Abstract
Although bereavement is associated with increased morbidity and mortality in the surviving spouse, some widow(er)s remain healthy. Genetic variability in expression of inflammatory markers in response to stress may be the key to this observation. The present study compares bereaved vs. married/partnered older adults, investigating the impact of bereavement status, pro-inflammatory cytokine single nucleotide polymorphisms (SNPs) on circulating markers of inflammation and hypothesizing a gene by environment (GxE) effect. The study sample included 64 older adults, of which 36 were widow(er)s. Circulating levels of inflammatory markers IL-6, IL-1RA and sTNFRII were measured. Participants were genotyped for SNPs in the IL-6 gene (IL-6 – 174 and –572), the IL-1β gene (IL-1β –511), and TNF-α gene (TNF-α –308). Grief severity was assessed with the Inventory of Complicated Grief. Bereaved participants had higher circulating levels of IL-1RA and IL-6. This increase could not be explained by pro-inflammatory genotype frequency differences, or Complicated Grief diagnosis. However, a GxE effect with the IL-6 –174 SNP moderated individual vulnerability to higher circulating levels of inflammation resulting from bereavement exposure. These results suggest a possible mechanism for the increase in morbidity and mortality in the surviving spouse. Genetic variability interacts with an environmental stressor, leading to increased inflammatory markers in genetically susceptible subjects only. For these patients, clinical interventions for bereavement-related stressor reduction might be crucial for overall health.
Keywords: Aging; Bereavement; Cytokines; Gene; Gene by environment; Grief; IL-1; IL-6, TNF-a; Inflammation
1. Introduction
The death of a spouse is one of the most distressing life events, and is associated with an increase in morbidity and mortality risk, independent of a host of covariates (Boyle et al., 2011). Stressful life events are linked to dysregulation of the immune system and increased cellular inflammatory signaling (Irwin and Cole, 2011). Because it is our long-term goal to determine the mechanism linking bereavement and morbidity/mortality, and because substantial evidence shows that increases in inflammatory markers such as interleukin (IL)-6 are associated with mortality risk (Ershler and Keller, 2000), we investigated whether responses to spousal bereavement are impacted by markers of inflammation.
Chronic stress as a result of caregiving (i.e., those taking care of a severely ill family member) or social isolation is associated with the up-regulated gene expression and production of systemic markers of inflammation (Kiecolt-Glaser et al., 2003; McDade et al., 2006), especially of IL-6, IL-1 and TNF-alpha (Dantzer, 2001). Further data indicate genetic variability in the expression of inflammatory markers in response to stress (Cole et al., 2010, 2011). For example, the presence of the guanine/cytosine (G/C) single nucleotide polymorphism (SNP) in the promoter of the IL-6 gene (IL-6) –174 bp upstream of the transcription start site affects the binding of a β-adrenergic-sensitive transcription factor, GATA-1 (Cole et al., 2010). Following an in vitro β-adrenergic stimulus, the IL-6 –174G SNP leads to increased IL-6 production, while the IL-6 – 174C SNP does not. In vivo, an association between depression and increased mortality risk was found only in IL-6 174GG homozygous patients (Cole et al., 2010), but levels of inflammatory markers were not characterized. Thus, it is not known whether genetic variability in cytokine gene polymorphisms alters the influence of life stress on the production of inflammatory markers in humans.
Given evidence that some widow(er)s show an increase in morbidity and mortality, but others remain healthy and thus appear to be protected from this “widowhood effect” (Buckley et al., 2011), the present study hypothesizes that bereavement may be a socio-environmental stress that interacts with pro-inflammatory genetic variation to produce modulated levels of inflammation. This gene by environment (GxE) interaction predicts that among widow(er)s, those with genotypes that are associated with higher expression of inflammatory markers would exhibit greater systemic signs of inflammation, e.g. higher circulating levels of inflammatory markers. Conversely, widow(er)s with low pro-inflammatory genotypes are hypothesized to have similar circulating levels as non-bereaved individuals.
The work reported here compares bereaved and healthy married/partnered older adults, first investigating the impact of bereavement status on circulating levels of inflammatory markers IL-6, IL-1RA as a marker of IL-1 activity (Arend et al., 1998) and soluble tumor necrosis factor receptor II (sTNFRII) as a surrogate marker of TNF-α levels (Diez-Ruiz et al., 1995; Schuld et al., 1999). Second, corresponding to our circulating measures, we investigated the impact of pro-inflammatory cytokine SNPs in the IL-6, IL-1β, and tumor necrosis factor alpha (TNF-α genes on the mentioned inflammatory markers and a possible interaction with bereavement status. Given evidence of in vitro data for the GxE pathway for the IL-6 –174 SNP, we primarily focused on the interaction between bereavement stress and this polymorphism.
2. Methods and materials
2.1. Participants
A total of 64 older adults (age 61–83) were recruited from the Los Angeles community. Thirty-six were widowed; on average they had experienced the death of their spouse or partner in the past 2 years (mean: 23.75 months; range 2–69 months). Widowhood was defined as the death of a co-habiting life partner of the same generation, although not necessarily legally married (the majority were married). The other 28 participants were non-bereaved control subjects who were married/partnered and had not lost a first-degree relative or spouse in the prior 36 months.
The sample of 64 participants was mainly of Caucasian descent (77%). Other ethnicities included African-American (13%), Hispanic (6%), Asian (2%), Pacific Islander (1%) and other (1%). For statistical analyses we created a dichotomous variable (Caucasian vs. Non-Caucasian).
To assess the level of stress resulting from life events among all participants, we used the revised Social Readjustment Rating Scale (SRRS-R) (Hobson and Delunas, 2001). The SRRS-R covers 51 life events that may have occurred in the past 12 months. In addition, perceived stress was assessed with the Perceived Stress Scale (PSS), a 14-item measure of the degree to which respondents appraise stressful situations that occurred during the past month (Cohen et al., 1983). In our study internal consistency was α = .82.
Exclusion criteria included: (a) presence of current major psychiatric disorder (e.g., Major Depressive Disorder, alcohol or substance dependence) as assessed with the DSM-IV SCID-I (Spitzer et al., 1994); (b) use of psychotropic medications initiated since the death event; (c) immunosuppressive medication (d) major medical illnesses (e.g., cancer); (e) current smokers (due to potential confounding effects on markers of inflammation) (O’Connor et al., 2009); (f) participants with IL-6 levels > 2 standard deviations above the mean, if there was self-reported illness in the past two weeks.
Bereaved participants were given the Inventory of Complicated Grief (ICG) (Prigerson et al., 1995). Consistent with prior studies we used an ICG total score of 30 or higher for diagnosis of Complicated Grief (Shear et al., 2005). Internal consistency was α = .90.
The Institutional Review Board of UCLA approved the study and after complete description of the study all participants gave written informed consent.
2.2. Levels of circulating markers of inflammation
EDTA plasma was collected and stored at −80 °C until all samples were obtained. IL-6 was measured using Quantikine High Sensitivity Immunoassay kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. IL-1RA and sTNFRII were measured with Quantikine Immunoassay kits (R&D Systems).
All samples were assayed in duplicate, and an internal quality control sample was included on every assay; the interassay and intraassay coefficients of variation were less than 8% and 5%, respectively. None of the circulating inflammatory markers were below the limit of detection. Personnel who performed immunoassays were blinded to the identity and diagnostic status of study participants.
2.3. DNA extraction and genotyping
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood samples using Ficoll separation and stored at −80 °C until processing. Genomic DNA was isolated with Wizard® Genomic DNA Purification Kit (Promega, Madison, WI) followed by determination of concentration and purity. SNPs in the genes of interest were determined with TaqMan® SNP Genotyping Assays (Applied Biosystems, Foster City, CA). Corresponding to our circulating measures we investigated SNPs that have been identified as influencing quantitative gene expression levels: IL-6 – 174 (Fishman et al., 1998; Olomolaiye et al., 1998) and –572 (Terry et al., 2000); IL-1β –511 (di Giovine et al., 1992); and TNF-α –308 (Wilson et al., 1992).
For the IL-6 –174 G/C and IL-6 –572 G/C polymorphisms we used Custom TaqMan® SNP Genotyping Assays; primer sequences are shown in Table 1. Custom assays were run with control DNA of known genotype, for confirmation of assay quality control. The other two SNPs (IL-1β –511 A/G, TNF-α –308 G/A) were genotyped using commercially available TaqMan® SNP Genotyping Assays. These assays were used according to the manufacturer’s instructions. Genotyping for all samples was done in duplicate (concordance >.99) with call rates of all SNPs being >.95. Investigators who performed SNP analyses were blinded to the identity and diagnostic status of the study participants.
Table 1.
Primer and probe sequences used for genotyping of IL-6 –174 and IL-6 –572 SNP
| Primer name | Sequence 5′–3′ |
| IL-6 –174 forward | GACGACCTAAGCTGCACTTTTC |
| IL-6 –174 reverse | GGGCTGATTGGAAACCTTATTAAGATTG |
| IL-6 –572 forward | GCCTTGAAGTAACTGCACGAAATT |
| IL-6 –572 reverse | CCAGTCATCTGAGTTCTTCTGTGTT |
| Probe name | Sequence 5′–3′I |
| IL-6 –174C VIC | CTTTAGCATGGCAAGAC |
| IL-6 –174G FAM | CTTTAGCATCGCAAGAC |
| IL-6 –572G VIC | TACAACAGCCCCTCACAG |
| IL-6 –572C FAM | AACAGCCGCTCACAG |
A = adenine, C = cytosine, G = guanine, T = thymine; VIC = VIC®; FAM = 6-carboxyfluorescein.
2.4. Statistical Analysis
Statistical analyses were carried out using SPSS 19 (SPSS, Chicago, IL, USA). Group comparisons were assessed by ANOVAs, or chi-square analyses, and t-tests were used for planned post hoc comparisons. Linear and multivariate logistic regression analyses analyzed relationships while controlling for demographic, medical, or biobehavioral confounds. Biomarker data were log-transformed for statistical analyses; untransformed data were used for tables and graphical purposes. Significance was defined as p-values <.05.
In order to provide meaningful statistics we grouped pro-inflammatory cytokine SNPs in accordance with their cytokine production profile (high vs. low producer, see Table 2). The IL-6–174 SNP was grouped according to the finding that the C allele is not sensitive to β-adrenergic activation of GATA1 and therefore –174C allele carriers (G/C or C/C) have less IL-6 production in response to sympathetic nervous system activation (Cole et al., 2010). Grouping for the IL-6 – 572 polymorphism was done according to the finding that –572C allele carriers (G/C or C/C) show increased IL-6 transcription (Brull et al., 2001; Ferrari, 2003; Gu et al., 2010). The majority of studies have linked subjects who are homozygous for the A allele at the IL-1β – 511 SNP (also described as homozygous T, referring to the complementing T allele) with higher production of IL-1β compared to G allele carriers (G/A or G/G) (Hall et al., 2004; Hwang et al., 2002; Pociot et al., 1992). Subjects who are homozygous for the –308G allele at the TNF-α – 308 SNP have consistently been shown to have significantly lower cytokine production than –308A allele carriers (A/G or A/A) (Kroeger et al., 2000; Mira et al., 1999; Wilson et al., 1997).
Table 2.
Genotype frequencies
| Bereaved (N = 36) |
Non-bereaved (N = 28) |
X2 | P-value | |||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| IL-6 –174GG (high) | 19 | 53 | 13 | 46 | 0.25 | 0.61 |
| IL-6 –174C carriers (low) | 17 | 47 | 15 | 54 | ||
| IL-6 –174GC | 14 | 39 | 11 | 40 | ||
| IL-6 –174CC | 3 | 8 | 4 | 14 | ||
| Allele frequency | 0.72G, 0.28C | 0.66G, 0.34C | ||||
| IL-6 –572C carriers (high) | 7 | 19 | 5 | 18 | 0.03 | 0.87 |
| IL-6 –572GC | 7 | 19 | 4 | 14 | ||
| IL-6 –572CC | 0 | 0 | 1 | 4 | ||
| IL-6 –572GG (low) | 29 | 81 | 23 | 82 | ||
| Allele frequency | 0.90G, 0.10C | 0.89G,0.11 | ||||
| IL-1β –511AA (high) | 10 | 28 | 4 | 14 | 1.68 | 0.2 |
| IL-1β –511G carriers (low) | 26 | 72 | 24 | 86 | ||
| IL-1β –511AG | 14 | 39 | 11 | 40 | ||
| IL-1β –511GG | 12 | 33 | 13 | 46 | ||
| Allele frequency | 0.47A, 0.53G | 0.32A, 0.68G | ||||
| TNF-α –308A carriers (high) | 10 | 28 | 2 | 7 | 4.4 | 0.04 |
| TNF-α –308GA | 8 | 22 | 2 | 7 | ||
| TNF-α –308AA | 2 | 6 | 0 | 0 | ||
| TNF-α –308GG (low) | 26 | 72 | 26 | 93 | ||
| Allele frequency | 0.83G, 0.17A | 0.96G, 0.04A | ||||
Genotype frequencies N (%), X2 value (chi square test). Genotype frequency statistic based on comparison (shown in bold) of GG vs. C carriers for IL-6 –174 and –572, AA vs. G carriers for IL-1β –511; GG vs. A carriers for TNF-α –308.
3. Results
Group comparisons on demographic characteristics using ANOVAs and Chi-square tests are shown in Table 3. There were no significant group differences between bereaved and non-bereaved study participants.
Table 3.
Demographic characteristics
| Bereaved (N = 36) |
Non-bereaved (N = 28) |
F-value/X2 | P-value | |||
|---|---|---|---|---|---|---|
| Mean/N | SD/% | Mean | SD/% | |||
| Age | 72.9 | 5.8 | 72.4 | 4.2 | 0.17 | 0.69 |
| Gender (female) | 23 | 64% | 15 | 54% | 0.7 | 0.4 |
| Ethnicity (non-Caucasian) | 10 | 28% | 5 | 18% | 0.86 | 0.35 |
| Employment (retired) | 23 | 64% | 15 | 54% | 0.7 | 0.4 |
| Education (post graduate) | 14 | 39% | 13 | 46% | 0.37 | 0.55 |
| Years married/partnered | 35.8 | 17.6 | 41.2 | 11.2 | 2 | 0.16 |
| Body mass index | 26.6 | 5.2 | 27.4 | 5.6 | 0.31 | 0.58 |
| Alcohol (drinks per week) | 2.1 | 4.3 | 1.7 | 2.9 | 0.18 | 0.67 |
Continuous variables: mean (±SD), F-value (ANOVA); categorical variables: N (%), X2 value (chi square test).
3.1. Group differences in levels of circulating markers of inflammation
Body mass index (BMI) and age have been shown to affect inflammatory marker production and therefore were included with bereavement status in regression analyses (O’Connor et al., 2009). Controlling for BMI and age, bereavement was a significant predictor of higher levels of IL-6 (2.8 ± 2.2 pg/ml vs. 1.9 ± 0.9 pg/ml, β = .26, p = .04), and IL1-RA (305 ± 167 pg/ml vs. 227 ± 94 pg/ml, β = .33, p = .01), but not for sTNFRII (2903 ± 1066 pg/ml vs. 2750 ± 725 pg/ml, β = .08, p = .55). In order to rule out that differences between the groups could be attributed to more current stressors other than bereavement, we correlated levels of pro-inflammatory cytokines with PSS score, SRRS-r score and time since bereavement. Linear correlations were all not significant.
3.2. Group differences in genotype frequencies
Genotype and allele frequencies are presented in Table 2. In spite of the ethnic diversity, genotype frequencies met Hardy–Weinberg equilibrium and were consistent with the literature (see HapMap allele frequencies at SNP database of the National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov/snp/).
None of the grouped genotypes differed in gender, but significant differences in ethnicity (Caucasian vs. non-Caucasian) were observed for the IL-6 –572 SNP (Fisher’s exact test, one-sided p < .01), and IL-1β –511 SNP (Fisher’s exact test, one-sided p = .01). Therefore ethnicity was controlled for in all analyses involving IL-6 –572 and IL-1β –511 SNPs.
As the reported differences in pro-inflammatory cytokine levels between bereaved and non-bereaved participants might be moderated by the difference of frequency of a high/low producing cytokine-genotype we investigated proportional genotype distribution between the two groups. Analyses revealed a significant difference in distribution between the groups only for TNF-α –308 (Fisher’s exact test, one-sided p = .04). Twenty-eight percent bereaved versus 7% non-bereaved participants were identified as carriers of the high-producing A-allele. Although this might have been due to sampling bias, as described above, circulating levels of sTNFRII did not differ between groups, so the unbalanced distribution of genotype appeared not have a detectable effect on circulating level.
3.3. Gene by Environment (GxE) effect
Given evidence of in vitro data for the GxE pathway for the IL-6 – 174 SNP, we primarily focused on this polymorphism. To determine whether genotype moderated IL-6 as a function of group, simple regressions were computed separately for bereaved/non-bereaved (Fig. 1 and Table 4). Despite the death of a spouse, bereaved IL-6 –174C allele carriers had virtually the same mean level of IL-6 as non-bereaved –174C carriers (controlling for BMI β = .06, p = .75). In contrast, bereaved IL-6 –174G homozygotes had over twofold higher mean levels of IL-6 compared to non-bereaved – 174G homozygotes (controlling for BMI β = .37, p = .03). Using regression analyses to investigate the relationship between bereaved/non-bereaved groups and IL-6 – 174 genotype revealed an interaction term that approached significance for predicting IL-6 levels (β = –0.38, p = 0.09), over and above the main effects of bereavement group and genotype.
Fig. 1.
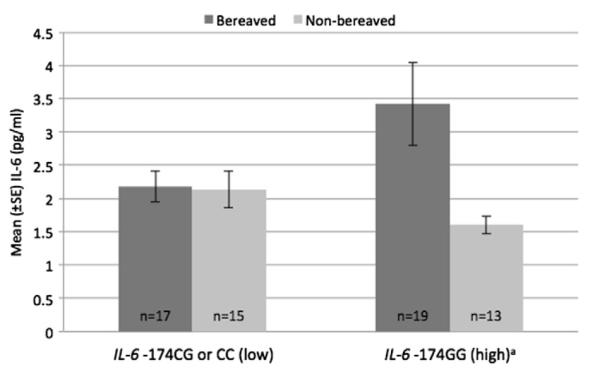
Gene (IL-6 –174) by environment (bereavement) effect on circulating IL-6. aSignificant difference in circulating IL-6 between bereaved and non-bereaved subjects with IL-6 –174GG genotype. F (1,30) = 5.09, p = .03.
Table 4.
Gene (SNP) by environment (bereavement) effect on inflammatory marker levels
| N | IL-6 pg/ml | SD | β | P-value | ||
|---|---|---|---|---|---|---|
| IL-6 –174C carrier | Bereaved | 17 | 2.2 | 1 | ||
| Non-bereaved | 15 | 2.1 | 1.1 | 0.06 | 0.75 | |
| IL-6 –174GG | Bereaved | 19 | 3.4 | 2.7 | ||
| Non-bereaved | 13 | 1.6 | 0.5 | 0.37 | 0.03 | |
| IL-6 –572GG | Bereaved | 29 | 2.7 | 1.9 | ||
| Non-bereaved | 23 | 2 | 0.9 | 0.18 | 0.2 | |
| IL-6 –572C carrier | Bereaved | 7 | 3.6 | 3 | ||
| Non-bereaved | 5 | 1.3 | 0.3 | 0.56 | 0.09 | |
| N | IL-1RA pg/ml | SD | β | P-value | ||
|
|
||||||
| IL-1β –511G carrier | Bereaved | 26 | 311.1 | 189 | ||
| Non-bereaved | 24 | 239.6 | 94.8 | 0.27 | 0.05 | |
| IL-1β –511AA | Bereaved | 10 | 289.7 | 91.5 | ||
| Non-bereaved | 4 | 149.7 | 29.8 | 0.63 | 0.01 | |
| N | sTNFRII pg/ml | SD | β | P-value | ||
|
|
||||||
| TNF-α –308GG | Bereaved | 26 | 2665 | 738 | ||
| Non-bereaved | 26 | 2764 | 750 | −0.06 | 0.7 | |
| TNF-α –308A carrier | Bereaved | 10 | 3522 | 481 | ||
| Non-bereaved | 2 | 2561 | 178 | 0.28 | 0.38 | |
β and P-values from linear regressions, controlling for BMI; biomarker data were log-transformed for statistical analyses.
Analyses were performed to investigate whether a similar GxE effect exists for the other polymorphisms (Table 4). First, bereaved IL-6 –572G allele (low IL-6 producing) homozygotes had similar mean levels of IL-6 as non-bereaved –572G homozygotes (controlling for BMI β = .18, p = .20). In contrast, bereaved IL-6 –572C allele carriers trended toward higher levels of IL-6 compared to non-bereaved –572C allele carriers (controlling for BMI β = .56, p = .09). Controlling for ethnicity in each of the preceding analyses that had sufficient power (n > 20, see Table 4) did not change the results. Second, bereaved IL-1β –511G allele (low IL-1 producing) carriers had different mean levels of IL-1RA as non-bereaved – 511G carriers (controlling for BMI β = .27, p = .05). Also, bereaved IL-1β –511A homozygotes had significantly higher mean levels of IL-1RA compared to non-bereaved 511A homozygotes (controlling for BMI β = .63, p = .01). Again, controlling for ethnicity in each of the preceding analyses that had sufficient power did not change the results. Third, no GxE effect could be seen for TNF-α –308 SNP. Bereaved TNF-α –308G allele (low TNF producing) homozygotes had similar mean levels of sTNFRII as non-bereaved 308G homozygotes (controlling for BMI β = .06, p = .70). Similarly, bereaved TNF-α –308A allele carriers had equal levels of sTNFRII compared to non-bereaved –308A allele carriers (controlling for BMI β = .28, p = .38).
3.4. Effects of grief severity (Complicated Grief)
The intensity of grief symptoms may influence the extent of the stress response and thus could also have an impact on cytokine production. We assessed all bereaved participants to determine whether they met criteria for Complicated Grief, a syndrome characterized by persistent separation distress with feelings of overwhelming yearning and preoccupation with the deceased (Prigerson et al., 2009; Shear et al., 2011). Regressing grief severity (ICG score) on levels of inflammatory markers did not reach significance for any of the markers, controlling for age and BMI (IL-6: F (3, 31) = 1.76, p = .18; IL-1RA: F (3, 31) = .49, p = .69; sTNFRII: F (3, 31) = 1.91, p = .15). Thirteen of the 36 bereaved subjects met diagnostic criteria for Complicated Grief. Not surprisingly, regressing the dichotomous subgroups Complicated Grief and Non-complicated Grief on the cytokine markers, and controlling for age and BMI, revealed that group did not predict any of the markers. Genotype frequencies between Complicated Grief and Non-complicated Grief subgroups were not significantly different, which may be due to lack of power due to small sample sizes rather than to biological differences.
In addition, BDI-II data was available on a subset of the sample: 23 bereaved subjects (9 Complicated Grief) and 15 non-bereaved subjects. Since we excluded for MDD the overall BDI scores were very low. We could not find a GxE effect for the IL-6 –174 SNP and depressive symptoms. This was also true for the other SNPs.
4. Discussion
To our knowledge, this is the first study to show a GxE effect for stress, genotype and circulating markers of inflammation in a human sample. First, IL-6 and IL-1RA pro-inflammatory cytokine levels are higher in bereaved individuals than in married/partnered controls. Secondly, the present data suggest that the IL-6 –174 SNP moderates individual vulnerability to higher circulating levels of inflammation resulting from bereavement exposure (a GxE effect).
Consistent with a recent report in other patient populations (Cole et al., 2010), sympathetic nervous system activation caused by bereavement-related distress may lead to GATA1-mediated upregulation of IL-6 production, but only in homozygous subjects with two GATA1-sensitive –174G alleles. In contrast, carrying the –174C allele (which renders the IL-6 promoter unresponsive to GATA1) appears to be protective. As hypothesized, bereaved –174C carriers had lower circulating IL-6 levels, which may provide protection from bereavement-related risk for morbidity and mortality. The present data support our GxE hypothesis and may help to explain the relationship between the “widowhood effect” and inflammatory disease (Boyle et al., 2011). The widowhood effect has been shown for ischemic disease, respiratory disease and cerebrovascular disease, including stroke (Martikainen and Valkonen, 1996). Because the mechanism between stress and inflammation is well worked out, sympathetic nervous system-induced inflammatory mechanisms specifically should also be investigated as a possible contributor to disease risk subsequent to bereavement.
The trend toward a GxE effect seen for the IL-6 –572 SNP has also not previously been described in the literature. The mechanism(s) that may make two copies of the IL-6 –572G allele protective for bereaved participants from higher levels of IL-6, remains to be elucidated. The latter effect could also be driven by the IL-6 – 174 SNP since both polymorphisms are located in proximity to each other and affect the same cytokine. Missing to detect a GxE effect for TNF-α –308 SNP could have also been due to lack of power as there were only 2 non-bereaved and 10 bereaved TNF-α – 308A carriers.
In comparison to the GxE effect, genotypes alone did not account for the observed significant differences in circulating levels of IL-6 between bereaved and non-bereaved participants, as there were no significant differences in frequencies of the high/low producing genotype of the corresponding polymorphism. However, the statistical power of the analyses needs to be considered because of the small group size in some subgroups (see Table 2).
The observation that cytokine levels do not differ between the two bereaved subgroups with and without Complicated Grief might be of importance, if (as our data suggest) this disorder does not share the pro-inflammatory cytokine pathway seen in other psychopathologies like Major Depressive Disorder (Miller et al., 2009). Clinical evidence shows that Complicated Grief is distinct from Major Depressive Disorder (Prigerson et al., 1995). Although provocative, we cannot conclude from a negative result that Complicated Grief is not associated with the investigated cytokine levels or cytokine polymorphisms.
4.1. Limitations
Limitations of the present study, particularly with regard to the genotyping data, include a small sample size. However, assuming the statistical models are stable, these significant results in a small sample size would likely be due to a strong effect size. While for the IL-6 –174 the GxE effect has been shown on a molecular level (Cole et al., 2010), our results for the other investigated SNPs remain preliminary and need replication in a larger sample. Furthermore, the range of time of bereavement was sizable, ranging from 2 to 69 months. However, effects of grief have been shown to have physiological effect for as long as nine years (Santić et al., 2006). The lack of correlations between levels of pro-inflammatory cytokines and PSS score, SRRS-r score or time since bereavement shows that the observed increase in levels of cytokines cannot be attributed to more current stressors. Therefore we assume the death of a spouse as the predominant stressful life event leading to SNS activation. One strength of the study sample was its inclusion of participants with different ethnicity, given that there were neither differences between the bereaved subgroup and the married/partnered controls (Caucasian vs. non-Caucasian) nor did ethnic diversity affect the genotype frequencies (Hardy–Weinberg equilibrium was met for all genotypes). Future research should focus on replicating these findings in a larger sample, using a longitudinal study design to investigate changes over the course of bereavement and elucidate underlying causality. We suggest further investigation of polymorphisms in pro- and anti-inflammatory genes including specially designed primers to allow precise haplotype analyses. Additional RNA transcription analyses would help to interpret the genotyping data by determining up- and down-regulated genes in response to the death of spouse.
Summary
To summarize, the present study found elevated levels of the inflammatory markers IL-1RA and IL-6 in the bereaved group. This increase in systemic inflammation as a response of the immune system to the death of spouse could not be explained by either pro-inflammatory genotype frequency differences, severity of grief or Complicated Grief. However, a GxE effect with a SNP at the IL-6 – 174 gene moderates individual vulnerability to higher circulating levels of inflammation resulting from bereavement exposure. These results pose implications for clinical interventions by demonstrating the importance of bereavement-related stressor reduction, particularly in genetically susceptible patients.
Acknowledgments
This research was supported by the National Institute of Aging (K01-AG028404), UCLA Older Americans Independence Center Inflammatory Biology Core (NIH/NIA Grant P30-AG028748) and the UCLA Cousins Center for Psychoneuroimmunology. We would like to thank Richard Olmstead for his comments on the statistical analyses, and Ryan Sadakane and Susanne Yoon for their technical assistance.
Footnotes
Conflict of Interest Statement All authors declare that there are no conflicts of interest.
References
- Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu. Rev. Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- Boyle PJ, Feng Z, Raab GM. Does widowhood increase mortality risk?: testing for selection effects by comparing causes of spousal death. Epidemiology (Cambridge, Mass.) 2011;22:1–5. doi: 10.1097/EDE.0b013e3181fdcc0b. [DOI] [PubMed] [Google Scholar]
- Brull DJ, Montgomery HE, Sanders J, Dhamrait S, Luong L, Rumley A, Lowe GDO, Humphries SE. Interleukin-6 gene -174G>C and -572G>C promoter polymorphisms are strong predictors of plasma interleukin-6 levels after coronary artery bypass surgery. Arterioscler. Thromb. Vasc. Biol. 2001;21:1458–1463. doi: 10.1161/hq0901.094280. [DOI] [PubMed] [Google Scholar]
- Buckley T, Mihailidou AS, Bartrop R, McKinley S, Ward C, Morel-Kopp M-C, Spinaze M, Tofler GH. Haemodynamic changes during early bereavement: potential contribution to increased cardiovascular risk. Heart Lung Circ. 2011;20:91–98. doi: 10.1016/j.hlc.2010.10.073. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Cole SW, Arevalo JMG, Manu K, Telzer EH, Kiang L, Bower JE, Irwin MR, Fuligni AJ. Antagonistic pleiotropy at the human IL6 promoter confers genetic resilience to the pro-inflammatory effects of adverse social conditions in adolescence. Dev. Psychol. 2011;47:1173–1180. doi: 10.1037/a0023871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Arevalo JMG, Takahashi R, Sloan EK, Lutgendorf SK, Sood AK, Sheridan JF, Seeman TE. Computational identification of gene-social environment interaction at the human IL6 locus. Proc. Natl. Acad. Sci. USA. 2010;107:5681–5686. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann. NY. Acad. Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Diez-Ruiz A, Tilz GP, Zangerle R, Baier-Bitterlich G, Wachter H, Fuchs D. Soluble receptors for tumour necrosis factor in clinical laboratory diagnosis. Eur. J. Haematol. 1995;54:1–8. doi: 10.1111/j.1600-0609.1995.tb01618.x. [DOI] [PubMed] [Google Scholar]
- Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu. Rev. Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- Ferrari SL. Two promoter polymorphisms regulating interleukin-6 gene expression are associated with circulating levels of C-reactive protein and markers of bone resorption in postmenopausal women. J. Clin. Endocrinol. Metab. 2003;88:255–259. doi: 10.1210/jc.2002-020092. [DOI] [PubMed] [Google Scholar]
- Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, Humphries S, Woo P. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J. Clin. Invest. 1998;102:1369–1376. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Giovine FS, Takhsh E, Blakemore AI, Duff GW. Single base polymorphism at -511 in the human interleukin-1 beta gene (IL1 beta) Hum. Mol. Genet. 1992;1:450. doi: 10.1093/hmg/1.6.450. [DOI] [PubMed] [Google Scholar]
- Gu W, Zeng L, Zhou J, Jiang D-P, Zhang L, Du D-Y, Hu P, Chen K, Liu Q, Wang Z-G, Jiang J-X. Clinical relevance of 13 cytokine gene polymorphisms in Chinese major trauma patients. Intensive Care Med. 2010;36:1261–1265. doi: 10.1007/s00134-010-1797-5. [DOI] [PubMed] [Google Scholar]
- Hall SK, Perregaux DG, Gabel CA, Woodworth T, Durham LK, Huizinga TWF, Breedveld FC, Seymour AB. Correlation of polymorphic variation in the promoter region of the interleukin-1 beta gene with secretion of interleukin-1 beta protein. Arthritis Rheum. 2004;50:1976–1983. doi: 10.1002/art.20310. [DOI] [PubMed] [Google Scholar]
- Hobson CJ, Delunas L. National norms and life-event frequencies for the revised social readjustment rating scale. Int. J. Stress Manage. 2001;8:299–314. [Google Scholar]
- Hwang I-R, Kodama T, Kikuchi S, Sakai K, Peterson LE, Graham DY, Yamaoka Y. Effect of interleukin 1 polymorphisms on gastric mucosal interleukin 1beta production in Helicobacter pylori infection. Gastroenterology. 2002;123:1793–1803. doi: 10.1053/gast.2002.37043. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat. Rev. Immunol. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the pro-inflammatory cytokine IL-6. Proc. Natl. Acad. Sci. USA. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger KM, Steer JH, Joyce DA, Abraham LJ. Effects of stimulus and cell type on the expression of the -308 tumour necrosis factor promoter polymorphism. Cytokine. 2000;12:110–119. doi: 10.1006/cyto.1999.0529. [DOI] [PubMed] [Google Scholar]
- Martikainen P, Valkonen T. Mortality after the death of a spouse: rates and causes of death in a large Finnish cohort. Am. J. Public Health. 1996;86:1087. doi: 10.2105/ajph.86.8_pt_1.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Hawkley LC, Cacioppo JT. Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: the Chicago health, aging, and social relations study. Psychosom. Med. 2006;68:376–381. doi: 10.1097/01.psy.0000221371.43607.64. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira JP, Cariou A, Grall F, Delclaux C, Losser MR, Heshmati F, Cheval C, Monchi M, Teboul JL, Riché F, Leleu G, Arbibe L, Mignon A, Delpech M, Dhainaut JF. Association of TNF2, a TNF-alpha promoter polymorphism, with septic shock susceptibility and mortality: a multicenter study. JAMA. 1999;282:561–568. doi: 10.1001/jama.282.6.561. [DOI] [PubMed] [Google Scholar]
- Olomolaiye O, Wood N, Bidwell J. A novel NlaIII polymorphism in the human IL-6 promoter. Eur. J. Immunogenet. 1998;25:267. [PubMed] [Google Scholar]
- O’Connor M-F, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, Thomas KS, Irwin MR. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav. Immun. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pociot F, Mølvig J, Wogensen L, Worsaae H, Nerup J. A TaqI polymorphism in the human interleukin-1 beta (IL-1 beta) gene correlates with IL-1 beta secretion in vitro. Eur. J. Clin. Invest. 1992;22:396–402. doi: 10.1111/j.1365-2362.1992.tb01480.x. [DOI] [PubMed] [Google Scholar]
- Prigerson HG, Maciejewski PK, Reynolds CF, Bierhals AJ, Newsom JT, Fasiczka A, Frank E, Doman J, Miller MD. Inventory of Complicated Grief: a scale to measure maladaptive symptoms of loss. Psychiatry Res. 1995;59:65–79. doi: 10.1016/0165-1781(95)02757-2. [DOI] [PubMed] [Google Scholar]
- Prigerson HG, Horowitz MJ, Jacobs SC, Parkes CM, Aslan M, Goodkin K, Raphael B, Marwit SJ, Wortman C, Neimeyer RA, Bonanno GA, Block SD, Kissane D, Boelen PA, Maercker A, Litz BT, Johnson JG, First MB, Maciejewski PK. Prolonged grief disorder: psychometric validation of criteria proposed for DSM-V and ICD-11. PLoS Med. 2009;6:e1000121. doi: 10.1371/journal.pmed.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santić Z, Lukić A, Sesar D, Milicević S, Ilakovac V. Long-term follow-up of blood pressure in family members of soldiers killed during the war in Bosnia and Herzegovina. Croat. Med. J. 2006;47:416–423. [PMC free article] [PubMed] [Google Scholar]
- Schuld A, Mullington J, Hermann D, Hinze-Selch D, Fenzel T, Holsboer F, Pollmacher T. Effects of granulocyte colony-stimulating factor on night sleep in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1999;276:R1149. doi: 10.1152/ajpregu.1999.276.4.R1149. [DOI] [PubMed] [Google Scholar]
- Shear MK, Frank E, Houck PR, Reynolds CF. Treatment of complicated grief: a randomized controlled trial. JAMA. 2005;293:2601–2608. doi: 10.1001/jama.293.21.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear MK, Simon NM, Wall M, Zisook S, Neimeyer R, Duan N, Reynolds CF, Lebowitz B, Sung S, Ghesquiere A, Gorscak B, Clayton P, Ito M, Nakajima S, Konishi T, Melhem NM, Meert K, Schiff M, O’Connor M-F, First MB, Sareen J, Bolton J, Skritskaya N, Mancini AD, Keshaviah A. Complicated grief and related bereavement issues for DSM-5. Depress. Anxiety. 2011;28:103–117. doi: 10.1002/da.20780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbons M, First MD. Structured Clinical Interview of the DSM-IV. American Psychiatric Press; Washington, DC: 1994. [Google Scholar]
- Terry CF, Loukaci V, Green FR. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J. Biol. Chem. 2000;275:18138–18144. doi: 10.1074/jbc.M000379200. [DOI] [PubMed] [Google Scholar]
- Wilson AG, di Giovine FS, Blakemore AI, Duff GW. Single base polymorphism in the human tumour necrosis factor alpha (TNF alpha) gene detectable by NcoI restriction of PCR product. Hum. Mol. Genet. 1992;1:353. doi: 10.1093/hmg/1.5.353. [DOI] [PubMed] [Google Scholar]
- Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc. Natl. Acad. Sci. USA. 1997;94:3195–3199. doi: 10.1073/pnas.94.7.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]


