Abstract
In order to characterize the effects of increasing phosphatidylinositol(4,5)bisphosphate (PtdIns(4,5)P2) on nuclear function, we expressed the human phosphatidylinositol (4)-phosphate 5-kinase (HsPIP5K) 1α in Nicotiana tabacum (NT) cells. The HsPIP5K-expressing (HK) cells had altered nuclear lipids and nuclear functions. HK cell nuclei had 2-fold increased PIP5K activity and increased steady state PtdIns(4,5)P2. HK nuclear lipid classes showed significant changes compared to NT (wild type) nuclear lipid classes including increased phosphatidylserine (PtdSer) and phosphatidylcholine (PtdCho) and decreased lysolipids. Lipids isolated from protoplast plasma membranes (PM) were also analyzed and compared with nuclear lipids. The lipid profiles revealed similarities and differences in the plasma membrane and nuclei from the NT and transgenic HK cell lines. A notable characteristic of nuclear lipids from both cell types is that PtdIns accounts for a higher mol % of total lipids compared to that of the protoplast PM lipids. The lipid molecular species composition of each lipid class was also analyzed for nuclei and protoplast PM samples. To determine whether expression of HsPIP5K1α affected plant nuclear functions, we compared DNA replication, histone 3 lysine 9 acetylation (H3K9ac) and phosphorylation of the retinoblastoma protein (pRb) in NT and HK cells. The HK cells had a measurable decrease in DNA replication, histone H3K9 acetylation and pRB phosphorylation.
Keywords: plant nuclei; phospholipids; histone; phosphatidylinositol (4,5) bisphosphate; plasma membrane
1. Introduction
The phosphoinositide (PI) pathway is important for many biological processes that affect plant basal metabolism and growth including vesicle trafficking, membrane structure, protein localization, enzyme activity, ion channel regulation, secondary messenger formation and organelle signaling [1–4]. The amount and cellular location of PI pathway lipids and/or enzymes respond to many internal and external cellular cues including heat stress, osmotic stress, cell expansion, cell type, differentiation, and cellular metabolism [1,5–14]. Altering the flux through the plant PI pathway by mutation of genes in the PI pathway, overexpression of genes or heterologous expression systems has been shown to affect plant growth and development [3,15–21]; however, the effects of PI metabolism on nuclear processes and nuclear lipids have not been studied in plants.
In yeast, animals, and plants, PI pathway enzymes or enzyme activities and their products have been found in the nucleus [9,12,22–29]. In animal cells, the nuclear localized PI pathway is regulated independently of the PM bound enzymes and lipids creating a novel intra-nuclear signaling mechanism composed of nuclear generated inositol phospholipids and inositol phosphates [5,9,26,30]. For example, the inositol phospholipid PtdIns(4,5)P2 has a variety of effects on nuclear proteins and processes including, but not limited to, removal of histone H1 from chromatin in vitro [31], regulating multiple remodeling complexes similar to the SWI/SNF complex like Brahma associated factor (BAF) [32,33], regulating a novel polyadenylate polymerase [34,35], and interacting with other nuclear proteins like actin binding proteins [7,8]. In D. melanogaster the nuclear phosphatidylinositol (4)-phosphate 5-kinase (PIP5K), Skittles, is essential for embryogenesis, and when skittles is mutated, histone H1 is hyperphosphorylated and chromatin is compacted [22].
Another inositol phospholipid, phosphatidylinositol(5)phosphate (PtdIns5P), interacts with human INhibitor of Growth 2 (ING2) to regulate a variety of chromatin remodeling complexes [36]. Nuclear localization and activity of phosphatidylinositol-phospholipase C (PI-PLC) β1 was required for cell cycle progression as demonstrated by G2/M blockade by PI-PLC inhibitors [37] and the dominant negative activity of a mutant PI-PLCβ1 without a nuclear localization signal [38]. In animals and yeast many other PI pathway inositol phospholipids, inositol phosphates and enzymes have been shown to modify nuclear functions [9,32,33,39,40] including cell cycle progression [5,41–43], germline development [44], and cell polarity [45].
In contrast, the plant cellular and nuclear PI pathways are still being characterized. Early reports described plant nuclear lipids based on [3H]ethanolamine [5] and [3H] myo-inositol labeling as well as by measuring PIP5K activity with [γ–32P]ATP labeling [24]. Further analysis of plant nuclei has shown specific nuclear lipid kinase activities including phosphatidylinositol 3-kinase (PI3K) [29] and phosphatidylinositol 4-kinase (PI4K) [46]. More recent data indicate that plant lipids, their modifying enzymes including PIP5Ks, and proteins with lipid binding domains will localize to the nucleus [12,47–53]. However, plant nuclear lipids are not well characterized, and to our knowledge, no one has studied the effects of increasing the flux through the nuclear PI pathway in plants.
We have employed a heterologous expression to assess whether increased cellular PtdIns(4,5)P2 affected nuclear lipids and functions. Im et al. [54] had shown that N-terminal GFP tagged Homo sapiens phosphatidylinositol 4-phosphate 5kinase 1α (E.C. 2.7.1.68) (hereafter denoted HsPIP5K1α) localized to the PM of Nicotiana tabacum (NT) cells and increased PM PIP5K specific activity and steady state PM PtdIns(4,5)P2 by 100-fold [54]. HsPIP5K1α is known to localize to both the PM and the nucleus in human cell lines [11]. Although in previous studies GFP fluorescence from expressed GFP-HsPIP5K1α was primarily detected in the plasma membrane of HsPIP5K1α-expressing (HK) cells [54], we hypothesized that expressing HsPIP5K1α would affect the plant nuclear PI pathway as well as the plasma membrane PI pathway. We show that expressing HsPIP5K1α increased nuclear PIP5K activity and PtdIns(4,5)P2. Isolated HK nuclei also have altered phospholipid composition compared to NT nuclei. Nuclei were isolated from protoplasts, and the analyzed protoplast PM and nuclei showed distinct differences in lipid classes in the NT and HK cell lines. We also assessed the effects of expressing HsPIP5K1α on several nuclear functions. DNA replication measured by Bromodeoxyuridine (BrdU) incorporation, histone acetylation on histone 3 lysine 9 and phosphorylation of the retinoblastoma protein (pRb) were all decreased in HK cells.
2. Methods
2.1 Protoplast isolation
Cells were harvested 4 d after transfer by gravity filtration using a 75 μm filter. Cells with a fresh weight (fw) between ~0.7 g to 1.6 g fw per 25 mL of medium were used to produce protoplasts for plasma membrane and nuclei isolation. To digest the cell wall, cells (0.7 to 1 g fw) were incubated at room temperature (RT) with shaking at 75 rpm in a 10 × 10 mm plastic Petri dish for 1 ½ to 2 h in 10 mL of cell wall digestion buffer (0.4 M sorbitol in 10 mM MES (pH 5.6) with 7.5 mg.mL−1 cellulase and 0.1 mg.mL−1 pectolyase). Cell wall digestion was monitored microscopically and was stopped by addition of 10 mL protoplast wash buffer (PWB: 0.4M D-sorbitol in 10 mM MES (pH 5.6)) when round protoplasts were evident and the remaining cells would release protoplasts when gentle pressure was applied to the microscope cover slip. To remove remaining cells and debris, the protoplasts were filtered through a 75 μm filter followed by 2 rinses with 10 mL of PWB and collected in the 50 mL Falcon tube. Filtered protoplasts were centrifuged at 100 g for 5 min. Each protoplast pellet was washed 2 times with 10 mL of PWB by gentle resuspension in PBW followed by centrifugation at 100 g.
2.2 Nuclei Isolation
Nuclei were isolated immediately from protoplasts. Protoplasts were incubated on ice for 5 min in PWB, the supernatant was removed with a Pasteur pipette, and protoplasts gently resuspended in 6 mL of ice cold nuclear isolation buffer 1 (NIB1) (0.7 molal D-sorbitol HB (5 mM HEPES, 10 mM MgCl2, 2 mM EGTA, pH 7) and incubated for 5 min on ice. Protoplasts were centrifuged at 100 g for 5 min at RT and returned to ice promptly. NIB1 was removed and protoplasts were gently resuspended in 6 mL of ice cold NIB2 (0.2 molal D-sorbitol HB, 0.025% Triton X-100, 1 μg.mL−1 leupeptin, 100 μM PMSF (phenylmethanesulfonyl fluoride) and incubated at least 5 min on ice. Protoplasts in NIB2 were passed through a 26 gauge double-sided needle fitted with 2, 10 mL syringes with rubber tipped plungers, 6 to 7 times. Broken protoplast mixture was filtered through 75 μm mesh and two layers of nylon mesh (150 μm) and rinsed through 2 times with 6 mL of ice cold NIB3 (NIB2 without Triton X-100) and collected into new 50 mL Falcon tubes. The filtered solution was centrifuged 5 min at 100 g at RT. A Pasteur pipette was used to carefully remove all the supernatant so as to not disturb the loose pellet. The pellet was gently washed with 6 mL of ice cold NIB3, centrifuged at 100 g for 5 min, supernatant was removed with a Pasteur pipette and the isolated nuclei were kept on ice for further procedures.
2.3 Plasma membrane isolation from protoplasts
For plasma membrane isolation, protoplasts were ground 25–30 times in a Dounce ground glass homogenizer on ice and centrifuged at 3,000 g for 10 min at 4° C to remove unbroken nuclei and other organelles. The supernatant was centrifuged at 40,000 g for 1 h to recover a microsomal pellet as previously described [54]. A plasma membrane-enriched fraction was isolated from the microsomal pellet by aqueous two-phase partitioning as previously described [55].
2.4 Protein quantification
Plasma membrane and nuclei protein was quantified using bicinchoninic acid (BCA) protein assay (microBCA kit from Pierce, Rockingham, IL) with BSA as a standard.
2.5 PtdIns(4,5)P2 mass measurement
Aliquots of nuclei containing at least 0.5mg of nuclear protein were added to 20% cold PCA (perchloric acid) in a 1:1 v/v ratio. Lipids were extracted from PCA precipitate, the headgroup was hydrolyzed, and Ins(1,4,5)P3 measured using the Ins(1,4,5)P3 binding assay (Amersham Inc.) as previously described [56].
2.6 Lipid Kinase Assay
Nuclei were assayed for PIK, PIP5K and DAGK activity with 10 μg of protein for 10 min using the conditions previously described for plasma membrane and microsome activity assays [54,55], with one modification. The ATP concentration was increased to 100 μM with 74 kBq of [γ–32 P]ATP .nmole−1 total ATP per assay because of competing reactions in the nuclear preparations. When exogenous substrate was added to the reaction mixture, the final concentration was 125μM in 0.01% Triton X-100. To monitor the effects of increasing ATP, the specific activity of the [γ –32 P]ATP was held constant (74 kBq.nmole−1 ATP); the reaction was incubated for 30 min and 20 μg of protein was used per assay (Supplemental Figure 2). While 100 μM ATP was not found to be saturating, in the interest of safety we did not use higher concentrations of ATP but rather lowered the amount of protein used for the subsequent kinase assays. The reactions were stopped and lipids were extracted and separated on Partisil LK5D TLC plates (Whatman Inc., Maidstone, England) with chloroform (CHCl3): methanol (MeOH): concentrated ammonia (NH4OH):water (90:90:7:22, v/v/v/v) as a solvent as previously described [54]. Incorporation of 32P was monitored with autoradiography, quantified using a BioScan Imaging scanner and reported as pmol.mg−1.min−1 and % of Total radioactive counts.
2.7 Lipid profiling
Lipids were isolated from 45 to 213 μg of plasma membrane protein and 50 to 4000 μg of nuclei protein using the protocol recommended at the Kansas State Lipidomics Web site for animal tissue samples (http://www.k-state.edu/lipid/lipidomics/animal-lipid-extraction.htm). Plasma membrane and nuclei samples were brought up to between 250 and 500 μL in volume with Tris buffer or NIB3, respectively. Briefly, volume of nuclei or plasma membrane used for lipid isolation was set as 0.8 part, and 1 part of CHCl3 and then 1 part of MeOH were added and the solution was vortexed immediately. An additional 1 part of CHCl3 was added to the mixture and vortexed briefly. The mixture was centrifuged at 1000 g for 10 min. The lower, CHCl3 layer was removed and placed in a clean test tube. The remaining upper layer was extracted 2 additional times by adding 1 part of CHCl3 followed by a 5 min centrifugation at 1000 g. The CHCl3 lower phase was removed after each centrifugation. The combined CHCl3 fraction was back-washed with 0.1 mL of 1M KCl by brief vortexing followed by centrifugation at 1000 g for 5 min. The top, aqueous KCl layer was removed. The remaining CHCl3 fraction was further washed with 0.1 mL H2O. After the H2O wash the CHCl3 layer was removed to a clean test tube and the remaining H2O was re-extracted with 1 part CHCl3. The combined CHCl3 solution was stored at −20°C for up to 1 week. CHCl3 extractions from nuclear and plasma membrane lipids from the same day were dried under a stream of N2 on the same day and stored under N2 at −20°C. Lipids were shipped on dry ice or ice to the Kansas Lipidomics Center for analysis.
2.8 Analysis of lipid profile
Lipids were divided into 11 lipid classes by headgroup (digalactosyldiacylglycerol (DGDG), monogalactosyldiacylglycerol (MGDG), lysophosphatidylcholine, (LysoPtdCho), phosphatidylglycerol (PtdGro), lysophosphatidylethanolamine (LysoPtdEtn), phosphatidylinositol (PtdIns), phosphatidic acid (PtdOH), phosphatidylethanolamine (PtdEtn), phosphatidylcholine (PtdCho), lysophosphatidylglycerol (LysoPtdGro), phosphatidylserine (PtdSer)) and each lipid class was divided into lipid molecular species by the headgroup and the fatty acid contents (ie. PtdIns 34:1, PtdGro 32:0). The lipid classes of each sample were screened with both the Q test and Grubbs test, and outlier samples for each lipid classes were removed (Supplemental Table 1). Lipid class and lipid molecular species data were analyzed by pair-wise two tailed Student T-tests with the assumption of equal variance similar to previously reported analysis [57] (Supplemental Table 2). Comparisons were made between the NT and HK lipid samples within each sample type (e.g. comparison of NT and HK nuclear lipid analysis) and between different sample types (i.e. PM to nuclei comparison from NT cells). For plasma membrane analysis, five different samples from 3 different biological replicates were averaged. For nuclear lipid analysis, 10 wild type samples from 7 different biological replicates and 12 HK samples from 8 different biological replicates were harvested.
2.9 BrdU labeling
Cells were harvested by gravity on a 75 μm filter. 0.1g of cells from flasks with similar weights (example flasks with fw between 0.7 to 1.2 g) were placed in 25mL of the same conditioned media (media in which the cells had been growing) and allowed to equilibrate for 30 minutes. BrdU (10 μM) was added for either 30 min or 1 h at RT at 75 rpm shaking. The cells were harvested by suction filtration to remove media, and divided into 50 μg aliquots before freezing. DNA was extracted from 50 μg of cells with Qiagen Plant Genomic DNA extraction kit. DNA concentration was measured and the samples were diluted so that the DNA concentration was equivalent for all cell lines. Three aliquots of increasing amounts of DNA were spotted onto nitrocellulose, cross-linked and the BrdU labeled DNA was detected using a BrdU specific antibody (Sigma, Inc.) and appropriate secondary antibody as previously described [58] and supersignal chemiluminescence (Pierce, Rockingham, IL).
2.10 Western Blot Analysis
Cells were harvested by gravity filtration 4 d after transfer and frozen at −80°C. Nuclear protein was isolated as described above and boiled in SDS-PAGE loading buffer.
2.10.1 Calreticulin and H+-ATPase immunoblots
Nuclei proteins (10 μg) were separated on 8% SDS-PAGE for H+-ATPase or 10% SDS-PAGE for calreticulin (CRT). Protein was transferred to PVDF membrane with 1x CAPS buffer. H+-ATPase, a marker for plasma membrane, was detected using a polyclonal primary antibody (described in [59]). A polyclonal antibody to the maize CRT was used to detect CRT as a marker for ER as previously described [54]. The antibodies were visualized using anti-rabbit secondary antibody and super signal chemiluminescence (Pierce, Rockingham, IL, according to the manufacturer’s directions).
2.10.2 Histone immunoblots
To enrich protein isolations for histones, proteins were acid extracted from frozen cells by grinding 25–30 times in a ground glass homogenizer (10 mM Tris-HCl, pH 7.5, 2mM EDTA, 0.25 M HCl, 5 mM 2-mercaptoethanol, 0.2 mM PMSF), precipitated with 25% TCA and washed 2x with ice cold 100% acetone as described by Tariq et al. (2003)[60]. The dried acetone protein pellets were resuspended in 5 % SDS overnight and protein quantified by BCA assay.
Proteins were separated by 15% SDS-PAGE and transferred to PVDF membranes with Tris-glycine transfer buffer. H3 lysine 9 acetylation (H3K9ac) and H3 were detected using primary antibodies to H3K9ac (Upstate, 07-352) and total H3 antibody (Abcam, ab1791) and were visualized with supersignal chemiluminescence (Pierce, Rockingham, IL). H3K9ac was compared to total H3 to ensure equal histone loading. ImageJ [61] was used to calculate a ratio of the H3K9 acetylation intensity to the H3 intensity of the same, reprobed blot. The NT H3K9ac /H3 ratio was normalized to 100% to compare HK samples to NT samples.
2.10.3 Retinoblastoma (pRb) immunoblots
Protein was isolated from frozen cell samples by grinding 25–30 times in a ground glass homogenizer in buffer containing (25 mM TRIS-HCl pH 7.6, 15 mM MgCl2, 15 mM EGTA, 75 mM NaCl, 60 mM β-glycerophosphate, 1 mM dithiothreitol (DTT), 0.1% NP-40, 0.1 mM Na3VO4, 1 mM NaF, 1 mM phenylmethylsulphonyl fluoride (PMSF), 10 mg.mL-1 protease inhibitors (Complete, Roche, Mannheim, Germany)) as in Abraham et al. [62]. Proteins were separated by 10% SDS-PAGE and transferred to PVDF membranes with Tris-glycine transfer buffer. Nicotiana tabacum phosphorylated pRb protein was detected using human polyclonal antibody (Phospho-Rb (Ser807/811) Antibody #9308, Cell Signaling Technology) and visualized with supersignal chemiluminescence (Pierce, Rockingham, IL).
3. RESULTS
3.1 Nuclei isolated from the NT and HK cells appear similar using light microscopy
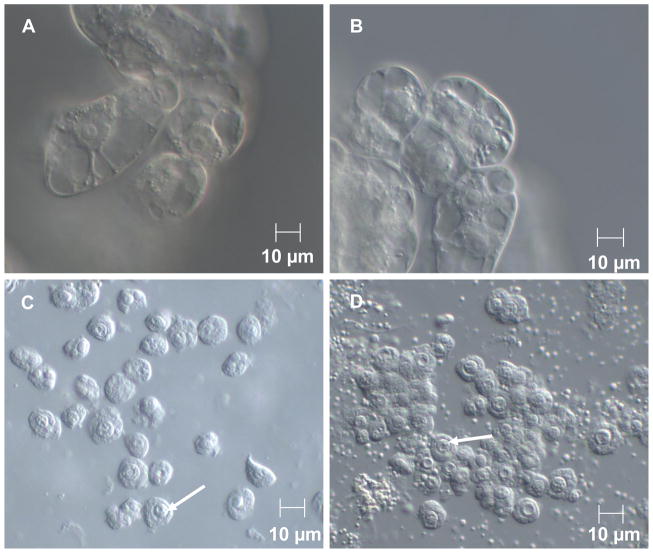
We hypothesized that expression of HsPIP5K1α in NT cells would affect the PIP5K activity in the nucleus. To test this hypothesis, we first isolated nuclei from NT and HK cells by rupturing protoplasts. Nuclei from both NT cells and HK cells have similar size (Figure 1). The HK cells contained more noticeable starch granules and these co-purify with nuclei (Figure 1). Visible starch granule contamination could be reduced by applying nuclei to Percoll gradients; however, recovered nuclei were less abundant and visually less stable so we did not use gradient purification.
Figure 1.
Nuclei isolated from NT and HK cells are intact and have similar size. NT (A) and HK (B) cells. HK cells are slightly smaller. NT (C) and HK (D) nuclei after isolation. Note Nucleolus denoted by arrows.
To assess the purity of nuclear preparations we detected plasma membrane contamination using antibodies to the plasma membrane H+-ATPase and ER contamination using antibodies to maize calreticulin (CRT) (Supplemental Figure 1A). There was no H+-ATPase detected in either the HK or NT nuclei fractions indicating little if any PM contamination. The ER is contiguous with the outer nuclear envelope and invaginations and ER associated proteins have been found within the nucleus [63–65]. Calreticulin (CRT), an ER associated protein, has been found associated with nucleus and outer nuclear envelope by fluorescent protein localization [66–68]. A small amount of CRT was detected in nuclei isolated from both HK and NT cells. The relative amount of CRT recovered increased in nuclei isolations applied to Percoll gradients, where the nuclei were disrupted, and as noted above, these preparations were not used for further experiments (Supplemental Figure 1B).
To assess plastid contamination and determine whether the increased starch granules resulted in increased plastid membrane contamination the lipids, MGDG and DGDG were compared between the two nuclei preparations. As shown in Figure 2 there was no difference in total MGDG or DGDG lipid class from nuclei isolated from the HK and NT cell lines even though the HK line had significantly higher number of starch granules. For comparison MGDG and DGDG were also assayed in the protoplast PM and increased MGDG, but not DGDG, was found in HK protoplast PM. This is in contrast to previous analysis of cell PM which showed decreased MGDG and DGDG in HK cells compared with NT cells [54] and may reflect differences in the plasma membrane lipids after cell wall digestion.
Figure 2.
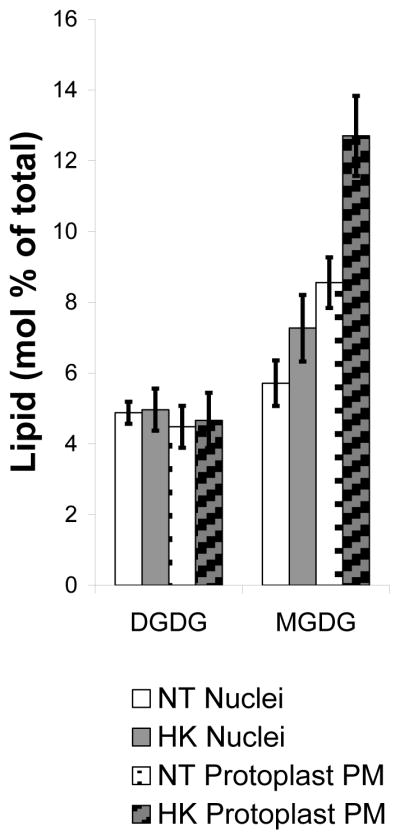
DGDG and MGDG from NT and HK nuclei and protoplast PM samples. NT nuclei (white), NT protoplast PM (white dotted), HK nuclei (grey) and HK protoplast PM (grey striped). Nuclei data are represented by the average mol % ± standard error (SE). NT nuclei data is the average of 10 samples from 7 different biological replicates and HK nuclei data is the average of 12 samples from 8 different biological replicates. Protoplast PM data are the average of 5 lipid extractions from 3 different biological replicates. Significant changes in the MGDG and DGDG were not found when comparing NT to HK nuclear lipid samples, but a significant increase was seen in the HK protoplast PM MGDG compared to the NT protoplast PM MGDG. In addition, there is a 1.4 fold increase in MGDG on a mol % basis in the PM of both types of protoplasts compared to the respective nuclei.
3.2 HK nuclei had increased PtdIns(4,5)P2 and PIP5K activity
3.2.1 HK nuclei had increased PtdIns(4,5)P2 based on mass 0measurements
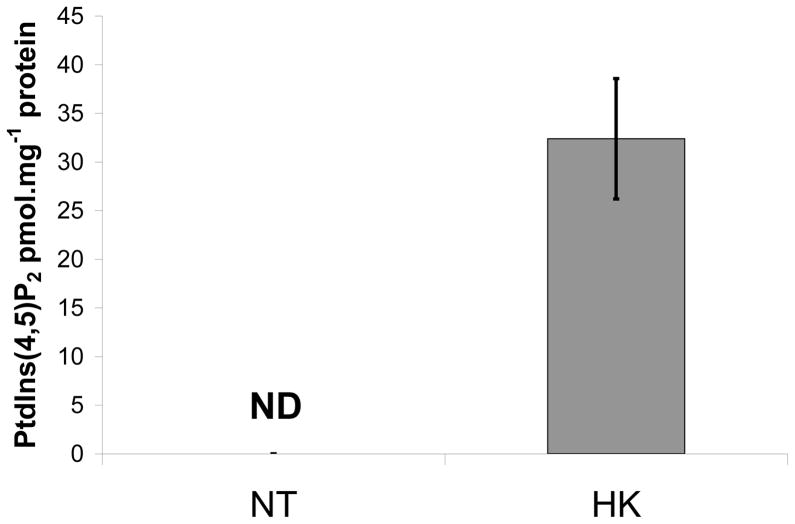
Mass measurements indicated a steady state increase in PtdIns(4,5)P2 in the HK nuclei. HK nuclei had 32.4 ± 6.2 pmol PtdIns(4,5)P2.mg−1 protein, while a NT nuclei (at least 0.5 mg of protein) did not have detectable PtdIns(4,5)P2 by mass measurement (Figure 3). Because the PtdIns(4,5)P2 in the NT nuclei was below the limits of detection with this assay method, we used in vitro PIPK assays to measure the potential to synthesize PtdIns(4,5)P2 from PtdInsP.
Figure 3.
HK nuclei have increased steady state PtdIns(4,5)P2. Lipid headgroup analysis of steady state PtdIns(4,5)P2 was measured from NT (white) and HK (grey) nuclei. Measurements are the average of 3 independent nuclei isolations ± Stdev. ND = not detected. At least 0.5 mg of nuclear protein was used per sample.
3.2.2 HK nuclei have increased PIP5K activity
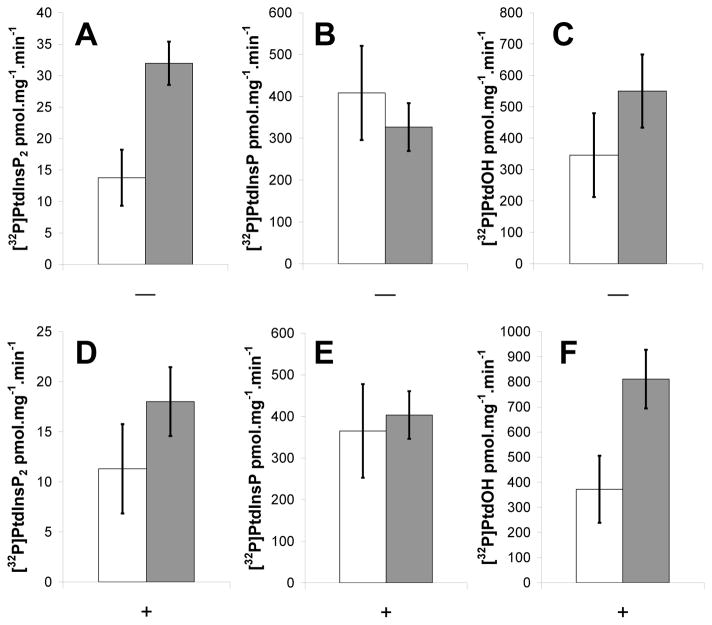
PIP5K activity assays were performed using isolated nuclei from HK and NT cells. Using endogenous substrate and 100μM ATP, both NT and HK nuclei had diacylglycerol kinase (DAGK), PtdIns kinase (PIK) and PIPK activity. The HK nuclei produced about 2-fold more [32P]PtdInsP2.mg−1 protein compared with NT nuclei. (Figure 4A). The increase is similar in fold-differences to what was reported for plasma membranes from the HK cells with endogenous substrate [54]. Unlike the plasma membrane; however, the PIPK activity did not increase significantly in the nuclei preparations when exogenous substrate (PtdIns4P 125 μM) was added. The lack of detectable increase may be because there were so many competing reactions in the nuclei that used the ATP and PtdIns4P. In support of this hypothesis, there tended to be less [32P]PtdInsP2 and more [32P]PtdOH recovered from HK nuclei when exogenous PtdIns4P was added (Figure 4). Furthermore, when PtdIns was added to the isolated nuclei, [32P]PtdInsP2 increased significantly suggesting that the enzyme preferred endogenously synthesized PtdInsP and that there may be a concerted reaction from PtdIns to PtdInsP2 (Supplemental Figure 3).
Figure 4.
HK nuclei have increased endogenous PIP5K activity. NT (white) and HK (grey) nuclei were assayed for endogenous lipid kinase activity without PtdIns4P added (−) and added PtdIns4P (+). [32P]PtdInsP2 (A, D), [32P]PtdInsP (B, E) and [32P]PtdOH (C, F) are reported as pmol.mg−1.min−1. Measurements are the average of 3 independent experiments ± SE.
3.3 Comparison of lipid classes and lipid molecular species between NT and HK nuclei and protoplast PM lipids
3.3.1 Nuclear lipid classes were different in the NT and HK lines
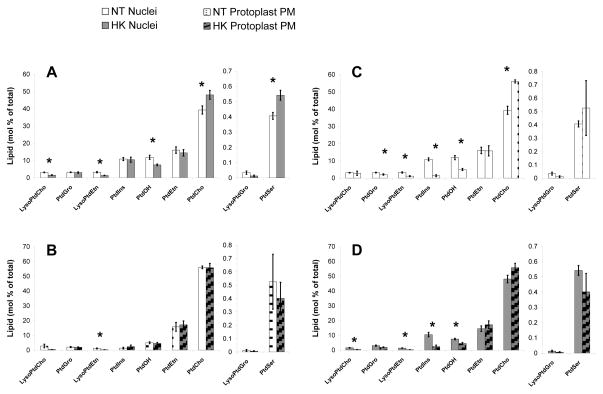
Nuclear lipids were extracted from NT and HK nuclei and analyzed by Kansas Lipidomics Research Center Analytical Laboratory as previously described [57]. Analysis of NT and HK nuclear lipids revealed significant differences in 5 out of 11 lipid classes (mol %, p-value of 0.05) (Figure 5 A). HK nuclear lipids had significantly decreased mol % of PtdOH, LysoPtdCho and LysoPtdEtn and increased PtdSer and PtdCho compared to NT nuclei. Even though the HK nuclei had high production of PtdInsP2, the mol % PtdIns was not significantly different.
Figure 5.
Lipid classes from NT and HK nuclei and protoplast PM show significant differences between cell types. A. Comparison of lipid classes from NT (white) and HK (grey) nuclei. B. Comparison of lipid classes from NT (white dotted) and HK (grey striped) protoplast PM. C. Comparison of lipid classes from NT nuclei (white) and NT protoplast PM (white dotted). D. Comparison of lipid classes from HK nuclei (grey) and HK protoplast PM (grey striped). * indicates a changed lipid classes at a p-value of 0.05. NT nuclei data are the average of 10 lipid extractions from 7 independent biological replicates. HK nuclei data are the average of 12 samples from 8 independent biological replicates. NT and HK protoplast PM data are the average of 5 lipid extractions from 3 independent biological replicates. All data are the average mol % ± SE.
3.3.2 Changes in lipid molecular species of NT and HK nuclear lipids
The 11 lipid classes analyzed can be subdivided into 153 unique lipid molecular species that have different fatty acid tail/headgroup combinations. Over 25% of the lipid molecular species (47 out of 153) showed significant changes between NT and HK nuclei at p-value of 0.05 (Supplemental Table 2). Half of the significantly changed lipid molecular species (28) were changed between NT and HK nuclei at a p-value of 0.01. In HK nuclei PtdCho 36:1, PtdIns 36:1 and PtdSer 36:1 increased about 2-fold on a mol % basis and PtdCho 32:0 and PtdOH 32:0, LysoPtdCho 18:2 and LysoPtdEtn 18:2 decreased over 2-fold on a mol % basis compared with NT nuclei. Many of the changes in lipid molecular species with similar fatty acid composition (carbon content: double bond number (i.e. 36:1)) had a similar direction and amount of change in the NT and HK nuclei comparison regardless of lipid molecular species headgroup. For example the lipid molecular species PtdCho 36:1 and PtdIns 36:1 have different headgroups, but both lipid molecular species increased about 2-fold based on mol % in HK nuclei compared with NT nuclei.
In general, the unsaturated fatty acids increased and saturated fatty acids decreased in HK nuclei. Many of the lipid molecular species with unsaturated fatty acids increased in HK nuclei compared to NT nuclei with a p-value of less than 0.01. Out of 10 lipid molecular species with saturated fatty acids that were measured, 7 decreased at least 1.5 times on a mol % basis in HK nuclei compared to NT nuclei. Most lipid molecular species derived directly from plastid fatty acids (16:0-16:0 fatty acid profile) decreased in HK nuclei, suggesting a shift in the origin of the fatty acids for lipid molecular species. The only plastid fatty acid lipid molecular species that increased in the HK nuclei was PtdIns 32:3, which increased 15 fold on a mol % basis, but is found in trace amounts compared to other PtdIns lipid molecular species composed of 16:0 fatty acids.
3.3.3 Analysis of lipid classes in nuclei and protoplast PM
PtdIns(4,5)P2 has been characterized as a signaling lipid, and is known to change the curvature of a lipid membrane [2]. Because HsPIPK1α localizes primarily to the PM and resulted in a 100-fold steady state increase in PtdIns(4,5)P2 from the PM of cells [54] and because we had to first isolate protoplasts in order to obtain intact nuclei, we wanted to characterize the impact of high PtdIns(4,5)P2 on the protoplast PM lipid composition. We compared 11 lipid classes from both nuclei and protoplast PM samples from both NT and HK cell lines (Figure 5). Molecular percentages (mol %) of the total lipids recovered of each lipid classes were compared between different nuclei samples and different protoplast PM samples as well as comparison of nuclei to protoplast PM lipids of each cell type.
3.3.4 Comparison of protoplast PM lipid classes between NT and HK cells
Total LysoPtdEtn decreased 5 fold on a mol % basis in HK protoplast PM (Figure 5B) and MDGD increased 1.4 fold on a mol % basis compared with NT protoplast PM (Figure 2). LysoPtdEtn is the only lipid class that was decreased significantly in both protoplast PM and nuclei of the HK protoplasts. Notably, LysoPtdEtn is a lipid with an inverted cone-shaped structure resulting from the loss of one fatty acid while PtdIns(4,5)P2 has a cone shape because of the large polar headgroup [2,69], so the decrease in LysoPtdEtn may reflect a compensatory mechanism to maintain membrane structure.
3.3.5 Comparison of protoplast PM lipid molecular species between NT and HK cells
The PM of HK protoplasts had 25 lipid molecular species significantly changed from PM of NT protoplasts (Supplemental Table 2). Ten lipid molecular species increased in the HK protoplast PM compared with NT protoplast PM. An increase of 1.5 times or more on a mol % basis was seen in HK protoplast PM PtdCho 36:1 and PtdEtn 36:1 and 7 other lipid molecular species. 15 lipid molecular species decreased in the HK protoplast PM compared with NT protoplast PM. These included a decrease of PtdCho 32:0 of 1.5 fold on a mol % basis in HK protoplast PM and a decrease of 3 fold on a mol % basis in PtdEtn 32:0.
3.3.6 Common and unique changes are found between nuclei and protoplast PM lipids in both NT and HK samples
Lipids in the nucleus and the plasma membrane originate from plastids and the endoplasmic reticulum and changes in the trafficking between the ER and plastids or to either the nucleus or PM could affect the lipid composition. PtdIns(4,5)P2 is an important molecule for signaling and trafficking, and has been shown to cause changes in cellular trafficking and membrane lipid composition. We have shown that changes in cellular PtdIns(4,5)P2 can also alter the lipid composition of nuclei and protoplast PM. To further understand how the nuclear lipids and protoplast PM lipids were affected by expression of the HsPIPK1α, we compared the nuclear and plasma membrane lipids of protoplasts within each cell line.
3.3.7 Comparison of lipid classes between protoplast PM and nuclei for each cell line
Both NT and HK cell lines showed similar differences between the protoplast PM and nuclear lipid profiles. The nuclear MGDG was 1.4 fold lower on a mol % basis and nuclear PtdIns, LysoPtdEtn and PtdOH were over 1.5 fold higher on a mol % basis compared to the protoplast plasma membrane in both cell lines (Figure 5 C, D). The cell lines also had distinct differences in the lipid comparisons between protoplast PM and nuclei. The NT nuclear samples had significantly more PtdGro and less PtdCho compared to the protoplast PM (Figure 5C) and the HK nuclei had more LysoPtdCho compared to the HK PM (Figure 5D).
3.3.8 Comparison of nuclei and protoplast PM lipid molecular species for both NT and HK cells
Analysis of NT and HK nuclei to protoplast PM lipid molecular species revealed 40 common changes at p-value of 0.05 (Supplemental Table 2). Nuclei had 23 lipid molecular species that account for increased mol % compared to the protoplast PM from both cell lines, including LysoPtdCho 18:2, LysoPtdEtn 18:2, and PtdCho 34:1. NT and HK nuclear lipids have 17 lipid molecular species accounting for a decreased mol % when compared with protoplast PM samples, including PtdIns 32:1 and PtdEtn 32:1.
NT samples had 16 distinct lipid molecular species that were different in the nuclei compared with protoplast PM, including 6 were over 1.5-fold higher on a mol % basis like PtdIns 32:1 and 7 that were over 1.5-fold lower on a mol % basis like PtdSer 40:2 (Supplemental Table 2). HK nuclei had 12 distinctly different lipid molecular species including 4 that were over 1.5-fold higher on a mol % basis e.g., PtdSer 38:2, and 8 that were over 1.5-fold lower on a mol % basis e.g., LysoPtdGro18:2 (Supplemental Table 2).
A comparison of nuclear and protoplast PM lipids showed distinct changes in NT and HK cell lines, but also show common lipid profiles characteristic of the nucleus and PM in both cell lines. Interestingly, trace amounts of very long chain fatty acid lipids with fatty acid composition 42-carbon in PtdEtn and PtdSer and 44-carbon in PtdSer were observed in the nuclei suggesting that, like yeast [70], the plant nuclei may employ very long chain fatty acids for specialized functions (These very long chain fatty acids were previously described in plants [71]). Our lab had previously analyzed PM lipids isolated from cells of the NT and HK lines [54]. To gain insight into the effects of the cell wall digestion process on PM lipid composition of cells with high PtdIns(4,5)P2, we compared our current protoplast PM data with the previously published cell PM data [54].
3.4 Comparison of lipid classes and lipid molecular species between protoplast and cell PM
3.4.1 Common and cell line specific changes in lipid classes during cell wall digestion of NT and HK cells
Not surprisingly, the cell PM from both NT and HK cell lines had similar changes in lipid classes after cell wall digestion (Supplemental Table 2). Significant changes in the total mol % of PtdCho, PtdEtn and PtdSer are found in both NT and HK protoplast PM lipids compared with cell PM lipids at a p-value of 0.05. For example, compared to the PM from cells, protoplast PM PtdCho was over 2-fold higher on a mol % basis, PtdEtn was over 2-fold lower on a mol % basis and PtdSer was over 9-fold lower based on mol % in the protoplast PM from both NT and HK lines.
Cell line specific changes are also detected, for example, NT protoplast PM had decreased DGDG (a 1.6-fold decrease in mol % basis) and PtdIns (3-fold decrease in mol %) compared to NT cell PM. In comparison, HK protoplast PM MGDG is 1.5-fold higher on a mol % basis compared to the HK cell PM.
3.4.2 Comparison of lipid molecular species from cell and protoplast PM
Differences between cell and protoplast PM lipid samples are also reflected in changes in composition of lipid molecular species. Comparison of protoplast PM and cell PM revealed 27 lipid molecular species changes in common between NT and HK samples (Supplemental Table 2). Increases of over 1.5-fold on a mol % basis were found in 7 lipid molecular species in protoplast PM, e.g. PtdEtn 34:1 and PtdCho 34:1, and decreases of 1.4 fold on a mol % basis were observed in 20 lipid molecular species in the protoplast PM, e.g. PtdEtn 34:2 and PtdSer 34:2.
3.5 Effects of increasedPtdIns(4,5)P2 on nuclear functions
Animal nuclear lipids have been implicated in multiple nuclear processes [7,8,26]. We have shown increased PtdIns(4,5)P2 and changes in the nuclear lipid profile in HK nuclei, and we wished to assess the effect of the cellular and nuclear lipid changes on other nuclear processes including DNA synthesis, chromatin modification and cell cycle regulation proteins.
3.5.1 BrdU incorporation is less in HK cells
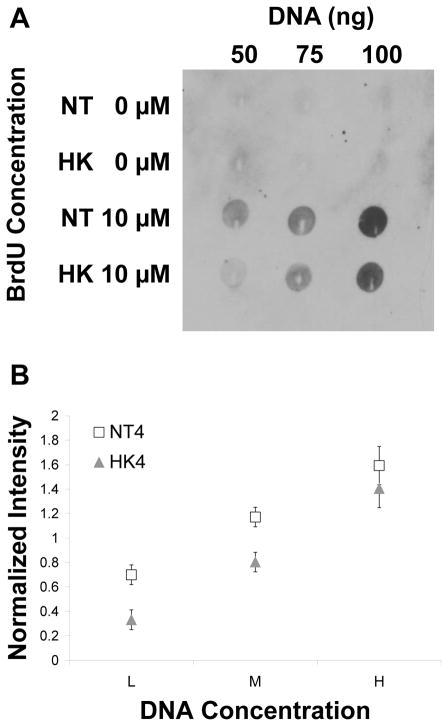
We compared BrdU incorporation into DNA in the NT and HK cells to assess the effect of lipid changes on DNA synthesis. Cells were incubated in equal amounts of BrdU, DNA isolated and three concentrations of DNA spotted on membranes for immunoblot detection of BrdU incorporation. Dot blot analysis of NT and HK BrdU containing DNA after incubation with BrdU showed significantly less BrdU incorporation in HK DNA (Figure 6A). Blot signals were normalized by dividing the intensity of an individual measurement (i.e. NT day 4 lowest DNA concentration) by the average of all three day 4 NT and HK samples to normalize the samples for the blot intensity. Normalized data for each data measurement including over three biological replicates of cell labeling experiments were averaged for NT and HK cells at three different DNA concentrations and plotted along with their standard error (Figure 6B). Significant changes at p-value of 0.05 were detected at the lower two DNA concentrations. Although the chemiluminescent signal saturated at the highest DNA concentrations, the average HK BrdU intensities were always lower than NT intensities at every concentration tested. Similar studies were done on cells at day 2 after transfer when DNA synthesis would be highest. The same trend of HK cells having lower BrdU incorporation compared to NT cells was observed, but a statistically significant difference was not detected.
Figure 6.
BrdU incorporation is decreased in HK cells compared with NT cells. A) Example of an immunoblot detecting BrdU incorporation in DNA isolated from NT and HK cells after BrdU treatment. Three different DNA concentrations are shown. B) A comparison of BrdU incorporation between NT and HK cells, normalized to total signal intensity (L=low, M=medium and H=high DNA, e.g. 50, 75 and 100 ng of DNA). Values are the average of more than three separate experiments ± SE.
3.5.2 H3K9ac is decreased in HK cells
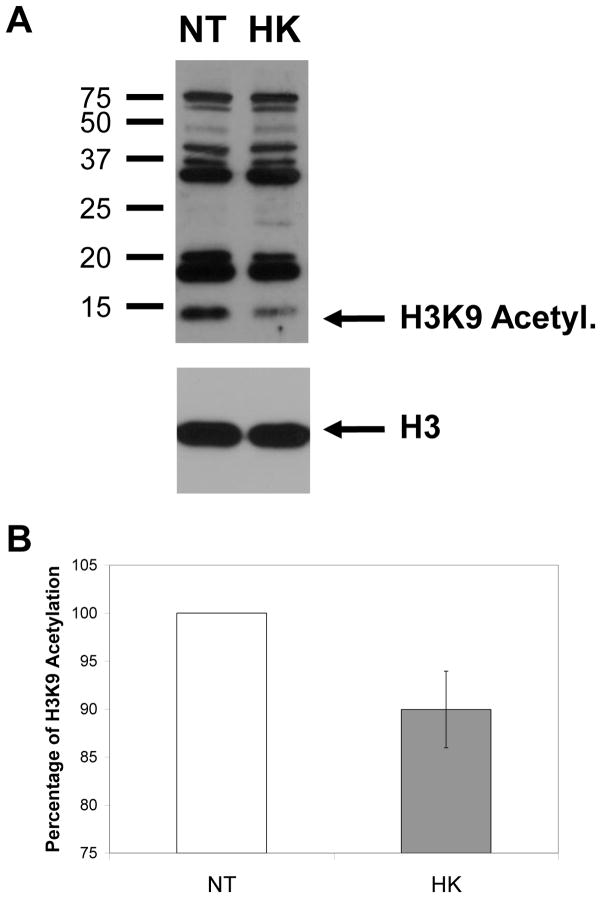
Histone acetylation is an integral part of the histone modification code. Previous papers have shown that lipids can effect chromatin remodeling complexes [72] including the Spt-Ada-Gcn5-Acetyltransferase (SAGA) complex which modifies H3K9 with acetylation [73,74]. We employed antibodies specific to acetylated H3K9 (H3K9ac) and reprobed the same blot with antibodies to total H3. Figure 7A shows a representative blot probed with both H3K9ac and H3 antibodies. HK cells have decreased H3K9ac compared to NT cells (arrow Figure 7A). To average multiple blots the ratio of the H3K9ac/total H3 was quantified, and the H3K9ac/H3 ratio of NT samples were normalized to 100%. Figure 7B shows the average relative ratio of H3K9ac/H3 from 3 blots for HK cells compared to normalized NT cells. All blots showed a decreased H3K9ac to H3 ratio for HK cells compared with NT cells.
Figure 7.
Histone H3 lysine 9 acetylation is decreased in HK cells compared with NT cells. A) Example of an immunoblot with H3K9ac-specific antibodies and H3-specific antibodies with NT and HK protein samples. B) Graph of the average ratio of H3K9ac/H3 ratio from HK cells compared to the NT H3K9ac/H3 ratio which was normalized to 100%.
3.5.3 Retinoblastoma protein (pRb) phosphorylation is decreased in HK cells
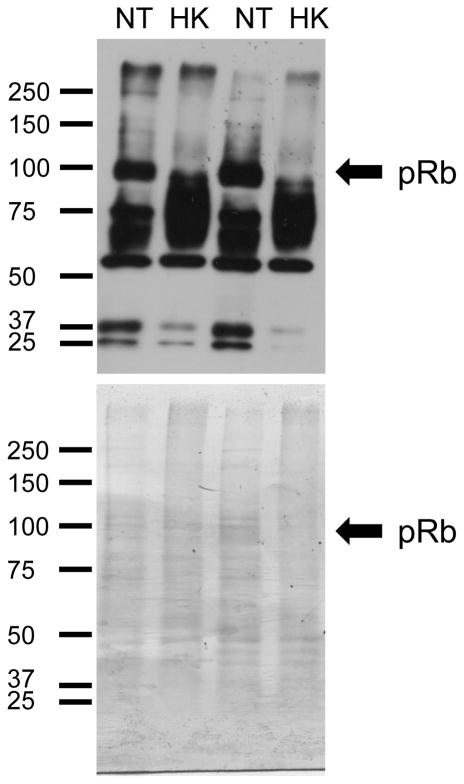
Total protein was isolated from NT and HK cells, and western blot analysis with the Ser 807/811 specific antibody (Phospho-Rb (Ser807/811) Antibody #9308) was performed as previously described [62]. Western blots showed decreased pRb phosphorylation (Figure 8 arrow) compared with stained total protein in two different protein isolations. Non-specific bands between 100 and 50 kDa increased and may simply result from non-specific cross reactivity with phosphoproteins in the HK lines or from increased proteolysis of the phosphorylated pRb protein. In either event there was less recovered full-length phosphorylated pRb.
Figure 8.
Phosphorylation of retinoblastoma protein (pRb) is decreased in HK cells. Immunoblot is shown using pRb phosphoSer807/811 specific antibodies of two biological replicates of NT and HK cell samples. Arrow indicates the migration of pRb at ~100kDa.
4. Discussion
Expression of HsPIP5K1α in NT suspension cell culture increased PM PIPK specific activity [54]. We show here that the total nuclear PtdIns(4,5)P2 recovered from isolated nuclei increased from levels that were below the limits of detection to 32.4 ± 6.2 pmoles.mg−1 of nuclear protein in the HsPIP5K1α expressing cells. Furthermore, the nuclear PIPK activity increased. There was 2-fold higher PIP5K activity in HK nuclei compared with NT nuclei with endogenous lipids as substrate. In the in vitro assays, [32P]PtdInsP2 increased even more when exogenous PtdIns was added to the reaction (Supplemental Figure 3) but not when PtdIns4P was added (Figure 4). These data suggest that there was a concerted reaction from PtdIns to PtdInsP2 or that the exogenously added PtdIns4P was degraded by phosphatases or lipases in the nuclei before it could be phosphorylated by PIPK. The fact that PtdOH increased significantly when PtdIns4P was added to the HK nuclei suggests that the PtdIns4P was being converted to DAG.
Of interest, there was no decreased in mol % PtdIns in the HK nuclei suggesting that the cells were compensating for the increase in PtdInsP2 biosynthesis by increasing PtdIns production. The changes in the lipid molecular species of the PtdIns (increased unsaturation) in the HK nuclei is also consistent with a change in flux in PtdIns biosynthetic pathways [14,75].
Nuclei were isolated in the presence of 0.025% Triton X-100 and markers for ER showed only minor contamination. The detection of CRT in nuclear protein from intact nuclei is to be expected as it has been found in nuclear fractions [67] and the ER is contiguous with the outer nuclear envelope [63,76,77]. A general increase in CRT has been reported for HK cells [54] so an increase in ER contamination in the HK nuclei would have increased the CRT significantly. Importantly, western blots indicated that the CRT in the HK nuclei was not significantly higher than that in the NT nuclei samples.
A major visual difference in the nuclei isolated from HK cells was the increase in cosedimenting starch grains. We concluded that the starch granules did not contribute to the changes in lipid composition for the following reasons: Vasanthan and Hoover et al. [78] showed that isolated dry starch granules did not yield significant lipids using an extraction method with a chloroform/methanol extraction similar to our method [78,79]. If we had plastid contamination in HK nuclei, the HK nuclear lipid analysis would have shown an enrichment of DGDG and MGDG lipid classes [79]. We did not detect significant differences in DGDG and MGDG in the nuclei samples between NT and HK cells.
PtdIns(4,5)P2 and PtdIns5P are important lipids in nuclear functions in animal cells [11,80]. A transient increase in PtdIns(4,5)P2 and/or an unregulated, continuous increase inPtdIns(4,5)P2 production during cell growth may have influenced phospholipid biogenesis and nuclear processes and thereby affected the lipid composition of the nucleus and PM. We found multiple changes in nuclear lipids and protoplast PM lipids when HsPIP5K1α expressing cells were compared with wild type, which is not surprising because of the interconnected lipid biosynthesis pathways [81–85].
Along with increased PtdCho, increased PtdSer and decreased lysolipids were characteristic of the HK nuclei. Both PtdSer and lysolipids have been hypothesized as signaling molecules in animals and plants, although the intracellular localization and effects of these signaling molecules are still not well understood [5,86,87]. Like PtdIns(4,5)P2, lysolipids are predicted to have an inverted-cone shape in a lipid bilayer [2,69]. It is likely that the decrease in lysolipids help compensate for the over accumulation of PtdIns(4,5)P2.
Major fatty acid differences were identified between the nuclei and protoplast PM. Total saturated fatty acids decreased in HK nuclei and protoplast PM and total unsaturated fatty acids increased. These changes in lipid saturation may reflect the increased flux through the PI signaling pathways because the PI signaling pools were observed to have increased unsaturated fatty acids [14]. Increased turnover of signaling PtdIns(4,5)P2 would also be predicted to increase unsaturation of other phospholipids through changing the PtdOH and DAG fatty acid backbones [14,53]. Increased PtdIns(4,5)P2 and PI pathway signaling may also change the localization, activity or substrate preference of lipid metabolizing enzymes, lipid binding proteins or change the types of isozymes that are active. Endogenous plant PtdIns(4,5)P2 signaling is increased during stress responses like heat and osmotic stress [12,88,89], and lipid remodeling, lipid isozyme changes, and changes in fatty acid compositions are known responses to heat and osmotic stress [90,91].
Changes in fatty acid composition between the NT and HK nuclei also may indicate that changes in the carbon metabolism in HK cells are affecting multiple cellular compartments. Fatty acid transport among the chloroplast, the mitochondria and the ER affects the fatty acid composition of phospholipids. The decrease in 16:0-16:0 fatty acid containing lipids suggests a change in chloroplast fatty acid trafficking. Lipids and lipid precursors with 16:0-16:0 fatty acids are synthesized in the chloroplast, and the decrease of multiple different lipids with 16:0-16:0 in the nucleus may indicate a general cellular decrease in utilization of plastid lipids and more of a reliance on the ER for lipid precursors. Schneiter et al. [70] identified a unique PtdIns lipid molecular species with a 26:0 very long chain fatty acid that was important for nuclear membrane formation and is hypothesized to be important for nuclear pore stability. Like the changes in lipid classes, the changes in nuclear lipid molecular species between the HK and NT nuclei also may be compensating for the effect of increased nuclear PtdIns(4,5)P2 in the HK nuclear membrane.
To isolate intact nuclei we used protoplasts which involved enzymatic digestion of the cell wall. Increased lipid kinase activity has been measured in embryogenic carrot cells during enzymatic cell wall digestion [92] and because others had shown that the lipid composition of PM isolated from protoplasts isolated with enzymatic digestion was different from that of cells in both plants and oomycetes [93,94], we compared the lipid composition of the protoplast PMs with that previously reported for cell PMs [54]. The NT and HK lines had multiple common changes when comparing their respective protoplast PM and cell PM lipid classes and lipid molecular species (Supplemental Table 2). In addition, multiple unique changes were found in comparing the protoplast to the cell PM within each cell type (Supplemental Table 2). The changes in the lipid molecular species composition of HK protoplast PM compared with the NT protoplast PM could have arisen from a differential response to cell wall digestion [13]. Changes in lipid saturation and lipid molecular species in HK protoplast PM, for example, the decrease in the lipid classes LysoPtdEtn and some LysoPtdCho lipid molecular species could help compensate for the effect of increased PtdIns(4,5)P2 on membrane fluidity [17] and stabilize the newly formed protoplast.
We hypothesized that changes in cellular and nuclear lipids could influence nuclear functions in HK cells since phospholipids have been shown to influence a variety of nuclear processes including DNA synthesis, chromatin remodeling and cell cycle progression [7]. For example eukaryotic DNA polymerases α, β and γ and type I and II Topoisomerases are inhibited by a variety of anionic phospholipids [95]. Chromatin remodeling complexes like the animal BAF complexes are influenced by the lipid PtdIns(4,5)P2 and its signaling products [32].
We found a decrease in DNA replication in HK cells as determined by BrdU incorporation. Decreased BrdU incorporation could have resulted from differences in cell cycle regulation or from DNA synthesis defects; however, a major defect in cell cycle was not observed as the cells divided and increased in fresh wt. Lewis et al. [96] recently identified direct binding of human Topoisomerase IIα to PtdIns(4,5)P2 and showed decreased topoisomerase activity when PtdIns(4,5)P2 was present [96]. PtdInsP2 or PtdSer in the nucleus of the HK cells could have inhibited the DNA polymerase activity and/or Topoisomerase activity, thus slowing down DNA polymerase progression. More extensive studies are needed to understand the exact mechanisms behind the changes in DNA replication in the HK cells.
H3K9ac was decreased in HK cells. We cannot rule out that changes in the H3K9ac may be responding to other metabolic cues in the HsPIP5K1α expressing cells, but there is evidence from both plants and animals to suggest a direct effect of nuclear lipids on chromatin remodeling complexes and their histone modification. A variety of chromatin remodeling complexes like the BAF are known to interact with PtdIns(4,5)P2, the phosphoinositide lipids and secondary signaling molecules produced by PtdIns(4,5)P2 hydrolysis like Ins(1,4,5)P3 in animals [72], and data for lipid-chromatin remodeling interactions in plants is growing [97–99].
One chromatin remodeling protein in plants that has been shown to be sensitive to the PI pathway lipid PtdIns5P is the histone trimethytransferase ATX1. Exogenously added PtdIns5P causes relocalization of ATX1 from the nucleus to the PM and subcellular vesicles. The result is a decrease in ATX1-dependent H3K4 trimethylation [98]. Studies of the interaction of chromatin modification in plants have shown that H3K4 trimethylation at different genes enhances H3K9 acetylation [100]. It is possible that expression of HsPIP5K1α and increasing PtdIns(4,5)P2 indirectly increased plasma membrane PtdIns5P as a result of the dephosphorylation of PtdIns(4,5)P2 by a PtdIns(4,5)P2 4-ptase. In addition, other chromatin remodeling complexes in the nucleus could respond to different lipid class changes in the nucleus of HK cells. For example, increased PtdSer in HK nuclei may inhibit the plant GCN5 homologue H3K9 acetylation activity through one of its co-activating Ada2 homologues similar to the mammalian SAGA complex [74].
PtdIns(4,5)P2 has also been found to be important for cell cycle regulation including mitotic spindle formation, cell plate formation, and regulation of retinoblastoma protein. We hypothesized that increased PtdIns(4,5)P2 could affect the phosphorylation of the retinoblastoma protein. Total protein isolated from HK cells showed decreased pRb phosphorylation with human pRb Ser 807/811 specific antibodies previously shown to detect plant phosphorylated pRb [62]. Decreased phosphorylated pRb could slow cell cycle progression, change DNA replication timing, and affect chromatin remodeling during cell division [101].
In summary, we have shown that expressing HsPIP5Kα in plants affected nuclear lipid PIPK activity and the composition of nuclear lipids. DNA replication, histone acetylation and retinoblastoma protein phosphorylation were all decreased. These results indicate that increasing PtdIns(4,5)P2 and altering nuclear lipids in plants can have pleiotropic effects on nuclear function. The effects of lipids on nuclear function in plants is in agreement with the research of lipids on animal nuclei. Lewis et al. [96] recently identified multiple nuclear proteins that bind directly to PtdIns(4,5)P2 thus increasing the possible protein targets in animals. Additional in vitro and in vivo studies targeting and identifying specific nuclear PtdIns(4,5)P2 binding proteins in plants are necessary to elucidate the direct effects of PtdIns(4,5)P2 and other lipids on the plant nuclear functions.
Supplementary Material
Supplemental Figure 1. NT and HK nuclei samples show limited contamination of H+-ATPase (A) and CRT (B). Plus (+) represents an equal amount of protoplast protein and was used as a positive control.
Supplemental Figure 2. NT nuclei from Day 2 and 4 were assayed for PIP5K activity for 30 minutes with PtdIns4P added and increasing ATP (50, 100 and 200μM at a specific activity of 74 kBq/nmole ATP). [32P]PtdInsP2 (A), [32P]PtdInsP (B) and [32P]PtdOH (C ) are reported as pmol.mg−1.min−1.
Supplemental Figure 3. HK nuclei produce more [32P]PtdInsP2 with added PtdIns. NT and HK nuclei were assayed for PIP5K activity with PtdIns4P and PtdIns added and lipids were extracted and separated by TLC. Phosphatidic acid (PtdOH), LysoPtdOH, PtdInsP, PtdInsP2 lipids and origin are indicated.
Supplemental Table 1. Lipid classes and lipid molecular species mol % raw data from NT and HK nuclei lipid samples, protoplast PM samples and cell PM samples. For each sample the submission identifier, name of sample, and all lipid class and lipid molecular species mol % data is provided. One worksheet provides the raw data, and the second worksheet represents the data that was used for further analysis with outliers removed with either the Q-test or Grubbs test.
Supplemental Table 2. Analysis of nuclei, protoplast PM, and cell PM lipid classes and lipid molecular species profiles by Student t-Test. Student t-Test data for the following comparisons is provided: NT and HK nuclei, NT and HK protoplast PM, NT nuclei and protoplast PM, HK nuclei and protoplast PM, NT and HK cell PM, NT cell PM and protoplast PM, and HK cell PM and protoplast PM. Significantly different comparisons (p-value ≤ 0.05) are highlighted for each comparison and the fold change between each mol% comparison is provided.
Acknowledgments
We appreciate the critical comments provided by Drs. Matthew Keogh and Mintu Kumar Desai. This work was supported in part by a grant from the National Science Foundation (MCB-0718452), by the North Carolina Agricultural Research Service, by an Initiative for Future Agriculture and Food Systems (IFAFS) Research Fellowship through the USDA and by a NIH/NCSU Molecular Biotechnology Training Program (MBTP) Fellowship. The lipid analyses described in this work were performed at the Kansas Lipidomics Research Center Analytical Laboratory. Kansas Lipidomics Research Center was supported by National Science Foundation (EPS 0236913, MCB 0455318, DBI 0521587), Kansas Technology Enterprise Corporation, K-IDeA Networks of Biomedical Research Excellence (INBRE) of National Institute of Health (P20RR16475), and Kansas State University.
Abbreviations
- ATX1
Arabidopsis homolog of Trithorax 1
- BAF
Brahma associated factor
- BCA
bicinchoninic acid
- Bromodeoxyuridine
5-bromo-2′-deoxyuridine or BrdU
- CRT
calreticulin
- fw
fresh weight
- H3K4
histone H3 lysine 4
- H3K9ac
histone H3 lysine 9 acetylation
- HsPIP5K1α
Homo sapiens phosphatidylinositol 4-phosphate 5kinase 1α
- HK
HsPIP5K1α-expressing
- NIB
nuclear isolation buffer
- PCA
perchloric acid
- PI
phosphoinositide
- PMSF
phenylmethanesulfonyl fluoride
- plasma membrane
PM
- PWB
protoplast wash buffer
- pRb
retinoblastoma protein
- SAGA
Spt-Ada-Gcn5-Acetyltransferase
Footnotes
Conflict of Interest Statement:
The authors have no conflicts of interest to declare.
References
- 1.Heilmann I. Towards understanding the function of stress-inducible PtdIns(4,5)P(2) in plants. Commun Integr Biol. 2008;1:204–206. doi: 10.4161/cib.1.2.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melser S, Molino D, Batailler B, Peypelut M, Laloi M, Wattelet-Boyer V, et al. Links between lipid homeostasis, organelle morphodynamics and protein trafficking in eukaryotic and plant secretory pathways. Plant Cell Reports. 2011;30:177–193. doi: 10.1007/s00299-010-0954-1. [DOI] [PubMed] [Google Scholar]
- 3.Szumlanski AL, Nielsen E. Phosphatidylinositol 4-Phosphate is Required for Tip Growth in Arabidopsis thaliana. In: Munnik T, editor. Lipid Signaling in Plants. Springer Berlin Heidelberg; Berlin, Heidelberg: 2010. pp. 65–77. [Google Scholar]
- 4.Thole JM, Nielsen E. Phosphoinositides in plants: novel functions in membrane trafficking. Current Opinion in Plant Biology. 2008;11:620–631. doi: 10.1016/j.pbi.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Albi E, Viola Magni MP. The role of intranuclear lipids. Biol Cell. 2004;96:657–667. doi: 10.1016/j.biolcel.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Balla T, Szentpetery Z, Kim YJ. Phosphoinositide signaling: new tools and insights. Physiology (Bethesda) 2009;24:231–244. doi: 10.1152/physiol.00014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barlow CA, Laishram RS, Anderson RA. Nuclear phosphoinositides: a signaling enigma wrapped in a compartmental conundrum. Trends in Cell Biology. 2010;20:25–35. doi: 10.1016/j.tcb.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doughman RL, Firestone AJ, Anderson RA. Phosphatidylinositol phosphate kinases put PI4,5P(2) in its place. J Membr Biol. 2003;194:77–89. doi: 10.1007/s00232-003-2027-7. [DOI] [PubMed] [Google Scholar]
- 9.Irvine RF. Nuclear lipid signalling. Nature Reviews Molecular Cell Biology. 2003;4:349–361. doi: 10.1038/nrm1100. [DOI] [PubMed] [Google Scholar]
- 10.King J, Keim M, Teo R, Weening KE, Kapur M, McQuillan K, et al. Genetic Control of Lithium Sensitivity and Regulation of Inositol Biosynthetic Genes. PLoS ONE. 2010;5:e11151. doi: 10.1371/journal.pone.0011151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mellman DL, Anderson RA. A novel gene expression pathway regulated by nuclear phosphoinositides. Advances in Enzyme Regulation. 2009;49:11–28. doi: 10.1016/j.advenzreg.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mishkind M, Vermeer JEM, Darwish E, Munnik T. Heat stress activates phospholipase D and triggers PIP 2 accumulation at the plasma membrane and nucleus. The Plant Journal. 2009;60:10–21. doi: 10.1111/j.1365-313X.2009.03933.x. [DOI] [PubMed] [Google Scholar]
- 13.Ischebeck T, Stenzel I, Heilmann I. Type B phosphatidylinositol-4-phosphate 5-kinases mediate Arabidopsis and Nicotiana tabacum pollen tube growth by regulating apical pectin secretion. The Plant Cell. 2008;20:3312–30. doi: 10.1105/tpc.108.059568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konig S, Mosblech A, Heilmann I. Stress-inducible and constitutive phosphoinositide pools have distinctive fatty acid patterns in Arabidopsis thaliana. The FASEB Journal. 2007;21:1958–1967. doi: 10.1096/fj.06-7887com. [DOI] [PubMed] [Google Scholar]
- 15.Böhme K, Li Y, Charlot F, Grierson C, Marrocco K, Okada K, et al. The Arabidopsis COW1 gene encodes a phosphatidylinositol transfer protein essential for root hair tip growth. Plant J. 2004;40:686–698. doi: 10.1111/j.1365-313X.2004.02245.x. [DOI] [PubMed] [Google Scholar]
- 16.Cowan AK. Phospholipids as Plant Growth Regulators. Plant Growth Regulation. 2006;48:97–109. [Google Scholar]
- 17.Furt F, Konig S, Bessoule JJ, Sargueil F, Zallot R, Stanislas T, et al. Polyphosphoinositides Are Enriched in Plant Membrane Rafts and Form Microdomains in the Plasma Membrane. Plant Physiology. 2010;152:2173–2187. doi: 10.1104/pp.109.149823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ischebeck T, Seiler S, Heilmann I. At the poles across kingdoms: phosphoinositides and polar tip growth. Protoplasma. 2010;240:13–31. doi: 10.1007/s00709-009-0093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Preuss ML, Schmitz AJ, Thole JM, Bonner HKS, Otegui MS, Nielsen E. A role for the RabA4b effector protein PI-4Kbeta1 in polarized expansion of root hair cells in Arabidopsis thaliana. J Cell Biol. 2006;172:991–998. doi: 10.1083/jcb.200508116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saavedra L, Balbi V, Lerche J, Mikami K, Heilmann I, Sommarin M. PIPKs are essential for rhizoid elongation and caulonemal cell development in the moss Physcomitrella patens. The Plant Journal. 2011;67:635–647. doi: 10.1111/j.1365-313X.2011.04623.x. [DOI] [PubMed] [Google Scholar]
- 21.Williams ME, Torabinejad J, Cohick E, Parker K, Drake EJ, Thompson JE, et al. Mutations in the Arabidopsis Phosphoinositide Phosphatase Gene SAC9 Lead to Overaccumulation of PtdIns(4,5)P2 and Constitutive Expression of the Stress-Response Pathway. Plant Physiology. 2005;138:686–700. doi: 10.1104/pp.105.061317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng MK, Shearn A. The Direct Interaction Between ASH2, a Drosophila Trithorax Group Protein, and SKTL, a Nuclear Phosphatidylinositol 4-Phosphate 5-Kinase, Implies a Role for Phosphatidylinositol 4,5-Bisphosphate in Maintaining Transcriptionally Active Chromatin. Genetics. 2004;167:1213–1223. doi: 10.1534/genetics.103.018721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cocco L, Gilmour R, Ognibene A, Letcher A, Manzoli F, Irvine R. Synthesis of polyphosphoinositides in nuclei of Friend cells. Evidence for polyphosphoinositide metabolism inside the nucleus which changes with cell differentiation. Biochemical Journal. 1987;248:765. doi: 10.1042/bj2480765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hendrix KW, Assefa H, Boss WF. The polyphosphoinositides, phosphatidylinositol monophosphate and phosphatidylinositol bisphosphate, are present in nuclei isolated from carrot protoplast. Protoplasma. 1989;151:6272. [Google Scholar]
- 25.Jones AME, MacLean D, Studholme DJ, Serna-Sanz A, Andreasson E, Rathjen JP, et al. Phosphoproteomic analysis of nuclei-enriched fractions from Arabidopsis thaliana. Journal of Proteomics. 2009;72:439–451. doi: 10.1016/j.jprot.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 26.jan Keune W, Bultsma Y, Sommer L, Jones D, Divecha N. Phosphoinositide signalling in the nucleus. Advances in Enzyme Regulation. 2011;51:91–99. doi: 10.1016/j.advenzreg.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Matsubara T. Nuclear Transportation of Diacylglycerol Kinase and Its Possible Function in the Nucleus. Journal of Biological Chemistry. 2006;281:6152–6164. doi: 10.1074/jbc.M509873200. [DOI] [PubMed] [Google Scholar]
- 28.Viola-Magni MP, Gahan PB, Pacy J. Phospholipids in plant and animal chromatin. Cell Biochemistry and Function. 1985;3:71–78. doi: 10.1002/cbf.290030113. [DOI] [PubMed] [Google Scholar]
- 29.Bunney TD, Watkins PAC, Beven AF, Shaw PJ, Hernandez LE, Lomonossoff GP, et al. Association of phosphatidylinositol 3-kinase with nuclear transcription sites in higher plants. The Plant Cell Online. 2000;12:1679–1688. doi: 10.1105/tpc.12.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzales ML, Anderson RA. Nuclear phosphoinositide kinases and inositol phospholipids. Journal of Cellular Biochemistry. 2006;97:252–260. doi: 10.1002/jcb.20655. [DOI] [PubMed] [Google Scholar]
- 31.Yu H, Fukami K, Watanabe Y, Ozaki C, Takenawa T. Phosphatidylinositol 4,5-bisphosphate reverses the inhibition of RNA transcription caused by histone H1. Eur J Biochem. 1998;251:281–287. doi: 10.1046/j.1432-1327.1998.2510281.x. [DOI] [PubMed] [Google Scholar]
- 32.Rando OJ, Zhao K, Janmey P, Crabtree GR. Phosphatidylinositol-dependent actin filament binding by the SWI/SNF-like BAF chromatin remodeling complex. Proceedings of the National Academy of Sciences. 2002;99:2824–2829. doi: 10.1073/pnas.032662899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao K, Wang W, Rando OJ, Xue Y, Swiderek K, Kuo A, et al. Rapid and phosphoinositol-dependent binding of the SWI/SNF-like BAF complex to chromatin after T lymphocyte receptor signaling. Cell. 1998;95:625–636. doi: 10.1016/s0092-8674(00)81633-5. [DOI] [PubMed] [Google Scholar]
- 34.Li W, Laishram RS, Ji Z, Barlow CA, Tian B, Anderson RA. Star-PAP Control of BIK Expression and Apoptosis Is Regulated by Nuclear PIPKIα and PKCδ Signaling. Molecular Cell. 2012;45:25–37. doi: 10.1016/j.molcel.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mellman DL, Gonzales ML, Song C, Barlow CA, Wang P, Kendziorski C, et al. A PtdIns4,5P2-regulated nuclear poly(A) polymerase controls expression of select mRNAs. Nature. 2008;451:1013–1017. doi: 10.1038/nature06666. [DOI] [PubMed] [Google Scholar]
- 36.Russell M, Berardi P, Gong W, Riabowol K. Grow-ING, Age-ING and DieING: ING proteins link cancer, senescence and apoptosis. Experimental Cell Research. 2006;312:951–961. doi: 10.1016/j.yexcr.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 37.Sun B, Murray NR, Fields AP. A role for nuclear phosphatidylinositol-specific phospholipase C in the G2/M phase transition. J Biol Chem. 1997;272:26313–26317. doi: 10.1074/jbc.272.42.26313. [DOI] [PubMed] [Google Scholar]
- 38.Cocco L, Faenza I, Fiume R, Maria Billi A, Gilmour RS, Manzoli FA. Phosphoinositide-specific phospholipase C (PI-PLC) beta1 and nuclear lipid-dependent signaling. Biochim Biophys Acta. 2006;1761:509–521. doi: 10.1016/j.bbalip.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Alcázar-Román AR, Wente SR. Inositol polyphosphates: a new frontier for regulating gene expression. Chromosoma. 2008;117:1–13. doi: 10.1007/s00412-007-0126-4. [DOI] [PubMed] [Google Scholar]
- 40.Zewail A, Xie MW, Xing Y, Lin L, Zhang PF, Zou W, et al. Novel functions of the phosphatidylinositol metabolic pathway discovered by a chemical genomics screen with wortmannin. Proc Natl Acad Sci U S A. 2003;100:3345–3350. doi: 10.1073/pnas.0530118100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Capitani S, Billi AM, Bertagnolo V, Previati M, Mazzoni M, Neri LM, et al. Inositol lipids in Friend erythroleukemia cells: evidence for changes in nuclear metabolism after differentiation. Cell Biochem Funct. 1991;9:135–145. doi: 10.1002/cbf.290090211. [DOI] [PubMed] [Google Scholar]
- 42.Faenza I. A Role for Nuclear Phospholipase Cbeta 1 in Cell Cycle Control. Journal of Biological Chemistry. 2000;275:30520–30524. doi: 10.1074/jbc.M004630200. [DOI] [PubMed] [Google Scholar]
- 43.Matteucci A, Faenza I, Gilmour RS, Manzoli L, Billi AM, Peruzzi D, et al. Nuclear but not Cytoplasmic Phospholipase C β1 Inhibits Differentiation of Erythroleukemia Cells. Cancer Research. 1998;58:5057–5060. [PubMed] [Google Scholar]
- 44.Hassan BA, Prokopenko SN, Breuer S, Zhang B, Paululat A, Bellen HJ. skittles, a Drosophila phosphatidylinositol 4-phosphate 5-kinase, is required for cell viability, germline development and bristle morphology, but not for neurotransmitter release. Genetics. 1998;150:1527–1537. doi: 10.1093/genetics/150.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Panbianco C, Weinkove D, Zanin E, Jones D, Divecha N, Gotta M, et al. A casein kinase 1 and PAR proteins regulate asymmetry of a PIP(2) synthesis enzyme for asymmetric spindle positioning. Dev Cell. 2008;15:198–208. doi: 10.1016/j.devcel.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okpodu CM. Characterization of a nuclear phosphatidylinositol 4-kinase in carrot suspension culture cells. Plant Physiology and Biochemistry. 1999;37:473–480. doi: 10.1104/pp.107.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mei Y, Jia W-J, Chu Y-J, Xue H-W. Arabidopsis phosphatidylinositol monophosphate 5-kinase 2 is involved in root gravitropism through regulation of polar auxin transport by affecting the cycling of PIN proteins. Cell Res. 2011 doi: 10.1038/cr.2011.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Froidure S, Canonne J, Daniel X, Jauneau A, Briere C, Roby D, et al. AtsPLA2- nuclear relocalization by the Arabidopsis transcription factor AtMYB30 leads to repression of the plant defense response. Proceedings of the National Academy of Sciences. 2010;107:15281–15286. doi: 10.1073/pnas.1009056107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lou Y, Gou JY, Xue HW. PIP5K9, an Arabidopsis Phosphatidylinositol Monophosphate Kinase, Interacts with a Cytosolic Invertase to Negatively Regulate Sugar-Mediated Root Growth. The Plant Cell Online. 2007;19:163–181. doi: 10.1105/tpc.106.045658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Minasbekyan LA, Yavroyan ZV, Darbinyan MR, Vardevanyan PO. The Phospholipid Composition of Nuclear Subfractions from Germinating Wheat Embryos. Russian Journal of Plant Physiology. 2004;51:708–712. [Google Scholar]
- 51.Minasbekyan LA, Yavroyan ZV, Darbinyan MR, Vardevanyan PO. Changes in the composition of phospholipids in nuclear subfractions of wheat seedlings treated with gibberellin. Russian Journal of Plant Physiology. 2008;55:372–377. [Google Scholar]
- 52.Drøbak BK, Heras B. Nuclear phosphoinositides could bring FYVE alive. Trends Plant Sci. 2002;7:132–138. doi: 10.1016/s1360-1385(01)02213-0. [DOI] [PubMed] [Google Scholar]
- 53.Konig S, Ischebeck T, Lerche J, Stenzel I, Heilmann I. Salt-stress-induced association of phosphatidylinositol 4,5-bisphosphate with clathrin-coated vesicles in plants. The Biochemical Journal. 2008;415:387–99. doi: 10.1042/BJ20081306. [DOI] [PubMed] [Google Scholar]
- 54.Im YJ, Perera IY, Brglez I, Davis AJ, Stevenson-Paulik J, Phillippy BQ, et al. Increasing plasma membrane phosphatidylinositol(4,5)bisphosphate biosynthesis increases phosphoinositide metabolism in Nicotiana tabacum. Plant Cell. 2007;19:1603–1616. doi: 10.1105/tpc.107.051367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perera IY, Love J, Heilmann I, Thompson WF, Boss WF. Up-regulation of phosphoinositide metabolism in tobacco cells constitutively expressing the human type I inositol polyphosphate 5-phosphatase. Plant Physiol. 2002;129:1795–1806. doi: 10.1104/pp.003426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heilmann I, Perera IY, Gross W, Boss WF. Plasma membrane phosphatidylinositol 4,5-bisphosphate levels decrease with time in culture. Plant Physiol. 2001;126:1507–1518. doi: 10.1104/pp.126.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Welti R, Li W, Li M, Sang Y, Biesiada H, Zhou HE, et al. Profiling membrane lipids in plant stress responses. Role of phospholipase D alpha in freezing-induced lipid changes in Arabidopsis. J Biol Chem. 2002;277:31994–32002. doi: 10.1074/jbc.M205375200. [DOI] [PubMed] [Google Scholar]
- 58.Ueda J, Saito H, Watanabe H, Evers BM. Novel and quantitative DNA dot-blotting method for assessment of in vivo proliferation. American Journal of Physiology -Gastrointestinal and Liver Physiology. 2005;288:G842–G847. doi: 10.1152/ajpgi.00463.2004. [DOI] [PubMed] [Google Scholar]
- 59.Morsomme P, Dambly S, Maudoux O, Boutry M. Single point mutations distributed in 10 soluble and membrane regions of the Nicotiana plumbaginifolia plasma membrane PMA2 H+-ATPase activate the enzyme and modify the structure of the C-terminal region. J Biol Chem. 1998;273:34837–34842. doi: 10.1074/jbc.273.52.34837. [DOI] [PubMed] [Google Scholar]
- 60.Tariq M. Erasure of CpG methylation in Arabidopsis alters patterns of histone H3 methylation in heterochromatin. Proceedings of the National Academy of Sciences. 2003;100:8823–8827. doi: 10.1073/pnas.1432939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abramoff Md, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- 62.Ábrahám E, Miskolczi P, Ayaydin F, Yu P, Kotogány E, Bakó L, et al. Immunodetection of retinoblastoma-related protein and its phosphorylated form in interphase and mitotic alfalfa cells. Journal of Experimental Botany. 2011;62:2155–2168. doi: 10.1093/jxb/erq413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Collings DA, Carter CN, Rink JC, Scott AC, Wyatt SE, Allen NS. Plant Nuclei Can Contain Extensive Grooves and Invaginations. The Plant Cell Online. 2000;12:2425–2440. doi: 10.1105/tpc.12.12.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gupton SL, Collings DA, Allen NS. Endoplasmic reticulum targeted GFP reveals ER organization in tobacco NT-1 cells during cell division. Plant Physiology and Biochemistry. 2006;44:95–105. doi: 10.1016/j.plaphy.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 65.Prunuske AJ, Ullman KS. The nuclear envelope: form and reformation. Curr Opin Cell Biol. 2006;18:108–116. doi: 10.1016/j.ceb.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Denecke J, Carlsson LE, Vidal S, Höglund AS, Ek B, van Zeijl MJ, et al. The tobacco homolog of mammalian calreticulin is present in protein complexes in vivo. Plant Cell. 1995;7:391–406. doi: 10.1105/tpc.7.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jia XY, Xu CY, Jing RL, Li RZ, Mao XG, Wang JP, et al. Molecular cloning and characterization of wheat calreticulin (CRT) gene involved in drought-stressed responses. Journal of Experimental Botany. 2008;59:739–751. doi: 10.1093/jxb/erm369. [DOI] [PubMed] [Google Scholar]
- 68.Napier RM, Trueman S, Henderson J, Boyce JM, Hawes C, Fricker MD, et al. Purification, sequencing and functions of calreticulin from maize. Journal of Experimental Botany. 1995;46:1603–1613. [Google Scholar]
- 69.Chernomordik LV, Kozlov MM. Mechanics of membrane fusion. Nat Struct Mol Biol. 2008;15:675–683. doi: 10.1038/nsmb.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schneiter R, BrüGger B, Amann CM, Prestwich GD, Epand RF, Zellnig G, et al. Identification and biophysical characterization of a very-long-chain-fatty-acid-substituted phosphatidylinositol in yeast subcellular membranes. Biochemical Journal. 2004;381:941. doi: 10.1042/BJ20040320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Devaiah SP, Roth MR, Baughman E, Li M, Tamura P, Jeannotte R, et al. Quantitative profiling of polar glycerolipid species from organs of wild-type Arabidopsis and a PHOSPHOLIPASE Dα1 knockout mutant. Phytochemistry. 2006;67:1907–1924. doi: 10.1016/j.phytochem.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 72.Jones DR, Divecha N. Linking lipids to chromatin. Current Opinion in Genetics & Development. 2004;14:196–202. doi: 10.1016/j.gde.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 73.Guha N, Desai P, Vancura A. Plc1p Is Required for SAGA Recruitment and Derepression of Sko1p-regulated Genes. Molecular Biology of the Cell. 2007;18:2419–2428. doi: 10.1091/mbc.E06-10-0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoke SMT, Genereaux J, Liang G, Brandl CJ. A Conserved Central Region of Yeast Ada2 Regulates the Histone Acetyltransferase Activity of Gcn5 and Interacts with Phospholipids. Journal of Molecular Biology. 2008;384:743–755. doi: 10.1016/j.jmb.2008.09.088. [DOI] [PubMed] [Google Scholar]
- 75.Lofke C, Ischebeck T, Konig S, Freitag S, Heilmann I. Alternative metabolic fates of phosphatidylinositol produced by phosphatidylinositol synthase isoforms in Arabidopsis thaliana. The Biochemical Journal. 2008;413:115–24. doi: 10.1042/BJ20071371. [DOI] [PubMed] [Google Scholar]
- 76.Brandizzi F, Irons SL, Evans DE. The plant nuclear envelope: new prospects for a poorly understood structure. New Phytologist. 2004;163:227–246. doi: 10.1111/j.1469-8137.2004.01118.x. [DOI] [PubMed] [Google Scholar]
- 77.Marius P, Guerra MT, Nathanson MH, Ehrlich BE, Leite MF. Calcium release from ryanodine receptors in the nucleoplasmic reticulum. Cell Calcium. 2006;39:65–73. doi: 10.1016/j.ceca.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 78.Vasanthan T, Hoover R. A comparative study of the composition of lipids associated with starch granules from various botanical sources. Food Chemistry. 1992;43:19–27. [Google Scholar]
- 79.Finnie SM, Jeannotte R, Morris CF, Giroux MJ, Faubion JM. Variation in polar lipids located on the surface of wheat starch. Journal of Cereal Science. 2010;51:73–80. [Google Scholar]
- 80.Bunce MW, Gonzales ML, Anderson RA. Stress-ING out: phosphoinositides mediate the cellular stress response. Sci STKE. 2006;2006:pe46. doi: 10.1126/stke.3602006pe46. [DOI] [PubMed] [Google Scholar]
- 81.Eastmond PJ, Quettier AL, Kroon JTM, Craddock C, Adams N, Slabas AR. PHOSPHATIDIC ACID PHOSPHOHYDROLASE1 and 2 Regulate Phospholipid Synthesis at the Endoplasmic Reticulum in Arabidopsis. The Plant Cell Online. 2010;22:2796–2811. doi: 10.1105/tpc.109.071423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ghosh R, Bankaitis VA. Phosphatidylinositol transfer proteins: Negotiating the regulatory interface between lipid metabolism and lipid signaling in diverse cellular processes. BioFactors. 2011;37:290–308. doi: 10.1002/biof.180. [DOI] [PubMed] [Google Scholar]
- 83.Testerink C, Munnik T. Molecular, cellular, and physiological responses to phosphatidic acid formation in plants. Journal of Experimental Botany. 2011;62:2349–2361. doi: 10.1093/jxb/err079. [DOI] [PubMed] [Google Scholar]
- 84.Villa-García MJ, Choi MS, Hinz FI, Gaspar ML, Jesch SA, Henry SA. Genome-wide screen for inositol auxotrophy in Saccharomyces cerevisiae implicates lipid metabolism in stress response signaling. Molecular Genetics and Genomics. 2010;285:125–149. doi: 10.1007/s00438-010-0592-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vincent P, Chua M, Nogue F, Fairbrother A, Mekeel H, Xu Y, et al. A Sec14p-nodulin domain phosphatidylinositol transfer protein polarizes membrane growth of Arabidopsis thaliana root hairs. J Cell Biol. 2005;168:801–812. doi: 10.1083/jcb.200412074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nardicchi V, Macchioni L, Ferrini M, Goracci G. The presence of a secretory phospholipase A2 in the nuclei of neuronal and glial cells of rat brain cortex. Biochimica Et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2007;1771:1345–1352. doi: 10.1016/j.bbalip.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 87.Manzoli FA, Cocco L, Maraldi NM, Capitani S, Barnabei O. Unfolding of nucleosome core induced by phosphatidylserine. Adv Enzyme Regul. 1987;26:271–283. doi: 10.1016/0065-2571(87)90018-5. [DOI] [PubMed] [Google Scholar]
- 88.Pical C, Westergren T, Dove SK, Larsson C, Sommarin M. Salinity and hyperosmotic stress induce rapid increases in phosphatidylinositol 4,5-bisphosphate, diacylglycerol pyrophosphate, and phosphatidylcholine in Arabidopsis thaliana cells. J Biol Chem. 1999;274:38232–38240. doi: 10.1074/jbc.274.53.38232. [DOI] [PubMed] [Google Scholar]
- 89.Westergren T, Dove SK, Sommarin M, Pical C. AtPIP5K1, an Arabidopsis thaliana phosphatidylinositol phosphate kinase, synthesizes PtdIns(3,4)P(2) and PtdIns(4,5)P(2) in vitro and is inhibited by phosphorylation. Biochem J. 2001;359:583–589. doi: 10.1042/0264-6021:3590583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Burgos A, Szymanski J, Seiwert B, Degenkolbe T, Hannah MA, Giavalisco P, et al. Analysis of shortterm changes in the Arabidopsis thaliana glycerolipidome in response to temperature and light. The Plant Journal. 2011;66:656–668. doi: 10.1111/j.1365-313X.2011.04531.x. [DOI] [PubMed] [Google Scholar]
- 91.Upchurch RG. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnology Letters. 2008;30:967–977. doi: 10.1007/s10529-008-9639-z. [DOI] [PubMed] [Google Scholar]
- 92.Chen Q, Boss WF. Short-Term Treatment with Cell Wall Degrading Enzymes Increases the Activity of the Inositol Phospholipid Kinases and the Vanadate-Sensitive ATPase of Carrot Cells 1. Plant Physiol. 1990;94:1820–1829. doi: 10.1104/pp.94.4.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aidemark M, Tjellström H, Sandelius A, Stålbrand H, Andreasson E, Rasmusson AG, et al. Trichoderma viride cellulase induces resistance to the antibiotic pore-forming peptide alamethicin associated with changes in the plasma membrane lipid composition of tobacco BY-2 cells. BMC Plant Biology. 2010;10:274. doi: 10.1186/1471-2229-10-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Briolay A, Bouzenzana J, Guichardant M, Deshayes C, Sindt N, Bessueille L, et al. Cell wall polysaccharide synthases are located in detergent-resistant membrane microdomains in oomycetes. Appl Environ Microbiol. 2009;75:1938–1949. doi: 10.1128/AEM.02728-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ishimaru C, Takeuchi T, Sugawara F, Yoshida H, Mizushina Y. Inhibitory effects of diacylglyceride phospholipids on DNA polymerase and topoisomerase activities, and human cancer cell growth. Med Chem. 2010;6:114–122. doi: 10.2174/1573406411006030114. [DOI] [PubMed] [Google Scholar]
- 96.Lewis AE, Sommer L, Arntzen MØ, Strahm Y, Morrice Na, Divecha N, et al. Identification of nuclear phosphatidylinositol 4,5-bisphosphate-interacting proteins by neomycin extraction. Molecular & Cellular Proteomics: MCP. 2011;10:M110.003376. doi: 10.1074/mcp.M110.003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alvarez-Venegas R, Avramova Z. Methylation patterns of histone H3 Lys 4, Lys 9 and Lys 27 in transcriptionally active and inactive Arabidopsis genes and in atx1 mutants. Nucleic Acids Res. 2005;33:5199–5207. doi: 10.1093/nar/gki830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Alvarez-Venegas R, Sadder M, Hlavacka A, Baluska F, Xia Y, Lu G, et al. The Arabidopsis homolog of trithorax, ATX1, binds phosphatidylinositol 5-phosphate, and the two regulate a common set of target genes. Proc Natl Acad Sci USA. 2006;103:6049–6054. doi: 10.1073/pnas.0600944103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ndamukong I, Jones DR, Lapko H, Divecha N, Avramova Z. Phosphatidylinositol 5-Phosphate Links Dehydration Stress to the Activity of ARABIDOPSIS TRITHORAX-LIKE Factor ATX1. PLoS ONE. 2010;5:e13396. doi: 10.1371/journal.pone.0013396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ha M, Ng DWK, Li WH, Chen ZJ. Coordinated histone modifications are associated with gene expression variation within and between species. Genome Research. 2011;21:590–598. doi: 10.1101/gr.116467.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Park JA, Ahn JW, Kim YK, Kim SJ, Kim JK, Kim WT, et al. Retinoblastoma protein regulates cell proliferation, differentiation, and endoreduplication in plants. The Plant Journal. 2005;42:153–163. doi: 10.1111/j.1365-313X.2005.02361.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. NT and HK nuclei samples show limited contamination of H+-ATPase (A) and CRT (B). Plus (+) represents an equal amount of protoplast protein and was used as a positive control.
Supplemental Figure 2. NT nuclei from Day 2 and 4 were assayed for PIP5K activity for 30 minutes with PtdIns4P added and increasing ATP (50, 100 and 200μM at a specific activity of 74 kBq/nmole ATP). [32P]PtdInsP2 (A), [32P]PtdInsP (B) and [32P]PtdOH (C ) are reported as pmol.mg−1.min−1.
Supplemental Figure 3. HK nuclei produce more [32P]PtdInsP2 with added PtdIns. NT and HK nuclei were assayed for PIP5K activity with PtdIns4P and PtdIns added and lipids were extracted and separated by TLC. Phosphatidic acid (PtdOH), LysoPtdOH, PtdInsP, PtdInsP2 lipids and origin are indicated.
Supplemental Table 1. Lipid classes and lipid molecular species mol % raw data from NT and HK nuclei lipid samples, protoplast PM samples and cell PM samples. For each sample the submission identifier, name of sample, and all lipid class and lipid molecular species mol % data is provided. One worksheet provides the raw data, and the second worksheet represents the data that was used for further analysis with outliers removed with either the Q-test or Grubbs test.
Supplemental Table 2. Analysis of nuclei, protoplast PM, and cell PM lipid classes and lipid molecular species profiles by Student t-Test. Student t-Test data for the following comparisons is provided: NT and HK nuclei, NT and HK protoplast PM, NT nuclei and protoplast PM, HK nuclei and protoplast PM, NT and HK cell PM, NT cell PM and protoplast PM, and HK cell PM and protoplast PM. Significantly different comparisons (p-value ≤ 0.05) are highlighted for each comparison and the fold change between each mol% comparison is provided.