Abstract
Kaempferol is a polyphenol antioxidant found in fruits and vegetables. Many studies have described the beneficial effects of dietary kaempferol in reducing the risk of chronic diseases, especially cancer. Epidemiological studies have shown an inverse relationship between kaempferol intake and cancer. Kaempferol may help by augmenting the body’s antioxidant defense against free radicals, which promote the development of cancer. At the molecular level, kaempferol has been reported to modulate a number of key elements in cellular signal transduction pathways linked to apoptosis, angiogenesis, inflammation, and metastasis. Significantly, kaempferol inhibits cancer cell growth and angiognesis and induces cancer cell apoptosis, but on the other hand, kaempferol appears to preserve normal cell viability, in some cases exerting a protective effect. The aim of this review is to synthesize information concerning the extraction of kaempferol, as well as to provide insights into the molecular basis of its potential chemo-preventative activities, with an emphasis on its ability to control intracellular signaling cascades that regulate the aforementioned processes. Chemoprevention using nanotechnology to improve the bioavailability of kaempferol is also discussed.
Keywords: Dietary flavonoid, Kaempferol, Angiogenesis, Apoptosis, Signal transduction, Metastasis, Nanotechnology
1. Introduction
Cancer manifests itself in a number of forms, all marked by the same unrestrained proliferation of cells. There currently exist many techniques to manage this leading cause of mortality. Surgery, radiation treatments, and chemotherapy have shown remarkable efficacy in cancer treatments, but they are not without serious shortcomings. None of these is a panacea for such a resilient disease. Cancer cells adapt to treatment; they have a stubborn inclination to mutate or metastasize. Once a tumor is eliminated, its remnants tend to linger. Chemotherapy is also notorious for inducing a plethora of adverse effects in patients. From vomiting to hair loss, quality of life can be severely compromised during rounds of chemotherapy.
Flavonoids are polyphenolic compounds commonly found in plants and constitute a significant part of the human diet (Wojdylo, Oszmianski, & Czemerys, 2007). The antioxidant and anti-inflammatory capacities of these compounds are well documented (Seifried et al., 2007), and many display cancer fighting potential. Flavonoids were reported to inhibit VEGF expression, cancer cell proliferation and angiogenesis (Luo, Jiang, King, & Chen, 2008). Of particular interest is the flavonoid kaempferol (Fig. 1). A member of the flavonols, kaempferol is abundantly found in tea, broccoli, apples, strawberries, and beans (Somerset and Johannot, 2008). It has been demonstrated to invoke several different mechanisms in the regulation of cancer cells. Not only is kaempferol a potent promoter of apoptosis (Ramos, 2007), but it also modifies a host of cellular signaling pathways. In addition, kaempferol is much less toxic to normal cells in comparison to standard chemotherapy drugs (Zhang, Chen, Li, Chen, & Yao, 2008). This review aims to catalogue the numerous anticancer properties of kaempferol and the cellular processes affected. An investigation into the bioavailability of kaempferol is also conducted.
Figure 1.
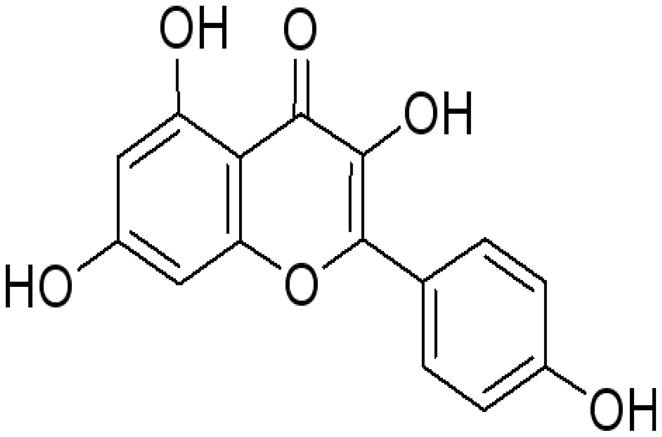
Chemical Structure of Kaempferol.
2. Extraction
An economic and low cost method for kaempferol preparation was proposed by enzymatic hydrolysis using two kaempferol glycosides in tea seed (Park, Rho, Kim, & Chang, 2006). The conventional organic solvent extraction method has been used to extract the two kaempferol glycosides (Sekine et al., 1991; 1993; Park et al., 2006). However, this procedure is time-consuming and labour-intensive. Handling of large volumes of hazardous solvents and extended concentration steps can result in the loss or degradation of target compounds.
Supercritical fluid extraction (SFE) is a rapid method for the extraction of natural bioactive compounds from plant materials (Marr and Gamse, 2000; Brunner, 2005; Huang, Li, Niu, Li, & Zhang, 2008; Lee, Charles, Kung, Ho, & Huang, 2010c). It is developed as an alternative extraction technology consuming less organic solvents to offset the rising solvent acquisition and disposal costs (Scalia, Giuffreda, & Pallado, 1999).
Carbon dioxide (CO2) is the most frequently used solvent because it is non-toxic, non-flammable, odourless, and easily separated from the extract. It also has a low critical temperature, which allows it to be used to extract thermally labile and reactive compounds (Liu et al., 2009a; Liu, Yang, Zhang, Ji, Hong, & Deng, 2009b). However, pure CO2 is not an appropriate extraction fluid for polar analytes and retentive matrices. The addition of a polar modifier to supercritical CO2 is the simplest and the most effective way to obtain the desired polarity of CO2 based fluids for the extraction polar organic compounds. One can readily manipulate the properties of the fluid by changing the type or amount of modifier (Scalia et al., 1999; Rostagno, Araújo, & Sandi, 2002). Ethanol is an ideal modifier for polar compounds in supercritical fluid extraction because of its low toxicity (Lang and Wai, 2001). Li, Xu, Jin, Wu, and Tu (2010) developed a rapid and simple method using an optimized SFE process to extract the two kaempferol glycosides from tea seed cake. Response surface methodology (RSM) was used to build a model from four independent factors including extraction time, pressure, temperature and ethanol content of modifier. Zhu, Lin, Chen, Xie, and Wang (2011) used a mechanochemical-assisted extraction (MCAE) method in extracting kaempferol from Camellia oleifera Abel. meal. Compared with the heat reflux extraction (HRE) method, the yield and antioxidant activities of the extracts from MCAE with water as the solvent were both higher and stronger.
3. Effect on apoptosis and growth inhibition
(a) Signal Transduction
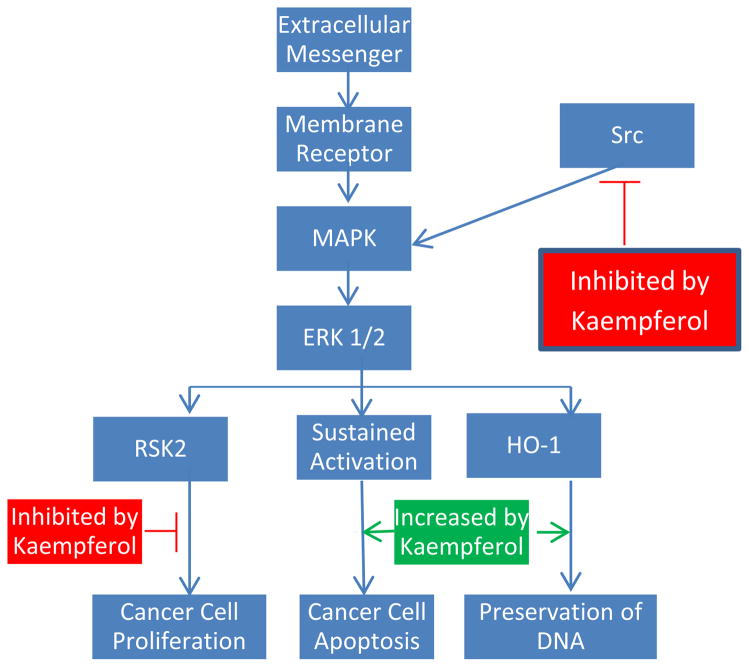
In the context of a multicellular organism, individual cells can only work in cohort when the proper channels of communication are open. Cells must be able to respond appropriately to a wide variety of external stimuli. There exist many pathways for translating chemical messages into physical changes in gene expression, one of which is the MAPK/ERK pathway. This signal route begins with the binding of an extracellular messenger to a specific membrane protein. The membrane protein can then activate Ras, which subsequently activates a series of kinases, triggering a phosphorylation cascade. Eventually, the message reaches the nucleus, where it can effect changes in transcription and translation. The MAPK pathway plays an integral role in both the promotion and regulation of cell growth. For example, ERK 1 and 2 activate the 90-κDa ribosomal S6 kinases, which are sensitive to numerous growth factors (Frodin and Gammeltoft, 1999). Of particular interest is the protein RSK2, which is thought to play a major role in driving growth and enhancing proliferation. RSK2 levels are noticeably higher in cancerous cells (Cho, Yao, Pugliese, Malakhova, Bode, & Dong, 2009). However, increasing evidence suggests that the MAPK signaling route is necessary for cell death as well (Nguyen et al., 2003). Brief activation of MAPK has been associated with surges of cell propagation, while sustained activation appears to promote the apoptosis machinery in a number of cell lines (Kim et al., 2008).
Many in vitro studies have catalogued the apoptosis inducing properties of kaempferol, which can at least be partially attributed to its effects on the MAPK pathway. Introduction of kaempferol to cancer cells is strongly linked to a prolonged activation of the signaling path. In fact, incorporation of MAPK blockers can significantly hinder apoptosis, even when combined with inhibition of pro-growth proteins (Kim et al., 2008). At least in MCF-7 and A549 cells, MAPK induction seems to be a necessary factor in kaempferol-initiated apoptosis. Furthermore, kaempferol-mediated MAPK activation can prevent DNA damage leading to cell transformation. The presence of kaempferol was found to increase haeme oxygenase (HO)-1 gene expression, which causes a surge in the antioxidant capacity of cells (Hong, Yen, Wang, Lo, Chen, & Wu, 2009). Kaempferol treatment dramatically heightened cell viability in response to oxidative stress, which includes unstable radicals prone to harm DNA. In this way, kaempferol-induced MAPK initiation protects healthy cells from transforming into cancerous ones. Importantly, these protective effects seem to only apply to normally functioning body cells. Administration of kaempferol actually increases oxidative stress in glioblastoma cells, increasing production of reactive oxygen species (ROS) in these cancerous cells (Sharma, Joseph, Ghosh, Agarwal, Mishra, & Sen, 2007). This heightened trauma induces apoptosis, while preserving healthy cells at the same time.
In addition to extended activation of the pathway, kaempferol also modifies a number of the proteins involved. RSK2 is a key suppressor of apoptosis. It has been found to down-regulate the apoptosis promoting protein BAD and up-regulate levels of Bcl-2 (Bonni, Brunet, West, Datta, Takasu, & Greenberg, 1999; Luo, Rankin, Li, Depriest, & Chen, 2011). Kaempferol has recently been shown to bind directly to the RSK2 protein, specifically at the Val82 and the Lys100 sites, positions which are critical in the function of RSK2 (Cho et al., 2009). Thereby kaempferol paralyzes the RSK2 protein. As expected, treatment was reported to drop Bcl levels and boost concentrations of tumor suppressor proteins BAD and p53 (Luo et al., 2011). Furthermore, kaempferol has been demonstrated to disrupt Src kinase activity as well (Lee et al., 2010b). Src is known to activate MAPK in the pro-growth context, which turns on the COX-2 protein, the presence of which is a warning marker for skin tumors (Athar et al., 2001). UVB radiation appears to be one of the major contributors to Src kinase activity. Kaempferol, on the other hand, shows great potential as a competitive inhibitor of Src, which requires the linking of a molecule of ATP in order to function. Kaempferol easily bonds to Src at its ATP site, disturbing its skin cancer promoting activity. The MAPK/ERK pathway is altered at several key locations by kaempferol (Fig. 2), and is only one of many cellular processes transformed.
Figure 2.
Kaempferol’s Effects on the MAPK Pathway.
Besides the MAPK channel, the PI3K/AKT pathway represents another signaling route implicated in cancer development. Over-activation of PI3K leads to an accumulation of AKT, which is sensitive to levels of epidermal growth factor (EGF) (Nomura, He, Koyama, Ma, Miyamoto, & Dong, 2003). AKT regulates a number of transcription factors. Heightened activation of AKT results in an increase in AP-1 and NF-κB activity, conducive to the genesis of tumors (Li, Westergaard, Ghosh, & Colburn, 1997). Activation of PI3K is counterproductive to apoptosis, which is why some cancer drugs focus on inhibiting this pathway.
Much in the same manner as it blocks Src function, kaempferol has been demonstrated to compete with ATP in binding to PI3K in mouse epidermal JB6 P+ cells (Lee et al., 2010a). By neutralizing PI3K, kaempferol inhibits subsequent downstream activity of AKT and its transcription factor targets. In the presence of kaempferol, AKT can no longer phosphorylate the apoptotic protein BAD, thereby dropping Bcl-2 levels in concert (Nguyen et al., 2003, Luo et al., 2011). With these suppressors subdued, apoptosis can be initiated at a much stronger pace. It should be noted that these cytotoxic effects seem to target cancer cells specifically. In fact, kaempferol appears to serve a protective role with respect to normal body cells. Kaempferol has been shown to neutralize the toxic properties of 7beta-hydroxycholesterol in rat smooth muscle cells, actually staving off apoptosis in these healthy cells (Ruiz, Padilla, Redondo, Gordillo-Moscoso, & Tejerina, 2006). The ability to discriminate between normal and malignant cells is a highly desirable property, a huge leap forward in the context of today’s chemotherapy drugs. In conclusion, through its effects on both MAPK and PI3K, kaempferol shows remarkable promise in manipulating cell signaling in the induction of apoptosis, while simultaneously leaving healthy cells alone.
(b) Cell Cycle
In order to propagate, cancer cells must, of course, pass through the cell cycle, which is tightly regulated by a host of proteins. Mitosis-promoting factor (MPF) is the chief agent responsible for the transition from the G2 phase to the M phase. Composed of a cyclin subunit and a kinase subunit, activated MPF serves a critical role in the passing of the G2 checkpoint. The kinase subunit is termed a cyclin-dependent kinase (CDK). To initiate mitosis, the CDK must bind to the cyclin regulatory protein. The concentration of the MPF cyclin steadily climbs throughout interphase and peaks during mitosis, where it drops suddenly. In this way, active MPF is only present to induce mitosis, after which it is quickly decomposed. Therefore, an effective cancer therapy may target MPF in an attempt to arrest the cell cycle and cellular growth.
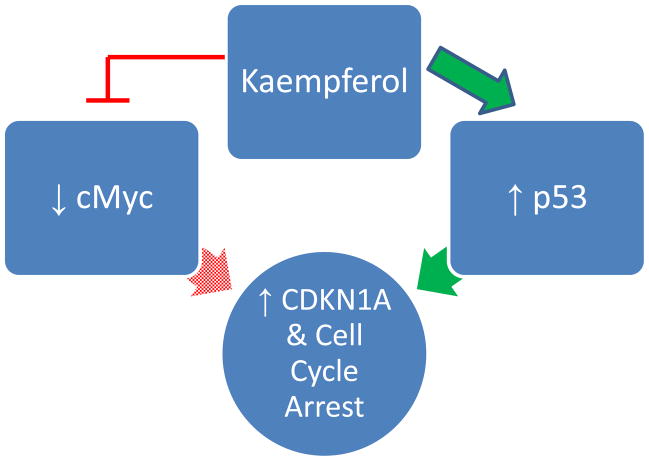
While the concentration of cyclin typically fluctuates over time, the concentration of CDK remains constant. However, the introduction of kaempferol has been shown to reduce CDK1 levels in human breast cancer MDA-MB-453 cells (Choi and Ahn, 2008). Consequently, kaempferol was able to inhibit proliferation by disrupting the cell cycle at the G2 checkpoint. Moreover, in cancer cells, the oncogene cMyc is generally overexpressed, leading to unrestrained proliferation (Jung, Menssen, Mayr, & Hermeking, 2008). Evidence supposes that increased levels of cMyc antagonize CDKN1A mRNA concentrations (Luo, Daddysman, Rankin, Jiang, & Chen, 2010). CDKN1A is a cyclin-dependent kinase inhibitor, which obstructs the cell cycle by binding to CDK complexes. Kaempferol, when administered with the chemotherapy drug cisplatin, appears to lower cMyc mRNA levels and heighten those of CDKN1A mRNA in ovarian cancer cells. Cisplatin itself fails to destroy cancer cells but, with addition of kaempferol, successfully induces apoptosis through hampering the expression of cMyc. Interestingly, inhibition of cMyc may not be the only mechanism through which kaempferol enhances CDKN1A levels. The presence of kaempferol seems to cause a surge in p53 levels of breast cancer MDA-MB-453 cells (Choi and Ahn, 2008). p53 is a well-known tumor suppressor protein commonly referred to as the guardian of the genome. When cellular DNA becomes damaged, repairs and subsequent growth arrest are usually mediated by this protein. Sensing injured genetic information, p53 works by activating p21, also known as CDKN1A. Following this stimulation, CDKN1A is free to paralyze CDK complexes, thereby halting the cell cycle. Kaempferol thus appears to modify a number of upstream regulators of CDKN1A (Fig. 3). Moreover, kaempferol resulted in the phosphorylation of p53 at serine 15, a phenomenon associated with apoptosis in cancer cells (Ito et al., 2004).
Figure 3.
Kaempferol’s Effects on the Cell Cycle.
In another study, in combination with the flavonoid quercetin, kaempferol treatment resulted in a reduction of total protein levels, most notably in Ki67 (Ackland, Van De Waarsenburg, & Jones, 2005). Ki67 is a protein generally associated with cellular growth and is normally absent from resting cells (Scholzen and Gerdes, 2000). Exposure to kaempferol led to cell cycle arrest and a marked decrease in proliferation. This is easily only the tip of the iceberg in the number of proteins kaempferol affects. Kaempferol is a versatile molecule with serious potential in upsetting cancer growth and warrants further inquiry into its effects on the cell cycle. A multipurpose chemoprophylactic agent, kaempferol seems to play a part in every aspect of cancer growth. Undoubtedly, there remain a host of kaempferol-sensitive genes waiting to be investigated.
(c) Energetic Impairment
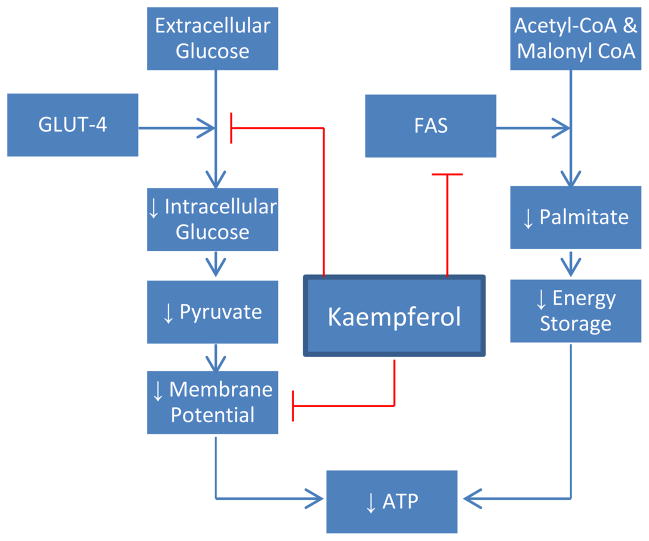
Perhaps the most direct method of promoting cell death is through nutrient starvation. Once unable to support the most vital cellular functions, cells typically activate the apoptosis machinery. In HeLa cells, kaempferol has been demonstrated to block cellular intake of glucose (Filomeni et al., 2010). Even in the presence of insulin, glucose transporter 4 is unable to maintain glucose influx while bound to kaempferol. To further compound the energy problem, kaempferol appears to interfere with oxidative phosphorylation through inhibiting ATP synthase and the respiratory chain activities (Li, Vik, & Tu, 2012). An independent study confirms that kaempferol treatment lowers mitochondrial membrane potential, which destroys the proton gradient ATP synthase requires (Huang et al., 2010). Introduction of pyruvate still leaves the HeLa cells energetically deficient, suggesting that the mitochondrial electron chain is hampered as well (Filomeni et al., 2010). Treatment with kaempferol seems to not only hinder glycolysis but also aerobic respiration. Finally, lipogenesis represents yet another vital cellular process disrupted by kaempferol treatment. The production of fats is necessary to the growth of any cell, which utilizes fatty acids not only for energy storage but also for membrane structure. Fatty acid synthase (FAS) is one the key enzymes in lipogenesis, where it converts acetyl-CoA and malonyl-CoA into palmitate. Kaempferol has been found to directly target FAS and palmitate synthesis (Brusselmans, Vrolix, Verhoeven, & Swinnen, 2005). In prostate cancer cells, where expression of FAS is excruciatingly high, administration of kaempferol was strongly associated with growth arrest and apoptosis. To confirm the FAS specificity, addition of palmitate curbed the cytotoxic action of kaempferol. Interestingly, lipogenesis of normal cells appears to be unaffected. Kaempferol was noted to only attack malignant cells, where levels of FAS were heightened. In summation, kaempferol’s cancer fighting effects can be observed in nutrient delivery, processing, and storage, a truly comprehensive crusade to starve out cancer cells (Fig. 4).
Figure 4.
Metabolic Effects of Kaempferol.
Unfortunately, cancer has a reputation as one of the more resilient diseases. Tumor cells adapt well to nutrient poor and hypoxic conditions. Perhaps most strikingly, HeLa cells are known to commence autophagy when energetically stressed (Filomeni et al., 2010). Mediated by AMP-activated protein kinase (AMPK), autophagy represents a survival mechanism wherein unnecessary cellular processes are shut down and cells begin degrading their disposable organelles for energy. Activation of autophagy significantly reduces the extent of apoptosis and allows for continued cancer proliferation. Early in vitro research has seen promise in combining kaempferol treatment with autophagy and AMPK inhibitors, but deeper investigation must be conducted before substantial results can be obtained.
4. Effect on angiogenesis
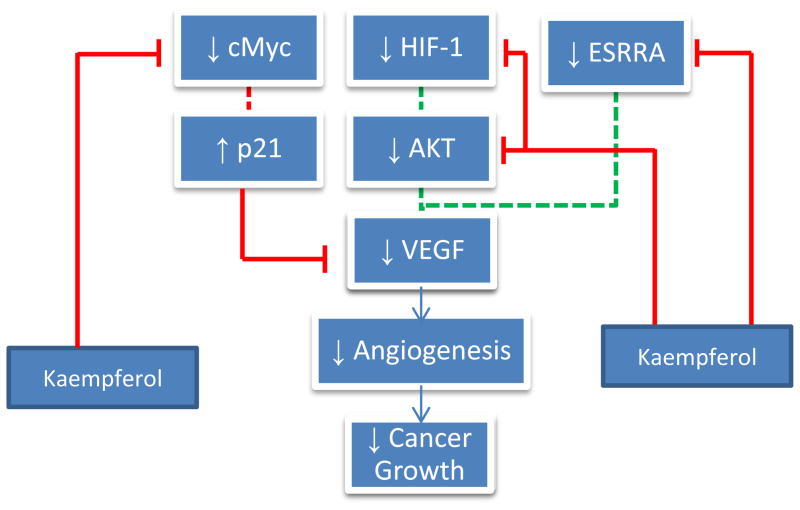
Like every other part of the body, cancer cells require a supply of oxygen and nutrients, furnished by a network of circulating blood vessels. The formation of new blood vessels designed to meet the growing needs of the tumor is termed angiogenesis, of which vascular endothelial growth factor (VEGF) is the primary mediator (Ferrara, 2004). Recent research efforts have shown the efficacy of kaempferol in impairing cancer angiogenesis both in vitro and in vivo through inhibiting VEGF secretion in human cancer cell lines (Luo, Rankin, Liu, Daddysman, Jiang, & Chen, 2009; Luo, Rankin, Juliano, Jiang, & Chen, 2012a). In MDA human breast cancer cells, kaempferol inhibited VEGF release (Schindler and Mentlein, 2006), and in ovarian cancer cells, VEGF mRNA concentrations were reduced. VEGF protein levels were most markedly affected, suggesting a mechanism of action centered on translation (Luo et al., 2009). Kaempferol appears to inhibit VEGF expression and angiogenesis through an ERK-NFκB-cMyc-p21 pathway (Luo et al., 2012a). Kaempferol administration has been shown to discourage ERK phosphorylation as well as NFκB and cMyc expression, the reduction of which, as stated earlier, promotes p21 (CDKN1A) expression. p21 is a tumor suppressor protein known to antagonize VEGF secretion (Luo et al., 2012a). Furthermore, kaempferol seems to exert effects on VEGF regulators, too. Restrictive oxygen conditions activate hypoxia-inducible factor 1 (HIF-1), which through the PI3K/AKT pathway stimulates VEGF expression. Not only does kaempferol lower HIF levels, but also it blocks AKT phosphorylation, impeding the signaling machinery that calls for increased VEGF production (Luo et al., 2009). Similarly, kaempferol blocks ESRRA activity by lowering its mRNA levels. A separate regulator of VEGF, ESRRA is associated with estrogen activity and is regarded as a promoter of cancer. Ostensibly, kaempferol is a thorough VEGF antagonist, attacking its synthesis from all directions (Fig. 5). If the current evidence can be corroborated, then certainly the antiangiogenesis character of kaempferol is less susceptible to a lone mutation in cancer cells. With such a broad range of activity, cells will need to institute sweeping changes to neutralize kaempferol’s anticancer properties.
Figure 5.
Effects of Kaempferol on Angiogenesis. Dashed lines represent previous processes that have been reduced by kaempferol.
5. Effect on metastasis
One of the most lethal aspects of cancer is its ability to disseminate to other parts of the body. As opposed to localized tumors, metastasized cancer presents a far more difficult problem to treat. Surgery can remove a primary tumor, but these secondary growths are prone to resurface once eliminated. In order to spread, malignant cells must first degrade their surrounding extracellular matrix (ECM), making their way through to the body’s vascular system for a free ride. To break down the neighboring ECM, cancer cells typically employ a host of enzymes, which include the matrix metalloproteinases (MMPs). High levels of MMP-3 especially are associated with increased levels of tumor invasion and poor prognoses (Coussens and Werb, 1996). MMP-3 is known to destroy vital ECM proteins such as fibronectin and collagen (Wu, Lark, Chun, & Eyre, 1991). Additionally, signal transduction of extracellular hepatocyte growth factor (HGF) has been implicated in the dissemination of medulloblastoma, a highly metastatic cancer of the brain (Li et al., 2008). Evidence suggests that HGF signaling results in the creation of actin-rich membrane ruffles, which are integral for cell migration (Labbé, Provencxal, Lamy, Boivin, Gingras, & Béliveau, 2009).
In the highly invasive breast cancer cell line MDA-MB-231, kaempferol has shown great promise in disrupting cancer metastasis. While secretion of MMP-3 was largely unaffected by flavonoid treatment, kaempferol did significantly inhibit MMP-3 protein activity in a dose-dependent manner (Phromnoi, Yodkeeree, Anuchapreeda, & Limtrakul, 2009). Most prominently, the presence of kaempferol blocked the in vitro migration of MDA-MB-231 cells, which proposes the use of kaempferol in managing tumor invasion. Furthermore, HGF/Met signaling in medulloblastoma line DAOY was upset by the introduction of kaempferol (Labbé et al., 2009). Not only did kaempferol block HGF-dependent phosphorylation of Met, a special receptor tyrosine kinase sensitive to HGF, but also it reduced downstream HGF-mediated activation of AKT, a finding already covered in the discussion of the PI3K/AKT pathway. Met activation has been implicated in cancer growth and angiogenesis, and in vitro administration of kaempferol has yielded positive results in inhibiting tumor migration. While deeper research in vivo is required, kaempferol has already proven to meaningfully lessen the extent of metastasis not only through the novel mechanisms explored here, but also through the antiangiogenesis previously investigated. Stunting the formation of new blood vessels blocks nutrient transport as well as a means for cancer cells to move around.
6. Effect on inflammation
The inflammatory response is a commonplace bodily process meant to help facilitate healing. The signs of inflammation are evoked by vasodilation and increased blood vessel permeability, which manifest as redness and swelling. The heightened blood flow allows extra blood cells to reach the site of injury to begin eliminating intruders, while simultaneously regenerating the original tissue. Inflammation is a highly regulated process built on the secretion of a plethora of chemical messengers. Leukocytes at the site of injury secrete numerous cytokines which help recruit more white blood cells and promote the inflammatory response. Among these messengers are various interleukins and tumor necrosis factor. When the proteins reach another leukocyte, they can activate transcription factors, initiating the synthesis of additional proteins which continue to propagate the inflammatory signal or prompt the differentiation of certain white blood cells. If inflammation is not tightly controlled, the body can easily end up damaging itself such as in rheumatoid arthritis or Crohn’s disease, where the immune system attacks normally functioning cells. Even worse, chronic inflammation is strongly linked to a predisposition to developing cancer (Rakoff-Nahoum, 2006). Peptic ulcers are associated with an increased risk for stomach cancer, and mesothelioma can be traced back to irritation brought on by asbestos exposure. A diet high in flavonols, especially kaempferol, has been found to correlate with reduced serum interleukin-6 levels, an inflammatory cytokine (Bobe et al., 2010). In the same study, a decrease in IL-6 levels was found to lower risk for advanced adenoma recurrence. This cancer correlation can be understood by examining the effects of inflammation. When faced with an infection, the body initiates the inflammatory response and creates free radicals, thought to act as antimicrobials (Hussain, Hofseth, & Harris, 2003). With their unpaired electrons, radicals are incredibly unstable compounds, prone to damage DNA and accelerate the process of human aging. An accumulation of radicals can help promote the transformation of healthy cells into cancerous ones. In addition, the body must replace cells destroyed by inflammatory stimuli. Consequently, signals are broadcast to surviving cells, encouraging them to initiate mitosis and commence tissue repair (Chen, Egan, Li, Greten, Kagnoff, & Karin, 2003). If any of the remaining cells are malignant, inflammation will create the ideal environment for a growing tumor. Studies have documented the efficacy of anti-inflammatory drugs such as corticosteroids in the prevention of tumors. There exists strong evidence that short circuiting the inflammatory response can be part of a viable cancer therapy (Ulrich, Bigler, & Potter, 2006).
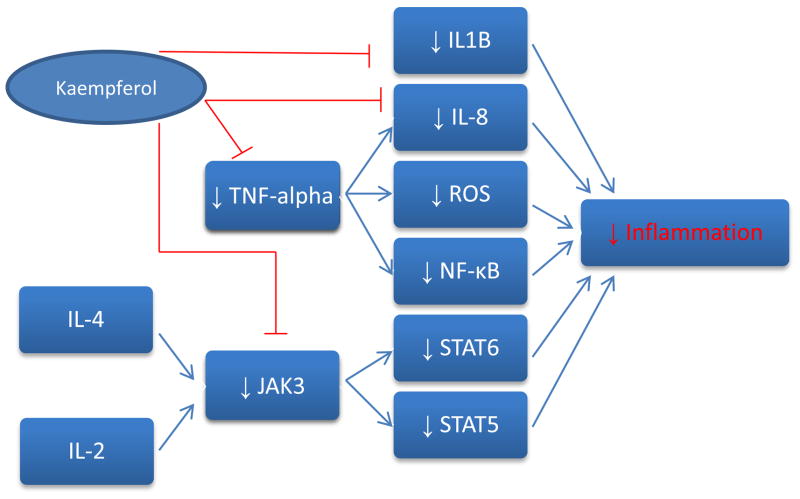
In vitro studies have shown kaempferol to substantially interfere in a number of inflammation mechanisms. In J744.2 macrophages, treatment with kaempferol blocks expression of both interleukin-1 beta and tumor necrosis factor (Kowalski, Samojedny, Paul, Pietsz, & Wilczok, 2005). These two cytokines promote the activation of certain enzymes and genes crucial to the inflammatory response. Kaempferol lowers the mRNA levels of both cytokines, inhibiting their transcription and their subsequent effects. TNF-alpha and IL1B are both linked to the development of a number of autoimmune disorders, including multiple sclerosis. Furthermore, kaempferol has been demonstrated to be a disruptor of TNF-mediated operations (Lee et al., 2009). TNF promotes activation of IL-8, another pro-inflammatory cytokine. In HEK 293 cells, kaempferol blocked not only TNF-induced IL-8 promoter activation, but also IL-8 gene expression. IL-8 has been found to be a potent enhancer of angiogenesis as well, once again displaying the wide variety of proteins kaempferol affects (Qazi, Tang, & Qazi, 2011). Also among the functions of TNF is activation of NF-κB, a transcription factor which prompts synthesis of inflammatory proteins. Administration of kaempferol blocked migration of NF-κB into the nucleus, thereby preventing induction of its gene targets (Lee et al., 2009). Finally, TNF stimulation appears to be associated with an increase in the number of reactive oxygen species (ROS). These molecules are of course unstable and are prone to damage DNA, possibly promoting transformation into malignant cells. Kaempferol treatment dramatically lowered production of ROS in normal HEK 293 cells (Lee et al., 2009). Ostensibly, kaempferol significantly handicaps TNF assembly in addition to its broad spectrum of inflammatory effects.
IL-4 represents another inflammatory-signaling cytokine, prominent for its effects on T cell differentiation. However, similar to all interleukins previously mentioned, IL-4 has been implicated in autoimmune disease and deregulation of inflammation (Finnegan et al., 2002). IL-4 manifests its effects through phosphorylating JAK3, a tyrosine kinase found in the cytoplasm. JAK3 successively phosphorylates STAT6, a transcription factor responsible for the synthesis of proteins tasked with actualizing the inflammatory response (Nelms, Keegan, Zamorano, Ryan, & Paul, 1999). Unsurprisingly, kaempferol was found to inhibit this signaling pathway, specifically by targeting JAK3 activity (Cortes, Perez-G, Rivas, & Zamorano, 2007). Upon introduction of kaempferol, JAK3 was unable to phosphorylate STAT6, effectively disrupting the secretion of STAT6 associated inflammatory proteins. Since JAK3 is upstream of a number of transcription factors, kaempferol has promise in upsetting many JAK3 controlled processes. Since the inflammatory cytokine IL-2 also employs JAK3 to activate STAT5, another transcription factor, kaempferol is a potent inhibitor of IL-2 mediated outcomes as well (Fig. 6).
Figure 6.
Anti-inflammatory Effects of Kaempferol.
Finally, kaempferol seems to hold therapeutic effects through the endocannabinoid system. The nervous system houses a host of cannabinoid receptors sensitive to specific neurotransmitters. Activation of the endogenous cannabinoid system regulates several physiological process including appetite, pain, and inflammation. Generally, induction of cannabinoid receptors seems to impair inflammation and nociception. However, the neurotransmitters responsible for their activation are usually short-lived and quickly captured by the neuron that released them. Fatty acid amide hydrolase (FAAH) functions in the breakdown of anandamide, an endocannabinoid capable in disrupting the inflammatory response (Deutsch and Chin, 1993). Kaempferol has been documented to inhibit FAAH activity, allowing anandamide to continue to exert its therapeutic effects (Thors, Belghiti, & Fowler, 2008). However, the concentrations of kaempferol needed to block FAAH are not achievable through dietary consumption, due to kaempferol’s poor bioavailability. It is questionable as to whether FAAH inhibition is important in relaying kaempferol’s in vivo effects, which may be established through other mechanisms. Deeper research is required before a judgment can be made concerning the role of kaempferol in the endocannabinoid system.
7. Bioavailability and epidemiology of anticarcinogenic effects
Up to this point, an incredible amount of research has been conducted detailing the in vitro effects of dietary flavonoids including kaempferol. The question still remains, though, as to whether kaempferol is effective in helping real patients suffering from cancer. Low intake of vegetables has been consistently associated with an increased risk of cancer (Banks, 2000). Encouragingly, a large number of population studies have confirmed that a diet high in flavonoids, namely kaempferol, reduces cancer risk in smokers (Bobe et al., 2008; Cui et al., 2008; Nöthlings et al., 2008). This can be partially explained by kaempferol’s interruption of the aryl hydrocarbon receptor (AHR) pathway. AHR is commonly activated by human carcinogens, such as those found in cigarette smoke (Denison and Nagy, 2003). These agonists form complexes with AHR, which translocate to the nucleus and induce expression of carcinogenic genes. Kaempferol works to block binding of AHR to the carcinogen, thereby inhibiting cell transformation brought on by cigarette usage (Puppala, Gairola, & Swanson, 2007). However, results from studies focusing on the nonsmoking population have been more ambivalent. Some prospective studies revealed that over decades, consumption of kaempferol dramatically reduced the risk of cancer in American female nurses (Gates, Tworoger, Hecht, Vivo, Rosner, & Hankinson, 2007). This finding suggests that kaempferol, as a nontoxic, inexpensive dietary component, is a promising agent for the chemoprevention of ovarian cancers, easily adopted into the lifestyles of most women. On the other hand, some have found extremely limited support for chemoprevention through a diet heavy in flavonoids (Gates et al., 2009), while others maintain no association exists between cancer risk and flavonoid content (Wang, Lee, Zhang, Blumberg, Buring, & Sesso, 2009). In light of the large catalogue of research documenting the impressive in vitro potential of kaempferol, the best of way of understanding these underwhelming findings is through the bioavailability of kaempferol and flavonoids in general. Like all substances consumed orally, flavonoids are subject to first-pass metabolism through the liver and intestinal wall (Barve, Chen, Hebbar, Desiderio, Saw, & Kong, 2009). Conjugation reactions typically involve modifying the original compound to a less chemically active one. Before kaempferol can even reach the main circulation, most of it has already been transformed into a handicapped metabolite. Furthermore, since flavonoids are recognized by the body as foreign substances, cells are equipped with a number of pumps designed to escort these alien chemicals outside of cells and membranes (Schinkel and Jonker, 2003). Kaempferol is poorly absorbed into the bloodstream and cannot force its way inside cells, where it can manipulate signaling pathways or inhibit certain protein functions. Because of this efflux, kaempferol’s anticancer effects may not be experienced by the body.
Recent advances, however, have imparted hope to overcoming these obstacles in bioavailability. Breast cancer resistance protein (Abcg2) is a transport protein tasked with removing a host of toxic compounds from the cell, including quercetin, another flavonol with promise in treating cancer. Apparently, kaempferol has a higher affinity for Abcg2 than quercetin does. Administration of both flavonols simultaneously revealed that kaempferol blocked the efflux of quercetin, allowing quercetin to remain inside and exert its effects (Guohua, Gallegos, & Morris, 2011). Therefore, kaempferol could possibly be combined with another substance with an even higher affinity for Abcg2, which would leave kaempferol inside to wreak havoc on the malignant cells. In addition, kaempferol has been found to lower mRNA levels of ABCC6, another ATP-binding cassette transporter (Luo et al., 2010). ABCC6 is involved in the transport of cisplatin, a chemotherapy drug, outside of the cell (Zhou et al., 2008). Introduction of kaempferol was found to significantly enhance cisplatin’s cytotoxic effects on cancer cells (Luo et al., 2010). Ostensibly, even if kaempferol itself experiences poor bioavailability, at least it seems to improve the bioavailability of other substances meant to fight cancer. These transport proteins are a good potential research focus for increasing the body’s access to kaempferol and other drugs.
8. Nanotechnology
Perhaps the most promising and innovative technique to improving bioavailability, though, is through nanotechnology. The coating of certain chemicals with a layer of nanoparticles increases the permeability and amount of that substance to reach systemic circulation. The capsule of nanoparticles can help shield kaempferol from efflux transporters and coax cells to transport the nanoparticle complex inwards, in addition to preserving its structural integrity. Research done on EGCG, another flavonoid with potential in cancer treatment, has already demonstrated promise in nanochemoprevention. EGCG was found to be easily coated with an external matrix of chitosan and caseinophosphopeptides, which significantly improves the in vitro transport of EGCG over cell monolayers (Hu, Ting, Yang, Tang, Zeng, & Huang, 2012). The nanoparticle coating seems to have low cytotoxicity to normal cells as well. Nano research has also been conducted focusing on enhancing the bioavailability of kaempferol specifically. Encapsulation with poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) (PEO-PPO-PEO) nanoparticles appears to significantly reduce cancer cell viability, as does coating with poly(DL-lactic acid-co-glycolic acid) (PLGA) nanoparticles (Luo, Jiang, Li, Li, Jiang, & Chen, 2012b). Both encapsulations are more potent than kaempferol treatment alone. The PEO-PPO-PEO formulation shows a higher effectiveness in lowering cancer viability, but the PLGA covered kaempferol preferentially kills malignant cells. Future research could possibly focus on utilizing a special targeting mechanism sensitive to folate, which cancerous cells tend to overproduce (Sunoqrot et al., 2012). Folate-targeted kaempferol complexes could be incorporated into potential nanoparticles to achieve high targeting efficacy against folate-overexpressing cancerous cells while limiting potential effects on normal cells.
A final reason for the meager bioavailability of kaempferol is its poor dissolution in a number of solvents. In order to be absorbed, substances must first be broken into particles in solution. A covering of nanoparticles allows for smaller, more soluble particles with a greater affinity for the surrounding excipient molecules (Tzeng et al., 2011). This formulation appears to augment the clinical properties of kaempferol, notably its antioxidant capacity. Nanochemoprevention represents an exciting field with many new avenues to explore, which means many unanswered questions. Work done in vivo is scarce, and it still remains to be seen whether nanoparticles can really help augment kaempferol’s anticancer effects in live cancer patients. Further investigation is a must before we can make sense of kaempferol’s true worth.
9. Conclusions
Upon examination of its remarkable catalogue of cancer fighting properties, it is plain to see that kaempferol is brimming with potential. In the in vitro setting, this flavonoid boasts a wide spectrum of cancer targeting effects in apoptosis, angiogenesis, metastasis, and inflammation. Most significantly, kaempferol is not a compound which concentrates its efforts in one area. If cancerous cells adapt to VEGF inhibition, they remain vulnerable to the other destructive effects of kaempferol. Also, kaempferol’s value in its ability to distinguish between healthy and malignant cells cannot be overstated. Modern chemotherapy treatments pose serious health risks, a problem kaempferol seems to have resolved. Though its importance as a cancer treatment remains questionable, it does appear to be a low risk option. Finally, although poor bioavailability epitomizes a major obstacle, nanotechnology has emerged as a promising means to overcoming this problem, revitalizing hope in employing kaempferol as a chemo-preventative agent.
Cancer ranks among the most imperative medical issues afflicting the human population, and chemoprevention strategies represent a promising approach in reducing incidence and mortality. Kaempferol, as a natural compound, may elicit great variability in its therapeutic results. Although a large amount of studies in vitro have been conducted, few clinical trials using precise concentrations of these compounds have been performed. More experiments and clinical studies focused on flavonoids need to be executed to clarify the worth of these molecules in cancer treatment. Though a wealth of information has been compiled, future inquiries must investigate the use of kaempferol as a treatment option for live cancer patients.
Highlights of the review.
Kaempferol reduces the risk of chronic diseases, especially cancer.
Kaempferol augments human body’s antioxidant defense against free radicals.
Kaempferol modulates apoptosis, angiogenesis, inflammation, and metastasis.
Nanotechnology can improve the bioavailability of kaempferol.
Acknowledgments
This research was supported by a West Virginia Experimental Program to Stimulate Competitive Research grant and an NIH grant (5P20RR016477 and 8P20GM104434) from the National Center for Research Resources awarded to the West Virginia IDeA Network of Biomedical Research Excellence.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackland ML, Van De Waarsenburg S, Jones R. Synergistic Antiproliferative Action of the Flavonols Quercetin and Kaempferol in Cultured Human Cancer Cell Lines. In Vivo. 2005;19:69–76. [PubMed] [Google Scholar]
- Athar M, An KP, Morel KD, Kim AL, Aszterbaum M, Longley J, Epstein EH, Jr, Bickers DR. Ultraviolet B(UVB)-induced cox-2 expression in murine skin: an immunohistochemical study. Biochemical and biophysical research communications. 2001;280:1042–7. doi: 10.1006/bbrc.2000.4201. [DOI] [PubMed] [Google Scholar]
- Banks E. The epidemiology of ovarian cancer. In: Bartlett JMS, editor. Ovarian cancer methods and protocols. Totowa: Humana Press; 2000. pp. 3–11. [Google Scholar]
- Barve A, Chen C, Hebbar V, Desiderio J, Saw CL, Kong AN. Metabolism, oral bioavailability and pharmacokinetics of chemopreventive kaempferol in rats. Biopharm Drug Dispos. 2009;30:7, 356–365. doi: 10.1002/bdd.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobe G, Albert PS, Sansbury LB, Lanza E, Schatzkin A, Colburn NH, Cross AJ. Interleukin-6 as a Potential Indicator for Prevention of High Risk Adenoma Recurrence by Dietary Flavonols in the Polyp Prevention Trial. Cancer Prev Res (Phila) 2010;3(6):764–775. doi: 10.1158/1940-6207.CAPR-09-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobe G, Weinstein SJ, Albanes D, Hirvonen T, Ashby J, Taylor PR, Virtamo J, Stolzenberg-Solomon RZ. Flavonoid Intake and Risk of Pancreatic Cancer in Male Smokers (Finland) Cancer Epidemiol Biomarkers Prev. 2008;17(3):553–62. doi: 10.1158/1055-9965.EPI-07-2523. [DOI] [PubMed] [Google Scholar]
- Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–62. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- Brunner G. Supercritical fluids: technology and application to food processing. J Food Eng. 2005;67:21–33. [Google Scholar]
- Brusselmans K, Vrolix R, Verhoeven G, Swinnen JV. Induction of Cancer Cell Apoptosis by Flavonoids Is Associated with Their Ability to Inhibit Fatty Acid Synthase Activity. The Journal of Biological Chemistry. 2005;280:7, 5636–45. doi: 10.1074/jbc.M408177200. [DOI] [PubMed] [Google Scholar]
- Chen LW, Egan L, Li ZW, Greten FR, Kagnoff MF, Karin M. The two faces of IKK and NF-kappaB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med. 2003;9(5):575–81. doi: 10.1038/nm849. [DOI] [PubMed] [Google Scholar]
- Cho YY, Yao K, Pugliese A, Malakhova ML, Bode AM, Dong Z. A Regulatory Mechanism for RSK2 NH2-Terminal Kinase Activity. Cancer Res. 2009;69(10):4398–4406. doi: 10.1158/0008-5472.CAN-08-4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EJ, Ahn WS. Kaempferol induced the apoptosis via cell cycle arrest in human breast cancer MDA-MB-453 cells. Nutrition Research and Practice. 2008;2(4):322–325. doi: 10.4162/nrp.2008.2.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes JR, Perez-G M, Rivas MD, Zamorano J. Kaempferol Inhibits IL-4-Induced STAT6 Activation by Specifically Targeting JAK3. The Journal of Immunology. 2007;179:3881–3887. doi: 10.4049/jimmunol.179.6.3881. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Matrix metalloproteinases and the development of cancer. Chem Biol. 1996;3:11, 895–904. doi: 10.1016/s1074-5521(96)90178-7. [DOI] [PubMed] [Google Scholar]
- Cui Y, Morgenstern H, Greenland S, Tashkin DP, Mao JT, Cai L, Cozen W, Mack TM, Lu QY, Zhang ZF. Dietary Flavonoid Intake and Lung Cancer— A Population-based Case-control Study. Cancer. 2008;112:2241–8. doi: 10.1002/cncr.23398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- Deutsch DG, Chin SA. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol. 1993;46:5, 791–796. doi: 10.1016/0006-2952(93)90486-g. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor as a target for anticancer therapy. Oncologist. 2004;9:1, 2–10. doi: 10.1634/theoncologist.9-suppl_1-2. [DOI] [PubMed] [Google Scholar]
- Filomeni G, Desideri E, Cardaci S, Graziani I, Piccirillo S, Rotilio G, Ciriolo MR. Carcinoma cells activate AMP-activated protein kinase-dependent autophagy as survival response to kaempferol-mediated energetic impairment. Autophagy. 2010;6:2, 202–216. doi: 10.4161/auto.6.2.10971. [DOI] [PubMed] [Google Scholar]
- Finnegan A, Grusby MJ, Kaplan CD, O’Neill SK, Eibel H, Koreny T, Czipri M, Mikecz K, Zhang J. IL-4 and IL-12 regulate proteoglycan-induced arthritis through Stat-dependent mechanisms. J Immunol. 2002;169:6, 3345–3352. doi: 10.4049/jimmunol.169.6.3345. [DOI] [PubMed] [Google Scholar]
- Frodin M, Gammeltoft S. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol Cell Endocrinol. 1999;151:65–77. doi: 10.1016/s0303-7207(99)00061-1. [DOI] [PubMed] [Google Scholar]
- Gates MA, Tworoger SS, Hecht JL, Vivo ID, Rosner B, Hankinson SE. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. International Journal of Cancer. 2007;121(10):2225–2232. doi: 10.1002/ijc.22790. [DOI] [PubMed] [Google Scholar]
- Gates MA, Vitonis AF, Tworoger SS, Rosner B, Titus-Ernstoff L, Hankinson SE, Cramer DW. Flavonoid intake and ovarian cancer risk in a population-based case-control study. Int J Cancer. 2009;124(8):1918–25. doi: 10.1002/ijc.24151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guohua A, Gallegos J, Morris ME. The Bioflavonoid Kaempferol Is an Abcg2 Substrate and Inhibits Abcg2-Mediated Quercetin Efflux. Drug Metabolism and Disposition. 2011;39:3, 426–32. doi: 10.1124/dmd.110.035212. [DOI] [PubMed] [Google Scholar]
- Hong JT, Yen JH, Wang L, Lo YH, Chen ZT, Wu MJ. Regulation of heme oxygenase-1 expression and MAPK pathways in response to kaempferol and rhamnocitrin in PC12 cells. Toxicol Appl Pharm. 2009;237:59–68. doi: 10.1016/j.taap.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Hu B, Ting Y, Yang X, Tang W, Zeng X, Huang Q. Nanochemoprevention by encapsulation of (—)-epigallocatechin-3-gallate with bioactive peptides/chitosan nanoparticles for enhancement of its bioavailability. Chem Commun. 2012;48:2421–3. doi: 10.1039/c2cc17295j. [DOI] [PubMed] [Google Scholar]
- Huang W, Li Z, Niu H, Li D, Zhang J. Optimization of operating parameters for supercritical carbon dioxide extraction of lycopene by response surface methodology. J Food Eng. 2008;89:298–302. [Google Scholar]
- Huang WW, Chiu YJ, Fan MJ, Lu HF, Yeh HF, Li KH, Chen PY, Chung JG, Yang JS. Kaempferol induced apoptosis via endoplasmic reticulum stress and mitochondria-dependent pathway in human osteosarcoma U-2 OS cells. Molecular Nutrition and Food Research. 2010;54:1–11. doi: 10.1002/mnfr.201000005. [DOI] [PubMed] [Google Scholar]
- Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:4, 276–85. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- Ito K, Nakazato T, Yamato K, Miyakawa Y, Yamada T, Hozumi N, Segawa K, Ikeda Y, Kizaki M. Induction of apoptosis in leukemic cells by homovanillic acid derivative, capsaicin, through oxidative stress: implication of phosphorylation of p53 at Ser-15 residue by reactive oxygen species. Cancer Res. 2004;64(3):1071–8. doi: 10.1158/0008-5472.can-03-1670. [DOI] [PubMed] [Google Scholar]
- Jung P, Menssen A, Mayr D, Hermeking H. AP4 encodes a c-MYC-inducible repressor of p21. Proc Natl Acad Sci USA. 2008;105(39):15046–51. doi: 10.1073/pnas.0801773105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BW, Lee ER, Min HM, Jeong HS, Ahn JY, Kim JH, Choi HY, Choi H, Kim EY, Park SP, Cho SG. Sustained ERK activation is involved in the kaempferol-induced apoptosis of breast cancer cells and is more evident under 3-D culture condition. Cancer Biology & Therapy. 2008;7:7, 1080–1089. doi: 10.4161/cbt.7.7.6164. [DOI] [PubMed] [Google Scholar]
- Kowalski J, Samojedny A, Paul M, Pietsz G, Wilczok T. Effect of apigenin, kaempferol and resveratrol on the expression of interleukin-1β and tumor necrosis factor-α genes in J774.2 macrophages. Pharmacological Reports. 2005;57:390–4. [PubMed] [Google Scholar]
- Labbé D, Provencxal M, Lamy S, Boivin D, Gingras D, Béliveau R. The Flavonols Quercetin, Kaempferol, and Myricetin Inhibit Hepatocyte Growth Factor-Induced Medulloblastoma Cell Migration. J Nutr. 2009;139:646–652. doi: 10.3945/jn.108.102616. [DOI] [PubMed] [Google Scholar]
- Lang Q, Wai CM. Supercritical fluid extraction in herbal and natural product studies— a practical review. Talanta. 2001;53:771–782. doi: 10.1016/s0039-9140(00)00557-9. [DOI] [PubMed] [Google Scholar]
- Lee KM, Lee DE, Seo SK, Hwang MK, Heo YS, Lee KW, Lee HJ. Phosphatidylinositol 3-kinase, a novel target molecule for the inhibitory effects of kaempferol on neoplastic cell transformation. Carcinogenesis. 2010a;31(8):1338–1343. doi: 10.1093/carcin/bgq102. [DOI] [PubMed] [Google Scholar]
- Lee KM, Lee KW, Jung SK, Lee EJ, Heo YS, Bode AM, Lubet RA, Lee HJ, Dong Z. Kaempferol inhibits UVB-induced COX-2 expression by suppressing Src kinase activity. Biochem Pharmacol. 2010b;80(12):2042–2049. doi: 10.1016/j.bcp.2010.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kim YJ, Kwon S, Lee Y, Choi SY, Park J, Kwon HJ. Inhibitory effects of flavonoids on TNF-α-induced IL-8 gene expression in HEK 293 cells. BMB reports. 2009;42(5):265–270. doi: 10.5483/bmbrep.2009.42.5.265. [DOI] [PubMed] [Google Scholar]
- Lee YH, Charles AL, Kung HF, Ho CT, Huang TC. Extraction of nobiletin and tangeretin from Citrus depressa Hayata by supercritical carbon dioxide with ethanol as modifier. Ind Crop Prod. 2010c;31:59–64. [Google Scholar]
- Li B, Xu Y, Jin YX, Wu YY, Tu YY. Response surface optimization of supercritical fluid extraction of kaempferol glycosides from tea seed cake. Industrial Crops and Products. 2010;32:123–128. [Google Scholar]
- Li B, Vik SB, Tu YY. Theaflavins inhibit the ATP synthase and the respiratory chain without increasing superoxide production. Journal of Nutritional Biochemistry. 2012;23:953–960. doi: 10.1016/j.jnutbio.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Westergaard C, Ghosh P, Colburn NH. Inhibitors of both nuclear factor-kappaB and activator protein-1 activation block the neoplastic transformation response. Cancer Res. 1997;57(16):3569–76. [PubMed] [Google Scholar]
- Li Y, Guessous F, Johnson EB, Eberhart CG, Li XN, Shu Q, Fan S, Lal B, Laterra J, Schiff D, Abounader R. Functional and molecular interactions between the HGF/c-Met pathway and c-Myc in large-cell medulloblastoma. Lab Invest. 2008;88:2, 98–111. doi: 10.1038/labinvest.3700702. [DOI] [PubMed] [Google Scholar]
- Liu W, Fu YJ, Zu YG, Tong MH, Wu N, Liu XL, Zhang S. Supercritical carbon dioxide extraction of seed oil from Opuntia dillenii Haw. and its antioxidant activity. Food Chem. 2009a;114:334–339. [Google Scholar]
- Liu S, Yang F, Zhang C, Ji H, Hong P, Deng C. Optimization of process parameters for supercritical carbon dioxide extraction of Passiflora seed oil by response surface methodology. J Supercrit Fluid. 2009b;48:9–14. [Google Scholar]
- Luo H, Jiang BH, King SM, Chen YC. Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutr Cancer. 2008;60(6):800–9. doi: 10.1080/01635580802100851. [DOI] [PubMed] [Google Scholar]
- Luo H, Rankin GO, Liu L, Daddysman MK, Jiang BH, Chen YC. Kaempferol Inhibits Angiogenesis and VEGF Expression Through Both HIF Dependent and Independent Pathways in Human Ovarian Cancer Cells. Nutr Cancer. 2009;61(4):554–563. doi: 10.1080/01635580802666281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Daddysman MK, Rankin GO, Jiang BH, Chen YC. Kaempferol enhances cisplatin’s effect on ovarian cancer cells through promoting apoptosis caused by down regulation of cMyc. Cancer Cell International. 2010;10:16. doi: 10.1186/1475-2867-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Rankin GO, Li Z, Depriest L, Chen YC. Kaempferol induces apoptosis in ovarian cancer cells through activating p53 in the intrinsic pathway. Food Chem. 2011;128(2):513–519. doi: 10.1016/j.foodchem.2011.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Rankin GO, Juliano N, Jiang BH, Chen YC. Kaempferol inhibits VEGF expression and in vitro angiogenesis through a novel ERK-NFκB-cMyc-p21 pathway. Food Chem. 2012a;130(2):321–328. doi: 10.1016/j.foodchem.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Jiang B, Li B, Li Z, Jiang BH, Chen YC. Kaempferol nanoparticles achieve strong and selective inhibition of ovarian cancer cell viability. International Journal of Nanomedicine. 2012b;7:3951–9. doi: 10.2147/IJN.S33670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr R, Gamse T. Use of supercritical fluids for different processes including new developments a review. Chem Eng Process. 2000;39:19–28. [Google Scholar]
- Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–38. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- Nguyen TTT, Tran E, Ong CK, Lee SK, Do PT, Huynh TT, Nguyen TH, Lee JJ, Tan Y, Ong CS, Huynh H. Kaempferol-Induced Growth Inhibition and Apoptosis in A549 Lung Cancer Cells Is Mediated by Activation of MEK-MAPK. Journal of Cellular Physiology. 2003;197:110–121. doi: 10.1002/jcp.10340. [DOI] [PubMed] [Google Scholar]
- Nomura M, He Z, Koyama I, Ma WY, Miyamoto KI, Dong Z. Involvement of the Akt/mTOR pathway on EGF-induced cell transformation. Molecular Carcinogenesis. 2003;38(1):25–32. doi: 10.1002/mc.10140. [DOI] [PubMed] [Google Scholar]
- Nöthlings U, Murphy SP, Wilkens LR, Boeing H, Schulze MB, Bueno-de-Mesquita HB, Michaud DS, Roddam A, Rohrmann S, Tjønneland A, Clavel-Chapelon F, Trichopoulou A, Sieri S, Rodriguez L, Ye W, Jenab M, Kolonel LN. A food pattern that is predictive of flavonol intake and risk of pancreatic cancer. Am J Clin Nutr. 2008;88:1653–62. doi: 10.3945/ajcn.2008.26398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Rho HS, Kim DE, Chang IS. Enzymatic preparation of kaempferol from green tea seed and its antioxidant activity. J Agric Food Chem. 2006;54:2951–2956. doi: 10.1021/jf052900a. [DOI] [PubMed] [Google Scholar]
- Phromnoi K, Yodkeeree S, Anuchapreeda S, Limtrakul P. Inhibition of MMP-3 activity and invasion of the MDA-MB-231 human invasive breast carcinoma cell line by bioflavonoids. Acta Pharmacologica Sinica. 2009;30:1169–1176. doi: 10.1038/aps.2009.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puppala D, Gairola CG, Swanson HI. Identification of kaempferol as an inhibitor of cigarette smoke-induced activation of the aryl hydrocarbon receptor and cell transformation. Carcinogenesis. 2007;28:3, 639–47. doi: 10.1093/carcin/bgl169. [DOI] [PubMed] [Google Scholar]
- Qazi BS, Tang K, Qazi A. Recent advances in underlying pathologies provide insight into interleukin-8 expression-mediated inflammation and angiogenesis. Int J Inflam. 2011;2011:908468. doi: 10.4061/2011/908468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S. Why Cancer and Inflammation? Yale Journal of Biology and Medicine. 2006;79:123–130. [PMC free article] [PubMed] [Google Scholar]
- Ramos S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J Nutr Biochem. 2007;18(7):427–42. doi: 10.1016/j.jnutbio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Rostagno MA, Araújo JMA, Sandi D. Supercritical fluid extraction of isoflavones from soybean flour. Food Chem. 2002;78:111–117. [Google Scholar]
- Ruiz E, Padilla E, Redondo S, Gordillo-Moscoso A, Tejerina T. Kaempferol inhibits apoptosis in vascular smooth muscle induced by a component of oxidized LDL. European Journal of Pharmacology. 2006;529:79–83. doi: 10.1016/j.ejphar.2005.10.061. [DOI] [PubMed] [Google Scholar]
- Scalia S, Giuffreda L, Pallado P. Analytical and preparative supercritical fluid extraction of Chamomile flowers and its comparison with conventional methods. J Pharmaceut Biomed. 1999;21:549–558. doi: 10.1016/s0731-7085(99)00152-1. [DOI] [PubMed] [Google Scholar]
- Schindler R, Mentlein R. Flavonoids and Vitamin E Reduce the Release of the Angiogenic Peptide Vascular Endothelial Growth Factor from Human Tumor Cells. J Nutr. 2006;136:1477–1482. doi: 10.1093/jn/136.6.1477. [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 2003;55:1, 3–29. doi: 10.1016/s0169-409x(02)00169-2. [DOI] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182(3):311–22. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Seifried HE, Anderson DE, Fisher EI, Milner JA. A review of the interaction among dietary antioxidants and reactive oxygen species. J Nutr Biochem. 2007;18(9):567–79. doi: 10.1016/j.jnutbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Sekine T, Arai Y, Ikegami F, Fujii Y, Shindo S, Yanagisawa T, Ishida Y, Okonogi S, Murakoshi I. Isolation of camelliaside C from — tea seed cake and inhibitory effects of its derivatives on arachidonate 5-lipoxygenase. Chem Pharm Bull. 1993;41:1185–1187. doi: 10.1248/cpb.41.1185. [DOI] [PubMed] [Google Scholar]
- Sekine T, Arita J, Yamaguchi A, Saito K, Okonogi S, Morisaki N, Iwasaki S, Murakoshi I. Two flavonol glycosides from seeds of Camellia sinensis. Phytochemistry. 1991;30:991–995. doi: 10.1016/0031-9422(91)85293-9. [DOI] [PubMed] [Google Scholar]
- Sharma V, Joseph C, Ghosh S, Agarwal A, Mishra MK, Sen E. Kaempferol induces apoptosis in glioblastoma cells through oxidative stress. Molecular Cancer Therapy. 2007;6:2544–2553. doi: 10.1158/1535-7163.MCT-06-0788. [DOI] [PubMed] [Google Scholar]
- Somerset SM, Johannot L. Dietary flavonoid sources in Australian adults. Nutr Cancer. 2008;60:442–449. doi: 10.1080/01635580802143836. [DOI] [PubMed] [Google Scholar]
- Sunoqrot S, Bae JW, Pearson RM, Shyu K, Liu Y, Kim DH, Hong S. Temporal control over cellular targeting through hybridization of folate-targeted dendrimers and PEG-PLA nanoparticles. Biomacromolecules. 2012;13(4):1223–30. doi: 10.1021/bm300316n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thors L, Belghiti M, Fowler CJ. Inhibition of fatty acid amide hydrolase by kaempferol and related naturally occurring flavonoids. British Journal of Pharmacology. 2008;155:244–252. doi: 10.1038/bjp.2008.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng CW, Yen FL, Wu TH, Ko HH, Lee CW, Tzeng WS, Lin CC. Enhancement of dissolution and antioxidant activity of kaempferol using a nanoparticle engineering process. J Agric Food Chem. 2011;59(9):5073–80. doi: 10.1021/jf200354y. [DOI] [PubMed] [Google Scholar]
- Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer. 2006;6(2):130–40. doi: 10.1038/nrc1801. [DOI] [PubMed] [Google Scholar]
- Wang L, Lee IM, Zhang SM, Blumberg JB, Buring JE, Sesso HD. Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. Am J Clin Nutr. 2009;89:905–12. doi: 10.3945/ajcn.2008.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojdyo A, Oszmianski J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chemistry. 2007;105:940–9. [Google Scholar]
- Wu JJ, Lark MW, Chun LE, Eyre DR. Sites of stromelysin cleavage in collagen types II, IX, X, and XI of cartilage. J Biol Chem. 1991;266:9, 5625–8. [PubMed] [Google Scholar]
- Zhang Y, Chen AY, Li M, Chen C, Yao Q. Ginkgo Biloba Extract Kaempferol Inhibits Cell Proliferation and Induces Apoptosis in Pancreatic Cancer Cells. J Surg Res. 2008;148(1):17–23. doi: 10.1016/j.jss.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SF, Wang LL, Di YM, Xue CC, Duan W, Li CG, Li Y. Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr Med Chem. 2008;15:20, 1981–2039. doi: 10.2174/092986708785132870. [DOI] [PubMed] [Google Scholar]
- Zhu XY, Lin HM, Chen X, Xie J, Wang P. Mechanochemical-assisted extraction and antioxidant activities of kaempferol glycosides from Camellia oleifera Abel. meal. J Agric Food Chem. 2011;59(8):3986–93. doi: 10.1021/jf1042689. [DOI] [PubMed] [Google Scholar]