Abstract
Acute myocardial infarction is still one of the leading causes of death in the industrial nations. Even after successful revascularization, myocardial ischemia results in a loss of cardiomyocytes and scar formation. Embryonic EPCs (eEPCs), retroinfused into the ischemic region of the pig heart, provided rapid paracrine benefit to acute and chronic ischemia in a PI-3K/Akt-dependent manner. In a model of acute myocardial ischemia, infarct size and loss of regional myocardial function decreased after eEPC application, unless cell pre-treatment with thymosin β4 shRNA was performed. Thymosin β4 peptide retroinfusion mimicked the eEPC-derived improvement of infarct size and myocardial function. In chronic ischemia (rabbit model), eEPCs retroinfused into the ischemic hindlimb enhanced capillary density, collateral growth, and perfusion. Therapeutic neovascularization was absent when thymosin β4 shRNA was introduced into eEPCs before application. In conclusion, eEPCs are capable of acute and chronic ischemia protection in a thymosin β4 dependent manner.
Keywords: thymosin β4, progenitor cells, ischemia/reperfusion, infarct size, angiogenesis
Introduction
In recent years progenitor cell therapy was introduced as a potential treatment option for myocardial injury after acute and chronic ischemia.1–3 Promising experimental studies reported successful use of progenitor/stem cells with respect to cardiovascular disease models.4–6 Asahara and co-workers showed that exogenous-applied endothelial progenitor cells (EPCs) selectively home and settle in the ischemic tissue and enhance neovascularization.6 A paracrine effect on neovascularization by cell therapy was described in several studies.5,7,8 To further investigate the cardioprotective effect of EPCs, we conducted an acute myocardial infarction model in pigs, where embryonic EPCs were regionally applied at the time of reperfusion. Analysis after 7 days of cell application displayed an enhanced capillary density in the ischemic tissue combined with a significant reduction of infarct size.9 This cardioprotection was at least partially driven by the PI3K/Akt signal transduction pathway, because the coapplication of Wortmanin abolished this effect.9 Among the eEPC-produced paracrine factors activating the PI3K-AKT pathway, proangiogenic factors such as vascular endothelial growth factor-A, platelet-derived growth factor-BB, and insulin-like growth factor-1 are expressed at low levels. In contrast, the highly expressed wnt agonists wnt7b and wnt11 that activate PI3K/AKT signaling are not known for cardioprotection (as opposed to the wnt antagonist FrzA10). On the other hand, thymosin β4 (Tβ4), which was found abundantly in eEPCs, displayed the potential to reduce infarct size in a chronic murine LAD occlusion model, requiring PI3K/AKT signaling.11 Moreover, Tβ4 is capable of promoting angiogenesis.12,13
We hypothesized that Tβ4 is mediating eEPC-derived protection after acute myocardial infarction (pig model) and chronic hindlimb ischemia (rabbit model). Therefore, in this study, we modulated the Tβ4 production of eEPCs by specific short hairpin RNA (shRNA) transfection or exogenously applied Tβ4 via retroinfusion and investigated the corresponding postischemic myocardial injury (pig model) and the proangiogenic potential (rabbit model).
Results
In an in vitro coculture with cardiomyocytes, embryonic EPCs reduce hypoxia/reoxygenation-dependent cell death, unless Tβ4 was reduced by shRNA transfection or a Tβ4 antibody was applied. To evaluate the role of Tβ4 in vivo, eEPCs with or without Tβ4 shRNA transfection or Tβ4 protein were applied in a pig model of acute myocardial infarction (Supporting Fig. S1A). As depicted in Supporting Fig. S2A, the transfection of the eEPCs with the Tβ4 shRNA decreased Tβ4 mRNA by 77%. Although regionally applied eEPCs into the area of ischemia by retroinfusion significantly reduced the infarct size (38 ± 4% vs. 54 ± 4% of area at risk in controls), Tβ4 reduction rendered the eEPC application inefficient (infarct size 62 ± 3%). In contrast, retroinfusion of Tβ4 protein alone revealed a similar reduction of infarct size (37 ± 3%) as the wild-type eEPC application (Fig. 1A & Supporting Fig. S2B). Consistently, wild-type eEPCs provided cardioprotection,which improved regional myocardial function as assessed by subendocardial segment shortening (Fig. 1B and Supporting Fig. S2C), was unaltered after application of Tβ4 shRNA treated eEPCs.
Figure 1.
Effect of Tβ4 shRNA on eEPC-mediated cardioprotection in vivo and infarct size measurement (percentage of AAR) 24 h after ischemia and retroinfusion of 5 × 106 eEPCs with Tβ4 shRNA. (A) Quantification revealed a significant decrease in infarct size after retroinfusion of eEPC transfected with scrambled (s.c.) shRNA or thymosin β4 protein (n = 9 per group; # P < 0.01 vs. control). (B) Subendocardial segment shortening (SES) in the apical LAD-perfused region (percentage of the nonischemic right circumflex region) at rest (n = 9 per group; # P < 0.05 vs. control and eEPC transfected with Tβ4 shRNA). Beside the influence on infarct size and myocardial function, Tβ4 moderates postischemic inflammation. (C) Quantitative adhesion of THP1 cells on activated endothelium without or with eEPC coincubation or thymosin β4 preincubation (n = 3; # P < 0.05 vs. control). (D) Myeloperoxidase (MPO) activity in infarcted regions after retroinfusion of eEPCs transfected with Tβ4 or scrambled (s.c.) shRNA Tβ4 shRNA or direct application of thymosin β4 protein (n = 6; # P < 0.05 vs. control).
The cellular detriment caused by ischemia and reperfusion, partially mediated by myocardial ischemia-reperfusion injury in postischemic inflammation. In vitro, eEPCs were capable of reducing the amount of adhesive inflammatory cells on an activated endothelial layer under flow(Fig. 1C), similar to the preincubation of the endothelial cells with Tβ4 peptide. Leukocyte influx into the infarcted region, assessed by myeloperoxidase activity, was limited after application of eEPCs and Tβ4 protein in vivo, but not Tβ4 shRNA eEPCs (Fig. 1D). Taken together, these results revealed that Tβ4, which has cardioprotective properties during hypoxia and reoxygenation in vitro, limits the extent of ischemia-reperfusion injury in vivo. Consistently, a reduction in Tβ4 expression by shRNA diminishes the cardioprotection achieved by an eEPC population, indicating that Tβ4 is an essential factor in the acute, eEPC-mediated cardioprotection in vitro and in vivo.
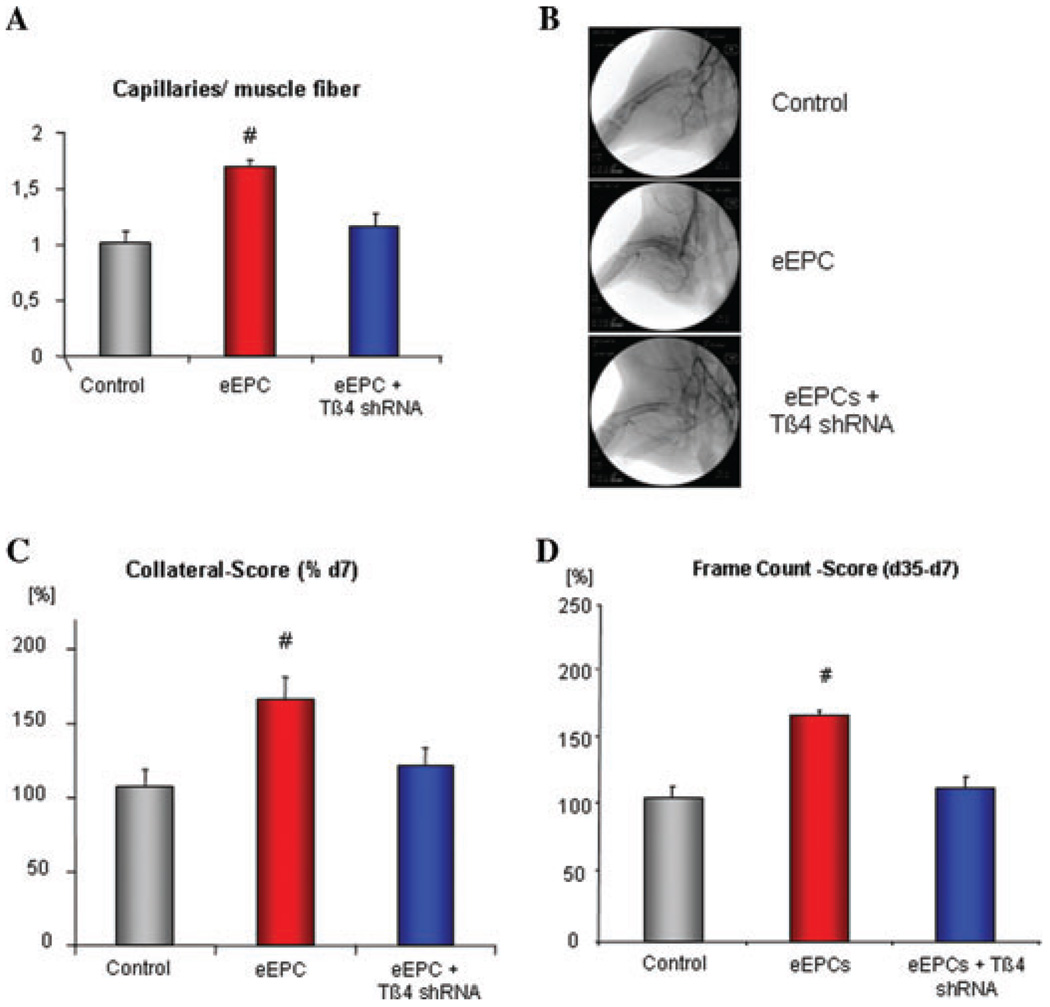
Besides the cardioprotective effect of endothelial progenitor cells, the induction of neovascularization by progenitor cell therapy has been frequently observed.14,15 Therefore, we investigated whether Tβ4 has a role for the proangiogenic effects of the eEPCs.16 In an in vitro tube formation assay, human microvascular endothelial cells (HMECs) were seeded on matrigel and incubated with the supernatant of eEPCs ± Tβ4 shRNA or transfected with Tβ4 cDNA(Fig. 2A & 2B).The incubation of HMEC with the eEPC supernatant enhanced tube formation (31 ± 2 vs. 15 ± 1 tubes/low power field in control) to the same extent as the Tβ4 overexpression (29 ± 0.7), whereas the absence of Tβ4 abolished this proangiogenic effect (11 ± 0.6 tubes/low power field). To further investigate the proangiogenic potential of eEPCs and the role of Tβ4 release in this effect, we conducted a study of chronic hindlimb ischemia in a rabbit model (Fig. 2A). At day 0, the femoral artery was excised. After 7 days of ischemia, eEPCs, without or with Tβ4 shRNA transfection, were retroinfused into the ischemic limb. Final assessment of the neovascularization potential was performed 4 weeks after cell treatment (Supporting Fig. S1B). The analysis of the capillary growth in the calf muscle, a key parameter of therapeutic angiogenesis, showed a significant increase of capillaries in the ischemic muscles after eEPC application. This proangiogenic effect was abolished through the downregulation of the Tβ4 expression. (Fig. 3A) In similar fashion, assessment of collateral growth, or macro-arteriogenesis, revealed a distinct increase of collaterals after regional application of the eEPCs, an effect abolished by downregulation of Tβ4 (Fig. 3B & 3C). The gain in hindlimb perfusion after eEPC application was prevented by pretreatment of eEPCs with Tβ4 shRNA (Fig. 3D).
Figure 2.
(A) Examples of endothelial sprouting in a matrigel assay. Incubation of human microvascular endothelial cells (HMECs) with conditioned media of eEPCs transfected ± thymosin β4 shRNA or transfected with thymosin β4 cDNA. (B) Quantitative analysis indicated a similar sprouting activity of VEGF stimulated HMECs (positive control) compared to eEPC conditioned media and addition of thymosin β4, whereas the conditioned media of eEPCs transfected with thymosin β4 shRNA lost this effect (n = 4 per group; # P < 0.05 vs. control).
Figure 3.
(A) Quantification of capillary/muscle fiber ratio of ischemic calf muscles revealed an increase of capillaries after regional eEPCs application, unless thymosin β4 was downregulated. (B, C) Collateral growth and (D) perfusion score were increased after retroinfusion of eEPCs unless the cells were transfected with thymosin β4 shRNA (n = 3; # P < 0.05 vs. control).
In summary, the regional application of eEPCs is capable of inducing therapeutic angiogenesis in a Tβ4-dependent manner. Because de novo vessel formation, as well as collateral growth, were both enhanced through eEPCs treatment, leading to increased perfusion score as long as Tβ4 expression was unaltered.
Discussion
In this study, we investigated the impact of murine eEPCs transplantation on acute and chronic ischemia.16 Given the recent finding that the PI3K/AKT signaling pathway is critically involved in eEPC-mediated limitation of ischemia-reperfusion injury, we screened the eEPC transcriptome for genes encoding secreted proteins capable of activating this pathway.16 One of the most highly expressed Akt-activating factors was the Tβ4, which is a small 43aa long G-actin-sequestering protein.17 Tβ4 is expressed in a wide variety of circulating and parenchymal cells17–19 and has been shown to have proangiogenic and cardioprotective effects.11,13 We investigated the impact of Tβ4 in acute and chronic ischemia by either applying the protein or reducing thymosin content in the cells via shRNA. In vitro Tβ4 incubation prevented cardiomyocyte cell death after hypoxia and reoxygenation, either as a peptide or as a paracrine factor released by eEPCs.20 Moreover, Tβ4 was capable of reducing endothelial cell apoptosis after the hypoxia-reoxygenation protocol, again similar to the Tβ4-containing eEPCs.20 In vivo, the decrease in infarct size and postischemic inflammation and the increase in left ventricular function achieved by eEPC retroinfusion were blocked by Tβ4 shRNA pretreatment of the eEPCs (Fig. 1). Confirming the relevance of eEPC-produced Tβ4, exogenous application of the peptide in vivo mimicked the cardioprotection achieved by eEPCs (Fig. 1).
Given the fact that eEPCs are capable of inducing therapeutic neovascularization in a chronic hindlimb model16 and that Tβ4 is inducing angiogenesis in vitro and in vivo,12,13 we investigated the role of Tβ4 in the eEPC-mediated angiogenesis. In vitro matrigel assays showed an enhanced tube formation in a paracrine, Tβ4-dependent manner. Incubation of the endothelial cells with the supernatant of eEPCs enhanced tube formation to the same extent as Tβ4 overexpression. In contrast, knockdown of Tβ4 expression in eEPCs with anti-Tβ4 shRNA abolished this effect (Fig. 2). The local application of the eEPCs into the tibial vein of a chronic hindlimb model significantly enhanced capillary growth 35 days after femoralis excision, even though the number of recovered eEPCs declined and was undetectable after 14 days.16 Moreover, eEPCs were capable of inducing robust arteriogenesis, resulting in an enhanced perfusion of the ischemic hindlimb. (Fig. 3) Consistently, the reduction of Tβ4 levels in the eEPCs abolished the genes of the cells followed by a reduced perfusion (Fig. 3).
In summary, our studies revealed a cardioprotective potential of embryonic endothelial progenitor cells during ischemia/reperfusion injury, which to a significant part appears to depend on Tβ4. Accordingly, similar protection may be achieved by local delivery of Tβ4 protein. Besides the acute cardioprotection, eEPCs are capable of inducing neovascularization in the setting of chronic ischemia, mediated by paracrine factors, such as Tβ4.
Supplementary Material
Figure 4.
Embryonic EPCs derived from a thrombomodulin-LacZ transgene mouse strain21 are capable of neovascularization of ischemic muscle tissue (late phase = requiring days to weeks). An early direct effect was found with respect to paracrine factors such as thymosin β4, which is a survival and activator signal for cardiomyocytes and endothelial cells. The receptor or internalization mechanism for thymosin β4 into myocytes or endothelial cells are not known to date.
Acknowledgments
The authors thank Tien Cuong Kieu for his excellent technical assistance. All animal experiments were conducted at the Walter Brendel Center of Experimental Medicine. The study was funded by a DFG grant KU1019/10-1 to C.K. This work was supported in part by the NIH grant HL083958 to A.K.H.
Footnotes
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Protocol of the acute myocardial infarction model (pig, A). Briefly, after baseline measurements the left descending artery (LAD) is occluded for 60 min. After 55 min of ischemia regional application of eEPCs/thymosin β4 protein is performed via selective pressure-regulated retroinfusion over 10 min.9,20 After 24 h of reperfusion, infarct size, global and regional myocardial functions are obtained. (B) Protocol of the hindlimb ischemia model (rabbit). At day 0 the femoral artery was excised and 7 days later baseline measurements for perfusion and collateralization were obtained. eEPCs with or without thymosin β4 shRNA transfection were retrogradely applied to the tibial vein in the ischemic hindlimb. Twenty-eight days after treatment final measurements for blood flow and collateral score were conducted and tissue was harvested for capillary staining. (NMR-image of right art, femoralis excision (rabbit). Courtesy of B. Wintersperger, Institute of Radiology, LMU Munich.)
Figure S2. (A) Example and quantification of Tβ4 expression reduction by shRNA. (B) AAR/left ventricle (LV) did not differ significantly between groups. (C) Subendocardial segment shortening (SES) in the apical LAD perfused region under increased heart rate (150-bpm atrial pacing, n = 9 per group; § P < 0.05 vs. control; # P < 0.05 vs. control and eEPC transfected with Tβ4 shRNA).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Assmus B, Schachinger V, Teupe C, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI) Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 2.Erbs S, Linke A, Schachinger V, et al. Restoration of microvascular function in the infarct-related artery by intracoronary transplantation of bone marrow progenitor cells in patients with acute myocardial infarction: the Doppler Substudy of the Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction (REPAIR-AMI) trial. Circulation. 2007;116:366–374. doi: 10.1161/CIRCULATIONAHA.106.671545. [DOI] [PubMed] [Google Scholar]
- 3.Schachinger V, Erbs S, Elsasser A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N. Engl. J. Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 4.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 5.Wollert KC. Cell therapy for acute myocardial infarction. Curr. Opin. Pharmacol. 2008;8:202–210. doi: 10.1016/j.coph.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Kawamoto A, Gwon HC, Iwaguro H, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 7.Kinnaird T, Stabile E, Burnett MS, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 8.Urbich C, Aicher A, Heeschen C, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J. Mol. Cell. Cardiol. 2005;39:733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Kupatt C, Hinkel R, Lamparter M, et al. Retroinfusion of embryonic endothelial progenitor cells attenuates ischemia-reperfusion injury in pigs: role of phosphatidylinositol 3-kinase/AKT kinase. Circulation. 2005;112(9 Suppl.):I117–I122. doi: 10.1161/CIRCULATIONAHA.104.524801. [DOI] [PubMed] [Google Scholar]
- 10.Barandon L, Couffinhal T, Ezan J, et al. Reduction of infarct size and prevention of cardiac rupture in transgenic mice overexpressing FrzA. Circulation. 2003;108:2282–2289. doi: 10.1161/01.CIR.0000093186.22847.4C. [DOI] [PubMed] [Google Scholar]
- 11.Bock-Marquette I, Saxena A, White MD, et al. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432:466–472. doi: 10.1038/nature03000. [DOI] [PubMed] [Google Scholar]
- 12.Philp D, Nguyen M, Scheremeta B, et al. Thymosin beta4 increases hair growth by activation of hair follicle stem cells. FASEB J. 2004;18:385–387. doi: 10.1096/fj.03-0244fje. [DOI] [PubMed] [Google Scholar]
- 13.Smart N, Risebro CA, Melville AA, et al. Thymosin beta-4 is essential for coronary vessel development and promotes neovascularization via adult epicardium. Ann. N.Y. Acad. Sci. 2007;1112:171–178. doi: 10.1196/annals.1415.000. [DOI] [PubMed] [Google Scholar]
- 14.Shantsila E, Watson T, Lip GY. Endothelial progenitor cells in cardiovascular disorders. J. Am. Coll. Cardiol. 2007;49:741–752. doi: 10.1016/j.jacc.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, Ip JE, Huang J, et al. Essential role of ICAM-1/CD18 in mediating EPC recruitment, angiogenesis, and repair to the infarcted myocardium. Circ. Res. 2006;99:315–322. doi: 10.1161/01.RES.0000235986.35957.a3. [DOI] [PubMed] [Google Scholar]
- 16.Kupatt C, Horstkotte J, Vlastos GA, et al. Embryonic endothelial progenitor cells expressing a broad range of proangiogenic and remodeling factors enhance vascularization and tissue recovery in acute and chronic ischemia. FASEB J. 2005;19:1576–1578. doi: 10.1096/fj.04-3282fje. [DOI] [PubMed] [Google Scholar]
- 17.Cassimeris L, Safer D, Nachmias VT, Zigmond SH. Thymosin beta 4 sequesters the majority of G-actin in resting human polymorphonuclear leukocytes. J. Cell Biol. 1992;119:1261–1270. doi: 10.1083/jcb.119.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huff T, Muller CS, Otto AM. beta-Thymosins, small acidic peptides with multiple functions. Int. J. Biochem. Cell. Biol. 2001;33:205–220. doi: 10.1016/s1357-2725(00)00087-x. [DOI] [PubMed] [Google Scholar]
- 19.Grant DS, Rose W, Yaen C, et al. Thymosin beta4 enhances endothelial cell differentiation and angiogenesis. Angiogenesis. 1999;3:125–135. doi: 10.1023/a:1009041911493. [DOI] [PubMed] [Google Scholar]
- 20.Hinkel R, El-Aouni C, Olson T, et al. Thymosin beta4 is an essential paracrine factor of embryonic endothelial progenitor cell-mediated cardioprotection. Circulation. 2008;117:2232–2240. doi: 10.1161/CIRCULATIONAHA.107.758904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatzopoulos AK, Folkman J, Vasile E, et al. Isolation and characterization of endothelial progenitor cells from mouse embryos. Development. 1998;125:1457–1468. doi: 10.1242/dev.125.8.1457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.