Abstract
Clinical evidence indicates that mutation/activation of EGF receptors (EGFRs) is mutually exclusive with the presence of K-RAS oncogenes in lung and colon tumors. We have validated these observations using genetically engineered mouse models. However, pancreatic ductal adenocarcinomas driven by K-Ras oncogenes are totally dependent on EGFR signaling. Similar results were obtained using human pancreatic tumor cell lines. EGFRs were also essential even in the context of pancreatic injury and absence of p16Ink4a/p19Arf. Only loss of p53 made pancreatic tumors independent of EGFR signaling. Additional inhibition of PI3K and STAT3 effectively prevented proliferation of explants derived from these p53–defective pancreatic tumors. These findings may provide the bases for more rational approaches to treat pancreatic tumors in the clinic.
INTRODUCTION
Patients with pancreatic ductal adenocarcinoma (PDAC) have an average survival of less than a year with fewer than 5% surviving more than five years (Vincent et al., 2011). Current standard of care for PDAC patients is Gemcitabine, a nucleoside analogue that only prolongs survival for few weeks (Burris et al., 1997; Li et al., 2004). Hence, there is an urgent medical need to find more effective therapeutic approaches to treat this deadly disease (Hidalgo, 2010).
PDAC is likely to stem from a process known as acinar to ductal metaplasia that involves either transdifferentiation of adult acinar cells or misdifferentiation of their progenitors into ductal-like cells. These cells can subsequently progress into malignant adenocarcinoma through a series of histopathological lesions known as pancreatic intraepithelial neoplasias (PanINs) (Maitra and Hruban, 2008). Early pancreatic lesions including low-grade PanINs already carry mutations in K-RAS oncogenes, along with loss or inactivation of the P16INK4a tumor suppressor (Kanda et al., 2012). High-grade lesions develop upon accumulation of further mutational events, mainly involving inactivation of other tumor suppressors such as TP53, SMAD4 or BRCA2 (Hong et al., 2011). Exome sequencing analysis of PDAC genomes has revealed an incredibly complex pattern of mutations affecting as many as twelve different signaling pathways (Jones et al., 2008). In a recent study describing the exomic sequence of different areas of a single PDAC tumor, Campbell et al., (2010) have illustrated the perverse molecular evolution of these tumors even before they spread to other organs.
In 2007, a clinical trial combining Gemcitabine with the EGFR inhibitor, Erlotinib, reported some responses in a limited number of PDAC patients (Moore et al., 2007). Yet, the overall results were not sufficiently significant for the FDA to recommend the combination of these two drugs as standard of care. These observations are intriguing since the EGFR is known to signal upstream of K-RAS and hence, its inhibition should have little or no effect on downstream K-RAS driven oncogenic signals (Yarden and Sliwkowski, 2001). Indeed, in non-small lung adenocarcinoma (NSCLC) mutations in EGFR and in K-RAS are mutually exclusive (Shigematsu et al., 2005). Likewise, a large clinical trial carried out in patients with advanced colorectal carcinomas (CRC) has determined that patients carrying tumors with K-RAS mutations do not benefit from treatment with Cetuximab, a monoclonal antibody that blocks EGFR signaling (Karepetis et al., 2008). In spite of these odds, we decided to interrogate by genetic means whether EGFRs might play a role in K-Ras oncogene-driven PDAC using a well-characterized genetically engineered mouse (GEM) model for this disease (Guerra et al., 2007; 2011).
RESULTS
Acinar to ductal metaplasia requires EGFR signaling even in the presence of K-Ras oncogenes
Pancreatic acinar to ductal metaplasia is a precursor of the preneoplastic PanIN lesions that eventually lead to PDAC development (Parsa et al., 1985). In normal mice, generation of acinar to ductal metaplasia is largely dependent on activation of EGFRs (Means et al., 2005). Since EGFRs signal through the Ras pathway, we examined whether expression of a constitutive active K-Ras oncoprotein could bypass the requirement for EGFR activity during the generation of metaplasia. Pancreatic cell explants obtained from K-Ras+/LSLG12Vgeo;Elas-tTA/tetO-Cre mice (from now on ElasK-RasG12V) in which the K-RasG12V oncogene is selectively expressed in acinar cells, efficiently transdifferentiated into metaplastic ductal-like cells leading to the generation of 5 to 10-fold more metaplastic structures than those not expressing the oncogene (Figure S1A available on line). Yet, K-RasG12V-driven metaplasia was still largely dependent on activation of EGFRs since addition of their cognate ligands EGF or TGFα, effectively increased the number of metaplastic figures (Figure S1B). Ablation of Egfr alleles significantly reduced, but did not eliminate the ability of acinar cell explants to generate metaplastic structures (Figure S1B-S1D). These observations suggest that EGF and TGFα may contribute to acinar to ductal metaplasia by activating additional receptors, at least in vitro. Pancreatic acinar cells also expressed high levels of amphiregulin, but not of other members of the EGFR family of ligands (Figure S1E).
Human and mouse pancreatic lesions express abundant EGFRs
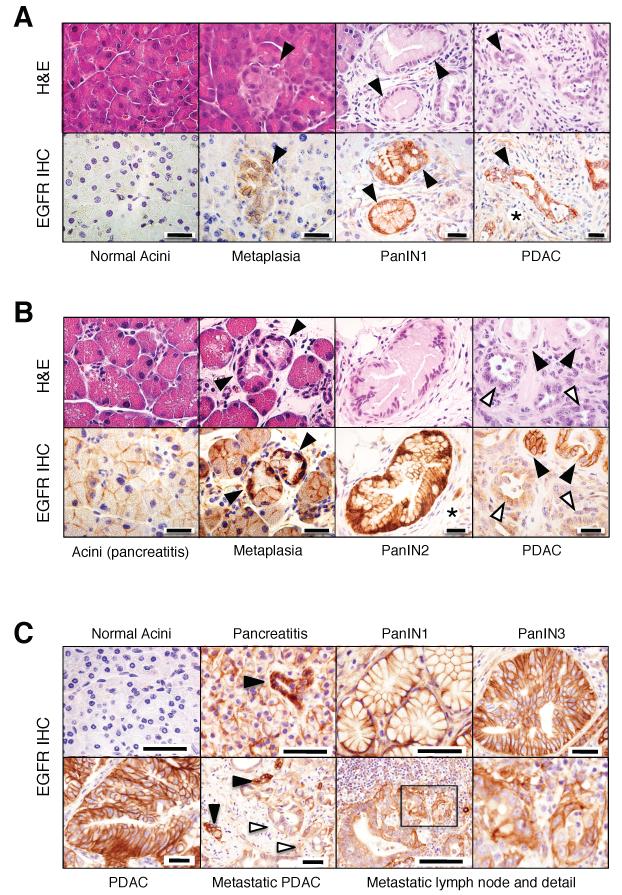
Mouse acinar cells did not express detectable levels of EGFRs regardless of whether they expressed a K-Ras oncogene or not (Figure 1A). In contrast, PanINs, regardless of their grade, were decorated with high levels of the receptor (Figure 1A) (Ueda et al., 2004; Hingorani et al., 2005). Elevated expression of EGFRs was maintained during tumor progression including well-differentiated glandular structures within PDAC tumors (Figure 1A). However, expression levels decreased in poorly differentiated tumor cells (Figure 1B) (Ueda et al., 2004; Hingorani et al., 2005). Human normal pancreata also displayed undetectable levels of EGFRs (Figure 1C). However, morphologically normal acinar cells of pancreatitis patients expressed significant levels of EGFRs in a manner highly reminiscent of the result obtained in pancreata derived from mice exposed to caerulein (Figure 1B and 1C).
Figure 1. Expression of EGFR in pancreas of ElasK-RasG12V mice and of patients with pancreatitis and PDAC.
(A) Serial paraffin sections obtained from ElasK-RasG12V mice not exposed to doxycycline depicting normal acini, acinar to ductal metaplasia, PanIN1 and PDAC were stained with hematoxylin and eosin (H&E) or with antibodies against the EGFR (EGFR IHC).
Lesions are indicated by solid arrowheads.
Asterisk indicates stroma cells positive for EGFR immunostaining.
Scale bars represent 20 μm.
(B) Serial paraffin sections obtained from ElasK-RasG12V mice exposed to doxycycline from conception to P60 and to caerulein from P90 to P180 depicting acini, acinar to ductal metaplasia, PanIN2 and PDAC were stained with H&E or with antibodies against the EGFR (EGFR IHC).
Lesions are indicated by solid arrowheads. Open arrowheads indicate less-differentiated glands within a PDAC.
Asterisk indicates stroma cells positive for EGFR expression.
Scale bars represent 20 μm.
(C) EGFR IHC of human pancreatic biopsies depicting normal pancreata, pancreata from patients with pancreatitis, PanIN lesions (PanIN1 and PanIN3), non metastatic PDAC and a metastatic lymph node with amplified detail.
Lesions are indicated by solid arrowheads. Open arrowheads indicate less-differentiated glands within the metastatic PDAC.
Scale bars represent 50 μm.
See also Figure S1
These observations are in agreement with an early study describing overexpression of EGFRs in patients with chronic pancreatitis (Korc et al., 1994). We also observed that metaplasias present in pancreatitis biopsies displayed elevated levels of EGFRs (Figure 1C). Low-grade and high-grade PanINs present in human PDAC tumors also expressed high levels of EGFRs (Figure 1C). Interestingly, their pattern of expression in tumored areas closely resembled that observed in mouse PDACs (Figure 1B). Whereas well-differentiated tumor glands were uniformly decorated with EGFR antibodies, less-differentiated glands expressed significantly lower levels of the receptors (Figure 1C). Finally, metastatic cells localized in a regional lymph node retained detectable, albeit somewhat attenuated levels of EGFRs (Figure 1C). These observations indicate that induction of EGFRs in acinar cells of injured pancreata as well as in PanIN and PDAC lesions is a common event in mouse and human pancreatic tissues.
EGFRs are essential for the generation of K-Ras oncogene-driven PanIN lesions
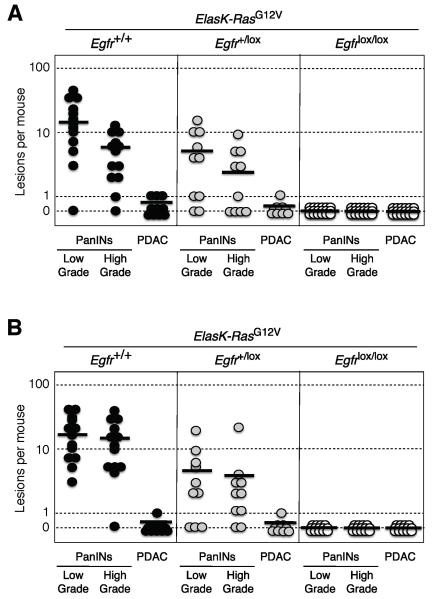
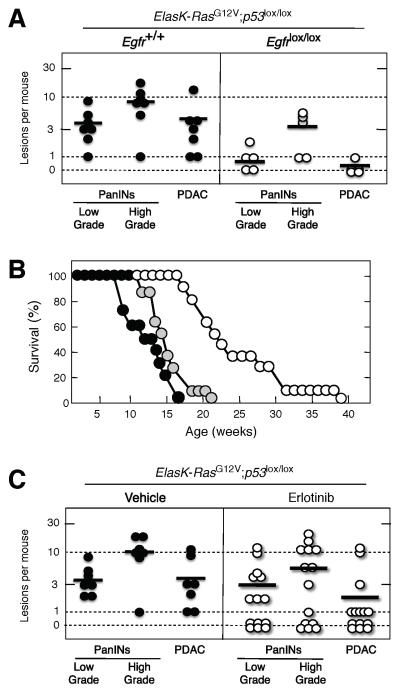
To determine whether development of PanIN lesions and PDAC tumors require EGFR signaling, we generated ElasK-RasG12V;Egfr+/+ and ElasK-RasG12V; Egfrlox/lox strains and analyzed their pancreata at one year of age. These mice were not exposed to doxycycline to allow expression of the Elastase-driven Cre recombinase during late embryonic development (E16.5). Cre-mediated recombination allowed concomitant expression of the resident K-RasG12V oncogene and ablation of the floxed Egfr alleles in acinar cells (Figure S2A). As illustrated in Figure 2A, control ElasK-RasG12V;Egfr+/+ littermates (12 out of 13 animals, 92%) exhibited abundant low- and high-grade PanIN lesions (average of 16 and 5 lesions per pancreata, respectively). Moreover, three animals (23%) displayed sizable PDAC tumors. Animals heterozygous for the Egfr locus also harbored low- and high-grade PanIN lesions albeit at reduced numbers (average of 5 and 2.5 lesions per pancreata, respectively). Likewise, only one out of ten heterozygous mice carried a PDAC tumor (Figure 2A).
Figure 2. Induction of PanINs and PDAC tumors by an endogenous K-RasG12V oncogene requires expression of the EGFR.
(A) Number of low- and high-grade PanINs and PDACs per mouse in untreated, one year old ElasK-RasG12V mice carrying the indicated Egfr alleles. ElasK-RasG12V;Egfr+/+ (solid circles), ElasK-RasG12V;Egfr+/lox (grey circles) and ElasK-RasG12V;Egfrlox/lox (open circles) mice. In these mice, Cre recombinase-mediated expression of the endogenous K-RasG12V oncogene and ablation of the conditional Egfrlox alleles took place in a percentage (30%) of acinar cells during late embryonic development.
(B) Number of low- and high-grade PanINs and PDACs per mouse in 14 month old ElasK-RasG12V mice carrying the indicated Egfr alleles. ElasK-RasG12V;Egfr+/+ (solid circles), ElasK-RasG12V;Egfr+/lox (grey circles) and ElasK-RasG12V;Egfrlox/lox (open circles) mice. These mice were exposed to doxycycline from conception to P60, a time at which Cre recombinase-mediated expression led to the concomitant activation of the resident K-RasG12V oncogene and ablation of the conditional Egfrlox alleles in acinar cells. Mice were subsequently treated with caerulein from P90 to P180.
Horizontal bars indicate the average number of lesions per mouse for each genotype. See also Figure S2
In contrast, careful analysis of serial sections of pancreata from one year old ElasK-RasG12V; Egfrlox/lox animals (n=24) only revealed the presence of a limited number of PanIN lesions (10 low-grade and 2 high-grade PanINs) in eight mice. More importantly, all of these lesions expressed EGFRs due to incomplete recombination of the floxed Egfr alleles (Figure S2B and 2C). Similar results were obtained in older mice sacrificed at 2 years of age (data not shown). These observations indicate that EGFRs are essential for the induction of PanINs and PDAC by K-Ras oncogenes.
Adult mice also require EGFR signaling for PDAC development
To exclude the possibility that these observations were due to developmental defects in acinar cells lacking EGFRs during embryonic development, we exposed ElasK-RasG12V;Egfr+/+ and ElasK-RasG12V;Egfrlox/lox littermates to doxycycline from conception until adulthood (8 weeks of age) to prevent expression of the Cre recombinase. As previously reported, induction of PanIN lesions in these mice requires a pancreatic insult (Guerra et al., 2007). Analysis of 14 month old ElasK-RasG12V;Egfrlox/lox mice (n=14) treated with caerulein for three months (P90-P180), that is, one year after turning on expression of the resident K-RasG12V oncogene, revealed complete absence of EGFR positive low- and high-grade PanIN lesions or PDAC tumors (Figure 2B). Only three mice carried PanIN lesions, all of which expressed EGFRs (data not shown). Mice examined at 2 years of age displayed a total of 9 low-grade and 3 high-grade PanIN lesions in three out of the seven mice analyzed, all of which retained EGFR expression (data not shown).
As summarized in Figure 2B, control ElasK-RasG12V;Egfr+/+ littermates exhibited the expected number of lesions (Guerra et al., 2011). All mice (n=13) developed low-grade PanINs (average of 18 lesions per pancreata) and more than 90% (12 out of 13) displayed high-grade PanINs (average of 16 lesions per pancreata). Only one mouse out of thirteen (8%) had a full-blown PDAC tumor (Figure 2B). Ablation of a single Egfr allele yielded similar results regarding the number of mice affected (80% of the animals carried PanIN lesions and 10% a PDAC tumor). However, the average number of lesions per pancreata was significantly lower (Figure 2B). These observations strongly support the concept that initiation of PDAC tumors requires at least two independent signaling inputs mediated by the EGFR and the K-Ras oncoprotein.
EGFRs cooperate with resident K-Ras oncogenes by activating AKT and STAT3 signaling pathways
In an attempt to shed light on the mechanism by which the EGFR cooperate with the resident K-RasG12V oncoprotein to induce pancreatic lesions, we examined the status of AKT, a well-known downstream effector of the PI3K/AKT survival pathway and STAT3, a mediator of inflammatory cytokines that has been recently implicated in PDAC development (Corcoran et al., 2011; Fukuda et al., 2011; Lesina et al., 2011). As illustrated in Figure S2D, pancreata of untreated ElasK-RasG12V mice display acinar cells that do not express either EGFR or detectable levels of phosphorylated AKT or STAT3. Thus indicating that expression of the resident K-RasG12V oncoprotein does not activate these pathways, at least in this cellular context. However, pancreata of ElasK-RasG12V mice treated with caerulein for three months exhibit robust expression of EGFRs along with nuclear phospho-AKT and phospho-STAT3 proteins, indicating that activation of these effector molecules is directly mediated by the induced EGFRs. Further support to this concept was provided by the uniform expression of EGFRs along with phospho-AKT and phospho-STAT3 proteins throughout the entire pancreata, since induction of K-RasG12V expression in these mice only takes place in about 30% of their acinar cells (Guerra et al., 2007, 2011). As expected, pancreatic lesions including metaplasias and PanINs, also display activated phospho-AKT and phophop-STAT3 molecules in response to EGFR expression, suggesting that activation of the PI3K/AKT and STAT3 signaling pathways play a role in the induction of these lesions (Figure S2E). Finally, the presence of nuclear phospho-AKT and phospho-STAT3 in PDAC tumors also suggests that activation of these effector molecules might be required for tumor progression (data not shown).
Human pancreatic ductal tumor cell lines are dependent on EGFR signaling regardless of the presence of K-RAS oncogenes
Next, we interrogated whether cell lines derived from human PDACs also depend on EGFR signaling for proliferation. We selected eight well-characterized tumor cell lines with different pattern of mutations. Six of them, AsPc1, CFPAC, IMIMPC-2, MIAPaCa, PANC1 and SKPC, harbor K-RAS oncogenes along with inactivation of P16INK4a and TP53 tumors suppressor genes (Table 1). CFPAC and SKPC cells also have a deleted SMAD4 locus. The remaining pancreatic tumor cell lines BxPc3 and T3M4, carry a wild type K-RAS locus. Yet, they also have mutated or silenced P16INK4a and TP53 loci and one of them, BxPc3, a mutated SMAD4 locus (Table 1). Knockdown of EGFRs using two independent shRNAs efficiently inhibited proliferation (>70%) of AsPc1, BxPc3, MIAPaCa and T3M4 cells. Two cell lines, PANC1 and IMIMPC-2 were only partially inhibited whereas the remaining cell lines, CFPAC and SKPC, were resistant (Table 1). Thus, the effect of EGFR signaling on proliferation appears to be independent of the presence of K-RAS oncogenes. Knockdown of EGFR expression only inhibited the PI3K pathway, as determined by phosphorylation of AKT, in those cells carrying a wild type K-RAS locus (Figure S3).
Table 1.
Human pancreatic ductal adenocarcinoma cell lines are sensitive to inhibition of EGFR and MEK signaling
| Mutation |
EGFR knockdown |
Erlotinib treatment |
PD325901 treatment |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Tumor | K-RAS | P16INK4a | TP53 | SMAD4 | % inhibition | IC50 | IC90 | IC50 | IC90 |
| AsPc1 | G12D | Frameshift | Frameshift | WT | 72.3% | >200.0 μM | >200 μM | 190.0 μM | >200 μM |
| CFPAC | G12V | Methylated | Mutation | Deletion | 5.2% | 20.0 μM | >200 μM | 22.5 μM | >200 μM |
| IMIMPC-2 | G12D | Deletion | Mutation | WT | 32.5% | >200.0 μM | >200 μM | 0.8 μM | >200 μM |
| MIAPaCa | G12C | Deletion | Mutation | WT | 97.0% | 66.8 μM | >200 μM | 8.5 μM | >200 μM |
| PANC1 | G12D | Deletion | Mutation | WT | 59.9% | >200.0 μM | >200 μM | >200.0 μM | >200 μM |
| SKPC | G12V | Methylated | Mutation | Deletion | 08.7% | 70.0 μM | >200 μM | 0.5 μM | >200 μM |
| BxPc3 | WT | Mutation | Mutation | Mutated | 81.0% | 23.5 μM | >200 μM | 0.3 μM | >200 μM |
| T3M4 | WT | Methylated | Mutation | WT | 90.1% | 12.2 μM | >200 μM | 0.1 μM | >200 μM |
WT, wild type
See also Figure S3
Five of these tumor cell lines, including K-RAS oncogene-positive CFPAC, MiaPaCa and SKPC cells and K-RAS oncogene-negative BxPc3 and T3M4 cells were partially sensitive to Erlotinib (Table 1). Erlotinib treatment did not result in complete inhibition of cell proliferation (IC90) even at concentrations as high as 200 μM. The SKPC cell line, whereas partially sensitive to Erlotinib, was refractory to EGFR knockdown (Table 1). This discrepancy might be explained by either the inability of the shRNAs to effectively knockdown the high levels of EGFRs present in this cell line or to the off-target effect of Erlotinib on related tyrosine protein kinase receptors (Figure S3). We also examined the effect of directly inhibiting the RAS pathway by using the MEK inhibitor, PD325901. Four cell lines carrying K-RAS oncogenes, CFPAC, IMIMPC-2, MiaPaCa and SKPC cells were sensitive to this inhibitor. Interestingly, the BxPc3 and T3M4 cell lines that have a wild type K-RAS locus, were also highly sensitive to the MEK inhibitor, suggesting that these cells may have activated their RAS pathway by mechanisms other than mutating their K-RAS locus (Table 1). Finally, AsPc1 and PANC1 cells were resistant to MEK inhibition in spite of carrying K-RAS oncogenes, suggesting that in these cells K-RAS oncogenes may no longer play an essential role in maintaining their proliferative properties (Table 1).
K-RASG12V-driven lung and intestinal tumors do not require EGFR signaling
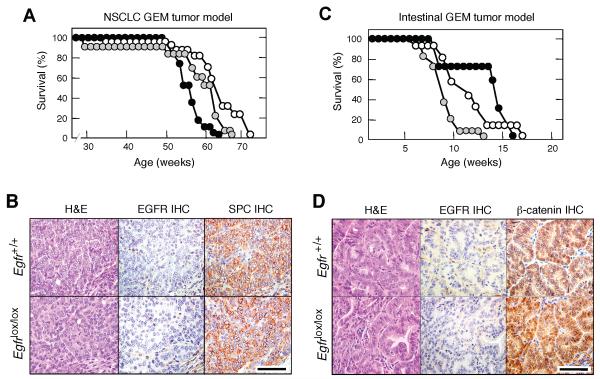
The above results, taken together, indicate that proliferation of pancreatic ductal tumor cells have a dual requirement for EGFR and K-RAS signaling. These observations are at odds with extensive clinical data in human NSCLCs, in which oncogenic mutations in the EGFR and K-RAS loci are mutually exclusive (Shigematsu et al., 2005). Likewise, CRC patients carrying K-RAS oncogenes do not benefit from treatments involving inhibition of EGFR signaling (Karepetis et al., 2008). To determine whether the results described above only occur in the context of mouse tumor models or are an intrinsic property of pancreatic tumors, we ablated the Egfr locus in two well-characterized GEM models of lung and intestinal tumors induced by the same endogenous K-RasG12V oncogene used to initiate pancreatic lesions. In these models, expression of the resident K-RasG12V oncogene is mediated by activation of a ubiquitously expressed Cre-ERT2 inducible recombinase knocked-in at the locus encoding the large subunit of RNA polymerase II (RERT strain, see Guerra et al., 2003). For the lung model, RERT;K-RasG12V;Egfr+/+ (n=17), RERT;K-RasG12V;Egfr+/lox (n=13) and RERT;K-RasG12V;Egfrlox/lox (n=25) littermates were treated at weaning with a single dose of 4-hydroxy-tamoxifen (4OHT) (Guerra et al., 2003; Puyol et al., 2010). As illustrated in Figure 3A, all mice died of lung tumors between 63 and 72 weeks of age. Mice displayed similar number of adenomas (15 per mouse) and adenocarcinomas (3 per mouse) regardless of genotype. None of the tumors analyzed expressed EGFRs by IHC analysis (Figure 3B). Moreover, PCR analysis of tumor DNA only revealed recombined Egfr null alleles (data not shown), thus indicating that tumor development had occurred in the absence of EGFRs.
Figure 3. Ablation of EGFRs has no effect on K-RasG12V driven lung and intestinal tumors.
(A) Survival of RERT;K-RasG12V;Egfr+/+ (solid circles), RERT;K-RasG12V;Egfr+/lox (grey circles) and RERT;K-RasG12V;Egfrlox/lox (open circles) mice treated with a single injection of 4OHT at P21 to induce NSCLCs.
(B) H&E staining and EGFR and pro-surfactant protein C (SPC) immunostaining (IHC) of consecutive paraffin sections showing representative adenocarcinoma lesions from nine month old RERTK-RasG12V mice carrying either (top) wild type Egfr or (bottom) conditional Egfr alleles.
Scale bar represents 50 μm.
(C) Survival of RERT;K-RasG12V;Apclox/lox;Egfr+/+ (solid circles), RERT;K-RasG12V;Apclox/lox;Egfr+/lox (grey circles) and RERT;K-RasG12V;Apclox/lox;Egfrlox/lox (open circles) mice treated with 4OHT (three days per week, for two weeks) at P21 to induce intestinal tumors.
(D) H&E staining and EGFR and β-catenin immunostaininig (IHC) of consecutive paraffin sections showing representative intestinal tumor lesions from two month old RERT;K-RasG12V;Apclox/lox mice carrying either (top) wild type Egfr or (bottom) conditional Egfr alleles.
Scale bar represents 50 μm.
Similar results were obtained in a GEM model of intestinal tumors. RERT;K-RasG12V;Apclox/lox;Egfrlox/lox mice (n=16) along with control RERT;K-RasG12V;Apclox/lox;Egfr+/lox (n=17) and RERT;K-RasG12V;Apclox/lox;Egfr+/+ (n=7) littermates were treated at weaning for two weeks (3 days per week) with 4OHT. These mice displayed similar tumor burden including adenomas and adenocarcinomas (data not shown) and did not survive beyond 20 weeks of age (Figure 3C). As expected, tumor cells, regardless of genotype, failed to express EGFRs (Figure 3D). These results, taken together, indicate that the requirement of EGFR signaling for the onset of neoplastic pancreatic lesions driven by K-Ras oncogenes is unique to this tumor type. Moreover, the similarity between the results obtained in clinical trials and in mouse models of lung and intestinal cancer reinforces the concept that GEM tumor models faithfully reproduce those events observed in cancer patients.
Loss of senescence does not override the need for EGFR signaling during PanIN and PDAC development
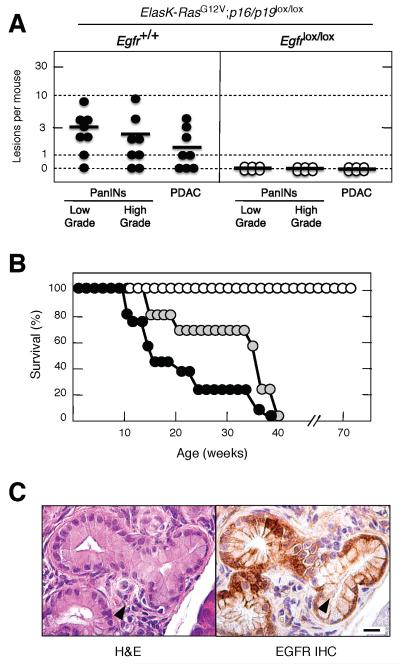
The EGFR is known to promote survival signals that might be essential to overcome the oncogene-induced senescence characteristic of the early stages of pancreatic tumor development (Collado et al., 2005; Guerra et al., 2011). Indeed, most human PDACs carry a mutated or silenced P16INK4a/P14ARF locus, an event likely to override senescence (Hong et al., 2011). Thus, we reasoned that ablation of the p16INK4A/p19ARF tumor suppressors (from now on p16/p19), might bypass the requirement for EGFR signaling during tumor initiation. Conditional floxed p16/p19 alleles were introduced into ElasK-RasG12V mice carrying wild type or floxed Egfr alleles and their pancreata examined at 16 weeks of age, before they displayed any obvious signs of overt tumor development. These mice were not exposed to doxycycline to allow expression of the resident K-RasG12V oncogene during late embryonic development (Guerra et al., 2007). As summarized in Figure 4A, six out of eight mice carrying wild type EGFRs displayed abundant low- and high-grade lesions. Moreover, five animals had developed at least a PDAC tumor at this time. In contrast, none of the mice carrying Egfrlox/lox alleles (n=6) displayed PanIN lesions or PDAC (Figure 4A).
Figure 4. Loss of p16/p19 tumor suppressors does not abrogate the need for EGFR expression during PanIN and PDAC development.
(A) Number of low- and high-grade PanINs and PDAC lesions per mouse in untreated, sixteen week old ElasK-RasG12V;p16/p19lox/lox mice carrying either wild type (solid circles) or conditional (open circles) Egfr alleles. In these mice, expression of a Cre recombinase in pancreatic acinar cells during late embryonic development results in the concomitant expression of the endogenous K-RasG12V oncogene and in the ablation of the conditional p16/p19 and Egfr alleles. PanIN lesions positive for EGFR expression in ElasK-RasG12V;p16/p19lox/lox;Egfrlox/lox mice (see below) were not scored.
Horizontal bars indicate the average number of lesions per mouse.
(B) Survival of untreated ElasK-RasG12V;p16/p19lox/lox; Egfr+/+ (solid circles), ElasK-RasG12V;p16/p19lox/lox;Egfr+/lox (grey circles) and ElasK-RasG12V;p16/p19lox/lox;Egfrlox/lox (open circles) mice.
(C) H&E (left) and EGFR IHC (right) of consecutive paraffin sections showing an occasional PanIN lesion observed in ElasK-RasG12V;p16/p19lox/lox;Egfrlox/lox animals.
Scale bar represents 50 μm.
Mice with the above genotypes were allowed to age. Littermates carrying wild type Egfr alleles, either in homozygocity (n=12) or heterozygocity (n=30) succumbed to the disease before they reached 10 months of age (Figure 4B). Postmortem analysis revealed multiple lesions including invasive and metastatic PDAC as well as anaplastic carcinomas that metastasized to multiple organs (Aguirre et al., 2003; Guerra et al., 2011). As expected, none of the low-grade PanIN lesions contained senescent cells as determined by staining for β-galactosidase activity (data not shown). In contrast, ElasK-RasG12V;p16/p19lox/lox;Egfrlox/lox mice (n=7) sacrificed at one year of age did not carry any PanIN lesion positive for EGFRs in spite of careful analysis of multiple serial sections (data not shown). Only four animals had low-grade (n=6) and high-grade (n=2) PanIN “escaper” lesions positive for EGFRs (Figure 4C). Seventeen additional ElasK-RasG12V;p16/p19lox/lox;Egfrlox/lox mice were allowed to age beyond one year. Thus far, all of them remain in good health condition at 70 weeks of age (Figure 4B). These observations indicate that abrogation of senescence by inactivation of the p16/p19 tumor suppressors does not relieve pancreatic tumor cells of their need for EGFR signaling.
Loss of p16/p19 tumor suppressors also accelerates tumor development in adult mice providing they have undergone chronic or temporary pancreatitis (Guerra et al., 2011). Analysis of pancreata of ElasK-RasG12V;p16/p19lox/lox;Egfrlox/lox mice (n=7) 12 months after turning on K-RasG12V expression (8 months after completing caerulein treatment), also failed to reveal EGFR-negative PanIN lesions or PDAC tumors. Control ElasK-RasG12V;p16/p19lox/lox;Egfr+/+ littermates (n=10) died at the expected age (40 weeks of median survival) and displayed multiple PanIN lesions as well as PDACs, in some cases with perineural invasion, invasion of the intestinal wall and lymph node metastasis, as previously described (Guerra et al., 2011). These observations indicate that the requirement for EGFR signaling during PanIN and PDAC development cannot be compensated by loss of the p16/p19 tumor suppressors even in the context of an inflammatory response induced by pancreatitis.
Loss of p53 triggers oncogenic pathways independent of EGFR signaling
Human PDAC tumors harbor mutations in classical tumor suppressor genes such as TP53, SMAD4 or BRCA2 (Hong et al., 2011). Likewise, mice expressing an endogenous K-Ras oncogene during embryonic development in the absence of a functional p53 protein develop aggressive PanINs and PDAC tumors that result in the death of the animals within their first four to five months of life (Hingorani et al., 2005; Guerra et al., 2007). Similar results have been obtained in the context of adult K-Ras oncogene expression followed by pancreatic damage (Guerra et al., 2011). To examine the effect of abrogating EGFR signaling in the absence of p53, we inserted conditional p53lox alleles in the ElasK-RasG12V strain in the presence of wild type or floxed Egfr alleles. Animals were sacrificed at 10 weeks of age before they showed signs of overt tumor development. As summarized in Figure 5A, control mice carrying wild type Egfr alleles (n=7) displayed abundant low- and high-grade lesions and PDAC, averaging 10 high grade PanINs and 4 PDAC tumors per mouse. Interestingly, mice carrying conditional Egfr alleles (n=5) also displayed neoplastic lesions but with reduced incidence (Figure 5A).
Figure 5. Loss of EGFRs delays but does not prevent PanIN and PDAC development in the absence of p53.
(A) Number of low- and high-grade PanINs and PDACs per mouse in untreated, ten week old ElasK-RasG12V;p53lox/lox mice carrying either wild type (solid circles) or conditional (open circles) Egfr alleles. In these mice, expression of a Cre recombinase in pancreatic acinar cells during late embryonic development results in the concomitant expression of the endogenous K-RasG12V oncogene and in the ablation of the conditional p53 and Egfr alleles.
Horizontal bars indicate the average number of lesions per mouse.
(B) Survival of untreated ElasK-RasG12V;p53lox/lox;Egfr+/+ (solid circles), ElasK-RasG12V;p53lox/lox;Egfr+/lox (grey circles) and ElasK-RasG12V;p53lox/lox;Egfrlox/lox (open circles) mice.
(C) Inhibition of EGFR signaling by Erlotinib treatment reduces the number of PanIN and PDAC lesions. Number of low- and high-grade PanINs and PDACs per mouse in six week old ElasK-RasG12V;p53lox/lox mice treated for four weeks with vehicle (solid circles) or Erlotinib (open circles).
Horizontal bars indicate the average number of lesions per mouse. The decrease in the number of PDAC tumors in the Erlotinib treated cohort was statistically significant (p<0.05).
See also Figure S4
When we allowed these mice to age, all animals carrying wild type Egfr alleles (n=10) succumbed to pancreatic tumors around 20 weeks of age with a median survival of 12 weeks (Figure 5B). Similar results were obtained with heterozygous mice (n=13) (Figure 5B). At the time of death or humane end point, these mice displayed multiple PanIN lesions and PDAC tumors including a lung metastasis in one of the animals. ElasK-RasG12V;p53lox/lox;Egfrlox/lox mice (n=13) also developed low- and high-grade PanINs as well as PDAC tumors (Figure 5B). Moreover, three of these mice also had macroscopic metastasis at different locations such as peritoneum, diaphragm, liver and lung (data not shown). However, mice in which the conditional Egfr alleles have been ablated died by 40 weeks of age and displayed a median survival 83% longer than that of littermates expressing EGFRs (22 vs. 12 weeks) (Figure 5B). Similar results were obtained with mice that expressed the resident K-RasG12V oncogene during adulthood and were treated for three months with caerulein. Whereas ElasK-RasG12V;p53lox/lox;Egfrlox/lox animals (n=26) died before reaching 60 weeks of age with a median survival of 38 weeks, control ElasK-RasG12V;p53lox/lox;Egfr+/lox mice (n=8) were dead at 35 weeks of age with a median survival of 27 weeks (data not shown). Thus, ablation of EGFR signaling resulted in an increased survival time of 40%.
Tumor development in mice carrying conditional Egfr alleles was not due to incomplete recombination since the large majority of the PanIN lesions and PDAC tumor did not express EGFRs when analyzed by IHC (Figure S4A). Moreover, PCR analysis of DNA isolated from tumor cells also failed to reveal unrecombined Egfrlox alleles in most lesions (data not shown). Finally, histopathological analysis of PanIN and PDAC tumors lacking p53 and EGFRs did not reveal significant differences with those present in control animals (Figure S4A). These observations, taken together, indicate that loss of p53 activates oncogenic pathways that bypass the requirement of EGFR signaling for tumor development.
Inhibition of EGFR signaling with Erlotinib interferes with PDAC development in vivo
Previous studies have shown that Gefitinib can slow down progression of pancreatic precursor lesions to PDAC (Mohammed, et al., 2010). To determine whether EGFRs are required for the progression of PanIN lesions in a more aggressive GEM model, six week old ElasK-RasG12V;p53lox/lox mice were treated with either vehicle or Erlotinib (100 mg/Kg) for four weeks. A small group of mice (n=3) analyzed at the start of the treatment revealed low- and high-grade PanINs in each of the animals. Moreover, two of the mice already carried small PDACs (Figure S4B). At the end of the four-week treatment, all mice that received vehicle (n=7) displayed a significant increase in the number of lesions (Figure 5C). In contrast, Erlotinib treatment led to disappearance of all lesions in 3 out of the 14 animals included in this cohort. Moreover, three additional Erlotinib-treated mice contained PanIN lesions but no PDAC tumors (Figure 5C). Overall, the Erlotinib-treated cohort had fewer lesions than the control group, indicating a limited but reproducible therapeutic effect of this EGFR inhibitor on PDAC development (Figure 5C).
IHC analysis of lesions in mice treated with Erlotinib for 4 weeks revealed robust inhibition of pAKT but not of pSTAT3 or pERK when compared with samples obtained from control mice treated with vehicle (Figure S4C). As an additional control, we examined the status of pAKT, pSTAT3 and pERK in ElasK-RasG12V;p53lox/lox mice carrying either wild type or conditional Egfr alleles. As expected, ablation of EGFR expression resulted in inhibition of pAKT but not of pSTAT3 or pERK (Figure S4D). Interestingly, full blown PDACs retained pAKT IHC (Fig. S4E), a result confirmed by Western blot analysis (Figure S5 and data not shown). These observations suggest that loss of p53 might activate the PI3K pathway by a mechanism independent of EGFR signaling.
EGFR signaling is required for proliferation of PDAC tumor cell explants
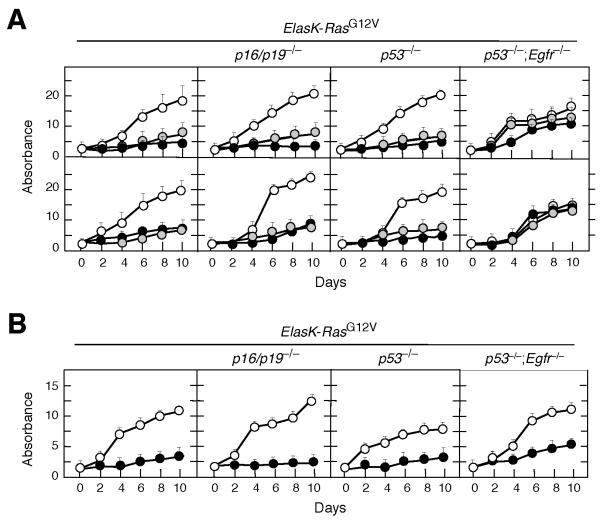
Knockdown of EGFR expression effectively slowed proliferation of cell explants derived from PDAC tumors isolated from ElasK-RasG12V mice (Figure 6A). Similar results were obtained using explants from tumors obtained from ElasK-RasG12V;p16/p19lox/lox and ElasK-RasG12V;p53lox/lox animals (Figure 6A). These tumor explants, regardless of genotype, were also sensitive to Erlotinib (Figure 6B). As expected, Erlotinib had a cytostatic effect since inhibition required continuous exposure to the drug (data not shown). Knockdown of EGFRs in these tumor explants significantly inhibited phosphorylation of STAT3 (Figure S5). Phosphorylation of ERK proteins was also ameliorated by EGFR knockdown (Figure S5), possibly an indirect consequence of the limited proliferation of these cells in the absence of EGFRs (Figure 6A). Interestingly, phosphorylation of AKT, a marker for the activation of the PI3K/AKT survival pathway was down-regulated in all explants except in those lacking p53 (Figure S5).
Figure 6. EGFR expression is required for proliferation of PDAC tumor explants in vitro.
(A) PDAC cell explants derived from tumors present in mice with the indicated genotypes were infected with lentiviral vectors expressing two independent shRNAs against the Egfr (solid and grey circles) or shRNA control (open circles).
Results are the average of two experiments carried out with two independent cell explants done in triplicate. Errors bars mean SD.
(B) PDAC cell explants derived from tumors present in mice with the indicated genotypes were either untreated (open circles) or treated with Erlotinib (solid circles). Erlotinib was used at a final concentration of 50 μM, a concentration that corresponds with the average IC90 for these cell explants.
Results are the average of two experiments carried out with two independent cell explants done in triplicate. Errors bars mean SD.
See also Figure S5
Cooperation between PI3K and STAT3 signaling pathways in the absence of EGFRs
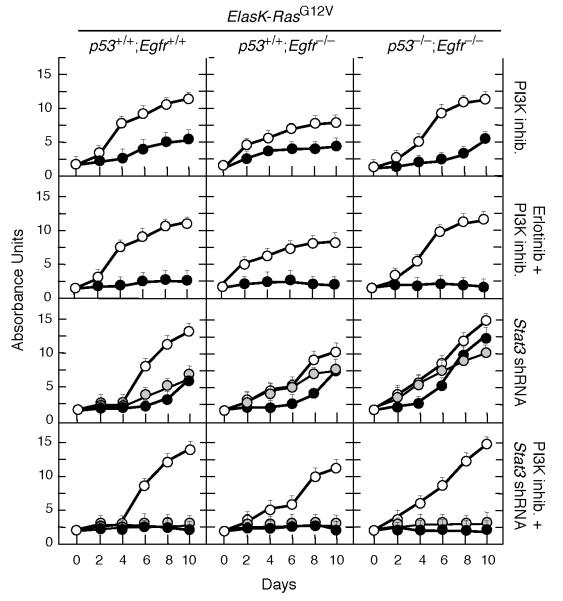
We reasoned that availability of mouse PDAC tumor explants lacking EGFRs and p53 may allow us to identify additional signaling pathways that contribute to tumor development. Unexpectedly, Erlotinib partially inhibited proliferation of PDAC explants from ElasK-RasG12V;p53lox/lox;Egfrlox/lox mice. These results are most likely due to off-target effects since shRNAs against the Egfr locus did not have any inhibitory effect on the proliferation rate of these cells (Figure 6A and 6B). As illustrated in Figure 7, inhibition of PI3K with ETP-46992 (Martínez-González et al., 2012) a selective inhibitor for the p110α and δ catalytic subunits only induced partial inhibition (Figure S6). However, combination of this inhibitor with Erlotinib resulted in robust inhibition of these tumor cell explants even in the absence of EGFRs and p53 (Figure 7).
Figure 7. Loss of p53 activates STAT3 and PI3K pathways.
PDAC cell explants derived from tumors present in mice with the indicated genotypes were treated with the indicated inhibitor(s) or infected with lentiviral vectors expressing two independent shRNA against Stat3 (solid and grey circles). Control cells were either left untreated or infected with a shRNA control (open circles). Erlotinib was used at a final concentration of 50 μM, a concentration that corresponds with the average IC90 for these cell explants. ETP-46992, a selective PI3Kp110α and p110δ inhibitor, was used at a final concentration of 20 μM, a concentration that corresponds to the average IC90 for these tumor explants. Each graph represents the average of two experiments carried out with two independent cell explants. Each sample was carried out in triplicate. Errors bars mean SD
See also Figure S6
As indicated above, ablation or inhibition of EGFRs in ElasK-RasG12V;p53lox/lox mice blocked AKT but not STAT3 phosphorylation (Figure S4C,D). Interestingly, knockdown of STAT3 expression did not inhibit proliferation of tumor cell explants, regardless of their genotype. However, addition of the PI3K inhibitor to the Stat3 shRNA robustly inhibited proliferation of all tumor cell explants (Figure 7). These observations indicate that the STAT3 pathway also contributes to tumor development at least in the absence of p53. Moreover, in this context, PI3K may signal by pathways independent of AKT (Vogt and Hart, 2011). Thus, successful treatment of PDAC tumors in the clinic may require compound inhibition of at least four distinct signaling cascades including those driven by K-RAS, EGFR, PI3K and STAT3.
DISCUSSION
Current dogma indicates that malignant progression of tumor cells selects against mutations in components of the same signaling pathway such as that driven by the EGFR and their downstream effectors, the RAS proteins. Indeed, clinical observations in NSCLCs, a tumor type that present frequent mutations in both EGFR and K-RAS, have indicated that they are mutually exclusive (Shigematsu et al., 2005). Similar results have been obtained in CRC patients. According to a large clinical trial, only patients containing non-mutated K-RAS genes benefit from treatment with EGFR inhibitors (Karepetis et al., 2008). This “dogma” however, might not apply to PDAC tumors. Although EGFR mutations have been found in a very small percentage (<3%) of human pancreatic cancers, they can co-exist with K-RAS mutations (Oliveira-Cunha et al., 2012). Moreover, as illustrated in this study, initiation of K-Ras oncogene-driven PanIN lesions and PDAC is absolutely dependent on EGFR signaling. This requirement is not abrogated even in the absence of the p16/p19 tumor suppressors, indicating that the absolute requirement for EGFR signaling in not involved in overcoming senescence. Only ablation of p53 overrules this requirement. Yet, neoplastic lesions lacking p53 take significantly longer to develop in the absence of EGFRs. Ongoing efforts to establish the mutational spectra of mouse PDAC exomic sequences might help us to identify those additional pathways activated by loss of p53.
Pancreatic injury results in the immediate induction of EGFR expression in acinar cells, leading to activation of the PI3K/AKT and STAT3 signaling pathways. EGFR expression, along with activation of AKT and STAT3, is maintained during PanIN progression to PDAC and only becomes attenuated in poorly differentiated glands of advanced PDAC tumors. The EGFR is also expressed in human biopsies obtained from patients suffering from pancreatitis and PDAC tumors, thus adding supporting the concept that GEM models faithfully reproduce PDAC development in an experimental setting. Previous studies have shown that overexpression of TGFα in the presence of a resident K-RasG12D oncogene accelerated progression of PanIN lesions to metastatic cancer and led to the development of cystic papillary lesions that resembled human intraductal papillary mucinous neoplasms (IPMN) (Siveke et al., 2007). These observations, taken together, suggest that upregulated expression of EGFRs leading to the activation of the PI3K/AKT and STAT3 pathways might be one of the early responses that predispose acinar cells to undergo neoplastic changes upon activation of K-Ras oncogenes. It is interesting to note that in acinar cells, activation of the PI3K/AKT pathway is mediated by induction of EGFRs and not by the resident K-Ras oncogenes. These observations may explain why, in spite of the presence of K-Ras oncogenes, EGFR signaling is essential to induce neoplastic lesions in pancreatic acinar cells.
Current GEM models of PDAC do not allow target ablation in existing tumors. Yet, our results demonstrating that expression of EGFRs are essential for the proliferation of tumor cell explants strongly suggest that EGFRs signaling is essential beyond the early stages of tumor development. Indeed, knockdown or pharmacological inhibition of EGFRs blocked proliferation of certain human pancreatic tumor cells lines. Likewise, EGFR inhibitors have been shown to limit progression of pancreatic lesions in mouse xenograft models (Ng et al., 2002; Durkin et al., 2006) as well as in K-Ras oncogene-driven GEM models (Mohammed et al., 2010; this study). These results suggest that clinical observations showing a limited beneficial effect of EGFR inhibitors in combination with Gemcitabine in patients with PDAC tumors need to be further explored (Moore et al., 2007).
Finally, our results using genetic as well as pharmacologic approaches, illustrate that blocking EGFR signaling only produces limited therapeutic benefit in the context of p53 inactivation, a mutation present in most human PDAC tumors. Availability of tumor cells lacking EGFRs and p53 has allowed us to demonstrate a synergistic activity between PI3K inhibitors and attenuation of STAT3 expression. These observations suggest that loss of TP53 might “re-activate” the PI3K/AKT and STAT3 signaling pathways by mechanisms independent of EGFR in human tumors. Further support for a key role of the EGFR/PI3K/AKT axis in PDAC development comes from preliminary studies in which ablation of the Pten tumor suppressor locus in ElasK-RasG12V;Egfrlox/lox mice leads to efficient tumor development (C.N.,I.H.,C.G.,M.B., unpublished observations). Recent observations regarding a limited but significant tumor inhibitory effect by inhibitors of the Notch pathway (Plentz et al., 2009; Cook et al., 2012) may open the door to design therapeutic strategies in combination with PI3K and STAT3 inhibitors. Yet, to induce complete regression of aggressive PDAC tumors it will be necessary to unveil additional signaling pathways amenable to pharmacological inhibition. Recent progress in overcoming the stromal barrier characteristic of PDAC tumors (Olive et al., 2009; Von Hoff et al., 2011; Frese et al., 2012; Jacobetz et al., 2012; Provenzano et al., 2012) should facilitate testing these drug combinations in relevant GEM models of pancreatic cancer as a preliminary step prior to their use in a clinical setting.
EXPERIMENTAL PROCEDURES
Mice
The ElasK-RasG12V strain has been previously described (Guerra et al., 2007). In these mice, the Elas-tTA/tetO-Cre transgenes drive expression of the bacterial Cre recombinase from the Elastase promoter under the negative control of doxycycline (Tet-off system). Other strains of mice used in this study include Egfrlox, p16/p19lox, p53lox, Apclox and RERT. The original references describing these strains appear in Supplemental Information. All experiments were approved by the CNIO Ethical Committee and performed in accordance with the guidelines for Ethical Conduct in the Care and Use of Animals as stated in The International Guiding Principles for Biomedical Research involving Animals, developed by the Council for International Organizations of Medical Sciences (CIOMS).
Mouse treatments
To prevent expression of the Elastase-driven Cre recombinase in ElasK-RasG12V mice, doxycycline (2 mg/ml, Sigma) was provided in the drinking water as a sucrose solution (5% w/v) to pregnant mothers from the time of conception and to their offspring until the time we activated expression of the resident K-RasG12V oncogene. Pancreatitis was induced by intraperitoneal injections of caerulein for three months (125 mg/Kg, 5 days per week, Sigma). For induction of NSCLC, RERT;K-RasG12V mice carrying wild type or floxed Egfr alleles were treated at P21 with a single dose of 4OHT (0.5 mg/ml in oil). For intestinal tumors, RERT;K-RasG12V;Apclox/lox mice carrying wild type or floxed Egfr alleles were treated 3 days per week for two weeks with 4OHT (0.5 mg/ml in oil) starting at P21. Erlotinib treatment was carried out in six week old ElasK-RasG12V;p53lox/lox mice by oral gavage (100 mg/Kg in 0.5% methylcellulose with 0.1% Tween80) for 4 weeks. Control mice received the same treatment without Erlotinib.
Histopathology and immunohistochemistry
Specimens were fixed in 10% buffered formalin and embedded in paraffin. For histopathological analysis, pancreata were serially sectioned (3 μm thick) and every 10 sections stained with hematoxylin and eosin (H&E). Remaining sections were kept for immunohistochemical studies with β-catenin (1:750, goat polyclonal, Santa Cruz Biotechnology, Sc-1496), pAKT (pS473) (1:175, rabbit monoclonal EP2109Y, Epitomics 2118-1), EGFR (1:100, rabbit monoclonal, Epitomics 1902-1), SPC (1:175, rabbit polyclonal, Millipore, AB3786) and pSTAT3 (Tyr705) (1:100, rabbit monoclonal (D3A7) Cell Signaling Technology, 9145) antibodies. Following incubation with the primary antibodies, positive cells were visualized using 3,3-diaminobenzidine tetrahydrochloride plus (DAB+) as a chromogen. For human samples (see below), immunostaining for EGFRs was preformed as described above.
PDAC cell explants
To generate mouse PDAC explants, freshly isolated tumors were minced with sterile razor blades, digested with collagenase P (1.5 μg/ml) in Hanks’ balanced salt solution (HBSS) for 30 minutes at 37°C, and cultured in DMEM with 10% of fetal bovine serum (FBS). After 48 hours, media was supplemented with Geneticin (75 μg/ml) to select for K-RasG12V expressing cells. All studies were done on cells cultivated for less than 10 passages. Their corresponding genotypes were verified by PCR analysis.
Cell culture and inhibitor treatments
Mouse embryonic fibroblasts (MEFs) were isolated from E13.5 embryos and propagated according to standard 3T3 protocols. Human tumor cell lines PANC1, MIAPaCa-2, SKPC, T3M4, were purchased from ATCC. BxPc3 was kindly provided by M. Hidalgo (CNIO). AsPc1, CFPAC and IMIMPC-2 by F.X. Real (CNIO). These cell lines as well as the PDAC explants were seeded in 96 well plates at a density of 1,000 cells/well or 300 cells/well, respectively and incubated for 24 hours DMEM media supplemented with 10% FBS, 2 mM L-glutamine, 50 U/ml penicillin and 50 μg/ml streptomycin (GIBCO-Invitrogen) before adding the corresponding inhibitor. Inhibitors, including EGFR inhibitor Erlotinib (LC laboratories), MEK inhibitor PD0325901 (Pfizer) and PI3K inhibitor ETP-46992 (CNIO) (Martínez-González et al., 2012), were dissolved in DMSO to yield the appropriate final concentrations. Three sets of control wells were included on each plate, containing either medium without drug or medium with the same concentration of DMSO. Cells were exposed to inhibitors for 10 to 14 days. Fresh drug was added every two days. For shRNA knockdown assays, human or mouse tumor cells were infected with MISSION shRNAs (Sigma) directed against human EGFR (TRC0000121206 and TRC0000121203), mouse Egfr (TRCN0000055218 and TRCN0000055221) and mouse Stat3 (TRCN0000071454 and TRCN0000071456) sequences. Non-Target shRNA Control vector (SHC002, Sigma) was used as a negative control. Cells were selected with puromycin (2 μg/ml) for 5 days before seeding and maintained in DMEM media supplemented with 10% FBS and puromycin. Proliferation rates were determined by the (4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Roche). The resulting absorbance was measured with a microplate reader at 544 nm (EnVision 2104 Multilabel Reader, Perkin Elmer, Waltham, USA). Results represent the average of three independent experiments in which all samples were assayed in triplicate.
Human Samples
Studies using human material were approved by the Ethics and Institutional Review Board of the Grupo Hospital de Madrid (CBBA/4 2008; REF: PI 275). All subjects gave informed consent.
Supplementary Material
SIGNIFICANCE.
Previous clinical studies have suggested a therapeutic benefit of Erlotinib, an EGFR inhibitor, in pancreatic ductal adenocarcinoma patients. Here we show that these observations may have a mechanistic base. EGFRs are expressed during pancreatic injury and in preneoplastic PanIN lesions. Loss of p53, but not of p16INK4a/p19ARF tumor suppressors, relieved the need of tumor cells to maintain EGFR signaling. Yet, loss of EGFRs increased tumor latency and survival. Tumor explants lacking p53 and EGFRs were sensitive to the combined inhibition of PI3K and STAT3. Thus, successful treatment of advanced human pancreatic tumors may require inhibition of at least four distinct signaling cascades including those driven by K-RAS, EGFRs, PI3K and STAT3.
HIGHLIGHTS.
Pancreatic injury induces expression of EGFRs in acinar cells
EGFR signaling is essential for PanIN and PDAC development induced by K-Ras oncogenes
Loss of senescence by ablation of p16Ink4a/p19Arf does not abrogate EGFR dependence
Loss of p53 makes PDAC tumors independent of EGFR signaling
ACKNOWLEDGEMENTS
We thank Howard C. Crawford (Mayo Clinic, Jacksonville, FL) and Jens Siveke (Technical University, Munich) for sharing their results prior to publication. We also thank I. Agudo, I. Aragón, M.C. González, M. Lamparero, M. San Román and R. Villar for excellent technical assistance. We value the excellent support provided by V. Alvarez, E. Gil, M. Gómez, P. González, and N. Matesanz with histopathology and M. Lozano with the laser-capture microscope. We thank J. Pastor and S. Martinez (CNIO) for providing a sample of the PI3K inhibitor, ETP-46992, E. Garcia, M. Hidalgo and F. X. Real (CNIO) for human samples and A. Means (Vanderbilt University Medical Centre, Nashville, TN) for her help with acinar to ductal transdifferentiation protocols. Work was supported by grants from the European Research Council (ERC-AG/250297-RAS AHEAD), EU-Framework Programme (LSHG-CT-2007-037665, HEALTH-F2-2010-259770 and HEALTH-2010-260791), Spanish Ministry of Science and Innovation (SAF2006-11773 and CSD2007-00017), Spanish Ministry of Economy and Competitiveness (SAF2011-30173), Autonomous Community of Madrid (GR/SAL/0587/2004 and S2006/BIO-0232) and Fundación de la Mutua Madrileña del Automóvil to M.B and by grants from Fondo de Investigación Sanitaria (PI042124 and PI08-1623), Autonomous Community of Madrid (GR/SAL/0349/2004) and Fundación Ramón Areces (FRA 01-09-001) to C.G. M.S. acknowledges funding by the Doctoral Program “Inflammation and Immunity” DK W1212, the EC program LSHC-CT-2006-037731 (Growthstop) and the Austrian Federal Government’s GEN-AU program “Austromouse” (GZ 200.147/1-VI/1a/2006 and 820966).
REFERENCES
- Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, Redston MS, DePinho RA. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, Stebbings LA, Morsberger LA, Latimer C, McLaren S, Lin ML, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109–1113. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, Benguría A, Zaballos A, Flores JM, Barbacid M, Beach D, Serrano M. Tumour biology: senescence inpremalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- Cook N, Frese KK, Bapiro TE, Jacobetz MA, Gopinathan A, Miller JL, Rao SS, Demuth T, Howat WJ, Jodrell DI, Tuveson DA. Gamma secretase inhibition promotes hypoxic necrosis in mouse pancreatic ductal adenocarcinoma. J. Exp. Med. 2012;209:437–444. doi: 10.1084/jem.20111923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran RB, Contino G, Deshpande V, Tzatsos A, Conrad C, Benes CH, Levy DE, Settleman J, Engelman JA, Bardeesy N. STAT3 plays a critical role in KRAS-induced pancreatic tumorigenesis. Cancer Res. 2011;71:5020–5029. doi: 10.1158/0008-5472.CAN-11-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin AJ, Osborne DA, Yeatman TJ, Rosemurgy AS, Armstrong C, Zervos EE. EGF receptor antagonism improves survival in a murine model of pancreatic carcinoma. J Surg Res. 2006;135:195–201. doi: 10.1016/j.jss.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Frese KK, Neesse A, Cook N, Tashinga E, Bapiro TE, Lolkema MO, Jodrell DI, Tuveson DA. nab-Paclitaxel Potentiates Gemcitabine Activity by Reducing Cytidine Deaminase Levels in a Mouse Model of Pancreatic Cancer. Cancer Discov. 2012;2:260–269. doi: 10.1158/2159-8290.CD-11-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda A, Wang SC, Morris J.Pt., Folias AE, Liou A, Kim GE, Akira S, Boucher KM, Firpo MA, Mulvihill SJ, Hebrok M. STAT3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell. 2011;19:441–455. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra C, Mijimolle N, Dhawahir A, Dubus P, Barradas M, Serrano M, Campuzano V, Barbacid M. Tumour induction by an endogenous K-ras oncogene is highly dependent on cellular context. Cancer Cell. 2003;4:111–120. doi: 10.1016/s1535-6108(03)00191-0. [DOI] [PubMed] [Google Scholar]
- Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Guerra C, Collado M, Navas C, Schuhmacher AJ, Hernandez-Porras I, Canamero M, Rodriguez-Justo M, Serrano M, Barbacid M. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell. 2011;19:728–739. doi: 10.1016/j.ccr.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Hong S-M, Park JY, Hruban RH, Goggins M. Molecular Signatures of Pancreatic Cancer. Arch Pathol Lab Med. 2011;135:716–727. doi: 10.5858/2010-0566-ra.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, Feig C, Nakagawa T, Caldwell ME, Zecchini HI, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2012 doi: 10.1136/gutjnl-2012-302529. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda M, Matthaei H, Wu J, Hong SM, Yu J, Borges M, Hruban RH, Maitra A, Kinzler K, Vogelstein B, Goggins M. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology. 2012;142:730–733. doi: 10.1053/j.gastro.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- Korc M, Friess H, Yamanaka Y, Kobrin MS, Buchler M, Beger HG. Chronic pancreatitis is associated with increased concentrations of epidermal growth factor receptor, transforming growth factor alpha, and phospholipase C gamma. Gut. 1994;35:1468–1473. doi: 10.1136/gut.35.10.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Kloppel G, Yoshimura A, Reindl W, Sipos B, Akira S, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19:456–469. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157–188. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-González S, Hernández AI, Varela C, Lorenzo M, Ramos-Lima F, Cendón E, Cebrián D, Aguirre E, Gomez-Casero E, Albarrán MI, et al. Rapid identification of ETP-46992, orally bioavailable PI3K inhibitor, selective versus mTOR. Bioorg Med Chem Lett. 2012;22:5208–5214. doi: 10.1016/j.bmcl.2012.06.093. [DOI] [PubMed] [Google Scholar]
- Means AL, Meszoely IM, Suzuki K, Miyamoto Y, Rustgi AK, Coffey RJ, Jr, Wright CV, Stoffers DA, Leach SD. Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development. 2005;132:3767–3776. doi: 10.1242/dev.01925. [DOI] [PubMed] [Google Scholar]
- Mohammed A, Janakiram NB, Li Q, Madka V, Ely M, Lightfoot S, Crawford H, Vernon E. Steele VE, Rao Ch.V. The Epidermal Growth Factor Receptor Inhibitor Gefitinib Prevents the Progression of Pancreatic Lesions to Carcinoma in a Conditional LSL-KrasG12D/+ Transgenic Mouse Model. Cancer Prev Res. 2010;3:1417–1426. doi: 10.1158/1940-6207.CAPR-10-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- Ng SS, Tsao MS, Nicklee T, Hedley DW. Effects of the epidermal growth factor receptor inhibitor OSI-774, Tarceva, on downstream signaling pathways and apoptosis in human pancreatic adenocarcinoma. Mol Cancer Ther. 2002;1:777–783. [PubMed] [Google Scholar]
- Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Cunha M, Hadfield KD, Siriwardena AK, Newman W. EGFR and KRAS Mutational Analysis and Their Correlation to Survival in Pancreatic and Periampullary Cancer. Pancreas. 2012;41:428–434. doi: 10.1097/MPA.0b013e3182327a03. [DOI] [PubMed] [Google Scholar]
- Parsa I, Longnecker DS, Scarpelli DG, Pour P, Reddy JK, Lefkowitz M. Ductal metaplasia of human exocrine pancreas and its association with carcinoma. Cancer Res. 1985;45:1285–1290. [PubMed] [Google Scholar]
- Plentz R, Park JS, Rhim AD, Abravanel D, Hezel AF, Sharma SV, Gurumurthy S, Deshpande V, Kenific C, Settleman J, et al. Inhibition Of g-Secretase Activity Inhibits Tumor Progression In A Mouse Model Of Pancreatic Ductal Adenocarcinoma. Gastroenterology. 2009;136:1741–1749. doi: 10.1053/j.gastro.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic Targeting of the Stroma Ablates Physical Barriers to Treatment of Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puyol M, Martín A, Dubus P, Mulero F, Pizcueta P, Khan G, Guerra C, Santamaría D, Barbacid M. A synthetic lethal interaction between K-Ras oncogenes and Cdk4 unveils a therapeutic strategy for non-small cell lung carcinoma. Cancer Cell. 2010;18:63–73. doi: 10.1016/j.ccr.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba, Fong KM, Lee H, Toyooka S, Shimizu N, et al. Clinical and biological features of epidermal growth factor receptor mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- Siveke JT, Einwachter H, Sipos B, Lubeseder-Martellato C, Kloppel G, Schmid RM. Concomitant Pancreatic Activation of KrasG12D and Tgfα Results in Cystic Papillary Neoplasms Reminiscent of Human IPMN. Cancer Cell. 2007;12:266–279. doi: 10.1016/j.ccr.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Ueda S, Ogata S, Tsuda H, Kawarabayashi N, Kimura M, Sugiura Y, Tamai S, Matsubara O, Hatsuse K, Mochizuki H. The correlation between cytoplasmic overexpression of epidermal growth factor receptor and tumor aggressiveness: poor prognosis in patients with pancreatic ductal adenocarcinoma. Pancreas. 2004;29:e1–8. doi: 10.1097/00006676-200407000-00061. [DOI] [PubMed] [Google Scholar]
- Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt PK, Hart JR. PI3K and STAT3: a New Alliance. Cancer Discov. 2011;1:481–486. doi: 10.1158/2159-8290.CD-11-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, Korn RL, Desai N, Trieu V, Iglesias JL, et al. Gemcitabine Plus nab-Paclitaxel Is an Active Regimen in Patients With Advanced Pancreatic Cancer: A Phase I/II Trial. J Clin Oncol. 2011;29:4548–4554. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.