Abstract
Introduction
Intrauterine growth-restriction (IUGR) alters fetal development and is associated with neurodevelopmental abnormalities. We hypothesized that growth-restriction from reduced intrauterine perfusion would predispose neonatal rats to subsequent inflammatory brain injury.
Methods
In the current study, IUGR was achieved by induced placental insufficiency in pregnant rats at 14 days of gestation. IUGR offspring and sham-operated control pups were subsequently injected with intracerebral lipopolysaccharide (LPS) as a model of PVL (perventricular leukomalacia).
Results
LPS similarly elevates proinflammatory cytokines in the brains of both IUGR and control rat pups. However, the chemokines cytokine-induced neutrophil chemoattractant-1 (CINC-1) and macrophage chemoattractant protein-1 (MCP-1), as well as microglia activation, were significantly higher in LPS-treated IUGR rat pups compared to LPS-treated controls. In addition to the unique brain inflammatory response, IUGR rat pups demonstrated enhanced brain damage with an increased number of apoptotic cells, larger lateral ventricular size, and more severe impairment of myelination.
Discussion
This study provides evidence that placental insufficiency may sensitize the innate immune system in the immature brain, and reveals a possible link between brain inflammation and injury.
Introduction
Intrauterine growth-restriction is failure of the fetus to achieve intrinsic growth potential leading to increased morbidity and mortality (1). A disorder of the feto-placental-maternal unit is the major etiology of compromised intrauterine growth in developed societies (2). In asymmetric growth restriction, fetal blood flow is redistributed to the brain resulting in an increase in brain mass relative to an overall reduction in body weight (3). There exists an apparent physical protection of the brain; however, in clinical practice IUGR infants demonstrate an increased risk for neurodevelopmental abnormalities including intracranial injury, cerebral palsy, psychologic disorders, cognitive defects and behavior problems (4,5). Chronic in utero stress and hypoxia associated with reduced intrauterine perfusion results in adaptive responses that can provide enhanced protection or an increased vulnerability to future sublethal insults (6,7). It remains to be elucidated if the brains of IUGR newborns are preconditioned and subsequently more tolerant to severe insults experienced in the perintal period, or sensitized and therefore more vulnerable (8). In our established model, intracerebral injection of lipopolysaccharaide (LPS) causes an inflammatory response in rat brain similar to the pathology seen in periventricular leukomalacia (PVL) (9-11). Clinically, hypoxia-ischemia and infection/inflammation often co-exist and interact with each other. Therefore, we hypothesize that chronic in utero stress from reduced intrauterine perfusion sensitizes the immature rat brain to subsequent inflammatory stress. We demonstrate an exacerbated inflammatory response to LPS in the growth-restricted rat brain that is associated with significantly enhanced brain injury relative to appropriately grown rat pups.
Methods
1. Chemicals
LPS (055:B5) was obtained from Sigma Chemical Co (St. Louis, MO). Antibodies for myelin basic protein (MBP), glial fibrillary acidic protein (GFAP) and ED1, and the TUNEL kit were from Millipore (Temecula, CA); The interleukin-1β (IL-1β), Macrophage chemoattractant protein-1 (MCP-1) and Cytokine-Induced Neutrophil Chemoattractant-1 (CINC-1) ELISA kits were obtained from R&D systems (Minneapolis, MN). Rat Cytokine Antibody Array 2 kit was from RayBiotech (Norcross, CA).
2. Animal models
All experimental procedures performed in this study were in accordance with National Institutes of Health guidelines for the use and care of animals, with approval of all protocols by the Animal Care and Use Committee at the University of Mississippi Medical Center.
A. IUGR model
Timed-pregnant Sprague-Dawley rats were purchased from Harlan Sprague-Dawley Inc (Indianapolis, IN) and housed individually. IUGR was induced by placement of a silver clip around the aorta below the renal arteries in pregnant rats at 14 days of gestation, as described in previously (12). Briefly, rats were anesthetized with 2% isoflorane (Butler Co, Edmonds, WA) in an anesthesia apparatus (Vaporizer for Forane Anesthetic, Ohio Medical Products, Gurnee, OH). A standardized silver clip (0.203-mm internal diameter) was placed around the isolated abdominal aorta above the iliac bifurcation, and two additional silver clips (0.100-mm internal diameter) were placed on both left and right branches of the uterine arteries. Sham-operated pregnant rats without clips served as controls.
All dams delivered at term with birth weights recorded within 12 hours. Overall, mean IUGR birth weight was significantly less than controls (6.1 +/- 0.1 versus 6.7 +/- 0.3 g, P<0.01), respectively, with asymmetric head-sparing growth as previously characterized (6). We defined IUGR as a birth weight less than the 10th percentile below the mean weight of the control rat pups born simultaneously. Control rat pups and previously defined IUGR rat pups were separately size-matched with 8 pups per dam. Growth-restricted pups with a birth weight greater than the defined 10th percentile (approximately 40% of total) and control rat pups with a birth weight less than this value were not utilized.
B. Intracerebral injection of LPS
The current study consisted of 4 experimental groups described as follows: control+saline, control+LPS, IUGR+saline, IUGR+LPS. Intracerebral injection of LPS was performed as previously described on postnatal day 5 (P5) (9,11) Briefly, pups were anesthetized with isoflorane (4% induction, 1.5% maintenance) and placed in a stereotaxic apparatus with an adapter for neonatal rats (David Kopf, Tujunga, CA). The intraventricular injection was performed using a 10 μl syringe using the following coordination: 1.0 mm posterior and 1.0 mm lateral to the bregma, and 3.0 mm deep to the skull surface. LPS (1 μg/animal) or sterile saline at a volume of 2 μl were injected into the left lateral ventricle over a period of 5 min. The intraventricular delivery was confirmed in series coronal frozen sections from the rat brains that were injected with trypan blue. For histological, immunohistochemistry and TUNEL analysis, six animals were included in each treatment group.
3. Cytokine array and ELISA
Whole brain tissue was homogenized with a glass homogenizer in 1ml ice-cold PBS (pH 7.2) with protease inhibitor. After centrifugation at 12,000×g for 20 minutes at 4 °C, the supernatant was collected and total protein was determined by the Pierce BCA method. Protein concentration was adjusted to 1 mg/ml. For the cytokine array, performed per manufacturer instructions, a total of 4 samples from the same treatment group were pooled. Signals were analyzed using ImageQuant software (Molecular Dynamics), and optical density of the target cytokine/chemokine was subtracted from the background and then expressed as a ratio to the positive control. A threshold of 2-fold change was considered to be significant. ELISA was performed following manufacture's instruction. Data was acquired using a 96-well plate reader (Bio-Tek Instruments, Inc., Winooski, VT). The cytokine contents were expressed as pg cytokines/mg total protein.
4. Quantification of histology and immunohistochemistry data
ED1 and GFAP immunostaining and TUNEL was quantified by cell counting. ED1+ and GFAP+ cells were counted in the entire periventricular white matter (including corpus callosum and cingular white matter) and TUNEL+ cells were counted in both the cortex and striatum. Four consecutive coronal sections at bregma level from each animal were selected for analysis. Six digital images (10x objective) from the cortex and periventricular white matter and 12 images from the striatum, which covered most areas with positive cells, were captured for each section. The number of positive cells from four sections were averaged for each animal. Data is presented as the number of cells/view field.
MBP immunohistochemistry was quantified using a grading method for each slide as previously described (11): 0, no staining; 1, detectable weak staining; 2, obvious staining, but less than 3 and stronger than 1; 3, normal strong positive stain. Consistency and unbiased scoring was ensured by evaluation from two different people blinded to treatment, and then averaged.
5. Histologic examination, immunohistochemistry and TUNEL
Fifty frozen consecutive sections (10 μm) were prepared at the level of bregma and dorsal hippocampus. Hematoxylin and eosin (H&E) and Nissl staining was used to examine gross histopathology.
Immunohistochemistry was performed using a standard protocol as previously described (10). After blocking, final concentrations of primary antibodies were diluted as follows: GFAP (1:300), ED1 (1:200), MBP (1:200). Images were captured with a CCD camera (Oly-750, Olympus), and superimposed using the Adobe Photoshop (version 7.0) software, if necessary. TUNEL staining, following the manufacture's instruction, was used to detect cell death.
To compare the size of the lateral ventricles in relation to overall brain size, H&E-stained coronal sections at the bregma level were scanned by a densitometer (Bio-Rad, Richmond,CA) and areas (cm2) of the left and right ventricles and whole brain were measured. Ventricular index size was calculated as the ratio of the area of each ventricle to whole brain.
6. Statistics
Unless otherwise indicated, results are represented as mean +/- SEM. Two Way Analysis of Variation followed by Student-Newman-Keuls test determined statistical significance among treatments, set at a p value of < 0.05.
Results
1. Brain Inflammatory responses to LPS
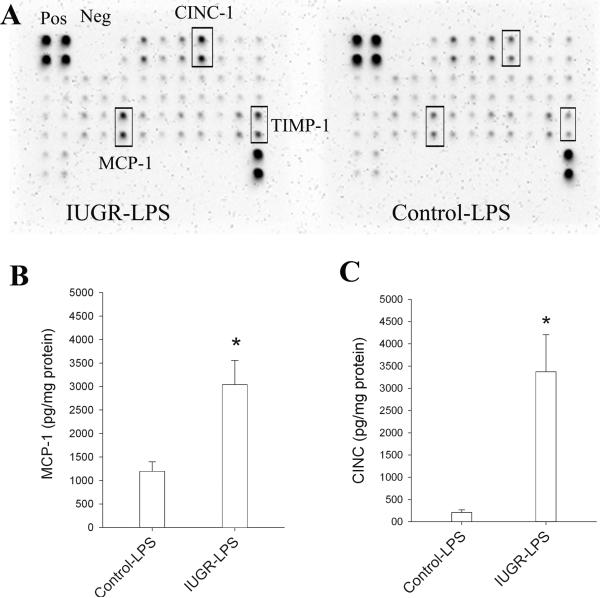
Cytokine array was used to identified differentially expressed cytokines/chemokines in the control and IUGR rats at both 6 and 24 hrs after LPS treatment. Major proinflammatory cytokines such as TNFα, IL-1 β, and IL-6 were elevated in IUGR and control rat pups to the same degree. However, two chemokines, MCP-1 and CINC-1, as well as tissue inhibitor of metalloproteinase-1 (TIMP-1), were significantly higher in IUGR-LPS rat pups compared to control-LPS rats at 24 hrs after LPS injection (Fig 1A). ELISA studies were conducted to evaluate for markers of inflammation as well as confirm and quantify cytokine array results. An increase in expression of the proinflammatory cytokines TNF-α and IL-6 was observed in the control and IUGR rats treated with LPS at both 6 and 24 hours (data not shown). At 24 hrs, IL-1β level was lower in IUGR-LPS compared to control-LPS (control-LPS: 706.8±92.3 pg/mg protein, n=8; IUGR-LPS: 523.6±38.9 pg/mg protein, n=10; Mean±SD); however, it did not reach statistical difference (P= 0.065). The levels of two chemokines, MCP-1 and CINC-1 (rat IL-8), were significantly higher in IUGR-LPS rats compared to control-LPS at 24 hrs. Specifically, the MCP-1 level was about 2.5 times higher in IUGR-LPS rats relative to control-LPS, while the increase in CINC-1 level was more than 16 times greater (Fig 1B&1C, respectively). Proinflammatory cytokines and chemokines were not detected from the brains of control or IUGR rats without LPS treatment.
Fig 1. Inflammatory response in rat brain after LPS injection.
A. Cytokine antibody array was used as an initial step to screen differentially expressed cytokines/ chemokines and quantify as described in Methods. Chemokines: CINC-1 and MCP-1, as well as TIMP-1, were found differentially expressed (in duplicate, labeled in boxes). B. ELISA was performed to further quantify major proinflammatory chemokines and cytokines. There were no significant differences in expression of acute phase cytokines (TNF, IL-1 and IL6) in control-LPS and IUGR-LPS; However, consistent with array data, chemokines MCP-1 and CINC-1 were significantly higher in IUGR-LPS than control-LPS. Proinflammatory cytokines, MCP-1 and CINC were undetectable in control and IUGR rat pups not treated with LPS. *p<0.01 vs control-LPS.
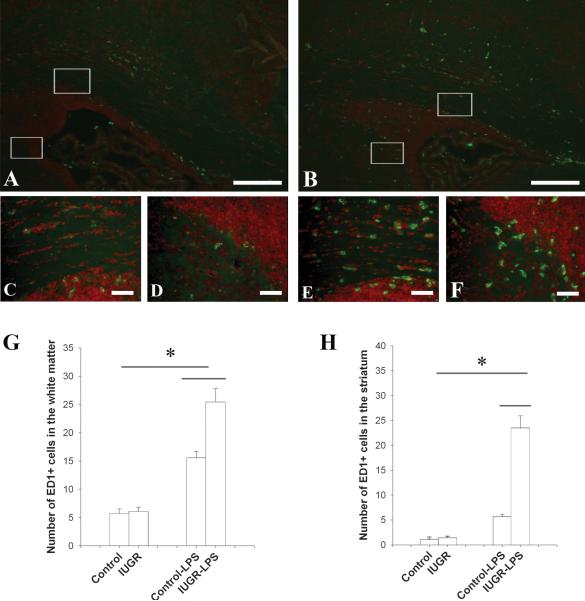
To determine differential responses of the microglia and astrocytes between IUGR and control rats following LPS treatment, immunohistochemistry studies using an ED1 antibody (a marker for activated microglia) revealed that microglia were activated in both control and IUGR rat brains following LPS exposure. However, IUGR-LPS showed marked enhancement of microglia activation compared to control-LPS at 24 hr (Fig.2). Although a few ED1+ cells were present in the periventricular white matter tracts in the brains of both groups without LPS treatment, there was no difference noted between the two (data not shown). Following LPS injection, ED1+ cells increased significantly in both groups, however the IUGR group demonstrated more enhanced activation compared to the control. Moreover, the enhancement demonstrated a regional difference with the striatum being more prominent (4.6 fold increase) than the periventricular white matter (1.6 fold increase; Fig 2 G & H). Enhanced activation of microglia was consistently observed in IUGR-LPS rat brain over time at P8 and P14 (data not shown).
Fig 2. Microglia response in rat brain after LPS injection.
A minimal number of ED1+ cells were detected in both control and IUGR rat brain without LPS exposure at P6. 24 hrs following intracerebral LPS injection, the number of ED1+ cells increased significantly in the periventricular white matter of both the control (A) and IUGR (B) rat brain, however, this response was intensified in IUGR-LPS rat brains. The highlighted areas within the white boxes at the corpus callosum (upper) and the junction between the subventricular zone and striatum (lower) in A were shown in higher magnifications in C and D, respectively, while that in B were shown as E and F. Scale bar: A, B: 200μm, C-F: 50μm. ED1+ cells were counted in the white matter and striatum at P6 and the data are shown in G and H respectively. *p<0.01, IUGR-LPS vs control-LPS; control or IUGR with LPS vs without LPS.
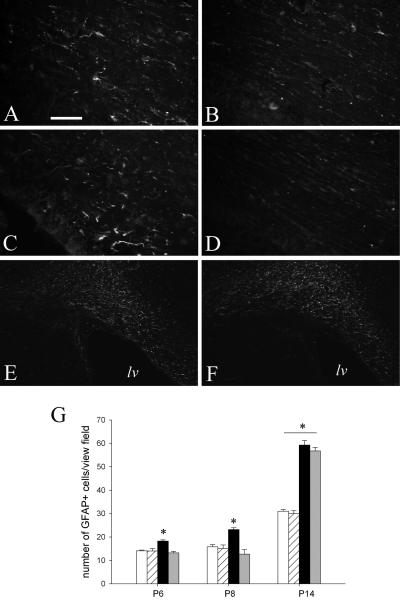
In contrast to a marked increase of microglia activation, astrocyte activation appeared delayed in IUGR rat brain after LPS treatment. There were significantly fewer GFAP+ cells in IUGR-LPS than control-LPS brain at P6 and P8, but this difference was no longer appreciated at P14 (Fig 3).
Fig 3. Delayed reactive astrogliosis in IUGR rat brain following LPS exposure.
Following intracerebral LPS injection, control rat brains demonstrated progressively increased GFAP immunostaining over time (A: P6, C: P8, and E: P14), compared to a delayed astrogliosis in IUGR rat brains that was found only significantly increased at P14 (F), but not at P6 (B) and P8 (D). GFAP+ cells were counted in the periventricular white matter and the data are shown in G (white bar: control; striped bar: IUGR; black bar: control-LPS; and gray bar: IUGR-LPS). Scale bar: A-D: 50μm, E and F: 200μm. lv: lateral ventricle. *p<0.01 control-LPS vs control (P6 and P8); control or IUGR with LPS vs without LPS.
2. Cell death following LPS exposure
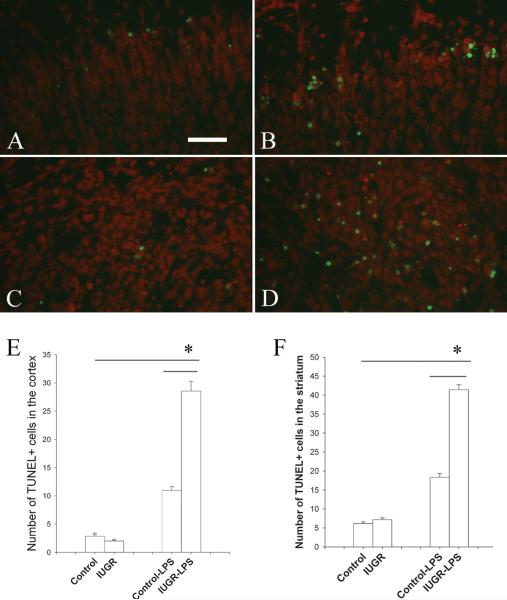
Cell death in response to LPS was examined using the TUNEL method. There were similar numbers of occasional scattered TUNEL positive cells in both groups without LPS treatment, but there appeared to be no difference between these two groups. Consistent with our previous observation, LPS treatment increased the number of TUNEL+ cells in the control rat brain tissue that was primarily located in layer I of the cortex after 24 hr of treatment. However, the increase of TUNEL+ cells in IUGR-LPS group more than doubled compared to control-LPS (p<0.001). In addition to the cortex, TUNEL+ cells were found to be significantly increased in other brain areas, most prominently in the striatum (Fig 4), of the IUGR-LPS rat group.
Fig 4. Cell death in the rat brain after LPS exposure.
TUNEL positive cells (green) with Propidium iodide counterstain (red) was utilized to visualize cell death in P6 rat brain at the bregma level. There was a marked increase of TUNEL+ cells in the cortex (A and B) and striatum (C and D) of both control-LPS (A,C) and IUGR-LPS (B,D) rat brain, but the increase was significantly greater in the IUGR-LPS group (A). Quantification of cell counting data in the cortex and striatum areas are shown in E and F, respectively. *p<0.001, IUGR-LPS vs control-LPS; control or IUGR with LPS vs without LPS.
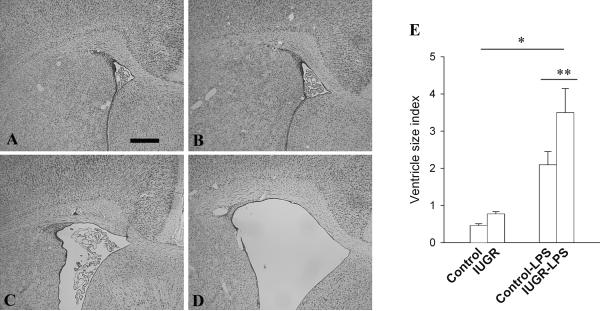
3. LPS-induced lateral ventricle dilation
A consistent gross pathological change in neonatal rat brain following LPS exposure is dilation of the lateral ventricles (13,14). As expected, the lateral ventricles were significantly enlarged in the control rat brain nine days after exposure to LPS (Fig 5). This effect, however, was further exaggerated in IUGR-LPS rats (p<0.05).
Fig 5. Changes of lateral ventricle size in rat brain after LPS injection.
H&E stained coronal sections of P14 rat brain at the bregma level demonstrated a dramatic dilation in lateral ventricle size in both control-LPS (C) and IUGR-LPS (D) rat brains compared to the control (A) and IUGR (B) without LPS exposure. The lateral ventricular (lv) size index at P14 was represented in E. Scale bar: A and B: 500μm, C and D: 200μm *p<0.001 vs control or IUGR with LPS vs without LPS, **p<0.05 IUGR-LPS vs control-LPS.
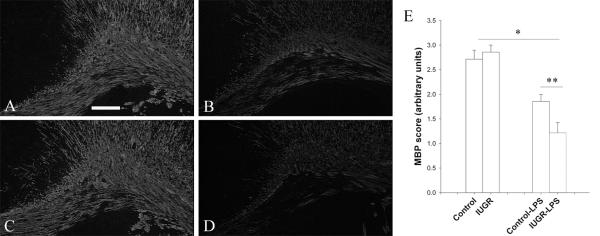
4. Reduction of myelination after LPS treatment
At P14, myelin is extensively detected in the white matter by MBP immunostaining, and the control and IUGR rat brains showed similar intensity and pattern of immunostaining. After LPS exposure, there was a significant decline of MPB immunostaining in both the groups compared to their corresponding non-LPS treated groups. However, IUGR-LPS rat brains showed an enhanced reduction compared to control-LPS (P<0.05, Fig 6).
Fig 6. Detection of myelination in the rat brain by MBP immunostaining.
At P14, control (A) and IUGR (B) rat brains demonstrate extensive baseline myelination of white matter that was significantly impaired by LPS treatment. However, decreased myelination (intensity and surface area) was affected more severely in IUGR-LPS (D) relative to control-LPS (C). Scale bar: 50 μm. A semi-quantitative method (see Method section) was employed to compare changes of myelination between the treatments and data is represented in (E). *p<0.01, control or IUGR with LPS vs without LPS; **p<0.05 IUGR-LPS vs control-LPS
Discussion
PVL is a prevalent form of brain injury in the premature infant that occurs following hypoxic-ischemic and inflammatory conditions (8). Utilizing an established model of neuroinflammation (10), we investigated whether IUGR enhances susceptibility to LPS insult. The major findings from our study indicate that IUGR rat pups produce an augmented inflammatory response and subsequent brain injury compared to control. To our knowledge, this is the first study that suggests placental insufficiency programs the infant brain as more vulnerable to postnatal inflammatory challenges.
Consistent with previous studies (13,14), we show similarly increased expression of proinflammatory cytokines following LPS exposure, but specific chemokines, including MCP-1 and CINC-1, were significantly elevated to a greater degree in growth-restricted rat pups. Activated macrophages/microglia,seen in the pathogenesis of PVL, aggregate towards MCP-1 chemotaxis (15), and their presence was significantly greater in the striatum and perventricular white matter of IUGR+LPS P6 rat brains. CINC-1 is a member of the interleukin-8 chemokine family, and its production by activated glial cells stimulates neutrophil recruitment. Upregulation of this chemokine is also involved in pathologic clinical conditions of brain injury including ischemia, meningitis, acidosis and oxidative stress (16,17).
Activation of glial cells results in complex downstream events, as they serve both protective and destructive roles through the production of trophic and toxic mediators (18). Our results show a different response in glial cells when conditioned with chronic hypoxia in utero followed by inflammatory stress compared to control. As expected, LPS resulted in reactive astrogliosis in both experimental groups similar to previous studies (10,19), and this type of pathologic change is associated with brain injury (20). However, astrogliosis is delayed in the IUGR+LPS group until P14 when it significantly increases and there is no longer a detectable difference relative to control-LPS. In LPS-treated brains from growth-restricted rat pups there is a robust activation of microglia, which affects astrogliosis through the release of cytokines and other soluble factors. In a previous in vitro study of glial cells by Guo (21), hypoxia and reoxygenation with LPS insult potentiated inducible nitric oxide synthase, TNF-alpha, and NFkB-dependent transcription production in microglia, which was not appreciated in astrocytes. However, the same combination of insults led to a substantial increase in a neuroprotective product, hemeoxygenase-1, in astrocytes, but not microglia. Thus, exacerbated activation of detrimental inflammatory products in microglia, and delayed astrogliosis in growth-restricted rat pups could lead to increased brain damage.
Literature supports that severe placental insufficiency causes brain damage, but the degree is affected by the timing, severity, and type of prenatal insult (6,7,22,23). In our model of relatively moderate placental blood flow reduction, there is a lack of abnormal pathology in the brains of growth-restricted rat pups without LPS exposure. This is a strength in our study, which preconditions the neonatal rat and demonstrates the susceptibility of IUGR rat brain to secondary inflammation following LPS treatment.
IUGR rat pups demonstrated region specific damage with an increased number of apoptotic cells primarily localized to the cortex and striatum twenty-four hours following LPS. TUNEL positive cells were not identified in the white matter tracts where oligodendrocytes are in abundance and injured in the pathogenesis of PVL. Knowledge of the basic anatomic regionalization of brain topography led us to believe the TUNEL positive cells were potentially neurons, however, co-localization techniques to identify the apoptotic cells were inconclusive (data not shown).
Prior studies demonstrate a lowered cerebral threshold towards postnatal hypoxic stress in fetal rats subjected to placental insufficiency (22). This vulnerability was related to alterations in the pro and anti-apoptotic mechanisms with decreased Bcl-2 and increased Bax gene expression following hypoxic insult in IUGR rat pups (24). We feel the increased apoptotic cell death in IUGR rat brains may be a result of modified apoptotic pathways and needs further investigation.
In our study, both control and IUGR rat pups demonstrate similar intensity of myelination. A secondary postnatal insult with intracerebral LPS decreased MBP stain in all experimental groups, but the hypomyelination is significantly more profound in IUGR rat pups. Clinical studies of growth-restricted infants suggest altered myelination patterns with increased prevalence of periventricular echodensities on neonatal ultrasounds and increased incidence of cerebral palsy (5,25). The timing of the LPS insult at P5 correlates with a vulnerable period in human neurodevelopment and oligodendrocyte maturation when PVL onset is prevalent (26). Thus, growth-restriction may sensitize immature oligodendrocytes to damage and impaired function following postnatal stress.
Ventriculomegaly is utilized in animal studies as a marker of brain injury, and can result from primary developmental failure or brain cell loss (10). Clinically, increased ventricular size volume correlates well with PVL and impaired cognitive and motor skills (27,28). At baseline, P14 IUGR rat pups trended towards increased ventricular dilation relative to control rat pups. Although this pattern is not statistically significant, Mallard et al demonstrated a significant size increase in the lateral ventricles of growth-restricted guinea pigs following late gestation placental insufficiency (23). Similar to previous studies (10, 18), LPS caused pathologic ventricular dilation in all animals exposed to intracerebral LPS, but the IUGR+LPS group was most profoundly affected. Increased TUNEL positive cells and a paucity of myelin immunostaining in IUGR+LPS suggest that the ventriculomegaly appreciated in these animals is secondary to cell loss and impaired brain structure.
In summary, we demonstrate that intrauterine growth-restriction sensitizes the immature rat brain to produce an amplified inflammatory response after LPS insult. Glial activation and the complex cascade of cytokine and chemokine production is modified in growth-restricted rat pups, which leads to increased apoptotic cell death, impaired myelination, and extensive ventricular dilation. An adverse intrauterine environment during critical stages of growth can modify gene expression and fetal development. There are multiple studies of LPS administration that demonstrate contrasting effects of both increased sensitization and protective preconditioning of the immature brain following hypoxic insult (29-31). However, this study is unique and investigates inflammatory effects only after exposure to prolonged hypoxia in utero.
We conclude that the immature brain in growth-restricted infants is more vulnerable to the consequences of neuroinflammation and activation of the innate immune system by LPS. The combination of chronic hypoxia and LPS-induced neuroinflammation likely works through synergistic pathways with an additive injurious effect on immature rat brains. Our model is clinically relevant, as IUGR infants are at risk for increased morbidity and mortality including infection and neurodevelopmental handicap (32,33). The differential response in IUGR rat pups relates to Barker's hypothesis of fetal programming in which an adverse environment experienced during critical developmental periods can predispose offspring to subsequent morbidities (1). Placental insufficiency creates a state of reduced oxygen availability, malnutrition, and altered endocrine status in the fetus. Delineation of how these stressors influence our results will be a focus of upcoming studies. Increased levels of CINC-1 and TIMP-1 production in growth-restricted rat pups after LPS insult lead us to hypothesize that polymorphonuclear cell recruitment and activation may be a key element in the development of observed brain damage. Additionally, IUGR-LPS rat brains have an increased number of TUNEL positive cells, which suggests alterations in apoptotic pathways of cellular death. Future research should explore the mechanisms and temporal relationship of LPS-induced sensitization in the neonatal IUGR rat brain to provide insight into potential neuroprotective interventions.
Acknowledgments
This study was supported by the NIH funded grant HL074927, and by funds from the Division of Newborn Medicine, University of Mississippi Medical Center
References
- 1.Barker D. In utero programming of chronic disease. Clin Sci. 1998;95:115–28. [PubMed] [Google Scholar]
- 2.Henriksen T, Clausen T. The fetal origins hypothesis: placental insufficiency and inheritance versus maternal malnutrition in well-nourished populations. Acta Obstet Gynecol Scand. 2002;81:112–14. doi: 10.1034/j.1600-0412.2002.810204.x. [DOI] [PubMed] [Google Scholar]
- 3.Laurini R, Laurin J, Manrsal K. Placental histology and fetal blood flow in intrauterine growth retardation. Acta Obstet Gynecol Scand. 1994;73:529–34. doi: 10.3109/00016349409006268. [DOI] [PubMed] [Google Scholar]
- 4.Geva R, Eshel R, Leitner Y, Valevski AF, Harel S. Neuropsychological outcome of children with intrauterine growth restriction: a 9-year prospective study. Pediatrics. 2006;118:91–100. doi: 10.1542/peds.2005-2343. [DOI] [PubMed] [Google Scholar]
- 5.Jarvis S, Glinianaia SV, Torrioli MG, et al. Cerebral palsy and intrauterine growth in single births: European collaborative study. Lancet. 2003;362:1106–11. doi: 10.1016/S0140-6736(03)14466-2. [DOI] [PubMed] [Google Scholar]
- 6.Rees S, Harding R, Walker D. An adverse environment: implications for injury and altered development of the brain. Int J Dev Neurosci. 2007;26:3–11. doi: 10.1016/j.ijdevneu.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Olivier P, Baud O, Bouslama M, Evrard P, Gressens P, Verney C. Moderate growth restriction: Deleterious and protective effects on white matter damage. Neurobiol Dis. 2007;26:253–62. doi: 10.1016/j.nbd.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50:553–62. doi: 10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Cai Z, Pang Y, Rhodes PG. Differential roles of tumor necrosis factor-alpha and interleukin-1 beta in lipopolysaccharide-induced brain injury in the neonatal rat. Brain Res. 2003;975:37–47. doi: 10.1016/s0006-8993(03)02545-9. [DOI] [PubMed] [Google Scholar]
- 10.Pang Y, Cai Z, Rhodes PG. Disturbance of oligodendrocyte development, hypomyelination and white matter injury in the neonatal rat brain after intracerebral injection of lipopolysaccharide. Brain Res Dev Brain Res. 2003;140:205–14. doi: 10.1016/s0165-3806(02)00606-5. [DOI] [PubMed] [Google Scholar]
- 11.Pang Y, Zheng B, Campbell LR, Fan LW, Cai Z, Rhodes PG. IGF-1 can either protect against or increase LPS-induced damage in the developing rat brain. Pediatr Res. 2010;67:579–84. doi: 10.1203/PDR.0b013e3181dc240f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension. 2003;41:457–62. doi: 10.1161/01.HYP.0000053448.95913.3D. [DOI] [PubMed] [Google Scholar]
- 13.Lee SC, Liu W, Dickson DW, Brosnan CF, Berman JW. Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1beta. J Immunol. 1993;150:2659–7. [PubMed] [Google Scholar]
- 14.Cai Z, Pan ZL, Pang Y, Evans OB, Rhodes PG. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr Res. 2000;47:64–72. doi: 10.1203/00006450-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Kadhim H, Tabarki B, Verellen G, De Prez C, Rona AM, Sebire G. Inflammatory cytokines in the pathogenesis of periventricular leukomalacia. Neurology. 2001;56:1278–83. doi: 10.1212/wnl.56.10.1278. [DOI] [PubMed] [Google Scholar]
- 16.Yamagami S, Tamura M, Hayashi M, et al. Differential production of MCP-1 and cytokine-induced neutrophil chemoattractant in the ischemic brain after transient focal ischemia in rats. J Leukoc Biol. 1999;65:744–9. doi: 10.1002/jlb.65.6.744. [DOI] [PubMed] [Google Scholar]
- 17.Katayama T, Tanaka H, Yoshida T, Uehara T, Minami M. Neuronal injury induces cytokine-induced neutrophil chemoattractant-1 (CINC-1) production in astrocytes. J Pharmacol Sci. 2009;109:88–93. doi: 10.1254/jphs.08298fp. [DOI] [PubMed] [Google Scholar]
- 18.Lehnardt S, Lachance C, Patrizi S, et al. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22:2478–86. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lodygensky GA, West T, Stump M, Holtzman DM, Inder TE, Neil JJ. In vivo MRI analysis of an inflammatory injury in the developing brain. Brain, Behavior Immun. 2010;24:759–67. doi: 10.1016/j.bbi.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deguchi K, Oguchi K, Takashima S. Characteristic neuropathology of leukomalacia in extremely low birth weight infants. Pediatr Neurol. 1997;16:296–300. doi: 10.1016/s0887-8994(97)00041-6. [DOI] [PubMed] [Google Scholar]
- 21.Guo G, Bhat NR. Hypoxia/reoxygenation differentially modulates NF-kappaB activation and iNOS expression in astrocytes and microglia. Antioxid Redox Signal. 2006;8:911–8. doi: 10.1089/ars.2006.8.911. [DOI] [PubMed] [Google Scholar]
- 22.Burke C, Sinclair K, Cowin G, et al. Intrauterine growth restriction due to uteroplacental vascular insufficiency leads to increased hypoxia-induced cerebral apoptosis in newborn pigs. Brain Res. 2006;1098:19–25. doi: 10.1016/j.brainres.2006.04.129. [DOI] [PubMed] [Google Scholar]
- 23.Mallard EC, Rehn A, Rees S, Tolcos M, Copolov D. Ventriculomegaly and reduced hippocampal volume following intrauterine growth-restriction: implications for the aetiology of schizophrenia. Schizophr Res. 1999;40:11–21. doi: 10.1016/s0920-9964(99)00041-9. [DOI] [PubMed] [Google Scholar]
- 24.Lane RH, Ramirez RJ, Tsirka AE, et al. Uteroplacental insufficiency lowers the threshold towards hypoxia-induced cerebral apoptosis in growth-retarded fetal rats. Brain Res. 2001;895:186–93. doi: 10.1016/s0006-8993(01)02074-1. [DOI] [PubMed] [Google Scholar]
- 25.Padilla-Gomes NF, Enriquez G, Acosta-Rojas R, Perapoch J, Hernandez-Andrade E, Gratacos E. Prevalence of neonatal ultrasound brain lesions in premature infants with and without intrauterine growth restriction. Acta Paediatr. 2007;96:1582–7. doi: 10.1111/j.1651-2227.2007.00496.x. [DOI] [PubMed] [Google Scholar]
- 26.Craig A, Ling Luo N, Beardsley DJ, et al. Quantitative analysis of perinatal rodent oligodendrocyte lineage progression and its correlation with human. Exp Neurol. 2003;181:231–40. doi: 10.1016/s0014-4886(03)00032-3. [DOI] [PubMed] [Google Scholar]
- 27.Baker LL, Stevenson DK, Enzmann DR. End-stage periventricular leukomalacia: MR evaluation. Radiology. 1988;168(3):809–815. doi: 10.1148/radiology.168.3.3406411. [DOI] [PubMed] [Google Scholar]
- 28.Melhem ER, Hoon AH, Jr, Ferrucci JT, Jr, et al. Periventricular leukomalacia: relationship between lateral ventricular volume on brain MR images and severity of cognitive and motor impairment. Radiology. 2000;214:199–204. doi: 10.1148/radiology.214.1.r00dc35199. [DOI] [PubMed] [Google Scholar]
- 29.Yang L, Sameshima H, Ikeda T, Ikenoue T. Lipopolysaccharide administration enhances hypoxic-ischemic brain damage in newborn rats. J Obstet Gynaecol Res. 2004;30:142–7. doi: 10.1111/j.1447-0756.2003.00174.x. [DOI] [PubMed] [Google Scholar]
- 30.Eklind S, Mallard C, Arvidsson P, Hagberg H. Lipopolysaccharide induces both a primary and a secondary phase of sensitization in the developing rat brain. Pediatric Res. 2005;58:112–6. doi: 10.1203/01.PDR.0000163513.03619.8D. [DOI] [PubMed] [Google Scholar]
- 31.Ikeda T, Yang Y, Ikenque T, Mallard C, Hagberg H. Endotoxin-induced hypoxic-ischemic tolerance is mediated by up-regulation of coricosterone in neonatal rat. Pediatr Res. 2006;59:56–60. doi: 10.1203/01.pdr.0000191140.87314.ce. [DOI] [PubMed] [Google Scholar]
- 32.Simchen MJ, Beiner ME, Strauss-Liviathan N, et al. Neonatal outcome in growth-restricted versus appropriately grown preterm infants. Am J Perinatol. 2000;17:187–92. doi: 10.1055/s-2000-9423. [DOI] [PubMed] [Google Scholar]
- 33.Garite TJ, Clark R, Thorp JA. Intrauterine growth restriction increases morbidity and mortality among premature neonates. Am J of Obstet Gynecol. 2004;191:481–7. doi: 10.1016/j.ajog.2004.01.036. [DOI] [PubMed] [Google Scholar]