was initially highlighted for its action on the hematopoietic system [1, 2]. EPO is also expressed in several non-hematopoietic tissues, where it protects from apoptosis and inflammation due to hypoxia, toxicity or injury [3, 4].
The human erythropoietin (hEPO) gene was first cloned in 1985 [5, 6]. Recombinant hEPO (rhEPO) has been available as a drug for over 25 years and is mainly used for treating anemia. In sports, its misuse has been suspected among athletes for many years [7]. rhEPO has a legitimate worldwide market of about 5 billion dollars per year, making it one of the leading biopharmaceutical products [8].
Active hEPO consists of a single 166 amino-acid polypeptide chain with three N-glycosylation sites at Asn24, Asn38, and Asn83, and one O-glycosylation site at Ser126. All sites are extensively glycosylated [9, 10] and the critical influence of this posttranslational modification for drug efficacy has been reported [11, 12]. Consequently, the oligosaccharide structures of native and rhEPO have been studied extensively. The hormone has been shown to have a large N-glycan microheterogeneity but an otherwise homogeneous protein backbone. Native and rhEPOs, isolated from urine and Chinese hamster ovary (CHO) cells, respectively, carry large amounts of elongated and branched structures (i.e. sialylated bi-, tri-, and tetra-antennary oligosaccharides). Native hEPO isolated from serum, which presents the physiologically active form of the hormone, exhibits in general the same type of N-linked oligosaccharides in terms of branching patterns as CHO-derived rhEPO but the relative amounts of the individual structures are different. A major difference is the presence of relatively large amounts of non-sialylated GnGnF structures and the absence of tetra-antennary oligosaccharides in circulatory hEPO [13]. Notably large glycan variations can occur between different individuals' urinary EPO [14]. These observations indicate that N-glycans actively contribute to the modulation of hEPO activities in vivo.
The importance of terminal sialic acid for the circulatory half-life of rhEPO is well documented [11, 15, 16], and many investigations have concentrated on enhancing the presence of this carbohydrate formation [17, 18]. Indeed, hyper-sialylated rhEPO with a prolonged half-life and subsequently enhanced drug efficacy has been reported [12]. Relatively little is known about the impact of other glycoforms. However, increased understanding is desirable in view of the large N-glycan heterogeneity of hEPO and the recent findings of the non-erythropoietic activities of the hormone. Accordingly, it would be extremely valuable to establish an rhEPO with a defined N-glycosylation pattern for future research.
Plants are a suitable alternative platform for the expression of complex human recombinant proteins. In recent years the ability to modulate the plant N-glycosylation profile toward human-like structures and thus alter the in vivo activities of therapeutic proteins has attracted great attention [19, 20]. Previous attempts to produce rhEPO in plants have resulted in the generation of a recombinant hormone exhibiting in vitro activity [21–24]. Unfortunately, most of these studies largely neglected the N-glycosylation status of the recombinant hormone, or reported the presence of unusual oligosaccharide structures [25]. Moreover, rhEPO expressed in tobacco cells failed to show in vivo activity, most probably due to the lack of sialic acid residues [26]. In addition, success in purifying the plant-derived recombinant hormone was also limited.
Here, we conducted a comprehensive study to evaluate the ability of plants to produce different glycoforms of rhEPO. Respective cDNA constructs were delivered to N. benthamiana and transiently expressed by a viral-based magnICON® expression system [27]. LC-electro-spray ionization-MS (LC-ESI-MS) used for N-glycosylation profiling, revealed that the recombinant hormone carries complex N-glycans with and without plant-specific epitopes (GnGnXF and GnGn), depending on whether the expression host is the wild type (WT) or the glycosylation mutant ΔXTFT (a mutant lacking the plant-specific N-glycan residues xylose and core α1,3 fucose, [28]). Synthesis of bisialylated rhEPO (NaNa) was achieved by co-expressing the protein with the mammalian genes necessary for in planta sialylation [29]. By fusing the short peptide tag ELDKWA to rhEPO, the recombinant hormone was purified via immunoaffinity chromatography and finally sialylated rhEPO was enriched with Sambucus nigra agglutinin (SNA) lectin chromatography. Cell-based receptor binding assay demonstrated the expression of a highly active hormone.
2 Material and methods
2.1 Construction of erythropoietin expression vectors
A 585-bp fragment containing the cDNA from hEPO (amino acids 28-194), codon optimized for dicot plants and with a C-terminal Strep II tag (WSHPQFEK) was purchased from Mr. Gene GmbH, Regensburg (Germany) (http://www.bionity.com). This sequence was used as a template in a set of PCR reactions using different primer combinations to amplify BsaI-BsaI hEPO fragments with a C- or N-terminal Strep II tag and fragments where the Strep II tag was replaced by the ELDKWA sequence (Table S1 of Supporting information). All four PCR products were digested with BsaI and cloned into a magnICON® tobacco mosaic virus-based 3'-module vector (pICH21595, Bayer BioScience NV Research, Gent, Belgium) containing two BsaI sites designed for directional cloning of the target gene [27]. The resulting vectors were named StreprhEPO, rhEPOStrep, ELDrhEPO, and rhEPOELD (Fig. 1A). The 5'-module (pICH20999) includes the signal peptide from the barley α-amylase sequence to direct proteins to the secretory pathway. A binary vector (pICH14011) expressing the recombinase was used to allow in planta assembly of the two viral modules.
Figure 1.
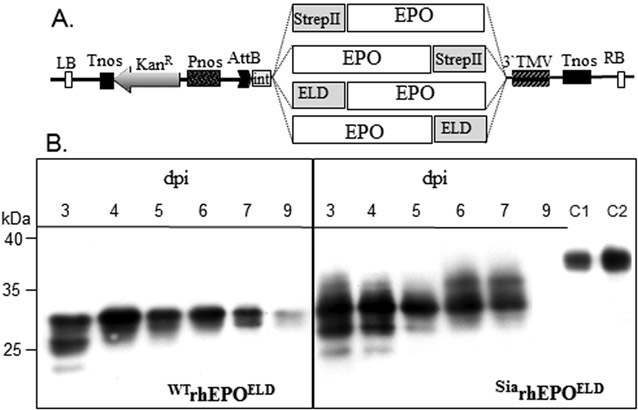
(A) Schematic representation of the 3'-modules of the TMV-based magnICON® vectors (pICH21595) used for the expression of the rhEPO. Pnos: nopaline synthase gene promoter; Tnos: nopaline synthase gene terminator; KanR: neomycin phosphotransferase II gene; 3'TMV: 3' untranslated region; AttB: recombination site; int: intron; Strep II: WSHPQFEK peptide sequence that binds specifically to streptavidin; ELD: ELDKWA peptide sequence from the HIV-1-gp41; EPO: plant codon optimized hEPO sequence lacking its native signal peptide sequence (see Section 2); LB: left border; RB: right border. (B) Timecourse analysis of rhEPOELDexpression in N. benthamiana. TSPs (2 μg) from infiltrated leaves were extracted on different days post-infiltration (dpi) and analyzed by western blotting with antibodies against hEPO. WTrhEPOELD:rhEPOELD expressed in WT plants. SiarhEPOELD:rhEPOELD co-expressed with the genes for the mammalian sialic acid pathway [29] in N. benthamiana Gal+ (mutant lacking β1,2-xylose and core α1,3-fucose, and carrying terminal β1,4-galactose, [30] ). C1 and C2: CHO-derived hEPO (50 and 100 ng, respectively). Data are representative of three independent experimental repeats. The sizes of molecular weight marker proteins are shown in kiloDalton.
2.2 Plant material and transient protein expression
WT N. benthamiana plants, the glycosylation mutants ΔXTFT (lacking plant-specific N-glycosylation, i.e. β1,2-xylose and core α1,3-fucose, [28]) and Gal+ (synthesizing β1,4-galactosylated structures, [30]), were grown at 22°C with a 16-h light: 8-h dark photoperiod. Four to five-week-old plants were used for agroinfiltration experiments as described previously [30]. To express the rhEPO, the 3'-magnICON® vector containing rhEPO cDNA was co-infiltrated with the corresponding 5'-vector carrying the signal peptide in combination with the binary vector containing the recombinase [27]. To modulate the N-glycosylation profiles, binary vectors containing the cDNA of the mammalian genes necessary for protein sialylation were co-infiltrated with the viral based vectors: namely, UDP-N-acetylglucosamine-2-epimerase/N-acetylmannosamine kinase (GNE), N-acetylneuraminic acid phosphate synthase (NANS), CMP-N-acetylneuraminic acid synthase (CMAS), CMP-sialic acid transporter (CST), and α2,6-sialyltransferase (ST) [29]. Agrobacteria carrying the magnICON® constructs were infiltrated using OD600 0.05–0.2 and those carrying the other binary constructs were infiltrated using OD600 0.05 (1.0 OD600 corresponds to 5 × 108 cells/mL).
2.3 Purification of SiarhEPO
2.3.1 2F5 immunoaffinity chromatography
Affinity chromatography was performed on a 2F5 antibody affinity column using the ÄKTAPurifier-10 FPLC system (GE Healthcare). 5 mg of purified 2F5 antibody (kindly supplied by Dr. Florian Rücker, Department of Biotechnology, University of Natural Resources and Life Sciences, Vienna, Austria) were coupled to a 1 mL pre-packed NHS-activated high performance Sepharose column (GE Healthcare) according to the manufacturer's instructions. Infiltrated leaf material (20 g) was ground in liquid nitrogen and thawed in an equal volume v/w of ice-cold extraction buffer (100 mM Tris-HCl pH 7.4, 30 mM ascorbic acid, 0.9% NaCl). The slurry was centrifuged (35 000 g for 30 min at 4°C) and the supernatant was vacuum filtrated (Macherey-Nagel filter circles MN619eh, 2–4 μm). The filtrate was further clarified by isoelectric precipitation at pH 4.5 (precipitation of ∼25% of total soluble proteins (TSPs) including RuBisCO), centrifuged as above and after adjustment to pH 7.4 incubated on ice for 15 min. Prior to loading on the chromatography column, the extract was passed through a 0.45 μm filter (Millipore Stericup Durapore low binding membrane PVDF, 73 mm/0.45 μm). The column was washed with 10 column volumes of binding buffer (PBS, pH 7.4) and the sample was applied onto the column at a flow rate of 0.9 mL/min. After washing with binding buffer, proteins were eluted with 50 mM glycine/HCl pH 2.8 and immediately neutralized with 0.5 M Tris. Column washing and elution were carried out at a flow rate of 1 mL/min. Prior to SDS-PAGE analysis the samples were precipitated in ice-cold acetone (4× sample volume) for 1 h at –20°C and centrifuged for 10 min at 16 000 g. The precipitate was air-dried, resuspended in 3× Laemmli buffer and heated at 96°C for 8 min.
2.3.2 SNA lectin affinity chromatography
Affinity chromatography was performed using the ÄKTAPurifier-10 FPLC system on a 1.6 mL SNA lectin affinity column (GALAB Technologies GmbH). The SiarhEPOELD-containing eluates from the 2F5 immunoaffinity chromatography were identified by dot blot using anti-hEPO antibodies and pooled. The sample was diluted with 2×SNA lectin binding buffer (40 mM Tris-HCl, 300 mM NaCl, 2 mM MnCl2, 2 mM CaCl2 2 mM MgCl2, pH 7.0) and slowly (0.4 mL/min) applied to the column using a P1 peristaltic pump (GE Healthcare). Sample loading and column washing steps were performed at 4°C. The binding fraction was eluted with 0.3 M lactose in 50 mM glycine/HCl pH 3.2 buffer at a flow rate of 0.8 mL/min at room temperature. Subsequently the eluates were analyzed by dot blot with anti-hEPO antibodies and the positive fractions were pooled. The buffer was exchanged by passing the sample through an LC-4 solid phase extraction (SPE) column (Supelclean – Supelco), washing it with water and eluting it in 2 mL of 60% ACN + 0.1% TFA. After drying in the speed-vac the samples were resuspended in water.
2.4 SDS-PAGE and immune-based analyses
Recombinant proteins fractionated by 12.5% SDS-PAGE were either stained with CBB G-250 [31] or used for western blot analysis. Those used for immunoblot analysis were blotted to Hybond-ECL® nitrocellulose membranes (Amersham), blocked for 1 h in 1×PBS supplemented with 3% w/v BSA and 1% v/v Tween 20 and detected using either anti-Strep II (IBA GmbH, Germany, 1:2000 dilution in PBST + 3% BSA), anti-hEPO (R&D systems, 1:3000 dilution in PBST), or 2F5 (1 μg 2F5/mL in PBS + 1% BSA) antibodies. The dot blot was prepared by spotting 10 μL of sample onto the nitrocellulose membrane and blocking for 1 h, and was detected with anti-hEPO antibodies as above.
Quantification of rhEPO was carried out by a double sandwich ELISA using mouse monoclonal hEPO antibodies (Genzyme, Art. Nr. AE-7A5) for coating (dilution 1:10.000) and biotinylated (according to GE healthcare, RPN2202) polyclonal rabbit hEPO Abs (R&D Systems; Art. Nr. AB-286-NA; 1:800 diluted) as a second antibody. Streptavidin-peroxidase (Boehringer Mannheim, Art. Nr. 1 096 044) was used for detection. The color reaction was measured by an ELISA Reader at 492/620 nm. The CHO-derived rhEPO standard was provided by Polymun Scientific GmbH (Vienna).
2.5 Profiling of N-glycopeptides
N-glycan analysis of rhEPO was carried out by LC-ESI-MS of tryptic glycopeptides as described [32]. Briefly, the purified samples were submitted to reducing SDS-PAGE and the immunoreactive bands were cut from the gel, S-alkylated, double-digested with trypsin and endoproteinase Glu-C, eluted from the gel fragment with 50% acetonitril and separated on a Biobasic C18 column (150 mm × 0.32 mm, Thermo Electron) with a gradient of 1–80% acetonitrile containing 65 mM ammonium formate, pH 3.0. This double digestion allows the discrimination of all three rhEPO glycopeptides:glycopeptide 1:E21AENITGCAE31; glycopeptide 2:H32CSLNENITVPDTK45; and glycopeptide 3:G77QALLVNSSQPWEPLQHLVDK97. Positive ions were detected with a quadrupole-time of flight (Q-TOF) Ultima Global mass spectrometer (Waters, Milford, MA, USA). Summed and deconvoluted spectra of the glycopeptides elution range were used for identification of glycoforms.
2.6 In vitro activity assay of rhEPO
The in vitro biological activity of rhEPOELD was measured in an UT-7 cell-based proliferation assay. Briefly, the UT-7 cell line [33] was maintained in RPMI 1640 (Biochrome AG) supplemented with 10% fetal calf serum (PAN Biotech.), 4 mM L-glutamine and 5 ng/mL EPO. The cells were washed with EPO-free culture medium and incubated for 4 h at 37°C and 7% CO2. Increasing amounts of CHO-derived rhEPO and plant-derived rhEPOELD (ranging from 0.03 to 30 000 ng/mL) were added to 100 μL of medium containing about 104 cells in a 96-well culture plate, resulting in a final rhEPO concentration of 0.01–10 000 ng/mL per well. After 4 days at 37°C and 7% CO2, 10 μL of an MTT (Thiazolyl Blue Tetrazolium Bromide; Sigma) solution (5 mg/mL) were supplied to each well and the plate was incubated for 4 h as before. Finally, 100 μL of 10% SDS (in 0.01 M HCl) were added to each well and mixed thoroughly at 37°C to dissolve blue crystals before absorbency was read at 570 nm (reference wavelength 690 nm). The results of the fivefold determination were evaluated using MS Excel Solver. The half-maximal effective UT-7 cell proliferation dose (ED50) was used to compare the activities of plant- and CHO-derived rhEPO.
3 Results
3.1 Vectors for rhEPO expression
Transient expression of rhEPO was achieved with magnICON® viral based vectors [27] designed to contain the respective cDNAs with a Strep II tag either at the N- or C-terminus (StreprhEPO, rhEPOStrep, Fig. 1A). Agrobacterium tumefaciens suspension cultures carrying the recombinant plasmids were delivered to the expression host N. benthamiana by agroinfiltration. Infiltrated leaves were harvested 5 days post-infiltration (dpi) and expression of the recombinant hormone was monitored by western blot analysis of TSPs. Antibodies against hEPO exhibited a strong signal at the expected size of 30-kDa for both constructs; however, no signal was detected when antibodies against the Strep II tag were used (data not shown). These results indicate that either the tag is cleaved off or it is not accessible at either of the termini. In search for an alternative purification strategy we used an immunoaffinity-based purification with the epitope ELDKWA (derived from HIV-1-gp41) in combination with the corresponding mAb 2F5 [34]. hEPO constructs with a N- or C-terminal tag (ELDrhEPO, rhEPOELD, Fig. 1A) were delivered to N. benthamiana and western blot analysis of TSP at 5 dpi showed strong signals at the expected size with both anti-hEPO and 2F5 antibodies (Supporting information Fig. S1). Consequently, we used the C-terminal-tagged rhEPO construct (rhEPOELD) in subsequent experiments.
3.2 Transient expression of rhEPOELD in N. benthamiana
Various infiltration experiments were carried out to explore the maximum expression levels of rhEPOELD in N. benthamiana. The OD600 of agrobacterial suspension was varied (0.5–0.05 OD600) and leaves were infiltrated at different developmental stages. In addition, timecourses were implemented. Western blot analyses revealed that the optimum rhEPOELD expression could be achieved upon infiltration of middle-aged leaves (from plants that carry six to eight leaves) using agrobacteria at a concentration of OD600 0.2. A timecourse from 2 to 11 dpi showed an expression maximum between 4 and 5 dpi (Fig. 1B). Western blot analysis with anti-hEPO antibodies showed a strong signal at the expected size of 30-kDa. In addition, two other bands were detected at positions 26- and 22-kDa (Fig. 1B). CHO-derived rhEPO was detected as an ∼40-kDa band (Fig. 1B, right panel C1, C2). This size discrepancy between plant- and CHO-derived rhEPO is most probably due to differences in N-glycosylation. The expression level of rhEPO, as determined by ELISA, varied from 50 to 85 mg rhEPO/kg fresh leaf material depending on the OD600 and dpi. With an OD600 of 0.2, a maximum expression level of 85 mg rhEPO/kg fresh leaf material was detected at 4 dpi, corresponding to ∼2% of TSP.
LC-ESI-MS analysis was performed to determine the N-glycosylation status of rhEPOELD expressed in WT N. benthamiana plants (WTrhEPOELD). Corresponding bands (22-, 26-, and 30-kDa) were excised from denaturing gels loaded with TSP and double digested with trypsin and endoproteinase Glu-C to release three N-glycopeptides (Gp1-Gp3). Subsequent MS analysis revealed that all three N-glycosylation sites of WTrhEPOELD carried a similar glycosylation pattern (Fig. 2 and Supporting information Fig. S2). The 26-kDa protein had a dominant single glycoform, i.e. GnGnXF, typical for plant-secreted proteins. This oligosaccharide formation was also present in the 30-kDa protein, however, the major fraction referred to ER-typical oligomannosidic structures (M8 and M9). Non-fucosylated complex glycans (GnGnX) and glycans containing galactose residues were detected in both bands. The galactosylated structures are most likely Lewis-a (Lea) carbohydrates (Gn(FA)XF), which have been previously reported on rhEPOFc expressed in N. benthamiana [35, 36] and are highly abundant on moss-derived rhEPO [24, 25]. MS analysis of the 22-kDa protein revealed non-glycosylated WTrhEPOELD, which accounts for less than 5% of the recombinant hormone. The differences in size upon gel electrophoresis are most probably associated with different glycosylation of the recombinant protein. Signals in immuno-blot analysis with 2F5 mAbs, specific for the ELD-Tag, indicate intact N- and C-termini of the recombinant protein (Supporting information Fig. S1). rhEPOELD was abundantly present in intercellular fluid (IF), indicating efficient secretion of rhEPOELD to this compartment. Glycan analysis of secreted rhEPOELD displayed a homogenous profile of plant-specific complex N-glycans (GnGnXF) and no significant amounts of oligomannosidic structures were detected (Fig. 2).
Figure 2.
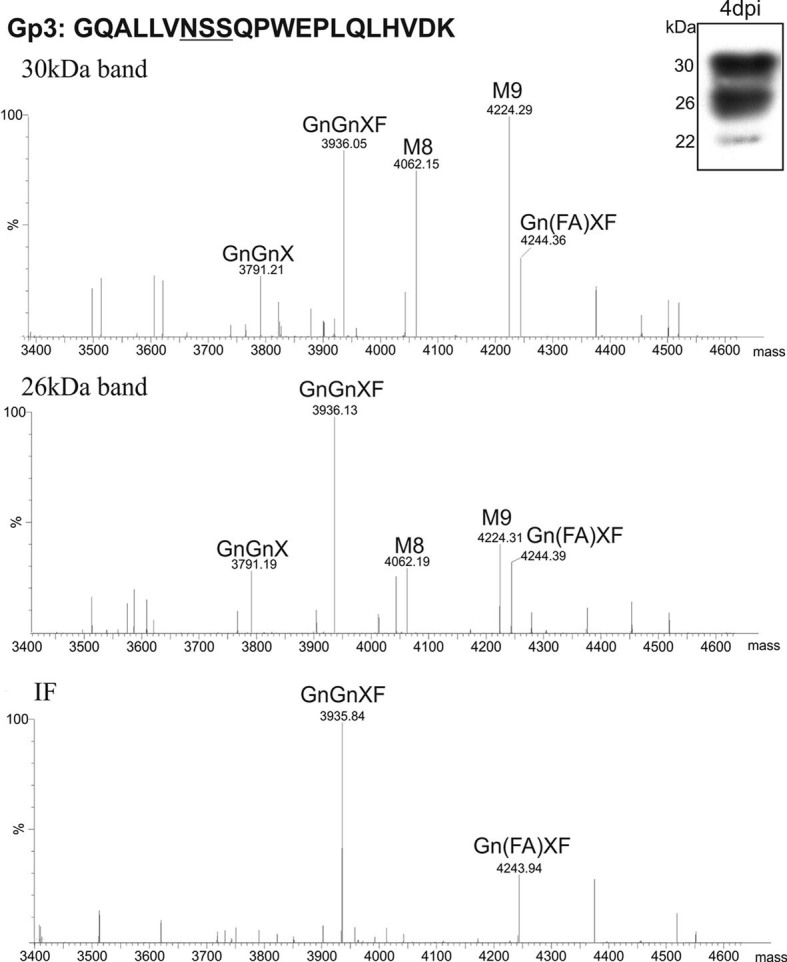
N-glycan profiles of rhEPOELD derived from N. benthamiana WT plants harvested at 4 dpi. At this time point western blot analysis of TSPs with anti-hEPO antibody shows three reactive bands (22-, 26-, and 30-kDa, top right cartoon). The N-glycan analysis was carried out by LC-ESI-MS of glycopeptides obtained by double digestion as described in Section 2. This allows discrimination of the three EPO glycopeptides (Gp1-Gp3). The MS profile of glycopeptide 3 (Gp3) is shown (see Supporting information Fig. S2 for analysis of glycopeptide 1 and 2). Top: profile of the 30-kDa band; middle: profile of the 26-kDa band; bottom: MS profile from rhEPOELD isolated from the IF. Two independent repeats of the experience were performed. Peak labels were made according to the ProGlycAn system (www.proglycan.com).
3.3 Modulation of rhEPOELD N-glycosylation
We used N. benthamiana ΔXTFT as a host in a first set of experiments aimed at generating rhEPOELD with human-like N-glycosylation, i.e. lacking plant specific core α1,3-fucose and β1,2-xylose residues. The recombinant EPO (ΔXTFTrhEPOELD) was produced in similar levels as WTrhEPOELD. Also, ΔXTFTrhEPOELD was detected in three immunoreactive bands by western blot, exhibiting distinct glycosylation profiles: the 30-kDa protein band carried GnGn structures accompanied by high levels of oligomannosidic N-glycans (M8, M9), the 26-kDa band mainly carried complex GnGn structures and the 22-kDa band corresponded to non-glycosylated ΔXTFTrhEPOELD (Fig. 3). No N-glycans carrying core α1,3-fucose or β1,2-xylose residues were detected. ΔXTFTrhEPOELD showed a largely homogeneous glycosylation profile.
Figure 3.
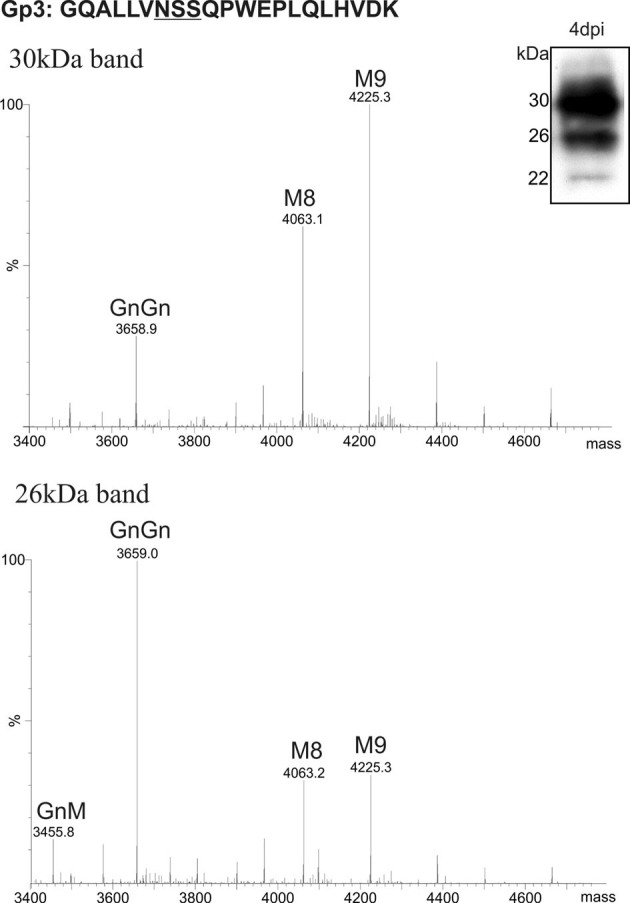
N-glycan profiles of rhEPOELD derived from N. benthamiana ΔXTFT harvested at 4 dpi. Western blot analysis of TSP extracts with anti-hEPO antibody shows three reactive bands (22-, 26-, and 30-kDa, top right cartoon). The glycosylation profile for glycopeptide 3 (Gp3) is shown. Top: MS profile corresponding to the 30-kDa band; bottom: MS profile corresponding to the 26-kDa band. Four independent repeats of the experience were performed. Peak labels were made according to the ProGlycAn system (www.proglycan.com).
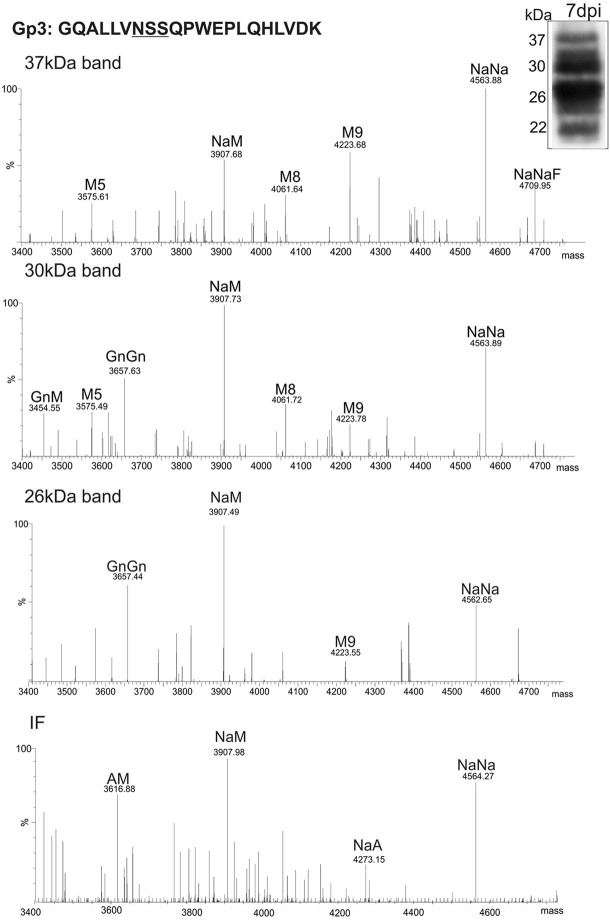
In a next step, we set out to generate rhEPOELD carrying sialylated N-glycans. Recently we showed in planta sialylation of recombinant mAbs through the overexpression of six genes involved in the mammalian sialic acid pathway [29]. We used N. benthamiana GalT+ as the expression host to accomplish sialylation in plant-derived rhEPOELD. This glycosylation mutant synthesizes mammalian-type terminal β1, 4-galactosylated structures, providing the acceptor substrate for subsequent sialylation [30]. Thus, rhEPOELD was co-expressed in N. benthamiana Gal+ plants with mammalian genes required for the synthesis of sialic acid (GNE, NANS, and CMAS), its transport into the Gogi (CST) and its transfer to terminally β1,4-galactosylated N-glycans (ST) (see Section 2). Subsequently, western blot analysis with antibodies against hEPO was used to monitor the expression of the recombinant hormone (SiarhEPOELD, Fig. 1B, right panel). The expression maximum was reached at 4 dpi, however, it seems that SiarhEPOELD is more stable than WTrhEPOELD over time (Fig. 1B, left panel). Moreover, immunoreactive bands shifted toward increased size over time (Fig. 1B), indicating accumulation of the sialylated recombinant hormone. Apart from the three reactive bands (22-, 26-, and 30-kDa) an additional 37-kDa band appeared at 6 dpi. A detailed N-glycan analysis of the proteins from individual bands revealed that the 22-kDa protein, which accounts for less than 5% of the recombinant hormone, was non-glycosylated. The three larger bands (26-, 30-, and 37-kDa) exhibited substantial fractions of sialylated N-glycans, with some variation in bi-sialylated (NaNa) and incompletely processed structures (NaM) (Fig. 4 and Supporting information, Fig. S3). In addition, significant amounts of GnGn and minor fractions of oligomanno-sidic structures (M8, M9) were also present. Notably, SiarhEPOELD derived from the apoplastic fluid was decorated almost exclusively with sialylated N-glycans (IF, Fig. 4).
Figure 4.
N-glycan profiles of rhEPOELD co-expressed with genes necessary for in planta sialylation in N. benthamiana Gal+, harvested at 7 dpi (SiarhEPOELD). Western blot analysis of TSP extracts with anti-hEPO antibody shows several reactive bands (22-, 26-, 30-, and 37-kDa, top right cartoon). The glycosylation profile (Gp3) of 37-, 30-, and 26-kDa immunoreactive bands are shown; bottom: MS profile of SiarhEPOELD isolated from the IF. (see Supporting information Fig. S3 for analysis of glycopeptide 1 and 2). Two independent repeats of the experience were performed. Peak labels were made according to the ProGlycAn system (www.proglycan.com).
3.4 Purification of SiarhEPOELD
Although the expression of rhEPO has been reported to be successful in various expression hosts, purification of the recombinant hormone remains a challenge. Previous attempts using a C-terminal His or Strep II tag to purify rhEPO proved unsuccessful. Although the expression levels of the hormone were similar to those obtained in this study, we were not able to purify the recombinant protein due to unspecific binding (His-tag) or loss of tag (Strep II tag) (our laboratory, unpublished data). In this study we explored immunoaffinity of the ELDKWA epitope to the mAb 2F5 [34] in view of establishing a new purification method. Five milligram of purified CHO-derived 2F5 were coupled to an NHS-Sepharose pre-packed chromatography column. Clarified leaf protein extracts from SiarhEPOELD were loaded onto the column and eluted at different pH conditions. A sharp elution peak was visible in eluates E5 and E6. These two eluates, with a total volume of 1 mL, contained about 90% of the purified product. We used CBB G-250 to detect the purified hormone as rhEPO is more sensitive to Brilliant Blue R-250 [31]. As already seen in immunoblots (Fig. 1B), the Coomassie-stained gels of purified recombinant hormone shows three protein bands (Fig. 5A) that react with anti-hEPO antibodies (Fig. 5B). Coomassie-stained gels upon loading 0.3 mL out of 0.5 mL eluate showed alongside the three major rhEPO specific bands, minor contamination with plant proteins (Supporting information, Fig. S4). Moreover significant amounts of the recombinant protein were detected in the flow through (FT, Fig. 5B), indicating that there is still room for optimization of the purification procedure. The N-glycosylation profile of the purified products largely resembled those observed in TSP extracts, where SiarhEPOELD is produced as a mixture of glycoforms including sialylated and high mannosidic structures. In our attempts to enrich the fraction of rEPOELD carrying sialylated glycoforms, we performed SNA lectin chromatography as a second purification step. A schematic representation of the experimental procedure is outlined in Supporting information Fig. S5. Briefly, samples from SiarhEPOELD purified by 2F5-based immunoaffinity were applied to the SNA lectin column, and elulates containing the recombinant protein were pooled and passed through an LC4 SPE reversed phase column to remove lactose. Subsequent N-glycosylation analysis of the product obtained revealed the presence of virtually exclusively sialylated N-glycan structures. An effective enrichment of bi-sialylated fractions accompanied by minor fractions of monoantennary sialylated structures was obtained (NaA, NaM, Fig. 5C, EL). As expected, rhEPOELD present in the FT carried exclusively oligomannosidic structures (Fig. 5C, FT), demonstrating the efficiency of the procedure. Note that although the SNA chromatography allowed isolation of fully sialylated rhEPOELD, this step caused a significant reduction in product yield, and thus may to be optimized.
Figure 5.
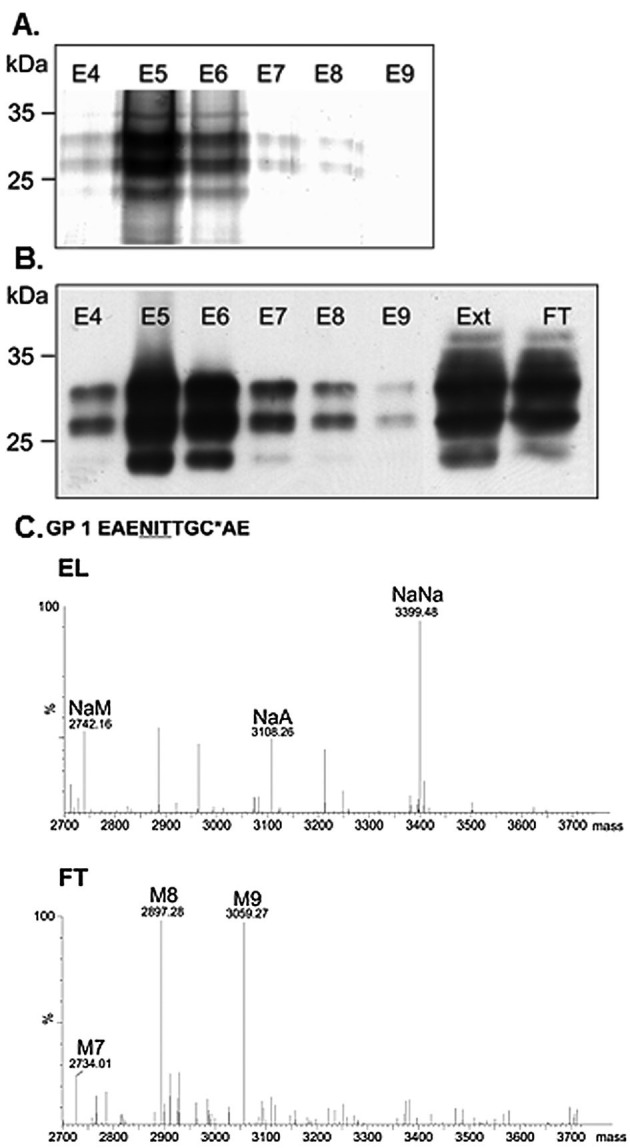
Analysis of 2F5 immunoaffinity-purified SiarhEPOELD. (A) SDS-PAGE Coomassie-stained gel of eluates 4–9 (E4–E9; no protein quantification was made), (B) corresponding western blot using antibodies against hEPO, Ext: TSP extract, FT: FT after immunoaffinity chromatography (5 μg TSP was loaded). Several independent repeats of the experience were performed. (C) N-glycan profiles of SiarhEPOELD purified by sequencial 2F5 immunoaffinity and SNA lectin affinity chromatography (see also Supporting information Fig. S4). The glycosylation profile for glycopeptide 1 in the eluate (EL) and FT is shown. Peak labels were made according to the ProGlycAn system (www.proglycan.com).
3.5 In vitro activity assay
The in vitro biological activity of plant-derived rhEPOELD was evaluated by a UT-7 cell-based proliferation assay and compared with CHO-derived sialylated rhEPO. The latter consisted mainly of tri- and tetrasialylated core fucosylated N-glycans (CHOrhEPO, Supporting information Fig. S6). Growth of the human leukemia cell line UT-7 is strictly correlated to the concentration of EPO in the culture medium. Cell-based in vitro assay indicated that plant-derived rhEPOELD was active (Supporting information, Fig. S7). SiarhEPOELD and CHOrhEPO showed similar half maximum effective doses (ED50 values 0.66 and 0.53 ng/mL, respectively). As expected, non-sialylated WTrhEPOELD exhibited increased activity (ED50 0.03 ng/mL) compared with its sialylated form.
4 Discussion
Erythropoietin is a multifunctional molecule produced and used by many tissues. Apart from its erythropoiesis action, other roles involve the acute and sub-acute biological responses to tissue damage [3, 4]. The extensive glycosylation of the protein is well characterized, and attempts have been made to generate hyperglycosylated forms of the protein in the course of developing drugs for treating anemia-associated conditions. However, considering the large N-glycan heterogeneity and multifunction of the hormone other glycoforms may be needed for new therapeutic applications [3, 4]. In the current study, we demonstrate the efficient expression of sialylated rhEPOELD in N. benthamiana, already at 4 dpi. This production speed provides advantages over other potential expression systems, such as mammalian cells. Terminal sialylation of rhEPOELD seemed to have a positive impact on the stability compared with WTrhEPOELD. Expression levels of up to 85 mg recombinant hormone/kg fresh leaf, corresponding to ∼2% of TSP, were achieved. Compared to previous studies where up to 0.02% expression levels of rhEPO in plants were reported [37] our results demonstrate a significant improvement. Nevertheless the expression of rhEPOELD was relatively low compared with expression levels of other recombinant proteins using similar transient production systems, e.g., monoclonal antibodies are produced up to 50% of TSP [38–41]. Similarly, the expression of a recombinant EPO fusion (rhEPOFc) using the same magICON® expression system resulted in the generation of a chimeric recombinant hormone accompanied by a five to ten times higher level of free Fc [35, 36]. The main goal of this study was to generate rhEPOELD carrying different N-glycosylation profiles. With this intent, WT N. benthamiana and the glycosylation mutants ΔXTFT and GalT+ were used as expression hosts. rhEPOELD derived from WT (WTrhEPOELD) was decorated mainly with plant-specific GnGnXF3 structures. These are the typical N-glycan formations expected on secreted plant glycoproteins. However, in addition significant amounts (ca. 30%) of oligomannosidic structures and Lea epitopes (ca. 15%) were detected. The presence of oligomannosidic structures was unexpected, they had not been detected previously in either native EPO or rhEPO expressed in mammalina cells [9, 11]. The fact that these structures were absent on IF-derived WTrhEPOELD, indicates that fractions of the protein are not properly secreted. It seems that part of rhEPOELD is sorted away from the secretory pathway and deposited in ER-derived compartments, a phenomenon observed previously upon expression of recombinant single chain antibodies in Arabidopsis seeds and N. benthamiana leaves [42, 43]. Lea epitopes were another N-glycan formation detected on WTrhEPOELD but not on native hEPO or CHO-derived rhEPO. Usually this glycoform accounts for only minor fractions of endogeneous Nicotiana proteins [28] and it had not been previously detected on plant-produced recombinant proteins [19, 20]. An exception is rhEPO expressed in moss cells, where large amounts of such oligosaccharide structures are present [25]. Lea structures have also been reported on a rhEPOFc fusion expressed in N. benthamiana [35, 36]. The reason for plant-derived rhEPO carrying relatively large amounts of Lea structures is currently not known.
N-glycans of rhEPOELDderived from ΔXTFT (ΔXTFTrhEPOELD) were essentially devoid of β1,2-xylose and core α1,3-fucose. Mainly two carbohydrate structures were present: complex GnGn and oligomannosidic structures. Surprisingly, no Lea epitopes were detected. Finally, using N. benthamiana GalT+ as the expression host, an efficiently sialylated glycoform was produced (SiarhEPOELD) by coexpressing hEPOELD with the genes necessary for in planta sialylation. Moreover, sialylation seemed to have a stabilization effect on the recombinant hormone as SiarhEPOELD accumulated up to 9 days after infiltration, contrasting with the gradual reduction over time of WTrhEPOELD expression.
Mammalian cell produced rhEPO is usually purified by ultrafiltration followed by ion exchange chromatography, reversed phase chromatography and gel filtration. These techniques are hardly applicable to crude plant leaf extracts. We evaluated different immunoaffinity tags in our attempts to establish an efficient purification protocol for plant produced rhEPO. A C-terminal His-tagged rhEPO showed similar expression levels to rhEPOELD but we were not able to efficiently purify the recombinant protein due to unspecific binding of the abundant endogeneous protein RuBisCO to Ni-Sepharose (our unpublished data). Another widely used tag (Strep II tag) failed as well because the tag was not detectable in western blot analysis, even though the hormone was nicely expressed. While searching for alternatives, we came across the mAb 2F5, which strongly binds to the epitope ELDKWA localized at the HIV-1 envelope protein gp41 [34]. We established a new immunoaffinity protocol that allowed purification of the recombinant hormone at great homogeneity. The sequencial immunoaffinity- and lectin affinity chromatography procedure allowed the enrichment of biantennary sialylated plant-derived rhEPOELD. Nevertheless, this procedure, particularly SNA treatment, caused a massive loss of the product, and finally only ca. 5% of the amount present in TSP could be recovered. The applicability of 2F5-ELDKWA-based immunoaffinity purification at industrial scale still needs to be evaluated and optimized. Previous studies reporting high level of 2F5 expression in CHO cells are an excellent basis for mass production of the mAb at reasonable costs [44, 45]. The fact that the immunoaffinity column could be reused several times indicates high stability of the 2F5-Sepharose. Finally, we demonstrated full activity of the plant-derived hormone. The degree of sialylation does not seem to influence receptor binding, as seen by similar binding affinities of bisialylated SiarhEPOELDand CHOrhEPO, which mainly consists of tri- and tetrasialylated structures. On the other side, a higher receptor binding affinity of non-sialylated rhEPOELD was observed, a phenomenon already described for hEPO [46]. These results suggest a targeted manipulation of biological activities by alteration of the N-glycosylation profile. The results presented here together with the generation of branched N-glycosylated rhEPO in plants (tri and tetra-antennary structures, [35, 36] ) serve as an excellent starting point for the synthesis of multi-sialylated rhEPO, the preferred glycoform currently used in anemia treatment (work is in progress). Bisialylated hEPO is an abundant glycoform of anemia-patients derived serum EPO. The function of this glycosylation form is not known. Our results allow structure-function studies on this (and other) individual glycoprofile(s), and thus may help to better understand the action of this multifunctional hormone.
In summary the ability to generate different rhEPOELD with a targeted N-glycosylation profile paves the way to further investigate the impact of N-glycosylation on the function of the hormone, which in turn could lead to the discovery of new hEPO functions. Given the speed and ease with which different glycovariants of rhEPOELD were produced, the plant-based expression platform presented in this study is of great use not only in the research and development of pharmaceutical proteins but also in basic scientific investigations.
Acknowledgments
We thank Pia Gattinger, Department of Applied Genetics and Cell Biology, University of Natural Resources and Life Sciences, Vienna, Austria, for excellent technical support and Bernhard Antes, f-star Biotechnologische Forschung Ges.m.b.H. Vienna, for helpful discussions. We also thank Elise Langdon-Neuner for language editing. The work was supported by the Laura Bassi Centres of Expertise (Grant Number 822757) and the Fonds zur Förderung der wissenschaftlichen Forschung FWF (Grant Number L575-B13).
The authors declare no conflict of interest.
Glossary
Abbreviations
- CBB
Coomassie brillant blue
- CHO
Chinese hamster ovary
- EPO
erythropoietin
- FT
flow through
- hEPO
human erythropoietin
- IF
intercellular fluid
- LC-ESI-MS
LC-electro-spray ionization-MS
- rhEPO
recombinant human erythropoietin
- SNA
Sambucus nigra agglutinin
- TSP
total soluble protein
- WT
wild type
Supplementary material
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- 1.Krantz SB. Erythropoietin. Blood. 1991;77:419–434. [PubMed] [Google Scholar]
- 2.Jelkmann W. Erythropoietin: Structure, control of production, and function. Physiol. Rev. 1992;72:449–489. doi: 10.1152/physrev.1992.72.2.449. [DOI] [PubMed] [Google Scholar]
- 3.Brines M, Patel NS, Villa P, Brines C, et al. Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin. Proc. Natl. Acad. Sci. USA. 2008;105:10925–10930. doi: 10.1073/pnas.0805594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chateauvieux S, Grigorakaki C, Morceau F, Dicato M, et al. Erythropoietin, erythropoiesis and beyond. Biochem. Pharmacol. 2011;82:1291–1303. doi: 10.1016/j.bcp.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs K, Schoemaker C, Rudersdorf R, Neill SD, et al. Isolation and characterization of genomic and cDNA clones of human erythropoietin. Nature. 1985;313:806–810. doi: 10.1038/313806a0. [DOI] [PubMed] [Google Scholar]
- 6.Lin FK, Suggs S, Lin CH, Browne JK, et al. Cloning and expression of the human erythropoietin gene. Proc. Natl. Acad. Sci. USA. 1985;82:7580–7584. doi: 10.1073/pnas.82.22.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thevis M, Schänzer W. Emerging drugs-potential for misuse in sport and doping control detection strategies. Mini Rev. Med. Chem. 2007;7:531–537. doi: 10.2174/138955707780619590. [DOI] [PubMed] [Google Scholar]
- 8.Jelkmann W. Biosimilar epoetins and other “follow-on” biologics: Update on the European experiences. Am. J. Hematol. 2010;85:771–780. doi: 10.1002/ajh.21805. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi M, Takasaki S, Miyazaki H, Kato T, et al. Comparative study of the asparagine-linked sugar chains of human erythropoietins purified from urine and the culture medium of recombinant Chinese hamster ovary cells. J. Biol. Chem. 1988;263:3657–3663. [PubMed] [Google Scholar]
- 10.Tsuda E, Goto M, Murakami A, Akai K, et al. Comparative structural study of N-linked oligosaccharides of urinary and recombinant erythropoietins. Biochemistry. 1988;27:5646–5654. doi: 10.1021/bi00415a038. [DOI] [PubMed] [Google Scholar]
- 11.Hokke CH, Bergwerff AA, Van Dedem GW, Kamerling JP, et al. Structural analysis of the sialylated N- and O-linked carbohydrate chains of recombinant human erythropoietin expressed in Chinese hamster ovary cells. Sialylation patterns and branch location of dimeric N-acetyllactosamine units. Eur. J. Biochem. 1995;228:981–1008. doi: 10.1111/j.1432-1033.1995.tb20350.x. [DOI] [PubMed] [Google Scholar]
- 12.Egrie JC, Browne JK. Development and characterization of novel erythropoiesis stimulating protein (NESP) Nephrol. Dial Transplant. 2001;16:3–13. [PubMed] [Google Scholar]
- 13.Skibeli V, Nissen-Lie G, Torjesen P. Sugar profiling proves that human serum erythropoietin differs from recombinant human erythropoietin. Blood. 2001;98:3626–3634. doi: 10.1182/blood.v98.13.3626. [DOI] [PubMed] [Google Scholar]
- 14.Sytkowski AJ, Feldman L, Zurbuch DJ. Biological activity and structural stability of N-deglycosylated recombinant human erythropoietin. Biochem. Biophys. Res. Commun. 1991;176:698–704. doi: 10.1016/s0006-291x(05)80240-2. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi M, Inoue N, Strickland TW, Kubota M, et al. Relationship between sugar chain structure and biological activity of recombinant human erythropoietin produced in Chinese hamster ovary cells. Proc. Natl. Acad. Sci. USA. 1989;86:7819–7822. doi: 10.1073/pnas.86.20.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuen CT, Storring PL, Tiplady RJ, Izquierdo M, et al. Relationships between the N-glycan structures and biological activities of recombinant human erythropoietins produced using different culture conditions and purification procedures. Br. J. Haematol. 2003;121:511–526. doi: 10.1046/j.1365-2141.2003.04307.x. [DOI] [PubMed] [Google Scholar]
- 17.Bragonzi A, Distefano G, Buckberry LD, Acerbis G, et al. A new Chinese hamster ovary cell line expressing alpha 2, 6-sialyltransferase used as universal host for the production of human-like sialylated recombinant glycoproteins. Biochim. Biophys. Acta. 2000;1474:273–282. doi: 10.1016/s0304-4165(00)00023-4. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Park JH, Park HH, Tan W, et al. Enhancement of recombinant human EPO production and sialylation in chinese hamster ovary cells through Bombyx mori 30Kc19 gene expression. Biotechnol. Bioeng. 2011;108:1634–1642. doi: 10.1002/bit.23091. [DOI] [PubMed] [Google Scholar]
- 19.Loos A, Steinkellner H. IgG-Fc glycoengineering in non-mammalian expression hosts. Arch. Biochem. Biophys. 2012;526:167–173. doi: 10.1016/j.abb.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castilho A, Steinkellner H. Glyco-engineering in plants towards human like structures. Biotechnol. J. 2012;7:1088–1098. doi: 10.1002/biot.201200032. [DOI] [PubMed] [Google Scholar]
- 21.Conley AJ, Mohib K, Jevnikar AM, Brandle JE. Plant recombinant erythropoietin attenuates inflammatory kidney cell injury. Plant Biotechnol. J. 2009;7:183–199. doi: 10.1111/j.1467-7652.2008.00389.x. [DOI] [PubMed] [Google Scholar]
- 22.Musa TA, Hung CY, Darlington DE, Sane DC, et al. Overexpression of human erythropoietin in tobacco does not affect plant fertility or morphology. Plant Biotechnol. Rep. 2009;3:157–165. [Google Scholar]
- 23.Kittur FS, Hung CY, Darlington DE, Sane DC, et al. N-Glycosylation engineering of tobacco plants to produce asialoerythropoietin. Plant Cell Rep. 2012;31:1233–1243. doi: 10.1007/s00299-012-1244-x. [DOI] [PubMed] [Google Scholar]
- 24.Parsons J, Altmann F, Arrenberg CK, Koprivova A, et al. Moss-based production of asialo-erythropoietin devoid of Lewis A and other plant-typical carbohydrate determinants. Plant Biotechnol. J. 2012;10:851–861. doi: 10.1111/j.1467-7652.2012.00704.x. [DOI] [PubMed] [Google Scholar]
- 25.Weise A, Altmann F, Rodriguez-Franco M, Sjoberg ER, et al. High-level expression of secreted complex glycosylated recombinant human erythropoietin in the Physcomitrella Delta-fuc-t Delta-xyl-t mutant. Plant Biotechnol. J. 2007;5:389–401. doi: 10.1111/j.1467-7652.2007.00248.x. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto S, Ikura K, Ueda M, Sasaki R. Characterization of a human glycoprotein (erythropoietin) produced in cultured tobacco cells. Plant Mol. Biol. 1995;27:1163–1172. doi: 10.1007/BF00020889. [DOI] [PubMed] [Google Scholar]
- 27.Marillonnet S, Thoeringer C, Kandzia R, Klimyuk V, et al. Systemic Agrobacterium tumefaciens-mediated transfection of viral replicons for efficient transient expression in plants. Nat. Biotechnol. 2005;23:718–723. doi: 10.1038/nbt1094. [DOI] [PubMed] [Google Scholar]
- 28.Strasser R, Stadlmann J, Schähs M, Stiegler G, et al. Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnol. J. 2008;6:392–402. doi: 10.1111/j.1467-7652.2008.00330.x. [DOI] [PubMed] [Google Scholar]
- 29.Castilho A, Strasser R, Stadlmann J, Grass J, et al. In planta protein sialylation through overexpression of the respective mammalian pathway. J. Biol. Chem. 2010;285:15923–15930. doi: 10.1074/jbc.M109.088401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strasser R, Castilho A, Stadlmann J, Kunert R, et al. Improved virus neutralization by plant-produced anti-HIV antibodies with a homogeneous beta1,4-galactosylated N-glycan profile. J. Biol. Chem. 2009;284:20479–20485. doi: 10.1074/jbc.M109.014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marchetti-Deschmann M, Kemptner J, Reichel C, Allmaier G. Comparing standard and microwave assisted staining protocols for SDS-PAGE of glycoproteins followed by subsequent PMF with MALDI MS. J. Proteomics. 2009;72:628–639. doi: 10.1016/j.jprot.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Stadlmann J, Pabst M, Kolarich D, Kunert R, et al. Analysis of immunoglobulin glycosylation by LC-ESI-MS of glycopeptides and oligosaccharides. Proteomics. 2008;8:2858–2871. doi: 10.1002/pmic.200700968. [DOI] [PubMed] [Google Scholar]
- 33.Komatsu N, Nakauchi H, Miwa A, Ishihara T, et al. Establishment and characterization of a human leukemic cell line with megakaryocytic features: Dependency on granulocyte-macrophage colony-stimulating factor, interleukin 3, or erythropoietin for growth and survival. Cancer Res. 1991;51:341–348. [PubMed] [Google Scholar]
- 34.Muster T, Steindl F, Purtscher M, Trkola A, et al. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castilho A, Gattinger P, Grass J, Jez J, et al. N-glycosylation engineering of plants for the biosynthesis of glycoproteins with bisected and branched complex N-glycans. Glycobiology. 2011;21:813–823. doi: 10.1093/glycob/cwr009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagels B, Van Damme EJ, Callewaert N, Zabeau L, et al. Biologically active, magnICON® expressed EPO-Fc from stably transformed Nicotiana benthamiana plants presenting tetra-antennary N-glycan structures. J. Biotechnol. 2012;160:242–250. doi: 10.1016/j.jbiotec.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Conley AJ, Zhum H, Le LC, Jevnikar AM, et al. Recombinant protein production in a variety of Nicotiana hosts: A comparative analysis. Plant Biotechnol. J. 2011;9:434–444. doi: 10.1111/j.1467-7652.2010.00563.x. [DOI] [PubMed] [Google Scholar]
- 38.Giritch A, Marillonnet S, Engler C, van Eldik G, et al. Rapid high-yield expression of full-size IgG antibodies in plants coinfected with noncompeting viral vectors. Proc. Natl. Acad. Sci. U. S. A. 2006;103:14701–14706. doi: 10.1073/pnas.0606631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castilho A, Bohorova N, Grass J, Bohorov O, et al. Rapid high yield production of different glycoforms of ebola virus monoclonal antibody. PLoS One. 2011;6:e26040. doi: 10.1371/journal.pone.0026040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bendandi M, Marillonnet S, Kandzia R, Thieme F, et al. Rapid, high-yield production in plants of individualized idiotype vaccines for non-Hodgkin's lymphoma. Ann. Oncol. 2010;21:2420–2427. doi: 10.1093/annonc/mdq256. [DOI] [PubMed] [Google Scholar]
- 41.Pogue GP, Vojdani F, Palmer KE, Hiatt E, et al. Production of pharmaceutical-grade recombinant aprotinin and a monoclonal antibody product using plant-based transient expression systems. Plant Biotechnol. J. 2010;8:638–654. doi: 10.1111/j.1467-7652.2009.00495.x. [DOI] [PubMed] [Google Scholar]
- 42.Loos A, Van Droogenbroeck B, Hillmer S, Grass J, et al. Expression of antibody fragments with a controlled N-glycosylation pattern and induction of endoplasmic reticulum-derived vesicles in seeds of Arabidopsis. Plant Physiol. 2011;155:2036–2048. doi: 10.1104/pp.110.171330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loos A, Van Droogenbroeck B, Hillmer S, Grass J, et al. Production of monoclonal antibodies with a controlled N-glycosylation pattern in seeds of Arabidopsis thaliana. Plant Biotechnol. J. 2011;9:179–192. doi: 10.1111/j.1467-7652.2010.00540.x. [DOI] [PubMed] [Google Scholar]
- 44.Kunert R, Steinfellner W, Purtscher M, Assadian A, et al. Stable recombinant expression of the anti HIV-1 monoclonal antibody 2F5 after IgG3/IgG1 subclass switch in CHO cells. Biotechnol. Bioeng. 2000;67:97–103. doi: 10.1002/(sici)1097-0290(20000105)67:1<97::aid-bit11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 45.Reisinger H, Steinfellner W, Stern B, Katinger H, et al. The absence of effect of gene copy number and mRNA level on the amount of mAb secretion from mammalian cells. Appl. Microbiol. Biotechnol. 2008;81:701–710. doi: 10.1007/s00253-008-1701-1. [DOI] [PubMed] [Google Scholar]
- 46.Darling RJ, Kuchibhotla U, Glaesner W, Micanovic R, et al. Glycosylation of erythropoietin affects receptor binding kinetics: Role of electrostatic interactions. Biochemistry. 2002;41:14524–14531. doi: 10.1021/bi0265022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.