Abstract
In the past three decades, mounting evidence has revealed that specification of the basic cortical neuronal classes starts at the time of their final mitotic divisions in the embryonic proliferative zones. This early cell determination continues during the migration of the newborn neurons across the widening cerebral wall, and it is in the cortical plate that they attain their final positions and establish species-specific cytoarchitectonic areas. Here, the development and evolutionary expansion of the neocortex is viewed in the context of the radial unit and protomap hypotheses. A broad spectrum of findings gave insight into the pathogenesis of cortical malformations and the biological bases for the evolution of the modern human neocortex. We examine the history and evidence behind the concept of early specification of neurons and provide the latest compendium of genes and signaling molecules involved in neuronal fate determination and specification.
Introduction
There is no disagreement that the cerebral cortex consists of distinct cytoarchitectonic areas, each serving a function(s) ranging from sensory perception and motor control to the symbolic thinking and language in humans. There is consensus among scientists that the human neocortex has evolved with a thousandfold increase in surface size and an increase in cytoarchitectonic areas since the emergence of a common mammalian ancestor. However, there is less than overwhelming agreement about the mechanisms involved in the development of the cortical map and how it became increasingly partitioned and elaborate during evolution.
Because cortical neurons are not generated within the cortex itself, one fundamental question is when and how postmitotic newborn neurons reach their appropriate areal and laminar position after being produced in the proliferative zones near to the cerebral ventricles. The other related question that is almost biblical in proportion is whether all cortical neurons are born equal and multipotent. If true, then distinct layer-, area- and species-specific molecular makeup (i.e. the morphology and connectivity of the individual neurons themselves) is elicited by input from the peripheral sensory receptors to the cortex (mostly via the thalamus). Alternatively, cortical neurons are specified at the time of their birth and then they migrate to their appropriate areal and laminar positions where they complete their differentiation. Final neuronal phenotypes are a result of their genetic makeup and interactions with specific afferents that they selectively attract. In this anniversary issue, it might be pertinent to mention that several basic aspects of the specification of cortical neurons have been discussed by the senior author (P.R.) in five review articles published in Trends in Neurosciences over the past 30 years [1–5]. During these three decades we have witnessed enormous progress in understanding the molecular and cellular mechanisms involved in the development and evolution of the cerebral cortex, much of which is outside of the scope of this review. Here, we will focus on the role of the last cell division in neuronal specification and on downstream implications that are relevant to the formation of cortical maps.
A little history of the big issues
Since two monumental publications delineated the cytoarchitectonic maps of the adult human cerebrum [6,7], scientists, philosophers and even the general public have pondered over the formation of these maps with respect to individual development and to evolution. The answer to this question at a biological level is not a simple task, in part because of one remarkable aspect of cortical development: none of the constituent neurons are generated within the cortex itself [8–11]. Rather, most cortical projection neurons originate in the proliferative ventricular (VZ) and subventricular (SVZ) zones that line the cerebral vesicle, then they migrate radially to their proper laminar and areal positions [12,13]. Likewise, interneurons, which in the rodent cortex originate almost entirely from the ganglionic eminence (GE) of the ventral telencephalon [14–19] and in primates (including humans) from both the GE and from an expanded SVZ [20,21], are also foreign born. How are these complex and dynamic cellular events that include cell commitment, allocation and migration orchestrated to create highly stereotyped but species-specific cortical maps?
Radial unit hypothesis
In all developing mammals, the migration of projection neurons from the ventricular surface to the developing cortical plate involves a remarkably uniform radial deployment, one that is crucially dependent on the shafts of radial glial cells (RGC) that span the entire fetal cerebral wall [12,22]. Such early RGC scaffolding is clearly evident even in the small and smooth embryonic rodent forebrain, but it is particularly prominent and stable in the large and convoluted primate cerebrum [23–27]. This transient RGC scaffolding explains how the convoluted cerebral cortex has enlarged predominately in surface as a uniform sheet, rather than in thickness, and has led to the postulation of the radial unit hypothesis of cortical development and its expansion during evolution [4,22].
According to the radial unit hypothesis, the tangential (areal or horizontal) coordinates of cortical neurons are determined by the relative positions of their precursors in the proliferative VZ, whereas their radial positions are a reflection of the time of their origin. After the completion of their last division cycles, postmitotic cells migrate along a common radial pathway to the developing cortical plate where they form ontogenetic columns (http://rakiclab.med.yale.edu; Figures 1 and 2). Although the relationship between ontogenetic and various functional columns has not been adequately investigated, it is clear that each functional column of the adult brain (depending on the species and/or areas under consideration) must consist of several ontogenetic columns that contain clonally related projection neurons originating from the VZ (Figure 1a,b). The polyclonal nature of ontogenetic columns was evident in DNA-labeling studies with radioactive thymidine that revealed the interspersion of labeled and unlabeled neurons within individual columns [22] (Figure 1d,e). Thus, a ‘functional column’ that responds to the same receptive field (e.g. neurons in the barrel fields) might consist of several ‘ontogenetic radial columns’ originating from the adjacent proliferative units in the VZ, which over the course of embryogenesis produce different neuronal and glial phenotypes [22].
Figure 1.
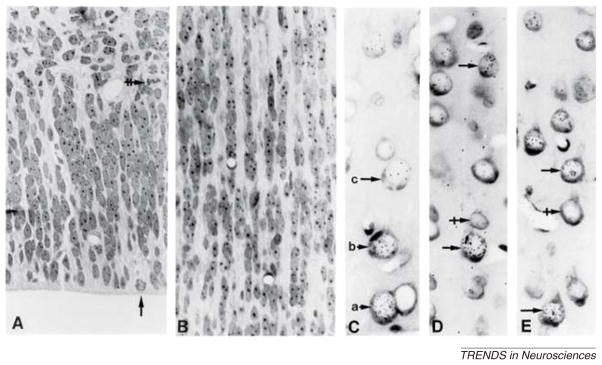
(A) Array of proliferative units in the VZ subjacent to the prospective primary visual cortex in the E91 monkey embryo as seen in epon-embedded tissue cut 1 μm stained with cresyl violet. Although most mitotic figures are located directly at the ventricular surface (vertical arrow), many can be found in the SVZ (horizontal double-crossed arrow). (B) Superjacent cortical plate of the same animal showing ontogenetic columns originated from the same proliferative units as illustrated in part (A). (C) Autoradiogram of an adult monkey exposed to [3H]-thymidine at E70, showing that the most intensely labeled cell (see arrow a) lies deeper in the cortex than the two progressively less labeled (arrows b and c), more superficially situated neurons, indicating inside-out sequence of their origin. (D,E) Unlabeled neurons (crossed arrows) might be interspersed among radioactive neurons (simple arrows) indicating that the ontogenetic columns originate from more than one progenitor. (Combined from Figures 1 and 2 in Ref. [22]).
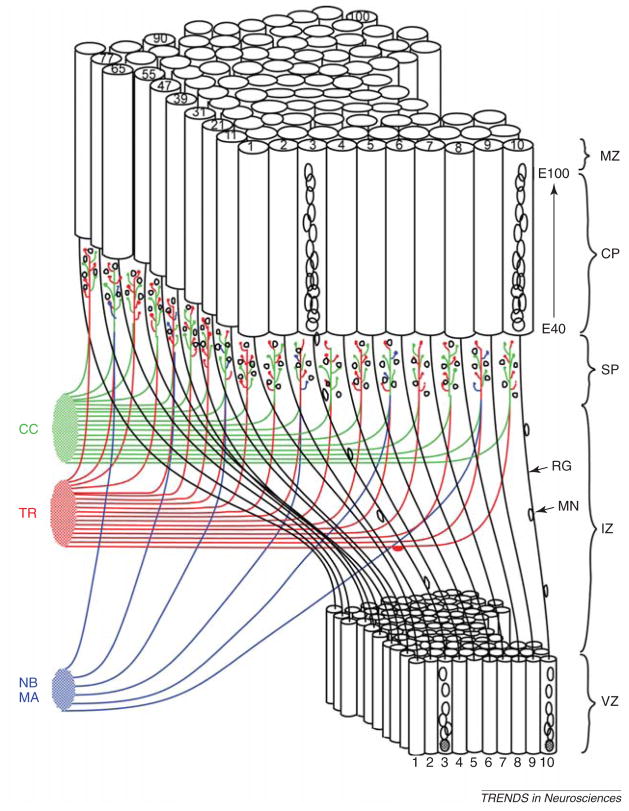
Figure 2.
3D reconstruction of migrating neurons, based originally on electron micrographs of serial sections of the monkey fetal cerebral wall modified from Ref. [4]. The more recent, animated version of this remonstration can be seen at: http://rakiclab.med.yale.edu/MigratingCorticalNeuron.html. Abbreviations: CC, corticocortical connection; CP, cortical plate; IZ, intermediate zone; MA, monoamine; MN, migrating neuron; MZ, marginal zone; NB, nucleus basalis; RG, radial glia; SP, subplate; TR, thalamic radiation; VZ, ventricular zone.
As summarized in Ref. [4], the radial unit hypothesis has also provided an explanation for cellular events underlying the enormous expansion of cortical surface during mammalian evolution. This concept is based on the biphasic kinetics of cell proliferation in the VZ.
In the first phase, which occurs before the onset of neurogenesis, neural stem cells are generated mostly by symmetric divisions [28]. Because each round of cell division duplicates the number of future radial units, growth in this phase is geometric. The length of the first phase divided by the average duration of each cell cycle determines not only the total number of cortical neurons [28] but also the number of radial units that participate in the overall expansion of cortical surface [4,22,29].
The second phase coincides with the onset of neurogenesis and is responsible for producing postmitotic neurons, predominantly (although not exclusively) through asymmetric cell divisions [21,30]. This linear growth during the second phase determines the resultant cortical thickness by regulating the number of neurons generated within each radial column until a termination by either symmetric cell division or programmed cell death [4]. Furthermore, because transcription factors controlling the cell cycle and the symmetric or asymmetric modes of cell division seem to be conserved across species, it was suggested that their expression could have been differentially modulated during cortical evolution by changes to the proliferation kinetics of the founder cells [1,4,31]. Hypothetically, the expansion of the progenitor pool can be achieved either by increasing the rate of cell division or by decreasing programmed cell death [32]. An increased number of radial columns in the developing cortical plate enhances the capacity for the cortex to establish new patterns of connectivity between cerebral and subcortical structures [33]. We have hypothesized that such new connections can be functionally validated through the process of natural selection [4]. Over three decades, the radial unit model has received support from in vivo retroviral gene transfer in the rodent and primate neocortex [30,31,34] and from experimental studies using chimeric and transgenic animals [29,33] for tracing cell lineages.
Protomap hypothesis
One issue related to the radial unit hypothesis is how the immature and seemingly homogeneous population of bipolar cells that arrives in the cortical plate differentiates into highly specialized classes that serve their area-specific functions in the mature cortex. Traditional thinking has, based on histological uniformity, assumed that the embryonic cortical plate is initially composed of equipotent cells that only later become specified by the input from the subcortical centers (the tabula rasa hypothesis). The concept of equipotentiality, effectively advocated by Otto Creutzfelt [35], grew to considerable popularity in the psychological, social and even political sciences during the 1980s because it leaves cortical elaboration in each individual and in each species to interactions with its environment and ecological niche. For example, the idea that all grains of wheat, just like the brains of all humans, are created equally – that the environment molds the phenotype of the wheat and the individuality, temperament or talents in humans – was a core of Soviet egalitarian ideology. In the case of the cerebral cortex, the environment indeed profoundly affects its development and refines the final pattern of synaptic organization in each individual according to their experience [36]. However, neuronal plasticity and the environmental effect (that cannot be carried to the next generation) was, at the time, often taken as evidence for the primary role of input in the specification of species-specific evolutionary changes in cortical cytoarchitecture.
An alternative hypothesis more consistent with the Morganian concept of genetic selection proposes that the basic phenotype, the laminar and areal specificity of cortical neurons, is determined at the time of their last cell divisions in the proliferative VZ. This hypothesis implies that cortical neurons and progenitors are targets of an evolutionary trajectory to endow them not only with the capacity for expansion but also with an intrinsic program for the development of species-specific cortical maps. According to this model, known as the protomap hypothesis [21], neural progenitors in the VZ form a mosaic of proliferative units that establish a primordial species-specific cortical map. The positional information of postmitotic neurons is maintained during their migration to the overlying cerebral cortex by the radial constraints inherent in the transient radial glial scaffolding [2,22,27,34]. Thus, this hypothesis explains how the convoluted cortical surface can expand whereas individual cells preserve their laminar and areal positions. Accordingly, differential expansion of cortical areas and the introduction of new areas during evolution might be the result of changes in the stem cells of the VZ.
In this vein, primordial cortical areas established in the cortical plate serve as a template to selectively attract afferents from the appropriate thalamic nuclei [22,37,38]. For example, the prospective primary visual cortex (area 17 or striate cortex) of the developing cortical plate in the macaque monkey specifically attracts afferents from the lateral geniculate nucleus, whereas the future primary somatosensory cortex (areas 1, 2 and 3) attracts inputs from the ventroposterolateral nuclei of the thalamus. One explanation for this selectivity might be that these attractions depend on specific sets of recognition molecules generated by the cortical neurons in each prospective area. The related ‘handshake hypothesis’ [39], an elaboration of the protomap hypothesis, proposes that the specified cortical neurons extend a descending process to meet with the specific thalamic input. Indeed, the idea that cortical neurons might have surface molecules that can distinguish between and choose among various thalamic inputs has been experimentally confirmed by several recently published studies [40,41].
The first experimental evidence that the prospective phenotype of cortical neurons is assigned at the time of their genesis in the VZ came from the spontaneous mutation of the reeler gene in inbred strains of mice [42]. Analysis of this mutant phenotype showed that neurons eventually assume their lamina-specific phenotypes and pattern of connectivity even if misplaced in inappropriate layers. Heterochronous transplantations of cortical VZ progenitors in ferrets confirmed these results by showing that donor neurons migrate to the appropriate layers and differentiate into the expected type of neuron [43]. Further evidence that the early embryonic cortical plate harbors a protomap of future functional areas came in the experimental ablation of projections from the lateral geniculate nucleus to the prospective V1 area in primate embryos. The results of binocular enucleation showed that some basic region-specific cytoarchitectonic features develop independently of thalamic input [22,37,44,45]. However, the study also revealed that the final size of the V1 area and its full molecular, cellular and synaptic characteristics can be achieved only through a cascade of reciprocal interactions between the afferents arriving from subcortical sources and the responsive cortical target neurons [37]. The prefix ‘proto’ was added to emphasize the primordial, malleable character of this map [22]. Still, the conversion of the embryonic protomap into the adult cyto- and synapto-architectonic maps can be accomplished only in cooperation with specific thalamic innervations [36,37,46,47]. Thus, the protomap model does not dispute the importance of the effect of the input on cortical differentiation [48] but, rather, implies that afferents can act only on pre-specified cortical neurons.
Evidence from molecular genetics
When examined by classical histological methods, the developing rodent cortex appears as a homogenous sheet of look-alike cells, without any trace of landmarks to delineate future areal boundaries. Although a more in-depth analysis reveals some regional differences in the embryonic mouse forebrain [49–51], histological differences between areas are more overt in the large fetal cerebrum of humans and non-human primates [22,52,53], thus contradicting the notion of the homogeneity of the embryonic neocortex. These regional differences even extend beyond the cortical plate to the complexity of the overlaying marginal zone (prospective layer I) and to differences in the kinetics of proliferation in the VZ and the pattern of lamination and thickness of the SVZ [46,54–57]. Still, greater genetic and molecular differences exist between regions, but these can be unmasked only by the sophisticated methods of modern molecular biology. In the following sections we review the data from molecular genetics and stem cell biology that pertain to the specification of cortical maps.
Morphogenetic gradients
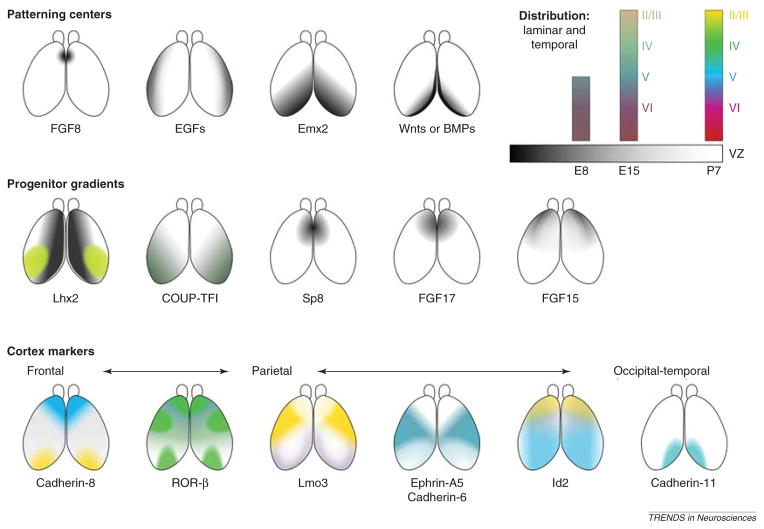
The first sign of cortical regionalization is the emergence of molecular gradients in the embryonic cerebral vesicles. The onset of diversification of neural stem cells in the proliferative VZ coincides with the appearance of patterning centers in and around the forebrain vesicle, which exert their influence over the entire rostro-caudal and medio-lateral extent of the cerebral hemispheres (Figure 3). As these gradients become refined, more distinct and discrete regional gene expression replaces them. The list of signaling and morphoregulatory candidate molecules has grown so extensively over the last decade that it is not practical to discuss all of them separately and/or give them deserved attention. Thus, we provide a concise diagram of their distribution within the embryonic forebrain (Figure 4) to convey the magnitude and complexity of the molecular machinery involved in cortical specification (a more exhaustive table is available at http://rakiclab.med.yale.edu/pages/molecules.php).
Figure 3.
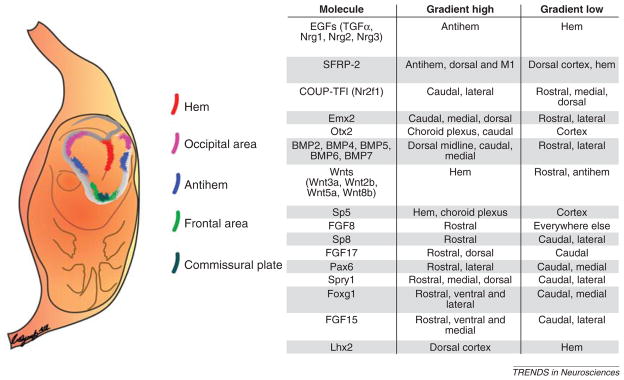
Patterning centers around the neocortex are responsible for establishing morphogenetic gradients that govern areal identity in the cortex. The table lists key molecules and their corresponding expression pattern. An extensive list is available at: http://rakiclab.med.yale.edu/pages/molecules.php. Abbreviations: Foxg1, forkhead box G1 (also known as brain factor 1 [BF1]); Nrg1, Nrg2 and Nrg3, neuregulin 1, 2 and 3; Otx2, orthodenticle homeobox 2; Spry1, sprouty homolog 1; TGFα, transforming growth factor α.
Figure 4.
Graded expression patterns of several factors thought to be responsible for shaping the ultimate neocortical landscape that is highly partitioned and specialized. A greater list is available at: http://rakiclab.med.yale.edu/pages/molecules.php. Abbreviations: Id2, Inhibitor of DNA binding 2; Lhx2, LIM homeobox 2; Lmo3, LIM domain only 3; ROR-β retinoid-related orphan receptor β.
Patterning centers confer absolute positional information and regulate the size of cortical areas by secreting members of the fibroblast growth factor (FGF), Wnt, and bone morphogenetic protein (BMP) families of signaling molecules, among others. The anterior patterning center at the commissural plate secretes FGF8 and FGF17, whereas the hem, the posteromedial border, secretes Wnts and BMPs. The lateral area of the telencephalon bordering the lateral GE, coined the now in-vogue term ‘antihem’, is thought to secrete Wnt antagonists secreted frizzled-related protein 2 (SFRP-2) and express FGF7 and the epidermal growth factor (EGF)-family members neuregulin-1, neuregulin-2, neuregulin-3 and transforming growth factor α [58].
Subsequently, expression gradients of numerous transcription factors are established in the telencephalic neuroepithelium under the general influence of secreted morphogenes. For example, anterior expression of FGF8 suppresses the posteriorly expressed chick ovalbumin upstream transcription factor I (COUP-TFI; also known as NR2F1) and empty spiracles homeobox 2 (EMX2) in telencephalic neuroepithelial cells [59–61], resulting in their lower anterior but higher posterior expression. The development of frontal and sensory subdivision is dependent on the graded action of FGF8 [62,63] and FGF17 [64]. Studies by Cholfin and Rubenstein [65,66] provide an example of how one cerebral lobe or area such as the frontal cortex can expand differentially and independently of the growth rate of the other areas.
It is important to remember that although transcription factor families are often the downstream targets of secreted signalers they are also responsible for the precise localization of the source of such diffusible ligands at the patterning centers. For example, the homeobox transcription factors Hex and Hex1 have been implicated in the initiation of FGF8 expression; however, the genes responsible for the temporal induction of FGF8 are not yet known [67]. Maintenance of FGF8 expression is coordinated by the action of the zinc-finger protein Sp8 [68], FGF8 auto-induction [69,70] and sonic hedgehog (Shh) signaling mechanisms [71]. Spatial restriction of FGF8 is thought to be mediated by transcriptional suppression of Sp8 [68] by Emx2 and by the antagonistic action of Wnts and BMPs from the hem region [71,72]. More broadly, interactions between FGF, BMP and Shh signaling can be dynamically balanced and mediated by intermediary molecules such as low-density lipoprotein-receptor-related protein (LRP2; also known as megalin) [71,73]. In LRP2−/− embryos, for example, BMP signaling is increased owing to a decrease in the clearance of BMPs. The absence of LRP2 causes an increase of BMP in the dorsal telencephalon and a decrease of Shh in the ventral telencephalon, resulting in extensive defects in both the dorsal and ventral telencephalon.
Spatiotemporal and dynamic regulation is also observed in experiments targeting other area-specific transcription factors. The transcription factor Emx2, which is expressed in a high posteromedial to low anterolateral gradient, has been implicated in the spatial delineation of posterior functional areas [74]. Manipulation of Emx2 expression in mice by genetic knockout causes an expansion of frontal and lateral regions (i.e. S1 and M1) at the expense of the visual cortex [74,75]. Furthermore, viral overexpression of Emx2 causes ectopic thalamocortical axon (TCA) projection from the lateral geniculate nucleus to areas outside the area normally conforming to V1 [76]. COUP-TFI, a high-posterolateral-to-low-anteromedial factor, functions to specify both somatosensory and visual areas and to suppress the expansion of anterior frontal and motor domains [60]. Interestingly, the expression of COUP-TFI seems to require FGF15, a ventral factor that emanates from the Shh-dependent rostral patterning center [77]. Taken together, this evidence directly supports the establishment of a proper cortical protomap to instruct the correct spatial expression of attractive cues necessary for TCA path finding.
These patterning mechanisms are not unique to neocortical development because regions ventral to the telencephalon also undergo extensive patterning by Shh and transcription factors such as forebrain embryonic zinc-finger (FEZF1), FEZ-like (FEZF2), iroquois homeobox 1 (Irx1) and glioma-associated oncogene homologs 1 and 2 (Gli1 and Gli2). It is in this manner that thalamic, prethalamic and hypothalamic nuclei [78–83] are produced. A wealth of evidence indicates that similar signaling mechanisms could be important in delineating functional regions elsewhere in the central nervous system [84].
From gradients to maps
The progressive sharpening of expression gradients into more discrete boundaries is an important aspect of corticogenesis [85,86]. Few, if any, molecules have been found with such discrete margins in the VZ [87] at the onset of corticogenesis. However, substantial evidence asserts that well-defined domains in the cortex emerge under the direction of proliferative zone patterning centers and do so entirely independently of input [60,62,64,87,88]. In agreement with this, many area markers are discretely partitioned at the time of thalamic input and earlier [85,89,90]. Such instances are exemplified by derangements of the primary cortical patterning centers after the birth of subplate neurons – they are often too late to affect the initial TCA projections to sensory areas [91]. However, all sensory pathways seem to exhibit plasticity (that alterations of input result in parallel changes to the cortical representations of those inputs) yet careful examination in nearly every case reveals some innate, and often stalwart, architectural governance. Barrels will not form properly in rodent S1 if the thalamic or brainstem representation of the whisker pad are disturbed [92,93] via any number of upstream lesions. Yet, the thalamic axons the terminals of which cluster to form barrels seem to travel to where they are directed in the developing cortex, as shown in the seminal work in which an exogenous FGF8 source causes barrel cortex duplication [61].
Interaction with input
The establishment of cellular patterns and diverse neuronal types in a variety of structures and species usually starts with a pronounced gradient of transcription factors and signaling molecules that become sharpened by cell–cell interactions. An instructive example is the development of the primary visual area (V1) in primates. In this area, the earliest ocular dominance columns occur before birth in monkey [94] and before eye-opening in neonatal ferrets. Although these early (‘proto’) columns seem to be immune to sensory imbalances [95], the final pattern is altered (i.e. after ferret P30) [96] if input is disrupted, and this depends on competition between the incoming inputs [25,97,98]. Moreover, many original histological [22] and architectural aspects [99] of primate area 17, especially those of the supragranular layers, are retained in early enucleated animals, even in the region that aberrantly becomes innervated by the pulvinar nucleus. Similarly, studies of auditory pathway lesions in the ferret, in which A1 is incorrectly given visual input from LGN, show that circuits in A1 partially organize to resemble those in V1; some features of the A1 cortex do remain (for a review, see Ref. [96]).
Transplantation studies [100,101] rest their case predominantly on a model of in situ differentiation of cortical neurons, as evidenced by donors acquiring properties of the host area; the host area of these studies was left mostly intact (i.e. able to recruit correct and organized TCA input; see barrel cortex notes earlier). In the same vein, sensory input perturbations have been shown to affect corticocortical and corticocallosal axon growth and targeting [100]. Fundamentally, these and other postnatal derangements cannot affect the cortical map per se but, rather, the local input cytoarchitecture of the cortex (for example, in monocular enucleation [96] or whisker row lesions; for review, see Ref. [93]). This plasticity might be a large component of the ability of the neocortex not only to grow considerably in size in evolution but also to accommodate changes in the quality and quantity of sensory input, given that it reduces the number of genetic alterations required to effect such changes.
Notwithstanding, TCAs have been shown to release mitogenic factors that act on cortical progenitors [102]. However, TCAs from the lateral geniculate nucleus project selectively to the portion of the VZ subjacent to the prospective area 17, indicating that this area already contains some region-specific signaling molecules for their exclusive attraction. It might be speculated that this TCA mitogenic influence on the VZ and SVZ is a solution to an area-specific evolutionary need (i.e. for more supragranular neurons in primate area 17). In fact, other mechanisms have apparently evolved in primates to fulfill the need for more complex circuitry; for example, the enlarged layer IV of V1 is partly due to influx of interneurons arriving from other sources via a migratory stream in the overlaying layer I [103].
The reports summarized earlier indicate that, although external inputs are undeniably crucial for proper neuronal maturation and indirectly shape functional areas, the cortical progenitors and their descendants are endowed with an intrinsic genetic program. A protomap (species specific) is thus established to attract appropriate inputs that, together with the intrinsic program, determine final cytoarchitectonic and functional maps. The multitude of domains and gradients derived from a few early patterning centers provide insight into how evolutionary adaptations could occur by simplifying the genetic changes necessary for enlarging and/or adding cytoarchitectonic areas [4,60,62,64,65,87,88].
Evidence from stem cell biology: the cortex and beyond
The issue of neuronal specification has received full attention only with the advent of modern stem cell biology, which has enabled tracing lineages of cortical neurons back to the place of their final division in the VZ (for review, see Ref. [104]). More recently, studies have shown that neural precursors in the proliferative compartments might have specific identities that govern their laminar contributions [105–109]. Despite enormous progress, the definition and identity of stem cells remains vague, with the preponderance of evidence indicating that the astroglial lineage, RGCs [110] and their derivatives, serves as the predominant neural stem cell in vivo [111–115]. (As discussed later, we thus use the term ‘stem cell’ with some reservation because the definition of ‘stem cell’ or ‘neural stem cell’ holds certain caveats, especially in the context of the protomap hypothesis.)
The most widely used definition of neural stem cell assumes that it should be capable of self-renewal and have the ability to generate the three major neural cell types in the brain: neurons, astrocytes and oligodendrocytes [116,117]. However, many researchers argue that a true neural stem cell should be capable of generating all types of neurons (i.e. pyramidal neurons, interneurons and Purkinje cells, etc.), astrocytes (i.e. protoplasmic astrocytes, hippocampal radial glia, Bergmann and Muller glia, etc.) and oligodendrocytes in the central nervous system [117,118]. There is no definitive evidence proving the existence of such potential in vivo or in vitro [117,119]. For this reason, and to enable the grouping of multipotent progenitors, lineage restricted progenitors and stem cells, the term ‘neural precursor cell’ is often substituted [117,118] and we use this terminology heretofore.
Protomap of the postnatal SVZ and astrocytes
After the existence of a protomap had been demonstrated in the early embryonic cerebrum, it was not entirely surprising to find evidence that the protomap ‘lives on’ in neurogenic niches that continue to generate neurons after birth. Indeed, several groups have now provided comprehensive lineage tracing and transplant experiments, which convincingly demonstrate that progenitors lining the lateral ventricle are heterogeneous and can be defined by their spatial location and the phenotypic differentiation of their progeny [120–125] (Figure 5a,b). Similar to the VZ of the embryonic cortex, where proliferative units generate ontogenetic columns [22], postnatal proliferative units generate ontogenic cohorts of olfactory bulb neurons.
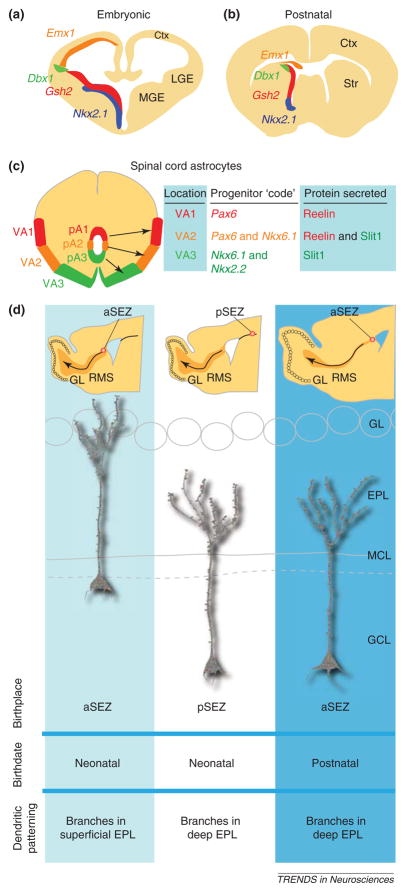
Figure 5.
Re-utilization of the protomap in the postnatal brain and glia. (a) Example of molecules involved in specification of the embryonic forebrain. (b) These molecules are similarly utilized in the postnatal forebrain for the generation of olfactory bulb (OB) neurons and glia in the OB and other forebrain regions as shown by several groups. (c) Summary of results from Ref. [126] showing that the combinatorial progenitor code (in this case, combinations of Pax6, Nkx6.1 and Nkx2.2 transcription factor expression) in the embryonic spinal cord pre-specify the eventual areal (VA1,VA2 or VA3) and secretion (reelin, Slit or both) subtype of astrocytes into three subclasses. (d) The location of generation of OB granule cells correlates with the location of the progenitor cells in the postnatal subependymal (SEZ) zone. Neonatal granule cells born in the anterior SEZ (aSEZ) typically have dendrites that branch in the superficial external plexiform layer (EPL), whereas posterior (SEZ)-derived granule cells branch in the deep EPL. Postnatally born aSEZ-derived granule cells preferentially branch in the EPL. See text for discussion of these results from Lois and colleagues [127]. Abbreviations: Ctx, cortex; Dbx1, developing brain homeobox 1; Emx1, empty spiracles homeobox 1; GCL, granule cell layer; GL, glomerular layer; Gsh2, genomic screen homeobox 2; LGE, lateral ganglionic eminence; MCL, mitral cell layer; MGE, medial ganglionic eminence; Nkx2.2, NK2 homeobox 1; Nkx6.1, NK6 homeobox 1; pA1, pA2 and pA3, progenitor domain astrocyte subtypes A1, A2 and A3; Pax6, paired box 6; pSEZ, posterior subependymal zone; RMS, rostral migratory stream; Str, striatum.
In addition, several lines of evidence indicate that glia have a similar protomap or at least reuse the developmental protomap. First, spinal cord astrocytes can be differentiated by their position and combinatorial expression of the secreted proteins reelin and Sli homolog 1 (SLIT1), much like neurons can be identified by position and neurotransmitter types [126]. Furthermore, these different astrocyte types are specified at the VZ surface by homeodomain transcription factors much in the same way that neurons are pre-specified in this region (Figure 5c).
As predicted by the protomap hypothesis, the areal identity (i.e. neuronal subtypes, shape of dendritic arbors and pattern of axon projections, etc.) of olfactory granule cells can be predicted by the positions of their progenitors in the SVZ (Figure 5d). This extraordinary finding was revealed using retroviral labeling of spatially and temporally discrete precursor populations and further supported by heterochronic and heterotopic transplants of enhanced green fluorescent protein (EGFP)-labeled neural precursors [127]. This degree of intrinsic specificity and predetermination in generating one specific type of neuron seems to argue against the concept that SVZ precursors are intrinsically pluripotent in their ability to generate different types of neurons, and thus adds a caution to the usage of the term ‘stem cells’. However, it is important to note that this particular phenomenon became apparent here owing to the protracted period of neurogenesis and the relative ease of labeling (compared with embryonic brains) and/or culturing populations from this region of the brain. The premise of the protomap hypothesis that cortical cells became committed to a basic cell type before the cessation of migration [22] indicates that any transplanted precursors should be matched to the phenotype and host region as closely as possible.
Conclusions
In the past 30 years we have witnessed an enormous amount of information on cortical specification to which we could not possibly give due attention in this review. To the contemporary reader, the initial resistance to the idea of the early commitment and decisive role of genes in the formation of cortical cytoarchitectonic maps might seem surprising. However, the overall cumulative impact of these studies is that the basic phenotype of cortical neurons and their species-specific laminar and areal identity is set at the time of their last cell division. Findings from neural stem cell biology and postnatal neurogenesis in other regions are consistent with these ideas. It is also clear that this basic protomap is shaped, modified and elaborated by interaction with inputs that at postnatal stages could be influenced by experience. This early specification can explain not only normal development and pathogenesis of congenital disorders but also cellular events that might underlie evolution of the most precious organ of the human body.
Acknowledgments
PR is supported by grants from the National Institute of Neurological Disorders and Stroke (www.ninds.nih.gov), the National Institute of Mental Health (www.nimh.nih.gov), the National Institute on Drug Abuse and the Kavli Institute for Neuroscience at Yale (http://kavli.yale.edu); A.E.A. is supported by a Patterson Trust Fellowship in Brain Circuitry (www.tmfnet.org/grantmake.html#patterson); J.B. is supported by the Connecticut Stem Cell Research Grants Program (www.ct.gov/dph/cwp/view.asp?a=3142&q=389702).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kuan CY, et al. Mechanisms of programmed cell death in the developing brain. Trends Neurosci. 2000;23:291–297. doi: 10.1016/s0166-2236(00)01581-2. [DOI] [PubMed] [Google Scholar]
- 2.Rakic P. Neuronal–glial interaction during brain development. Trends Neurosci. 1981;4:184–187. [Google Scholar]
- 3.Rakic P. Mechanism of ocular dominance segregation in the lateral geniculate nucleus: competitive elimination hypothesis. Trends Neurosci. 1986;9:11–15. [Google Scholar]
- 4.Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18:383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- 5.Sarkisian MR, et al. Trouble making the first move: interpreting arrested neuronal migration in the cerebral cortex. Trends Neurosci. 2008;31:54–61. doi: 10.1016/j.tins.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Johann Ambrosius Barth Verlag; 1909. [Google Scholar]
- 7.Economo C, Koskinas GN. Die Cytoarchitektonik der Hirnrinde des erwachsenen Menschen. Julius Springer; 1925. [Google Scholar]
- 8.Angevine JB, Jr, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192:766–768. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- 9.His W. Unsere Körperform und das physiologische Problem ihrer Entstehung; Briefe an einen befreundeten Naturforscher. Vogel; 1874. [Google Scholar]
- 10.Cajal SRy. Les nouvelles idées sur la structure du système nerveux chez l’homme et chez les vertébrés. C. Reinwald; 1894. [Google Scholar]
- 11.Rakic P, Sidman RL. Supravital DNA synthesis in the developing human and mouse brain 1. J Neuropathol Exp Neurol. 1968;27:246–276. [PubMed] [Google Scholar]
- 12.Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- 13.Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974;183:425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- 14.Ang ES, Jr, et al. Four-dimensional migratory coordinates of GABAergic interneurons in the developing mouse cortex. J Neurosci. 2003;23:5805–5815. doi: 10.1523/JNEUROSCI.23-13-05805.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson SA, et al. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- 16.de Carlos JA, et al. Dynamics of cell migration from the lateral ganglionic eminence in the rat. J Neurosci. 1996;16:6146–6156. doi: 10.1523/JNEUROSCI.16-19-06146.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavdas AA, et al. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J Neurosci. 1999;19:7881–7888. doi: 10.1523/JNEUROSCI.19-18-07881.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polleux F, et al. Control of cortical interneuron migration by neurotrophins and PI3-kinase signaling. Development. 2002;129:3147–3160. doi: 10.1242/dev.129.13.3147. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y, et al. Distinct molecular pathways for development of telencephalic interneuron subtypes revealed through analysis of Lhx6 mutants 1. J Comp Neurol. 2008;510:79–99. doi: 10.1002/cne.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petanjek Z, et al. Distinct origin of GABA-ergic neurons in forebrain of man, nonhuman primates and lower mammals. Coll Antropol. 2008;32 (Suppl 1):9–17. [PubMed] [Google Scholar]
- 21.Letinic K, Rakic P. Telencephalic origin of human thalamic GABAergic neurons. Nat Neurosci. 2001;4:931–936. doi: 10.1038/nn0901-931. [DOI] [PubMed] [Google Scholar]
- 22.Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 23.deAzevedo LC, et al. Cortical radial glial cells in human fetuses: depth-correlated transformation into astrocytes. J Neurobiol. 2003;55:288–298. doi: 10.1002/neu.10205. [DOI] [PubMed] [Google Scholar]
- 24.Levitt P, Rakic P. Immunoperoxidase localization of glial fibrillary acidic protein in radial glial cells and astrocytes of the developing rhesus monkey brain. J Comp Neurol. 1980;193:815–840. doi: 10.1002/cne.901930316. [DOI] [PubMed] [Google Scholar]
- 25.Rakic P. Development of visual centers in the primate brain depends on binocular competition before birth. Science. 1981;214:928–931. doi: 10.1126/science.7302569. [DOI] [PubMed] [Google Scholar]
- 26.Schmechel DE, Rakic P. A Golgi study of radial glial cells in developing monkey telencephalon: morphogenesis and transformation into astrocytes. Anat Embryol (Berl) 1979;156:115–152. doi: 10.1007/BF00300010. [DOI] [PubMed] [Google Scholar]
- 27.Zecevic N. Specific characteristic of radial glia in the human fetal telencephalon. Glia. 2004;48:27–35. doi: 10.1002/glia.20044. [DOI] [PubMed] [Google Scholar]
- 28.Caviness VS, Jr, et al. Cell output, cell cycle duration and neuronal specification: a model of integrated mechanisms of the neocortical proliferative process. Cereb Cortex. 2003;13:592–598. doi: 10.1093/cercor/13.6.592. [DOI] [PubMed] [Google Scholar]
- 29.Chenn A, Walsh CA. Increased neuronal production, enlarged forebrains and cytoarchitectural distortions in β-catenin overexpressing transgenic mice. Cereb Cortex. 2003;13:599–606. doi: 10.1093/cercor/13.6.599. [DOI] [PubMed] [Google Scholar]
- 30.Luskin MB, et al. Cell lineage in the cerebral cortex of the mouse studied in vivo and in vitro with a recombinant retrovirus. Neuron. 1988;1:635–647. doi: 10.1016/0896-6273(88)90163-8. [DOI] [PubMed] [Google Scholar]
- 31.Luskin MB, et al. Neurons, astrocytes, and oligodendrocytes of the rat cerebral cortex originate from separate progenitor cells: an ultrastructural analysis of clonally related cells. J Neurosci. 1993;13:1730–1750. doi: 10.1523/JNEUROSCI.13-04-01730.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuida K, et al. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 33.Haydar TF, et al. The role of cell death in regulating the size and shape of the mammalian forebrain. Cereb Cortex. 1999;9:621–626. doi: 10.1093/cercor/9.6.621. [DOI] [PubMed] [Google Scholar]
- 34.Kornack DR, Rakic P. Radial and horizontal deployment of clonally related cells in the primate neocortex: relationship to distinct mitotic lineages. Neuron. 1995;15:311–321. doi: 10.1016/0896-6273(95)90036-5. [DOI] [PubMed] [Google Scholar]
- 35.Creutzfeldt OD. Generality of the functional structure of the neocortex. Naturwissenschaften. 1977;64:507–517. doi: 10.1007/BF00483547. [DOI] [PubMed] [Google Scholar]
- 36.Catalano SM, Shatz CJ. Activity-dependent cortical target selection by thalamic axons. Science. 1998;281:559–562. doi: 10.1126/science.281.5376.559. [DOI] [PubMed] [Google Scholar]
- 37.Rakic P, et al. A novel cytoarchitectonic area induced experimentally within the primate visual cortex. Proc Natl Acad Sci U S A. 1991;88:2083–2087. doi: 10.1073/pnas.88.6.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Leary DD, Borngasser D. Cortical ventricular zone progenitors and their progeny maintain spatial relationships and radial patterning during preplate development indicating an early protomap. Cereb Cortex. 2006;16 (Suppl 1):i46–i56. doi: 10.1093/cercor/bhk019. [DOI] [PubMed] [Google Scholar]
- 39.Molnar Z, Blakemore C. How do thalamic axons find their way to the cortex? Trends Neurosci. 1995;18:389–397. doi: 10.1016/0166-2236(95)93935-q. [DOI] [PubMed] [Google Scholar]
- 40.Torii M, Levitt P. Dissociation of corticothalamic and thalamocortical axon targeting by an EphA7-mediated mechanism. Neuron. 2005;48:563–575. doi: 10.1016/j.neuron.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 41.Yun ME, et al. EphA family gene expression in the developing mouse neocortex: regional patterns reveal intrinsic programs and extrinsic influence. J Comp Neurol. 2003;456:203–216. doi: 10.1002/cne.10498. [DOI] [PubMed] [Google Scholar]
- 42.Caviness VS, Jr, Rakic P. Mechanisms of cortical development: a view from mutations in mice. Annu Rev Neurosci. 1978;1:297–326. doi: 10.1146/annurev.ne.01.030178.001501. [DOI] [PubMed] [Google Scholar]
- 43.McConnell SK. Fates of visual cortical neurons in the ferret after isochronic and heterochronic transplantation. J Neurosci. 1988;8:945–974. doi: 10.1523/JNEUROSCI.08-03-00945.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakagawa Y, et al. Graded and areal expression patterns of regulatory genes and cadherins in embryonic neocortex independent of thalamocortical input. J Neurosci. 1999;19:10877–10885. doi: 10.1523/JNEUROSCI.19-24-10877.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyashita-Lin EM, et al. Early neocortical regionalization in the absence of thalamic innervation. Science. 1999;285:906–909. doi: 10.1126/science.285.5429.906. [DOI] [PubMed] [Google Scholar]
- 46.Dehay C, Kennedy H. Cell-cycle control and cortical development. Nat Rev Neurosci. 2007;8:438–450. doi: 10.1038/nrn2097. [DOI] [PubMed] [Google Scholar]
- 47.O’Leary DD, et al. Area patterning of the mammalian cortex. Neuron. 2007;56:252–269. doi: 10.1016/j.neuron.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 48.Catalano SM, Shatz CJ. Activity-dependent cortical target selection by thalamic axons. Science. 1998;281:559–562. doi: 10.1126/science.281.5376.559. [DOI] [PubMed] [Google Scholar]
- 49.Donoghue MJ, Rakic P. Molecular gradients and compartments in the embryonic primate cerebral cortex. Cereb Cortex. 1999;9:586–600. doi: 10.1093/cercor/9.6.586. [DOI] [PubMed] [Google Scholar]
- 50.Kolk SM, et al. A unique subpopulation of Tbr1-expressing deep layer neurons in the developing cerebral cortex. Mol Cell Neurosci. 2006;32:200–214. doi: 10.1016/j.mcn.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 51.Polleux F, et al. The timetable of laminar neurogenesis contributes to the specification of cortical areas in mouse isocortex. J Comp Neurol. 1997;385:95–116. [PubMed] [Google Scholar]
- 52.Poliakov GI. Development of the cerebral neocortex during first half of intrauterine life. In: Sarkisov SA, editor. Development of the Child’s Brain. Medicina: 1965. pp. 22–52. [Google Scholar]
- 53.Sidman RL, Rakic P. Neuronal migration, with special reference to developing human brain: a review. Brain Res. 1973;62:1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- 54.Kornack DR, Rakic P. Changes in cell-cycle kinetics during the development and evolution of primate neocortex. Proc Natl Acad Sci U S A. 1998;95:1242–1246. doi: 10.1073/pnas.95.3.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lukaszewicz A, et al. G1 phase regulation, area-specific cell cycle control, and cytoarchitectonics in the primate cortex. Neuron. 2005;47:353–364. doi: 10.1016/j.neuron.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zecevic N, Rakic P. Development of layer I neurons in the primate cerebral cortex. J Neurosci. 2001;21:5607–5619. doi: 10.1523/JNEUROSCI.21-15-05607.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Letinic K, et al. Origin of GABAergic neurons in the human neocortex. Nature. 2002;417:645–649. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- 58.Assimacopoulos S, et al. Identification of a Pax6-dependent epidermal growth factor family signaling source at the lateral edge of the embryonic cerebral cortex. J Neurosci. 2003;23:6399–6403. doi: 10.1523/JNEUROSCI.23-16-06399.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garel S, et al. Molecular regionalization of the neocortex is disrupted in Fgf8 hypomorphic mutants. Development. 2003;130:1903–1914. doi: 10.1242/dev.00416. [DOI] [PubMed] [Google Scholar]
- 60.Armentano M, et al. COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas. Nat Neurosci. 2007;10:1277–1286. doi: 10.1038/nn1958. [DOI] [PubMed] [Google Scholar]
- 61.Fukuchi-Shimogori T, Grove EA. Neocortex patterning by the secreted signaling molecule FGF8. Science. 2001;294:1071–1074. doi: 10.1126/science.1064252. [DOI] [PubMed] [Google Scholar]
- 62.Storm EE, et al. Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development. 2006;133:1831–1844. doi: 10.1242/dev.02324. [DOI] [PubMed] [Google Scholar]
- 63.Garel S, et al. Molecular regionalization of the neocortex is disrupted in Fgf8 hypomorphic mutants. Development. 2003;130:1903–1914. doi: 10.1242/dev.00416. [DOI] [PubMed] [Google Scholar]
- 64.Cholfin JA, Rubenstein JL. Patterning of frontal cortex subdivisions by Fgf17. Proc Natl Acad Sci U S A. 2007;104:7652–7657. doi: 10.1073/pnas.0702225104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dominguez MH, Rakic P. Neuroanatomy of the FGF system. J Comp Neurol. 2008;509:141–143. doi: 10.1002/cne.21748. [DOI] [PubMed] [Google Scholar]
- 66.Cholfin JA, Rubenstein JL. Frontal cortex subdivision patterning is coordinately regulated by Fgf8, Fgf17, and Emx2. J Comp Neurol. 2008;509:144–155. doi: 10.1002/cne.21709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martinez-Barbera JP, Beddington RS. Getting your head around Hex and Hesx1: forebrain formation in mouse. Int J Dev Biol. 2001;45:327–336. [PubMed] [Google Scholar]
- 68.Sahara S, et al. Sp8 exhibits reciprocal induction with Fgf8 but has an opposing effect on anterior-posterior cortical area patterning. Neural Dev. 2007;2:10. doi: 10.1186/1749-8104-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crossley PH, et al. Midbrain development induced by FGF8 in the chick embryo. Nature. 1996;380:66–68. doi: 10.1038/380066a0. [DOI] [PubMed] [Google Scholar]
- 70.Crossley PH, et al. Coordinate expression of Fgf8, Otx2, Bmp4, and Shh in the rostral prosencephalon during development of the telencephalic and optic vesicles. Neuroscience. 2001;108:183–206. doi: 10.1016/s0306-4522(01)00411-0. [DOI] [PubMed] [Google Scholar]
- 71.Ohkubo Y, et al. Coordinate regulation and synergistic actions of BMP4, SHH and FGF8 in the rostral prosencephalon regulate morphogenesis of the telencephalic and optic vesicles. Neuroscience. 2002;111:1–17. doi: 10.1016/s0306-4522(01)00616-9. [DOI] [PubMed] [Google Scholar]
- 72.Shimogori T, et al. Embryonic signaling centers expressing BMP, WNT and FGF proteins interact to pattern the cerebral cortex. Development. 2004;131:5639–5647. doi: 10.1242/dev.01428. [DOI] [PubMed] [Google Scholar]
- 73.Spoelgen R, et al. LRP2/megalin is required for patterning of the ventral telencephalon. Development. 2005;132:405–414. doi: 10.1242/dev.01580. [DOI] [PubMed] [Google Scholar]
- 74.Hamasaki T, et al. EMX2 regulates sizes and positioning of the primary sensory and motor areas in neocortex by direct specification of cortical progenitors. Neuron. 2004;43:359–372. doi: 10.1016/j.neuron.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 75.Leingartner A, et al. Cortical area size dictates performance at modality-specific behaviors. Proc Natl Acad Sci U S A. 2007;104:4153–4158. doi: 10.1073/pnas.0611723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leingartner A, et al. Cloning and cortical expression of rat Emx2 and adenovirus-mediated overexpression to assess its regulation of area-specific targeting of thalamocortical axons. Cereb Cortex. 2003;13:648–660. doi: 10.1093/cercor/13.6.648. [DOI] [PubMed] [Google Scholar]
- 77.Borello U, et al. FGF15 promotes neurogenesis and opposes FGF8 function during neocortical development. Neural Dev. 2008;3:17. doi: 10.1186/1749-8104-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hirata T, et al. Zinc-finger genes Fez and Fez-like function in the establishment of diencephalon subdivisions. Development. 2006;133:3993–4004. doi: 10.1242/dev.02585. [DOI] [PubMed] [Google Scholar]
- 79.Hashimoto-Torii K, et al. Differential activities of Sonic hedgehog mediated by Gli transcription factors define distinct neuronal subtypes in the dorsal thalamus. Mech Dev. 2003;120:1097–1111. doi: 10.1016/j.mod.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 80.Stamataki D, et al. A gradient of Gli activity mediates graded Sonic Hedgehog signaling in the neural tube. Genes Dev. 2005;19:626–641. doi: 10.1101/gad.325905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kiecker C, Lumsden A. Hedgehog signaling from the ZLI regulates diencephalic regional identity. Nat Neurosci. 2004;7:1242–1249. doi: 10.1038/nn1338. [DOI] [PubMed] [Google Scholar]
- 82.Vieira C, et al. Positional regulation of Pax2 expression pattern in mesencephalic and diencephalic alar plate. Neuroscience. 2006;137:7–11. doi: 10.1016/j.neuroscience.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 83.Scholpp S, et al. Otx1l, Otx2 and Irx1b establish and position the ZLI in the diencephalon. Development. 2007;134:3167–3176. doi: 10.1242/dev.001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 85.Liu Q, et al. Differential expression of COUP-TFI, CHL1, and two novel genes in developing neocortex identified by differential display PCR. J Neurosci. 2000;20:7682–7690. doi: 10.1523/JNEUROSCI.20-20-07682.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rubenstein JL, et al. Genetic control of cortical regionalization and connectivity. Cereb Cortex. 1999;9:524–532. doi: 10.1093/cercor/9.6.524. [DOI] [PubMed] [Google Scholar]
- 87.O’Leary DD, Nakagawa Y. Patterning centers, regulatory genes and extrinsic mechanisms controlling arealization of the neocortex. Curr Opin Neurobiol. 2002;12:14–25. doi: 10.1016/s0959-4388(02)00285-4. [DOI] [PubMed] [Google Scholar]
- 88.Fukuchi-Shimogori T, Grove EA. Emx2 patterns the neocortex by regulating FGF positional signaling. Nat Neurosci. 2003;6:825–831. doi: 10.1038/nn1093. [DOI] [PubMed] [Google Scholar]
- 89.Sestan N, et al. Independent parcellation of the embryonic visual cortex and thalamus revealed by combinatorial Eph/ephrin gene expression. Curr Biol. 2001;11:39–43. doi: 10.1016/s0960-9822(00)00043-9. [DOI] [PubMed] [Google Scholar]
- 90.Polleux F, et al. Transcriptional regulation of vertebrate axon guidance and synapse formation. Nat Rev Neurosci. 2007;8:331–340. doi: 10.1038/nrn2118. [DOI] [PubMed] [Google Scholar]
- 91.Shimogori T, Grove EA. Fibroblast growth factor 8 regulates neocortical guidance of area-specific thalamic innervation. J Neurosci. 2005;25:6550–6560. doi: 10.1523/JNEUROSCI.0453-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Datwani A, et al. Lesion-induced thalamocortical axonal plasticity in the S1 cortex is independent of NMDA receptor function in excitatory cortical neurons. J Neurosci. 2002;22:9171–9175. doi: 10.1523/JNEUROSCI.22-21-09171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Inan M, Crair MC. Development of cortical maps: perspectives from the barrel cortex. Neuroscientist. 2007;13:49–61. doi: 10.1177/1073858406296257. [DOI] [PubMed] [Google Scholar]
- 94.Rakic P. Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature. 1976;261:467–471. doi: 10.1038/261467a0. [DOI] [PubMed] [Google Scholar]
- 95.Crowley JC, Katz LC. Early development of ocular dominance columns. Science. 2000;290:1321–1324. doi: 10.1126/science.290.5495.1321. [DOI] [PubMed] [Google Scholar]
- 96.Sur M, Rubenstein JL. Patterning and plasticity of the cerebral cortex. Science. 2005;310:805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- 97.Rakic P, Riley KP. Regulation of axon number in primate optic nerve by prenatal binocular competition. Nature. 1983;305:135–137. doi: 10.1038/305135a0. [DOI] [PubMed] [Google Scholar]
- 98.Catalano SM, Shatz CJ. Activity-dependent cortical target selection by thalamic axons. Science. 1998;281:559–562. doi: 10.1126/science.281.5376.559. [DOI] [PubMed] [Google Scholar]
- 99.Bourgeois JP, Rakic P. Synaptogenesis in the occipital cortex of macaque monkey devoid of retinal input from early embryonic stages. Eur J Neurosci. 1996;8:942–950. doi: 10.1111/j.1460-9568.1996.tb01581.x. [DOI] [PubMed] [Google Scholar]
- 100.O’Leary DD, Koester SE. Development of projection neuron types, axon pathways, and patterned connections of the mammalian cortex. Neuron. 1993;10:991–1006. doi: 10.1016/0896-6273(93)90049-w. [DOI] [PubMed] [Google Scholar]
- 101.Schlaggar BL, O’Leary DD. Potential of visual cortex to develop an array of functional units unique to somatosensory cortex. Science. 1991;252:1556–1560. doi: 10.1126/science.2047863. [DOI] [PubMed] [Google Scholar]
- 102.Dehay C, et al. Cell-cycle kinetics of neocortical precursors are influenced by embryonic thalamic axons. J Neurosci. 2001;21:201–214. doi: 10.1523/JNEUROSCI.21-01-00201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zecevic N, Rakic P. Synaptogenesis in monkey somatosensory cortex. Cereb Cortex. 1991;1:510–523. doi: 10.1093/cercor/1.6.510. [DOI] [PubMed] [Google Scholar]
- 104.Temple S. The development of neural stem cells. Nature. 2001;414:112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- 105.Cubelos B, et al. Cux-2 controls the proliferation of neuronal intermediate precursors of the cortical subventricular zone. Cereb Cortex. 2008;18:1758–1770. doi: 10.1093/cercor/bhm199. [DOI] [PubMed] [Google Scholar]
- 106.Shen Q, et al. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat Neurosci. 2006;9:743–751. doi: 10.1038/nn1694. [DOI] [PubMed] [Google Scholar]
- 107.Chen B, et al. Fezl regulates the differentiation and axon targeting of layer 5 subcortical projection neurons in cerebral cortex. Proc Natl Acad Sci U S A. 2005;102:17184–17189. doi: 10.1073/pnas.0508732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen JG, et al. Zfp312 is required for subcortical axonal projections and dendritic morphology of deep-layer pyramidal neurons of the cerebral cortex. Proc Natl Acad Sci U S A. 2005;102:17792–17797. doi: 10.1073/pnas.0509032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Molyneaux BJ, et al. Fezl is required for the birth and specification of corticospinal motor neurons. Neuron. 2005;47:817–831. doi: 10.1016/j.neuron.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 110.Noctor SC, et al. Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. J Neurosci. 2002;22:3161–3173. doi: 10.1523/JNEUROSCI.22-08-03161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Doetsch F, et al. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 112.Johansson CB, et al. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- 113.Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 114.Garcia AD, et al. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 115.Coskun V, et al. CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain. Proc Natl Acad Sci U S A. 2008;105:1026–1031. doi: 10.1073/pnas.0710000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 117.Sohur US, et al. Adult neurogenesis and cellular brain repair with neural progenitors, precursors and stem cells. Philos Trans R Soc Lond B Biol Sci. 2006;361:1477–1497. doi: 10.1098/rstb.2006.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Breunig JJ, et al. Everything that glitters isn’t gold: a critical review of postnatal neural precursor analyses. Cell Stem Cell. 2007;1:612–627. doi: 10.1016/j.stem.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 119.Stiles CD. Lost in space: misregulated positional cues create tripotent neural progenitors in cell culture. Neuron. 2003;40:447–449. doi: 10.1016/s0896-6273(03)00683-4. [DOI] [PubMed] [Google Scholar]
- 120.Hack MA, et al. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci. 2005;8:865–872. doi: 10.1038/nn1479. [DOI] [PubMed] [Google Scholar]
- 121.Kohwi M, et al. Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. J Neurosci. 2005;25:6997–7003. doi: 10.1523/JNEUROSCI.1435-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Willaime-Morawek S, et al. Embryonic cortical neural stem cells migrate ventrally and persist as postnatal striatal stem cells. J Cell Biol. 2006;175:159–168. doi: 10.1083/jcb.200604123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Long JE, et al. Dlx-dependent and -independent regulation of olfactory bulb interneuron differentiation. J Neurosci. 2007;27:3230–3243. doi: 10.1523/JNEUROSCI.5265-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Merkle FT, et al. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- 125.Young KM, et al. Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J Neurosci. 2007;27:8286–8296. doi: 10.1523/JNEUROSCI.0476-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hochstim C, et al. Identification of positionally distinct astrocyte subtypes whose identities are specified by a homeodomain code. Cell. 2008;133:510–522. doi: 10.1016/j.cell.2008.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kelsch W, et al. Distinct mammalian precursors are committed to generate neurons with defined dendritic projection patterns. PLoS Biol. 2007;5:e300. doi: 10.1371/journal.pbio.0050300. [DOI] [PMC free article] [PubMed] [Google Scholar]