Abstract
Hyperhomocysteinemia is associated with aortic aneurysm, however, the mechanisms are unclear. We hypothesize that the expression level of genes involved in extracellular matrix (ECM) remodeling, oxidative stress, and enzymes involved in homocysteine metabolism pathway in aortic aneurysm and hyperhomocysteinemia are differentially regulated by DNA methylation. We studied the mRNA levels of MTHFR, SAHH, MMP-1, -9, TIMP-1, -4, peroxiredoxin, NOX-2, -3 (NAPDH oxidase subunits), collagen and elastin in normal and aortic aneurysm tissues from humans and aorta tissue from HHcy (Cystathionine beta synthase heterozygote knockout, CBS+/-) mice treated with high methionine diet. The total RNA was extracted using Trizol method and RT-PCR was performed. Protein expression of MTHFR, H3K9 (trimethyl) and TIMP4 were studied in mice using immunohistochemistry. MTHFR and TIMP4 expression was seen to be increasing in both human aneurysm samples as well as HHcy CBS+/- mice. There was increased expression of MMP9, peroxiredoxin and decreased expression of MMP1, Collagen I and IV was noted in thoracic aortic aneurysm samples. Increased Collagen IV and decreased Collagen I levels were seen in CBS +/- HHcy mice compared to their wild type controls. Since DNA methylation regulates gene expression of enzymes in Hcy metabolism pathway, we also measured the mRNA levels of DNMTs, MBD2 and H3K9. The results suggest an increase in the levels of DNMT1, 3a, MBD2 and H3K9 in CBS +/- aorta compared to their wild type controls. Our findings suggest a possible role of methylation in regulation of expression of genes involved in matrix remodeling and homocysteine metabolism.
Keywords: Homocysteine, aortic aneurysm, CBS, MTHFR, MMP, TIMP, collagen
Introduction
Atherosclerosis, aortic aneurysms and dissecting aneurysms, are linked to hyperhomocysteinemia. In order to gain an understanding of the relationship between homocysteine and aortic diseases, it is important to first explain the nature of these diseases. An aneurysm is an abnormal, irreversible dilation caused by arterial wall weakness due to a decrease in the elastin content. Abdominal aortic aneurysms (AAA) are known to be associated with atherosclerosis, hypertension, hyperhomocysteinemia and smoking. Interestingly, increased plasma homocysteine levels have been observed in AAA patients (15.7±6.5; n=58) when compared to their healthy controls (9.6±3.9; n=60) [1]. In addition infections, congenital defects, and trauma were also found to be some plausible etiologic factors of AAA. The most common site for an aneurysm to develop is the abdominal aorta. Most AAA occurs below the level of the renal arteries. They may affect the bifurcation of the aorta as well as the iliac arteries. Aneurysms often increase in size; they can rupture and lead to hemorrhage and death, if not treated. If an aneurysm is greater than 5 cm, surgery is generally recommended. Ruptured aneurysms occur in approximately 5 out of 10,000 people. In the descending thoracic and abdominal aorta, aneurysms result from severe intimal atherosclerosis, increased elastin and collagen degrading enzymes and pro-inflammatory cytokines, chronic inflammation, and remodeling of the elastic media [1].
Thoracic aortic aneurysms (TAA), like AAA, are caused due to atherosclerosis, trauma, and hypertension. Other unique factors which have been found to play a major role in causing TAA are syphilis, Marfan syndrome (MFS), and bicuspid aortic valves. Aneurysms in the ascending aorta are due to degeneration of the aortic media through Cystic Medial Necrosis (CMN): loss of smooth muscle cells and degeneration of elastic fibers. Accelerated CMN has been known to be associated with genetic syndromes, like MFS [1].
Dissection is a condition than is distinct from aneurysm. It develops after a tear in the intima with separation in the layers of the aortic wall and forms a passageway for blood. This diverts blood throughout the aorta in the chest and abdomen and can result in ischemia. Dissection results in acute aortic dilatation and frequently ruptures to death.
Bicuspid aortic valves only have two leaflets that usually are not equal in size [2,3]. This congenital abnormality is most commonly identified as a systolic ejection click during exam. It is present in about 1-2% of the population and the anomaly often has no symptoms, though the coronary arteries may be abnormal and sometimes the aortic root is dilated. An existence of a causal relationship between aortic aneurysm and bicuspid aortic valves has been hypothesized [4].
Homocysteine (Hcy) is a sulfur containing amino acid formed during methionine metabolism. The normal range of homocysteine in the blood is around 4–17 μmol/L, though it is important to note than the normal range varies with age and sex as well as nutritional determinants [5]. In cases of hyperhomocysteinemia (HHcy) in humans, various pathologies result. HHcy is a risk factor for cardiovascular diseases, like stroke, ischemic heart disease, and peripheral vascular disease [6].
Eleven genes have been examined in this study. They are organized into three groups: defects in homocysteine metabolism, remodeling, and oxidative stress. It is hypothesized that high levels of Hcy due to defects in methionine metabolism causes oxidative stress which induces remodeling of the aortic wall.
The first group was made of genes dealing with methionine metabolism. S - Adenosyl-L-homocysteine hydrolase (SAHH) and Methylenetetrahydrofolate reductase (MTHFR) genes were chosen. Intracellular Hcy is made by the enzyme SAHH from S-adenosylhomocysteine [7]. MTHFR is the enzyme involved in folate metabolism, catalyzing the reduction of 5, 10-methylenetetrahydrofolate to 5 methyltetrahydrofolate. Because folate is a cofactor in re-methylation of homocysteine, without MTHFR, homocysteine levels in the plasma increase. CBS removes Hcy from the methionine cycle permanently by catalyzing the synthesis of cystathionine from Hcy and serine [7]. Lack of SAHH causes a decrease in intracellular homocysteine, while lack of MTHFR and CBS causes an increase in homocysteine levels. CBS +/- mice have been used in the current study to analyze the genes in HHcy.
In the remodeling group are: Collagen Type I, -IV, Elastin, Matrix metalloproteinase -1, -9 (MMP-1, -9), Tissue inhibitors -1, -4 (TIMP-1, -4). These genes were chosen to learn more about the composition of the aortic wall in the various groups. MMP-1 initiates cleavage of fibrillar collagens at a single site so that other MMPs can further degrade collagen [8]. MMP-9, a 92kDa gelatinase, has been known to be present in high amounts in the plasma of patients with AAAs [9]. Both collagen and elastin make up the wall of the aorta. Collagen type I is 90% of all human collagen and it forms fibers while collagen type IV forms networks because it makes up the basal lamina. Tissue inhibitors of matrix metalloproteinases (TIMPs) prevent aortic wall destruction and prevent aneurysm development by inhibiting MMPs [10]. TIMP-1 is a collagenase inhibitor while TIMP-4 is secreted extracellularly primarily from heart tissue and it may be involved with extracellular matrix homeostasis [11].
The final group is oxidative stress which includes NADPH Oxidase (NOX), Peroxiredoxin (PRDX) genes. PRDX is a redox enzyme that has a cysteine in the active site. The cysteine can be over oxidized under oxidative stress conditions. PRDX may detoxify reactive oxygen species [12]. NOX has a different purpose than PRDX; it generates O2- in both phagocytic cells and non-phagocytic cells [13]. Oxidative stress is increased in hyperhomocysteinemia; hence PRDX is decreased and NOX is increased in this disease state.
DNA methylation is known to be an important epigenetic mechanism in transcriptional regulation of many genes. The methyl group is usually attached to the cytosine residue in the nucleotide sequence with the help of specific enzymes called DNA methyl transferases (DNMT) [14] There are two types of methylation involved – de novo methylation facilitated by DNMT 3a and DNMT 3b and maintenance methylation, handled by DNMT1. Methyl binding domain (MBDs) proteins like MBD2 have been known to bind themselves to these methylated CpG islands and down regulate transcription of downstream sequence by recruiting co-repressor complexes [15]. These co-repressor complexes are proteins which are bound to chromatin remodeling proteins like Histone 3 tri-methylated at lysine 9 (H3K9) [16] HDACs etc. Homocysteine induced DNA methylation has been found to play an important role in atherosclerosis [17]. In the current study, aortic tissue samples from wild type (C57BL/6J), CBS +/- (high methionine diet) mice were taken and cDNA analysis of various genes involved in methylation like DNMTs, MBD2, H3K9 was performed to study the levels of DNA methylation in hyperhomocysteinemia.
Methods
Tissue procurement from human aortic samples
Aneurysmal aortic specimens (n=22) were obtained at the time of operative AAA repair from patients ranging in age from 49-77 years. All aneurysm tissues were obtained from regions 3-6 cm below the renal arteries. Normal aortic specimens (n=5) were obtained from organ donors ranging in age from 39-59 years at the time of harvest. These specimens were obtained from the infra renal or suprarenal abdominal aorta. Experiments were conducted under NIH guidelines with the approval of the Institutional Animal Care and Use Committee (IACUC). All the tissue samples were frozen in liquid nitrogen and stored at -80°C until use. A description of the groups is given as follows: 1) Normal aorta with normal aortic valve (Normal), 2) Aortic aneurysm with diseased tricuspid aortic valve, abdominal aortic aneurysm (AAA), 3) Aortic aneurysm with bicuspid aortic valve, Thoracic aortic aneurysm (TAA) and 4) Normal aortas with bicuspid aortic valve (Normal+ BiAV).
Mouse samples
CBS +/- (B6129P2) were fed with high methionine diet for three weeks in order to create hyperhomocysteinemia condition. Aortic tissue was isolated from wild type (C57BL/6J) and CBS +/- (B6129P2) treated mice, obtained from Jackson Laboratories.
Histopathological analysis
Reverse transcription polymerase chain reaction (PCR)
Total RNA was isolated from approximately 1-2g (total wet weight) of aortic tissue using Trizol reagent (Invitrogen) [18]. The quality of RNA was determined by electrophoresis on 1.5% agarose-formaldehyde gels that were stained with ethidium bromide. Only RNA samples that showed clear 18s and 28s rRNA bands were used for the study. 1 μg of total RNA was reverse transcribed using the Reverse Transcription System (Promega), according to the manufacturer’s instruction. Primers, to amplify specific gene sequences, were designed using Primer 3 design. The list of primers for amplifying human and murine samples is given in Table 1 (a), (b) and (c). PCR was performed in the Biorad DNA Engine® Thermocycler for 35 cycles (denaturation at 94°C for 60 min, annealing varies with different genes ,extension at 72°C for 120 min carried out in a volume of 25 μl under the following cycling conditions: 94°C for 1min), PCR products were analyzed by gel electrophoresis in 1% or 1.5% agarose gels. The gel images were documented using Bio-Rad ChemiDoc™ XRS+ System and analyzed using Image Pro analysis (Bio-Rad).
Table 1.
List of primers used in (A) human samples and (B) murine aortic samples
| A | ||
|
| ||
| Gene | Primer pair sequence (5’-3’) | Product size (bp) |
|
| ||
| Collagen - I | GACGGGAGTTTCTCCTCGGGGTC GAGTCTCCGGATCATCCACGTC | 398 |
| Collagen - IV | ATGTCAATGGCACCCATCAC CTTCAAGGTGGACGGCGTAG | 382 |
| Elastin | AAAGCAGCAGCAAAGTTCGG ACCTGGGACAACTGGAATCC | 288 |
| SAHH | TGTTGCTTTTATGTCTCTCTGG GCTTGGCATTCTCTTAAACC | 470 |
| MTHFR | GCAATTGTGGGATGTCCTCT TTCTGAGCTTGTGCATTTGG | 570 |
| MMP1 | ACCCCAAGGACATCTACAGC CACCTTCTTTGGACTCACACC | 786 |
| MMP9 | ACCTGGATGCCGTCGTGGAC TGTGGCAGCACCAGGGCAGC | 475 |
| TIMP1 | GGGGCTTCACCAAGACCTACAC AAGAAAGATGGGAGTGGGAACA | 506 |
| TIMP4 | CGGCCAGTGCAGACCCTGCTG TGAGAGGCAGGTGGCCCCGGT | 317 |
| PRDX-2 | CTTGCCTGGTGTCGGTGGTTAGT CGGCTGAATCTGAAGTCTTGGTTTT | 278 |
| NOX-2 | ATATTTTGGAATTGCAGATGAACA ATATTGAGGAAGAGACGGTAG | 650 |
| GAPDH | ACCACAGTCCATGCCATCAC TCCACCACCCTGTTGCTGTA | 903 |
|
| ||
| B | ||
|
| ||
| Gene | Primer pair sequence (5’-3’) | Product size (bp) |
|
| ||
| Collagen - I | AGAACTTTGCTTCCCAGATG CTATCTGTACCACCCCCTTG | 162 |
| Collagen - IV | GCTGCTAAGAACTTGCCTTC GGGGACAGAGAAGATGTCAC | 105 |
| Elastin | TGACAGTATAGGGCTGAGCA GAGTTGTTGTGGGTGAGACA | 238 |
| SAHH | CATGGGGTAGGAAAAGATTG ACCTCCTCACCAATGTCCTA | 144 |
| MTHFR | ACCTGAAGCATTTGAAGGAG GATAGGGCAAGAGATGCCTA | 128 |
| MMP1 | CACACTGTTCCAGCTTTACG GCTCTTGAACAGCCCATACT | 101 |
| MMP9 | CATGTCACTTTCCCTTCACC TTGCCGTCCTTATCGTAGTC | 158 |
| TIMP1 | CTCAAATGGGAGAAGCTGTT AAGTGACGGCTCTGGTAGTC | 299 |
| TIMP4 | CTGGTAAGTGAAGGGGCTAA ACCAGGTTCATGGAGGTAGA | 289 |
| PRDX-2 | TGTGCTCCATACCAGAGCTA CCTCTTCAAATGGCTTCACT | 221 |
| NOX-3 | CAGAACTTGTGTGTCCACTG CCCCTTTCTCCTTAAATTGT | 237 |
| GAPDH | TAAATTTAGCCGTGTGACCT AGGGGAAAGACTGAGAAAAC | 177 |
| DNMT1 | GGGTCTCACCAAGTATCTCA GGTGTGTGACTCCAGTTTTT | 235 |
| DNMT3a | GGGAGAGAGGGAAAATTCTA GGTTTTCTTCAAGGTTTCCT | 298 |
| DNMT3b | GACTGCCTGGAGTTCAGTAG ACAGGCAAAGTAGTCCTTCA | 285 |
| MBD2 | TAGCGCATCAGATGTAACAG AGAGACTTGTCCTGTGATGG | 199 |
| H3K9 | CTGTCTCAAAGAAGGAGGTG GGACAGACAAACACCAAAGT | 279 |
| HDAC1 | GACTGGACCCTCTGTCATTA TTTCTCCTAAAGTGCAGCTC | 248 |
Immunohistochemistry
Mice aortic tissue was collected and made into blocks in a Peel-A-Way disposable plastic tissue embedding molds (Polysciences inc., Warrington, PA., USA) containing tissue freezing media (Triangle Biomedical Sciences, Durham, N.C., USA) and stored at -80°C until further use. 5 μm thickness tissue sections were made using Cryocut (Leica CM 1850) and placed on Super frost plus microscope slides, air-dried and processed for immunohistochemistry (IHC). Following reagents were used for the IHC: rabbit polyclonal anti-TIMP4, mouse polyclonal anti-Histone 3 tri-methyl K9, rabbit polyclonal anti NOX2 and mouse monoclonal anti MTHFR. The primary antibodies were bought from Abcam (Cambridge, MA). The following secondary fluorescent antibodies were used: Texas Red raised in rabbit, Alexa Fluor 488 raised in mouse from Invitrogen (Carlsbad, CA). IHC was performed as described previously [19]. Briefly, after tissue fixation and permeabilization, primary antibodies were applied overnight followed by fluorescent secondary antibodies. The stained slides were mounted and visualized with a laser scanning confocal microscope (Olympus FluoView1000) with appropriate filters.
Image analysis
Images obtained after immunohistochemistry were analyzed with Image Proplus software. Image analysis was done using Image-pro software in confocal microscope images taking different optical fields at random into consideration.
Statistical analysis
Statistical analysis was performed using GraphPad InStat to compare data collected from groups. Differences between groups and controls were determined by one-way analysis of variance (ANOVA). A probability level (p<0.05) is considered statistically significant. All values will be presented as mean ± SEM.
Results
Increased expression of metabolites involved in homocysteine metabolism pathway and remodeling proteins in human aortic tissue samples
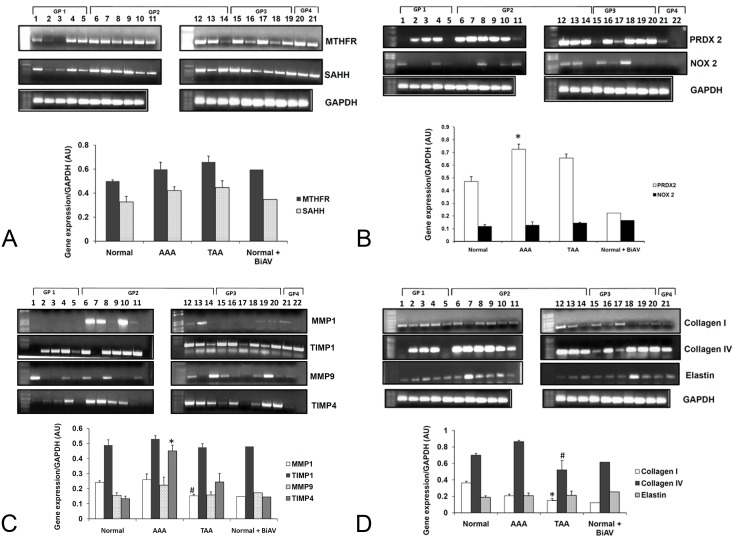
Expression of MTHFR and SAHH was found to be higher in AAA aortic tissue samples when compared to the healthy controls (Figure 1A). The expression level of peroxiredoxin 2 was found to be greater in abdominal and thoracic aortic aneurysm tissue samples when compared to controls (Figure 1B). There was no significant fluctuation in the levels of NOX2 in AAA and TAA samples with respect to their controls. There was an increase in the level of TIMP4 in aneurysm samples, whereas a slight increase in MMP9 level was seen in AAA samples when compared to the controls (Figure 1C). Collagen I levels were found to decrease whereas Collagen IV levels were found to increase in AAA samples and decrease in TAA when compared to their healthy controls (Figure 1D). No significant change was noticed in the elastin levels.
Figure 1.
Reverse transcription polymerase chain reaction. RNA extracted from various aortic tissue samples were reverse transcribed and amplified using primers designed for (A) MTHFR, SAHH (B) PRDX2 and NOX2, *p<0.05 versus normal controls in group 1 (n=8) (C) MMP1, TIMP1, MMP9, TIMP4, *p<0.05 versus normal controls in group 1 (n=4), #p<0.05 versus AAA samples in group 2 (n=4) (D) Collagen1, Collagen4, Elastin, *p<0.05 versus healthy controls in group1 (n=4), #p<0.05 versus AAA samples in group 2 (n=4).
Expression of metabolites involved in homocysteine metabolism pathway and remodeling proteins in murine aortic tissue samples
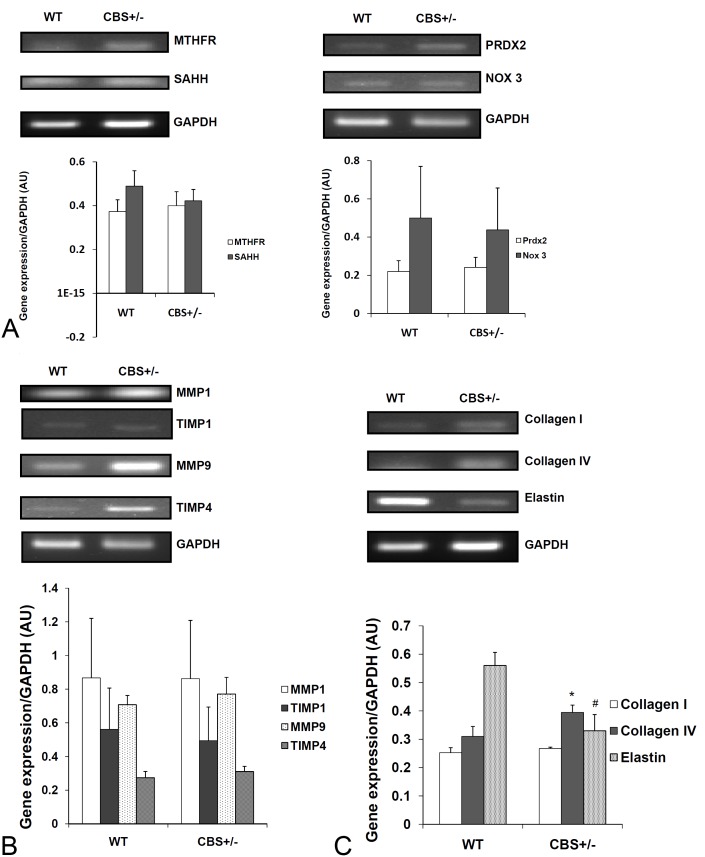
A slight increase in MTHFR and a slight decrease in SAHH levels were seen in tissue samples from CBS+/- mice (Figure 2A). Peroxiredoxin levels were found to be slightly increasing for CBS +/- tissue samples. There is no significant difference in NOX3 levels between WT and CBS +/-. There was no significant difference in MMP1 and TIMP1 levels across WT and CBS +/-. A slight increase in MMP9 and TIMP4 levels were noted in CBS+/- samples compared to the wild type samples (Figure 2B). A significant increase was seen in Collagen IVa1 levels and a mild increase was seen in Collagen Ia1 levels in CBS +/- mouse samples. The level of Elastin was found to be decreasing in CBS +/- samples when compared to WT (Figure 2C).
Figure 2.
Reverse transcription polymerase chain reaction analysis. Reverse transcription was carried out on RNA extracted from aorta from wild type (WT) and CBS +/- mice. The expression levels of (A) MTHFR, SAHH, PRDX2, NOX3 (B) MMP1, TIMP1, MMP9, TIMP4 (C) Collagen I, Collagen IV, and Elastin were analyzed. *p<0.05 versus wild type controls, #p<0.05 versus wild type controls (n=4).
Expression levels of genes involved in global methylation in murine aortic samples
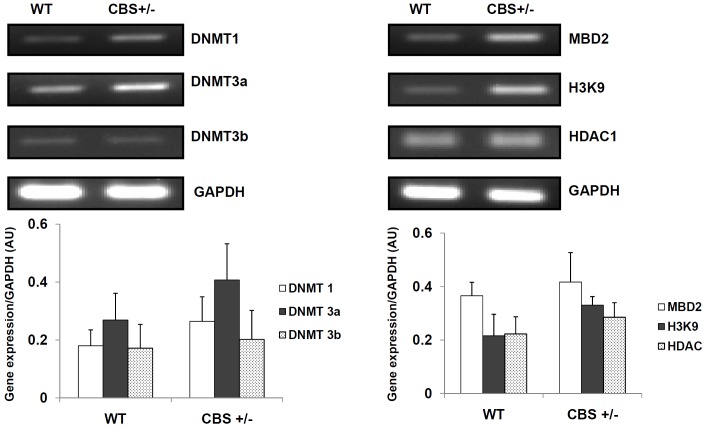
DNMT 1 and 3a levels were found to increase in CBS +/- mice to a considerable extent when compared to WT mice (Figure 3). DNMT 3b levels did not show any great increase between WT and CBS +/- mice samples. MBD2 and Histone 3 trimethylation in lysine 9 (H3K9) expression levels were found to increase in CBS +/- mice when compared to WT. However, there was no significant difference in the Histone deacetylase 1 (HDAC1) levels.
Figure 3.
The mRNA expression levels of DNMT1, DNMT 3a, DNMT 3b, MBD2, H3K9 and HDAC1 were studied in aorta from wild type (WT) and CBS +/- mice.
Protein expression in mouse aorta
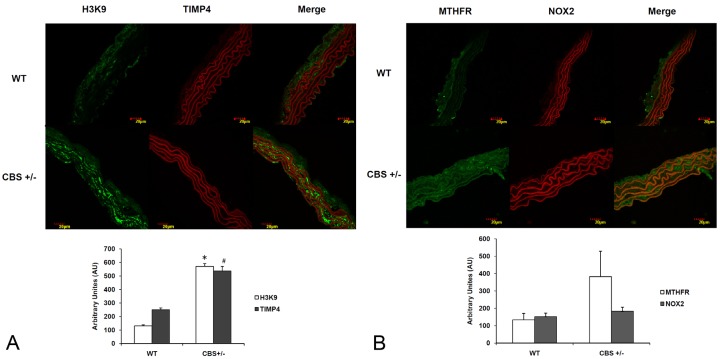
Immunohistochemistry of CBS+/- mouse aorta sections displayed an increased expression of H3K9, MTHFR and TIMP4 proteins when compared to WT controls (Figure 4A and 4B). There was no significant change in the expression of NOX2 levels between the groups.
Figure 4.
Immunohistochemistry. The protein expression was seen as (A) H3K9, TIMP4 and (B) MTHFR, NOX2 were studied. H3K9 and MTHFR are stained with green fluorescence intensity and expression of TIMP4 and NOX2 are seen as red fluorescence intensity. *p<0.01 versus wild type, #p<0.02 versus wild type.
Discussion
Increased activity of MMPs/TIMPs has been implicated in ECM alterations in vascular diseases such as aortic aneurysm, dissection and atherosclerosis. Homocysteine is one of the well-known risk factors for aortic dissection [20]. Since increased plasma homocysteine levels have previously been correlated with aortic aneurysm [1,21], aorta tissue from CBS+/- mice, serve as HHcy samples. In the present study, we performed an analysis of various genes involved in homocysteine metabolism pathway and ECM remodeling in human aortic aneurysm samples. The human aorta samples consist of four groups: normal aortas with normal aortic valve as a control; aortic aneurysm with normal or diseased tricuspid aortic valve (AAA); aortic aneurysm with bicuspid aortic valve (TAA); and normal aortas with bicuspid aortic valve.
Human thoracic and abdominal aortic samples showed similar increase in MTHFR and SAHH levels (Figure 1A), whereas samples from WT and CBS+/- mice did not differ significantly (Figures 2A, 4B). MTHFR is generally known to be involved in the methylation of homocysteine to methionine and CBS is known to metabolize homocysteine to cysteine. An increase in the homocysteine level could have triggered the remethylation pathway to enhance the metabolism of homocysteine (Figure 5). This can be a possible explanation for the increase in MTHFR in aneurysmal and CBS+/- aorta samples. A decreased relative expression of SAHH in CBS +/- can be accounted to similar decrease in SAHH levels reported in vascular smooth muscle cells under HHcy conditions [17].
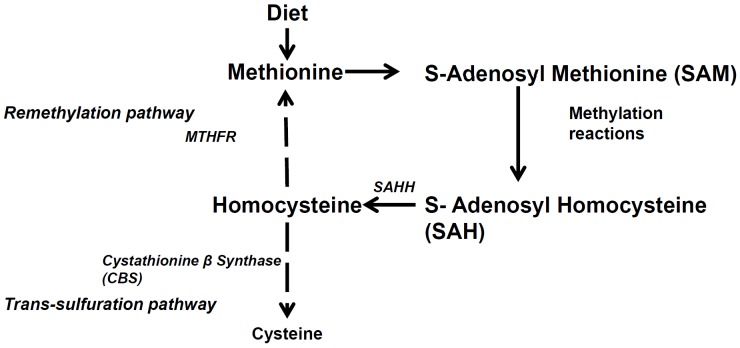
Figure 5.
Schematic representation of the genes involved in homocysteine metabolism pathway.
Although peroxiredoxin -2 levels were increased in human samples (Figure 1B) their levels in CBS +/- animals were decreased (Figure 2A). It has been previously reported that peroxiredoxin -1 and 2 levels increased when wild type mice were subjected to dietary induced HHcy conditions when compared to CBS +/- mice [22]. Our findings concur with these reports.
Since increased serum levels of MMP3 and MMP9 are associated with ascending aortic aneurysm [23], we measured MMP9 and its inhibitor TIMP4 which is widely expressed in abdominal aortic aneurysm and found a significant elevation in their levels (Figure 1C). A similar increase was observed in CBS +/- aorta samples (Figures 2B and 4A). MMP1 and TIMP1 levels were found to be elevated in abdominal aortic aneurysm samples and decreased in thoracic aortic aneurysm (Figure 1C) reflecting a regional difference in (ECM) regulation. MMP1 levels have been previously reported to be increased in abdominal aortic aneurysm [24] and decreased TIMP1 levels have been shown to increase thoracic aortic aneurysm progression [25]. A contradictory increased TIMP1 levels in AAA samples and decreased MMP1 levels in TAA samples can be due to compensatory effects on their respective MMP1 and TIMP1 levels. TIMP1 and TIMP4 are known to be endogenous inhibitors of MMP1 and MMP9 respectively [26,27]. Hence an increase in MMP1 and MMP9 levels could have caused an increase in their respective TIMP1 and TIMP4 levels in order to bring the levels of MMPs under control. Decreased Collagen I has been reported in wild type mice with AAA [28]. This result is concurrent with decreased Collagen I expression in human AAA, TAA samples (Figure 1D). Collagen IV levels were seen to increase in AAA samples and decrease in TAA samples. There was a potential increase in Collagen I and IV levels in CBS +/- mouse aorta samples (Figure 2C). There was also a significant decrease in elastin levels in CBS +/- mice but no fluctuations were seen in aneurysmal condition. Decreased elastin levels have been previously reported in CBS +/- mice accounting for arterial remodeling [29]. Our results correlate the enzymes that are involved in aneurysm susceptibility during HHcy.
DNA methylation has been known to play a predominant role in regulating gene expression. CBS knockout mice have shown tissue specific fluctuation in their DNA methylation levels [30]. In human vascular smooth muscle cells, homocysteine has been known to demethylate promoter region of MTHFR, thereby causing an upregulation of the gene expression [31].
Expression levels of DNA methyltransferase (DNMT), MBD2 and H3K9 was performed to check their expression in HHcy mouse. Levels of DNMT 1 and DNMT 3a expression were found to increase in CBS +/- mice indicating an increase in global methylation levels in HHcy (Figure 3). Increased methylation pattern further concurred with an increase in the expression of Methyl Binding Domain protein, MBD2 and Histone 3 tri-methylated in Lysine 9 residue (H3K9) proteins (Figures 3, 4A). No significant changes were seen in the levels of HDAC 1. Since HHcy has previously been correlated with aortic aneurysm, a decline in gene expression in aortic aneurysm is possibly due to an increase in methylation levels. Although there is evidence suggesting an association of gene promoter specific hyper-methylation in atherosclerosis [32], the role of methylation in aortic aneurysm is yet to be studied.
Limitations
(i) Though human samples have been collected from individuals with aneurysm, the ambiguities in the data between samples from individuals within the same group is possibly due to the differential affect. Here, we have taken the mean of the frequently occurring patterns in a particular group and considered it as the expression pattern representing that particular group.
(ii) The lack of error bars in group 4 is due to the small sample size. This data was included in our results to serve as a basis for future studies.
(iii) The difference in the case numbers between the control and disease patient groups is due to the limited availability of human samples.
Acknowledgements
This work was supported in part by NIH grants: HL – 74185 and HL – 108621.
Abbreviations
- MTHFR
5,10 Methylene Tetrahydrofolate Reductase
- AAA
Abdominal aortic aneurysm
- CBS
Cystathionine beta synthase
- DNMT
DNA methyltransferase
- ECM
Extracellular Matrix
- H3K9
Histone 3 trimethylated at Lysine 9 residue
- Hcy
Homocysteine
- HHcy
Hyperhomocysteinemia
- MMP
Matrix metalloproteinase
- MBD2
Methyl binding domain protein 2
- NOX
NADPH oxidase
- PRDX
Peroxiredoxin
- SAHH
S – adenosyl homocysteine hydrolase
- TAA
Thoracic aortic aneurysm
- TIMP
Tissue inhibitor metalloproteinase
Disclosure
There is no conflict of interests to disclose.
References
- 1.Giusti B, Marcucci R, Lapini I, Sestini I, Lenti M, Yacoub M, Pepe G. Role of hyperhomocysteinemia in aortic disease. Cell Mol Biol (Noisy-le-grand) 2004;50:945–952. [PubMed] [Google Scholar]
- 2.Roberts WC. Morphologic aspects of cardiac valve dysfunction. Am Heart J. 1992;123:1610–1632. doi: 10.1016/0002-8703(92)90817-f. [DOI] [PubMed] [Google Scholar]
- 3.Roberts WC. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am J Cardiol. 1970;26:72–83. doi: 10.1016/0002-9149(70)90761-7. [DOI] [PubMed] [Google Scholar]
- 4.Michelena HI, Khanna AD, Mahoney D, Margaryan E, Topilsky Y, Suri RM, Eidem B, Edwards WD, Sundt TM III, Enriquez-Sarano M. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306:1104–1112. doi: 10.1001/jama.2011.1286. [DOI] [PubMed] [Google Scholar]
- 5.Refsum H, Smith AD, Ueland PM, Nexo E, Clarke R, McPartlin J, Johnston C, Engbaek F, Schneede J, McPartlin C, Scott JM. Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem. 2004;50:3–32. doi: 10.1373/clinchem.2003.021634. [DOI] [PubMed] [Google Scholar]
- 6.Austin RC, Lentz SR, Werstuck GH. Role of hyperhomocysteinemia in endothelial dysfunction and atherothrombotic disease. Cell Death Differ. 2004;11(Suppl 1):S56–S64. doi: 10.1038/sj.cdd.4401451. [DOI] [PubMed] [Google Scholar]
- 7.James SJ, Melnyk S, Pogribna M, Pogribny IP, Caudill MA. Elevation in S-adenosylhomocysteine and DNA hypomethylation: potential epigenetic mechanism for homocysteine-related pathology. J Nutr. 2002;132:2361S–2366S. doi: 10.1093/jn/132.8.2361S. [DOI] [PubMed] [Google Scholar]
- 8.Redlich M, Roos H, Reichenberg E, Zaks B, Grosskop A, Bar KI, Pitaru S, Palmon A. The effect of centrifugal force on mRNA levels of collagenase, collagen type-I, tissue inhibitors of metalloproteinases and beta-actin in cultured human periodontal ligament fibroblasts. J Periodontal Res. 2004;39:27–32. doi: 10.1111/j.1600-0765.2004.00700.x. [DOI] [PubMed] [Google Scholar]
- 9.Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, Ennis TL, Shapiro SD, Senior RM, Thompson RW. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105:1641–1649. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadoglou NP, Liapis CD. Matrix metalloproteinases: contribution to pathogenesis, diagnosis, surveillance and treatment of abdominal aortic aneurysms. Curr Med Res Opin. 2004;20:419–432. doi: 10.1185/030079904125003143. [DOI] [PubMed] [Google Scholar]
- 11.Greene J, Wang M, Liu YE, Raymond LA, Rosen C, Shi YE. Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J Biol Chem. 1996;271:30375–30380. doi: 10.1074/jbc.271.48.30375. [DOI] [PubMed] [Google Scholar]
- 12.Chevallet M, Wagner E, Luche S, van DA, Leize-Wagner E, Rabilloud T. Regeneration of peroxiredoxins during recovery after oxidative stress: only some overoxidized peroxiredoxins can be reduced during recovery after oxidative stress. J Biol Chem. 2003;278:37146–37153. doi: 10.1074/jbc.M305161200. [DOI] [PubMed] [Google Scholar]
- 13.Perner A, Andresen L, Pedersen G, Rask-Madsen J. Superoxide production and expression of NAD(P)H oxidases by transformed and primary human colonic epithelial cells. Gut. 2003;52:231–236. doi: 10.1136/gut.52.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet. 2010;11:204–220. doi: 10.1038/nrg2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berger J, Bird A. Role of MBD2 in gene regulation and tumorigenesis. Biochem Soc Trans. 2005;33:1537–1540. doi: 10.1042/BST0331537. [DOI] [PubMed] [Google Scholar]
- 16.Kouzarides T. Histone methylation in transcriptional control. Curr Opin Genet Dev. 2002;12:198–209. doi: 10.1016/s0959-437x(02)00287-3. [DOI] [PubMed] [Google Scholar]
- 17.Yideng J, Jianzhong Z, Ying H, Juan S, Jinge Z, Shenglan W, Xiaoqun H, Shuren W. Homocysteine-mediated expression of SAHH, DNMTs, MBD2, and DNA hypomethylation potential pathogenic mechanism in VSMCs. DNA Cell Biol. 2007;26:603–611. doi: 10.1089/dna.2007.0584. [DOI] [PubMed] [Google Scholar]
- 18.Dai XD, Yin M, Jing W, DU HQ, Ye HY, Shang YJ, Zhang L, Zou YY, Qu ZP, Pan J. [Expressions of atherosclerosis-related genes in aorta in young apoE/LDLR double knockout mice] . Sheng Li Xue Bao. 2008;60:43–50. [PubMed] [Google Scholar]
- 19.Givvimani S, Munjal C, Tyagi N, Sen U, Metreveli N, Tyagi SC. Mitochondrial division/mitophagy inhibitor (Mdivi) ameliorates pressure overload induced heart failure. PLoS One. 2012;7:e32388. doi: 10.1371/journal.pone.0032388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takagi H, Umemoto T. Homocysteinemia is a risk factor for aortic dissection. Med Hypotheses. 2005;64:1007–1010. doi: 10.1016/j.mehy.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Moroz P, Le MT, Norman PE. Homocysteine and abdominal aortic aneurysms. ANZ J Surg. 2007;77:329–332. doi: 10.1111/j.1445-2197.2007.04052.x. [DOI] [PubMed] [Google Scholar]
- 22.DiBello PM, Dayal S, Kaveti S, Zhang D, Kinter M, Lentz SR, Jacobsen DW. The nutrigenetics of hyperhomocysteinemia: quantitative proteomics reveals differences in the methionine cycle enzymes of gene-induced versus diet-induced hyperhomocysteinemia. Mol Cell Proteomics. 2010;9:471–485. doi: 10.1074/mcp.M900406-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsarouhas K, Tsitsimpikou C, Apostolakis S, Haliassos A, Tzardi M, Panagiotou M, Tsatsakis A, Spandidos DA. Homocysteine and metalloprotease-3 and -9 in patients with ascending aorta aneurysms. Thromb Res. 2011;128:e95–e99. doi: 10.1016/j.thromres.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Wilson WR, Anderton M, Choke EC, Dawson J, Loftus IM, Thompson MM. Elevated plasma MMP1 and MMP9 are associated with abdominal aortic aneurysm rupture. Eur J Vasc Endovasc Surg. 2008;35:580–584. doi: 10.1016/j.ejvs.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Ikonomidis JS, Gibson WC, Butler JE, McClister DM, Sweterlitsch SE, Thompson RP, Mukherjee R, Spinale FG. Effects of deletion of the tissue inhibitor of matrix metalloproteinases-1 gene on the progression of murine thoracic aortic aneurysms. Circulation. 2004;110:II268–II273. doi: 10.1161/01.CIR.0000138384.68947.20. [DOI] [PubMed] [Google Scholar]
- 26.Li YY, Feldman AM, Sun Y, McTiernan CF. Differential expression of tissue inhibitors of metalloproteinases in the failing human heart. Circulation. 1998;98:1728–1734. doi: 10.1161/01.cir.98.17.1728. [DOI] [PubMed] [Google Scholar]
- 27.Mishra PK, Chavali V, Metreveli N, Tyagi SC. Ablation of MMP9 induces survival and differentiation of cardiac stem cells into cardiomyocytes in the heart of diabetics: a role of extracellular matrix. Can J Physiol Pharmacol. 2012;90:353–360. doi: 10.1139/y11-131. [DOI] [PubMed] [Google Scholar]
- 28.Cho BS, Roelofs KJ, Ford JW, Henke PK, Upchurch GR Jr. Decreased collagen and increased matrix metalloproteinase-13 in experimental abdominal aortic aneurysms in males compared with females. Surgery. 2010;147:258–267. doi: 10.1016/j.surg.2009.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar M, Tyagi N, Moshal KS, Sen U, Kundu S, Mishra PK, Givvimani S, Tyagi SC. Homocysteine decreases blood flow to the brain due to vascular resistance in carotid artery. Neurochem Int. 2008;53:214–219. doi: 10.1016/j.neuint.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choumenkovitch SF, Selhub J, Bagley PJ, Maeda N, Nadeau MR, Smith DE, Choi SW. In the cystathionine beta-synthase knockout mouse, elevations in total plasma homocysteine increase tissue S-adenosylhomocysteine, but responses of S-adenosylmethionine and DNA methylation are tissue specific. J Nutr. 2002;132:2157–2160. doi: 10.1093/jn/132.8.2157. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Zhang J, Wang S. [Demethylation in the promoter region of MTHFR gene and its mRNA expression in cultured human vascular smooth muscle cells induced by homocysteine] . Wei Sheng Yan Jiu. 2007;36:291–294. [PubMed] [Google Scholar]
- 32.Krishna SM, Dear AE, Norman PE, Golledge J. Genetic and epigenetic mechanisms and their possible role in abdominal aortic aneurysm. Atherosclerosis. 2010;212:16–29. doi: 10.1016/j.atherosclerosis.2010.02.008. [DOI] [PubMed] [Google Scholar]