SUMMARY
Toll-like receptor 11 (TLR11) recognizes T. gondii profilin (TgPRF) and is required for interleukin-12 production and induction of immune responses that limit cyst burden in Toxoplasma gondii-infected mice. However, TLR11 only modestly affects survival of T. gondii-challenged mice. We report that TLR12, a previously uncharacterized TLR, also recognized TgPRF. TLR12 was sufficient for recognition of TgPRF by plasmacytoid dendritic cells (pDCs), whereas TLR11 and TLR12 were both required in macrophages and conventional DCs. In contrast to TLR11, TLR12-deficient mice succumb rapidly to T. gondii infection. TLR12-dependent induction of IL-12 and IFN-α in pDCs led to production of IFN-γ by NK cells. Consistent with this observation, the partial resistance of Tlr11−/− mice is lost upon pDC or NK cell depletion. Thus, TLR12 is critical for the innate immune response to T. gondii, and this TLR may promote host resistance by triggering pDC and NK cell function.
INTRODUCTION
Infectious diseases are one of the leading causes of morbidity and mortality worldwide. Toxoplasma gondii is an obligate intracellular apicomplexan parasite that has been documented to infect more than 60% of the worldwide population and has the ability to infect a wide range of warm-blooded hosts (Pappas et al., 2009). T. gondii is the causative agent of toxoplasmosis, which is considered to be the third leading cause of death attributed to food-borne illnesses in the United States (Mead et al., 1999). T. gondii infection during pregnancy is of significant concern because it can lead to abortion or congenital toxoplasmosis (Jones et al., 2007). Currently there is no vaccine available to prevent toxoplasmosis in humans. Therefore, understanding how protective immune responses are mounted against these parasites is crucial to developing effective vaccination strategies or better therapeutics.
TLRs play an important role in the initial recognition of micro-organisms through detection of unique pathogen-associated molecular patterns (PAMPs) (West et al., 2006). Activation of most TLRs via their respective ligands results in rapid nuclear translocation of the transcription factor nuclear factor κB (NF-κB) and consequent synthesis and secretion of proinflammatory cytokines and chemokines. Previous studies have shown that interleukin-12 (IL-12), produced by dendritic cells (DCs), macrophages, and neutrophils in infected animals, is responsible for the induction of T helper 1 (Th1) cell responses and T. gondii clearance (Gazzinelli et al., 1993; Reis e Sousa et al., 1997; Yap et al., 2000). IL-12 supports the induction of interferon-γ (IFN-γ) during T. gondii infection (Gazzinelli et al., 1993, 1994), and IFN-γ is essential for induction of T cell responses and resistance to T. gondii (Suzuki et al., 1988; Trinchieri, 2003). A major source of IL-12 during T. gondii infection is thought to be CD8+ DCs (Liu et al., 2006). Indeed, studies with mouse models selectively targeting DC subsets confirmed that the bulk of the IL-12 produced during T. gondii infection with tachyzoites was produced by CD8+ DCs (Mashayekhi et al., 2011). However, deletion of all conventional DCs, including CD8+ DCs, clearly indicates that there is also a cDC-independent component of the innate response to T. gondii (Meredith et al., 2012).
In mice, an intermediate host for T. gondii, resistance to death is dependent upon IL-12 production in response to signaling through myeloid differentiation factor 88 (MyD88)-dependent pathways (Scanga et al., 2002). MyD88 is an adaptor molecule involved in most TLR signaling cascades and it is, therefore, believed that TLR recognition of T. gondii is crucial for host resistance. However, although multiple TLRs have been suggested to recognize T. gondii, deletions of these TLRs do not recapitulate the lethality observed in MyD88-deficient mice. Recognition of glycosylphosphatidyl inositol (GPI)-anchored proteins, by TLRs 2 and 4, and profilin-like protein from T. gondii (TgPRF) by TLR11, induce potent cytokine responses (Debierre-Grockiego et al., 2007; Yarovinsky et al., 2005). However, mice deficient for TLR2, TLR4, or TLR11 survive T. gondii infection (Debierre-Grockiego et al., 2007; Scanga et al., 2002; Yarovinsky et al., 2005). These findings led us to hypothesize that MyD88-dependent recognition of T. gondii by the innate immune system depends upon additional TLR. We now report that a previously uncharacterized TLR, TLR12, is required for mounting an effective protective response against T. gondii. TLR12 can act alone in pDCs and with TLR11 in cDCs and macrophages to specifically recognize and respond to T. gondii profilin. Therefore, our studies provide evidence for a functional role for TLR12 in host defense as well as an example of heterodimerization in the TLR family of pattern recognition receptors.
RESULTS
TLR12 Resembles TLR11 but Is Expressed More Selectively In Vivo
In mammals, 12 TLRs (10 in humans and 12 in mice) have been identified. Prior studies have delineated the specificity of different TLRs for various PAMPs from a diverse array of pathogens such as bacteria (by TLR2, TLR4, TLR5, TLR9, and TLR11), viruses (by TLR3, TLR7, TLR8, and TLR9), fungi (TLR2, TLR4), and parasites (TLR9 and TLR11) (Akira et al., 2006; Alexopoulou et al., 2001; Hayashi et al., 2001; Heil et al., 2004; Hemmi et al., 2000; Medzhitov et al., 1997; Takeuchi et al., 2000; Zhang et al., 2004). However, the specificity of TLR12 and its biological role remained to be determined.
Analysis of the putative open reading frame of TLR12 (Gen-Bank accession number NP_991388.1) predicted the existence of a 927 amino acid protein with all the hallmarks of known mammalian TLRs: a trans-membrane protein with multiple leucine-rich repeat (LRR) motifs in the extracellular domain, a single-span trans-membrane segment, and a cytoplasmic signaling domain homologous to that of the interleukin 1 receptor (IL-1R), termed the Toll-IL-1R homology (TIR) domain. TLR12 sequences are present in the genomes of rodents, horses, and lemurs, but could not be detected in humans.
A comparison of TLR12 to other TLRs reveals 96% similarity to TLR11, with an E value of 1 × 10−132, demonstrating that these receptors share significant homology to each other compared to other mammalian TLRs (Figure 1A). Notable differences between the receptors lie within the transmembrane region and within the box 2 region of their TIR domains, where TLR12 has an isoleucine instead of the highly conserved proline residue (Figure S1A available online). Phylogenetic analysis of TLR12 revealed close similarity to TLR5 and TLR11, indicating that these TLRs form a subgroup within the TLR family (Figure 1B). Notably, TLR5 and TLR11 are the two mammalian TLRs known to recognize protein ligands (Hayashi et al., 2001; Zhang et al., 2004). This suggested that TLR12 might also recognize a protein ligand. Based on the knowledge that TLRs 1, 2, and 6 are homologous to each other, form a subgroup within the TLR family, and can function as heterodimers, we hypothesized that TLR11 and TLR12 might form heterodimers capable of recognizing common ligands.
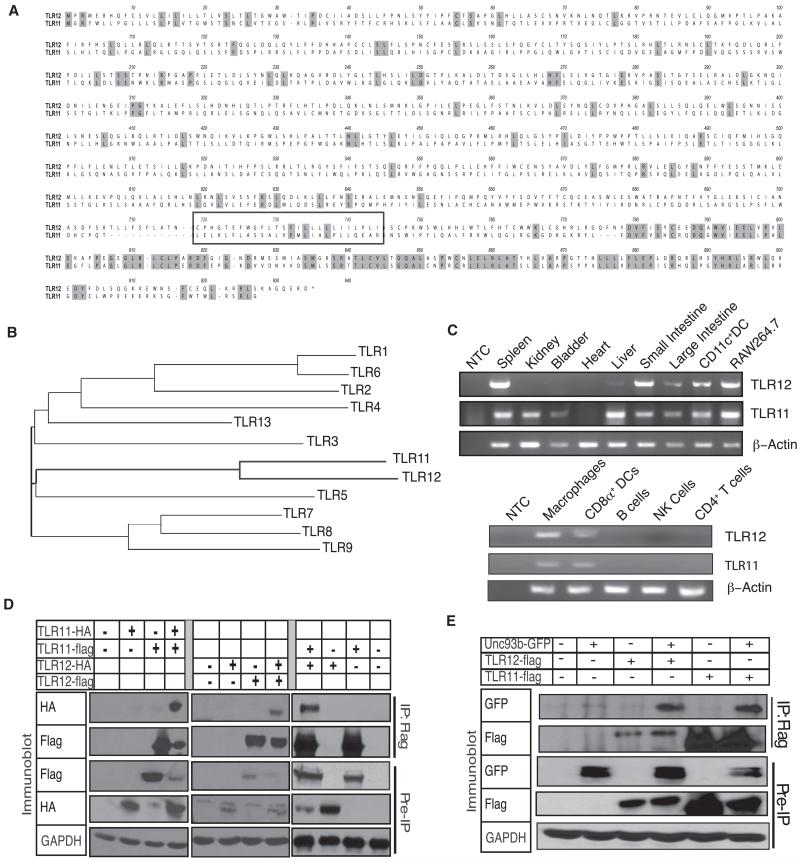
Figure 1. TLR12 Can Form Homodimers and Heterodimers with TLR11 but Has a More Restricted Expression Pattern.
(A) Alignment of amino acid sequences of TLR12 and TLR11. The transmembrane region is boxed and darker gray shading indicates amino acid residues that are identical.
(B) Phylogenetic analysis of known TLRs with TLR12 was performed with CLUSTALW software with the fullamino acid sequences of murine TLRs 1–9 and TLRs11–12.
(C) RT-PCR was used to determine TLR11 and TLR12 mRNA levels in multiple tissues and purified hematopoietic cell populations. A β-actin probe was used as a control for RNA loading. NTC, no template control. Data are representative of three independent experiments.
(D) TLR12 can form homodimers with itself and a heterodimer with TLR11. Flag-TLR12 and HA-TLR12 were transfected into HEK293 cells. Cells were lysed and coimunoprecipitation with either anti-flag or anti-HA, followed by SDS-PAGE and protein blot analysis with antibodies specific for Flag and HA. Data are representative of three independent experiments.
(E) TLR12 and TLR11 interact with Unc-93B1. HEK293 cells were transiently transfected with TLR12-Flag or TLR11-Flag and Unc-93B1-GFP as indicated, lysed, and subjected to anti-flag immunoprecipitation, SDS-PAGE, and protein blotting with antibodies specific for Flag and GFP. Data are representative of two independent experiments.
See also Figures S1 and S2.
Reverse-transcription polymerase chain reactions (RT-PCR) via oligonucleotide primers specific for TLR11 or TLR12 revealed that the expression of TLR12 was substantially more limited than that of TLR11. TLR12 expression was restricted to myeloid cells such as cDCs and pDCs, macrophages, and lymphoid cells including T cells and B cells (Figure 1C). In situ hybridization experiments confirmed the RT-PCR results and showed strong TLR12 expression primarily in the spleen and very weak expression in other tissues such as the kidney (Figure S1B). Thus, although there is overlap in TLR12 and TLR11 expression, most notably in macrophages and DCs, TLR12 exhibits a broader distribution of expression in hematopoietic lineages whereas TLR11 is more expressed at epithelial surfaces.
TLR12 Can Form Homodimers or Heterodimers with TLR11
The TLR12 gene (which included the signal sequence found in the first 30 amino acids) was cloned from RAW264.7 cDNA into the pCMV-14 vector. Standard subcloning was used to generate vectors encoding TLR12 with Flag or hemagglutinin (HA) tags. Coimmunoprecipitation experiments were performed by transfecting Flag-TLR12 and HA-TLR12 into HEK293 cells. Immunoprecipitation of Flag-TLR12, followed by immunoblotting of the precipitates for the HA-epitope, revealed that TLR12, like TLR11, can form homodimers (Figure 1D). In addition, after cotransfection of Flag-TLR11 and HA-TLR12, immunoblotting of anti-flag immunoprecipitates revealed the presence of HA-tagged TLR12, demonstrating that TLR11 and TLR12 can form a heterodimer (Figure 1D). Therefore, consistent with overlapping but distinct tissue expression patterns, these results suggest that TLR11 and TLR12 can form both homo- and heterodimers.
To determine the intracellular location of the two TLRs, immunofluorescence analysis of HeLa cells transiently transfected with a C-terminal Flag-tagged TLR11 and a C-terminal HA-tagged TLR12 was performed. This analysis suggested that both receptors colocalize to similar endosomal compartments (Figure S2). We previously reported that TLR11 was localized to an endosomal compartment (Zhang et al., 2004) and a recent report has demonstrated that its localization is dependent on Unc-93B1 (Pifer et al., 2011), which is consistent with the behavior of TLRs known to reside on endosomal compartments (Melo et al., 2010; Pifer et al., 2011; Tabeta et al., 2006). Therefore, to determine whether TLR12 could also interact with Unc-93B1, we performed coimmunoprecipitation experiments by transfecting TLR12-Flag with Unc-93B1-GFP in HEK293 cells (Figure 1E). Immunoprecipitation of Flag-TLR12, followed by immunoblotting with GFP antibodies, revealed that TLR12, like TLR11, can interact with Unc-93B1, suggesting that TLR12 also is guided to the endolysosomal system by Unc-93B1. Given the high degree of sequence similarity, evolutionary conservation, and the ability to heterodimerize and colocalize, we hypothesized that TLR11 and TLR12 probably recognize common ligands and act synergistically in innate immune responses.
TLR12 Recognizes and Triggers IL-12 Production in Response to TgPRF
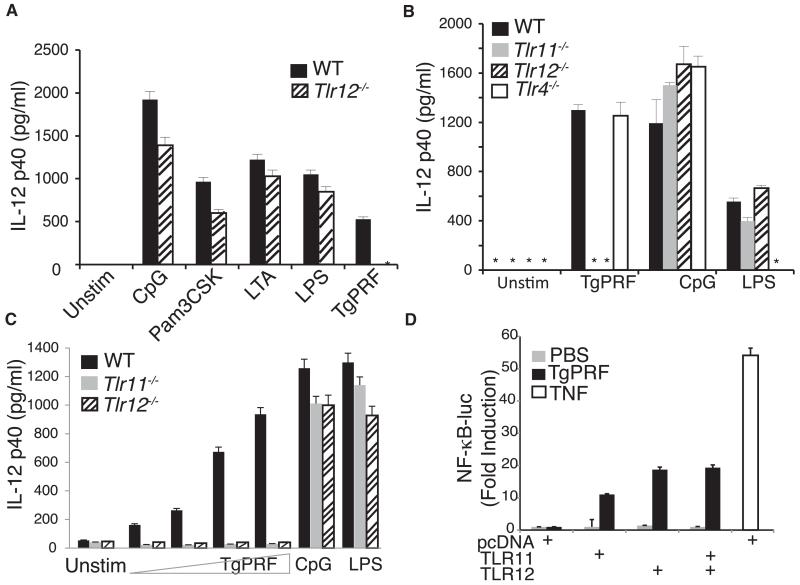
To test the hypothesis that TLR12 might work synergistically with TLR11 to recognize similar protein ligands, we generated mice lacking TLR12 (Figure S3). We backcrossed heterozygous Tlr12+/− mice for ten generations into the C57BL/6 background and obtained viable homozygous Tlr12−/− mice at close to the expected Mendelian ratios. Macrophages from Tlr12−/− mice were analyzed to assess the requirement for TLR12 in responding to different TLR ligands: lipopolysaccharide (LPS), lipoteichoic acid (LTA), Pam(3)CSK(4) synthetic lipoprotein, CpG DNA, and TgPRF (Figure 2A). Tlr12−/− macrophages produced similar amounts of IL-12p40 compared to wild-type (WT) macrophages in response to stimulation with most known TLR ligands except for TgPRF, which induced significantly lower amounts of IL-12p40 in Tlr12−/− macrophages. To rule out cell type-specific effects or unintended consequences of thioglycollate elicitation, we similarly tested the requirement of TLR12 in CD11c+ splenocyte responses to TgPRF, which we had previously established required TLR11 (Yarovinsky et al., 2005). Consistent with the results obtained with peritoneal macrophages, Tlr12−/− splenic CD11c+ cells stimulated with the TLR9 and TLR4 ligands CpG and LPS produced IL-12 at amounts similar to wild-type cells, whereas TgPRF was unable to induce significant amounts of IL-12p40 (Figure 2B). As expected, the response to TgPRF was also abrogated in the absence of TLR11. For confirmation, CD11c+ cells were stimulated with increasing amounts of TgPRF to determine whether TLR12-dependent IL-12 production exhibited dose dependence (Figure 2C). Compared to WT cells, Tlr12−/− cells, similar to Tlr11−/− cells, failed to produce IL-12 in response to increasing amounts of TgPRF. Thus, TLR12 is required for recognition of TgPRF and both TLR11 and TLR12 are required for IL-12 production in macrophages and CD11c+ cells, because absence of either receptor abolishes production of IL-12.
Figure 2. TLR12 Recognizes TgPRF and Is Required for Production of IL-12 in Macrophages and CD11c+ Cells.
(A) Thioglycollate-elicited peritoneal macrophages were isolated from wild-type control (WT), Tlr11−/−, and Tlr12−/− mice and were stimulated with 10 ng/ml LPS, 1 μM CpG, 10 μg/ml LTA, 10 μg/ml Pam3CSK4, or 1.5 μg/ml TgPRF for 24 hr. The concentrations of IL-12p40 in the supernatants were measured by ELISA.
(B) CD11c+ splenic cells from WT, Tlr11−/−, Tlr12−/−, and Tlr4−/− mice were stimulated with 1 μM CpG or 2 μg/ml TgPRF or 10 ng/ml LPS for 24 hr and concentrations of IL-12p40 was measured by ELISA (asterisk indicates values below the limit of detection).
(C) CD11c+ splenic cells from WT, Tlr11−/−, and Tlr12−/− mice were stimulated with either CpG or increasing amounts of TgPRF (0.25–25 μg/ml) for 24 hr, and the concentrations of IL-12p40 was measured by ELISA.
(D) Recognition of TgPRF by either TLR11 or TLR12 can induce NF-κB activity. HEK293 cells stably expressing an NF-κB luciferase reporter construct were transfected with vectors encoding Flag-TLR11 or Flag-TLR12 and later stimulated with 6 μg/ml of TgPRF or 10 ng/ml of TNF as indicated, for 7 hr. n = 3; error bars are plus and minus one standard deviation [SD]; data are representative of three independent experiments. See also Figure S3.
TgPRF stimulation of TLR11 can trigger activation of NF-κB (Yarovinsky et al., 2005). To explore the contribution of TLR12 to NF-κB activation, TLR12 was overexpressed in a NF-κB luciferase reporter HEK293 cell line that lacks endogenous expression of either TLR12 and TLR11 (Figure 2D). Cells transfected with either TLR11 or TLR12 were able to induce NF-κB activity in response to TgPRF stimulation, suggesting that either receptor can induce NF-κB transcriptional activity, although our analysis of primary cells suggests that both receptors are needed to induce IL-12 in macrophages and CD11c+ cells.
TLR12 Selectively Recognizes Apicomplexan Profilin
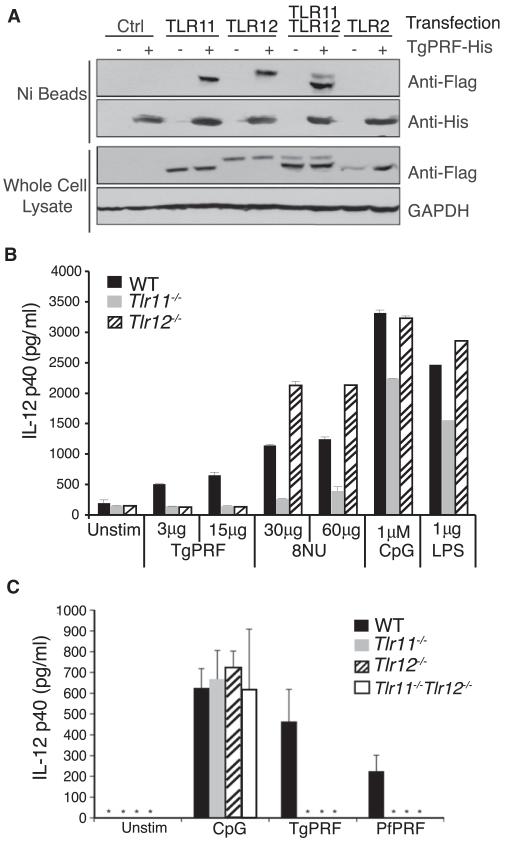
To further explore the specificity of ligand recognition by TLR12 versus TLR11, we performed receptor pull-down assays with recombinant TgPRF. We expressed TLR11 and TLR12 in HEK293 cells, lysed the cells, added His-tagged TgPRF to the lysates, and used Ni-affinity resin to pull down the His-tagged TgPRF. Both TLR11 and TLR12 could be pulled down with TgPRF, suggesting that both receptors can recognize profilin from the parasite (Figure 3A). TLR11 was initially discovered to be involved in the recognition of an uropathogenic bacterial strain, E. coli 8NU (Zhang et al., 2004). We had previously hypothesized that the protection from UPEC mediated by TLR11 was at least in part related to TLR11 expression by cells within the urinary epithelial compartment (Zhang et al., 2004). The lack of TLR12 expression in epithelial tissues led us to wonder whether recognition of the UPEC PAMP would also be shared between TLR11 and TLR12. Therefore, splenocytes from Tlr12−/− mice were stimulated with heat-killed lysates from E. coli 8NU. Tlr12−/− splenocytes produced normal amounts of IL-12 when stimulated with 8NU lysate, LPS, and CpG, but were completely unresponsive to TgPRF stimulation (Figure 3B). These experiments demonstrate that TLR11 and TLR12 have overlapping but nonredundant functions: whereas TLR12 and TLR11 are both triggered by the protein PAMP TgPRF, TLR11 alone recognizes an uncharacterized PAMP present in lysates from the UPEC 8NU.
Figure 3. TLR12 Specifically Recognizes Profilin from Apicomplexan Parasites.
(A) Both TLR11 and TLR12 can pull down TgPRF, either as homodimers or heterodimers. HEK293 cells transiently expressing Flag-TLR12 and Flag-TLR11 were lysed and incubated with His-TgPRF. His-TgPRF was subjected to Ni-affinity resin pull down and analyzed for TLR12 and TLR11 binding by protein blotting with antibodies specific for Flag and then His.
(B) Tlr12−/− cells can respond to stimulation by uropathogenic E. coli 8NU. CD11c+ splenocytes were isolated from WT, Tlr11−/−, and Tlr12−/− and stimulated overnight with TgPRF, 8NU, and LPS. Amounts of IL-12p40 in the supernatants were measured by ELISA (n = 3; error bars are plus and minus 1 SD).
(C) TLR12 can recognize profilin from Plasmodium falciparum. Thioglycollate-elicited peritoneal macrophages from wild-type (WT), Tlr11−/−, Tlr12−/−, and Tlr11−/−Tlr12−/− mice were stimulated for 24 hr with 1.0 μM of CpG, 3.0 μg/ml of TgPRF, and 3.0 μg/ml of P. falciparum profilin (PfPRF). After 24 hr supernatant was removed, and IL-12(p40) was measured by ELISA (n = 3; error bars are plus and minus 1 SD; asterisk indicates values below the limit of detection). Data in all panels are representative of at least three independent experiments.
To further confirm our hypothesis that the primary role of TLR12 is in recognition of protozoa, we tested a profilin from another apicomplexan parasite, Plasmodium falciparum. Profilin from T. gondii and other apicomplexan parasites are well conserved but divergent from mammalian profilins (Yarovinsky et al., 2005). TLR11 recognizes unique protein motifs found in TgPRF and Plasmodium falciparum profilin that are absent in mammalian profilins (Kucera et al., 2010). Therefore we tested whether TLR12 could also recognize P. falciparum profilin (PfPRF). PfPRF triggered a lower IL-12 response than TgPRF. Nevertheless, Tlr12−/− and Tlr11−/−Tlr12−/− macrophages failed to produce significant amounts of IL-12 in response to PfPRF, suggesting that TLR12 might be involved more broadly in the recognition of apicomplexan parasites (Figure 3C).
TLR11 and TLR12 Are Required for Optimal Activation of NF-κB in Response to TgPRF
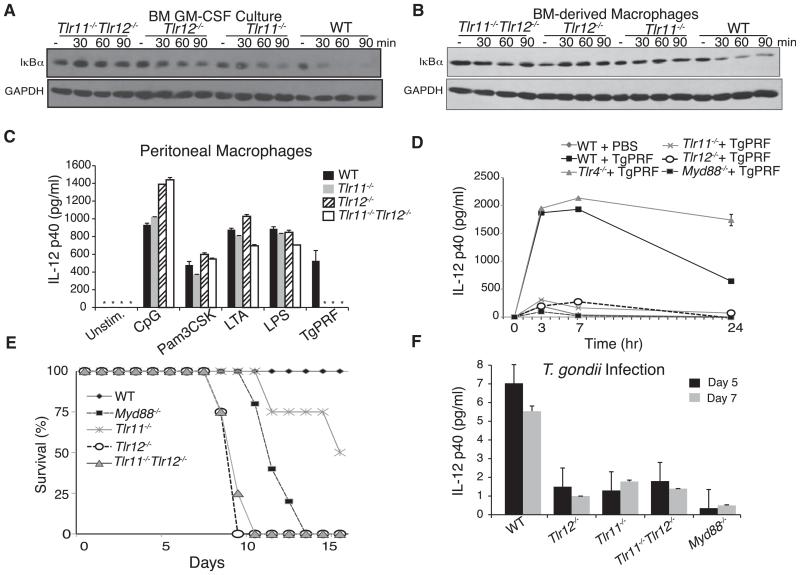
Overexpression of TLR12 activates NF-κB, but it remains unclear why in CD11c+ DCs and macrophages both TLR11 and TLR12 are required for production of IL-12 (Figure 2B). If loss of TLR11 or TLR12 led to a failure to recognize TgPRF, then TLR signaling, and especially NF-κB activation, should be abrogated in the absence of these TLRs. To explore the contribution of TLR11 and TLR12 to NF-κB activation in primary cells, TgPRF was used to stimulate bone-marrow-derived, GM-CSF-induced dendritic cells (GM-CSF BMDCs), which are primarily conventional DCs (CD11c+MHCII+) from WT control, Tlr11−/−, Tlr12−/−, and Tlr11−/−Tlr12−/− cells (Figure 4A). Activation of NF-κB by TLRs requires the phosphorylation and degradation of the typical IκB protein, IκBα, the key negative regulator of canonical NF-κB transcriptional activity. Immunoblot analysis of GM-CSF BMDCs stimulated with TgPRF revealed that in control cells, IκBα degradation begins after about 30 min. IκBα degradation was impaired in both Tlr11−/− and Tlr12−/− cells (Figure 4A). The substantial difference in activation of the NF-κB pathway in control cells and the TLR11- and TLR12-deficient cells may explain why deleting either TLR abolishes IL-12 production by conventional DCs. We also analyzed bone-marrow-derived macrophages isolated from WT control, Tlr11−/−, Tlr12−/−, and Tlr11−/−Tlr12−/− cells to determine NF-κB activity in response to stimulation by TgPRF in macrophages (Figure 4B). Immunoblot analysis revealed similar results where, in control cells, IκBα degradation begins after 30 min but is impaired in both Tlr11−/− and Tlr12−/− cells. Therefore, in both conventional DCs and macrophages, TLR11 and TLR12 act as obligate heterodimers to activate NF-κB and produce IL-12 in response to TgPRF. Finally, we analyzed the response of peritoneal macrophages from wild-type, Tlr11−/−, Tlr12−/−, and Tlr11−/−Tlr12−/− mice to different TLR ligands, and once again we observed complete abolishment of IL-12 production in response to TgPRF in the absence of either TLR11 or TLR12 (Figure 4C), consistent with the results on IκBα degradation (Figures 4A and 4B).
Figure 4. TLR12 Is Required for Protection against T. gondii Infection.
(A and B) TLR11 and TLR12 are required for optimal NF-κB activation in response to TgPRF stimulation of (A) bone marrow-derived dendritic cell culture (GM-CSF) and (B) bone-marrow-derived macrophages isolated from WT, Tlr11−/−, Tlr12−/−, and Tlr11−/−Tlr12−/− mice. GM-CSF cultures were stimulated with 3 μg/ml of TgPRF for indicated times and bone-marrow-derived macrophages were stimulated with 6 μg/ml of TgPRF. Cells were harvested and analyzed by protein blotting for IκBα degradation. Data are representative of two independent experiments.
(C) Tlr11−/−Tlr12−/− thioglycollate-elicited peritoneal macrophages fail to produce IL-12 in response to stimulation from PFTG. Macrophages from WT, Tlr11−/−, Tlr12−/−, and Tlr11−/−Tlr12−/− mice were isolated and stimulated with 10 ng/ml LPS, 500 μM CpG, 10 μg/ml LTA, 10 μg/ml Pam3CSK4, and 1.5 μg/ml TgPRF for 24 hr. The levels of IL-12p40 in the supernatants were measured by ELISA (n = 3; error bars are plus and minus 1 SD; asterisk indicates values below the limit of detection; representative of three independent experiments).
(D) Low levels of IL-12p40 are detected in the serum of Tlr11−/− and Tlr12−/− after injection with TgPRF. WT, Tlr11−/−, Tlr12−/− mice (n = 3 per group) received intraperitoneal injection with 10 μg/ml TgPRF and were bled at the time points indicated. Serum levels of IL-12(p40) were measured by ELISA (n = 3; error bars are plus and minus 1 SD; representative of three independent experiments).
(E) Myd88−/−, Tlr11−/−Tlr12−/−, and Tlr12−/− mice succumb to T. gondii infection. WT, Tlr11−/−, Tlr12−/−, and Tlr11−/−Tlr12−/− mice were infected with 20 cysts of T. gondii (ME49 strain) and monitored for survival (representative of three independent experiments).
(F) Tlr11−/−, Tlr12−/−, and Tlr11−/−Tlr12−/− mice have significantly lower serum IL-12 compared to WT mice during T. gondii infection. Measurement of serum IL-12 by ELISA from infected animals at post infection days 5 and 7 (error bars are plus and minus 1 SD; representative of three independent experiments).
Both Tlr12−/− and Tlr11−/−Tlr12−/− Mice Are Highly Susceptible to T. gondii Infection
Based on the requirement for both TLR11 and TLR12 in NF-κB responses and IL-12 production in response to TgPRF, we hypothesized that TLR12-deficient mice would be a phenocopy of TLR11-deficient mice when challenged with T. gondii or TgPRF. To determine the role of TLR12 in vivo, we first tested whether TLR12 was required for the induction of IL-12 after injection of mice with TgPRF. Tlr12−/−, Tlr11−/−, Tlr4−/−, Myd88−/−, and WT control mice were injected with TgPRF, or PBS as control, and IL-12p40 serum concentrations were determined by ELISA (Figure 4D). Both Tlr12−/− and Tlr11−/− mice had significantly lower amounts of IL-12 in response to TgPRF injection compared to control mice, confirming that both receptors are required for optimal IL-12 response in vivo and suggesting that TLR11 and TLR12 may indeed function together in response to T. gondii.
Next, we tested whether TLR12, like TLR11, is required for optimal IL-12 production and host resistance during T. gondii infection. WT, Tlr11−/−, Tlr12−/−, Tlr11−/−Tlr12−/−, and Myd88−/− mice were infected with ME-49, an avirulent T. gondii strain (Figure 4E). In contrast to our prediction that TLR12 and TLR11 function as an obligate heterodimer to mount the innate response during T. gondii infection, the Tlr12−/− mice were significantly more susceptible to T. gondii infection (Figure 4E). Tlr11−/−Tlr12−/− mice failed to display increased susceptibility to infection relative to Tlr12−/− mice, suggesting that TLR11 provides no additional protection against T. gondii infection in the absence of TLR12. Systemic IL-12p40 levels after infection were similarly decreased in Tlr11−/−, Tlr12−/−, and Tlr11−/− Tlr12−/− mice (Figure 4F). Although both Tlr11−/− and Tlr12−/− mice had similarly lower amounts of IL-12 after 7 days of infection, and although in agreement with previous results Tlr11−/− mice had higher brain cyst counts compared to control animals (not shown), only Tlr12−/− and Tlr11−/−Tlr12−/− mice died early in infection. This suggests an additional contribution of TLR12 in innate resistance to T. gondii.
TLR12-Dependent Induction of IL-12 and IFN-α in pDCs Leads to Activation of Natural Killer Cells and Production of IFN-γ
Given that TLR11 and TLR12 could interact with TgPRF independently and that overexpression experiments in HEK293 cells suggested that TLR12 could induce NF-κB activation by TgPRF in the absence of TLR11, we wished to test whether TLR12 function in a specific cell subset could explain the difference in the susceptibility of Tlr11−/− and Tlr12−/− animals to T. gondii infection-induced lethality. We analyzed TgPRF-induced activation of TLR11- and TLR12-expressing cells: namely, macrophages and bone marrow-derived Flt3L-induced dendritic cells (Flt3L BMDCs), which contain a mixture of conventional DCs that are CD8 like (CD11c+) and pDCs, both of which express TLR12 (Figure 5A) and TLR11 (Figure 5B). The Flt3L BMDCs were sorted into two separate populations based on CD11c and Siglec-H staining (Figure S4A) and analyzed for Tg-PRF-induced IL-12 production. Consistent with our previous results, Tlr11−/− and Tlr12−/− Flt3-derived CD11c+ cells failed to produce IL-12 in response to TgPRF, unlike WT control cells (Figure 5C). This suggests that in macrophages and conventional DCs, TLR11 and TLR12 must both be present to produce IL-12 in response to TgPRF stimulation.
Figure 5. TLR12, but Not TLR11, Mediates Recognition of TgPRF and Production of IL-12 by pDCs.
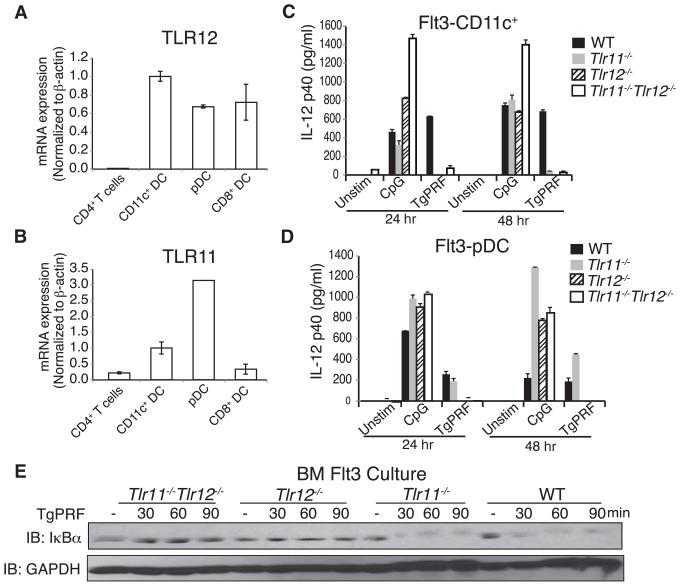
(A and B) Quantitative PCR analysis of TLR12 (A) and TLR11 (B) mRNA in total CD11c+ conventional DCs, plasmacytoid dendritic cells (pDC; Singlec-HhiCD11cint), CD8α+ dendritic cells (CD8α+; CD11chi, MHC class II+, CD8α+ population), and CD4+ T cells. PCR results were normalized with primers specific for β-actin.
(C) FACS-sorted CD11c+ Flt3L BMDCs were stimulated with either 1 μM CpG or 3 μg/ml TgPRF for 24 hr. Supernatants were collected and analyzed by IL-12 ELISA.
(D) FACS-sorted pDCs from Flt3L BMDC cultures were stimulated with CpG or TgPRF. Supernatant IL-12p40 was analyzed by ELISA.
(E) Protein blotting analysis of Flt3L BMDC culture reveals that only TLR12 is required for NF-κB activation. Flt3L BMDCs isolated from WT, Tlr11−/−, Tlr12−/−, and Tlr11−/−Tlr12−/− mice were stimulated with 3 μg/ml of TgPRF for indicated times. Cells were harvested, lysed, and analyzed by protein blotting for IκBα degradation.
Error bars are plus and minus 1 SD; data representative of three independent experiments.
Similarly, we found that both Tlr11−/−Tlr12−/− and Tlr12−/− Flt3-derived pDCs fail to produce IL-12 in response to TgPRF stimulation. Surprisingly, for pDCs, which express relatively high amounts of TLR11 mRNA (Figure 5B), Tlr11−/− cells respond to TgPRF stimulation and produce IL-12 similar to WT control cells (Figure 5D). Therefore, consistent with overexpression experiments in HEK293 cells (Figure 2C), TLR12 in pDCs can function in the absence of TLR11 in the recognition of TgPRF and induction of cytokine responses, probably through TLR12 homodimers. Immunoblot analysis of Flt3L BMDCs stimulated with TgPRF revealed that in WT and Tlr11−/− cells, IκBα degradation occurs after 30 min (Figure 5E), whereas in the absence of TLR12 (either Tlr12−/− or Tlr11−/−Tlr12−/− cells), IκBα degradation induced by TgPRF was blocked.
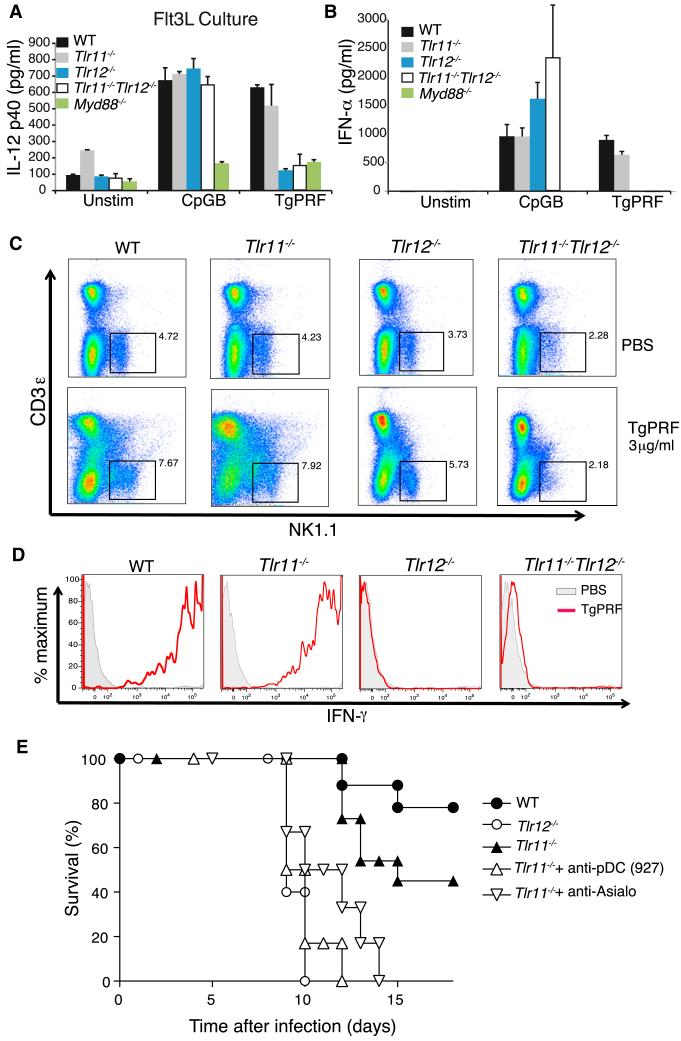
Prior studies have shown that in pDCs, induction of cytokines in response to TLR signaling is MyD88 dependent (Reizis et al., 2011). Therefore we analyzed Flt3L BMDCs stimulated with TgPRF from Myd88−/−, Tlr11−/−, Tlr12−/−, and Tlr11−/−Tlr12−/− mice to determine whether MyD88 is required for IL-12 production. We confirmed that TgPRF induction of IL-12 in Flt3L BMDCs is MyD88 dependent (Figure 6A) as it is in the GM-CSF BMDCs (Figure S4B). pDCs are known to be potent producers of IFN-α, so we measured IFN-α concentrations of primary CD11c+ splenic cells after stimulation with TgPRF by ELISA. WT and Tlr11−/− cells produce IFN-α in response to TgPRF stimulation. This response is completely abolished in Tlr12−/− and Tlr11−/− Tlr12−/− cells (Figure 6B). Taken together, these data suggest that engagement of TLR12 alone induces production of IL-12 and IFN-α in pDCs in response to TgPRF stimulation. These differences in activation of the NF-κB pathway, IL-12 induction, and IFN-α production between WT and Tlr11−/− pDCs compared to Tlr12−/− cells offer a potential explanation for why Tlr12−/− mice are more susceptible to T. gondii infection.
Figure 6. TLR12-Dependent Production of IL-12 and IFN-α Is Required for Activation of NK Cells and Production of IFN-γ.
(A) TgPRF induction of IL-12 in pDCs is TLR11 independent but TLR12 and MyD88 dependent. Flt3L BMDC cultures from WT, Tlr11−/−, Tlr12−/−, Tlr11−/−Tlr12−/−, and Myd88−/− mice were stimulated with 1 μM CpG and 3 μg/ml TgPRF for 24 hr, and supernatants were analyzed by ELISA (error bars are plus and minus 1 SD; representative of three independent experiments).
(B) TgPRF stimulation can induce IFN-α in primary CD11c+ splenocytes. Cells were stimulated with either CpG or TgPRF for 48 hr. Supernatants were analyzed after 24 hr by ELISA (error bars are plus and minus 1 SD; representative of three independent experiments).
(C) WT, Tlr11−/−, Tlr12−/−, and Tlr11−/−Tlr12−/− mice were injected intraperitoneally with 4 μg/ml TgPRF and single-cell suspension of splenocytes were prepared. Splenocytes were stained for anti-NK1.1 and anti-IFN-γ and analyzed by flow cytometry.
(D) Histograms showing amounts of intercellular IFN-γ in NK1.1+ cells after TgPRF injection. The black lines represent PBS-injected and the red line represents TgPRF-injected animals. Data are representative of at least two independent experiments.
(E) Tlr11−/− mice were injected i.p. with either anti-CD317 (mAb 927) or control ascites (0.5 μg Ig/mouse) or i.v. with 50 μl of anti-AsialoGM1 on day −1 and +3 relative to infection, and their survival was monitored daily.
See also Figure S4.
It has previously been reported that low amounts of IFN-α in conjunction with IL-12 can provide an enhancing effect on natural killer (NK) cell-mediated cytotoxicity, blastogenesis, and proliferation (Biron, 2001) and can also promote IFN-γ production by NK cells (Hunter et al., 1997). Furthermore, it has been shown that NK cells can promote induction of CD8+ T cell responses to T. gondii (Combe et al., 2005) and can produce IFN-γ during T. gondii infection (Yarovinsky et al., 2008) and that NK cell IFN-γ promotes IL-12 production by macrophage-derived DCs (Goldszmid et al., 2012). Indeed, production of IFN-γ by NK cells during T. gondii infection is maintained in Tlr11−/− mice (Yarovinsky et al., 2008). Therefore, we compared IFN-γ levels produced by NK cells in Tlr11−/−, Tlr12−/−, and Tlr11−/−Tlr12−/− mice in response to injected TgPRF. After 16 hr, spleens were harvested and cells were stained and gated for NK cells (Figure 6C). Control and Tlr11−/− mice show increased amounts of IFN-γ in response to TgPRF, whereas Tlr12−/− and DKO mice have nearly undetectable levels of IFN-γ (Figure 6D). Therefore, these data provide strong evidence that production of IFN-γ by NK cells is TLR12 dependent. Given that IL-12 and IFN-α promote NK cell IFN-γ production, that NK cell expansion during T. gondii infection is IL-12 dependent (Combe et al., 2005), and that Tlr11−/− pDCs can produce both IFN-α and IL-12 in response to TgPRF, we predicted that depletion of either pDCs or NK cells should negate the relative resistance of TLR11 mice, in comparison to Tlr12−/− mice, to T. gondii challenge. Indeed, when pDCs or NK cells were depleted, we observed a significant increase in the sensitivity of TLR11 mice to T. gondii challenge (Figure 6E). Taken together, these results suggest that TLR12-pDC axis contributes to protection against T. gondii infection.
DISCUSSION
The requirement for innate recognition of T. gondii via the TLR pathway has been well established. Most notably, MyD88-deficient mice exhibit dramatic susceptibility to T. gondii infection (Scanga et al., 2002). Although numerous PAMPs have been identified in apicomplexan parasites, mice in which one or more of the TLRs known to recognize these PAMPs are deleted fail to recapitulate the phenotype of MyD88-deficient mice (Debierre-Grockiego et al., 2007; Melo et al., 2010; Pifer and Yarovinsky, 2011; Scanga et al., 2002; Yarovinsky et al., 2005). These findings suggested that the innate immune system of mice has an additional means of detecting and mounting a response to T. gondii infection.
We previously characterized T. gondii profilin-like protein as an important apicomplexan PAMP recognized by murine TLR11. When infected with T. gondii, mice lacking TLR11 have dramatically reduced production of IL-12, a cytokine known to be important for induction of the Th1 cell responses integral to the effective immune response to T. gondii. Although TLR11-deficient mice also show increased parasite cyst loads after infection, surprisingly they do not succumb to infection like Myd88−/− mice. These data suggested that there are alternative MyD88-dependent pathways that contribute to a protective response against T. gondii.
We now demonstrate that TLR12, a heretofore uncharacterized member of the mammalian TLR family, plays a pivotal role in the control of T. gondii infection. TLR12 is highly homologous to TLR11 and, similar to TLR11-deficient cells, Tlr12−/− macrophages and conventional dendritic cells fail to respond to TgPRF stimulation, demonstrating that TLR12 can recognize the same protein ligand as TLR11. However, these receptors clearly also mediate distinct aspects of the innate immune response. TLR11 and TLR12 exhibit overlapping but unique tissue expression patterns. Notably, the expression of TLR12 appears more restricted to hematopoietic cells. TLR11 is more broadly expressed, especially at epithelial surfaces and hematopoietic cells. Consistent with the expression of TLR11, but not TLR12, at epithelial surfaces, the response to heat-killed 8NU UPEC lysates depends only on TLR11. Therefore, although these receptors can recognize the protein ligand from T. gondii, they can also work individually as in the TLR11-dependent response to UPECs.
The ability of TLR11 and TLR12 to interact with each other, and their shared localization in endosomes, suggests that they can work as heterodimers. This hypothesis is also supported by our analysis of bone-marrow-derived macrophages and GM-CSF BMDCs in which both TLR11 and TLR12 are required for NF-κB activation and IL-12 production in response to TgPRF stimulation. Given that TLR11 and TLR12 are capable of forming a heterodimer, these findings suggest that TLR11 and TLR12 act as an obligate heterodimer in the recognition of, and/or response to, TgPRF in macrophages and DCs. Therefore, it was surprising that Tlr12−/− mice succumb to T. gondii infection at rates similar to Myd88−/− mice. However, the increased brain cyst burden and decreased IL-12 production in Tlr11−/− mice demonstrate that TLR12 by itself cannot provide complete protection. Therefore, we concluded that although TLR11-mediated responses to T. gondii require TLR12, there are also TLR11-independent, but TLR12-dependent, aspects of the innate response to T. gondii.
Plasmacytoid DCs are a rare cell type (0.3%–0.5% of human blood and lymphoid organs) and are considered to be potent secretors of type I interferons (IFN-α and IFN-β) in response to foreign nucleic acids (Reizis et al., 2011). This pDC function is linked to their expression of TLR7 and TLR9, and not other TLRs. The discovery of TgPRF, a protein ligand that can induce IL-12 and IFN-α in a TLR12-dependent manner in pDCs provides additional insight into the potential role of this myeloid cell population. It has been previously reported that after T. gondii infection, there is an expansion of pDCs at day 2 postinfection and that these cells upregulate MHC class II and costimulatory molecules that are critical for priming CD4+ T cells (Pepper et al., 2008). Protective responses against T. gondii are dependent on the induction of Th1 cell responses driven by IL-12 and IFN-γ. NK cells are an important source of IFN-γ, although the mechanism by which they might be activated during T. gondii infection is unclear. NK cells do not express TLR12 and do not respond directly to TgPRF. Instead, TLR12 is expressed in pDCs and, in these cells, can mediate responses to TgPRF in a TLR11-independent manner. A previous report had demonstrated that pDC activation by TgPRF was dependent on TLR11 (Pepper et al., 2008), which differs from the results reported here. However, a careful comparison of the experimental conditions used reveals significant differences. Pepper et al. (2008) used substantially lower concentrations of STAg (50 ng/ml), which is an unpurified source of TgPRF, to stimulate the FLt3L-bone-marrow-derived cells overnight whereas we used 5 μg/ml of purified TgPRF and stimulated the cells for 24 to 48 hr. At 24 hr, low amounts of IL-12 are produced from the Tlr11−/− cells compared to Tlr12−/− and Tlr11−/−Tlr12−/−. However, after 48 hr of TgPRF stimulation, Tlr11−/− pDCs exhibited robust production of IL-12 in vitro. Therefore, although our studies do not rule out a role for TLR11 in pDC responses (Pepper et al., 2008), they do establish that in pDCs TLR12 can mediate a TLR11-independent response that may contribute to the resistance of Tlr11−/− mice to T. gondii.
TgPRF induces weak IFN-α and substantial IL-12 production by pDCs. Localized production of IL-12 by this relatively rare cell type is unlikely to significantly alter systemic serum IL-12 levels. However, locally elevated IL-12 production in conjunction with IFN-α may be crucial for the induction of IFN-γ. Our results suggest that type I interferons contribute to the protective response, but only synergistically with proinflammatory cytokines such as IL-12, through activation of NK cells and enhanced NK cell production of IFN-γ. It has been documented that type I interferons can induce potent immunomodulatory effects and that even low-level basal IFN-α production increases the production of IFN-γ by NK cells (Hunter et al., 1997). Taken together, these data suggest that despite severely limited innate responses to T. gondii in TLR11-knockout mice, these animals can mount a protective response through TLR12-dependent pDC recognition of TgPRF leading to enhanced NK cell IFN-γ production. In strong support of this model, the resistance of TLR11-knockout animals is abrogated upon pDC or NK cell depletion.
In summary, our studies show that mice possess two pattern recognition receptors, TLR11 and TLR12, which are specialized for the recognition of profilin from apicomplexan parasites. Mice are a major intermediate host for the T. gondii life cycle, because they are the hosts through which T. gondii infects feline species. As a result, mice, and rats, have strong selective pressure to maintain robust innate recognition of T. gondii and this benefits the parasite by promoting transmission through increased host survival. It is possible that in humans and other higher mammals, expression of TLR11 and TLR12 may have been selected against because of the potentially lethal proinflammatory effects of TLR recognition of profilin. This interesting dichotomy between mouse and human TLR expression may have relevance in understanding the differential response of rodents and humans to other apicomplexan protozoa as well as mucosal bacterial pathogens.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
Antibodies used were anti-Flag M2 (Sigma), anti-Ha (Roche), anti-IκBα, anti-GFP (Santa Cruz), anti-GAPDH (Fitzgerald), anti-IFN-α/β RMMA, anti-IFN-α/β (PBL Interferon Source), APC-conjugated CD11c+, PE-conjugated Siglec-H, FITC-conjugated anti-NK1.1, APC-conjugated anti-CD3ε, and PE-conjugated anti-IFN-γ (eBioscience). Antibodies used for immunodepletion experiments were anti-AsialoGM1 (Wako Pure Chemicals, Richmond, VA) and ascites of anti-CD317 mAb 927 specific for pDC marker PDCA-1 was matched with control mAb rat IgG2b (G. Trinchieri). LPS was isolated from Salmonella entrica (Sigma). CpG1826 (sequence 5′-TCCATGACGTTCCTGACGTT-3′) was synthesized at the W.M. Keck Oligonucleotide Synthesis facility at Yale University. Synthesized polyinosinc-polyctidylic acid [poly(I:C)] was from Sigma whereas Pam3CysSerLys4 (Pam3CSK) was obtained from InvivoGen. Lipoteichic acid (LTA) isolated from S. aureus was purchased from InvivoGen. The uropathogenic E. coli 8NU was kindly provided by D. Klumpp (Northwestern University, IL), and heat-killed preparations were generated as described previously (Zhang et al., 2004).
TgPRF Protein Expression and Purification
T. gondii profilin was expressed in BL21 E. coli cells with the pET28 vector. PFTG expression was induced, based on empirically determined conditions, with 0.2 mM IPTG at 37°C for 4 hr. The culture was pelleted and incubated in 50 mM phosphate buffer (pH 8.0), 300 mM NaCl (Buffer A) with 100 μg/ml lysozyme, and 0.5 mM PMSF for 90 min on ice. The culture was sonicated (VirTis) and pelleted and the cleared lysates were incubated with 5 ml of a 50% slurry of Ni-NTA agarose washed four times in Buffer A at 4°C for 1 hr. The lysates was loaded onto a column and washed with 5 column volumes of Buffer A followed by 5 column volumes each of Buffer A with 10 and 30 mM imidazole. TgPRF was eluted with 2 column volumes of 200 mM imidazole. Wash and eluate fractions were examined by SDS-PAGE and commassie brilliant blue staining. Eluate fractions containing the TgPRF peak, judged to be >95% pure, were pooled and dialyzed (2.5 l × 2) into PBS with 5% glycerol. Endotoxin was removed by passing over two polymyxin-B columns. TgPRF was sterilized with a 0.22 μM syringe filter; aliquot were snap frozen and stored at −80°C.
Cloning and Sequence Analysis
The mouse TLR12 open reading frame was amplified from cDNA made from RNA isolated from RAW 264.7 cells with the primers 5′-AAGCTTGCGGCCGCGGTCAGCATGCCAAGG-3′ and 5′-GCTCGCGGATCCTTCCTGCAGTCC TTAATC- 3′, and was cloned into pFlag-CMV14 vector (Sigma) containing an C-terminal Flag epitope tag. TLR12 was additionally cloned with a HA-epitope tag on the C-terminal end. TLR11 open reading frame was also cloned into pFlag-CMV14 with the following primers: 5′-CGGGAATTCACCATGGGCAGGTACTGGCTG-3′ and 5′-CGGTCTAGACCCTAGCCTGCTCCTCAG-3′. TLR11 was additionally cloned with a HA-epitope tag at the C-terminal end.
RT-PCR and qPCR Analysis
Total RNA was isolated from multiple murine tissues with RNeasy Mini kit (QIAGEN) and from specific FACS-sorted cells with RNA-Bee (Tel-Test). 1 μg of RNA was used for reverse transcription with Superscript III reverse transcription kit (Invitrogen). 100 ng of this reaction was used as a template for PCR amplification with HotStar Taq polymerase (QIAGEN) according to manufacturer’s guidelines. The sequences of the TLR12 primers were 5′-ATTCATTTTCCCTCCCTGCG-3′ and 5′-CACACCAGATAAAGAAGTGCTCCAG-3′, and the TLR11 primers were 5′-TTGAGGGTATGGGGTGCTGGAAAC-3′ and 5′-TGGGTTATGGACTGAAGCGACG-3′. For qPCR analysis, we followed PerfeCta SYBR Green FastMix protocol (Quanta Bioscience) and used β-actin primers 5′- GCTGTGCTGTCCCTGTATGCCTCT-3′ and 5′- CCTCTCAGCTGTGGTGGTGAAGC-3′ as control.
Experimental Animals
C57BL/6 and Tlr4−/− strains were obtained from Taconic Farms. Myd88−/− strains were generously provided by R. Medzhitov. Tlr12−/− strains were backcrossed for ten generations into C57BL/6 for the experiments described. The Tlr11−/− and Tlr12−/− strains were both backcrossed into C57BL/6 and then bred together to produce the DKO strain. All animals used were maintained at an American Association of Laboratory Animal Care-accredited animal facilities, initially at Yale University School of Medicine and then at Columbia University College of Physicians and Surgeons. All the animals were age and sex matched and either WT littermates or congenic strains were used as controls.
Peritoneal Macrophage Isolation
Mice were injected i.p. with 2 ml of 4% thioglycollate. After 5 days, mice were euthanized and their peritoneal cavities were lavaged with PBS. The PBS solution was harvested by syringe, centrifuged for 5 min at 1,500 rpm, and resuspended into DMEM with 5% FBS. Cells were than plated.
CD11c+ Splenocyte Isolation
Spleens were harvested from WT, Tlr11−/−, Tlr12−/−, and Tlr4−/− mice. These tissues were minced and run through a 70 mm nylon strainer. Cells were resuspended in Red Blood Cell lysis buffer (Sigma) and then washed with RPMI with 10% FBS. Cells were spun and resuspended in RPMI with 10% FBS. Cell suspensions were then overlaid over Lympholyte-M (Cedar Line). This suspension was then centrifuged for 20 min at 2,500 rpm at 4°C. The lymphocyte layer was harvested, washed with ice-cold PBS, and resuspended with RPMI with 10% FBS. Cells were than plated.
ELISA Analysis
Total splencotyes, CD11c+ cells, and peritoneal macrophages were plated at 5 × 104 cells per well on a 96-well plate. Cells were stimulated with 1.5 μg/ml of TgPRF, 10 ng/ml of LPS, and 500 μM CpG for 24 hr. Supernatant was harvested and analyzed for production of IL-12 (p40) by ELISA according to manufacturer’s instruction (R&D System). For IFN-α ELISA, 1 × 106 CD11c+ cells were plated per well on a 96-well plate and stimulated with 3 μg/ml of TgPRF for 48 hr. IFN-α concentration was measured by ELISA with anti-murine IFN-α antibodies and recombinant standard (PBL Interferon Source).
NF-κB Luciferase Assays
HEK293-LUC cells stably transfected with a NF-κB luciferase reporter construct pBIIX-luc were used. Cells were stimulated with 6.0 μg/ml of TgPRF, 1 μM CpG DNA, or 10 ng/ml of TNF-α for 6.5 hr and luciferase activity was measured with the Luciferase Reporter Assay System (Promega).
Bone-Marrow-Derived Dendritic Cells and Macrophages
WT, Tlr11−/−, Tlr12−/−, DKO, and Myd88−/− mouse bone marrow was harvested from femurs and hind leg bones. Cells were washed with PBS and resuspended in DMEM with 10% FBS. Depending on cell lineage, different growth factors were supplemented. For conventional DCs, 10 ng/ml of recombinant GM-CSF (PeproTech) and recombinant IL-4 (eBioscience) was added; for pDCs, 100 ng/ml of recombinant Flt3L (eBioscience) was added; and for macrophages, 30% L929 conditional medium was added to DMEM. Cells were incubated for 8 days for DCs and 6 days for macrophages with conditional media and then harvested and plated into 6-well plates at 2 × 106 cells per well for immunoblot analysis and into 96-well plate for ELISA analysis.
FACS Analysis and Sorting
Flt3L BMDC culture was stained directly with APC-conjugated CD11c+ and PE-conjugated Siglec-H and sorted with FACSAria (BD Biosciences). Gates were set on forward and side scatter and were sorted into two populations. Purity of sorted cells typically exceeded 92%.
Protein Immunoblot Analysis
An additional 5 μl of 2-mercaptoethanol was added to 100 μl of 6× SDS loading dye before protein samples were boiled. 30–50 μg of total protein were run on either 10% SDS-polyacrylamide gel electrophoresis gel or 6%–18% polyacrylamide gels. After transfer to polyvinylidene fluoride membrane (Millipore), immunoblotting was performed as per the manufacturer’s protocol.
In Vivo Analysis of TgPRF and Infection Experiments
5 μg/ml of TgPRF was injected i.p. into WT, Tlr12−/−, Tlr11−/−, Tlr4−/−, or Myd88−/− mice and bled at 2, 6, and 24 hr. IL-12p40 levels were then measured in the serum samples by ELISA as described above. To assess resistance to infection, the same strains of mice were infected i.p. with an average of 20 cysts of T. gondii (ME49 strain) and survival monitored. At day 30 the surviving animals were sacrificed and brain cyst counts were measured for parasite load. In the same experiments, serum IL-12p40 levels were assessed at day 5 and day 7 postinfection by ELISA.
Measurement of Intracellular IFN-γ
Intracellular IFN-γ was measured in NK cells stimulated with 3 μg/ml TgPRF for 16 hr. Spleens were harvested and to inhibit release of IFN-γ, cells were treated with monesin (Sigma). Cells were then stained with FITC-anti-NK1.1 and APC-CD3ε. Next cells were fixed with 1% formaldehyde overnight. After being washed twice in PBS supplemented with 5% calf serum, cells were permeabilized by resuspending in 0.5% saponin in PBS supplemented. Intracellular IFN-γ was stained by incubating the permeabilized cells with PE-anti-IFN-γ antibody (eBiosciences). After washing with supplemented PBS, stained cells were identified by flow cytometry.
Supplementary Material
ACKNOWLEDGMENTS
We thank B. Beulter (Scripps Research Institue) for the Unc93b-GFP plasmid, R. Medzhitov (Yale University) for MyD88-deficient mice, Y. Modis (Yale University) for PfPRF, and G. Trinchieri for kindly providing CD317 mAb and control ascites. The studies in this manuscript were supported by grants from NIH (RO1-59440 and R37-33443) and institutional support from Columbia University. M.S.H. is supported by ARRA P30 AR058886-01. This work was supported in part by the intramural research program of NIAID, NIH.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes four figures and can be found with this article online at http://dx.doi.org/10.1016/j.immuni.2012.09.016.
REFERENCES
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Biron CA. Interferons alpha and beta as immune regulators—a new look. Immunity. 2001;14:661–664. doi: 10.1016/s1074-7613(01)00154-6. [DOI] [PubMed] [Google Scholar]
- Combe CL, Curiel TJ, Moretto MM, Khan IA. NK cells help to induce CD8(+)-T-cell immunity against Toxoplasma gondii in the absence of CD4(+) T cells. Infect. Immun. 2005;73:4913–4921. doi: 10.1128/IAI.73.8.4913-4921.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debierre-Grockiego F, Campos MA, Azzouz N, Schmidt J, Bieker U, Resende MG, Mansur DS, Weingart R, Schmidt RR, Golenbock DT, et al. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J. Immunol. 2007;179:1129–1137. doi: 10.4049/jimmunol.179.2.1129. [DOI] [PubMed] [Google Scholar]
- Gazzinelli RT, Hieny S, Wynn TA, Wolf S, Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc. Natl. Acad. Sci. USA. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli RT, Wysocka M, Hayashi S, Denkers EY, Hieny S, Caspar P, Trinchieri G, Sher A. Parasite-induced IL-12 stimulates early IFN-γamma synthesis and resistance during acute infection with Toxoplasma gondii. J. Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- Goldszmid RS, Caspar P, Rivollier A, White S, Dzutsev A, Hieny S, Kelsall B, Trinchieri G, Sher A. NK cell-derived interferon-γ orchestrates cellular dynamics and the differentiation of monocytes into dendritic cells at the site of infection. Immunity. 2012;36:1047–1059. doi: 10.1016/j.immuni.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- Hunter CA, Gabriel KE, Radzanowski T, Neyer LE, Remington JS. Type I interferons enhance production of IFN-γ by NK cells. Immunol. Lett. 1997;59:1–5. doi: 10.1016/s0165-2478(97)00091-6. [DOI] [PubMed] [Google Scholar]
- Jones JL, Kruszon-Moran D, Sanders-Lewis K, Wilson M. Toxoplasma gondii infection in the United States, 1999 2004, decline from the prior decade. Am. J. Trop. Med. Hyg. 2007;77:405–410. [PubMed] [Google Scholar]
- Kucera K, Koblansky AA, Saunders LP, Frederick KB, De La Cruz EM, Ghosh S, Modis Y. Structure-based analysis of Toxoplasma gondii profilin: a parasite-specific motif is required for recognition by Toll-like receptor 11. J. Mol. Biol. 2010;403:616–629. doi: 10.1016/j.jmb.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Fan YT, Dias A, Esper L, Corn RA, Bafica A, Machado FS, Aliberti J. Cutting edge: dendritic cells are essential for in vivo IL-12 production and development of resistance against Toxoplasma gondii infection in mice. J. Immunol. 2006;177:31–35. doi: 10.4049/jimmunol.177.1.31. [DOI] [PubMed] [Google Scholar]
- Mashayekhi M, Sandau MM, Dunay IR, Frickel EM, Khan A, Goldszmid RS, Sher A, Ploegh HL, Murphy TL, Sibley LD, Murphy KM. CD8α(+) dendritic cells are the critical source of interleukin-12 that controls acute infection by Toxoplasma gondii tachyzoites. Immunity. 2011;35:249–259. doi: 10.1016/j.immuni.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV. Food-related illness and death in the United States. Emerg. Infect. Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- Melo MB, Kasperkovitz P, Cerny A, Könen-Waisman S, Kurt-Jones EA, Lien E, Beutler B, Howard JC, Golenbock DT, Gazzinelli RT. UNC93B1 mediates host resistance to infection with Toxoplasma gondii. PLoS Pathog. 2010;6:e1001071. doi: 10.1371/journal.ppat.1001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MM, Liu K, Darrasse-Jeze G, Kamphorst AO, Schreiber HA, Guermonprez P, Idoyaga J, Cheong C, Yao KH, Niec RE, Nussenzweig MC. Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J. Exp. Med. 2012;209:1153–1165. doi: 10.1084/jem.20112675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas G, Roussos N, Falagas ME. Toxoplasmosis snap-shots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int. J. Parasitol. 2009;39:1385–1394. doi: 10.1016/j.ijpara.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Pepper M, Dzierszinski F, Wilson E, Tait E, Fang Q, Yarovinsky F, Laufer TM, Roos D, Hunter CA. Plasmacytoid dendritic cells are activated by Toxoplasma gondii to present antigen and produce cytokines. J. Immunol. 2008;180:6229–6236. doi: 10.4049/jimmunol.180.9.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pifer R, Yarovinsky F. Innate responses to Toxoplasma gondii in mice and humans. Trends Parasitol. 2011;27:388–393. doi: 10.1016/j.pt.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pifer R, Benson A, Sturge CR, Yarovinsky F. UNC93B1 is essential for TLR11 activation and IL-12-dependent host resistance to Toxoplasma gondii. J. Biol. Chem. 2011;286:3307–3314. doi: 10.1074/jbc.M110.171025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis e Sousa C, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J. Exp. Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid dendritic cells: recent progress and open questions. Annu. Rev. Immunol. 2011;29:163–183. doi: 10.1146/annurev-immunol-031210-101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanga CA, Aliberti J, Jankovic D, Tilloy F, Bennouna S, Denkers EY, Medzhitov R, Sher A. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J. Immunol. 2002;168:5997–6001. doi: 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- Tabeta K, Hoebe K, Janssen EM, Du X, Georgel P, Crozat K, Mudd S, Mann N, Sovath S, Goode J, et al. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat. Immunol. 2006;7:156–164. doi: 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 2000;165:5392–5396. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- West AP, Koblansky AA, Ghosh S. Recognition and signaling by toll-like receptors. Annu. Rev. Cell Dev. Biol. 2006;22:409–437. doi: 10.1146/annurev.cellbio.21.122303.115827. [DOI] [PubMed] [Google Scholar]
- Yap G, Pesin M, Sher A. Cutting edge: IL-12 is required for the maintenance of IFN-γamma production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J. Immunol. 2000;165:628–631. doi: 10.4049/jimmunol.165.2.628. [DOI] [PubMed] [Google Scholar]
- Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, Sher A. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- Yarovinsky F, Hieny S, Sher A. Recognition of Toxoplasma gondii by TLR11 prevents parasite-induced immunopathology. J. Immunol. 2008;181:8478–8484. doi: 10.4049/jimmunol.181.12.8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522–1526. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
Note Added in Proof
After acceptance of this manuscript, our paper was published describing flagellin as the PAMP recognized by TLR11 in UPECs and Salmonella:
- Mathur R, Oh H, Zhang D, Park S-G, Seo J, Koblansky A, Hayden MS, Ghosh S. A mouse model of Salmonella Typhi infection. Cell. 2012;151:590–602. doi: 10.1016/j.cell.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.