Abstract
Background
Schizophrenia is a heterogeneous disorder that may consist of multiple etiologies and disease processes. Auditory hallucinations (AH), which are common and often disabling, represent a narrower and more basic dimension of psychosis than schizophrenia. Previous studies suggest that abnormal primary auditory cortex activity is associated with AH pathogenesis. We thus investigated functional connectivity, using a seed in primary auditory cortex, in schizophrenia patients with and without AH and healthy controls, to examine neural circuit abnormalities associated more specifically with AH than the myriad other symptoms that comprise schizophrenia.
Methods
Using resting-state fMRI (rsfMRI), we investigated functional connectivity of the primary auditory cortex, located on Heschl’s gyrus, in schizophrenia spectrum patients with AH. Participants were patients with schizophrenia, schizoaffective disorder, or schizophreniform disorder with lifetime AH (n=27); patients with the same diagnoses but no lifetime AH (n=14); and healthy controls (n=28).
Results
Patients with AH vulnerability showed increased left Heschl’s gyrus functional connectivity with left frontoparietal regions and decreased functional connectivity with right hippocampal formation and mediodorsal thalamus compared to patients without lifetime AH. Furthermore, among AH patients, left Heschl’s gyrus functional connectivity covaried positively with AH severity in left inferior frontal gyrus (Broca’s area), left lateral STG, right pre- and postcentral gyri, cingulate cortex, and orbitofrontal cortex. There were no differences between patients with and without lifetime AH in right Heschl’s gyrus seeded functional connectivity.
Conclusions
Abnormal interactions between left Heschl’s gyrus and regions involved in speech/language, memory, and the monitoring of self-generated events may contribute to AH vulnerability.
Keywords: psychosis, resting-state fMRI, Broca’s area, hippocampus, mediodorsal thalamus, cingulate cortex
1.INTRODUCTION
Schizophrenia (SZ) is a heterogeneous disorder, possibly consisting of several distinct though related disease processes. Here, we focused on the symptom dimension of auditory hallucinations (AH), a narrower and more basic phenotypic unit of analysis than SZ, with the goal to identify neural circuit abnormalities that are associated more specifically with AH than the myriad other symptoms seen in SZ. Though AH can also be phenomenologically heterogeneous, SZ patients with AH likely represent a more homogeneous group than all SZ patients. AH are common in SZ, can be disabling, and increase suicide risk (Falloon and Talbot, 1981). While antipsychotic medications can reduce their severity, AH often persist despite treatment.
Abnormalities of the primary auditory cortex (PAC), located on the superior temporal gyrus (STG), have been implicated in AH pathogenesis. SZ patients with AH have STG volume reductions (Allen et al., 2008; McCarley et al., 1999; Modinos et al., 2012), and the PAC in SZ contains morphologically abnormal pyramidal cells (Sweet et al., 2004; Sweet et al., 2009). Several, though not all, groups have reported increased activity in the dominant hemispheric PAC during AH (Dierks et al., 1999; Lennox et al., 2000; Matsuda et al., 1989; Suzuki et al., 1993; van de Ven et al., 2005). A perceptual basis for AH is supported by evidence that AH-related activity in the left STG competes with the processing of external sound (Ford et al., 2009; Kompus et al., 2011; Woodruff et al., 1997), and is consistent with patients’ experience of AH as real.
Neuroimaging studies also implicate other brain regions [e.g., medial temporal lobe (Liddle et al., 1992), anterior cingulate cortex (Lahti et al., 2006), Broca’s area (McGuire et al., 1993), thalamus, parahippocampal gyrus, ventral striatum, orbitofrontal cortex (Silbersweig et al., 1995)] during AH, suggesting that AH are associated with abnormal activity in a distributed network that includes multiple cortical and subcortical brain regions. The study of distributed brain abnormalities in AH may offer more broadly applicable insights into the neurobiology of SZ because subtle neurodevelopmental abnormalities (e.g., in neuronal migration, synaptogenesis, pruning) may lead to abnormal connectivity, and ultimately to symptom formation.
Low frequency (<0.1 Hz) spontaneous fluctuations in activity occur continually in the human brain (Fox and Raichle, 2007). These fluctuations have been observed under anesthesia, suggesting that they reflect intrinsic properties of functional brain organization (Vincent et al., 2007). Several studies have used resting state fMRI (rsfMRI) to study these fluctuations in AH. One study suggested abnormal interhemispheric PAC connectivity (Gavrilescu et al., 2009); three highlighted altered connectivity in speech-related (Vercammen et al., 2010; Wolf et al., 2011) or default mode (Rotarska-Jagiela et al., 2010) networks and their association with limbic regions; and a fifth study reported abnormal connectivity between speech-related areas and the putamen (Hoffman et al., 2011). None explicitly focused on the PAC’s connectivity with other brain circuits.
In this study, we investigated the functional connectivity (FC) of Heschl’s gyrus (HG) in SZ patients with and without vulnerability to AH and healthy controls, using rsfMRI. This is the first study to examine the PAC’s connectivity with distributed brain regions. We used HG as an anatomical proxy for PAC, as the PAC is consistently located in HG across the range of common morphological variants (Da Costa et al., 2011). We hypothesized that patients with lifetime AH, even if not currently hallucinating, will have abnormal FC in a network of HG and cortical and subcortical brain regions processing speech, language, affect, and source attribution.
2.METHODS
2.1.Participants
Following approval by the McLean Hospital IRB, we studied 69 men and women, ages 18–65 years, in three groups: (i) 27 auditory hallucinators (AH; SZ, schizoaffective, or schizophreniform disorder patients with lifetime AH); (ii) 14 never-auditory hallucinators (NAH; patients with the above diagnoses who have never experienced AH); and (iii) 28 healthy controls (HC; individuals with no psychiatric illness). We administered the Structured Clinical Interview for DSM-IV-TR (SCID) to establish axis I diagnoses, and used SCID item B16 (“Did you ever hear things that other people couldn’t, such as noises, or the voices of people whispering or talking?”) to categorize patients. Patients scoring 3 (threshold/true) on B16 were coded AH, and all others NAH.
Patients were recruited from inpatient and outpatient services at McLean Hospital, and HC through community advertisements. We excluded individuals with substance abuse or dependence in the previous three months, electroconvulsive treatment in the past year, or significant medical or neurological illness. We collected medication information, and calculated chlorpromazine (CPZ) equivalents for antipsychotics (Table 1).
Table 1.
Participant Characteristics
| AH | NAH | HC | Statistic | Significance | |
|---|---|---|---|---|---|
| Sample Size (N=69) | 27 fMRI (25 DTI) | 14 fMRI (13 DTI) | 28 fMRI (27 DTI) | ||
| Dataset1 (n = 21) | 7 fMRI (6 DTI) | 4 fMRI (4 DTI) | 10 fMRI (9 DTI) | ||
| Dataset2 (n = 48) | 20 fMRI (19 DTI) | 10 fMRI (9 DTI) | 18 fMRI (18 DTI) | ||
| DSM-IV Diagnosis | χ2 = 1.046 | p = 0.59 | |||
| Schizophrenia, No. (%) | 14 (52) | 5(36) | -- | ||
| Schizoaffective, No. (%) | 12 (44) | 8(57) | -- | ||
| Schizophreniform, No. (%) | 1(14) | 1(7) | -- | ||
| Age, mean ± SD, y | 40 ± 11(21–59) | 37 ± 10(25–55) | 39 ± 9 (24–54) | F = 0.383 | p = 0.68 |
| Female Sex, No. (%) | 12 (44) | 5(36) | 11(39) | χ2 = 0.324 | p = 0.85 |
| Handedness | χ2 = 1.171 | p = 0.56 | |||
| Right, No. (%) | 23 (85) | 10(71) | 23(82) | ||
| Left, No. (%) | 4(15) | 4(29) | 5(18) | ||
| Parental Education, No. (%)1 | 17(63) | 8(57) | 15(55) | χ2 = 0.503 | p = 0.78 |
| Illness Duration, mean ± SD (range), y | 18 ± 12(0–36) | 14 ± 10(0–28) | -- | t = 0.961 | p = 0.34 |
| Inpatient Hospitalized, No. (%) | 17(63) | 10(71) | -- | χ2 = 0.294 | p = 0.59 |
| Lifetime Substance Use | 10(37) | 6(43) | 5(18) | χ2 = 3.668 | p = 0.16 |
| Disorder, No. (%)2 | |||||
| SAPS3 | 39.8 ± 19.0 | 32.7 ± 16.7 | -- | t = 0.996 | p = 0.33 |
| SANS3 | 26.1 ± 18.4 | 20.2 ± 11.0 | -- | t = 0.921 | p = 0.37 |
| YMRS | 14.1 ± 7.9 | 15.6 ± 7.5 | -- | t = −0.584 | p = 0.56 |
| MADRS | 15.1 ± 8.0 | 13.6 ± 9.1 | -- | t = 0.532 | p = 0.60 |
| Fagerstrom3 | 1.2 ± 2.9 | 1.5 ± 3.2 | -- | t = −0.237 | p = 0.82 |
| Chlorpromazine | 611 ± 664 | 435 ± 231 | -- | t = 1.15 | p = 0.26 |
| Equivalent | mg | mg | |||
| Antipsychotic-Free, No. (%)4 | 2(7) | 0(0) | -- | ||
| Typical Antipsychotic, No. (%) | 4(15) | 2(14) | -- | χ2 = 0.002 | p = 1.00 |
| Haloperidol | 1(4) | 1(7) | -- | ||
| Fluphenazine | 1(4) | 0(0) | -- | ||
| Perphenazine | 2(7) | 0(0) | -- | ||
| Loxapine | 0(0) | 1(7) | -- | ||
| Atypical Antipsychotic, No. (%) | 23 (85) | 13(93) | - | χ2 = 0.507 | p = 0.65 |
| Clozapine | 10(37) | 2(14) | -- | ||
| Olanzapine | 2(7) | 5(36) | -- | ||
| Risperidone | 7(26) | 1(7) | -- | ||
| Paliperidone | 0(0) | 1(7) | -- | ||
| Quetiapine | 6(22) | 2(14) | -- | ||
| Aripiprazole | 2(7) | 4(29) | -- | ||
| Antiepileptic, No. (%) | 6(22) | 3(21) | -- | Fisher's exact | p = 1.00 |
| Lithium, No. (%) | 6(22) | 4(29) | -- | Fisher's exact | p = 0.71 |
| Benzodiazepine, No. (%) | 8(30) | 6(43) | -- | χ2 = 0.717 | p = 0.40 |
| Antidepressant, No. (%) | 11(41) | 5 (36) | -- | χ2 = 0.098 | p = 0.75 |
At least one parent with a college degree.
No participants had current (i.e., in the previous 3 months) substance abuse or dependence.
Data not available for participants from 2006–2007 (Dataset 1).
No patients were antipsychotic-naïve.
To enhance sample size, we combined two datasets: Dataset1 (n=21) from Feb2006-Apr2007, previously reported in an unrelated project (Ongur et al., 2010), and Dataset 2 (n=48) from Oct2008–Mar2010 acquired for this study (Table 1).
We used the Psychotic Symptom Rating Scale for AH (PSYRATS-AH) (Haddock et al., 1999) to measure AH severity. PSYRATS is unavailable for Datset1. See Supplementary Materials for details about additional clinical assessments that were administered.
2.2.Image Acquisition
Using a Siemens Trio 3-Tesla MRI scanner, we acquired a T1-weighted whole-brain anatomical image (MPRAGE, 256 × 256 voxels, 1 × 1.3mm2 in-plane resolution, 1.3mm slice thickness), followed by a T2-weighted functional scan (interleaved EPI sequence, 42 oblique slices, flip angle 82°, TE/TR=24/2500ms, 3.5mm isotropic voxels, matrix 128 × 128, 224mm2 FOV, 240 volumes over 600s). Participants were scanned at rest, and instructed to stay awake, keep their eyes open, and think of nothing in particular. The scanner underwent an upgrade during the study period, but there were no statistically significant differences in image quality related to the upgrade (Supplementary Materials). For Dataset2 participants, after scanning, we conducted a debriefing interview (Supplementary Material) to ascertain the presence of AH and other symptoms during image acquisition.
2.3.Image Analysis
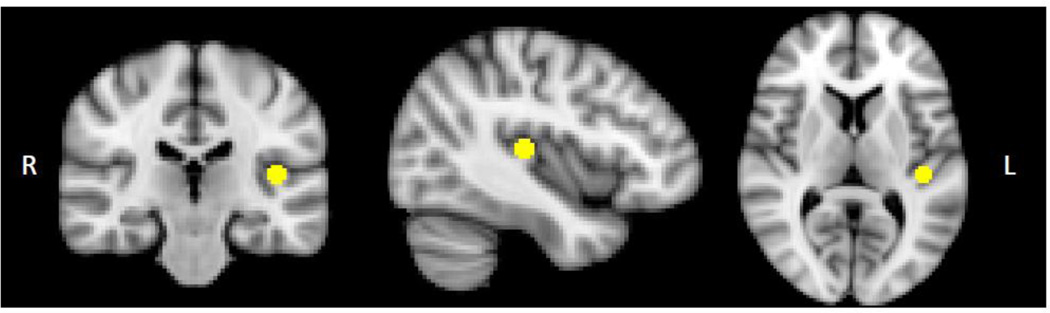
We used the FMRIB Software Library (FSL v4.1.6) (Smith et al., 2004) for image analyses. Images were slice-time and motion corrected (Jenkinson et al., 2002), intensity normalized, smoothed with a 6mm Gaussian kernel, and affine registered to Montreal Neurological Institute (MNI) space. Images were low-pass filtered to retain frequencies slower than 0.08Hz and reduce high-frequency nonneuronal noise. We placed a 10mm diameter sphere on left Heschl’s gyrus (LHG; −42, −26, 10) (Figure 1), the putative location of left PAC in humans (Da Costa et al., 2011). We selected coordinates with reference to a PAC probabilistic map (Rademacher et al., 2001) followed by adjustment based on macroanatomical landmarks on the MNI152 brain. We inspected the LHG sphere overlaid on each participant’s registered image to ensure appropriate placement on individual brains (Figure S1). The BOLD time course from LHG was extracted and entered into a general linear model (GLM) using FEAT (www.fmrib.ox.ac.uk/fsl/feat5), with signal from white matter, CSF, and motion correction parameters regressed. Data from first-level analyses were entered into a mixed-effects group analysis using the Bayesian estimation techniques in FLAME (Woolrich et al., 2004). We included de-meaned CPZ equivalents, handedness, acquisition period (Dataset1 vs. Dataset2), and scanner upgrade (pre- vs. post-upgrade) as covariates. We initially used a p<0.01 voxel threshold, whole-brain corrected with a p<0.05 cluster threshold. As no between-group differences could be detected at this level, we used an uncorrected threshold of p<0.001, and report grey matter clusters ≥20 voxels. SZ NAH patients are relatively difficult to recruit, and this group was small. We used a relatively liberal statistical threshold to account for this fact.
Figure 1.
Left Heschl's gyrus seed, 10 mm in diameter (x=-42, y=-26, z=10).
In addition to group-wise comparisons, which assume relative homogeneity within each group, we analyzed the data with AH as a continuous variable. For Dataset2 patients with current AH (n=16/20), the de-meaned PSYRATS-AH subscale, was entered as a covariate in the GLM. (Dataset1 AH patients not included because PSYRATS unavailable.) Because of the small sample size, only one covariate-of-no-interest (CPZ equivalents) was included in this analysis. For this covariate analysis, we used a p<0.01 voxel threshold, whole-brain corrected with a p<0.05 cluster threshold.
To assess for partial volume effects, we segmented the T1 image using FAST (Zhang et al., 2001) in FSL. For quality control, we also assessed between-group differences in head motion (mean absolute displacement of each brain volume compared to the previous volume) (Jenkinson et al., 2002; Van Dijk et al., 2012).
To test the specificity of abnormalities in the left HG, we performed connectivity analysis with a right HG seed (RHG; 46, −20, 8), using the same approach as for LHG (See Figure S2 for RHG seed on individual brains).
3.RESULTS
3.1.Participant Characteristics
The participant groups were well-matched demographically. AH and NAH patient groups had comparable clinical characteristics (Table 1).
Nearly all AH patients experienced verbal AH (n=25/27; unable to confirm for two Dataset1 patients). Twelve of the 27 (44.4%) reported running commentary (SCID B17) or voices conversing (SCID B18). Individual PSYRATS-AH items are presented in Table 2. Of participants for whom scanner debriefing data were available, six AH patients (31.6%) reported experiencing AH in the scanner. Group means for all other debriefing items are presented in Table 3.
Table 2.
PSYRATS Auditory Hallucinations Items (*n = 20/27 AH Participants)
| PSYRATS-AH Item |
Range | Mean ± SD |
|---|---|---|
| Frequency | 0–4 | 2.2 ± 1.5 |
| Duration | 0–4 | 2.2 ± 1.6 |
| Location | 0–4 | 1.9 ± 1.4 |
| Loudness | 0–4 | 1.6 ± 1.2 |
| Beliefs regarding origin of voices | 0–4 | 2.2 ± 1.6 |
| Amount of negative content of voicesf | 0–4 | 1.8 ± 1.4 |
| Degree of negative content | 0–4 | 2.2 ± 1.6 |
| Amount of distress | 0–4 | 1.6 ± 1.3 |
| Intensity of distress | 0–4 | 1.8 ± 1.6 |
| Disruption of life caused by voices | 0–4 | 1.5 ± 1.3 |
| Controllability of voices | 0–4 | 1.8 ± 1.6 |
| Total | 0–44 | 20.4 ± 12.8 |
| PSYRATS-AH | ||
| score | ||
| Minimum | 0 | |
| PSYRATS-AH | ||
| Maximum | 37 | |
| PSYRATS-AH | ||
| Median | 25 | |
| PSYRATS-AH |
Mean ± SD ratings are from the 20 AH patients who have PSYRATS data (Dataset2). The group includes 4 patients with lifetime but no current AH (all PSYRATS-AH items 0). PSYRATS data not available for the 7AH participants from 2006-2007(Dataset1).
Table 3.
Scanner Debriefing Interview (n = 47†)
| Item | Range | Mean ± SD (No. reporting yes) | ||
|---|---|---|---|---|
| AH (n = 19†) | NAH (n = 10†) | HC (n = 18†) | ||
| Physical discomfort | 0–5 | 1.6 ± 1.2 (14) | 1.5 ± 1.2 (7) | 1.2 ± 1.1 (12) |
| Anxiety | 0–5 | 0.7 ± 1.0 (8) | 0.9 ± 1.6 (4) | 0.3 ± 0.5 (5) |
| Delusional thought content | 0–5 | 1.2 ± 1.8 (7) | 1.0 ± 1.7 (4) | 0.0 ± 0.0 (0) |
| Auditory hallucinations | 0–5 | 0.8 ± 1.4 (6) | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) |
| Visual hallucinations | 0–5 | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) |
| Somatic hallucinations | 0–5 | 0.3 ± 0.6 (4) | 0.5 ± 0.7 (4) | 0.1 ± 0.3 (2) |
| Speed of thoughts | ||||
| Slow | 0–2 | 0.5 ± 0.6 (8) | 0.2 ± 0.4 (2) | 0.2 ± 0.4 (3) |
| Fast | 0–2 | 0.6 ± 0.7 (3) | 0.2 ± 0.4 (2) | 0.1 ± 0.2 (1) |
| Mood in the previous 2 weeks | ||||
| Low | 0–2 | 0.6 ± 0.7 (9) | 0.4 ± 0.5 (4) | 0.0 ±0.0 (0) |
| Elevated | 0–2 | 0.1 ± 0.3 (3) | 0.1 ± 0.3 (1) | 0.1 ± 0.2 (1) |
| Mood in the scanner | ||||
| Low | 0–2 | 0.1 ± 0.3 (2) | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) |
| Elevated | 0–2 | 0.0 ± 0.0 (0) | 0.1 ± 0.3 (1) | 0.1 ± 0.2 (1) |
| Total Debriefing Score | 0–36 | 6.1 ± 6.0 | 4.9 ± 4.0 | 1.9 ± 1.6 |
Scanner debriefing data not available for participants from 2006–2007 (Dataset1) and also missing for one AH patient from Dataset2.
3.2.Resting state fMRI
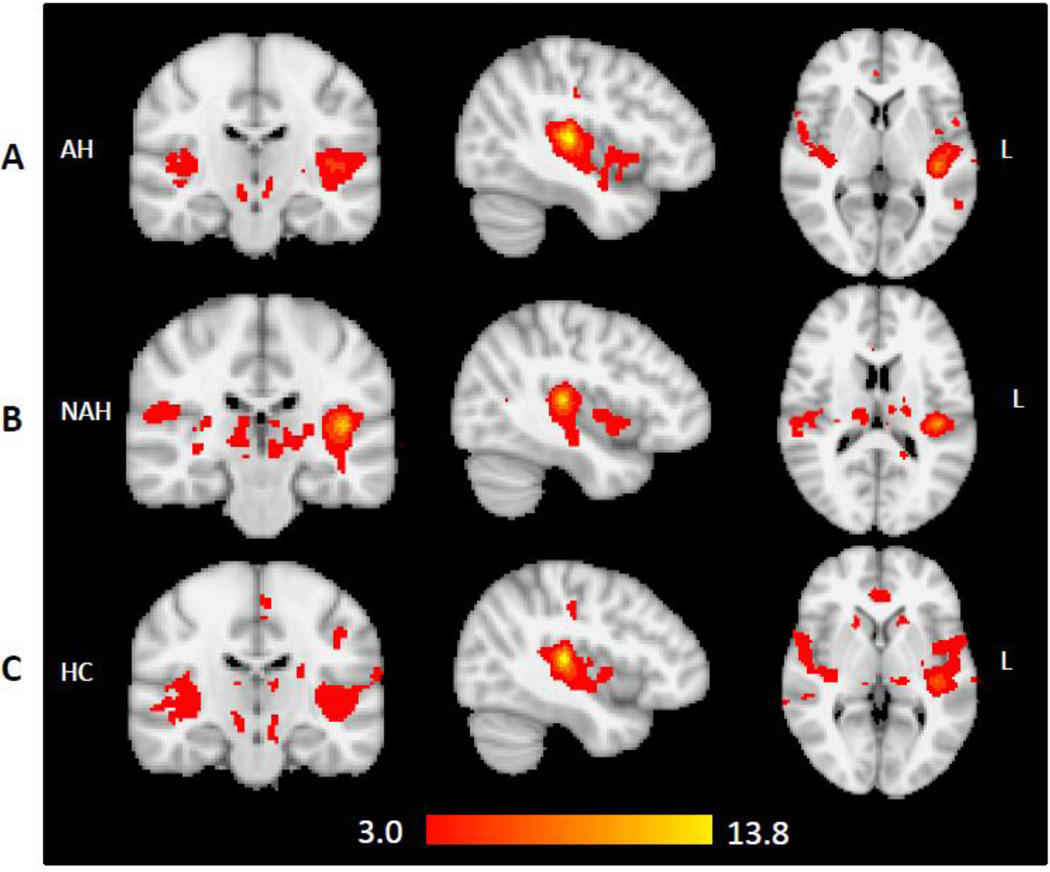
The three group maps (Figure 2) of LHG FC approximate the resting-state auditory network published by other groups (Beckmann et al., 2005; Cordes et al., 2001; Damoiseaux et al., 2006), and suggest that our LHG seed appropriately captured the network of interest.
Figure 2.
Group maps of brain areas that are temporally correlated with the left Heschl's gyrus seed in (A) patients with lifetime auditory hallucinations (AH, n=27); (B) patients with no lifetime auditory hallucinations (NAH, n=14); and (C) healthy controls (HC, n=28).
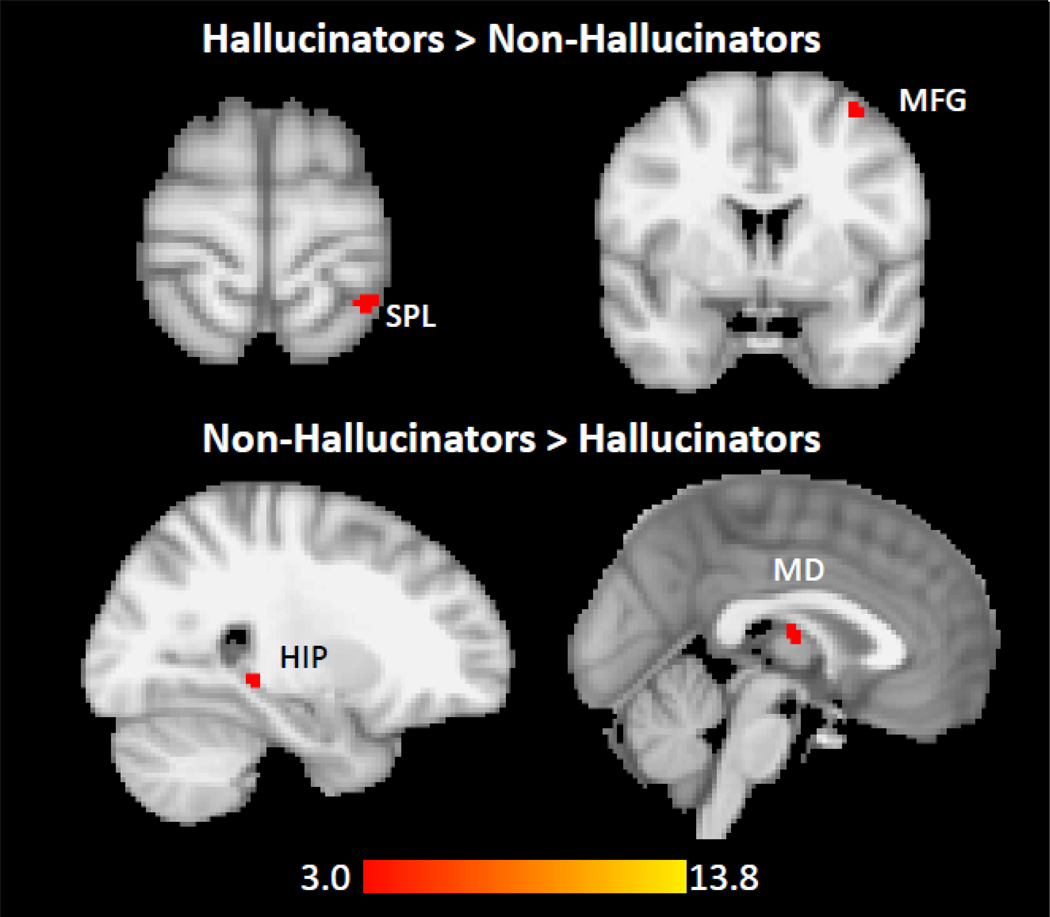
Relative to NAH, the AH group showed increased LHG FC with left superior parietal lobule (SPL) and left middle frontal gyrus (MFG), and decreased LHG FC with right hippocampal/parahippocampal gyrus (HIP/PHG) and mediodorsal (MD) thalamus (Table 4, Figure 3). Increased LHG-left SPL FC and decreased LHG-MD thalamus FC were also present when comparing AH and HC. These group-wise findings did not survive whole-brain correction.
Table 4.
Whole-Brain Analysis with Left Heschl’s Gyrus Seed (n = 27 AH, 14 NAH, 28 HC)
| Contrast | Region | BA | Hemisphere | k | Z-max | Peak coordinates (mm) | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| AH > NAH | Superior parietal lobule | 7 | Left | 25 | 3.80 | −32 | −46 | +70 |
| Middle frontal gyrus | 6 | Left | 20 | 3.50 | −34 | +2 | +60 | |
| NAH > AH | Hippocampus tail and parahippocampal gyrus | -- | Right | 52 | 4.06 | +18 | −38 | −4 |
| Mediodorsal thalamus | -- | Mid | 36 | 3.58 | 0 | −12 | +12 | |
| AH > HC | Precuneus | 7 | Left | 145 | 4.05 | −14 | −76 | +36 |
| Superior parietal lobule | 7 | Left | 50 | 4.02 | −18 | −66 | +46 | |
| Inferior parietal lobule (supramarginal gyrus) | 40 | Left | 27 | 3.97 | −54 | −38 | +32 | |
| HC > AH | Precuneus | 7 | Right | 56 | 4.26 | +12 | −50 | +48 |
| Mediodorsal thalamus | -- | Mid | 52 | 3.82 | −2 | −16 | +12 | |
| Parietal operculum | 4 | Left | 37 | 3.81 | −54 | −4 | +10 | |
| NAH > HC | No findings. | -- | -- | -- | -- | -- | -- | -- |
| HC > NAH | Anterior cingulated gyrus | 32 | Left | 72 | 3.98 | −8 | +34 | +28 |
| Superior temporal gyrus | 22 | Left | 47 | 4.17 | −62 | 0 | −4 | |
Controlled for CPZ equivalents, handedness, period of data acquisition (Dataset1 vs. Dataset2), and pre- vs. post scanner upgrade.
Threshold p < 0.001, uncorrected. (There are no findings that survive whole-brain correction.)
k = cluster size, in voxels
Figure 3.
Relative to patients with no lifetime auditory hallucinations (NAH, n=14), patients with lifetime auditory hallucinations (AH, n=27) have increased functional connectivity between LHG and (A) left superior parietal lobule, and (B) the middle frontal gyrus. Compared to NAH patients, AH patients have reduced functional connectivity between LHG and (C) right hippocampus/parahippocampal gyrus and (D) mediodorsal thalamus. Threshold p<0.001, uncorrected. The images have not been masked.
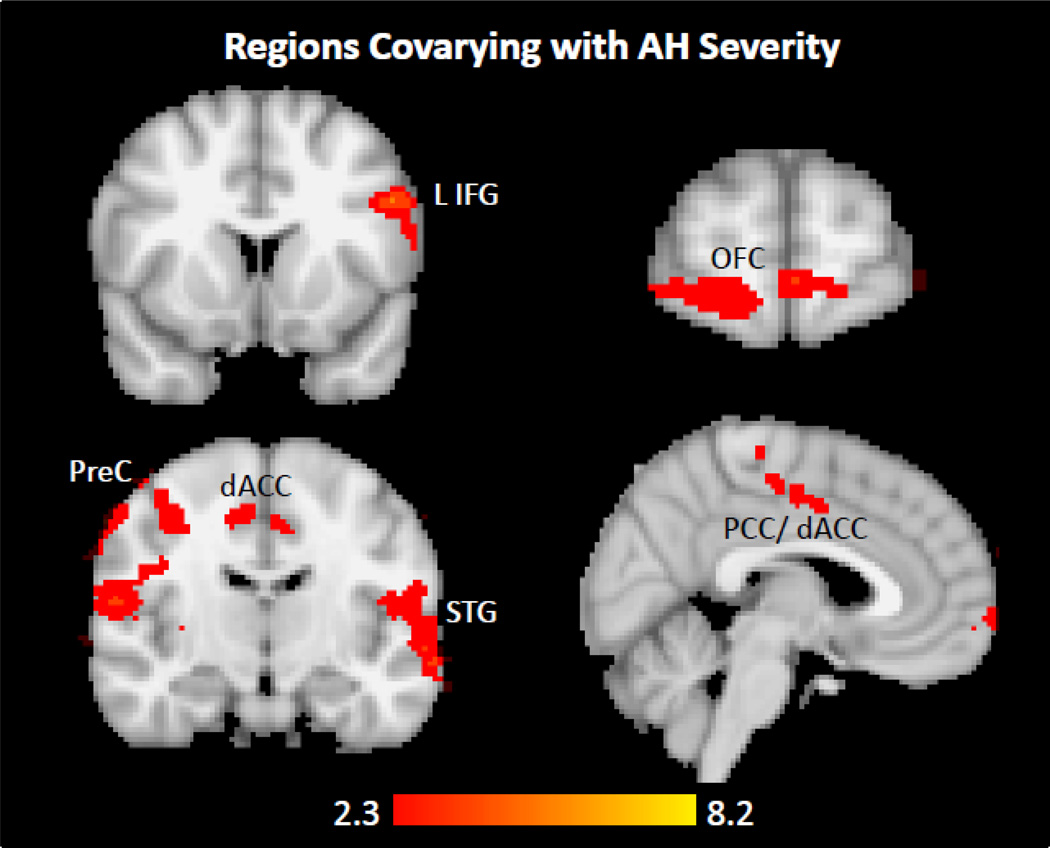
Among patients reporting AH on the PSYRATS, LHG FC covaried positively with AH severity in left inferior frontal gyrus (IFG, i.e., Broca’s area), left lateral STG, right pre- and postcentral gyri, cingulate cortex [dorsal anterior (dACC) and posterior (PCC)], and right orbitofrontal cortex (OFC) (Table 5, Figure 4). These results survived whole-brain cluster correction at p<0.05.
Table 5.
Regions that Covary with AH Severity (Among Dataset2 Patients Reporting AH on the PSYRATS, n = 16)
| Contrast | Region | BA | Hemisphere | k | Z- max |
Peak coordinates (mm) |
||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Covariation with PSYRATS-AH |
Inferior frontal gyrus/Superior temporal gyrus |
44/22 | Left | 1863 | 4.83 | −50 | +12 | +30 |
| Postcentral gyrus/Precentral gyrus |
1/4 | Right | 1787 | 4.83 | +64 | −18 | +46 | |
| Frontal pole/Orbitofrontal cortex |
10/11 | Left | 1180 | 4.03 | −22 | +76 | 10 | |
| Cingulate gyrus, posterior and anterior |
31/24 | Left | 689 | 3.83 | −6 | −32 | +42 | |
PSYRATS data not available for participants from 2006–2007 (Dataset1).
Controlled for CPZ equivalents.
Threshold p < 0.01, corrected using a cluster significance threshold of p < 0.05.
k = cluster size, in voxels
Figure 4.
Among Dataset2 patients with lifetime but no current auditory hallucinations (n=16), LHG functional connectivity co-varies with severity of AH (PSYRATS-AH subscale score) in (A) left inferior frontal gyrus, i.e., Broca’s area, (B) orbitofrontal cortex, (C) left lateral superior temporal gyrus, right postcentral gyrus, and cingulate cortex. Both dorsal anterior and posterior aspects of cingulate cortex are shown in (D). Threshold p<0.01, whole-brain corrected using a cluster significance threshold of p<0.05. The images have not been masked.
There were no AH vs. NAH differences in RHG FC (Table 6). Post-hoc analysis showed no LHG FC differences between the 6 patients who had AH during scanning and the 13 patients who reported no AH in the scanner debriefing interview. Partial volume effects are unlikely, as there were no betweengroup differences in the percentages of GM, WM, and CSF in the LHG ROI (Table S3). There were no statistically significant between-group differences in mean motion (in millimeters: AH 0.116 ± 0.066, NAH 0.138 ± 0.072, HC 0.133 ± 0.159; F=0.227, p=0.797).
Table 6.
Whole-Brain Analysis with Right Heschl’s Gyrus (RHG) Seed (n = 27 AH, 14 NAH, 28 HC)
| Contrast | Region | BA | Hemisphere | k | Z-max | Peak coordinates (mm) | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| AH-NAH | No findings | -- | -- | -- | -- | -- | -- | -- |
| NAH-AH | No findings | -- | -- | -- | -- | -- | -- | -- |
| AH-HC | Cerebellum | -- | Right | 71 | 4.12 | +10 | −62 | −16 |
| Midbrain | -- | Left | 25 | 3.77 | −4 | −16 | −8 | |
| Visual cortex | 17 | Left | 22 | 3.53 | −10 | −94 | −2 | |
| HC-AH | No findings | -- | -- | -- | -- | -- | -- | -- |
| NAH-HC | Inferior temporal gyrus | 37 | Right | 63 | 4.57 | +56 | −50 | −22 |
| Temporal fusiform gyrus | 36 | Right | 39 | 4.01 | +32 | −34 | −18 | |
| Visual cortex | 18 | Left | 24 | 3.65 | −24 | −56 | +6 | |
| HC-NAH | Superior frontal gyrus | 8 | Left | 55 | 3.74 | −8 | +40 | +38 |
| Middle frontal gyrus | 8 | Right | 41 | 3.96 | +32 | +28 | +40 | |
| 8 | Left | 31 | 3.58 | −22 | +20 | +42 | ||
| Anterior cingulate gyrus | 24 | Left | 23 | 3.85 | −4 | −6 | +42 | |
Controlled for CPZ equivalents, handedness, period of data acquisition (Dataset1 vs. Dataset2), and pre- vs. post scanner upgrade.
4.DISCUSSION
We investigated the FC of the circuitry underlying AH vulnerability in patients with SZ spectrum disorders. In contrast to AH activity studies which capture brain activity during AH (state), we examined abnormalities in connectivity between brain regions in patients with AH proneness (trait). With rsfMRI using a LHG seed, we found that AH-prone patients have reduced coupling of LHG with MD thalamus and right HIP/PHG but increased coupling of LHG with left SPL and left MFG, compared to patients with no lifetime AH. Additionally, we observed that coupling between LHG and several brain regions—including left IFG, left lateral STG, right precentral gyrus, dACC and PCC—was associated with greater AH severity. These findings point to dysfunctional interactions between LHG and thalamocortical circuits in AH-vulnerable patients, particularly inappropriately elevated coupling of LHG with a number of cortical regions at the expense of coordination with thalamic and hippocampal regions.
This is the first study to explicitly examine the PAC’s connectivity with distributed brain regions. Previous AH rsfMRI studies have used varying methods and asked different questions about AH-related connectivity.Gavrilescu et al. (2009) found that AH patients had reduced interhemispheric connectivity in primary and secondary auditory cortices.Vercammen et al. (2010), using a left temporoparietal seed, found reduced FC with right Broca’s homologue, and with ACC and amygdala in association with AH severity. Rotarska-Jagiela et al. (2010) examined five resting-state networks (RSN) using independent component analysis (ICA) and multiple regression, and found an inverse correlation between AH and left HIP connectivity in the default mode network (DMN).Wolf et al. (2011), looking at four RSN’s using ICA, found that within a speech-related network AH patients had reduced FC of the cingulate cortex and stronger FC of bilateral temporal regions.Hoffman et al. (2011), using a bilateral Wernicke’s seed, found increased FC along a corticostriatal loop consisting of Wernicke’s, left IFG, and putamen in AH patients. Notably, three of the studies did not include a NAH control group (Rotarska-Jagiela et al., 2010; Vercammen et al., 2010; Wolf et al., 2011), making it difficult to interpret whether findings are related to AH or SZ.
In the present study, we found that LHG recruitment of left IFG (Broca’s area) increases with greater AH severity. We also found abnormal FC between LHG and various task-positive frontoparietal regions, including left SPL, left MFG (premotor cortex), right precentral (motor cortex) and right postcentral (somatosensory cortex) gyri. These regions, with Broca’s area, comprise part of a neural system involved in the motor expression of language, especially relating to the assembly of phonemes into words and sentences (Mesulam, 2000) and to auditory monitoring and feedback of speech (Price, 2010). In healthy individuals, thinking in words involves the generation and silent articulation of inner speech, and is associated with activity in Broca’s area (McGuire et al., 1996). Broca’s area has been implicated in AH activity studies (Dierks et al., 1999; McGuire et al., 1993; Shergill et al., 2000) and survived in a meta-analysis of AH activity (Jardri et al., 2010), lending support to the idea that AH may be related to inner speech (Frith and Done, 1988; Jones and Fernyhough, 2007a, b; Seal et al., 2004). While some studies have found more evidence for the involvement of right Broca’s homologue in AH [e.g., (Sommer et al., 2008; Vercammen et al., 2010)], we found only left IFG to be abnormally connected with LHG.
The reduced coupling we observed between LHG and HIP/PHG suggests abnormalities in encoding or retrieval of episodic memories in relation to auditory processing. The HIP and/or PHG were activated in several AH symptom capture studies (Copolov et al., 2003; Dierks et al., 1999; Shergill et al., 2000; Silbersweig et al., 1995). Two recent studies showed that deactivation of the PHG consistently preceded AH (Diederen et al., 2010; Hoffman et al., 2008). AH may arise from an inability to inhibit auditory mental representations from intruding into consciousness and an inability to bind contextual cues to form complete representations of source memory (Waters et al., 2006), consistent with reduced connectivity between HIP/PHG and LHG.
AH patients showed reduced LHG FC with MD thalamus compared to both NAH and HC. The MD thalamus is a higher-order thalamic nucleus that is reciprocally connected to the prefrontal cortex (dorsolateral, OFC, medial frontal/ACC). This region shows a range of anatomical, chemical, metabolic, and functional abnormalities in SZ (Hazlett et al., 1999; Hazlett et al., 2004; Jakary et al., 2005; Pakkenberg, 1990), and the MD thalamus may play a critical role in SZ pathophysiology (Andreasen et al., 1999; Cohen and Yurgelun-Todd, 2001). Previous studies found increased activations in the thalamus during AH (Shergill et al., 2000; Silbersweig et al., 1995) and correlations between hallucinations and grey matter reductions in the thalamus (Neckelmann et al., 2006).
Given the reciprocal connections between MD-thalamus and prefrontal cortex, it is significant that we found AH patients have abnormal connectivity of LHG with OFC and cingulate cortex in association with greater AH severity. These areas are involved in error monitoring (Carter et al., 1998; Schoenbaum et al., 2009), and previous AH-related studies found increased activation in both OFC (Parellada et al., 2008; Silbersweig et al., 1995) and ACC (Allen et al., 2007; McGuire et al., 1993; Parellada et al., 2008; Shergill et al., 2000; Silbersweig et al., 1995; Suzuki et al., 1993; Szechtman et al., 1998). The ACC plays an important role in combined top-down and bottom-up models of AH (Allen et al., 2008). In the model by Hugdahl (2009), AH are bottom-up phenomena originating in the left temporal lobe but surfacing to attention because of a failure of top-down inhibition mediated by ACC and other prefrontal regions. Our findings provide evidence for abnormal interactions between left temporal lobe and higher-order modulatory centers, but the tighter LHG-cingulate coupling here contrasts with the concept of attenuated ACC functioning, and may be more consistent with the idea that heightened auditory expectancy increases susceptibility to AH (Hoffman, 2010; Hoffman et al., 2008). Northoff and Qin (Northoff and Qin, 2011) suggest that elevated resting-state activity in the PAC and abnormal interactions between PAC and DMN predispose to AH. While we did not specifically examine DMN, our data suggest that in AH the PAC has abnormal FC with PCC and HIP/PHIP, which are components of DMN.
Waters et al. (Waters et al., 2012) recently proposed an integrative cognitive model of AH involving interactions between signals arising from hyperactivation in auditory functional networks and several top-down mechanisms including (i) increased detection of ambiguous or salient signals due to deficits in signal detection mechanisms, (ii) faulty intentional inhibition leading to failure to suppress the acceptance of these signals as real and meaningful., (iii) the formation over time of hypervigilance, expectations, and bias, causing AH-prone patients to “tune in” more to aberrant auditory signals, and (iv) state and trait factors such as emotions, insight, and beliefs about oneself and the world that influence how these experiences are interpreted. Our study addresses select features of this theoretical model from a bottom-up perspective, focusing on the functional networks involving PAC. Our finding of increased connectivity between left PAC and brain areas involved in speech/language and memory is consistent with possible generation of auditory signals shaped by inner speech. Increased connectivity between left PAC and brain regions that are involved in monitoring, like cingulate cortex and OFC, may reflect elevated levels of perceptual expectation and vigilance, leading to increased biases in auditory perception. Finally, reduced connectivity between left PAC and right hippocampal formation may be consistent with a failure to access source memory in relation to auditory processing, which may lead to confusion about where a voice or phrase was originally heard.
This study has several limitations. First, we examined FC with only LHG and RHG seeds. However, PAC was not identified in all AH symptom capture studies [e.g.,(Hoffman et al., 2008; McGuire et al., 1993; Shergill et al., 2000; Silbersweig et al., 1995; Sommer et al., 2008)] and was not significant in a meta-analysis of cortical activations during AH in SZ (Jardri et al., 2010). The absence of PAC findings in these symptom capture studies does not diminish the potential role of PAC in AH pathophysiology, especially given the abundant evidence for PAC morphological and volumetric abnormalities [e.g., see meta-analysis by (Modinos et al., 2012)]. Nonetheless, future studies should directly compare FC using seeds in auditory association and language-related areas in addition to PAC.
Second, the seeds in this study were not functionally determined via AH symptom capture or an auditory or speech-sensitive task. However, there is a tight anatomical-functional relationship between PAC and HG (Da Costa et al., 2011), making mismatch between our anatomically selected HG seed and the PAC unlikely. Moreover, the seeds were appropriately positioned on individually registered brains, and the group maps resulting from our LHG seed are consistent with published resting-state auditory networks.
Third, the NAH group, a rarer and thus more challenging population to recruit, was small and we may be underpowered to detect biological differences. To address Type II error risk, we present uncorrected findings in the group-wise comparisons, and the findings from this categorical analysis (vs. the more robust findings from the PSYRATS covariate analysis) should be cautiously interpreted. The lack of more robust findings in the AH vs. NAH group-wise comparisons may also reflect the phenomenological heterogeneity of AH even within SZ (Jones, 2010), and future studies, employing larger sample sizes, should investigate the link between brain measures like functional connectivity and specific phenomenological features of AH.
Fourth, PSYRATS data were unavailable for some patients; this reduced our sample size for the PSYRATS covariate analysis. Fifth, PSYRATS relies on self-report. The boundary between hallucinations and delusions is not always clear (Strauss, 1969), and we found that distinguishing between AH and thought insertion could be particularly challenging in some patients. Furthermore, in our debriefing interview, we assessed for the presence of hallucinations, delusions, physical discomfort, racing or slowed thoughts, and depressed or elated mood in the scanner, but did not ask about certain other aspects of experience like inner speech, which would be relevant for the interpretation of our speech/language-related findings.
Lastly, we studied mostly chronic, medicated patients. However, the patient groups were wellmatched for illness duration, there was no statistically significant difference in CPZ equivalents between AH and NAH, and the rsfMRI results remained after controlling for CPZ equivalents.
In conclusion, we found evidence for abnormal FC between LHG and forebrain thalamocortical regions in AH-prone patients. Our data highlight the importance of interactions between left PAC and forebrain circuitry involved in speech/language, memory, and the monitoring of self-generated events.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to the McLean Hospital Brain Imaging Center staff, the patients who participated in this study, and Drs. Randy Buckner, PhD and Dara Manoach, PhD for helpful discussions.
Sources of funding: Shervert Frazier Research Institute to BMC. NIMH (K23MH079982 and R01MH094594) to DO. Unrestricted educational grants from the APA Program for Minority Research Training in Psychiatry (PMRTP) (NIH 5T32MH019126-19); Harvard Psychiatry Dupont-Warren Fellowship; and Harvard-MIT Health Sciences and Technology/Beth Israel Deaconess Medical Center (BIDMC) Clinical Investigator Training Program (CITP), in collaboration with Merck & Co. and Pfizer Inc. to AKS
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONTRIBUTORS
AKS, BMC, and DO designed the study. AKS and DO wrote the protocol. AKS performed the literature searches. AKS and JTB analyzed the data. AKS and DO wrote the first draft of the manuscript. All authors contributed to and approved the final draft of the manuscript.
CONFLICTS OF INTEREST
All four authors report no conflicts of interest relevant to this study. Research support came from the Shervert Frazier Research Institute to BMC and NIMH (K23MH079982 and R01MH094594) to DO. AKS was supported by unrestricted educational grants from the APA Program for Minority Research Training in Psychiatry (PMRTP) (NIH 5T32MH019126-19); Harvard Psychiatry Dupont-Warren Fellowship; and Harvard-MIT Health Sciences and Technology/Beth Israel Deaconess Medical Center (BIDMC) Clinical Investigator Training Program (CITP), in collaboration with Merck & Co. and Pfizer Inc. DO is a principal investigator in a research contract with Rules Based Medicine, Inc.
REFERENCES
- Allen P, Amaro E, Fu CH, Williams SC, Brammer MJ, Johns LC, McGuire PK. Neural correlates of the misattribution of speech in schizophrenia. Br J Psychiatry. 2007;190:162–169. doi: 10.1192/bjp.bp.106.025700. [DOI] [PubMed] [Google Scholar]
- Allen P, Laroi F, McGuire PK, Aleman A. The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neurosci Biobehav Rev. 2008;32(1):175–191. doi: 10.1016/j.neubiorev.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Nopoulos P, O'Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol Psychiatry. 1999;46(7):908–920. doi: 10.1016/s0006-3223(99)00152-3. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Cohen BM, Yurgelun-Todd D. Alterations of thalamic activity in schizophrenia and in response to antipsychotic drugs: studies in the legacy of Seymour S. Kety. Neuropsychopharmacology. 2001;25(3):305–312. doi: 10.1016/S0893-133X(01)00277-9. [DOI] [PubMed] [Google Scholar]
- Copolov DL, Seal ML, Maruff P, Ulusoy R, Wong MT, Tochon-Danguy HJ, Egan GF. Cortical activation associated with the experience of auditory hallucinations and perception of human speech in schizophrenia: a PET correlation study. Psychiatry Res. 2003;122(3):139–152. doi: 10.1016/s0925-4927(02)00121-x. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in "resting-state" data. AJNR Am J Neuroradiol. 2001;22(7):1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Da Costa S, van der Zwaag W, Marques JP, Frackowiak RS, Clarke S, Saenz M. Human primary auditory cortex follows the shape of Heschl's gyrus. J Neurosci. 2011;31(40):14067–14075. doi: 10.1523/JNEUROSCI.2000-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederen KM, Neggers SF, Daalman K, Blom JD, Goekoop R, Kahn RS, Sommer IE. Deactivation of the parahippocampal gyrus preceding auditory hallucinations in schizophrenia. Am J Psychiatry. 2010;167(4):427–435. doi: 10.1176/appi.ajp.2009.09040456. [DOI] [PubMed] [Google Scholar]
- Dierks T, Linden DE, Jandl M, Formisano E, Goebel R, Lanfermann H, Singer W. Activation of Heschl's gyrus during auditory hallucinations. Neuron. 1999;22(3):615–621. doi: 10.1016/s0896-6273(00)80715-1. [DOI] [PubMed] [Google Scholar]
- Falloon IR, Talbot RE. Persistent auditory hallucinations: coping mechanisms and implications for management. Psychol Med. 1981;11(2):329–339. doi: 10.1017/s0033291700052144. [DOI] [PubMed] [Google Scholar]
- Ford JM, Roach BJ, Jorgensen KW, Turner JA, Brown GG, Notestine R, Bischoff-Grethe A, Greve D, Wible C, Lauriello J, Belger A, Mueller BA, Calhoun V, Preda A, Keator D, O'Leary DS, Lim KO, Glover G, Potkin SG, Mathalon DH. Tuning in to the voices: a multisite FMRI study of auditory hallucinations. Schizophr Bull. 2009;35(1):58–66. doi: 10.1093/schbul/sbn140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Frith CD, Done DJ. Towards a neuropsychology of schizophrenia. Br J Psychiatry. 1988;153:437–443. doi: 10.1192/bjp.153.4.437. [DOI] [PubMed] [Google Scholar]
- Gavrilescu M, Rossell S, Stuart GW, Shea TL, Innes-Brown H, Henshall K, McKay C, Sergejew AA, Copolov D, Egan GF. Reduced connectivity of the auditory cortex in patients with auditory hallucinations: a resting state functional magnetic resonance imaging study. Psychol Med. 2009;40(7):1149–1158. doi: 10.1017/S0033291709991632. [DOI] [PubMed] [Google Scholar]
- Haddock G, McCarron J, Tarrier N, Faragher EB. Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS) Psychol Med. 1999;29(4):879–889. doi: 10.1017/s0033291799008661. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Byne W, Wei TC, Spiegel-Cohen J, Geneve C, Kinderlehrer R, Haznedar MM, Shihabuddin L, Siever LJ. Three-dimensional analysis with MRI and PET of the size, shape, and function of the thalamus in the schizophrenia spectrum. Am J Psychiatry. 1999;156(8):1190–1199. doi: 10.1176/ajp.156.8.1190. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Kemether E, Bloom R, Platholi J, Brickman AM, Shihabuddin L, Tang C, Byne W. Abnormal glucose metabolism in the mediodorsal nucleus of the thalamus in schizophrenia. Am J Psychiatry. 2004;161(2):305–314. doi: 10.1176/appi.ajp.161.2.305. [DOI] [PubMed] [Google Scholar]
- Hoffman RE. Revisiting Arieti's "listening attitude" and hallucinated voices. Schizophr Bull. 2010;36(3):440–442. doi: 10.1093/schbul/sbq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman RE, Anderson AW, Varanko M, Gore JC, Hampson M. Time course of regional brain activation associated with onset of auditory/verbal hallucinations. Br J Psychiatry. 2008;193(5):424–425. doi: 10.1192/bjp.bp.107.040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman RE, Fernandez T, Pittman B, Hampson M. Elevated functional connectivity along a corticostriatal loop and the mechanism of auditory/verbal hallucinations in patients with schizophrenia. Biol Psychiatry. 2011;69(5):407–414. doi: 10.1016/j.biopsych.2010.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakary A, Vinogradov S, Feiwell R, Deicken RF. N-acetylaspartate reductions in the mediodorsal and anterior thalamus in men with schizophrenia verified by tissue volume corrected proton MRSI. Schizophr Res. 2005;76(2–3):173–185. doi: 10.1016/j.schres.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Jardri R, Pouchet A, Pins D, Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am J Psychiatry. 2010;168(1):73–81. doi: 10.1176/appi.ajp.2010.09101522. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jones SR. Do we need multiple models of auditory verbal hallucinations? Examining the phenomenological fit of cognitive and neurological models. Schizophr Bull. 2010;36(3):566–575. doi: 10.1093/schbul/sbn129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Fernyhough C. Neural correlates of inner speech and auditory verbal hallucinations: a critical review and theoretical integration. Clin Psychol Rev. 2007a;27(2):140–154. doi: 10.1016/j.cpr.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Jones SR, Fernyhough C. Thought as action: inner speech, self-monitoring, and auditory verbal hallucinations. Conscious Cogn. 2007b;16(2):391–399. doi: 10.1016/j.concog.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Kompus K, Westerhausen R, Hugdahl K. The "paradoxical" engagement of the primary auditory cortex in patients with auditory verbal hallucinations: a meta-analysis of functional neuroimaging studies. Neuropsychologia. 2011;49(12):3361–3369. doi: 10.1016/j.neuropsychologia.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Carpenter WT, McMahon R. Correlations between rCBF and symptoms in two independent cohorts of drug-free patients with schizophrenia. Neuropsychopharmacology. 2006;31(1):221–230. doi: 10.1038/sj.npp.1300837. [DOI] [PubMed] [Google Scholar]
- Lennox BR, Park SB, Medley I, Morris PG, Jones PB. The functional anatomy of auditory hallucinations in schizophrenia. Psychiatry Res. 2000;100(1):13–20. doi: 10.1016/s0925-4927(00)00068-8. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Friston KJ, Frith CD, Jones T, Hirsch SR, Frackowiak RSJ. Patterns of cerebral blood flow in schizophrenia. British Journal of Psychiatry. 1992;160:179–186. doi: 10.1192/bjp.160.2.179. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Gyobu T, Hisada K, Ii M. SPECT imaging of auditory hallucination using 123IIMP. Adv Funct Neuroim. 1989;2(4):9–16. [Google Scholar]
- McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, Shenton ME. MRI anatomy of schizophrenia. Biological Psychiatry. 1999;45:1099–1119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire PK, Shah GM, Murray RM. Increased blood flow in Broca's area during auditory hallucinations in schizophrenia [see comments] Lancet. 1993;342(8873):703–706. doi: 10.1016/0140-6736(93)91707-s. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Silbersweig DA, Murray RM, David AS, Frackowiak RS, Frith CD. Functional anatomy of inner speech and auditory verbal imagery. Psychol Med. 1996;26(1):29–38. doi: 10.1017/s0033291700033699. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M. Principles of Behavioral and Cognitive Neurology. 2 ed. New York, NY: Oxford University Press; 2000. [Google Scholar]
- Modinos G, Costafreda SG, van Tol MJ, McGuire PK, Aleman A, Allen P. Neuroanatomy of auditory verbal hallucinations in schizophrenia: A quantitative meta-analysis of voxel-based morphometry studies. Cortex. 2012 doi: 10.1016/j.cortex.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Neckelmann G, Specht K, Lund A, Ersland L, Smievoll AI, Neckelmann D, Hugdahl K. Mr morphometry analysis of grey matter volume reduction in schizophrenia: association with hallucinations. Int J Neurosci. 2006;116(1):9–23. doi: 10.1080/00207450690962244. [DOI] [PubMed] [Google Scholar]
- Northoff G, Qin P. How can the brain's resting state activity generate hallucinations? A 'resting state hypothesis' of auditory verbal hallucinations. Schizophr Res. 2011;127(1–3):202–214. doi: 10.1016/j.schres.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Ongur D, Lundy M, Greenhouse I, Shinn AK, Menon V, Cohen BM, Renshaw PF. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res. 2010;183(1):59–68. doi: 10.1016/j.pscychresns.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkenberg B. Pronounced reduction of total neuron number in mediodorsal thalamic nucleus and nucleus accumbens in schizophrenics. Arch Gen Psychiatry. 1990;47(11):1023–1028. doi: 10.1001/archpsyc.1990.01810230039007. [DOI] [PubMed] [Google Scholar]
- Parellada E, Lomena F, Font M, Pareto D, Gutierrez F, Simo M, Fernandez-Egea E, Pavia J, Ros D, Bernardo M. Fluordeoxyglucose-PET study in first-episode schizophrenic patients during the hallucinatory state, after remission and during linguistic-auditory activation. Nucl Med Commun. 2008;29(10):894–900. doi: 10.1097/MNM.0b013e328302cd10. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Rademacher J, Morosan P, Schormann T, Schleicher A, Werner C, Freund HJ, Zilles K. Probabilistic mapping and volume measurement of human primary auditory cortex. Neuroimage. 2001;13(4):669–683. doi: 10.1006/nimg.2000.0714. [DOI] [PubMed] [Google Scholar]
- Rotarska-Jagiela A, van de Ven V, Oertel-Knochel V, Uhlhaas PJ, Vogeley K, Linden DE. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res. 2010;117(1):21–30. doi: 10.1016/j.schres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nat Rev Neurosci. 2009;10(12):885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal ML, Aleman A, McGuire PK. Compelling imagery, unanticipated speech and deceptive memory: neurocognitive models of auditory verbal hallucinations in schizophrenia. Cogn Neuropsychiatry. 2004;9(1–2):43–72. doi: 10.1080/13546800344000156. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Brammer MJ, Williams SC, Murray RM, McGuire PK. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry. 2000;57(11):1033–1038. doi: 10.1001/archpsyc.57.11.1033. [DOI] [PubMed] [Google Scholar]
- Silbersweig DA, Stern E, Frith C, Cahill C, Holmes A, Grootoonk S, Seaward J, McKenna P, Chua SE, Schnorr L, Jones T, Frackowiak RSJ. A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995;378(6553):176–179. doi: 10.1038/378176a0. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Diederen KM, Blom JD, Willems A, Kushan L, Slotema K, Boks MP, Daalman K, Hoek HW, Neggers SF, Kahn RS. Auditory verbal hallucinations predominantly activate the right inferior frontal area. Brain. 2008;131(Pt 12):3169–3177. doi: 10.1093/brain/awn251. [DOI] [PubMed] [Google Scholar]
- Strauss JS. Hallucinations and delusions as points on continua function. Rating scale evidence. Arch Gen Psychiatry. 1969;21(5):581–586. doi: 10.1001/archpsyc.1969.01740230069010. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Yuasa S, Minabe Y, Murata M, Kurachi M. Left superior temporal blood flow increases in schizophrenic and schizophreniform patients with auditory hallucination: a longitudinal case study using 123I-IMP SPECT. European Archives of Psychiatry & Clinical Neuroscience. 1993;242(5):257–261. doi: 10.1007/BF02190383. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Bergen SE, Sun Z, Sampson AR, Pierri JN, Lewis DA. Pyramidal cell size reduction in schizophrenia: evidence for involvement of auditory feedforward circuits. Biol Psychiatry. 2004;55(12):1128–1137. doi: 10.1016/j.biopsych.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34(2):374–389. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szechtman H, Woody E, Bowers KS, Nahmias C. Where the imaginal appears real: a positron emission tomography study of auditory hallucinations. Proc Natl Acad Sci U S A. 1998;95(4):1956–1960. doi: 10.1073/pnas.95.4.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Ven VG, Formisano E, Roder CH, Prvulovic D, Bittner RA, Dietz MG, Hubl D, Dierks T, Federspiel A, Esposito F, Di Salle F, Jansma B, Goebel R, Linden DE. The spatiotemporal pattern of auditory cortical responses during verbal hallucinations. Neuroimage. 2005;27(3):644–655. doi: 10.1016/j.neuroimage.2005.04.041. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercammen A, Knegtering H, den Boer JA, Liemburg EJ, Aleman A. Auditory hallucinations in schizophrenia are associated with reduced functional connectivity of the temporoparietal area. Biol Psychiatry. 2010;67(10):912–918. doi: 10.1016/j.biopsych.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Waters F, Allen P, Aleman A, Fernyhough C, Woodward TS, Badcock JC, Barkus E, Johns L, Varese F, Menon M, Vercammen A, Laroi F. Auditory hallucinations in schizophrenia and nonschizophrenia populations: a review and integrated model of cognitive mechanisms. Schizophr Bull. 2012;38(4):683–693. doi: 10.1093/schbul/sbs045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters FA, Badcock JC, Michie PT, Maybery MT. Auditory hallucinations in schizophrenia: intrusive thoughts and forgotten memories. Cogn Neuropsychiatry. 2006;11(1):65–83. doi: 10.1080/13546800444000191. [DOI] [PubMed] [Google Scholar]
- Wolf ND, Sambataro F, Vasic N, Frasch K, Schmid M, Schonfeldt-Lecuona C, Thomann PA, Wolf RC. Dysconnectivity of multiple resting-state networks in patients with schizophrenia who have persistent auditory verbal hallucinations. J Psychiatry Neurosci. 2011;36(4) doi: 10.1503/jpn.110008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff PW, Wright IC, Bullmore ET, Brammer M, Howard RJ, Williams SC, Shapleske J, Rossell S, David AS, McGuire PK, Murray RM. Auditory hallucinations and the temporal cortical response to speech in schizophrenia: a functional magnetic resonance imaging study. Am J Psychiatry. 1997;154(12):1676–1682. doi: 10.1176/ajp.154.12.1676. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21(4):1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.