Abstract
Purpose
Systemic exposure to mercaptopurine (MP) is critical for durable remissions in children with acute lymphoblastic leukemia (ALL). Nonadherence to oral MP could increase relapse risk and also contribute to inferior outcome in Hispanics. This study identified determinants of adherence and described impact of adherence on relapse, both overall and by ethnicity.
Patients and Methods
A total of 327 children with ALL (169 Hispanic; 158 non-Hispanic white) participated. Medication event-monitoring system caps recorded date and time of MP bottle openings. Adherence rate, calculated monthly, was defined as ratio of days of MP bottle opening to days when MP was prescribed.
Results
After 53,394 person-days of monitoring, adherence declined from 94.7% (month 1) to 90.2% (month 6; P < .001). Mean adherence over 6 months was significantly lower among Hispanics (88.4% v 94.8%; P < .001), patients age ≥ 12 years (85.8% v 93.1%; P < .001), and patients from single-mother households (80.6% v 93.1%; P = .001). A progressive increase in relapse was observed with decreasing adherence (reference: adherence ≥ 95%; 94.9% to 90%: hazard ratio [HR], 4.1; 95% CI,1.2 to 13.5; P = .02; 89.9% to 85%: HR, 4.0; 95% CI, 1.0 to 15.5; P = .04; < 85%: HR. 5.7; 95% CI, 1.9 to 16.8; P = .002). Cumulative incidence of relapse (± standard deviation) was higher among Hispanics (16.5% ± 4.0% v 6.3% ± 2.2%; P = .02). Association between Hispanic ethnicity and relapse (HR, 2.6; 95% CI, 1.1 to 6.1; P = .02) became nonsignificant (HR, 1.8; 95% CI, 0.6 to 5.2; P = .26) after adjusting for adherence and socioeconomic status. At adherence rates ≥ 90%, Hispanics continued to demonstrate higher relapse, whereas at rates < 90%, relapse risk was comparable to that of non-Hispanic whites.
Conclusion
Lower adherence to oral MP increases relapse risk. Ethnic difference in relapse risk differs by level of adherence—an observation currently under investigation.
INTRODUCTION
Acute lymphoblastic leukemia (ALL) is the most common childhood malignancy.1 Although a majority of children with ALL enter remission after induction, 20% relapse within 5 years.2 Furthermore, Hispanics have significantly inferior outcomes compared with non-Hispanic whites,3 a difference not entirely explained by clinical3 or genetic factors.4,5 Durable remissions require a 2-year maintenance phase that includes oral mercaptopurine (MP).6,7 Low erythrocyte levels of the MP metabolite (thioguanine nucleotide [TGN]) correlate with relapse.8 Significant interpatient variability in TGN levels has been observed,9 even after adjusting for inherited differences in thiopurine methyltransferase (TPMT) activity,9 and could be the result of failure to adhere to prescribed therapy.10 Lack of adherence to oral MP has been reported in children with ALL.10–12 However, reports on nonadherence have relied on small cohorts of patients and been based largely on self-report. Furthermore, the impact of nonadherence on relapse is not known. Finally, the role of nonadherence in explaining the observed ethnic difference in relapse remains unexplored.
We hypothesized that nonadherence to oral MP during maintenance would result in low systemic exposure to oral MP, thus increasing the risk of relapse. Furthermore, given the influence of sociodemographic factors on medication adherence in nononcologic populations,13–15 we hypothesized that ethnic differences in adherence would contribute to the inferior outcome in Hispanics. We tested this hypothesis by: measuring adherence to oral MP in Hispanic and non-Hispanic white children receiving maintenance therapy for ALL, and examining sociodemographic determinants of adherence; determining the impact of adherence on risk of relapse in the entire cohort and among Hispanics and non-Hispanic whites; and examining the extent to which adherence to oral MP explained ethnic difference in relapse.
PATIENTS AND METHODS
Study Participants
Participating institutions (Appendix, online only) contributed patients after obtaining approval from local institutional review boards for the study. Written informed consent/assent (in English or Spanish) was obtained from all participating patients and/or parents or legal guardians. Eligibility criteria included diagnosis of ALL at age ≤ 21 years; in first continuous remission; belonging to one of two self-reported ethnic/racial groups (Hispanic or non-Hispanic white); and receiving maintenance chemotherapy that included self- or parent- or caregiver-administered oral MP. Rationales for inclusion and exclusion criteria are described in Appendix Table A1 (online only).
Measurement of Adherence
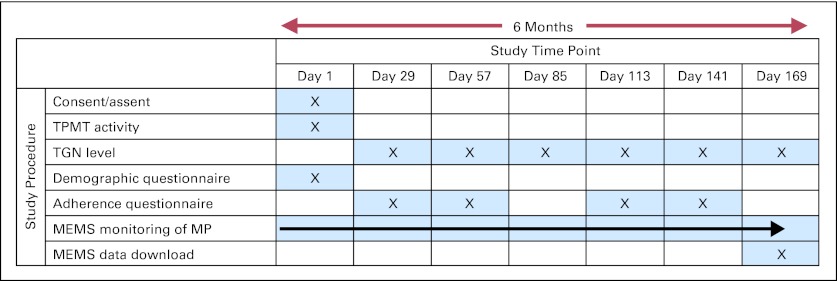
The study called for 6 months of adherence monitoring (study design illustrated in Appendix Fig A1, online only) using an electronic monitoring device (MEMS TrackCap; Aprex Corporation, Union City, CA). The MEMS cap uses microelectronic technology to record date and time of each pill bottle opening (Appendix Fig A2, online only). Patients/parents were informed about the purpose of MEMS and were instructed to take all doses of MP from the MEMS bottle. No compensation was provided for study participation. At the end of 6 months, data were downloaded (data download example illustrated in Appendix Fig A3, online only).
Erythrocyte TGN levels in pmol/8 × 108 erythrocytes were measured every month for the 6-month study period (Appendix Fig A1, online only) to demonstrate that MEMS bottle openings were accompanied by MP ingestion.16 TGN levels reflect chronic systemic exposure to MP over the past 30 days and therefore depend on the prescribed dose, adherence, and inherited variability in TPMT activity. TPMT activity was measured at least 90 days after red cell transfusion.17 Patients with TPMT activity ≥ 15th percentile for the cohort were assumed to have homozygous wild-type genotype based on known frequency of variant alleles.7,9,17,18
Demographic Questionnaire
A demographic questionnaire elicited self-reported information regarding parents' education, annual household income, number of adult caregivers, and race/ethnicity. Hispanics included patients of Mexican, Mexican-American, Chicano, Cuban, Puerto Rican, or other Spanish/Hispanic/Latino ethnicities, whereas non-Hispanic whites included patients of European, North African, or Middle Eastern ancestry.
Health Care Provider Reports
Participating institutions submitted monthly reports for each patient, detailing prescribed MP dose for each day of the preceding month and dates when the prescriber held MP for toxicity or illness. After completion of the 6-month adherence monitoring, participating institutions submitted biannual clinical status reports, detailing dates of last visit, relapse, or death and cause of death (if applicable) during the interim period.
Statistical Analyses
Adherence to oral MP.
Adherence rate was defined as the ratio of the number of days with MEMS cap openings (X) to the number of days MP was prescribed (N), reported as a percentage (X/N × 100). Days when MP was withheld by the prescriber were removed from the denominator (N). Adherence rate was computed for each of the 6 months of adherence monitoring. Longitudinal binomial regression was conducted using generalized estimating equation methods by modeling monthly adherence rate as an unstructured mean model using five indicator variables of time for the 6 study months. Time in months was also treated as a continuous variable to explore temporal trends in adherence rate. Compound symmetry was assumed as the working correlation matrix over time.19 Covariates considered for adjustment included those hypothesized to be predictors of adherence (sex, age at study participation [< 12 v ≥ 12 years], ethnicity [non-Hispanic whites v Hispanics], family structure [multiple caregivers v single mother], annual household income [≥ $20,000 v < $20,000], parental education [> high school v ≤ high school], time since start of maintenance, National Cancer Institute [NCI] risk classification [based on age at diagnosis and presenting white cell count],20 blast chromosomal abnormalities,21 and MP dose-intensity). MP dose-intensity was defined as the ratio of MP dose actually prescribed to the planned protocol dose of 75 mg/m2/d for the entire 6 months of adherence monitoring. Detailed definition of these variables is provided in the Appendix (online only). A relationship between MEMS-based adherence and erythrocyte TGN levels was sought using generalized estimating equation for normally distributed data by determining the association between the square root of TGN levels and MP dose-intensity–adjusted MEMS-based adherence rate.
Adherence to oral MP and risk of relapse.
All patients were in complete continuous first remission at entry into the adherence-monitoring study. Cumulative incidence of first relapse (at any site) was calculated, treating death as competing risk.22 Cox proportional hazards regression models were constructed to understand the impact of adherence on relapse. The following variables were included in the model: sex, ethnicity, parental education and annual household income, NCI risk classification, blast chromosomal abnormalities, TPMT activity (low/absent v normal), MP dose-intensity, time from ALL diagnosis to entry into adherence study, and adherence rate. Adherence rate was treated as a time-varying covariate, updating the rate each month by cumulating the values of X and N. The overall 6-month adherence rate was used for maintenance remaining after completion of adherence study. Adherence rate was categorized as: ≥ 95%, 94.9% to 90%, 89.9% to 85%, and < 85%. Proportional hazards assumption, examined by testing the interaction of adherence rate with time, was nonsignificant (P = .81).
Adherence to oral MP and ethnic difference in risk of relapse.
To test the hypothesis that MP adherence contributed to ethnic difference in relapse risk, Cox regression was performed with variables organized into four conceptual models. Model 1 included the clinical variables known to be associated with relapse (sex, NCI risk classification, blast chromosomal abnormalities, TPMT activity [low/absent v normal], MP dose-intensity, ethnicity, and time from ALL diagnosis to entry into adherence study). Model 2 added adherence rate to model 1. Model 3 replaced adherence in model 2 with parental education and annual household income. Finally, model 4 included all variables in models 1, 2, and 3.
Modifying effect of ethnicity on adherence-associated relapse.
Relapse risk among Hispanics and non-Hispanic whites was examined for varying categories of adherence (≥ 95%, 94.9% to 90%, 89.9% to 85%, and < 85%); non-Hispanics whites with adherence rate ≥ 95% served as referent group. Next, the modifying effect of ethnicity on relapse was examined by stratifying on categories of adherence.
All missing data were addressed with multiple imputation (Appendix, online only).23 PROCs GENMOD, LIFETEST, PHREG, MI, and MIANALYZE of SAS 9.1 (SAS Institute, Cary, NC) were used for analysis (Appendix, online only). Two-sided tests with P < .05 were considered statistically significant.
RESULTS
Patient Characteristics
Three hundred twenty-seven patients (169 Hispanics; 158 non-Hispanic whites) contributed 53,394 person-days for MP adherence monitoring. This sample size provided adequate power to address the proposed aims (Appendix, online only). All patients received treatment according to Children's Oncology Group therapeutic protocols (Appendix Table A2, online only). The distribution of Hispanics and non-Hispanic whites on low/standard-risk and high-risk protocols was comparable (Appendix, online only). Hispanic and non-Hispanic white participants were also comparable with respect to disease characteristics, MP dose-intensity, and TPMT activity (Table 1). However, Hispanics were more likely to report lower household income (< $20,000: 73 [45.6%] v 10 [6.5%]; P < .001), lower levels of parental education (≤ high school: 103 [62.8%] v 24 [15.2%]; P < .001), and higher prevalence of households with single mothers (26 [15.8%] v 10 [6.3%]; P = .007).
Table 1.
Demographics and Clinical Characteristics of the Study Participants
| Characteristic | Entire Cohort |
Non-Hispanic Whites |
Hispanics |
P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Cohort size | 327 | 100 | 158 | 48.3 | 169 | 51.7 | — |
| Age, years | .91 | ||||||
| Median | 4.0 | 4.5 | 4.0 | ||||
| Range | 1-19 | 1-19 | 1-18 | ||||
| Age at study participation, years | .85 | ||||||
| Median | 6.0 | 6.0 | 6.0 | ||||
| Range | 2-20 | 2-20 | 2-20 | ||||
| Male sex | 218 | 66.7 | 104 | 65.8 | 114 | 67.5 | .75 |
| WBC count at diagnosis≥ 50,000/μL | 61* | 18.8* | 31 | 19.6 | 30* | 18.0* | .70 |
| High-risk disease (NCI criteria) | 124* | 38.2* | 63 | 39.9 | 61* | 36.5* | .53 |
| Cytogenetics | |||||||
| Unfavorable† | 15* | 4.9* | 9* | 6.0* | 6* | 3.8* | .37 |
| Favorable† | 135* | 43.8* | 64* | 42.7* | 71* | 44.9* | .69 |
| Daily MP dose-intensity‡ | .18 | ||||||
| Mean | 82.3 | 80.6 | 83.8 | ||||
| SD | 21.3 | 21.4 | 21.2 | ||||
| TPMT activity | .16 | ||||||
| Median | 17.4* | 17.9* | 17.1* | ||||
| Range | 0.5-31.4* | 0.5-31.4* | 7.7-26.9* | ||||
| Person-days of adherence monitoring | 53,394 | 26,123 | 27,271 | — | |||
| Annual household income < $20,000 | 83* | 26.5* | 10* | 6.5* | 73* | 45.6* | < .001 |
| Paternal education ≤ high school | 159* | 50.2* | 42 | 26.6 | 117* | 73.6* | < .001 |
| Maternal education ≤ high school | 140* | 43.6* | 32 | 20.3 | 108* | 66.3* | < .001 |
| Parental education ≤ high school | 127* | 39.4* | 24 | 15.2 | 103* | 62.8* | < .001 |
| Single mother | 36* | 11.2* | 10 | 6.3 | 26* | 15.8* | .007 |
| Monolingual, Spanish speaking | 79* | 24.2* | NA | NA | 79* | 47.0* | — |
| Time from start of maintenance to study entry, years | .65 | ||||||
| Median | 0.8 | 0.8 | 0.9 | ||||
| Range | 0.2-2.2 | 0.2-2.1 | 0.2-2.2 | ||||
| Time from diagnosis to study entry, years | .56 | ||||||
| Median | 1.6 | 1.6 | 1.6 | ||||
| Range | 0.9-3.0 | 0.9-2.9 | 0.9-3.0 | ||||
| Length of follow-up from diagnosis, years | < .001 | ||||||
| Median | 5.3 | 5.9 | 4.6 | ||||
| Range | 1.3-10.3 | 1.5-10.3 | 2.0-10.1 | ||||
| Length of follow-up from study entry, years | < .001 | ||||||
| Median | 3.7 | 4.3 | 2.9 | ||||
| Range | 0.4-8.8 | 0.4-8.1 | 0.4-8.8 | ||||
| Length of follow-up from study exit, years | < .001 | ||||||
| Median | 3.3 | 3.8 | 2.4 | ||||
| Range | 0-8.5 | 0-8.0 | 0-8.5 | ||||
| Relapse | 27 | 8.3 | 8 | 5.1 | 19 | 11.2 | .04 |
Abbreviations: MP, mercaptopurine; NA, not applicable; NCI, National Cancer Institute; SD, standard deviation; TPMT, thiopurine methyltransferase.
Statistics were calculated for this table by excluding patients with missing values for the characteristics.
Unfavorable chromosomal abnormalities included t(9;22), t(4;11), hypodiploidy, or extreme hypodiploidy; favorable cytogenetics included one or more of the following: t(12;21), hyperdiploidy, trisomy 4 and 10, or trisomy 4, 10, and 17.
MP dose-intensity: mean MP dose (mg/m2 body surface area) prescribed over the No. of days that the drug was prescribed divided by the planned daily protocol dosage (75 mg/m2).
Adherence to Oral MP
Adherence to oral MP declined with time on study from 94.7% at the end of month 1 to 90.2% at the end of month 6 (P < .001; linear trend P = .001; Fig 1A). Multivariate analysis (Table 2) revealed Hispanic ethnicity (adjusted adherence rate: 88.4% v 94.8%; P < .001; Fig 1B), age ≥ 12 years at study entry (85.8% v 93.1%; P < .001; Fig 1C), and single-mother households (80.6% v 93.1%; P = .001; Fig 1D) to be associated with lower adherence. Sex (P = .51), parental education (P = .41), and annual household income (P = .93) did not influence adherence. Among patients with normal TPMT activity, each 1% increase in MEMS-measured adherence was associated with an 11.1-unit (pmol/8 × 108 erythrocytes) increase in MP dose- intensity–adjusted TGN levels (P < .001).
Fig 1.
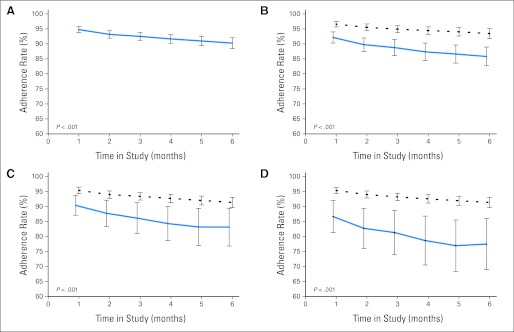
Adherence rates (A) for the entire cohort over the 6 months of observation, (B) over time according to ethnicity (solid and dashed lines represent estimated values for Hispanics and non-Hispanic whites, respectively), (C) over time according to age at study participation (solid and dashed lines represent estimated values for older [age ≥ 12 years] and younger [age < 12 years] patients, respectively), and (D) over time according to family structure (single mother v multiple caregivers; solid and dashed lines represent estimated values for single-mother and multiple-caregiver households, respectively). In each panel, 95% CIs of model estimates are presented on the plots.
Table 2.
Variables Associated With Adherence to Oral Mercaptopurine (N = 327)
| Variable | Parameter Estimate* | 95% CI | Estimated Adherence (%) | P |
|---|---|---|---|---|
| Intercept | 3.85 | 3.34 to 4.35 | — | < .001 |
| Time on study, months | < .001 | |||
| 1 | — | 94.7 | — | |
| 2 | −0.29 | −0.46 to −0.13 | 93.1 | < .001 |
| 3 | −0.41 | −0.61 to −0.22 | 92.5 | < .001 |
| 4 | −0.52 | −0.71 to −0.32 | 91.6 | < .001 |
| 5 | −0.59 | −0.81 to −0.37 | 91.0 | < .001 |
| 6 | −0.66 | −0.89 to −0.43 | 90.2 | < .001 |
| Age at study participation, years | < .001 | |||
| < 12 | — | 93.1 | ||
| ≥ 12 | −0.81 | −1.28 to −0.34 | 85.8 | |
| Household structure | .001 | |||
| Multiple caregivers | — | 93.1 | ||
| Single mother | −0.86 | −1.39 to −0.33 | 80.6 | |
| Ethnicity | < .001 | |||
| Non-Hispanic whites | — | 94.8 | ||
| Hispanics | −0.86 | −1.20 to −0.52 | 88.4 |
NOTE. All models were adjusted for time from initiation of maintenance to study entry. Other variables examined but not found to be significantly associated with adherence included: sex (P = .51), National Cancer Institute criteria for disease risk (P = .44), chromosomal abnormalities (P = .51), parental education (P = .41), and annual household income (P = .93).
More negative values of the parameter estimates indicate worse adherence rate when compared with the referent level, which is indicated by —.
Adherence to Oral MP and Risk of Relapse
With a median follow-up of 3.7 years (range, 0.4 to 8.8 years) from adherence study entry, 27 patients experienced relapse of their primary disease, and three patients died (second malignancies [n = 2]; relapse [n = 1]); the remainder were alive and in complete remission at last contact. The cumulative incidence of relapse (± standard deviation) for the entire cohort was 11.0% ± 2.1% at 4 years from adherence study entry. The median adherence rate over 6 months was significantly lower among those who relapsed (88.2%) compared with those who remained in complete continuous remission (96.2%; P = .001). After adjusting for sex, NCI risk classification, blast chromosomal abnormalities, MP dose-intensity, TPMT activity, ethnicity, time from diagnosis to adherence study entry, parental education, and household income, there was a progressive increase in risk of relapse with decreasing levels of adherence (reference: adherence ≥ 95%; 94.9% to 90%: HR, 4.1; 95% CI, 1.2 to 13.5; P = .02; 89.9% to 85%: HR, 4.0; 95% CI, 1.0 to 15.5; P = .04; < 85%: HR, 5.7; 95% CI, 1.9 to 16.8; P = .002; overall P = .01; df = 3; Table 3; model 4).
Table 3.
Cox Regression Analysis for Determinants of Relapse Risk in Children With ALL
| Variable | Model 1 |
Model 2 |
Model 3 |
Model 4 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| NCI criteria for disease risk | ||||||||||||
| Standard | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||
| High | 1.9 | 0.8 to 4.3 | .13 | 1.6 | 0.7 to 3.8 | .24 | 2.0 | 0.9 to 4.6 | .10 | 1.7 | 0.8 to 4.0 | .20 |
| Sex | ||||||||||||
| Male | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||
| Female | 1.0 | 0.4 to 2.4 | .95 | 1.1 | 0.5 to 2.7 | .78 | 1.0 | 0.4 to 2.4 | .94 | 1.1 | 0.5 to 2.8 | .76 |
| MP dose-intensity | ||||||||||||
| Per unit increase | 1.0 | 0.99 to 1.02 | .68 | 1.0 | 0.98 to 1.01 | .62 | 1.0 | 0.99 to 1.02 | .53 | 1.0 | 0.98 to 1.02 | .81 |
| TPMT activity | ||||||||||||
| Normal | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||
| Low | 0.9 | 0.3 to 3.2 | .91 | 0.8 | 0.2 to 3.1 | .76 | 0.9 | 0.2 to 3.2 | .89 | 0.8 | 0.2 to 3.1 | .75 |
| Ethnicity | ||||||||||||
| Non-Hispanic whites | 1.0 | 1.0 | 1.0 | 1.0 | ||||||||
| Hispanics | 2.6 | 1.1 to 6.1 | .02 | 2.3 | 0.9 to 5.7 | .07 | 2.1 | 0.8 to 5.7 | .14 | 1.8 | 0.6 to 5.2 | .26 |
| Adherence to oral MP* | ||||||||||||
| ≥ 95% | 1.0 | 1.0 | ||||||||||
| 94.9% to 90% | 4.0 | 1.2 to 13.2 | .02 | 4.1 | 1.2 to 13.5 | .02 | ||||||
| 89.9% to 85% | 3.6 | 1.0 to 13.5 | .05 | 4.0 | 1.0 to 15.5 | .04 | ||||||
| < 85% | 5.5 | 1.9 to 16.2 | .002 | 5.7 | 1.9 to 16.8 | .002 | ||||||
| Parental education | ||||||||||||
| > High school | 1.0 | 1.0 | ||||||||||
| ≤ High school | 1.6 | 0.6 to 4.0 | .35 | 1.8 | 0.7 to 4.4 | .23 | ||||||
| Annual household income | ||||||||||||
| ≥ $20,000 | 1.0 | 1.0 | ||||||||||
| < $20,000 | 1.0 | 0.4 to 2.5 | .99 | 0.9 | 0.4 to 2.3 | .85 | ||||||
NOTE. Adjusted for chromosomal abnormalities and time from diagnosis to study entry. Bold font indicates significance.
Abbreviations: ALL, acute lymphoblastic leukemia; HR, hazard ratio; MP, mercaptopurine; NCI, National Cancer Institute; TPMT, thiopurine methyltransferase.
P values for overall effect of adherence are .02 and .01 for models 2 and 4, respectively.
Clinically relevant adherence.
Nonadherence forms a continuum from the occasional missed dose to total refusal, creating a need to identify a clinically relevant level of adherence below which the risk of relapse is unacceptable. Using data from Table 3, model 4, we identified adherence rate < 95% to be associated with increased risk of relapse. Using this definition, 44% of the study participants were identified as nonadherent. The cumulative incidence of relapse (± standard deviation) was significantly greater among nonadherent patients (17.0% ± 3.7%) compared with adherent patients (4.9% ± 1.9%; P = .001; Fig 2A). The median absolute neutrophil count (MP dose-intensity adjusted) during the 6 months of adherence monitoring was significantly higher among the nonadherent patients (2.3 v 2.1; P < .04). After adjusting for all relevant prognosticators, nonadherent patients were 2.5-fold more likely to relapse (95% CI, 1.8 to 11.6; P = .002) compared with adherent patients. Finally, the adjusted risk of relapse attributable to nonadherence was 58.8%.24
Fig 2.
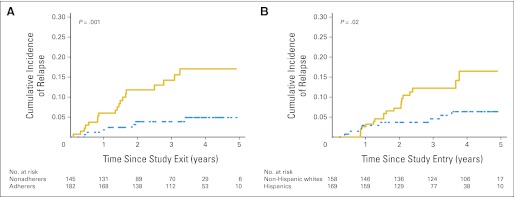
Cumulative incidence of relapse in a cohort of 327 children with ALL according to (A) adherence to oral mercaptopurine (solid and dashed lines represent nonadherers [< 95%] and adherers [≥ 95%], respectively) and (B) ethnicity (solid and dashed lines represent Hispanics and non-Hispanic whites, respectively).
Adherence to Oral MP and Ethnic Difference in Risk of Relapse
The cumulative incidence of relapse (± standard deviation) at 4 years was significantly higher among Hispanics (16.5% ± 4.0%) compared with non-Hispanic whites (6.3% ± 2.2%; P = .02; Fig 2B). To understand the contribution of adherence to ethnic difference in relapse risk, variables were introduced into the regression analysis serially (Table 3). In model 1, after adjusting for conventional prognosticators (sex, NCI risk classification, blast chromosomal abnormalities, MP dose-intensity, TPMT activity) and time from diagnosis to study entry, Hispanic ethnicity was significantly associated with relapse risk (HR, 2.6; 95% CI, 1.1 to 6.1; P = .02). Adding adherence to the model (model 2) partially mitigated the association between ethnicity and relapse risk (HR, 2.3; 95% CI, 0.9 to 5.7; P = .07). Replacing adherence with parental education and income (model 3) also mitigated the association between ethnicity and relapse risk (HR, 2.1; 95% CI, 0.8 to 5.7; P = .14). Finally, inclusion of adherence, parental education, and income to model 1 further mitigated the impact of ethnicity on relapse risk (Hispanics: HR, 1.8; 95% CI, 0.6 to 5.2; P = .26). Of note, the association between adherence and relapse remained similar in models 2 and 4, whereas the association between education/income and relapse were never statistically significant (model 3 or 4).
Importantly, a stratified analysis by ethnicity revealed a large association between adherence and relapse for non-Hispanic whites (adherence rate < 95%: HR, 11.6; 95% CI, 1.4 to 96.5; P = .02); the association was more modest for Hispanics (HR, 3.3; 95% CI, 1.1 to 9.8; P = .03]; Appendix Table A3, online only). This led to an evaluation of interaction between ethnicity and adherence-related relapse risk.
Modifying effect of ethnicity on adherence-related relapse.
As seen in Table 4, at adherence rates ≥ 90%, Hispanics were at higher risk of relapse compared with non-Hispanic whites (≥ 95%: HR, 5.3; 95% CI, 0.6 to 49.2; P = .14; 94.9% to 90%: HR, 10.5; 95% CI, 1.1 to 102.3; P = .04). However, at adherence rates < 90%, the ethnic difference in relapse risk was abrogated (89.9% to 85%: HR, 0.9; 95% CI, 0.1 to 7.8; P = .94; < 85%: HR, 0.5; 95% CI, 0.1 to 2.9; P = .37).
Table 4.
Relapse Risk in Children With ALL: Interaction Between Ethnicity and Adherence
| Adherence | Risk of Relapse for All Patients* |
Risk of Relapse by Ethnicity, Stratified by Adherence Level† |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| ≥ 95% | ||||||
| Non-Hispanic white | 1.0 | — | 1.0 | — | ||
| Hispanic | 5.3 | 0.6 to 49.2 | .14 | 5.3 | 0.6 to 49.2 | .14 |
| 90% to 94.9% | ||||||
| Non-Hispanic white | 2.8 | 0.2 to 45.7 | .46 | 1.0 | — | |
| Hispanic | 29.7 | 3.0 to 291.5 | .004 | 10.5 | 1.1 to 102.3 | .04 |
| 85% to 89.9% | ||||||
| Non-Hispanic white | 13.6 | 1.2 to 154.1 | .03 | 1.0 | — | |
| Hispanic | 12.6 | 1.0 to 162.6 | .05 | 0.9 | 0.1 to 7.8 | .94 |
| < 85% | ||||||
| Non-Hispanic white | 30.9 | 3.3 to 286.9 | .003 | 1.0 | — | |
| Hispanic | 16.5 | 1.8 to 148.7 | .01 | 0.5 | 0.1 to 2.9 | .37 |
NOTE. Adjusted for National Cancer Institute criteria of disease risk, sex, mercaptopurine dose-intensity, parental education, annual household income, blast chromosomal abnormalities, and time from diagnosis to adherence study entry.
Abbreviations: ALL, acute lymphoblastic leukemia; HR, hazard ratio.
Reference group: non-Hispanic whites with adherence ≥ 95%.
Reference group: non-Hispanics whites for the corresponding adherence level.
DISCUSSION
This report is the first to demonstrate conclusively that adherence to oral MP influences risk of relapse in children with ALL. There was a progressive increase in relapse risk with decreasing levels of adherence after adjusting for known clinical prognosticators. Furthermore, we identified an adherence rate of < 95% to be accompanied by a significant increase in relapse risk; patients who consumed < 95% of the prescribed MP were 2.5-fold more likely to suffer a relapse when compared with those who consumed > 95%. Using < 95% to define nonadherence, the cumulative incidence of relapse was 17% at 4 years among nonadherers and only 5% among adherers.
The current study confirmed adolescents to be vulnerable to nonadherence25–27; drift in adherence over time was also confirmed.26 However, to our knowledge, the current study is the first to document a significantly lower adherence rate among Hispanics as compared with non-Hispanic whites. Additionally, patients from single-mother households were at increased risk for nonadherence. Thus, the lower adherence among Hispanics could in part be explained by a household structure in which effective supervision by a single caregiver is not always possible, and adolescents assume increasing responsibility for self-medication.
Hispanics were at a 2.6-fold increased risk of relapse compared with non-Hispanic whites, confirming the previously reported inferior outcome experienced by Hispanics.3,28,29 The significantly lower level of adherence along with lower socioeconomic status among Hispanics contributed to the ethnic differences in relapse risk. However, ethnic difference in risk of relapse differed by the level of adherence. Thus, at adherence rates exceeding 90%, Hispanics continued to demonstrate a higher risk of relapse, whereas at adherence rates < 90%, the risk of relapse was comparable to that of non-Hispanic whites. These findings suggest that in the presence of adequate systemic exposure to MP, underlying genetic factors possibly influence relapse risk among Hispanics. On the other hand, at lower levels of adherence, relapse risk is comparable for Hispanics and non-Hispanic whites, suggesting that low systemic exposure to MP possibly supersedes the biologic differences. Supporting the genetic basis for the ethnic difference in outcome are publications demonstrating genomic variation that cosegregated with Native American ancestry and was associated with relapse5 and demonstrating the association between rearrangement of CRLF2, mutation of JAK kinases, alteration of IKZF1, Hispanic ethnicity, and outcome.4 However, neither study measured adherence to oral MP as cocontributor to relapse. A comprehensive evaluation of both the genetic and socioeconomic/cultural/behavioral underpinnings of the ethnic differences in survival is clearly needed and is currently under way.
Adherence to oral medications has been conventionally monitored by self-report and pill counts.30 Self-report is subject to social desirability bias,31 and pill counts to patient interference.32 Previous studies have reported lack of adherence in 10% to 33% of children with ALL.10–12 These studies were limited by poor quality of adherence measures (mostly self-report), small sample sizes, brief periods of observation, lack of association between adherence and relapse, and absence of a clinical basis for defining nonadherence. The current study used the MEMS device to monitor adherence for 53,394 person-days and used disease outcome to define a clinically relevant level of adherence. The MEMS device utilizes microelectronic technology to record each time the container is opened and provides the most accurate data in adherence research.33 Erythrocyte TGN levels are reflective of chronic (past 30 days) exposure10 but do not allow measurement of day-to-day variation in adherence. On the other hand, the MEMS device does not allow monitoring of pill ingestion, once the cap is opened. In the current study, TGN levels correlated with MEMS-based adherence rate, demonstrating that MEMS was an accurate method for assessing adherence.
Although every attempt was made to approach all eligible patients, this was logistically difficult to enforce at the 78 participating institutions. We addressed the issue of participation bias by comparing salient clinical characteristics of the adherence study participants with those of the corresponding therapeutic studies and demonstrated that there were no systematic differences between the two groups (Appendix Table A4, online only).
Hispanics are projected to account for 29% of the US population by 2050. According to the Institute of Medicine, Hispanics are vulnerable to adverse outcomes because of socioeconomic and cultural issues that create barriers to health care access and influence the understanding of prescribed treatment.34 This study describes for the first time to our knowledge differences in adherence to oral MP by ethnicity and the contribution of adherence to ethnic differences in ALL outcome.
Of all medication-related hospital admissions in the United States, up to 69% result from poor medication adherence, with a resultant cost of $100 billion per year.35 Our study demonstrates that 44% of children with ALL are consuming < 95% of prescribed MP and are consequently at increased risk of relapse. In fact, we demonstrate that 59% of all relapses were attributable to nonadherence. Outpatient oral MP treatment is inexpensive, whereas salvage treatment for recurrent ALL is expensive and largely unsuccessful. The current study identifies vulnerable populations that could benefit from targeted interventions to improve adherence, with resultant improvement in long-term survival and elimination of disparities in cancer outcomes. The current study also emphasizes the need to examine adherence to oral medications in other clinical situations; similar studies are currently planned for adolescent and young adult patients with ALL.
Acknowledgment
Presented as a podium presentation at the 52nd Annual Meeting of the American Society of Hematology, Orlando, FL, December 4-7, 2010.
Appendix
Sample Size and Power
The study enrolled 327 children with acute lymphoblastic leukemia (ALL; 169 Hispanic; 158 non-Hispanic white). With these sample sizes, the study had a power ranging from 82% to 100% to detect a difference of 0.15 to 0.30 in the proportion of nonadherers between Hispanics and non-Hispanic whites, assuming the proportion of nonadherers to be 35% to 40% in Hispanics and 10% to 25% in non-Hispanic whites. Furthermore, assuming the proportion of nonadherers to be 20%, a sample size of 327 enabled us to detect a hazard ratio of at least 1.6 between nonadherers and adherers, assuming percent loss of 5% to 10%.
On average, a Children's Oncology Group center sees five to 10 new patients with ALL each year. Of these, approximately 77% are non-Hispanic white, approximately 12% are Hispanics, approximately 6% are African Americans, approximately 2% are Asians, and approximately 3% are of mixed/other races. We therefore needed 78 participating institutions to accomplish the target enrollment, because a relatively small number of Hispanic patients are seen at each institution.
Variables Included in the Multivariate Analyses
National Cancer Institute risk criteria.
A composite term composed of white cell count at presentation and age at diagnosis. Two categories are as follows: standard risk: age at diagnosis of ALL 1 to 10 years and presenting WBC < 50,000; high risk: age at diagnosis of ALL < 1 year or age at diagnosis > 10 years or WBC at presentation > 50,000; by definition a dichotomous variable.
Chromosomal abnormalities.
Two categories are as follows: unfavorable cytogenetics: t(9;22); t(4;11), hypodiploidy, or extreme hypodiploidy; favorable cytogenetics: one or more of the following: t(12;21), hyperdiploidy, trisomy 4 and 10, or trisomy 4, 10, and 17.
Sex.
Males/females.
Mercaptopurine dose-intensity.
Continuous variable defined as the ratio of mercaptopurine (MP) dose actually prescribed to the planned protocol dose of 75 mg/m2/d for the entire duration (6 months) of the adherence monitoring.
Thiopurine methyltransferase activity.
Dichotomized; patients with thiopurine methyltransferase (TPMT) activity in the highest 85th percentile for the cohort were assumed to have homozygous wild-type genotype based on known frequency of variant alleles.
Ethnicity.
Dichotomous variable: Hispanic and non-Hispanic whites. Hispanics included patients of Mexican, Mexican-American, Chicano, Cuban, Puerto Rican, or other Spanish/Hispanic/Latino ethnicities, whereas non-Hispanic whites included patients of European, North African, or Middle Eastern ancestry.
Adherence.
Adherence rate was defined as the ratio of the number of days with MEMS cap openings (X) to the number of days MP was prescribed (N), reported as a percentage (X/N × 100). Days when MP was withheld by the prescriber were removed from the denominator (N). Adherence rate was computed monthly for each of the 6 months of adherence monitoring.
Age at study participation.
We examined age at study participation as a continuous variable and demonstrated an increasing risk of nonadherence with increasing age. However, to arrive at a clinically meaningful cut point that was also behaviorally and socially plausible, we created a categorical variable using 12 years as the age at which we believed a large majority of children were likely to achieve independence (from their parents) in terms of taking medication.
Parental education.
This was collected as a categorical variable and therefore was included in the analysis as a categorical variable. The parental education variable was computed by computing the mean of the maternal and paternal education levels.
Annual household income.
This was collected as a categorical variable and therefore was included in the analysis as a categorical variable (annual household income < $20,000 v ≥ $20,000).
Single caregiver versus multiple caregivers.
By definition a categorical variable.
Variables Included in the Multivariate Analysis Examining Determinants of Adherence
Variables included: time on study (month 1 to month 6), age at study participation, sex, ethnicity, parental education, household income, and household structure (single v multiple caregivers).
Variables Included in the Multivariate Analysis Determining Impact of Adherence on Relapse Risk and Ethnic Difference in Relapse Risk
Model 1.
National Cancer Institute (NCI) risk criteria (composite term composed of white cell count at presentation and age at diagnosis), sex, MP dose-intensity, TPMT activity, ethnicity, chromosomal abnormalities, and time from diagnosis to study entry.
Model 2.
All variables in model 1 plus adherence.
Model 3.
All variables in model 1 plus parental education and annual household income.
Model 4.
All variables in model 1 plus adherence plus parental education and annual household income.
MP Dose-Intensity–Normalized Thioguanine Nucleotide Levels
MP dose-intensity–normalized thioguanine nucleotide (TGN) level was calculated at each monthly time point by dividing the TGN levels by dose-intensity and then averaging across all time points. TGN level obtained within 90 days of red cell transfusion was not included in the calculation.
Multiple Imputation Procedure
Missing data (NCI risk classification [n = 2], chromosomal abnormalities [n = 19], parental education [n = 5], household income [n = 14], family structure [n = 4], language preference for completing questionnaire [n = 1], and TPMT activity [n = 19]) were addressed with multiple imputation (MI). A Markov chain Monte Carlo method assuming multivariate normality was used to fill in missing values, creating five complete data sets. Additionally included in the MI procedure were adherence rate, age at study entry, sex, dose-intensity, ratio of TGN to dose-intensity, ethnicity, recurrence/death, and time from start of maintenance to adherence study entry. Separate analyses were conducted for each complete data set; results were combined using MI inference procedures.
Statistical Analytic Techniques
PROCs GENMOD, LIFETEST, PHREG, MI, and MIANALYZE of SAS 9.1 (SAS Institute, Cary, NC) were used for analysis; t test was used to compare means of normally distributed variables; Wilcoxon rank sum test for non-normally distributed data, χ2 test for frequencies, and Wald's test for tests of significance of generalized estimating equation parameter estimates; two-sided tests with P < .05 were considered statistically significant.
Participating Institutions
All Children's Hospital, Allan Blair Cancer Centre, British Columbia's Children's Hospital, Brooklyn Hospital Center, Children's Healthcare of Atlanta, Emory University, Childrens Hospital & Clinics Minneapolis & St Paul, Children's Hospital and Regional Medical Center, Children's Hospital Central California, Children's Hospital Los Angeles, Children's Hospital of Austin, Children's Hospital of Michigan, Children's Hospital of Philadelphia, Children's Hospital of Pittsburgh, Children's Hospital of Western Ontario, Children's Hospital San Diego, Children's National Medical Center—DC, Children's of New Orleans/Louisiana State University Community Clinical Oncology Program, City of Hope National Medical Center, Columbus Children's Hospital, Connecticut Children's Medical Center, DeVos Children's Hospital, Driscoll Children's Hospital, Duke University Medical Center, Eastern Maine Medical Center, Geisinger Medical Center, Georgetown University Medical Center, Hackensack University Medical Center, Hurley Medical Center, Inova Fairfax Hospital, Joe DiMaggio Children's Hospital at Memorial, Kingston General Hospital/Kingston Regional Cancer, Lutheran General Children's Medical Center, Marshfield Clinic, McGill University Health Center—Montreal Children's Hospital, Midwest Children's Cancer Center, Miller Children's Hospital/Harbor—University of California at Los Angeles (UCLA), Nationwide Children's Hospital, New York Medical College, New York University Medical Center, Presbyterian Hospital, Primary Children's Medical Center, Princess Margaret Hospital for Children, Raymond Blank Children's Hospital, Rush—Presbyterian St Luke's Medical Center, Sacred Heart Children's Hospital, Saint Barnabas Medical Center, South Carolina Cancer Center, Southern California Permanente Medical Group, Southwest Texas Methodist Hospital, St Vincent Hospital—Wisconsin, Stanford University Medical Center, State University of New York at Stony Brook, Sunrise Childrens Hospital, Sunrise Hospital & Medical Center, SUNY Upstate Medical University, T.C. Thompson Children's Hospital, Tampa Children's Hospital, Texas Tech University Health Sciences Center—Amarillo, The Children's Hospital—Denver, The Children's Hospital of Southwest Florida, Lee M. Toledo Children's Hospital, UCLA School of Medicine, University of California at San Francisco School of Medicine, University of Alabama, University of California at Davis, University of Illinois, University of Minnesota Cancer Center, University of Nebraska Medical Center, University of New Mexico School of Medicine, University of Oklahoma Health Sciences Center, University of Texas Health Science Center at San Antonio, University of Vermont College of Medicine, University of Texas Southwestern Medical Center, Vanderbilt Children's Hospital, Virginia Commonwealth University Health System—Medical College of Virginia, Wake Forest University School of Medicine, Warren Clinic, West Virginia University Health Sciences Center—Charleston, and Yale University School of Medicine.
Table A1.
Inclusion and Exclusion Criteria With Rationale
| Criteria* | Rationale |
|---|---|
| Inclusion | |
| Diagnosis of ALL at age ≤ 21 years, in first remission | The study aimed to explore the underlying causes of ethnic differences in outcomes in children with ALL diagnosed at age ≤ 21 years |
| This age cutoff was used because the current study was attempting to understand the causes of racial/ethnic differences in survival that had been observed by us (and others) in previous studies that had also used age at diagnosis of 21 years as the cutoff (Bhatia S, Sather HN, Heerema NA, et al: Blood 100:1957-1964, 2002; Kadan-Lottick NS, Ness KK, Bhatia S, et al: JAMA 290:2008-2014, 2003) | |
| Belongs to the following self-reported ethnic/racial categories: non-Hispanic white or Hispanic† | The study aimed to explore the underlying causes of differences in outcomes in Hispanic and non-Hispanic white children with ALL |
| Has completed the first 6-month block of maintenance therapy for ALL | This allows patients to become accustomed to the routine of maintenance therapy; it also increases the probability that > 90 days have elapsed since last red cell transfusion, avoiding contamination of TGN and TPMT activity by transfusion (majority of transfusions administered during maintenance therapy are within the first 6 months) |
| ≥ 6 months of maintenance therapy remain at enrollment | The last 6-month block of maintenance therapy is needed to ensure adequate time for adherence monitoring during study enrollment |
| Receiving oral MP during maintenance therapy for ALL | The study is designed to measure adherence to oral MP |
| Signed assent/consent by the patient/parent | To ensure that all Protection of Human Subjects issues are addressed |
| Exclusion* | |
| Patients of multiethnic/multiracial backgrounds | The study aimed to explore the underlying causes of differences in outcomes in children with ALL belonging to the following self-reported race/ethnicities: Hispanic and non-Hispanic white; the outcome for patients of multiethnic/multiracial backgrounds was not included in this observational study; this will be the focus of future study |
| Patients who are unable to use MEMS TrackCap (eg, using pillbox or liquid MP) | MEMS TrackCap was the primary method of assessing adherence; use of a pillbox or liquid MP would have prevented the use of MEMS TrackCap |
Abbreviations: ALL, acute lymphoblastic leukemia; MP, mercaptopurine; TGN, thioguanine nucleotide; TPMT, thiopurine methyltransferase.
Eligibility is not affected by the patient or parent/caregiver's ability to speak English.
Non-Hispanic white: white or light-skinned patients of European, North African, or Middle Eastern ancestry; Hispanic: Mexican, Mexican American, Chicano, Cuban, Puerto Rican, or other Hispanic/Spanish/Latino ethnicities.
Table A2.
AALL03N1 Cohort: Treatment by COG Protocol by Ethnicity
| Protocol | Non-Hispanic White |
Hispanic |
Entire Cohort |
P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Low/standard risk | .87 | ||||||
| POG 9904 | 12 | 7.6 | 0 | 0.0 | 12 | 3.7 | |
| POG 9905 | 6 | 3.8 | 2 | 1.2 | 8 | 2.4 | |
| CCG 1922 | 0 | 0.0 | 1 | 0.6 | 1 | 0.3 | |
| CCG 1991 | 73 | 46.2 | 40 | 23.7 | 113 | 34.6 | |
| AALL0331 | 11 | 7.0 | 65 | 38.5 | 76 | 23.2 | |
| Total | 102 | 64.6 | 108 | 63.9 | 210 | 64.2 | |
| High risk | .87 | ||||||
| POG 9906 | 4 | 2.5 | 0 | 0.0 | 4 | 1.2 | |
| CCG 1961 | 26 | 16.5 | 21 | 12.4 | 47 | 14.4 | |
| AALL0031 | 0 | 0.0 | 1 | 0.6 | 1 | 0.3 | |
| AALL0232 | 25 | 15.8 | 34 | 20.1 | 59 | 18.0 | |
| AALL0434 | 0 | 0.0 | 3 | 1.8 | 3 | 0.9 | |
| Total | 55 | 34.8 | 59 | 34.9 | 114 | 34.8 | |
Abbreviations: AALL, acute lymphoblastic leukemia trial; CCG, Children's Cancer Group; COG, Children's Oncology Group; POG, Pediatric Oncology Group.
Table A3.
Cox Regression Analysis for Determinants of Relapse Risk in Children With ALL, Stratified by Ethnicity
| Adherence | Non-Hispanic Whites |
Hispanics |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Adherence rate | ||||||
| ≥ 95% | 1.0 | — | 1.0 | — | ||
| 94.9% to 90% | 2.8 | 0.2 to 45.7 | .46 | 5.6 | 1.5 to 21.2 | .01 |
| 89.9% to 85% | 13.6 | 1.2 to 154.1 | .03 | 2.4 | 0.4 to 13.5 | .33 |
| < 85% | 30.9 | 3.3 to 286.9 | .003 | 3.1 | 0.9 to 10.9 | .08 |
| Adherence rate | ||||||
| ≥ 95% | 1.0 | — | 1.0 | — | ||
| < 95% | 11.6 | 1.4 to 96.5 | .02 | 3.3 | 1.1 to 9.8 | .03 |
NOTE. Adjusted for National Cancer Institute criteria of disease risk, sex, mercaptopurine dose-intensity, average parental education, annual household income, chromosomal abnormalities, and time from diagnosis to study entry.
Abbreviations: ALL, acute lymphoblastic leukemia; HR, hazard ratio.
Table A4.
Comparison of Clinical Characteristics Between AALL03N1 Participants and Patients Treated on Parent Therapeutic Protocols
| Study | Race Group | Variable | Therapeutic Trial |
AALL03N1 |
P | ||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| 1961 | Both | Male | 1,033 of 1,724 | 59.92 | 35 of 47 | 74.47 | .0493 |
| 1961 | Both | Hispanic | 332 of 1,724 | 19.26 | 21 of 47 | 44.68 | < .001 |
| 1961 | Both | Median age | 11.53 | 11.15 | .8082 | ||
| Range | 1.00-21.14 | 1.79-17.94 | |||||
| 1961 | Both | Median WBC | 53.54 | 35.60 | .9572 | ||
| Range | 0.01-9,999.98 | 0.30-591.90 | |||||
| 1961 | White | Male | 825 of 1,392 | 59.27 | 17 of 26 | 65.38 | .6876 |
| 1961 | White | Median age | 11.69 | 10.78 | .3940 | ||
| Range | 1.00-20.43 | 2.34-16.33 | |||||
| 1961 | White | Median WBC | 51.10 | 71.25 | .3357 | ||
| Range | 0.11-9,999.98 | 1.30-377.00 | |||||
| 1961 | Hispanic | Male | 208 of 332 | 62.65 | 18 of 21 | 85.71 | .0352 |
| 1961 | Hispanic | Median age | 10.73 | 12.22 | .3573 | ||
| Range | 1.06-21.14 | 1.79-17.94 | |||||
| 1961 | Hispanic | Median WBC | 61.57 | 20.80 | .1462 | ||
| Range | 0.01-1,154.00 | 0.30-591.90 | |||||
| 1991 | Both | Male | 1,384 of 2,549 | 54.30 | 76 of 112 | 67.86 | .0048 |
| 1991 | Both | Hispanic | 546 of 2,549 | 21.42 | 40 of 112 | 35.71 | < .001 |
| 1991 | Both | Median age | 4.01 | 3.84 | .7372 | ||
| Range | 1.00-9.98 | 1.28-9.64 | |||||
| 1991 | Both | Median WBC | 6.90 | 6.90 | .4022 | ||
| Range | 0.02-49.80 | 0.50-43.60 | |||||
| 1991 | White | Male | 1,095 of 2,003 | 54.67 | 50 of 72 | 69.44 | .0154 |
| 1991 | White | Median age | 3.93 | 3.88 | .8757 | ||
| Range | 1.00-9.94 | 1.47-9.64 | |||||
| 1991 | White | Median WBC | 6.92 | 6.50 | .2073 | ||
| Range | 0.02-49.80 | 0.60-43.60 | |||||
| 1991 | Hispanic | Male | 289 of 546 | 52.93 | 26 of 40 | 65.00 | .1882 |
| 1991 | Hispanic | Median age | 4.24 | 3.75 | .3192 | ||
| Range | 1.03-9.98 | 1.28-8.28 | |||||
| 1991 | Hispanic | Median WBC | 6.45 | 8.30 | .6597 | ||
| Range | 0.48-49.30 | 0.50-35.20 | |||||
| AALL0232 | Both | Male | 1,373 of 2.513 | 54.64 | 40 of 59 | 67.80 | .0474 |
| AALL0232 | Both | Hispanic | 694 of 2,513 | 27.62 | 34 of 59 | 57.63 | < .001 |
| AALL0232 | Both | Median age | 12.17 | 10.01 | .1307 | ||
| Range | 1.00-30.68 | 1.46-19.02 | |||||
| AALL0232 | Both | Median WBC | 25.40 | 57.50 | .2569 | ||
| Range | 0.25-1,500.00 | 0.50-676.00 | |||||
| AALL0232 | White | Male | 983 of 1,819 | 54.04 | 17 of 25 | 68.00 | .2248 |
| AALL0232 | White | Median age | 12.18 | 8.22 | .2483 | ||
| Range | 1.01-30.68 | 1.46-19.02 | |||||
| AALL0232 | White | Median WBC | 19.61 | 58.30 | .3236 | ||
| Range | 0.25-1,500.00 | 1.50-173.40 | |||||
| AALL0232 | Hispanic | Male | 390 of 694 | 56.20 | 23 of 34 | 67.65 | .2170 |
| AALL0232 | Hispanic | Median age | 12.07 | 10.08 | .3221 | ||
| Range | 1.00-29.24 | 1.62-18.47 | |||||
| AALL0232 | Hispanic | Median WBC | 47.44 | 53.85 | .9973 | ||
| Range | 0.60-900.00 | 0.50-676.00 | |||||
| AALL0331 | Both | Male | 2,232 of 4,209 | 53.03 | 46 of 76 | 60.53 | .2039 |
| AALL0331 | Both | Hispanic | 1,026 of 4,209 | 24.38 | 65 of 76 | 85.53 | < .001 |
| AALL0331 | Both | Median age | 3.96 | 4.13 | .5078 | ||
| Range | 1.00-9.98 | 1.50-9.95 | |||||
| AALL0331 | Both | Median WBC | 7.30 | 8.05 | .5794 | ||
| Range | 0.30-49.98 | 0.80-47.10 | |||||
| AALL0331 | White | Male | 1,681 of 3,183 | 52.81 | 7 of 11 | 63.64 | .5554 |
| AALL0331 | White | Median age | 3.93 | 3.73 | .6365 | ||
| Range | 1.00-9.96 | 1.67-9.95 | |||||
| AALL0331 | White | Median WBC | 7.10 | 8.30 | .6945 | ||
| Range | 0.30-49.98 | 1.20-28.60 | |||||
| AALL0331 | Hispanic | Median age | 4.03 | 4.16 | .5350 | ||
| Range | 1.00-9.98 | 1.50-9.34 | |||||
| AALL0331 | Hispanic | Median WBC | 7.55 | 7.10 | .7437 | ||
| Range | 0.30-49.70 | 0.80-47.10 | |||||
| AALL0331 | Hispanic | Male | 551 of 1,026 | 53.70 | 39 of 65 | 60.00 | .3696 |
NOTE. AALL03N1 patients were largely drawn from the following four Children's Oncology Group (COG) protocols: 1961, 1991, AALL0232, and AALL0331. To demonstrate that the AALL03N1 population was representative of the COG study protocols from which it was drawn, we compared the distribution of the following variables in the parent therapeutic trial with the participants in the AALL03N1 study from the corresponding protocols. The results are summarized in this table. Because there was a deliberate attempt to overenroll Hispanics, there is a significantly higher proportion of Hispanics participating in the AALL03N1 study when compared with the parent therapeutic study. In terms of other prognostic variables, there was no difference by age at study participation or presenting WBC count or sex. The only exceptions were as follows: a higher proportion of Hispanic males treated per CCG 1961 participated in the AALL03N1 study (85.7% v 62.6%; P = .04), and a higher proportion of white males treated per CCG 1991 participated in the AALL03N1 study (69.9% v 55%; P = .01). These variables were taken into account in the multivariable analysis. These results demonstrate that the AALL03N1 study participants are representative of the parent therapeutic protocols from which they were drawn. Bold font indicates significance.
Fig A1.
Study design. MP, mercaptopurine; TGN, thioguanine nucleotide; TPMT, thiopurine methyltransferase.
Fig A2.
MEMS medication bottle with (arrow) TrackCap (Aprex, Union City, CA).
Fig A3.
Examples of adherence output using MEMS device from two patients: (A) adherent and (B) nonadherent.
Footnotes
Support information appears at the end of this article.
The National Institutes of Health and the American Lebanese Syrian Associated Charities had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: Mary V. Relling, Prometheus
AUTHOR CONTRIBUTIONS
Conception and design: Smita Bhatia, Mary V. Relling
Financial support: Smita Bhatia
Administrative support: Smita Bhatia, Lindsey Hageman, Nancy M. Kornegay, William L. Carroll, Mary V. Relling
Provision of study materials or patients: Smita Bhatia, William L. Carroll
Collection and assembly of data: Smita Bhatia, Wendy Landier, Lindsey Hageman, Alexandra N. Schaible, Andrea R. Carter, Cara L. Hanby, Nancy M. Kornegay, Leo Mascarenhas, A. Kim Ritchey, Jacqueline N. Casillas, David S. Dickens, William L. Carroll
Data analysis and interpretation: Smita Bhatia, Wendy Landier, Muyun Shangguan, Cara L. Hanby, Wendy Leisenring, Yutaka Yasui, Jane Meza, William L. Carroll, Mary V. Relling, F. Lennie Wong
Manuscript writing: All authors
Final approval of manuscript: All authors
Support
Supported in part by National Institutes of Health Grants No. R01 CA096670, M01-RR00043, U10 CA098543, U10 CA095861, R37 CA36401, and CA 21765, and by the American Lebanese Syrian Associated Charities.
REFERENCES
- 1.Linabery AM, Ross JA. Trends in childhood cancer incidence in the U.S. (1992–2004) Cancer. 2008;112:416–432. doi: 10.1002/cncr.23169. [DOI] [PubMed] [Google Scholar]
- 2.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia S, Sather HN, Heerema NA, et al. Racial and ethnic differences in survival of children with acute lymphoblastic leukemia. Blood. 2002;100:1957–1964. doi: 10.1182/blood-2002-02-0395. [DOI] [PubMed] [Google Scholar]
- 4.Harvey RC, Mullighan CG, Chen IM, et al. Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood. 2010;115:5312–5321. doi: 10.1182/blood-2009-09-245944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang JJ, Cheng C, Devidas M, et al. Ancestry and pharmacogenomics of relapse in acute lymphoblastic leukemia. Nat Genet. 2011;43:237–241. doi: 10.1038/ng.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koren G, Ferrazini G, Sulh H, et al. Systemic exposure to mercaptopurine as a prognostic factor in acute lymphocytic leukemia in children. N Engl J Med. 1990;323:17–21. doi: 10.1056/NEJM199007053230104. [DOI] [PubMed] [Google Scholar]
- 7.Relling MV, Hancock ML, Boyett JM, et al. Prognostic importance of 6-mercaptopurine dose intensity in acute lymphoblastic leukemia. Blood. 1999;93:2817–2823. [PubMed] [Google Scholar]
- 8.Lennard L, Lilleyman JS. Variable mercaptopurine metabolism and treatment outcome in childhood lymphoblastic leukemia. J Clin Oncol. 1989;7:1816–1823. doi: 10.1200/JCO.1989.7.12.1816. [DOI] [PubMed] [Google Scholar]
- 9.Lennard L, Lilleyman JS, Van Loon J, et al. Genetic variation in response to 6-mercaptopurine for childhood acute lymphoblastic leukaemia. Lancet. 1990;336:225–229. doi: 10.1016/0140-6736(90)91745-v. [DOI] [PubMed] [Google Scholar]
- 10.Lennard L, Welch J, Lilleyman JS. Intracellular metabolites of mercaptopurine in children with lymphoblastic leukaemia: A possible indicator of non-compliance? Br J Cancer. 1995;72:1004–1006. doi: 10.1038/bjc.1995.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies HA, Lennard L, Lilleyman JS. Variable mercaptopurine metabolism in children with leukaemia: A problem of non-compliance? BMJ. 1993;306:1239–1240. doi: 10.1136/bmj.306.6887.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lau RC, Matsui D, Greenberg M, et al. Electronic measurement of compliance with mercaptopurine in pediatric patients with acute lymphoblastic leukemia. Med Pediatr Oncol. 1998;30:85–90. doi: 10.1002/(sici)1096-911x(199802)30:2<85::aid-mpo3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Thumula V, Pace PF, et al. Predictors of medication nonadherence among patients with diabetes in Medicare Part D programs: A retrospective cohort study. Clin Ther. 2009;31:2178–2188. doi: 10.1016/j.clinthera.2009.10.002. discussion 50–51. [DOI] [PubMed] [Google Scholar]
- 14.Trinacty CM, Adams AS, Soumerai SB, et al. Racial differences in long-term adherence to oral antidiabetic drug therapy: A longitudinal cohort study. BMC Health Serv Res. 2009;9:24. doi: 10.1186/1472-6963-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy DA, Sarr M, Durako SJ, et al. Barriers to HAART adherence among human immunodeficiency virus-infected adolescents. Arch Pediatr Adolesc Med. 2003;157:249–255. doi: 10.1001/archpedi.157.3.249. [DOI] [PubMed] [Google Scholar]
- 16.Su Y, Hon YY, Chu Y, et al. Assay of 6-mercaptopurine and its metabolites in patient plasma by high-performance liquid chromatography with diode-array detection. J Chromatogr B Biomed Sci Appl. 1999;732:459–468. doi: 10.1016/s0378-4347(99)00311-4. [DOI] [PubMed] [Google Scholar]
- 17.McLeod HL, Lin JS, Scott EP, et al. Thiopurine methyltransferase activity in American white subjects and black subjects. Clin Pharmacol Ther. 1994;55:15–20. doi: 10.1038/clpt.1994.4. [DOI] [PubMed] [Google Scholar]
- 18.Relling MV, Hancock ML, Rivera GK, et al. Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus. J Natl Cancer Inst. 1999;91:2001–2008. doi: 10.1093/jnci/91.23.2001. [DOI] [PubMed] [Google Scholar]
- 19.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 20.Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14:18–24. doi: 10.1200/JCO.1996.14.1.18. [DOI] [PubMed] [Google Scholar]
- 21.Mrozek K, Heerema NA, Bloomfield CD. Cytogenetics in acute leukemia. Blood Rev. 2004;18:115–136. doi: 10.1016/S0268-960X(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 22.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: Wiley; 1987. [Google Scholar]
- 24.Benichou J. A review of adjusted estimators of attributable risk. Stat Methods Med Res. 2001;10:195–216. doi: 10.1177/096228020101000303. [DOI] [PubMed] [Google Scholar]
- 25.Smith SD, Rosen D, Trueworthy RC, et al. A reliable method for evaluating drug compliance in children with cancer. Cancer. 1979;43:169–173. doi: 10.1002/1097-0142(197901)43:1<169::aid-cncr2820430125>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 26.Tebbi CK, Cummings KM, Zevon MA, et al. Compliance of pediatric and adolescent cancer patients. Cancer. 1986;58:1179–1184. doi: 10.1002/1097-0142(19860901)58:5<1179::aid-cncr2820580534>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 27.MacDougall LG, Wilson TD, Cohn R, et al. Compliance with chemotherapy in childhood leukaemia in Africa. S Afr Med J. 1989;75:481–484. [PubMed] [Google Scholar]
- 28.Pollock BH, DeBaun MR, Camitta BM, et al. Racial differences in the survival of childhood B-precursor acute lymphoblastic leukemia: A Pediatric Oncology Group Study. J Clin Oncol. 2000;18:813–823. doi: 10.1200/JCO.2000.18.4.813. [DOI] [PubMed] [Google Scholar]
- 29.Kadan-Lottick NS, Ness KK, Bhatia S, et al. Survival variability by race and ethnicity in childhood acute lymphoblastic leukemia. JAMA. 2003;290:2008–2014. doi: 10.1001/jama.290.15.2008. [DOI] [PubMed] [Google Scholar]
- 30.Pritchard MT, Butow PN, Stevens MM, et al. Understanding medication adherence in pediatric acute lymphoblastic leukemia: A review. J Pediatr Hematol Oncol. 2006;28:816–823. doi: 10.1097/01.mph.0000243666.79303.45. [DOI] [PubMed] [Google Scholar]
- 31.Marlowe D, Crowne DP. Social desirability and response to perceived situational demands. J Consult Psychol. 1961;25:109–115. doi: 10.1037/h0041627. [DOI] [PubMed] [Google Scholar]
- 32.Cramer JA, Mattson RH, Prevey ML, et al. How often is medication taken as prescribed? A novel assessment technique. JAMA. 1989;261:3273–3277. [PubMed] [Google Scholar]
- 33.Urquhart J. The electronic medication event monitor: Lessons for pharmacotherapy. Clin Pharmacokinet. 1997;32:345–356. doi: 10.2165/00003088-199732050-00001. [DOI] [PubMed] [Google Scholar]
- 34.Smedley BD, Stith AY, Nelson A, editors. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academies Press; 2002. [PubMed] [Google Scholar]
- 35.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]