Abstract
Antimitochondrial antibodies (AMA) directed against the lipoyl domain of the E2 subunit of pyruvate dehydrogenase (PDC-E2) are detected in 95% of patients with PBC and are present before onset of clinical disease. The recent demonstration that AMA recognize xenobiotic modified PDC-E2 with higher titers than native PDC-E2, raises the possibility that the earliest events involved in loss of tolerance are related to xenobiotic modification. We hypothesized that reactivity to such xenobiotics would be predominantly IgM and using sera from a large cohort of PBC patients and controls (n=516), we examined in detail sera reactivity against either SAc-conjugated bovine serum albumin (BSA), recombinant PDC-E2 (rPDC-E2) or BSA alone. Further, we also defined the relative specificity to the SAc moiety using inhibition ELISA; SAc conjugate and rPDC-E2 specific affinity purified antibodies were also examined for antigen specificity, isotype and cross-reactivity. Reactivity to SAc conjugates is predominantly IgM; such reactivity reflects a footprint of previous xenobiotic exposure. Indeed, this observation is supported by both direct binding, cross reactivity, and inhibition studies. In both early and late stage PBC, the predominant Ig isotype to SAc is IgM, with titers higher with advanced stage disease. We also note that there was a higher level of IgM reactivity to SAc in early stage versus late stage PBC. Interestingly, this finding is particularly significant in light of the structural similarity between SAc and the reduced form of lipoic acid, a step which is similar to the normal physiological oxidation of lipoic acid. We submit that specific modifications of the disulfide bond within the lipoic-acid-conjugated PDC-E2 moiety, i.e. by an electrophilic agent renders PDC-E2 immunogenic in a genetically susceptible host.
Keywords: Tolerance, electrophiles, lipoic acid, environment and autoimmunity
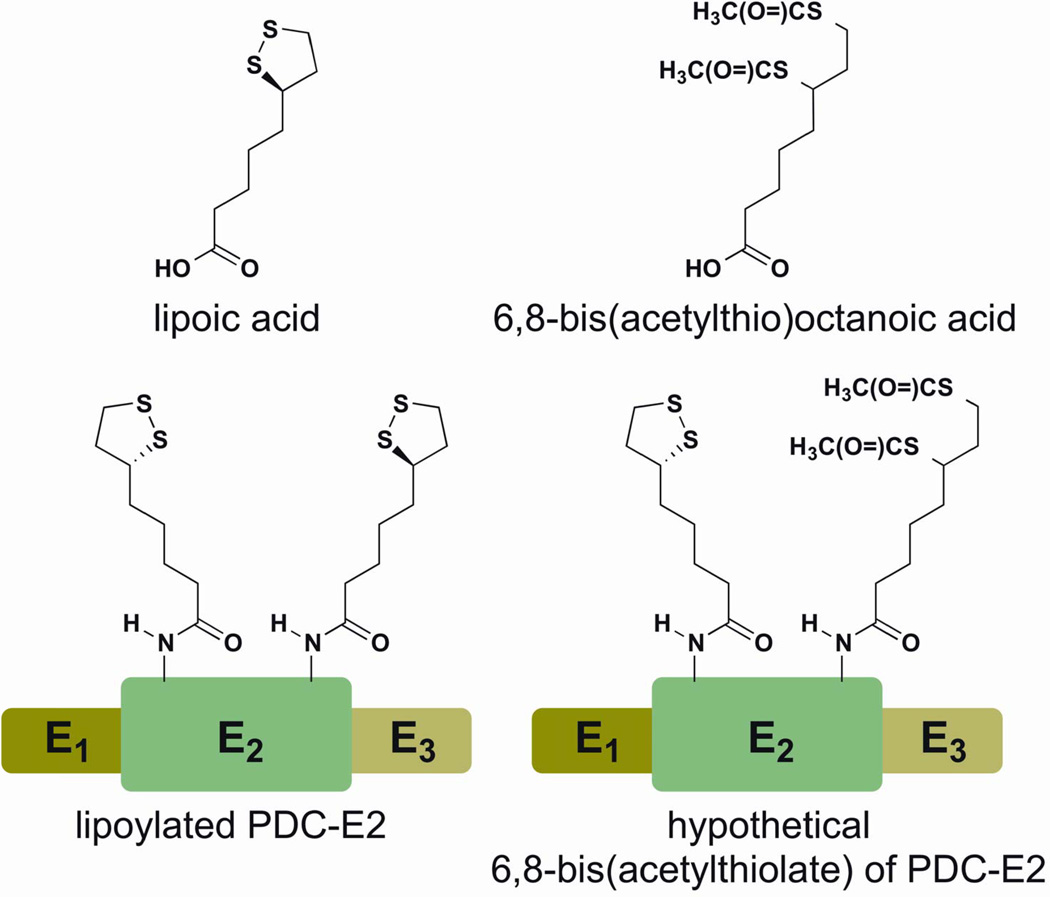
Anti-mitochondrial autoantibodies (AMA) to the E2 subunit of the pyruvate dehydrogenase complex (PDC-E2) are the serological hallmark of PBC (1–4). Previous analysis of the antibody specificity of anti-PDC-E2 revealed a number of subpopulations of anti-PDC-E2 antibodies that recognized either the PDC peptide, PDC peptide conjugated with lipoic acid or lipoic acid itself (5–7). Interestingly, PDC-E2 specific antibodies are present long before the onset of clinical symptoms and may represent a relic of initiating immunological events (8). Recent studies by quantitative structure-activity relationship (QSAR) analysis demonstrated that AMA-positive PBC sera, but not controls, reacted to a number of xenobiotic modified PDC-E2 structures (9–11), with a particularly striking level of reactivity against 6,8-bis(acetylthio) octanoic acid (SAc)-PDC-E2 (12). This observation is critical because SAc is a modified form of lipoic acid in which both sulfur atoms of the disulfide bond of the lipoyl ring are modified by acetyl groups (Figure 1), thereby maintaining PDC-E2 in a reduced state by preventing disulfide bond formation; this reduced state facilitates xenobiotic modification of PDC-E2 (13). We hypothesized that the presence of antibodies directed against the SAc-PDC-E2 conjugate in sera from PBC patients suggests that this structure is involved in loss of tolerance. Such data would also support the thesis that chemical modification of self-proteins play an important role in autoimmunity (7, 14–16), exemplified by minocycline-induced autoimmunity, whereby minocycline binding to self macromolecules produces immunogenic self antigens that become the target of disease generating, cross-reactive autoantibodies (17, 18).
Figure 1.
Chemical structure of lipoic acid and 6,8-bis(acetylthio) octanoic acid. Note that SAc resembles a modified form of lipoic acid in which both sulfur atoms of the disulfide bond of the lipoyl ring structure are modified by an acetyl group. SAc modification of the PDC-E2 lipoic acid renders PDC-E2 in a reduced state by preventing disulfide bond formation; this reduced state facilitates xenobiotic modification of PDC-E2.
Thus, to address our hypothesis and define the antibody reactivity to the SAc moiety, we have studied the serological reactivity of 241 AMA-positive PBC patients, 34 AMA-negative PBC patients, 86 patients with primary sclerosing cholangitis (PSC), 95 patients with autoimmune hepatitis (AIH), and 60 healthy controls against SAc conjugated BSA, 2-octynoic acid (2OA) conjugated BSA, recombinant PDC-E2 (rPDC-E2), and BSA itself. Importantly, we have mapped specific reactivities of a nested subset of 24 AMA-positive SAc-BSA-positive PBC sera, including use of various affinity-purified antisera and inhibition studies. Interestingly, our data suggest that IgM reactivity to SAc reflects the footprints of xenobiotic modification of PDC-E2. Finally, we report herein that the IgM reactivity to SAc persists from early to late stage PBC with only minimal IgG reactivity.
Materials and Methods
Serum samples
Sera samples were obtained from the Tufts New England Medical Center, the University of California School of Medicine at Davis, and Humanitas Clinical and Research Center, Milan Italy, including 241 AMA-positive patients with PBC, 34 AMA-negative patients with PBC, 86 PSC patients, 95 AIH patients and 60 healthy controls were used herein following appropriate informed consent. The clinical diagnosis of all patients was verified using published criteria (19–22) and the protocol approved by the Institutional Review Board of the University of California at Davis.
Synthesis of diacyl modified lipoic succinimidyl ester (SAc-NHS) analog
Lipoic acid (4.8 mmol) was placed in a round bottom flask and dissolved in water (24 mL), followed by the addition of NaHCO3 (4.8 mmol). The solution was placed in a sonicator until the solid dissolved, and the solution turned yellow. The solution was cooled to 0°C and solid NaBH4 (9.6 mmol) was slowly added. The reaction was stirred for 30 minutes at 0°C and thence an additional 30 minutes at room temperature. 2M HCl was added slowly until a pH of approximately 1 was reached. This solution was extracted with chloroform under an inert atmosphere. The combined extracts were dried over sodium sulfate and concentrated to deliver 6,8-dimercaptooctanoic acid (78%).
6,8-dimercaptooctanoic acid (4.8 mmol) was dissolved in 30 mL of acyl chloride and heated to 60°C for 4 hours. The reaction was quenched by the addition of 250 mL of ice water. This aqueous solution was extracted with ethyl acetate. The combined extracts were washed with water, brine, dried over sodium sulfate, and concentrated to derive acyl modified 6,8-di-mercaptooctanoic acid.
The crude acyl modified 6,8-di-mercaptooctanoic acid (3.4 mmol), N-hydroxysuccinimide (17.0 mmol), and dicyclohexylcarbodiimide (DCC) (17.0 mmol) were added to 10 mL of dry tetrahydrofuran (THF). The reaction was stirred at room temperature for 24 hours. The reaction mixture was gravity filtered and rinsed with additional dry THF. The filtrate was concentrated and dissolved in ethyl acetate. This organic solution was washed with water, brine, dried over sodium sulfate, and concentrated. The solid residue was purified by flash chromatography to yield the desired NHS ester (SAc-NHS), an amorphous solid (69 % over two steps).
Preparation of SAc modified albumin
In 1.75 mL of purified water, 83 mg of BSA was dissolved. SAc-NHS (0.29 mmol) dissolved in 200 µL dimethyl sulfoxide (DMSO) was then added drop-wise to the slowly vortexing BSA solution. The solution was allowed to react for 3 hours. This crude mixture was purified by HPLC. MALDI-TOF MS analysis showed a conjugation of 18–20 SAc molecules per BSA molecule. SAC-RSA was prepared similarly.
Preparation of 2OA-BSA
2OA-BSA was synthesized as previously described (23). Briefly, 2-octynoic acid (Sigma Aldrich) was conjugated to BSA as follows. First, 2-octynoic acid (1.00 mL, 6.86 mmol) was dissolved in dry diethyl ether (20 mL). N-hydroxysuccinimide (0.868 g, 7.54 mmol) was then added and the solution cooled to 0°C and stirred for 20 minutes. Dicyclohexylcarbodiimide (1.56 g, 7.54 mmol) was then added and the mixture allowed to warm to ambient temperature overnight. The solution was filtered, concentrated by roto-evaporation under reduced pressure, re-dissolved with diethyl ether (40 mL), washed with water (40 mL), NaHCO3 (1 M, 40 mL), brine (40 mL), dried over magnesium sulfate, filtered, and concentrated. The product was then purified using flash chromatography (30% ethyl acetate/hexanes). NHS-activated 2-octynoic acid was dissolved in DMSO and then coupled to the lysine residues of BSA (EMD Chemicals, Gibbstown, NJ). The solution was allowed to react for 3 hours followed by HPLC purification. MALDI-TOF analysis demonstrated a loading of 30 to 32 molecules of 2OA per BSA molecule.
Preparation of rPDC-E2
Overnight E. coli cultures expressing the human PDC-E2 lipoyl domain in plasmid pGEX4T-1 (24) were diluted 1:10 with fresh Lauria-Bertani medium (50ug/mL ampicillin) until the optical density (OD) was 0.7 to 0.8 and induced with 1mM isopropyl-β-thiogalactopyranoside for an additional 3 to 4 hours at 37°C. Cells were pelleted, re-suspended in PBS containing 1% Triton X-100 and 1% Tween 20 (Sigma Chemical Co., St. Louis, MO), and sonicated. The sonicated extract was centrifuged at 10,000× g for 15 minutes at 4°C; the supernatant was collected and incubated with glutathione agarose beads (Sigma, St. Louis, MO) for 2 hours at room temperature. Gluthathione-agarose-beads were washed 3 times with PBS and the fusion protein was eluted by competition with 50 mM Tris HCl pH 8.0 containing 20 mM reduced glutathione (Sigma, St. Louis, MO). Protein concentrations of the eluates were determined by bicinchoninic acid (BCA) assay (Thermo Scientific), and specificity of the purified recombinant proteins was verified by immunoblotting with anti-PDC-E2 monoclonal antibodies. Positive and negative controls were included throughout (25).
Enzyme-linked immunosorbent assay (ELISA)
96-well ELISA plates were coated with either rPDC-E2, SAc-BSA, 2OA-BSA or BSA (10 microgram/mL) in carbonate coating buffer at 4°C overnight, blocked with 3% non-fat dry milk in PBS and incubated with 1:500 dilution of the serum samples to be tested for 1 hour. The plates were then washed with PBS containing 0.05% Tween 20 and incubated for 1 hour with a predetermined optimized dilution of horse-radish peroxidase (HRP) conjugated anti-human IgG, IgM and IgA (Invitrogen, Carlsbad, CA), washed and developed with BD OptEIA Substrate (BD Biosciences, San Diego, CA) (26).
To determine if the specificity of the Ig reactivity against SAc-BSA was localized to the hapten and not the result of cross-reactivity with PDC-E2, a nested study of 24 random PBC serum that reacted with SAc-BSA and rPDC-E2 but not BSA were selected. Each serum sample at each dilution (1:250 to 1:2,000) was individually pre-incubated with either 100 microgram of rPDC-E2, SAc-BSA, or SAc-RSA per mL of diluted human serum sample at 4°C overnight, centrifuged and the supernatant analyzed for antibody reactivity against rPDC-E2, SAc-BSA, and SAc-RSA by ELISA. Similarly, aliquots of the serum samples were similarly pre-incubated with either BSA or another irrelevant protein Met e 1 (27) overnight at 4°C overnight. Thereafter, the serum samples were centrifuged and the supernatant fluids collected to be included as negative controls throughout.
Affinity purification of antibodies
To further determine the hapten specificities of the antibody population, rPDC-E2, SAc-BSA and SAc-RSA affinity purified antibodies from 10 of the 24 AMA-positive SAc-BSA-positive PBC human sera were prepared. Briefly, the target protein was conjugated to cyanogen bromide (CNBr)-activated sepharose beads (28). The PBC sera were centrifuged at 3800 rpm and the supernatant was diluted to 1:20 with 10 mM Tris pH 7.5. The diluted human serum was passed through the column 3 times. The bound antibodies were eluted off with 100 mM glycine pH 2.5 and neutralized immediately with 1M Tris pH 8.0. The concentrations of the purified antibodies were determined using the BCA assay (Thermo Scientific). These affinity-purified antibodies were assayed for reactivity against rPDC-E2, SAc-BSA, and SAc-RSA. Reactivity to an irrelevant protein Mete l (27) was used as a control throughout.
The Ig class of affinity-purified antibodies to SAc conjugates and rPDC-E2 was determined by ELISA as described above. Briefly, SAc-BSA-, SAc-RSA-, or rPDC-E2-coated ELISA plates were incubated with SAc-conjugate-purified antibodies or rPDC-E2-purified antibodies and probed with goat HRP-conjugated anti-human IgG, IgM and IgA antibodies (Invitrogen).
Anti-SAc antibodies of the IgM isotype and stages of PBC
To evaluate the specific Ig reactivity to SAc in early versus late stage of PBC, we performed a nested study involving a cohort of 50 patients with stage 1–2 PBC and 50 stages 3–4. These included 43 AMA positive and 7 AMA negative in the stage 1–2 group and a comparable number in the stage 3–4 group. Sera from each of these patients were studied for IgG and IgM reactivity to recombinant PDC-E2 and SAc-BSA as outlined above.
Statistical analysis
Averages and standard error of the mean (SEM) of Ig reactivity against antigens utilizing ELISAs, inhibition ELISAs, and affinity purified antibody ELISAs were calculated. A two-tailed unpaired t-test with Welch’s correction was used to analyze the Ig reactivity against xenobiotic-modified proteins for sera from AMA-positive patients with PBC, AMA-negative PBC patients, PSC patients, AIH patients, and healthy controls. Statistical significance for inhibition ELISAs and affinity purified antibody ELISAs between SAc-conjugate-specific and rPDC-E2 specific antibodies was also determined by a two-tailed unpaired t-test with Welch’s correction.
Results
Specific serological reactivity to SAc-BSA
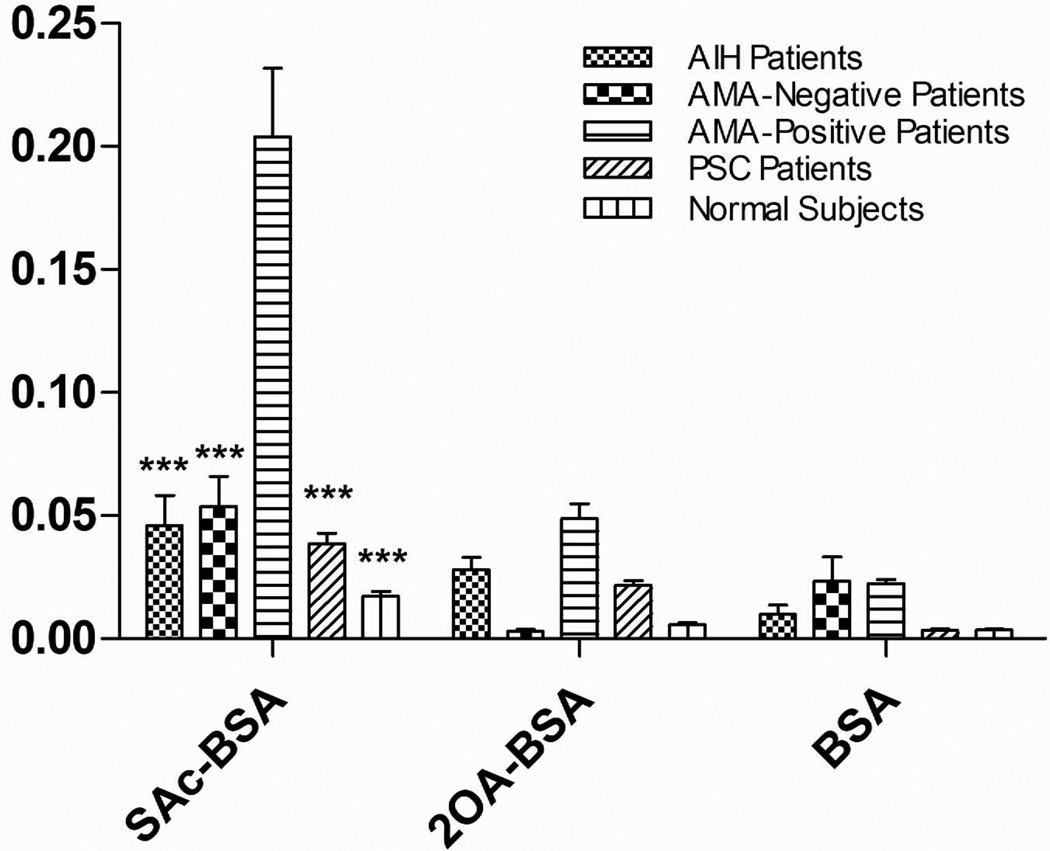
207 out of 241 AMA-positive PBC sera recognized SAc-BSA and 76 of the same 207 AMA-positive PBC sera also reacted to 2OA-BSA, whereas none of the sera reacted to BSA. Importantly, the mean Ig (comprising of IgG, IgA and IgM) reactivity against SAc-BSA of sera from AMA-positive PBC patients is significantly higher (p < 0.0001) than sera from AMA-negative PBC, AIH, PSC, and healthy controls (Figure 2). There was no further clinical data available in this cohort.
Figure 2.
IgG, IgM and IgA reactivity against SAc-BSA, 2OA-BSA, and BSA of AIH patients (n=95), AMA-negative PBC patients (n=20), AMA-positive PBC patients (n=155), PSC patients (n=86), and normal subjects (n=60). Data presented as mean ± SEM. Asterisks indicate significant differences as compared to the reactivity of AMA-positive PBC patients against SAc-BSA (***, p < 0.0001; two-tailed unpaired t-test with Welch’s correction).
Antibody cross-reactivity between SAc-BSA and rPDC-E2 distinguishes two different recognition patterns
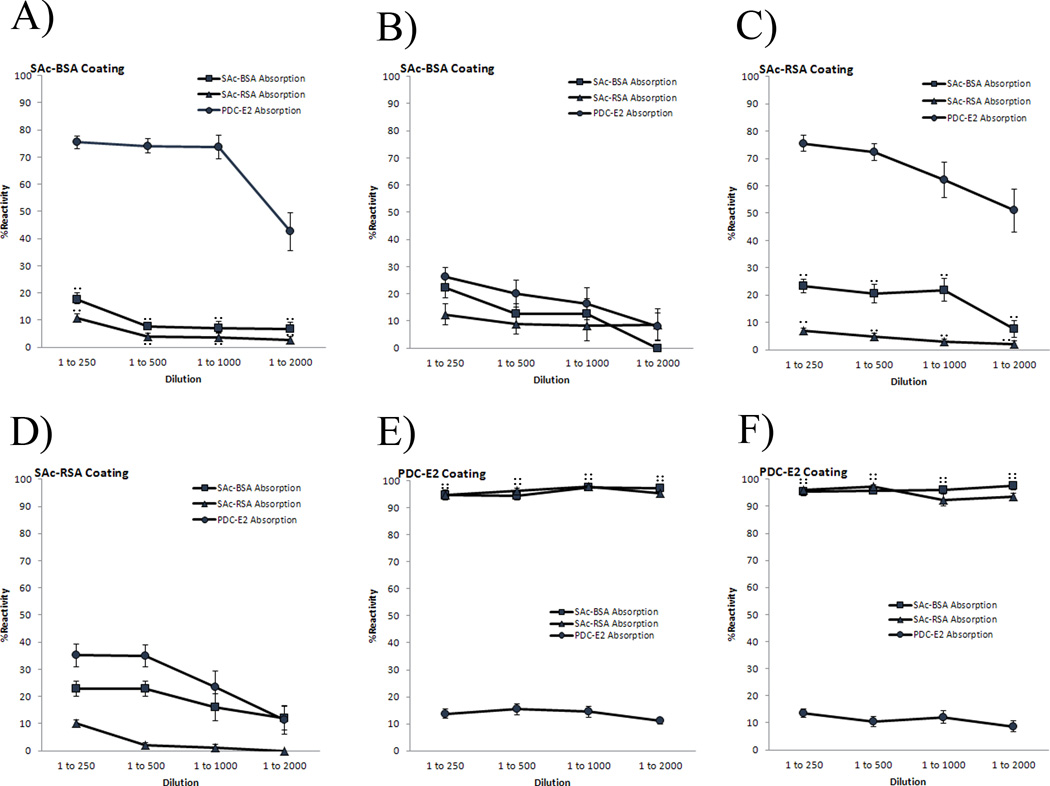
To determine if there are cross-reactive antibodies against SAc-BSA and rPDC-E2 in sera of AMA-positive PBC patients, 24 serum samples that recognized both SAc-BSA and rPDC-E2 were studied in detail by inhibition ELISA. Individual serum samples were first incubated with either rPDC-E2, SAc-BSA or SAc-RSA to absorb reactivity and then assayed for reactivity against the three substrates by ELISA. As negative controls, serum samples were pre-incubated with BSA and another irrelevant protein Met e 1 (27) and assayed for reactivity against rPDC-E2, SAc-BSA and SAc-RSA. Interestingly, two distinct patterns of antibody reactivity were found. Pre-absorption of 14/24 sera with rPDC-E2 did not remove reactivity to the SAc-conjugated proteins and most reactivity was retained (Figure 3A and 3C). For the other, 10/24 PBC sera, pre-absorption with rPDC-E2 ablated reactivity against SAc-BSA or SAc-RSA as well as against rPDC-E2 (Figure 3B and 3D). In all cases, pre-absorption with SAc-BSA or SAc-RSA led to loss of reactivity to SAc-conjugated proteins at 1:250, 1:500, 1:1000, and 1:2000 serum dilutions. Similarly pre-absorption of sera with rPDC-E2 ablated reactivity against rPDC-E2 at 1:250, 1:500, 1:1000, and 1:2000 serum dilutions. In the cross over experiment, when both populations were absorbed with SAc-conjugated proteins, they both retained their antibody recognition to rPDC-E2 at all dilutions (Figure 3E and 3F). When sera were absorbed independently with BSA and another irrelevant control protein Met e1, they retained > 97% reactivity against rPDC-E2, SAc-BSA, SAC-RSA at 1:250, 1:500, 1:1000 and 1:2000 sera dilution (Figure 3).
Figure 3.
IgG, IgM and IgA reactivity of AMA-positive PBC serum samples against SAc-BSA (A,B), SAc-RSA (C,D), and rPDC-E2 (E,F). 1st population (n=14) of AMA-positive PBC patients is represented in graphs A, C, and E; 2nd population (n=10) of AMA-positive PBC patients is shown in graphs B, D, and F. After absorption with SAc-BSA, SAc-RSA, rPDC-E2, BSA and Met e 1, reactivity is determined by ELISA at 1:250, 1:500, 1:1000, and 1:2000 serum dilutions. Note that 1st population (A,C) binds rPDC-E2 independently of SAc-conjugated proteins (p < 0.001). 2nd population (B,D) is cross-reactive between rPDC-E2 and SAc-conjugated proteins. Both 1st and 2nd populations retain their Ig reactivity against rPDC-E2 when absorbed with SAc-conjugated proteins (E,F). Both populations retain their reactivities to SAc-BSA, SAc-RSA, rPDC-E2 when absorbed with either BSA or Met e 1 (A–F). Data presented as mean ± SEM. Asterisks indicate significant differences of SAc-BSA absorption as compared to PDC-E2 absorption (**, p < 0.001; two-tailed unpaired t-test with Welch’s correction). Number signs indicate significant differences of SAc-RSA absorption compared to PDC-E2 absorption (##, p < 0.001; two-tailed unpaired t-test with Welch’s correction).
Affinity purified rPDC-E2 antibodies do not react with SAc-conjugated proteins
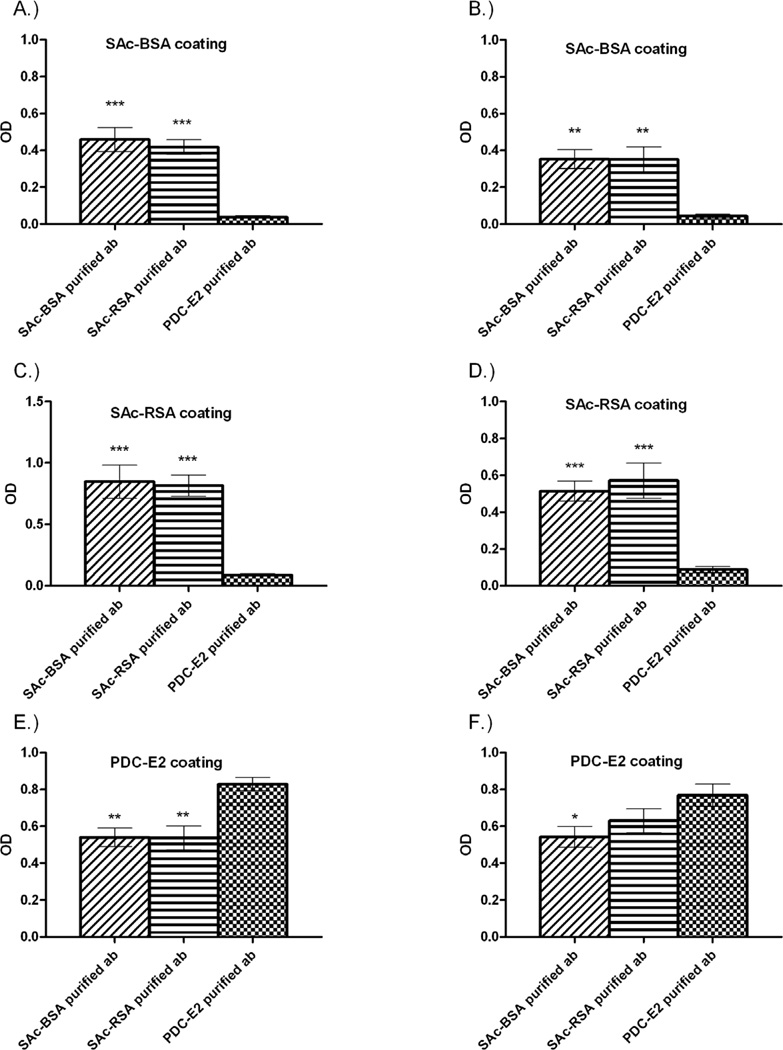
To further determine the hapten specificities of the antibody population, affinity-purified antibodies against rPDC-E2, SAc-BSA, and SAc-RSA were prepared from a subset of 24 AMA-positive SAc-BSA-positive PBC sera (5/10 of rPDC-E2 ablation group and 5/14 of the rPDC-E2 non-ablation group). The affinity purified antibodies against rPDC-E2 from both populations bound to only rPDC-E2 and not to SAc-BSA or SAc-RSA (Figure 4). In contrast, SAc-conjugate affinity purified antibodies from both populations reacted to both SAc-conjugates and rPDC-E2. The differences between the levels of reactivity against SAc-conjugates by SAc-conjugate-purified antibodies and rPDC-E2-purified antibodies are statistically significant in both populations (Figure 4A–4D).
Figure 4.
Reactivity of SAc-BSA-, SAc-RSA-, and rPDC-E2-purified antibodies against SAc-BSA, SAc-RSA, and rPDC-E2. Graphs A, C, and E represent select AMA-positive PBC patients (n=5) from 1st population. Graphs B, D, and F represent select AMA-positive PBC patients (n=5) from 2nd population. Note that rPDC-E2-purified antibodies in both populations do not react with SAc-BSA and SAc-RSA. Data presented as mean ± SEM. Asterisks indicate significant differences as compared with rPDC-E2-purified antibody (*, p < 0.05; **, p < 0.001; ***, p < 0.0001; two-tailed unpaired t-test with Welch’s correction).
Affinity purified antibodies of the IgG isotype show specificity for rPDC-E2
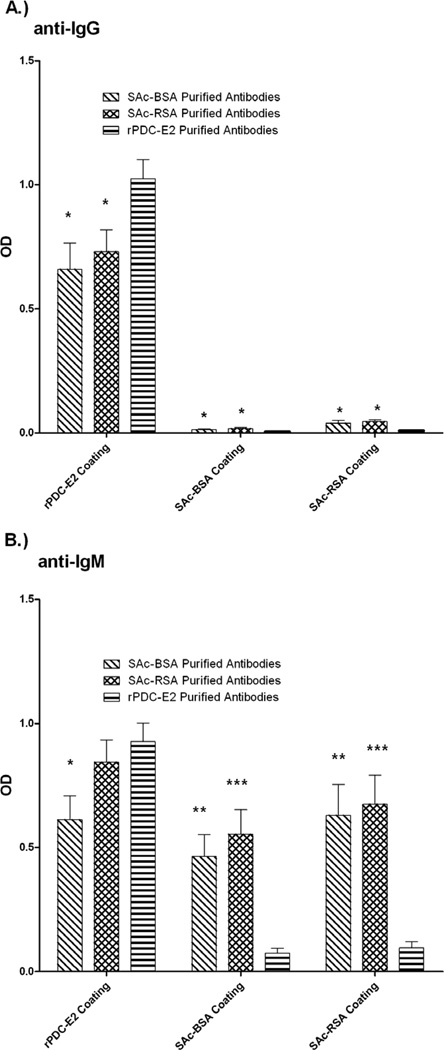
Isotyping was performed on the affinity purified antibodies to determine the major Ig classes. The affinity purified SAc-conjugate reactive antibodies and the affinity purified rPDC-E2 reactive antibodies displayed IgG reactivities against rPDC-E2 (Figure 5A). Little to no SAc-conjugate-purified and rPDC-E2-purified antibodies were detected against SAc-conjugated proteins using anti-IgG secondary antibodies. However, when SAc-conjugate-purified anitibodies were tested against rPDC-E2 the binding population was found to be predominantly IgG (p < 0.0001).
Figure 5.
IgG (A) and IgM (B) reactivity of SAc-BSA-, SAc-RSA-, and rPDC-E2-purified antibodies against SAc-BSA, SAc-RSA, and rPDC-E2. SAc-BSA- and SAc-RSA-purified antibodies are predominantly of IgM and recognize SAc-BSA, SAc-RSA, and rPDC-E2. rPDC-E2-purified antibodies are both IgG and IgM, and recognize only rPDC-E2. Data presented as mean ± SEM. Asterisks indicate significant differences as compared to rPDC-E2-purified antibody in their respective coating (*, p < 0.05; **, p < 0.001; ***, p < 0.0001; two-tailed unpaired t-test with Welch’s correction). ††† represents significant differences (p < 0.0001) between SAc-conjugate binding and rPDC-E2 binding of SAc-BSA-purified antibodies. ‡‡‡ represents significant differences (p < 0.0001) between SAc-conjugate binding and rPDC-E2 binding of SAc-RSA-purified antibodies.
Anti- SAc affinity-purified antibodies of the IgM isotype show specificity for both rPDC-E2 and SAc-conjugates
When an anti-IgM was used as a developing antibody, SAc-conjugate affinity-purified antibodies displayed reactivity against SAc-conjugates at levels that were significantly higher (SAc-BSA-purified antibodies, p < 0.001; SAc-RSA-purified antibodies, p < 0.0001) than did rPDC-E2-purified antibodies (Figure 5B). rPDC-E2-purified antibodies reactivity to SAc-conjugates (0.074±0.020 against SAc-BSA; 0.095±0.024 against SAc-RSA) is negligible. SAc-RSA-purified antibodies and rPDC-E2-purified antibodies reacted to rPDC-E2 to a similar degree, but SAc-BSA-purified antibodies bound rPDC-E2 significantly less than rPDC-E2 purified antibodies (p < 0.01).
Reactivity to SAc in early and late stage PBC
Of the 100 studied PBC sera, 50 were stage 1–2 and 50 were stage 3–4. In these two groups, there were 7 AMA negative sera each and in all AMA negative cases we confirmed the absence of reactivity to both SAc-BSA and to PDC-E2. Importantly, 30/43 early stage and 33/43 reacted to both SAc and recombinant PDC-E2. Interestingly, however, there was a slight and statistically significant increase in IgM reactivity when comparing early PBC (OD 0.164 ± 0.025) and late PBC (OD 0.205 ± 0.027) with respect to reactivity to SAc. IgG reactivity to SAc in both groups was insignificant. We do note however that in the early stage group, there were 6/43 patients with IgM reactivity to SAc that were higher than their IgM titers to PDC-E2; this pattern was not seen in any late stage sera (Table 1).
Table 1.
Clinical characteristics and antibody reactivity of early and late stage PBC sera.
| Early Stage PBC (n=50) |
Late Stage PBC (n=50) |
|
|---|---|---|
| AMA positive | 43 | 43 |
| AMA negative and SAc negative | 7 | 7 |
| ALP (IU/L) | 386 ± 36 | 456 ± 54 |
| Liver cirrhosis | 0/50 | 48/50 |
| IgM to SAc > rPDC-E2* | 6 | 0 |
OD of IgM to SAc 0.206±0.02, OD of IgM to rPDC-E2: 0.134±0.021
Discussion
In this study, we demonstrated that antibodies to the SAc-moiety are present in the majority of AMA-positive PBC patients. Two patterns of patient responsiveness are seen, one where AMAs cross-react with both SAc-conjugated proteins and rPDC-E2, and the other where AMAs show little cross-reactivity between the two antigens. Cross-reactivity predominantly resides with SAc-conjugate affinity-purified antibodies and not rPDC-E2-affinity purified antibodies. IgG from SAc-conjugate affinity-purified antibodies binds rPDC-E2 significantly more than SAc-conjugated proteins whereas IgM from SAc-conjugate-purified antibodies binds both rPDC-E2 and SAc-conjugated proteins (Figure 6). The presence of high amounts of IgM, a hallmark characteristic of PBC, leads us to speculate that these SAc-conjugate-purified IgM antibodies are footprints induced by xenobiotic exposure at the very early stages of development of PBC.
Figure 6.
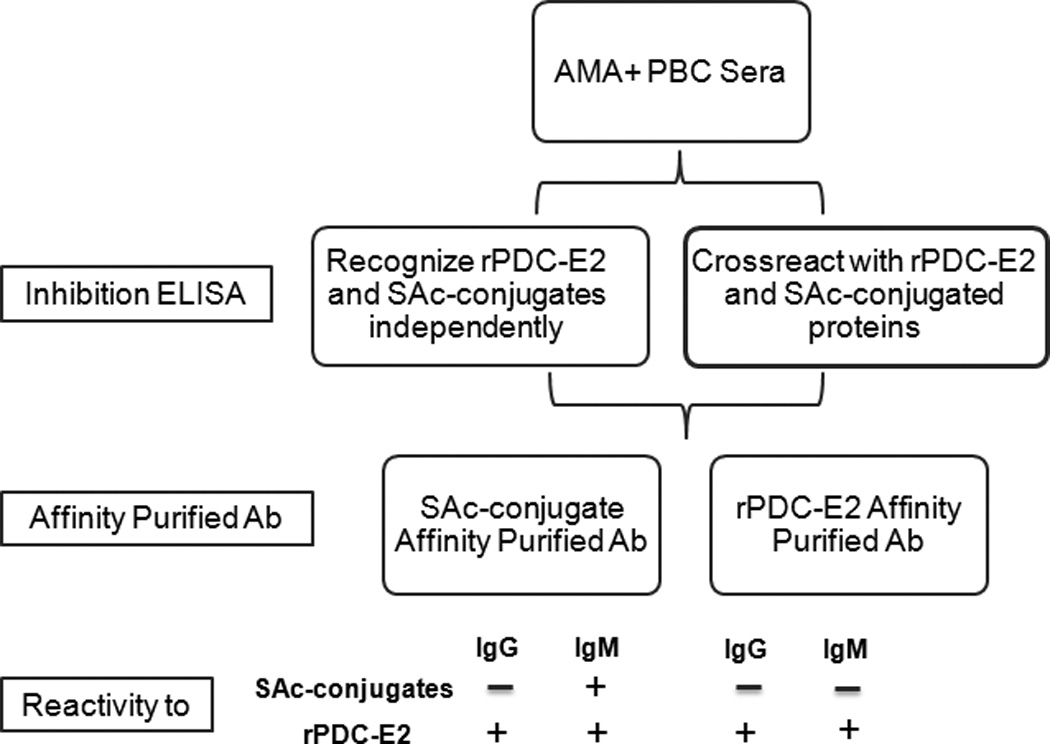
Reactivity patterns of SAc reactive and rPDC-E2 reactive antibodies and their isotypes in AMA positive PBC sera.
How might this occur? The immunodominant epitope of PDC-E2 is localized to the inner lipoyl domain (29–31), and the main function of the disulfide bond of the PDC-E2-lipoyl domain is electron transfer during ATP synthesis (32). This is achieved by constant forming and breaking of the disulfide bond with subsequent electron transfer of an acetyl group from pyruvate to coenzyme A (CoA) to produce acetyl-CoA. We have recently shown that the modification of this disulfide bond renders PDC-E2 more immunogenic and have proposed that this modification can interrupt ATP synthesis, causing cell death and exposing modified PDC-E2 to the immune system. This could initiate breakdown of self-tolerance to native PDC-E2 in genetically susceptible individuals by presentation of a cross-reactive moiety (12). This finding is supported by the observation that PDC-E2 is more immunogenic in its reduced and unmasked form (33), a structure equivalent to SAc-PDC-E2. Additionally, diacetyl derivatives of PDC-E2 (SAc-PDC-E2) cannot participate in the enzymatic reaction to form acetyl-CoA as efficiently as monoacetyl derivatives, the physiological form of PDC-E2 (34), again rendering the cell more susceptible to exogenous damage. Thus, direct alteration of the lipoyl ring – i.e., disruption of the S-S linkage – renders the lipoic acid “activated” and receptive to xenobiotic modification, which in turn presents a cross-reactive neo-epitope. Although it is not clear how xenobiotics or the modified cellular proteins initiate autoimmunity in PBC, analysis of serum samples from subjects with acute liver failure indicate that a severe liver oxidant injury can lead to AMA production (21, 35). In particular, AMA with the same antigen and epitope specificity as in patients with PBC was found in almost 35% of individuals poisoned by ingesting excessive amounts of acetyl-para-aminophenol (commonly known as acetaminophen) suggesting that the PDC-E2 lipoyl domain is likely a target of acetaminophen induced reactive oxygen species. Thus, in genetically susceptible individuals, prolonged exposure to electrophilic agents such as acetaminophen may initiate and/or enhance the breakdown of self-tolerance to PDC-E2 (13). We propose such modified-self comes to the attention of the immune system in apoptotic blebs from biliary epithelial cells during the normal turnover of the cellular lining of intrahepatic bile ducts in an environment of xenobiotic exposure (36).
Previous work has demonstrated that protein modification can be an initiating point to the breach of tolerance. Indeed, in one study it was estimated that the majority of human proteins are susceptible to post-translational modification, including, for example, (i.e. acetylation, lipidation, citrullination and glycosylation) (37, 38). The clinical significance of these modifications has been demonstrated in rheumatoid arthritis, Sjogren's syndrome, systemic lupus and celiac disease (39–42). The earliest work reflecting the clinical significance of xenobiotics with respect to modification and environmental factors was the relative induction of lupus-like diseases experimentally by mercury (43–46). In this respect, our own work reflecting the potential for modification of PDC-E2 takes on additional significance (47, 48).
What other aspects of autoantibody development are revealed in this study? The finding of cross-reactivity being greater with IgM than IgG antibodies is consistent with the general properties of IgM that tend to be lower affinity (49). The likely scenario is that the xenobiotic-modified self protein (in this case SAc-conjugated proteins) induces IgM antibody production that is originally specific for xenobiotic-modified self protein. Second, due to the close structural similarity between xenobiotic-modified self protein and native self protein, the immune system of genetically susceptible individuals starts to generate IgM antibodies that are cross-reactive or specific to native self protein (in this case PDC-E2) through affinity maturation and epitope spreading mechanisms. Third, at the same time with affinity maturation, isotype switching occurs and this process generates IgG antibodies that are more specific to native self protein (PDC-E2) than xenobiotic-modified self protein (SAc-conjugated proteins) which may have by that time disappeared. Thus, IgG antibodies mainly show the reactivity against native self protein and demonstrate very low reactivity against the xenobiotics. Fourth, the affinity maturation with repeated exposure of the native self protein continues to increase the affinity of IgM and IgG antibodies against the native self protein. Eventually, some clones of these IgM and IgG antibodies become highly specific for only native self protein with diminished reactivity against modified self protein. Due to the high affinity of these clones compared to the cross-reactive clones, most rPDC-E2-purified antibodies obtained in our experiment could only bind to rPDC-E2, but not SAc-conjugated proteins. This phenomenon can also explain the results of our inhibition ELISA experiments and why the SAc-conjugated protein absorption could not inhibit the serum reactivity to rPDC-E2 in both AMA populations.
Patient AMAs may be categorized on the basis of two distinct profiles of cross-reactivity such that PDC-E2 absorption either removes or leaves anti-SAc antibodies present in PBC sera. This may relate to the degree of polyclonality of the sera and perhaps to levels of IgM, which are known generally of lower specificity. Whether this reflects two mechanisms by which autoantibodies are induced or different subsequent histories is unknown. It should be noted that cross-reactivity is being detected many years after the initiating event and the time at which tolerance is broken. Thus it may be that the cross-reactivity is more readily detected early in the course of disease and that it disappears later. It would be of interest to perform a correlative study in which patient parameters such as duration of disease, age of diagnosis, severity, IgM levels and rate of progression are correlated with the type of cross-reactivity pattern present in sera. More importantly, however, would be to obtain patient sera prior to the onset of any symptoms as well as between symptomatic and asymptomatic patients. AMAs are now known to appear for several years longer before the onset of clinical disease or diagnosis. The presence of IgM reactivity to SAc throughout all stages of PBC is consistent with data that the onset of clinical disease occurs several years or longer after the first appearance of autoantibodies. In fact, elevated IgM throughout all stages of PBC is well known to occur in patients with PBC (50). This indeed appears to be the case for other autoimmune diseases, but given the frequency of PBC, this becomes a formidable task.
It is interesting to note that among the 50 early stage and the 50 late stage PBC sera studied, 7 of the early stage and 7 of the late stage sera were AMA neagtive and SAc negative. Of interest, IgM reactivity to SAc-BSA in 6/43 of the PDC-E2 positive early stage PBC sera were higher than IgM reactivity to PDC-E2; this pattern was not noted in the late stage group. The significance of this can only be extrapolated. We do not propose that there will be any specific clinical significance to the IgM reactivity other than the importance that it reflects a potential footprint of the earliest events that may lead to breach of tolerance. Indeed, all the data herein supports the concept that molecular mimicry between SAc and the lipoyl moiety of PDC-E2 is an important mechanism in induction of autoantibodies to PDC-E2. Finally, we should emphasize that we believe that multiple agents may be capable of similar modification of PDC-E2 through either their electrophilic properties and the creation of neoantigens or perhaps direct molecular mimicry.
We propose the following hypothetical etiology of PBC. Initial exposure to chemicals such as xenobiotic-modified PDC-E2 leads to a primary IgM specific immune response against the antigen, e.g. SAc. Subsequently, the similarity between the lipoyl domain of PDC-E2 and the xenobiotic-modified lipoyl domain of PDC-E2 (SAc-moiety) generates cross-reactive immune responses against the self-antigen, leading to the self-tolerance breakdown to the lipoyl domain of mitochondrial PDC-E2. Later, through the process of affinity maturation and isotype switching, the secondary immune response produces IgGs that are highly specific for mitochondrial PDC-E2. Once self tolerance to PDC-E2 is broken, the immune destruction is restricted to BECs due to their unique physiology and exacerbated by the retention of PDC-E2 in apoptotic blebs from the apoptosis of BECs (51, 52).
Acknowledgments
This work is supported in part by funding from National Institutes of Health grants DK39588 and DK067003
List of Abbreviations
- 2OA
2-octynoic acid
- AIH
autoimmune hepatitis
- AMA
antimitochondrial antibodies
- BEC
biliary epithelial cells
- BSA
bovine serum albumin
- ELISA
enzyme linked immunosorbent assay
- PBC
primary biliary cirrhosis
- PDC-E2
E2 subunit of pyruvate dehydrogenase
- PSC
primary sclerosing cholangitis
- rPDC-E2
recombinant protein of PDC-E2
- RSA
rabbit serum albumin
- SAc
6,8-bis(acetylthio) octanoic acid
- SAc-NHS
diacyl modified lipoic succinimidyl ester
Contributor Information
Richy C.Y. Chen, Email: rcychen@ucdavis.edu.
Phornnop Naiyanetr, Email: phornnop.nai@mahidol.ac.th.
Shang-An Shu, Email: sshu@ucdavis.edu.
Jinjun Wang, Email: yjwang@ucdavis.edu.
Guo-Xiang Yang, Email: gxyang@ucdavis.edu.
P. Kenny Thomas, Email: tpkenny@ucdavis.edu.
Kathryn C. Guggenheim, Email: kgguggenheim@ucdavis.edu.
Jeffrey D. Butler, Email: jdbutler@ucdavis.edu.
Christopher Bowlus, Email: clbowlus@ucdavis.edu.
Mi-Hua Tao, Email: bmtao@ibms.sinica.edu.tw.
Mark J. Kurth, Email: mjkurth@ucdavis.edu.
Aftab A. Ansari, Email: pathaaa@emory.edu.
Marshall Kaplan, Email: mkaplan@tuftsmedicalcenter.org.
Ross L. Coppel, Email: Ross.Coppel@monash.edu.
Ana Lleo, Email: ana.lleo@humanitas.it.
M. Eric Gershwin, Email: megershwin@ucdavis.edu.
Patrick S.C. Leung, Email: psleung@ucdavis.edu.
References
- 1.Gershwin ME, Mackay IR, Sturgess A, Coppel RL. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J Immunol. 1987;138:3525–3531. [PubMed] [Google Scholar]
- 2.Hirschfield GM, Heathcote EJ, Gershwin ME. Pathogenesis of cholestatic liver disease and therapeutic approaches. Gastroenterology. 2010;139:1481–1496. doi: 10.1053/j.gastro.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Selmi C, Mackay IR, Gershwin ME. The autoimmunity of primary biliary cirrhosis and the clonal selection theory. Immunol Cell Biol. 2011;89:70–80. doi: 10.1038/icb.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gershwin ME, Mackay IR. The causes of primary biliary cirrhosis: Convenient and inconvenient truths. Hepatology. 2008;47:737–745. doi: 10.1002/hep.22042. [DOI] [PubMed] [Google Scholar]
- 5.Bruggraber SF, Leung PS, Amano K, Quan C, Kurth MJ, Nantz MH, Benson GD, et al. Autoreactivity to lipoate and a conjugated form of lipoate in primary biliary cirrhosis. Gastroenterology. 2003;125:1705–1713. doi: 10.1053/j.gastro.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 6.Cha S, Leung PS, Coppel RL, Van de Water J, Ansari AA, Gershwin ME. Heterogeneity of combinatorial human autoantibodies against PDC-E2 and biliary epithelial cells in patients with primary biliary cirrhosis. Hepatology. 1994;20:574–583. [PubMed] [Google Scholar]
- 7.Selmi C. The worldwide gradient of autoimmune conditions. Autoimmun Rev. 2010;9:A247–S250. doi: 10.1016/j.autrev.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Benson GD, Kikuchi K, Miyakawa H, Tanaka A, Watnik MR, Gershwin ME. Serial analysis of antimitochondrial antibody in patients with primary biliary cirrhosis. Clinical & developmental immunology. 2004;11:129–133. doi: 10.1080/10446670410001722113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amano K, Leung PS, Rieger R, Quan C, Wang X, Marik J, Suen YF, et al. Chemical xenobiotics and mitochondrial autoantigens in primary biliary cirrhosis: identification of antibodies against a common environmental, cosmetic, and food additive, 2-octynoic acid. J Immunol. 2005;174:5874–5883. doi: 10.4049/jimmunol.174.9.5874. [DOI] [PubMed] [Google Scholar]
- 10.Long SA, Quan C, Van de Water J, Nantz MH, Kurth MJ, Barsky D, Colvin ME, et al. Immunoreactivity of organic mimeotopes of the E2 component of pyruvate dehydrogenase: connecting xenobiotics with primary biliary cirrhosis. J Immunol. 2001;167:2956–2963. doi: 10.4049/jimmunol.167.5.2956. [DOI] [PubMed] [Google Scholar]
- 11.Rieger R, Leung PS, Jeddeloh MR, Kurth MJ, Nantz MH, Lam KS, Barsky D, et al. Identification of 2-nonynoic acid, a cosmetic component, as a potential trigger of primary biliary cirrhosis. J Autoimmun. 2006;27:7–16. doi: 10.1016/j.jaut.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Naiyanetr P, Butler JD, Meng L, Pfeiff J, Kenny TP, Guggenheim KG, Reiger R, et al. Electrophile-modified lipoic derivatives of PDC-E2 elicits anti-mitochondrial antibody reactivity. J Autoimmun. 2011 doi: 10.1016/j.jaut.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung PSC, Lam K, Kurth MJ, Coppel RL, Geshwin ME. Xenobiotics and Autoimmunity: Does acetamimnophen cause primary biliary cirrhosis? Trends in Molecular Medicine. 2012 doi: 10.1016/j.molmed.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller FW. Environmental agents and autoimmune diseases. Adv Exp Med Biol. 2011;711:61–81. doi: 10.1007/978-1-4419-8216-2_6. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt CW. Questions persist: environmental factors in autoimmune disease. Environ Health Perspect. 2011;119:A249–A253. doi: 10.1289/ehp.119-a248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rieger R, Gershwin ME. The X and why of xenobiotics in primary biliary cirrhosis. Journal of autoimmunity. 2007;28:76–84. doi: 10.1016/j.jaut.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang C, Gershwin ME. Drugs and autoimmunity--a contemporary review and mechanistic approach. J Autoimmun. 2010;34:J266–J275. doi: 10.1016/j.jaut.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Angulo JM, Sigal LH, Espinoza LR. Coexistent minocycline-induced systemic lupus erythematosus and autoimmune hepatitis. Semin Arthritis Rheum. 1998;28:187–192. doi: 10.1016/s0049-0172(98)80035-8. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–1273. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 20.Bambha K, Kim WR, Talwalkar J, Torgerson H, Benson JT, Therneau TM, Loftus EV, Jr, et al. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology. 2003;125:1364–1369. doi: 10.1016/j.gastro.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Leung PS, Rossaro L, Davis PA, Park O, Tanaka A, Kikuchi K, Miyakawa H, et al. Antimitochondrial antibodies in acute liver failure: implications for primary biliary cirrhosis. Hepatology. 2007;46:1436–1442. doi: 10.1002/hep.21828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stravitz RT, Lefkowitch JH, Fontana RJ, Gershwin ME, Leung PS, Sterling RK, Manns MP, et al. Autoimmune acute liver failure: proposed clinical and histological criteria. Hepatology. 2011;53:517–526. doi: 10.1002/hep.24080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wakabayashi K, Lian ZX, Leung PS, Moritoki Y, Tsuneyama K, Kurth MJ, Lam KS, et al. Loss of tolerance in C57BL/6 mice to the autoantigen E2 subunit of pyruvate dehydrogenase by a xenobiotic with ensuing biliary ductular disease. Hepatology. 2008;48:531–540. doi: 10.1002/hep.22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moteki S, Leung PS, Coppel RL, Dickson ER, Kaplan MM, Munoz S, Gershwin ME. Use of a designer triple expression hybrid clone for three different lipoyl domain for the detection of antimitochondrial autoantibodies. Hepatology. 1996;24:97–103. doi: 10.1002/hep.510240117. [DOI] [PubMed] [Google Scholar]
- 25.Oertelt S, Rieger R, Selmi C, Invernizzi P, Ansari AA, Coppel RL, Podda M, et al. A sensitive bead assay for antimitochondrial antibodies: Chipping away at AMA-negative primary biliary cirrhosis. Hepatology. 2007;45:659–665. doi: 10.1002/hep.21583. [DOI] [PubMed] [Google Scholar]
- 26.Liu H, Norman GL, Shums Z, Worman HJ, Krawitt EL, Bizzaro N, Vergani D, et al. PBC screen: an IgG/IgA dual isotype ELISA detecting multiple mitochondrial and nuclear autoantibodies specific for primary biliary cirrhosis. Journal of autoimmunity. 2010;35:436–442. doi: 10.1016/j.jaut.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Leung PS, Chu KH, Chow WK, Ansari A, Bandea CI, Kwan HS, Nagy SM, et al. Cloning, expression, and primary structure of Metapenaeus ensis tropomyosin, the major heat-stable shrimp allergen. The Journal of allergy and clinical immunology. 1994;94:882–890. doi: 10.1016/0091-6749(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 28.Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. The Journal of biological chemistry. 1970;245:3059–3065. [PubMed] [Google Scholar]
- 29.Cha S, Leung PS, Gershwin ME, Fletcher MP, Ansari AA, Coppel RL. Combinatorial autoantibodies to dihydrolipoamide acetyltransferase, the major autoantigen of primary biliary cirrhosis. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:2527–2531. doi: 10.1073/pnas.90.6.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van de Water J, Fregeau D, Davis P, Ansari A, Danner D, Leung P, Coppel R, et al. Autoantibodies of primary biliary cirrhosis recognize dihydrolipoamide acetyltransferase and inhibit enzyme function. Journal of immunology. 1988;141:2321–2324. [PubMed] [Google Scholar]
- 31.Van de Water J, Gershwin ME, Leung P, Ansari A, Coppel RL. The autoepitope of the 74-kD mitochondrial autoantigen of primary biliary cirrhosis corresponds to the functional site of dihydrolipoamide acetyltransferase. The Journal of experimental medicine. 1988;167:1791–1799. doi: 10.1084/jem.167.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mao TK, Davis PA, Odin JA, Coppel RL, Gershwin ME. Sidechain biology and the immunogenicity of PDC-E2, the major autoantigen of primary biliary cirrhosis. Hepatology. 2004;40:1241–1248. doi: 10.1002/hep.20491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Odin JA, Huebert RC, Casciola-Rosen L, LaRusso NF, Rosen A. Bcl-2-dependent oxidation of pyruvate dehydrogenase-E2, a primary biliary cirrhosis autoantigen, during apoptosis. J Clin Invest. 2001;108:223–232. doi: 10.1172/JCI10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Connor TP, Roche TE, Paukstelis JV. 13C nuclear magnetic resonance study of the pyruvate dehydrogenase-catalyzed acetylation of dihydrolipoamide. J Biol Chem. 1982;257:3110–3112. [PubMed] [Google Scholar]
- 35.Bernal W, Meda F, Ma Y, Bogdanos DP, Vergani D. Disease-specific autoantibodies in patients with acute liver failure: the King's College London Experience. Hepatology. 2008;47:1096–1097. doi: 10.1002/hep.22179. author reply 1097. [DOI] [PubMed] [Google Scholar]
- 36.Lleo A, Bowlus CL, Yang GX, Invernizzi P, Podda M, Van de Water J, Ansari AA, et al. Biliary apotopes and anti-mitochondrial antibodies activate innate immune responses in primary biliary cirrhosis. Hepatology. 2010;52:987–998. doi: 10.1002/hep.23783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doyle HA, Mamula MJ. Post-translational protein modifications in antigen recognition and autoimmunity. Trends in immunology. 2001;22:443–449. doi: 10.1016/s1471-4906(01)01976-7. [DOI] [PubMed] [Google Scholar]
- 38.Doyle HA, Mamula MJ. Autoantigenesis: the evolution of protein modifications in autoimmune disease. Current opinion in immunology. 2012;24:112–118. doi: 10.1016/j.coi.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molberg O, McAdam SN, Korner R, Quarsten H, Kristiansen C, Madsen L, Fugger L, et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nature medicine. 1998;4:713–717. doi: 10.1038/nm0698-713. [DOI] [PubMed] [Google Scholar]
- 40.Stea EA, Routsias JG, Samiotaki M, Panayotou G, Papalambros E, Moutsopoulos HM, Tzioufas AG. Analysis of parotid glands of primary Sjogren's syndrome patients using proteomic technology reveals altered autoantigen composition and novel antigenic targets. Clinical and experimental immunology. 2007;147:81–89. doi: 10.1111/j.1365-2249.2006.03262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van den Steen PE, Proost P, Brand DD, Kang AH, Van Damme J, Opdenakker G. Generation of glycosylated remnant epitopes from human collagen type II by gelatinase B. Biochemistry. 2004;43:10809–10816. doi: 10.1021/bi0493665. [DOI] [PubMed] [Google Scholar]
- 42.Yang ML, Gee AJ, Gee RJ, Zuritalopez CI, Khare S, Clarke S, Mamula MJ. Lupus autoimmunity altered by cellular methylation metabolism. Autoimmunity. 2012 doi: 10.3109/08916934.2012.732133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cooper GS, Parks CG, Treadwell EL, St Clair EW, Gilkeson GS, Dooley MA. Occupational risk factors for the development of systemic lupus erythematosus. The Journal of rheumatology. 2004;31:1928–1933. [PubMed] [Google Scholar]
- 44.Pollard KM, Lee DK, Casiano CA, Bluthner M, Johnston MM, Tan EM. The autoimmunity-inducing xenobiotic mercury interacts with the autoantigen fibrillarin and modifies its molecular and antigenic properties. Journal of immunology. 1997;158:3521–3528. [PubMed] [Google Scholar]
- 45.Pollard KM, Pearson DL, Bluthner M, Tan EM. Proteolytic cleavage of a self-antigen following xenobiotic-induced cell death produces a fragment with novel immunogenic properties. Journal of immunology. 2000;165:2263–2270. doi: 10.4049/jimmunol.165.4.2263. [DOI] [PubMed] [Google Scholar]
- 46.Vas J, Monestier M. Immunology of mercury. Annals of the New York Academy of Sciences. 2008;1143:240–267. doi: 10.1196/annals.1443.022. [DOI] [PubMed] [Google Scholar]
- 47.Leung PS, Park O, Tsuneyama K, Kurth MJ, Lam KS, Ansari AA, Coppel RL, et al. Induction of primary biliary cirrhosis in guinea pigs following chemical xenobiotic immunization. J Immunol. 2007;179:2651–2657. doi: 10.4049/jimmunol.179.4.2651. [DOI] [PubMed] [Google Scholar]
- 48.Leung PS, Quan C, Park O, Van de Water J, Kurth MJ, Nantz MH, Ansari AA, et al. Immunization with a xenobiotic 6-bromohexanoate bovine serum albumin conjugate induces antimitochondrial antibodies. J Immunol. 2003;170:5326–5332. doi: 10.4049/jimmunol.170.10.5326. [DOI] [PubMed] [Google Scholar]
- 49.Makela O, Ruoslahti E, Seppala IJ. Affinity of IgM and IgG antibodies. Immunochemistry. 1970;7:917–932. doi: 10.1016/0019-2791(70)90053-4. [DOI] [PubMed] [Google Scholar]
- 50.Lleo A, Liao J, Invernizzi P, Zhao M, Bernuzzi F, Ma L, Lanzi G, et al. Immunoglobulin M levels inversely correlate with CD40 ligand promoter methylation in patients with primary biliary cirrhosis. Hepatology. 2012;55:153–160. doi: 10.1002/hep.24630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lleo A, Selmi C, Invernizzi P, Podda M, Coppel RL, Mackay IR, Gores GJ, et al. Apotopes and the biliary specificity of primary biliary cirrhosis. Hepatology. 2009;49:871–879. doi: 10.1002/hep.22736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lleo A, Shimoda S, Ishibashi H, Gershwin ME. Primary biliary cirrhosis and autoimmune hepatitis: apotopes and epitopes. J Gastroenterol. 2011;46(Suppl 1):29–38. doi: 10.1007/s00535-010-0303-8. [DOI] [PubMed] [Google Scholar]