Abstract
Purpose of review
Choline is an essential nutrient and the liver is a central organ responsible for choline metabolism. Hepatosteatosis and liver cell death occur when humans are deprived of choline. In the last few years there have been significant advances in our understanding of the mechanisms that influence choline requirements in humans and in our understanding of choline’s effects on liver function. These advances are useful in elucidating why non-alcoholic fatty liver disease (NAFLD) occurs and progresses sometimes to hepatocarcinogenesis.
Recent findings
Humans eating low choline diets develop fatty liver and liver damage,. This dietary requirement for choline is modulated by estrogen and by single nucleotide polymorphisms (SNPs) in specific genes of choline and folate metabolism. The spectrum of choline’s effects on liver range from steatosis to development of hepatocarcinomas, and several mechanisms for these effects have been identified. They include abnormal phospholipid synthesis, defects in lipoprotein secretion, oxidative damage caused by mitochondrial dysfunction, and endoplasmic reticulum (ER) stress. Furthermore, the hepatic steatosis phenotype and can be characterized more fully via metabolomic signatures and is influenced by the gut microbiome. Importantly, the intricate connection between liver function, one carbon metabolism, and energy metabolism is just beginning to be elucidated.
Summary
Choline influences liver function, and the dietary requirement for this nutrient varies depending on an individual’s genotype and estrogen status. Understanding these individual differences is important for gastroenterologists seeking to understand why some individuals develop NAFLD and others do not, and why some patients tolerate total parenteral nutrition and others develop liver dysfunction.
Keywords: choline, metabolomics, microbiome, non-alcoholic-fatty liver disease, single nucleotide polymorphisms
Introduction
The primary focus of this article will be to relate new understanding about the role of choline metabolism in sustaining normal liver function, development of nonalcoholic fatty liver disease (NAFLD), and hepatocarcinogenesis. The recent discoveries that the dietary requirement for choline varies substantially among individuals due to genetics [1, 2], gender [3], and microbiome composition [4] make it easier to consider the clinical implications of choline in diseases dealt with by gastroenterologists. This review will focus on recent advances on the importance of choline in the liver.
Choline biology
Choline is a constituent of cell and mitochondrial membranes and of the neurotransmitter acetylcholine. Given its essentially ubiquitous incorporation into cellular components and pathways, it is not surprising that this nutrient influences diverse processes such as lipid metabolism [5], signaling through lipid second messengers [6], methylation-dependent biosynthesis of molecules (including epigenetic regulation of gene expression) [7–9], activation of nuclear receptors [10, 11], enterohepatic circulation of bile and cholesterol [12], plasma membrane fluidity [13], and mitochondrial bioenergetics [14].
The two major fates for choline are to be phosphorylated and used to make phospholipids, or to be oxidized and used as a donor of methyl-groups. An especially important choline metabolite in liver is phosphatidylcholine, which is necessary for the packaging and export of triglycerides in very low density lipoprotein (VLDL) [15] and for the solubilization of bile salts for secretion [16]. Aberrant VLDL– mediated secretion of triglycerides is a central mechanism in hepatic steatosis [17]. The role of bile homeostasis in liver physiology is also quite evident, and mostly relates to the causes of gallstones, fibrosis, and hepatocarcinomas [18]. However, new functions attributed to bile salts, including regulation of energy and glucose metabolism [19–21], makes it likely that phosphatidylcholine plays a role in modulating these functions as well.
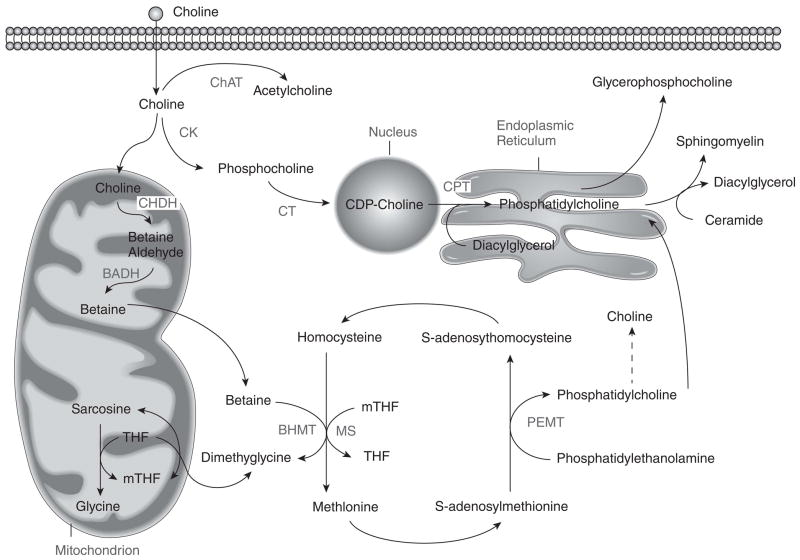
Choline, folate and methionine metabolism are interrelated as all influence the production of S-adenosylmethionine, the universal donor of methyl-groups in biological reactions [5] (Figure 1). Deficiency in one nutrient is associated with an increase in flux of the other nutrients towards methyl donation [5].
Figure 1. Choline, folate and homocysteine metabolism are closely interrelated.
The pathways for the metabolism of these three nutrients intersect at the formation of methionine from homocysteine.
BADH=betaine aldehyde dehydrogenase; BHMT=betaine homocysteine methyltransferase; ChAT=choline acetyltransferase; CHDH=choline dehydrogenase; CK=choline kinase; CPT=choline phosphotransferase; CT=CTP:phosphocholine cytidylytransferase; MS=methionine synthase; mTHF=methyl tetrahydrofolate PEMT=phosphatidylethanolamine-N-methyltransferase; THF=tetrahydrofolate
From: Present Knowledge in Nutrition Volume 10, with permission.
Individual choline requirements
Choline is found in a variety of foods, but it is particularly abundant in egg yolks and animal sources of protein (see www.ars.usda.gov/SP2UserFiles/Place/12354500/Data/Choline/Choln02.pdf). Many of these high-choline foods are high in fats or cholesterol (e.g. eggs) and are being avoided by many people who then do not achieve the recommended dietary Adequate Intake for choline [22, 23]. For example, several recent epidemiologic studies reported that 25% of Americans ate diets very low in choline (<203 mg/d in the Framingham Heart Study [24], <217 mg/d in the Atherosclerosis Risk In Communities study [25, 26] and <293 mg/d in the Nurse’s Health Study [27]; the Adequate Intake is 450–550 mg/day [22]).
Choline was once believed to be a dispensable nutrient because there is a pathway for endogenous formation of phosphatidylcholine catalyzed by phosphatidylethanolamine N-methyltransferase (PEMT). However, controlled clinical feeding studies demonstrated unequivocally that choline is an essential nutrient; humans deprived of choline developed either fatty liver and liver cell death or developed skeletal muscle damage [1, 2]. These findings were reinforced by clinical evidence that patients fed with total parenteral nutrition solutions low in choline developed fatty liver and liver damage [28].
There are two major sources for variation in human dietary requirements for choline: estrogen status and genetic variation. As mentioned earlier, the dietary requirement for choline can be spared by endogenous biosynthesis of phosphatidylcholine in liver, catalyzed by PEMT [5]. Expression of the gene PEMT is induced by estrogen [29], and therefore most premenopausal women [30] and postmenopausal women who are treated with estrogen [30] have a diminished dietary requirement for choline. However, more than 40% of women have a genetic polymorphism in PEMT (rs12325817) that makes this gene unresponsive to estrogen, and these women have the same high choline requirement as men [2, 29, 30]. There are other genetic polymorphisms that modify choline requirements by different mechanisms; choline dehydrogenase (CHDH) rs12676 and rs9001 [2], and methylene tetrahydrofolate dehydrogenase 1 (MTHFD1) rs2236225 [1].
Choline and non-alcoholic fatty liver disease
Liver is an important organ for metabolism and storage of choline, and liver is dependent on a source of choline [5]. Choline deficient diets, including those that are also deficient in methionine, have long been utilized to study the mechanisms of fatty liver disease and its progression because such diets recapitulate many of the phenotypes seen in humans with NAFLD, including an accumulation of triglycerides in the liver [5, 31]. (Figure 2) Although in many cases NAFLD maintains a benign course, hepatic steatosis is an early manifestation of liver dysfunction that sometimes progresses to steatohepatitis, fibrosis, cirrhosis and liver cancer [32].
Figure 2. Summary of Choline Deficiency Mediated Mechanisms of Liver Dysfunction.
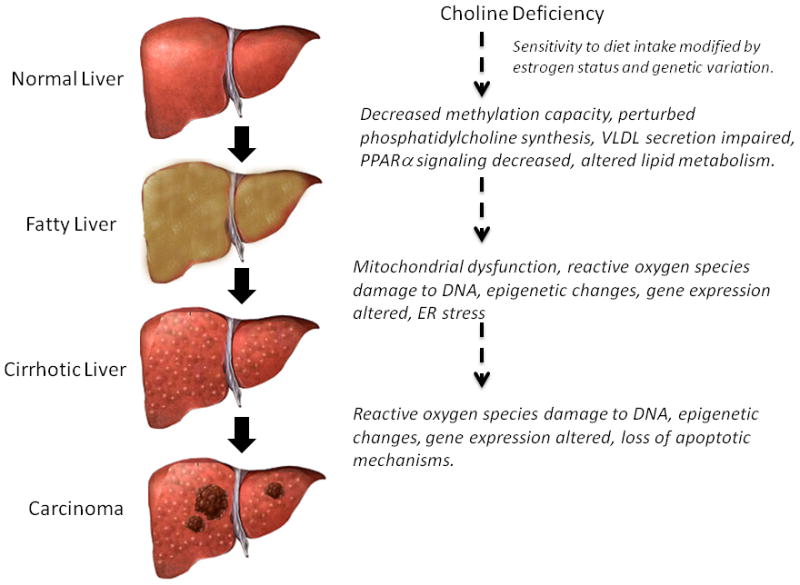
The progression of NAFLD from simple steatosis to hepatocarcinoma is influenced by multiple cholinemediated mechanisms.
Several mouse models with deletion of choline-related genes have given insight into the mechanisms of NAFLD. In several mouse models, deletion of genes needed to use choline as a methyl donor (Bhmt [33], Chdh [34]), deletion of genes needed to form the choline moiety endogenously (Pemt [35]) or deletion of genes needed to make S-adenosylmethionine (Mat1 [36]) result in fatty liver. In humans, polymorphisms in PEMT [37, 38] are associated with NAFLD. These observations suggest that the methyl-donation function of choline is important in the mechanism of NAFLD. Earlier, we discussed the hypothesis that phosphatidylcholine was required for normal VLDL secretion from liver. The genetic data suggest that it is phosphatidylcholine that is derived from the PEMT methylation pathway that is important (rather than phosphatidylcholine derived from preformed choline); mouse studies support this conclusion [39].
Although much can be gleaned by studying the genetic mechanisms of disease, several other important levels of control of fatty liver could be concurrently important. Metabolomics, especially when used in combination with other methods to define phenotype, has advanced our understanding of the role of choline in fatty liver. Humans who develop fatty liver on a choline deficient diet exhibit a metabolomic profile at baseline: altered choline metabolites, lipids (including acylcarnitines), and amino acids. This metabolomic profiling (done while people are eating normal diets) accurately predicted which humans would develop fatty liver when fed low choline diets [40]. It is interesting that plasma metabolomic profiling of NAFLD patients (independent of the cause of NAFLD) demonstrated that carnitines, choline metabolites, and bile acids could differentiate healthy controls from NAFLD or non-alcoholic steatohepatitis cases [41], suggesting that similar pathways are involved in NAFLD and choline deficiency.
Choline is an important part of the mitochondrial membrane and mitochondrial dysfunction is a central mechanism in the pathogenesis of NAFLD [32]. Low choline may be important in NAFLD pathophysiology because it perturbs mitochondrial bioenergetics [14] and fatty acid beta oxidation [42]. Choline deficiency alters the composition of mitochondrial membranes; cardiolipin in these membranes is oxidized, and membrane concentrations of phosphatidylethanolamine and phosphatidylcholine are decreased [43, 44]. These membrane changes result in mitochondrial decreased membrane potential [14, 45] and in reduced activity of complex I of the respiratory chain [44, 46]. Decreased ATP production by mitochondria occurs in rats fed a choline deficient diet [47] or a choline-methionine deficient diet [48]. Proteins involved in choline metabolism and transport also influence mitochondrial function. CHDH is a mitochondrial matrix protein that catalyzes the conversion of choline to betaine. Mice with deleted Chdh have abnormal mitochondrial function in multiple tissues [34]. Interestingly, CHDH is upregulated in the mitochondrial proteome of rats with fatty liver induced by alcohol [49]. This could be a compensatory response, since betaine is believed to have a hepato-protective effect [50].
Endoplasmic reticulum (ER) stress is a condition whereby excess unfolded proteins lead to a cascade of stress responses. If stress is chronic, cell death can occur. ER stress is believed to play a role in the pathogenesis of NAFLD [51]. In mice fed methionine-choline deficient diets for up to 21 days, hepatic steatosis was associated with inducing specific ER stress cascades upstream of the unfolded protein response. The integrated ER stress response was unable to cause liver injury in the absence of steatosis, suggesting a coordinated mechanism is necessary for liver disease progression [52]. Another link between choline, NAFLD, and ER stress was found when metabolomic and proteomic studies in obese, leptin deficient mice revealed that the obese phenotype is characterized by ER stress, increased expression of proteins involved in lipogenesis and phospholipid metabolism (including PEMT), and a distinct lipid profile characterized by increased monounsaturated fatty acids and an increased phosphatidylcholine to phosphatidylethanolamine concentration ratio. This altered ratio impairs calcium signaling and ER homeostasis [53].
Choline is a potent modifier of epigenetic marks on genes [7, 8]. It is likely that there are specific epigenetic outcomes that influence NAFLD under choline deprivation. Several genes that are central to the pathophysiology of metabolic disease, such as leptin [54] and PPAR gamma [55], are known to be epigenetically regulated. The specific mechanisms linking choline, epigenetics and NAFLD are areas of active investigation.
The study of the influence of the gut microbiome on human health has advanced tremendously. The gut microbiome integrates many important pathways, including those related to enterohepatic circulation of bile, cholesterol and phospholipids [56]. The gut flora modulates host immunity [57], glucose, lipid, and energy metabolism [58], and choline availability [59], all of which play a role in NAFLD [60]. Gut microbiome composition is influenced by multiple factors such as maternal diet, lifelong diet, environmental exposures, and genetics [61]. Gammaproteobacteria and Erysipelotrichi within the gut microbiome were directly associated with changes in liver fat in humans during choline depletion. Levels of these bacteria, change in amount of liver fat, and a single nucleotide polymorphism (PEMT rs12325817) that affects choline were combined into a model that accurately predicted the degree to which subjects developed fatty liver on a choline-deficient diet [4]. This suggests that understanding the effects of the microbiome can enhance current paradigms defining NAFLD risk and progression.
Choline and progression of fatty liver disease
We need to understand more about the factors that influence the progression of fatty liver disease to more severe liver injury and cancer. Choline and methionine deficiency has been a useful model for identifying potential mechanisms. In rodents, choline deficient diets caused progressive hepatic disease much like what is seen in some humans with fatty liver: steatosis → fibrosis → cirrhosis → hepatocellular carcinoma [6, 62]. This progression from fatty liver to hepatocarcinoma is also seen when a gene in choline metabolism is knocked out in mice. The Bhmt−/− mouse, discussed earlier, develops fatty liver and liver injury (elevated ALT and gamma glutamyltransferase 1) at 5 weeks of age and this progresses to hepatocarcinomas by 52 weeks of age [33]. Overall, the hepatic phenotype in the Bhmt −/− mouse suggests this model will be a valuable tool to characterize fatty liver progression as related specifically to choline deficiency, altered methylation potential, and perhaps other stress responses that could manifest in mitochondrial dysfunction and ER stress [33].
Multiple mechanisms have been identified that may explain why choline deficiency progresses to hepatocarcinoma. The primary mechanism involves damage to DNA, as assessed by the formation of 8- oxodeoxyguanosine [63, 64], apurinic/apyrimidinic sites [65] and Ogg1-sensitive sites [65] in DNA that accumulate when rats are deprived of choline. Choline deficient hepatocytes overproduce free radicals because their mitochondria become leaky [43, 45, 66–68]. In addition, death of hepatocytes that occurs in choline deprivation [69] causes an inflammatory response with an associated neutrophil/macrophage-mediated generation of reactive oxygen and nitrogen species [70].
Choline, NAFLD and metabolic syndrome
NAFLD is tightly linked to obesity and insulin resistance [32]. There is good reason to believe that choline and 1-carbon metabolism influence obesity and insulin resistance. In mice fed an obesogenic diet, which causes weight gain and hepatic steatosis, a plasma and liver metabolomic approach identified phosphatidylcholine, lysophosphatidylcholine, and betaine as metabolites that differentiated the obese versus lean phenotype [71]. The role of one carbon genes was also prominent in a study that merged genomic and metabolomic data sets to characterize diet-induced obesity in mice [72]. In human studies aiming to characterize insulin sensitivity and diabetes, choline metabolites have also been repeatedly identified as important for distinguishing metabolic states [73–75]. Conversely, altering genes in choline metabolism modifies responses to obesity. Pemt knockout mice are protected from obesity due to a high calorie/high fat diet [76]. It is interesting that in obese, leptin receptor deficient mice, genes involved in production of phosphatidylcholine, including Pemt, were upregulated [53]. In this mouse, hepatic steatosis was reduced if Pemt was silenced [53]. These data suggest that there are phosphatidylcholine-mediated mechanisms that influence responses to obesity.
PPARα, part of the peroxisome-proliferator family of nuclear receptors, is highly expressed in the liver and is involved in fatty acid metabolism, lipoprotein assembly [77] and gluconeogenesis [78]. The endogenous ligand for the PPARα receptor is a specific form of phosphatidylcholine (1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine). Infusion of this phosphatidylcholine recapitulates the protection from fatty liver seen with PPAR agonists [10]. Another nuclear receptor, liver receptor homolog 1 (LRH-1), had no known endogenous ligand until a specific phosphatidylcholine (dilauroyl phosphatidylcholine-DLPC) was identified as the agonist [11]. This receptor is involved in bile acid biosynthesis and activation promotes bile acid synthesis, lowers triglycerides in liver, and decreases serum glucose concentrations [11].
Conclusion
Our understanding of the mechanisms by which choline, and related metabolites, impact liver physiology and of the individual requirements for these nutrients is advancing rapidly. Progress in the utilization of advanced methods, such as metabolomics, and emerging science, such as the area of gut microbiome-host interactions, to broaden our appreciation of the mechanisms by which the multiple functions of choline converge in specific liver phenotypes is particularly exciting. The relatively unexplored non-canonical functions of some genes in the choline metabolism pathway along with the very recent observations linking one carbon and energy metabolism hold much promise for unraveling some of the mysteries of complex metabolic disease while possibly elucidating prevention and treatment targets for NAFLD.
Key points.
Choline is an essential nutrient with multiple mechanistic roles in NAFLD and its progression including VLDL export, enterohepatic metabolism of bile, mitochondrial function, epigenetics, ER Stress, and VLDL export.
Choline deficiency in humans is associated with liver dysfunction and susceptibility is dependent on factors, including genetics, gender, and the gut microbiome, which influence choline requirements.
Recent evidence has identified a prominent role for choline and one carbon metabolism in metabolic syndrome.
Applying knowledge of individual choline requirements into gastroenterology clinical practice has the potential to improve outcomes.
Acknowledgments
Work in the corresponding author’s laboratory is supported by the National Institutes of Health (R01 DK55865 and P30DK056350).
References and recommended reading
- 1.Kohlmeier M, da Costa KA, Fischer LM, et al. Genetic variation of folate-mediated one-carbon transfer pathway predicts susceptibility to choline deficiency in humans. Proc Natl Acad Sci U S A. 2005;102:16025–16030. doi: 10.1073/pnas.0504285102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.da Costa KA, Kozyreva OG, Song J, et al. Common genetic polymorphisms affect the human requirement for the nutrient choline. Faseb J. 2006;20:1336–1344. doi: 10.1096/fj.06-5734com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeisel SH. Nutritional genomics: defining the dietary requirement and effects of choline. J Nutr. 2011;141:531–534. doi: 10.3945/jn.110.130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4*.Spencer MD, Hamp TJ, Reid RW, et al. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology. 2011;140:976–986. doi: 10.1053/j.gastro.2010.11.049. Studies, in humans fed low choline diets, the relationship between gut microbiome and development of fatty liver. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. 2006;26:229–250. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.da Costa KA, Garner SC, Chang J, et al. Effects of prolonged (1 year) choline deficiency and subsequent refeeding of choline on 1,2,-sn-diradylglycerol, fatty acids and protein kinase C in rat liver. Carcinogenesis. 1995;16:327–334. doi: 10.1093/carcin/16.2.327. [DOI] [PubMed] [Google Scholar]
- 7*.Mehedint MG, Niculescu MD, Craciunescu CN, et al. Choline deficiency alters global histone methylation and epigenetic marking at the Re1 site of the calbindin 1 gene. FASEB J. 2010;24:184–195. doi: 10.1096/fj.09-140145. In mouse brain, maternal diets low in choline alter histone methylation in fetal brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J. 2006;20:43–49. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niculescu MD, Yamamuro Y, Zeisel SH. Choline availability modulates human neuroblastoma cell proliferation and alters the methylation of the promoter region of the cyclin-dependent kinase inhibitor 3 gene. J Neurochem. 2004;89:1252–1259. doi: 10.1111/j.1471-4159.2004.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakravarthy MV, Lodhi IJ, Yin L, et al. Identification of a physiologically relevant endogenous ligand for PPARalpha in liver. Cell. 2009;138:476–488. doi: 10.1016/j.cell.2009.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JM, Lee YK, Mamrosh JL, et al. A nuclear-receptor-dependent phosphatidylcholine pathway with antidiabetic effects. Nature. 2011;474:506–510. doi: 10.1038/nature10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawson PA, Lan T, Rao A. Bile acid transporters. J Lipid Res. 2009;50:2340–2357. doi: 10.1194/jlr.R900012-JLR200. Ver 10-7-11 submitted to Current Opinion In Gastroenterology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caballero F, Fernandez A, Matias N, et al. Specific contribution of methionine and choline in nutritional nonalcoholic steatohepatitis: impact on mitochondrial S-adenosyl-L-methionine and glutathione. J Biol Chem. 2010;285:18528–18536. doi: 10.1074/jbc.M109.099333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teodoro JS, Rolo AP, Duarte FV, et al. Differential alterations in mitochondrial function induced by a choline-deficient diet: understanding fatty liver disease progression. Mitochondrion. 2008;8:367–376. doi: 10.1016/j.mito.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Noga AA, Vance DE. A gender-specific role for phosphatidylethanolamine N-methyltransferase-derived phosphatidylcholine in the regulation of plasma high density and very low density lipoproteins in mice. J Biol Chem. 2003;278:21851–21859. doi: 10.1074/jbc.M301982200. [DOI] [PubMed] [Google Scholar]
- 16.Li Z, Agellon LB, Vance DE. Phosphatidylcholine homeostasis and liver failure. J Biol Chem. 2005;280:37798–37802. doi: 10.1074/jbc.M508575200. [DOI] [PubMed] [Google Scholar]
- 17.Vance DE. Role of phosphatidylcholine biosynthesis in the regulation of lipoprotein homeostasis. Curr Opin Lipidol. 2008;19:229–234. doi: 10.1097/MOL.0b013e3282fee935. [DOI] [PubMed] [Google Scholar]
- 18.Baghdasaryan A, Fickert P, Fuchsbichler A, et al. Role of hepatic phospholipids in development of liver injury in Mdr2 (Abcb4) knockout mice. Liver Int. 2008;28:948–958. doi: 10.1111/j.1478-3231.2008.01758.x. [DOI] [PubMed] [Google Scholar]
- 19.Hylemon PB, Zhou H, Pandak WM, et al. Bile acids as regulatory molecules. J Lipid Res. 2009;50:1509–1520. doi: 10.1194/jlr.R900007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gadaleta RM, van Mil SW, Oldenburg B, et al. Bile acids and their nuclear receptor FXR: Relevance for hepatobiliary and gastrointestinal disease. Biochim Biophys Acta. 2010;1801:683–692. doi: 10.1016/j.bbalip.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 22.Institute of Medicine, National Academy of Sciences USA: Choline. Dietary reference intakes for folate, thiamin, riboflavin, niacin, vitamin B12, panthothenic acid, biotin, and choline. Washington D.C: National Academy Press; 1998. pp. 390–422. [PubMed] [Google Scholar]
- 23.Jensen HH, Batres-Marquez SP, Carriquiry A, et al. Choline in the diets of the U.S. population: NHANES, 2003–2004. FASEB J. 2007;21:lb219. [Google Scholar]
- 24.Cho E, Zeisel SH, Jacques P, et al. Dietary choline and betaine assessed by food-frequency questionnaire in relation to plasma total homocysteine concentration in the Framingham Offspring Study. Am J Clin Nutr. 2006;83:905–911. doi: 10.1093/ajcn/83.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bidulescu A, Chambless LE, Siega-Riz AM, et al. Usual choline and betaine dietary intake and incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. BMC Cardiovasc Disord. 2007;7:20. doi: 10.1186/1471-2261-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bidulescu A, Chambless LE, Siega-Riz AM, et al. Repeatability and measurement error in the assessment of choline and betaine dietary intake: the Atherosclerosis Risk in Communities (ARIC) study. Nutr J. 2009;8:14. doi: 10.1186/1475-2891-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho E, Willett WC, Colditz GA, et al. Dietary choline and betaine and the risk of distal colorectal adenoma in women. J Natl Cancer Inst. 2007;99:1224–1231. doi: 10.1093/jnci/djm082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buchman AL. Complications of long-term home total parenteral nutrition: their identification, prevention and treatment. Dig Dis Sci. 2001;46:1–18. doi: 10.1023/a:1005628121546. [DOI] [PubMed] [Google Scholar]
- 29**.Resseguie ME, da Costa KA, Galanko JA, et al. Aberrant estrogen regulation of PEMT results in choline deficiency-associated liver dysfunction. J Biol Chem. 2011;286:1649–1658. doi: 10.1074/jbc.M110.106922. The gene encoding the enzyme needed for endogenous synthesis of phosphatidylcholine is regulated by estrogen and humans with a SNP in this gene are unresponsive to estrogen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30**.Fischer LM, da Costa KA, Kwock L, et al. Dietary choline requirements of women: effects of estrogen and genetic variation. Am J Clin Nutr. 2010;92:1113–1119. doi: 10.3945/ajcn.2010.30064. The dietary requirement for choline is reduced in women with estrogen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hebbard L, George J. Animal models of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2011;8:35–44. doi: 10.1038/nrgastro.2010.191. [DOI] [PubMed] [Google Scholar]
- 32.Cheung O, Sanyal AJ. Recent advances in nonalcoholic fatty liver disease. Curr Opin Gastroenterol. 2010;26:202–208. doi: 10.1097/MOG.0b013e328337b0c4. [DOI] [PubMed] [Google Scholar]
- 33*.Teng YW, Mehedint MG, Garrow TA, et al. Deletion of murine betaine-homocysteine S-methyltransferase in mice perturbs choline and 1-carbon metabolism, resulting in fatty liver and hepatocellular carcinoma. J Biol Chem. 2011 doi: 10.1074/jbc.M111.265348. jbc.M111.265348. in press. Mice with a gene of choline metabolism (Bhmt) deleted develop hepatocarcinomas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34*.Johnson AR, Craciunescu CN, Guo Z, et al. Deletion of murine choline dehydrogenase results in diminished sperm motility. Faseb J. 2010;24:2752–2761. doi: 10.1096/fj.09-153718. Mice with a gene of choline metabolism (Chdh) deleted develop mitochondrial dysfunction and have sperm that are not motile. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waite KA, Cabilio NR, Vance DE. Choline deficiency-induced liver damage is reversible in Pemt(−/−) mice. J Nutr. 2002;132:68–71. doi: 10.1093/jn/132.1.68. [DOI] [PubMed] [Google Scholar]
- 36.Cano A, Buque X, Martinez-Una M, et al. Methionine adenosyltransferase 1A gene deletion disrupts hepatic VLDL assembly in mice. Hepatology. 2011 doi: 10.1002/hep.24607. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song J, da Costa KA, Fischer LM, et al. Polymorphism of the PEMT gene and susceptibility to nonalcoholic fatty liver disease (NAFLD) Faseb J. 2005;19:1266–1271. doi: 10.1096/fj.04-3580com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeisel S. People with fatty liver are more likely to have the PEMT rs7946 SNP, yet populations with the mutant allele do not have fatty liver. Faseb J. 2006;20:2181–2182. [Google Scholar]
- 39.Noga AA, Zhao Y, Vance DE. An unexpected requirement for phosphatidylethanolamine N-methyltransferase in the secretion of very low density lipoproteins. J Biol Chem. 2002;277:42358–42365. doi: 10.1074/jbc.M204542200. [DOI] [PubMed] [Google Scholar]
- 40*.Sha W, da Costa KA, Fischer LM, et al. Metabolomic profiling can predict which humans will develop liver dysfunction when deprived of dietary choline. Faseb J. 2010;24:2962–2975. doi: 10.1096/fj.09-154054. Metabolomic profiling was helpful in predicting which humans were going to develop fatty liver when fed a low choline diet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalhan SC, Guo L, Edmison J, et al. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism. 2011;60:404–413. doi: 10.1016/j.metabol.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serviddio G, Giudetti AM, Bellanti F, et al. Oxidation of Hepatic Carnitine Palmitoyl Transferase-I (CPT-I) Impairs Fatty Acid Beta-Oxidation in Rats Fed a Methionine-Choline Deficient Diet. PLoS One. 2011;6:e24084. doi: 10.1371/journal.pone.0024084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vrablic AS, Albright CD, Craciunescu CN, et al. Altered mitochondrial function and overgeneration of reactive oxygen species precede the induction of apoptosis by 1-O-octadecyl-2-methyl- rac-glycero-3-phosphocholine in p53-defective hepatocytes. FASEB J. 2001;15:1739–1744. doi: 10.1096/fj.00-0300com. [DOI] [PubMed] [Google Scholar]
- 44.Petrosillo G, Portincasa P, Grattagliano I, et al. Mitochondrial dysfunction in rat with nonalcoholic fatty liver Involvement of complex I, reactive oxygen species and cardiolipin. Biochim Biophys Acta. 2007;1767:1260–1267. doi: 10.1016/j.bbabio.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 45.Guo WX, Pye QN, Williamson KS, et al. Mitochondrial dysfunction in choline deficiency-induced apoptosis in cultured rat hepatocytes. Free Radic Biol Med. 2005;39:641–650. doi: 10.1016/j.freeradbiomed.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 46.Hensley K, Kotake Y, Sang H, et al. Dietary choline restriction causes complex I dysfunction and increased H(2)O(2) generation in liver mitochondria. Carcinogenesis. 2000;21:983–989. doi: 10.1093/carcin/21.5.983. [DOI] [PubMed] [Google Scholar]
- 47.James SJ, Cross DR, Miller BJ. Alterations in nucleotide pools in rats fed diets deficient in choline, methionine and/or folic acid. Carcinogenesis. 1992;13:2471–2474. doi: 10.1093/carcin/13.12.2471. [DOI] [PubMed] [Google Scholar]
- 48.Serviddio G, Bellanti F, Tamborra R, et al. Uncoupling protein-2 (UCP2) induces mitochondrial proton leak and increases susceptibility of non-alcoholic steatohepatitis (NASH) liver to ischaemia-reperfusion injury. Gut. 2008;57:957–965. doi: 10.1136/gut.2007.147496. [DOI] [PubMed] [Google Scholar]
- 49*.Andringa KK, King AL, Eccleston HB, et al. Analysis of the liver mitochondrial proteome in response to ethanol and S-adenosylmethionine treatments: novel molecular targets of disease and hepatoprotection. Am J Physiol Gastrointest Liver Physiol. 2010;298:G732–745. doi: 10.1152/ajpgi.00332.2009. Chdh is upregulated in the mitochondrial proteome of rats with fatty liver induced by alcohol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50*.Kathirvel E, Morgan K, Nandgiri G, et al. Betaine improves nonalcoholic fatty liver and associated hepatic insulin resistance: a potential mechanism for hepatoprotection by betaine. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1068–1077. doi: 10.1152/ajpgi.00249.2010. The choline metabolite betaine has a hepato-protective effect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54:795–809. doi: 10.1016/j.jhep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soon RK, Jr, Yan JS, Grenert JP, et al. Stress signaling in the methionine-choline-deficient model of murine fatty liver disease. Gastroenterology. 2010;139:1730–1739. 1739, e1731. doi: 10.1053/j.gastro.2010.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53*.Fu S, Yang L, Li P, et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473:528–531. doi: 10.1038/nature09968. Endoplasmic reticulum stress iis an important component of obesity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stoger R. In vivo methylation patterns of the leptin promoter in human and mouse. Epigenetics. 2006;1:155–162. doi: 10.4161/epi.1.4.3400. [DOI] [PubMed] [Google Scholar]
- 55.Fujiki K, Kano F, Shiota K, et al. Expression of the peroxisome proliferator activated receptor gamma gene is repressed by DNA methylation in visceral adipose tissue of mouse models of diabetes. BMC Biol. 2009;7:38. doi: 10.1186/1741-7007-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Musso G, Gambino R, Cassader M. Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annu Rev Med. 2011;62:361–380. doi: 10.1146/annurev-med-012510-175505. [DOI] [PubMed] [Google Scholar]
- 57.Kau AL, Ahern PP, Griffin NW, et al. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Velagapudi VR, Hezaveh R, Reigstad CS, et al. The gut microbiota modulates host energy and lipid metabolism in mice. J Lipid Res. 2010;51:1101–1112. doi: 10.1194/jlr.M002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dumas ME, Barton RH, Toye A, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci U S A. 2006;103:12511–12516. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abu-Shanab A, Quigley EM. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2010;7:691–701. doi: 10.1038/nrgastro.2010.172. [DOI] [PubMed] [Google Scholar]
- 61.Spor A, Koren O, Ley R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol. 2011;9:279–290. doi: 10.1038/nrmicro2540. [DOI] [PubMed] [Google Scholar]
- 62.Ghoshal AK, Rushmore TH, Farber E. Initiation of carcinogenesis by a dietary deficiency of choline in the absence of added carcinogens. Cancer Lett. 1987;36:289–296. doi: 10.1016/0304-3835(87)90022-x. [DOI] [PubMed] [Google Scholar]
- 63.Yoshiji H, Nakae D, Mizumoto Y, et al. Inhibitory effect of dietary iron deficiency on inductions of putative preneoplastic lesions as well as 8-hydroxydeoxyguanosine in DNA and lipid peroxidation in the livers of rats caused by exposure to a choline-deficient L-amino acid defined diet. Carcinogenesis. 1992;13:1227–1233. doi: 10.1093/carcin/13.7.1227. [DOI] [PubMed] [Google Scholar]
- 64.Pogribny IP, Shpyleva SI, Muskhelishvili L, et al. Role of DNA damage and alterations in cytosine DNA methylation in rat liver carcinogenesis induced by a methyl-deficient diet. Mutat Res. 2009;669:56–62. doi: 10.1016/j.mrfmmm.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 65.Powell CL, Kosyk O, Bradford BU, et al. Temporal correlation of pathology and DNA damage with gene expression in a choline-deficient model of rat liver injury. Hepatology. 2005;42:1137–1147. doi: 10.1002/hep.20910. [DOI] [PubMed] [Google Scholar]
- 66.Banni S, Corongiu FP, Dessi MA, et al. Free radicals and lipid peroxidation in liver of rats kept on a diet devoid of choline. Free Radic Res Commun. 1989;7:233–240. doi: 10.3109/10715768909087947. [DOI] [PubMed] [Google Scholar]
- 67.Ghoshal AK, Farber E. Liver biochemical pathology of choline deficiency and of methyl group deficiency: a new orientation and assessment. Histology & Histopathology. 1995;10:457–462. [PubMed] [Google Scholar]
- 68*.Pacelli C, Coluccia A, Grattagliano I, et al. Dietary choline deprivation impairs rat brain mitochondrial function and behavioral phenotype. J Nutr. 2010;140:1072–1079. doi: 10.3945/jn.109.116673. Mitochondrial dysfunction is a consequence of choline deficiency. [DOI] [PubMed] [Google Scholar]
- 69.Albright CD, Zeisel SH. Choline deficiency causes increased localization of TGFβ1 signaling proteins and apoptosis in rat liver. Pathobiology. 1997;65:264–270. doi: 10.1159/000164137. [DOI] [PubMed] [Google Scholar]
- 70.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71*.Kim HJ, Kim JH, Noh S, et al. Metabolomic analysis of livers and serum from high-fat diet induced obese mice. J Proteome Res. 2011;10:722–731. doi: 10.1021/pr100892r. In mice fed an obesogenic diet, metabolomic profiling identified phosphatidylcholine, lysophosphatidylcholine, and betaine as metabolites that differentiated the obese versus lean phenotype. [DOI] [PubMed] [Google Scholar]
- 72*.Rubio-Aliaga I, Roos B, Sailer M, et al. Alterations in hepatic one-carbon metabolism and related pathways following a high-fat dietary intervention. Physiol Genomics. 2011;43:408–416. doi: 10.1152/physiolgenomics.00179.2010. Expression of genes of 1-carbon metabolism changed following a high-fat diet. [DOI] [PubMed] [Google Scholar]
- 73.Suhre K, Meisinger C, Doring A, et al. Metabolic footprint of diabetes: a multiplatform metabolomics study in an epidemiological setting. PLoS One. 2010;5:e13953. doi: 10.1371/journal.pone.0013953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang X, Wang Y, Hao F, et al. Human serum metabonomic analysis reveals progression axes for glucose intolerance and insulin resistance statuses. J Proteome Res. 2009;8:5188–5195. doi: 10.1021/pr900524z. [DOI] [PubMed] [Google Scholar]
- 76*.Jacobs RL, Zhao Y, Koonen DP, et al. Impaired de novo choline synthesis explains why phosphatidylethanolamine N-methyltransferase-deficient mice are protected from diet-induced obesity. J Biol Chem. 2010;285:22403–22413. doi: 10.1074/jbc.M110.108514. Mice with a gene of choline metabolism (Pemt) deleted are resistant to high fat diets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fruchart JC. Peroxisome proliferator-activated receptor-alpha (PPARalpha): at the crossroads of obesity, diabetes and cardiovascular disease. Atherosclerosis. 2009;205:1–8. doi: 10.1016/j.atherosclerosis.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 78.Im SS, Kim MY, Kwon SK, et al. Peroxisome proliferator-activated receptor {alpha} is responsible for the up-regulation of hepatic glucose-6-phosphatase gene expression in fasting and db/db Mice. J Biol Chem. 2011;286:1157–1164. doi: 10.1074/jbc.M110.157875. [DOI] [PMC free article] [PubMed] [Google Scholar]