Abstract
Objective
To assess ictal adiponectin (ADP) levels before and after acute abortive treatment in women episodic migraineurs.
Methods
Peripheral blood specimens were collected from women episodic migraineurs before and after acute abortive treatment with sumatriptan/naproxen sodium (suma/nap) versus placebo. Univariate and multivariate models were utilized to examine the relationship between serum total-ADP (T-ADP), ADP oligomers (high molecular weight [HMW], middle molecular weight [MMW], and low molecular weight [LMW]-ADP) and ADP ratio levels and pain severity. Paired-t tests and random intercept longitudinal models were utilized to assess the mean changes in T-ADP, ADP oligomers and ratios over time in treatment responders and non-responders.
Results
Twenty participants (11 responders, 9 non-responders) have been studied to date. In all participants, increases in the HMW:LMW adiponectin ratio were associated with an increase in pain severity. For every 1 point increase in the HMW:LMW ratio, pain severity increased by 0.22 (CI: 0.07, 0.37; p=0.004). In contrast, for every 0.25 µg/mL increase in LMW-ADP, pain severity decreased by 0.20 (CI: -0.41, -0.002; p=0.047). In treatment responders, T-ADP levels were reduced at 30 min (12.52 ± 3.4; p=0.03), 60 min (12.32 ± 3.2; p=0.017) and 120 min (12.65 ± 3.2; p=0.016) after treatment as compared to onset (13.48 ± 3.8). Additionally, in responders, the HMW:LMW ratio level was greater at pain onset (3.70 ±1.9 µg/mL) as compared to non-responders (2.29 ± 0.71 µg/mL), p=0.050. Responders also showed a decrease in the HMW:LMW ratio at 60 min (2.37 ± 1.1; p=0.002) and 120 min (2.76 ± 1.4; p=0.02) after treatment as compared to onset (3.70 ± 1.9). These changes in responders remained significant after adjusting for covariates, including measured BMI (m-BMI). Although non-responders showed no significant changes in unadjusted T-ADP or ADP oligomer or ratio levels, the HMW:LMW ratio was increased in non-responders after adjustments, (p=0.025).
Conclusion
In this pilot study of women episodic migraineurs, the HMW:LMW adiponectin ratio level was associated with migraine severity and predictive of acute treatment response. ADP and the HMW:LMW ratio of adiponectin represent potential novel biomarkers and drug targets for episodic migraine.
Keywords: adiponectin, biomarker, headache, migraine
INTRODUCTION
Multiple lines of research have shown that adipose tissue secretes a variety of adipocytokines that modulate inflammation. Adiponectin (ADP) is one such adipocytokine.1 While ADP has been most often reported as having anti-inflammatory properties, (based on the early observations that ADP is reduced in obesity and type I diabetes), recent studies have shown that ADP may be elevated in inflammatory disorders, (eg. inflammatory bowel disease).2
ADP’s ability to exert both pro and anti-inflammatory properties is primarily determined by the form of ADP involved. ADP undergoes oligomerization and is released in the circulation as trimers (low molecular weight [LMW]), hexamers (middle molecular weight [MMW]), larger complexes (high molecular weight [HMW] multimers) and a globular fraction formed by proteolytic cleavage of the full length monomer, called globular adiponectin (gADP).1 The MMW and HMW complexes are the major forms of circulating ADP. The lower concentrations of LMW-ADP and gADP may be related to their shorter half-lives.3
In humans, HMW and MMW-ADP have been shown to activate the NFkβ pathways. Further, while HMW-ADP induces interleukin (IL)-6 secretion, LMW-ADP reduces IL-6 secretion.4,5
Given the sex differences in migraine prevalence and in ADP serum levels, as well as the previous research demonstrating that IL-6 and NFkβ are increased during acute migraine attacks,6–8 the aim of our study was to evaluate ictal levels of ADP and its oligomers before and after acute abortive treatment in women migraineurs. We hypothesized that: 1) increases in HMW-ADP would be associated with increases in pain severity and would decrease in treatment responders, and that 2) increases in LMW-ADP would be associated with decreases in pain severity and would increase in treatment responders.
METHODS
This is an ongoing, multi-center, randomized, double-blind, placebo-controlled, pilot study evaluating ictal serum ADP levels in women episodic migraineurs, before and after treatment with sumatriptan/naproxen sodium (suma/nap) versus placebo. Subjects were recruited from three tertiary care headache clinics between December 2009 to January 2012. The study was approved by the institutional review board from each site; and registered at ClinicalTrials.gov, (NCT01138150). The primary objective of this study was to evaluate serum levels of ADP as well as its oligomers (HMW, MMW, LMW) and ratios (HMW:LMW, HMW:T-ADP, LMW:T-ADP) in episodic migraineurs at onset of moderate to severe pain as compared to serum levels after acute abortive treatment at 30, 60, and 120 minutes. After the final blood draw, rescue therapy (eg. suma/nap, ketorolac, and/or diphenhydramine and metoclopramide) was offered to any participant who continued to report pain.
Participants
Women were eligible for the study if they were ≥ 18 years of age, had a diagnosis of migraine fulfilling the International Classification of Headache Disorders - 2nd edition criteria as determined by a headache specialist, and an attack frequency >1and ≤ 12 headache days per month. Exclusion criteria included: inflammatory, infectious, autoimmune, metabolic, renal, gastrointestinal, and cardiovascular disease, pain disorders other than migraine, change of migraine prophylactic medications within one month of the study, pregnancy or lactation, allergy or contraindication to a triptan or non-steroidal medication.
Study Protocol
All participants had vital signs and neurological examinations, and completed baseline and ictal standardized forms to identify demographics (eg. race, marital status), medical history, headache characteristics (eg. migraine disability (headache impact test [HIT]-6)9, and covariates as described below.10
Following completion of at least one 28-day prospectively-maintained headache calendar, a Johns Hopkins research pharmacist randomized participants to active drug or placebo with a 1:1 allocation in blocks of four. Participants and treating research staff at all institutions were blinded to treatment assignments.
Participants were instructed to return within 4 hours of onset of a moderate to severe acute migraine attack. Upon presentation with an acute attack, an intravenous catheter was placed in an antecubital or forearm vein. Blood was drawn before treatment, upon confirmation of moderate to severe pain (T0), and at 30, 60, and 120 minutes after treatment. Pain severity was assessed using a verbal numerical rating scale (NRS), from 0 (no pain) to 10 (most severe pain). Treatment responders were defined as those participants with a reduction of pain at T0 from moderate to severe (≥4/10 on the NRS) to no to mild (≤3/10 on the NRS) pain at 120 minutes after treatment with either suma/nap or placebo. Thus, treatment responders included all suma/nap and placebo responders; and non-responders included all suma/nap and placebo non-responders.
Covariates
Headache Impact Test (HIT-6)
Headache-related disability was evaluated using the HIT-6. The HIT-6 is a validated questionnaire which demonstrates good reliability and validity across various levels of headache impact.10 The scale consists of six items that reflect health-related quality of life (eg. social functioning). For each item, points are assigned to the response provided. Higher scores indicate a greater impact.
Body Composition
Total body obesity was estimated based on measured height and weight. Measured BMI (m-BMI) was then categorized as normal (18.5 to 24.9 kg/m2) or overweight (>24.9 kg/m2). Abdominal obesity was estimated based on measured waist circumference (WC) in centimeters (cm). WC was measured using an anthropometric tape over skin or light clothing, at the minimum circumference between the iliac crest and the rib cage. Abdominal obesity status was categorized as normal (<80 cm) or overweight (WC ≥80 cm).11
Patient Health Questionnaire (PHQ)-9
Given that depression is associated with both migraine and obesity, depression was evaluated using the PHQ-9.12,13 The PHQ-9 is a self-reported diagnostic measure for depression that utilizes the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria.10 Previous research has shown that a score of ≥15 on the PHQ-9 is associated with a 68% sensitivity and 95% specificity in diagnosing “major depressive disorder” using the DSM-IV criteria.
Laboratory Methods
After sampling in serum-separating tubes, blood was centrifuged, aliquoted and stored at −80° C until assayed. All analyses were conducted in the Core Laboratory of the Center for Clinical and Translational Science, Nutrition Obesity Research Center, and Diabetes Research and Training Center at the University of Alabama Birmingham.
Adiponectin
Total adiponectin (T-ADP) was determined in duplicate by a radioimmunoassay (Millipore, ST. Charles, MO) which utilizes a polyclonal antibody directed against the oligomeric forms of ADP, (ie. It will not detect monomeric or globular ADP). Minimum assay sensitivity was 0.92 ng/mL, with an inter-assay CV of 10.87% and intra-assay CV of 4.80%. ADP oligomers (HMW and combined HMW + MMW) were assessed in duplicate by an enzyme immunosorbent assay (EIA), (ALPCO, Salem, NH), which utilizes a monoclonal antibody against the dimer form of ADP. Digestion and separation steps allow for differentiation of HMW, MMW, and LMW species. Assay sensitivity was 0.019 ng/mL, inter-assay CV 7.01% and intra-assay CV 5.77%. All assays were performed in duplicate according to the manufacturers' instructions.
Additionally, ADP ratios were calculated utilizing the ALPCO EIA. Given that recent data suggests that the ratio of individual oligomers to T-ADP may be a more significant predictor of disease than T-ADP alone,14,15 the ratios of the anti-inflammatory oligomer, LMW-ADP, to total ADP (LMW:T-ADP) and of the pro-inflammatory oligomer, HMW-ADP, to total ADP (HMW:T-ADP) were calculated for all participants. Finally, given the opposing role of HMW-ADP and LMW-ADP in inflammation, the HMW:LMW ratio was also calculated.
Glucose, Insulin, & Cholesterol
Glucose levels were determined using the glucose oxidase method on a SIRRUS analyzer, (Stanbio Laboratory, Boerne, TX,) with an inter-assay coefficient of variation (CV) of 1.5% and an intra-assay CV of 1.3%. Insulin was assayed by immunofluorescence using a TOSOH AIA-600 II analyzer, (TOSOH Bioscience, South San Francisco, CA), with an inter-assay CV of 4.4% and intra-assay CV of 1.5%. Total cholesterol was measured colorimetrically using the SIRRUS analyzer.
Sex hormones
Given that ADP has been shown to be modulated by sex hormones8 estradiol, estrone, progesterone and testosterone were determined by immunofluorescence using the TOSOH with the following CV and sensitivities: Estradiol: 5.38% inter-assay CV, 6.01% intra-assay CV, 25 pg/ml minimum sensitivity; Estrone: 6.21% inter-assay CV, 4.69% intra-assay CV, 10 pg/ml minimum sensitivity; Progesterone: 5.09% inter-assay CV, 5.33% intra-assay CV, 0.1 ng/ml minimum sensitivity; Testosterone: 2.73% inter-assay CV, 2.43% intra-assay CV, 10 ng/dL minimum sensitivity.
Statistical Analysis
Statistical analyses were performed using Stata Statistical Software: Release 11, 2009, (College Station, TX: StataCorp LP). Baseline demographic and clinical characteristics were compared in treatment responders and non-responders using independent t-tests and chi-squared analyses. Univariate and multivariate linear models were fit to examine the association between T-ADP, ADP oligomers and ratios, and pain severity, (based on the NRS), in all participants. Additionally, in treatment responders and non-responders, the mean levels of T-ADP, ADP oligomers (HMW, MMW, LMW) and ADP ratios (LMW:T-ADP, HMW:T-ADP and HMW:LMW) at 30, 60 and 120 minutes were compared to pain onset (T0) utilizing paired t-tests; random intercept longitudinal models were utilized to assess the mean change in T-ADP and ADP oligomers and ratios over time (to account for the differences in baseline levels), adjusting for age, BMI, race and study site. Sensitivity analyses were performed excluding one participant determined to have a history of untreated arthritis after completion of blood draws. No significant differences in outcomes were observed; and thus, final models included this participant.
RESULTS
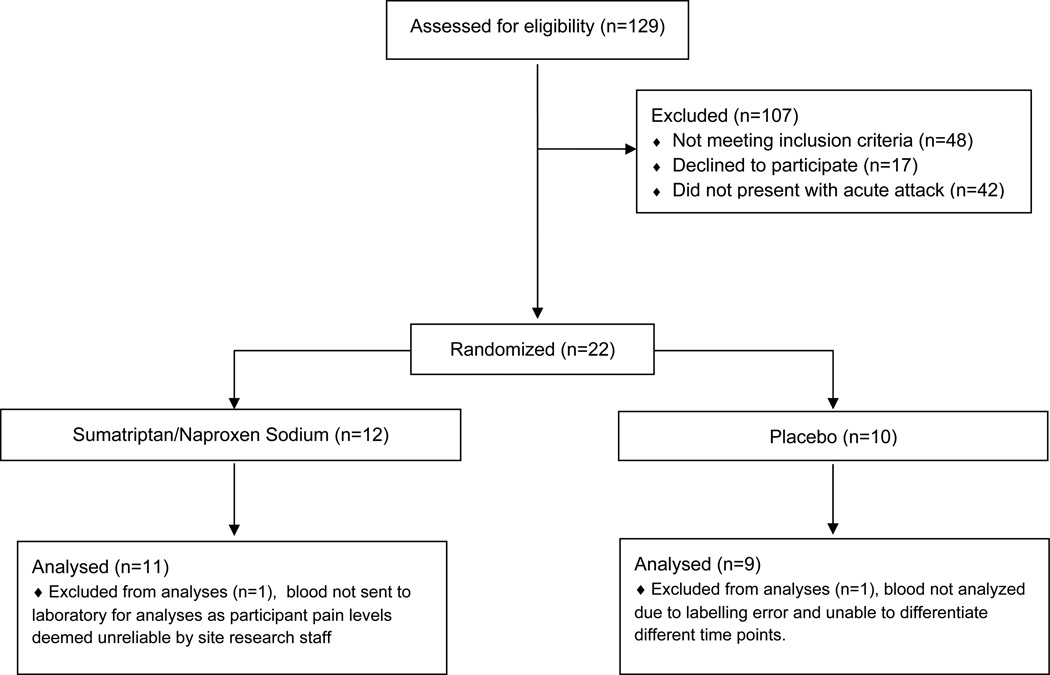
A total of 20 episodic migraineurs were randomized to receive treatment, of which 11 received suma/nap and 9 placebo, (see Figure 1). Eleven participants (6 suma/nap & 5 placebo) were identified as treatment responders. Participant demographics, headache characteristics, body composition, and standard laboratories including insulin, glucose, sex hormones and cholesterol levels are presented in Table 1.
Figure 1. Women Episodic Migraineurs Eligibility, Randomization, & Enrollment.
Table 1.
Demographic & Laboratory Characteristics of Episodic Women Migraineurs by Treatment Response
| Total (N=20) |
Treatment Responders (n=11) |
Treatment Non-Responders (n=9) |
p-value* | |
|---|---|---|---|---|
| Treatment | ||||
| Suma/Nap | 11 (55.0) | 6 (54.5) | 5 (55.6) | 0.96 |
| Placebo | 9 (45.0) | 5 (45.5) | 4 (44.4) | |
| Age | 34.5 (9.1) | 32.8 (8.6) | 36.4 (9.6) | 0.39 |
| Race – n (%) | ||||
| Caucasian | 17 (85.0) | 9 (81.8) | 8 (88.9) | 0.66 |
| African American | 3 (15.0) | 2 (18.2) | 1 (11.1) | |
| Marital Status - n (%) | ||||
| Single | 14 (70.0) | 8 (72.7) | 6 (66.7) | 0.77 |
| Married | 6 (30.0) | 3 (27.3) | 3 (33.3) | |
| Education - n (%) | ||||
| HS or College | 13 (65.0) | 7 (63.6) | 6 (66.7) | 0.89 |
| Post Grad | 7 (35.0) | 4 (36.4) | 3 (33.3) | |
| Income- n (%) | ||||
| <50 K | 8 (40.0) | 4 (36.4) | 4 (44.4) | 0.71 |
| >50 K | 12 (60.0) | 7 (63.6) | 5 (55.6) | |
| Physician Dx - n (%) | ||||
| MO | 11 (55.0) | 7 (63.6) | 4 (44.4) | 0.39 |
| MA | 9 (45.0) | 4 (36.4) | 5 (55.6) | |
| HA Frequency (1 mo) | 5.8 (2.5) | 6.0 (2.4) | 5.4 (2.8) | 0.61 |
| HA Disability (HIT-6) | 65.6 (4.9) | 64.8 (5.5) | 66.6 (4.1) | 0.44 |
| Pain Intensity: NRS 0-10 (mean ±SD) | ||||
| T0 | 6.1 (1.2) | 5.8 (1.3) | 6.3 (1.1) | 0.399 |
| 30 Min | 5.5 (2.0) | 4.5 (2.0) | 6.7 (1.3) | 0.011 |
| 60 Min | 4.6 (2.9) | 2.6 (2.5) | 6.9 (1.4) | 0.0001 |
| 120 Min | 3.7 (2.9) | 1.4 (1.3) | 6.6 (1.3) | <0.0001 |
| # Daily Meds - n (%) | ||||
| 0 | 7 (35.0) | 4 (36.4) | 3 (33.3) | 0.140 |
| 1 | 7 (35.0) | 2 (18.2) | 5 (55.6) | |
| 2 | 6 (30.0) | 5 (45.5) | 1 (11.1) | |
| Abdominal Obesity – n (%) (WC ≥ 80 cm) | 11 (55.0) | 6 (54.6) | 5 (55.6) | 0.96 |
| m-BMI (kg/m2) | 26.2 (6.4) | 25.9 (6.8) | 26.5 (6.4) | 0.86 |
| Total Body Obesity – n (%) (≥ 24.9 m-BMI) | 10 (50.0) | 7 (63.6) | 3 (33.3) | 0.18 |
| PHQ-9 ≥15- n (%) | 3 (15.0) | 1 (9.1) | 2 (22.2) | 0.413 |
| Smoking Hx(pack/day) | 0.15 (0.49) | 0 | 0.33 (0.71) | - |
| Arthritis - n (%) | 1 (5.0) | 1 (9.1) | 0 | 0.35 |
| Study Site - n (%) | ||||
| UT | 5 (25.0) | 5 (45.5) | 0 | 0.05 |
| DM | 1 (5.0) | 0 | 1 (11.1) | |
| JH | 14 (70.0) | 6 (54.5) | 8 (88.9) | |
| T0 Laboratories | ||||
| Glucose (mg/dL) | 92.5 (14.2) | 93.0 (15.1) | 91.8 (13.7) | 0.54 |
| Insulin (uU/mL) | 14.8 (14.1) | 17.7 (17.6) | 11.4 (8.0) | 0.34 |
| Cholesterol (mg/dL) | 178.9 (38.6) | 188.0 (35.7) | 167.7 (41.1) | 0.25 |
| E2 (pg/mL) | 84.3 (72.1) | 93.5 (77.6) | 73.0 (67.5) | 0.54 |
| E1 (pg/mL) | 215.2 (142.5) | 210.2 (112.5) | 221.1 (179.8) | 0.87 |
| Progesterone (ng/mL) | 1.88 (2.98) | 2.6 (3.8) | 0.97 (1.2) | 0.23 |
| Testosterone (ng/dL) | 29.5 (23.5) | 24.1 (14.2) | 36.2 (31.1) | 0.26 |
Note: Responders include all treatment responders (ie. suma/nap responders and placebo responders); non-responders include all suma/nap and placebo non-responders; Data is presented as the mean and standard deviation except where indicated as n (%);
p-value for X2 tests and student’s t-test;
m-BMI = measured body mass index; DM = Dartmouth; E1 = estrone; E2=estradiol; HA = headache; HIT-6 = headache impact test-6;HS = high school; JH = Johns Hopkins; MA = migraine with aura; MO=migraine without aura; NRS=numerical rating scale; PHQ-9 = patient healthcare questionnaire-9; suma/nap = sumatriptan/ naproxen sodium; T0 = time point 0 (moderate to severe pain onset); UT = University of Toledo; WC = waist circumference
Adipopnectin and Pain Severity
In all participants (n=20), incremental changes in T-ADP, HMW-ADP, and MMW-ADP were not significantly associated with a reduction or increase of pain severity trajectories on the NRS of 0 (no pain) to 10 (most severe pain), after adjusting for treatment, BMI, age and time since pain onset, (Table 2). However for each 0.25 µg/mL increase in LMW-ADP, pain severity on the NRS declined by 0.20 (CI: -0.41, -0.0002; p=0.047). Additionally, for each 1 point increase in the HMW:LMW ratio, pain severity increased by 0.22 (CI: 0.07, 0.37; p=0.004), (Table 2),
Table 2.
Unadjusted & adjusted† pain severity trajectories‡ in relationship to changes in total adiponectin, its oligomers and the HMW:LMW adiponectin ratio in all female episodic migraine participants, (n=20)
| Unadjusted estimates of Pain Intensity | Adjusted estimates of Pain Intensity * | |||||
|---|---|---|---|---|---|---|
| Coef.* | 95% CI | p-value | Coef.* | 95% CI | p-value | |
| Adiponectin | ||||||
| T-ADP (per 1 µg/ml increase) | 0.12 | (−0.16, 0.20) | 0.844 | −0.05 | (−0.24, 0.14) | 0.628 |
| HMW-ADP (per 0.25 µg/ml increase) | −0.02 | (−0.15, 0.11) | 0.734 | −0.05 | (−0.16, 0.07) | 0.408 |
| MMW-ADP (per 0.25 µg/ml increase) | 0.05 | (−0.17, 0.27) | 0.641 | 0.07 | (−0.13, 0.28) | 0.485 |
| LMW-ADP (per 0.25 µg/ml increase) | −0.25 | (−0.43, −0.06) | 0.010 | −0.20 | (−0.41, −0.002) | 0.047 |
| HMW:LMW (per 1 point increase) | 0.17 | (−0.08, 0.43) | 0.187 | 0.22 | (0.07, 0.37) | 0.004 |
Trajectories of pain severity assessed by linear mixed models with random intercepts;
Pain severity score measured by numeric rating scale, from 0 (no pain) to 10 (most severe pain);
Multivariate models adjusted for treatment, BMI, age and time after pain onset;
Coef.: Coefficient represents the population average change in pain scores;
T-ADP=total adiponectin; HMW-ADP=high molecular weight adiponectin; MMW-ADP=middle molecular weight adiponection; LMW-ADP=low molecular weight adiponectin
Adiponectin and Treatment Response
T-ADP
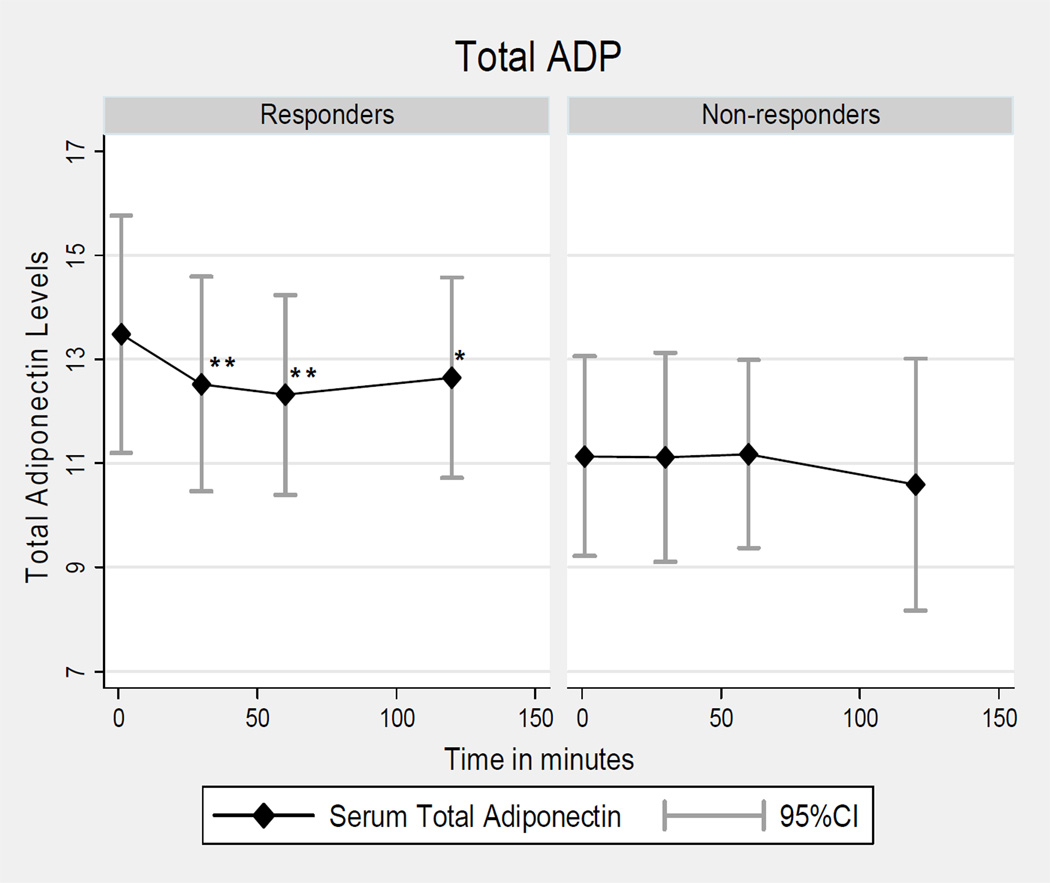
In responders, unadjusted T-ADP levels were significantly reduced at 30 min (12.52 ± 3.4 µg/mL; p=0.030), 60 min (12.32 ± 3.2 µg/mL; p=0.017) and 120 min (12.65 ± 3.2 µg/mL; p=0.016) as compared to onset (13.48 ± 3.8 µg/mL; see Table 3, Figure 2). After adjustments for BMI, age, race, and study site, the mean change in T-ADP levels at 30 min was -0.96 µg/mL (95% CI: -1.63, -0.28; p=0.005), -1.16 µg/mL (95% CI: -1.84, -0.49; p=0.001) at 60 min and -0.84 µg/mL (95% CI: -1.51, -0.16; p=0.02) at 120 min, (Table 3). In non-responders T-ADP unadjusted and adjusted levels did not significantly change over time, (Tables 3 and 4).
Table 3.
Unadjusted ADP &ADP ratio levels in women episodic migraine responders/non-responders before & after treatment.
| ADP & Oligomers |
Time-Point |
All Treatment Responders (n=11) |
All Treatment Non-Responders (n=9) |
ADP Ratios |
Time-Point | All Treatment Responders (n=11) |
All Treatment Non-Responders (n=9) |
|---|---|---|---|---|---|---|---|
| Total ADP (µg/mL) | T0 | 13.48 | 11.14 | HMW : T-ADP | T0 | 0.60 | 0.54 |
| (3.79) | (2.89) | (0.09) | (0.07) | ||||
| 30 min | 12.52* | 11.12 | 30 min | 0.58 | 0.56 | ||
| (3.42) | (3.01) | (0.09) | (0.07) | ||||
| p=0.030 | p=0.97 | p=0.24 | p=0.96 | ||||
| 60 min | 12.32* | 11.18 | 60 min | 0.55* | 0.56 | ||
| (3.18) | (2.72) | (0.08) | (0.10) | ||||
| p=0.017 | p=0.92 | p=0.02 | p=0.43 | ||||
| 120 min | 12.65* | 10.59 | 120 min | 0.55 | 0.58 | ||
| (3.19) | (3.63) | (0.10) | 0.14 | ||||
| p=0.016 | p=0.16 | p=0.98 | p=0.21 | ||||
| HMW (µg/mL) | T0 | 4.37 | 3.30 | HMW : LMW | T0 | 3.70‡ | 2.29 |
| (1.68) | (0.96) | (1.90) | (0.71) | ||||
| 30 min | 4.08 | 3.20 | 30 min | 3.22 | 3.14 | ||
| (1.38) | (1.01) | (1.82) | (1.71) | ||||
| p=0.131 | p=0.97 | p=0.47 | p=0.15 | ||||
| 60 min | 3.98* | 3.18 | 60 min | 2.37** | 3.39 | ||
| (1.35) | (1.03) | (1.10) | (2.30) | ||||
| p=0.048 | p=0.79 | p=0.002 | p=0.15 | ||||
| 120 min | 3.98 | 3.25 | 120 min | 2.76* | 4.22^ | ||
| (1.43) | (1.10) | (1.41) | (3.87) | ||||
| p=0.099 | p=0.498 | p=0.02 | p=0.17 | ||||
| LMW(µg/mL) | T0 | 1.37 | 1.44 | LMW : T-ADP | T0 | 0.20 | 0.25 |
| (0.54) | (0.36) | (0.10) | (0.05) | ||||
| 30 min | 1.55 | 1.21 | 30 min | 0.23 | 0.23 | ||
| (0.66) | (0.52) | (0.11) | (0.11) | ||||
| p=0.35 | p=0.27 | p=0.29 | p=0.47 | ||||
| 60 min | 1.80** | 1.14 | 60 min | 0.26** | 0.20 | ||
| (0.46) | (0.53) | (0.08) | (0.10) | ||||
| p=0.013 | p=0.17 | p=0.01 | p=0.20 | ||||
| 120 min | 1.62 | 1.20 | 120 min | 0.24* | 0.22 | ||
| (0.55) | (0.65) | (0.10) | (0.12) | ||||
| p=0.16 | p=0.36 | p=0.04 | p=0.44 |
Note: Total adiponectin (T-ADP) was significantly reduced after treatment in all responders at 30, 60, and 120 minutes after treatment as compared to T0 (before treatment) and remained so after adjustments. In responders, the HMW:LMW ratio was significantly reduced after treatment in all responders at 60, and 120 minutes after treatment as compared to T0, (before treatment) and remained so after adjustments. While unadjusted ratio levels were not increased in non-responders, after adjustments the HMW:LMW ratio was increased in non-responders at 120 min. The following p-values are comparisons to T0:
p≤0.05;
p≤0.01,
<0.05 after adjustments;
p=0.050 for responders at T0 as compared to non-responders at T0.
ADP=adiponectin; HMW=high molecular weight; LMW=low molecular weight; SumaNap=sumatriptan/naproxen.
Figure 2. Total adiponectin (ADP) levels in Women Episodic Migraine Responders & Non-Responders.
LMW= low molecular weight; ** p≤ 0.005, * p=0.02 after adjustments
Table 4.
Adjustedŧ Random Intercept Longitudinal Model for All Responders and Non-Responders
| Total Adiponectin | HMW-ADP | MMW-ADP | LMW-ADP | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient (SE) | 95% CI |
p- value |
Coefficient (SE) | 95% CI |
p- value |
Coefficient (SE) | 95% CI |
p- value |
Coefficient (SE) | 95% CI |
p- value |
|||
| Responders | Time-Point | |||||||||||||
| T0 (Onset) | Reference | Reference | Reference | Reference | ||||||||||
| 30 min | −0.96 (0.34) | −1.63, −0.28 | 0.005 | −0.30 (0.17) | −0.63, 0.03 | 0.08 | −0.08 (0.17) | −0.41, 0.25 | 0.63 | 0.18 (0.19) | −0.19, 0.55 | 0.33 | ||
| 60 min | −1.16 (0.34) | −1.84, −0.49 | 0.001 | −0.39 (0.17) | −0.72, −0.06 | 0.02 | −0.08 (0.17) | −0.41, 0.25 | 0.63 | 0.43 (0.19) | 0.06, 0.80 | 0.02 | ||
| 120 min | −0.84 (0.34) | −1.51, −0.16 | 0.02 | −0.40 (0.17) | −0.72, −0.07 | 0.02 | 0.14 (0.17) | −0.19, 0.46 | 0.41 | 0.25 (0.19) | −0.12, 0.62 | 0.19 | ||
| Total Adiponectin | HMW-ADP | MMW-ADP | LMW-ADP | |||||||||||
| Coefficient (SE) | 95% CI |
p- value |
Coefficient (SE) | 95% CI |
p- value |
Coefficient (SE) | 95% CI |
p- value |
Coefficient (SE) | 95% CI |
p- value |
|||
| Non-Responders | Time-Point | |||||||||||||
| T0 (Onset) | Reference | Reference | Reference | Reference | ||||||||||
| 30 min | −0.02 (0.42) | −0.85, 0.81 | 0.962 | 0.01 (0.12) | −0.22, 0.23 | 0.964 | −0.05 (0.15) | −0.36, 0.25 | 0.726 | −0.22 (0.19) | −0.59, 0.15 | 0.238 | ||
| 60 min | 0.04 (0.42) | −0.79, 0.87 | 0.923 | −0.02 (0.12) | −0.25, 0.21 | 0.894 | 0.06 (0.15) | −0.24, 0.37 | 0.674 | −0.26 (0.19) | −0.63, 0.11 | 0.164 | ||
| 120 min | −0.55 (0.42) | −1.38, 0.28 | 0.196 | 0.06 (0.12) | −0.17, 0.29 | 0.612 | −0.12 (0.15) | −0.43, 0.18 | 0.419 | −0.24 (0.19) | −0.61, 0.13 | 0.205 | ||
Table 3: Mean adjusted change in adiponectin (ADP) and ADP oligomer levels in episodic women migraineurs after treatment, stratified by response. In responders, total adiponectin, (at all time points,) and HMW-ADP levels, (at 60 and 120 min,) were decreased while LMW-ADP levels were increased, (at 60 min,) after treatment as compared to before treatment at pain onset (T0). All p-values are comparisons to T0. T0=pain onset;
All results were adjusted for BMI, age, race, and study site. Among responders, no participants were enrolled from the DM site and thus omitted from the model. Among non-responders, JH participants were dropped due to collinearity with the outcome.
CI=confidence interval; SE=standard error; HMW-ADP=high molecular weight adiponectin; MMW-ADP=middle molecular weight adiponectin; LMW-ADP=low molecular weight adiponectin
HMW-ADP
In responders, unadjusted serum HMW-ADP levels were non-significantly reduced at 30 min (4.08 ± 1.4 µg/mL; p=0.131), reached significance at 60 min, (3.98 ± 1.35 µg/mL; p=0.048) and were non-significantly reduced at 120 min (3.98 ± 1.43 µg/mL; p=0.099) as compared to onset (4.37 ± 1.7; see Table 3). After adjustments, HMW-ADP levels were significantly decreased in responders (-0.39 µg/mL; 95% CI: -0.72, -0.06; p=0.02) at 60 min; and 120 min (-0.39 µg/mL; 95% CI: -0.72, -0.07; p=0.02) after treatment as compared to onset, (Table 3). In non-responders, unadjusted and adjusted HMW-ADP levels did not significantly change over time, (Tables 3 and 4).
MMW-ADP
In responders, unadjusted MMW-ADP levels did not significantly change at 30 min (1.31 ± 0.5 µg/mL; p=0.5), 60 min (1.31 ± 0.7 µg/mL; p=0.5) or 120 min (1.52 ± 0.77 µg/mL; p=0.5) as compared to onset (1.39 ± 0.5 µg/mL) and remained non-significant after adjustments. In non-responders, unadjusted MMW-ADP levels were not significantly modulated at 30 min (1.20 ±0.6 µg/mL; p=0.66), 60 min (1.32 ± 0.61 µg/mL; p=0.60) or 120 min (1.12 ± 0.5 µg/mL; p=0.60) as compared to onset (1.25 ± 0.5 µg/mL) and remained non-significant after adjustments, (Table 4).
LMW-ADP
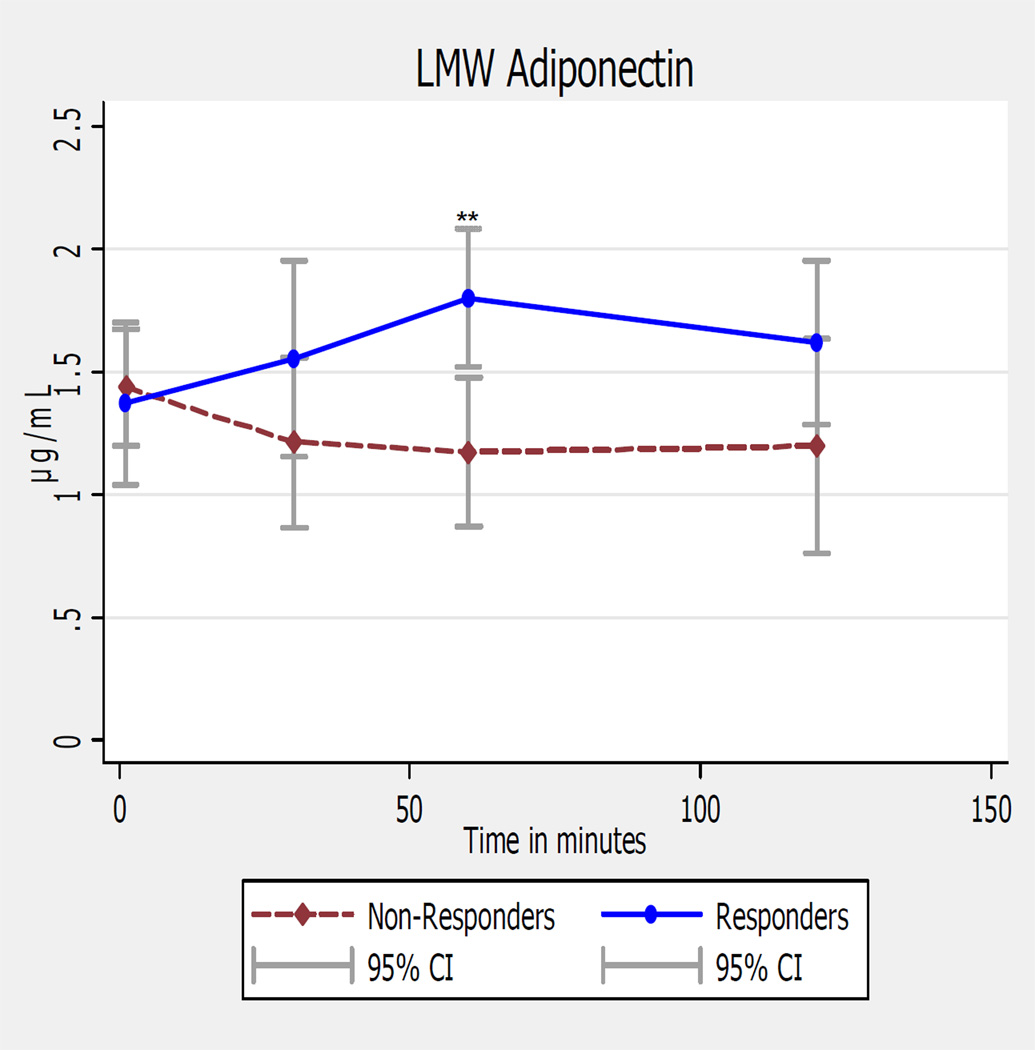
In responders, unadjusted LMW-ADP was non-significantly increased at 30 min (1.55 ± 0.7 µg/mL; p=0.35), reached significance at 60 min (1.80 ± 0.5 µg/mL; p=0.029), and was non-significantly increased at 120 min (1.62 ± 0.55 µg/mL; p=0.16) as compared to onset (1.37 ± 0.5 µg/mL), with similar findings after adjustments, (Figure 3). In non-responders, LMW-ADP levels did not significantly change over time, (Tables 3 and 4).
Figure 3. Ictal LMW adiponectin levels in Women Episodic Migraine Responders & Non-Responders.
LMW= low molecular weight; ** p≤ 0.02 for comparison between T0 and 60 min time points before & after adjustments
HMW:LMW
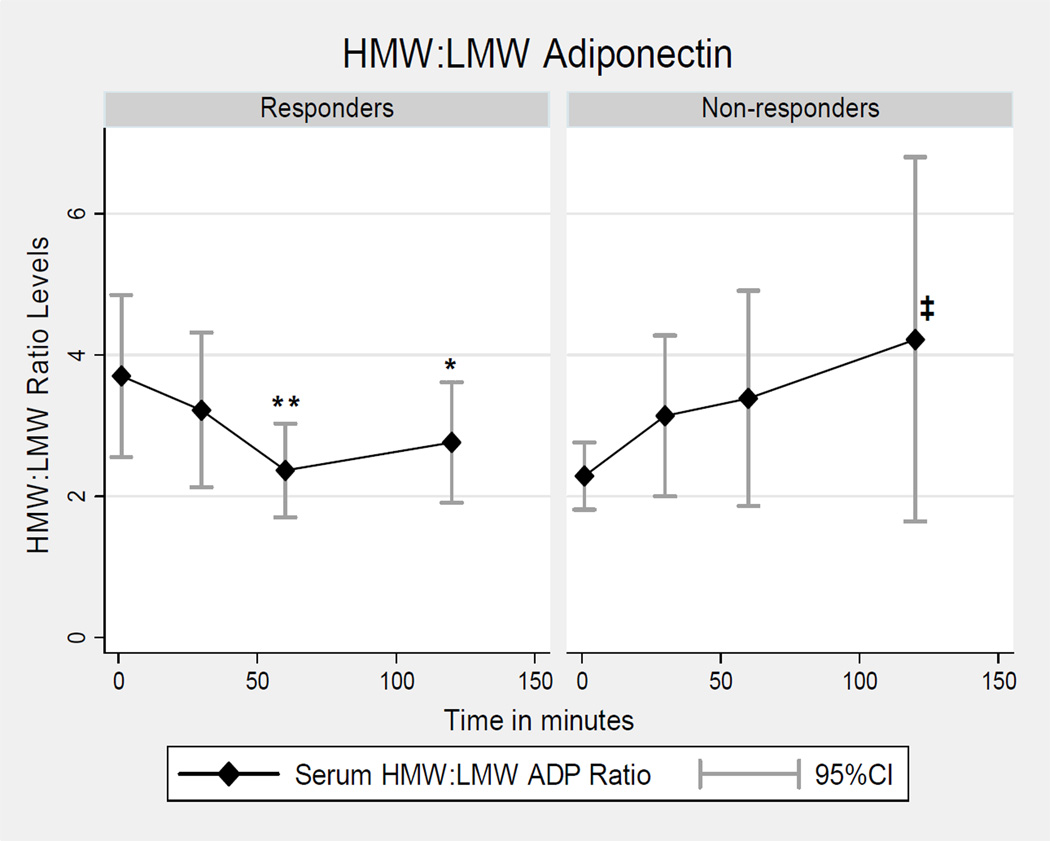
The ratio of the proinflammatory ADP oligomer, HMW-ADP, to the anti-inflammatory ADP oligomer, LMW-ADP, (HMW:LMW ratio) was decreased in responders at 60 min (2.37 ± 1.1 µg/mL; p=0.002) and 120 min (2.76 ± 1.41 µg/mL; p=0.021) as compared to onset (3.70 ±1.9 µg/mL), (Figure 4); and remained so after adjustments, (Tables 3 and 5). In non-responders the unadjusted HMW:LMW ADP ratio trended up non-significantly at 30 min (3.14 ± 1.71 µg/mL; p=0.15), 60 min (3.39 ± 2.30 µg/mL; p=0.015), and 120 min (4.22 ± 3.87 µg/mL, p=0.17) as compared to onset (2.29 ± 0.71 µg/mL); however, after adjustments the HMW:LMW ADP ratio was significantly increased by 1.93 µg/mL at 120 min (95% CI: 0.24, 3.62; p=0.025), in non-responders (Table 5). Finally, the HMW:LMW ratio level was greater at pain onset (T0) in responders (3.70 ±1.9 µg/mL) as compared to non-responders (2.29 ± 0.71 µg/mL) at T0, p=0.050.
Figure 4. Ictal HMW:LMW Adiponectin Levels in Women Episodic Migraine Responders & Non-responders.
All p-values are for comparisons between T0 and subsequent time points; **p≤0.003, *p≤0.04 before and after adjustments; ‡p=0.03 after adjustments only. LMW= low molecular weight; HMW=high molecular weight
Table 5.
Adjustedŧ Adiponectin Ratios in Women Episodic Migraine Responders and Non-Responders Before and After Treatment
| HMW : T-ADP | LMW : T-ADP | LMW : T-ADP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient (SE) | 95% CI | p-value | Coefficient (SE) | 95% CI | p-value | Coefficient (SE) | 95% CI | p-value | |||
| Responders | Time-Point | ||||||||||
| T0 (Onset) | Reference | Reference | Reference | ||||||||
| 30 min | −0.02 (0.02) | −0.06, 0.02 | 0.33 | 0.48 (0.45) | −1.37, 0.41 | 0.29 | 0.03 (0.02) | −0.02, 0.08 | 0.22 | ||
| 60 min | −0.05 (0.020) | −0.09, −0.01 | 0.02 | −1.34 (0.45) | −2.23, −0.45 | 0.003 | 0.06 (0.02) | 0.02, 0.11 | 0.008 | ||
| 120 min | −0.05 (0.02) | −0.09, −0.01 | 0.02 | −0.94 (0.45) | −1.83, −0.05 | 0.04 | 0.03 (0.02) | −0.01, 0.08 | 0.14 | ||
| HMW : T−ADP | LMW : T−ADP | LMW : T−ADP | |||||||||
| Coefficient (SE) | 95% CI | p−value | Coefficient (SE) | 95% CI | p−value | Coefficient (SE) | 95% CI | p−value | |||
| Non-Responders | Time-Point | ||||||||||
| T0 (Onset) | Reference | Reference | Reference | ||||||||
| 30 min | 0.02 (0.03) | −0.03, 0.07 | 0.396 | 0.85 (0.86) | −0.85, 2.54 | 0.327 | −0.02 (0.03) | −0.08, 0.03 | 0.434 | ||
| 60 min | 0.02 (0.03) | −0.03, 0.07 | 0.485 | 1.10 (0.86) | −0.59, 2.79 | 0.204 | −0.04 (0.03) | −0.09, 0.02 | 0.188 | ||
| 120 min | 0.04 (0.03) | −0.008, 0.092 | 0.096 | 1.93 (0.86) | 0.24, 3.62 | 0.025 | −0.03 (0.03) | −0.08, 0.02 | 0.284 | ||
Table 4: Mean adjusted change in adiponectin (ADP) ratio levels in episodic women migraineurs after treatment, stratified by response. HMW:LMW ADP levels were decreased in responders and increased in non-responders after adjustments. All p-values are comparisons to T0. T0=pain onset;
Results adjusted for BMI, age, race, and study site. Among responders, no participants were enrolled from the DM site and thus omitted from the model. Among non-responders, JH participants were dropped due to colinearity with the outcome.
CI=confidence interval; SE=standard error; HMW-ADP=high molecular weight adiponectin; MMW-ADP=middle molecular weight adiponectin; LMW-ADP=low molecular weight adiponectin
HMW:T-ADP
In responders, the unadjusted ratio of the proinflammatory ADP oligomer, HMW-ADP, to T-ADP (HMW:T-ADP) was significantly decreased at 60 min (0.55 ± 0.08 µg/mL; p=0.02), as compared to onset (0.60 ± 0.09 µg/mL; see Table 3); after adjustments the HMW:T-ADP ratio was significantly reduced at both 60 min (p=0.02) and 120 min (p=0.02), in responders, (Table 5). In non-responders, HMW-T-ADP ratio levels were not significantly modulated after treatment, (Tables 3 and 5).
LMW:T-ADP
The ratio of LMW-ADP to T-ADP (LMW:T-ADP) was significantly increased at 60 min (0.26 ± 0.08 µg/mL; p=0.013) and 120 min (0.24 ± 0.1 µg/mL; p=0.043) in responders as compared to onset (0.20 ±0.1 µg/mL; see Table 3). However, after adjustments, the ratio of LMW:T-ADP was significantly increased only at 60 min, (p=0.008; see Table 5). In non-responders, no significant differences in the LMW:T-ADP ratios were found after treatment as compared to onset, (Tables 3 and 5).
DISCUSSION
ADP has been reported to have both pro and anti-inflammatory effects, which may be explained by the differential effects of ADP oligomers.3,11 In the current study we evaluated ictal, serum levels of ADP, its oligomers and ratios in women episodic migraineurs within 4 hours of onset of a moderate to severe migraine attack and at 30, 60 and 120 minutes after treatment with sumatriptan/naproxen sodium versus placebo. This pilot study has two main findings. The first is that ictal changes in LMW-ADP and the HMW:LMW ratio are associated with changes in pain severity in women episodic migraineurs. In all participants, increases in the anti-inflammatory adiponectin oligomer, LMW-ADP, were associated with significant decreases in pain severity on the NRS. In contrast, increases in the HMW:LMW ADP ratio were associated with increases in pain severity. Second, treatment responders showed decreased levels of T-ADP as early as 30 minutes and up to 2 hours after treatment, and these effects were not seen in non-responders. Finally, changes in the ratios of the ADP oligomers to each other and to T-ADP over time were also modulated by treatment response. Specifically, those migraineurs who had a reduction or resolution of pain exhibited changes in the HMW:LMW ADP ratio towards an anti-inflammatory state. The opposite was observed in non-responders; the HMW:LMW ADP ratio was increased in those who continued to have pain after treatment, corresponding to a worsening inflammatory state. These findings suggest that the HMW:LMW ADP ratio may be a potential novel biomarker of acute treatment response in women migraineurs.
Limited data has previously suggested that HMW-ADP is elevated in women at baseline level of pain in those with chronic migraine as compared to controls.11 Our data extend this to suggest that the HMW:LMW ADP ratio may be increased with the presence of active migraine pain - be it acute onset or chronically active migraine pain. If the HMW:LMW ratio is also elevated in episodic migraineurs when pain-free, this would suggest drugs targeting a reduction of the HMW:LMW ratio may be effective migraine preventives; and if not, drugs targeting a reduction in the HMW:LMW ADP could represent acute abortive migraine agents.
The regulation of ADP expression and secretion of its oligomers into the circulation is complex. However, (and similar to migraine prevalence), after puberty a sexual dimorphism in adiponectin expression becomes evident, with women having higher total and HMW-ADP levels than males, and lower LMW-ADP levels.8 This shift to greater levels of the pro-inflammatory ADP oligomer, HMW-ADP, and decrease in the anti-inflammatory LWM-ADP in women, may create or contribute to an internal milieu placing women at greater risk of migraine at baseline than men. Our current study is unable to examine the validity of this hypothesis given that only women migraineurs were examined and that pain-free levels were not. However future analyses that are able to include comparisons of ADP levels stratified by sex in pain-free and acute pain states may assist in answering this hypothesis.
How ADP and its oligomers may be linked to migraine is not known. It is known that activation of the hypothalamus and modulation of the cerebral vasculature occur in migraine; and adiponectin receptors have been identified in mouse cerebral microvessels and the human hypothalamus, including in NPY neurons.16,17 Further, ADP binding to ADP receptors is associated with activation of manifold downstream signaling responses, several of which have also been implicated in migraine pathophysiology. Specifically ADP binding to ADP receptors has been shown to be associated with stimulation of the AMP activated protein kinase (AMPK) pathway and modulation of endothelial nitric oxide synthase, stimulation of the p38-mitogen activated protein kinase signaling pathway (which is activated by a variety of environmental stresses and cytokines), as well as stimulation of the proinflammatory NFkβ pathway.3,18
There are several limitations of our current study, including the small sample size and the use of a combination acute abortive migraine therapy. Although we observed similar trends in responders and non-responders when stratified by treatment sub-groups (suma/nap and placebo), as in the total group of all responders and non-responders, (See Appendices A-B), due to limited power as a result of small sample size limitations within these sub-groups, we cannot draw definitive or reliable conclusions as to the effect of the individual treatments with respect to response. A larger study is required to directly test the individual treatment (suma/nap vs placebo) responder status as a moderator of changes in ADP during the onset and resolution of migraine. Additionally, the use of a combination abortive migraine agent, sumatriptan/naproxen sodium, entirely inhibits our ability to examine if post-treatment changes in adiponectin are related to the triptan or the non-steroidal anti-inflammatory drug component or both. Finally, given our inclusion of only female migraineurs, these findings cannot be generalized to male migraineurs.
Despite the above limitations, the present study demonstrates for the first time that changes in adiponectin, and specifically the ratio of the proinflammatory ADP oligomer (HMW-ADP) to the anti-inflammatory oligomer (LMW-ADP), are associated with changes in migraine severity and are decreased in women responders after treatment and not in non-responders. These findings suggest that the “balance” or ratio of pro and anti-inflammatory ADP oligomers, may be as important, if not more, as T-ADP levels in evaluating migraine severity and acute treatment response. Larger and confirmatory trials are needed to determine if ADP, its oligomers and the HMW:LMW ratio are true operational biomarkers of migraine and if ADP modulating drugs are effective for migraine therapy.
Supplementary Material
Acknowledgments
Study Funding: Investigator initiated grant to Dr. Peterlin through GlaxoSmithKline and a NINDS contract, K23-10896737.
Abbreviations
- ADP
Adiponectin
- CV
Coefficient of Variation
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders-IV
- EIA
Enzyme Immunoassay
- HMW
High Molecular Weight
- HIT-6
Headache Impact Test-6
- IL-6
Interleukin-6
- LMW
Low Molecular Weight
- MMW
Middle Molecular Weight
- m-BMI
Measured Body Mass Index
- NFkβ
Nuclear Factor Kappa Beta
- NRS
Numerical Rating Scale
- T-ADP
Total Adiponectin
- T0
Time-point 0 (Time of onset of moderate to severe pain before study treatment)
- WC
Waist Circumference
- Suma/nap
Sumatriptan/naproxen sodium
Footnotes
Conflict of Interest Statement:
B. Lee Peterlin
This study was funded by an investigator initiated grant to Dr. Peterlin through GlaxoSmithKline. In the past two years Dr. Peterlin has not served as a consultant or speaker with any pharmaceutical company.Dr. Peterlin receives salary support through a mentored patient-oriented career research award (K23 10896737) from the National Institute of Neurological Disorders; and is an associate editor at the journal, Headache.
Gretchen Tietjen
Dr. Tietjen has received research support from GSK and has been on the advisory board of MAP Pharmaceuticals. Dr. Tietjen is an associate editor at the journal, Headache. She owns common stock in J&J and Stryker
Barbara A. Gower
No disclosures.
Thomas A. Ward
Dr. Ward has received research grant support from GSK; and is a consultant with Cowen and Co. Dr. Ward the Editor-in-Chief of Headache.
Stewart J. Tepper
Dr Tepper has received research support (no personal compensation from ATI (active), Boston Scientific (active), BristolMyerSquibb (active), DepoMed (active), GSK (active), MAP (active), Merck (active), NuPathe (active), OptiNose (active), & Zogenix (active). He has worked as a consultant for Allergan (active), ATI (active), GSK in the past, Helsinn in the past, MAP (active), Merck in the past, Nautilus (active), NuPathe (active), & Zogenix (active). He as served as a speaker for Allergan (active), ATI (active), GSK in the past,, Merck in the past, Nautilus (active), & Zogenix (active). He has served as an advisor to Allergan (active), ATI (active), GSK in the past, Merck in the past, MAP (active), Nautilus (active), NuPathe (active), Pfizer (active), USWorldMeds (active), & Zogenix (active). He has stock options in ATI. He receives royalties from University of Mississippi Press, Peoples Publishing House of Peking, & Springer. Dr. Tepper is an associate editor at the journal, Headache and Editor-in-Chief of Headache Currents.
Linda W. White
No Disclosures
Paul D. Dash
Dr. Dash has served as speaker for Teva Pharmaceuticals Industries.
Edward R. Hammond
No disclosures.
Jennifer Haythornthwaite
No disclosures.
REFERENCES
- 1.Peterlin BL, Bigul M, Tepper SJ, Urakaze M, Sheftell FD, Rapoport AM. Migraine and adiponectin: Is there a connection? Cephalalgia. 2007;27(5):435–446. doi: 10.1111/j.1468-2982.2007.01306.x. [DOI] [PubMed] [Google Scholar]
- 2.Karmiris K, Koutroubakis IE, Xidakis C, Polychronaki M, Voudouri T, Kouroumalis EA. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12(2):100–105. doi: 10.1097/01.MIB.0000200345.38837.46. [DOI] [PubMed] [Google Scholar]
- 3.Thundyil J, Pavlovski D, Sobey CG, Arumugam TV. Adiponectin receptor signalling in the brain. Br J Pharmacol. 2012;165(2):313–327. doi: 10.1111/j.1476-5381.2011.01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tilg H, Moschen AR. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6(10):772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 5.Neumeier M, Weigert J, Schaffler A, et al. Different effects of adiponectin isoforms in human monocytic cells. J Leukoc Biol. 2006;79(4):803–808. doi: 10.1189/jlb.0905521. [DOI] [PubMed] [Google Scholar]
- 6.Perini F, D'Andrea G, Galloni E, et al. Plasma cytokine levels in migraineurs and controls. Headache. 2005;45(7):926–931. doi: 10.1111/j.1526-4610.2005.05135.x. [DOI] [PubMed] [Google Scholar]
- 7.Sarchielli P, Floridi A, Mancini ML, et al. NF-kappaB activity and iNOS expression in monocytes from internal jugular blood of migraine without aura patients during attacks. Cephalalgia. 2006;26(9):1071–1079. doi: 10.1111/j.1468-2982.2006.01164.x. [DOI] [PubMed] [Google Scholar]
- 8.Combs TP, Berg AH, Rajala MW, et al. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes. 2003;52(2):268–276. doi: 10.2337/diabetes.52.2.268. [DOI] [PubMed] [Google Scholar]
- 9.Kosinski M, Bayliss MS, Bjorner JB, et al. A six-item short-form survey for measuring headache impact: The HIT-6. Qual Life Res. 2003;12(8):963–974. doi: 10.1023/a:1026119331193. [DOI] [PubMed] [Google Scholar]
- 10.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peterlin BL, Alexander G, Tabby D, Reichenberger E. Oligomerization state-dependent elevations of adiponectin in chronic daily headache. Neurology. 2008;70(20):1905–1911. doi: 10.1212/01.wnl.0000312278.40250.6e. [DOI] [PubMed] [Google Scholar]
- 12.Simon GE, Von Korff M, Saunders K, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63(7):824–830. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breslau N, Lipton RB, Stewart WF, Schultz LR, Welch KM. Comorbidity of migraine and depression: Investigating potential etiology and prognosis. Neurology. 2003;60(8):1308–1312. doi: 10.1212/01.wnl.0000058907.41080.54. [DOI] [PubMed] [Google Scholar]
- 14.Katsuki A, Suematsu M, Gabazza EC, et al. Decreased high-molecular weight adiponectin-to-total adiponectin ratio in sera is associated with insulin resistance in japanese metabolically obese, normal-weight men with normal glucose tolerance. Diabetes Care. 2006;29(10):2327–2328. doi: 10.2337/dc06-1239. [DOI] [PubMed] [Google Scholar]
- 15.Mangge H, Almer G, Haj-Yahya S, et al. Nuchal thickness of subcutaneous adipose tissue is tightly associated with an increased LMW/total adiponectin ratio in obese juveniles. Atherosclerosis. 2009;203(1):277–283. doi: 10.1016/j.atherosclerosis.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Spranger J, Verma S, Gohring I, et al. Adiponectin does not cross the blood-brain barrier but modifies cytokine expression of brain endothelial cells. Diabetes. 2006;55(1):141–147. [PubMed] [Google Scholar]
- 17.Kos K, Harte AL, da Silva NF, et al. Adiponectin and resistin in human cerebrospinal fluid and expression of adiponectin receptors in the human hypothalamus. J Clin Endocrinol Metab. 2007;92(3):1129–1136. doi: 10.1210/jc.2006-1841. [DOI] [PubMed] [Google Scholar]
- 18.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26(3):439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.