Abstract
Cis-trans isomerization of proteins phosphorylated by proline-directed kinases is proposed to control numerous signaling molecules, and is implicated in the pathogenesis of Alzheimer’s and other diseases. However, there is no direct evidence for the existence of cis-trans protein isomers in vivo, or for their conformation-specific function or regulation. Here we develop peptide chemistries that allow the generation of cis and trans-specific antibodies, and use them to raise antibodies specific for isomers of phosphorylated tau. Cis, but not trans, p-tau appears early in the brains of humans with mild cognitive impairment, and accumulates exclusively in degenerated neurons and localizes to dystrophic neurites during Alzheimer’s progression. Unlike trans p-tau, the cis isomer cannot promote microtubule assembly, is more resistant to dephosphorylation and degradation, and is more prone to aggregation. Pin1 converts cis to trans p-tau to prevent Alzheimer’s tau pathology. Isomer-specific antibodies and vaccines may therefore have value for the early diagnosis and treatment of Alzheimer’s disease
INTRODUCTION
The reversible protein phosphorylation on certain serine or threonine residues preceding a proline (pSer/Thr-Pro) is a central signaling mechanism in diverse cellular processes in physiology and disease (Blume-Jensen and Hunter, 2001; Lu et al., 2002; Nigg, 2001). Notably, certain pSer/Thr-Pro motifs in phosphopeptides exist in two distinct cis and trans conformations (Yaffe et al., 1997) and their isomerization is especially important because Pro-directed kinases and phosphatases are cis or trans conformation-specific (Brown et al., 1999; Zhou et al., 2000). Moreover, phosphorylation further slows down their isomerization rate, and also renders the peptide bond resistant to conventional peptidyl-prolyl cis-trans isomerase (PPIases) (Yaffe et al., 1997; Zhou et al., 2000). As a unique PPIase (Lu et al., 1996), Pin1 binds to and isomerizes specific pSer/Thr-Pro motifs derived from a subset of proteins, leading us to hypothesize a novel signaling mechanism, whereby Pin1 catalytically regulates its substrate conformation after phosphorylation to control protein function (Lu et al., 1999b; Ranganathan et al., 1997; Shen et al., 1998; Yaffe et al., 1997; Zhou et al., 2000; Zhou et al., 1999).
Subsequent studies have provided supporting evidence for this new concept of post-phosphorylation conformational regulation (Liou et al., 2011; Lu and Zhou, 2007). For example, Pin1 greatly accelerates isomerization of the APP intracellular domain between the two distinct conformations, as visualized by NMR (Pastorino et al., 2006) and has profound effects on a spectrum of activities in numerous signaling molecules (Girardini et al., 2011; Liou et al., 2011; Lu and Zhou, 2007; Theuerkorn et al., 2011; Tun-Kyi et al., 2011; Yuan et al., 2011). Functionally, Pin1 regulates many cellular processes involving Pro-directed phosphorylation, with an emerging theme that Pin1 often acts on multiple targets to synergistically drive certain cellular processes to one direction (Liou et al., 2011; Lu et al., 2007; Lu and Zhou, 2007). Importantly, Pin1 deregulation contributes to an increasing number of diseases, notably cancer and Alzheimer’s disease (AD) (Butterfield et al., 2006; Lee et al., 2011b; Lu and Zhou, 2007). These Pin1 functions are abolished by catalytically inactivating mutations (Lu and Zhou, 2007) or DAPK1-mediated inhibitory phosphorylation (Lee et al., 2011a), suggesting the importance of Pin1 catalytic activity. However, without a tool to directly detect cis or trans-specific protein conformation in the cell, there is no in vivo evidence for such two conformations for any protein, their conformation-specific function or regulation (Liou et al., 2011; Lu and Zhou, 2007).
The neuropathological hallmarks of AD are tangles made of hyperphosphorylated tau (p-tau) and plaques composed of amyloid beta-peptides (Aβ) derived from amyloid precursor protein (APP) (Ballatore et al., 2007; Goedert and Spillantini, 2006; Mattson, 2004; Spires-Jones et al., 2009). It is increasingly evident that tau pathology in AD may result from the combination of the detrimental effects from losses of tau normal function to promote microtubule (MT) assembly and toxic gains-of-function acquired by p-tau aggregates (Ballatore et al., 2007). A defining early event that disrupts tau MT function and precedes tangle formation and neurodegeneration in AD is increased tau phosphorylation, especially on Ser/Thr-Pro motifs (Ballatore et al., 2007; Goedert and Spillantini, 2006; Mattson, 2004; Spires-Jones et al., 2009). Many kinases or phosphatases are deregulated in AD brains, and modulating these enzymes can affect AD-related phenotypes (Ballatore et al., 2007; Cruz and Tsai, 2004; Dolan and Johnson, 2010). However, it is not clear how such phosphorylation becomes pathogenic and how to control it.
Recently, we have found a pivotal role for Pin1 in protecting against age-dependent neurodegeneration in AD (Lee et al., 2011b). Pin1 binds to and isomerizes the pThr231-Pro motif in tau and the pThr668-Pro motif in APP in vitro (Lu et al., 1999a; Pastorino et al., 2006; Zhou et al., 2000). Furthermore, Pin1 restores p-tau MT function and also promotes p-tau dephosphorylation and degradation (Lim et al., 2008; Liou et al., 2003; Lu et al., 1999a; Zhou et al., 2000). Pin1 also reduces amyloidogenic APP processing and toxic Aβ secretion (Pastorino et al., 2006) as well as promotes pThr668-APP degradation (Ma et al., 2011). Consequently, Pin1 knockout mice develop age-dependent tau- and Aβ pathologies, and neurodegeneration, resembling many aspects of human AD (Liou et al., 2003; Pastorino et al., 2006). By contrast, Pin1 overexpression in postnatal neurons effectively inhibits tau pathology and neurodegeneration in AD mouse models overexpressing human wild-type tau (Lim et al., 2008). Significantly in human brains, Pin1 is highly expressed in most neurons, but is inhibited in MCI (mild cognitive impairment) and AD neurons by multiple mechanisms (Butterfield et al., 2006; Liou et al., 2003; Lu et al., 1999a), whereas the Pin1 SNP that prevents its down-regulation is associated with delayed onset of AD (Ma et al., 2010). Notably, Thr231 phosphorylation is on top of the sequential phosphoepitopes (pThr231→TG3→AT8→AT100→Alz50) during pretangle formation in AD (Luna-Munoz et al., 2007). Thus, Pin1 might accelerate cis to trans isomerization to protect against tau and Aβ pathology in AD (Lu et al., 2007; Lu and Zhou, 2007). However, there is no evidence that Pin1 actually regulates protein conformations in vivo in AD or other processes.
To detect Pin1-catalyzed conformational changes, we have developed novel peptide chemistry to generate the first antibodies (Abs) that can distinguish cis from trans pThr231-Pro conformation in p-tau and provided the first evidence that Pin1 accelerates cis to trans conversion to prevent the accumulation of the pathogenic cis p-tau in AD. Our findings develop the first tool to directly detect cis-trans prolyl isomerization in vivo, and suggest novel conformation-specific vaccines and Abs for treating or even preventing AD at early stages.
RESULTS
Novel Peptide Chemistry Enables to Generate Cis and Trans Conformation-Specific Abs
Proline-directed phosphorylation is a central signalling mechanism in the cell, but it is unknown whether such a phosphoprotein exists in cis and trans conformations in vivo. To address this question, we developed a novel strategy to generate the Abs that can distinguish cis from trans pThr231-Pro conformation in p-tau. NMR analysis showed that the pThr231-Pro motif in a synthetic tau peptide contained only 9% cis (Figure 1A and Figure S1A), making it difficult to produce cis specific Ab. Therefore, our strategy is to immunize rabbits with a modified pThr231-Pro tau peptide that contains a minimal structural change, but has both cis and trans contents high enough to produce cis- and trans-specific Abs, followed by separating them using affinity purification and counter-purification procedures to remove potential contamination with cis and trans locked peptides, respectively (Figure 1B).
Figure 1. Novel Peptide Chemistry Enables to Generate Abs that Distinguish Cis from Trans pThr231-Pro Conformation in p-Tau.
(A) Proline modifications increase the prevalence of the cis isomer, as determined by NMR.
(B) The scheme for Ab purification.
(C, D) Specific recognition of the cis and trans pThr231-Pro tau Abs by cis (pThr231-Dmp) and trans (pThr231-Ala) peptides, respectively, while both Abs recognize wild-type pThr231-Pro (cis+trans) tau peptide.
(E) T231A point mutation abolishes the ability of cis or trans pThr231-Pro tau Ab to recognize tau in SY5Y cells.
(F) Cis and trans-pThr231-Pro tau Abs are tau- and phosphorylation-specific, as shown in Tau-Tg brains after dephosphorylation by CIP or in Tau knockout mouse brains.
(G) Cis and trans pThr231-Pro tau Abs recognize tau in AD brains. White arrows and arrowheads indicate total tau-positive neurons with and without cis or trans pThr231-Pro tau expression, respectively. Red arrows indicate the neurons shown in insets. Yellow arrows point to dystrophic neurites labeled with the cis, and blue arrow to almost exclusive neuronal body localization for the trans.
(H) Cis and trans Abs are conformation-specific with little cross-reactivity in AD brain sections. Only the cis and trans Abs that were pre-absorbed with trans and cis peptides, respectively, show signals. Scale bar, 20 µm.
See also Figure S1.
Since the unique five-membered carbonyl ring of Pro in all natural amino acids renders Pro to adopt cis and trans conformations, we reasoned that replacing the five-membered carbonyl ring of Pro with a six-membered ring having one additional methylene group, as seen in homoproline (Pip), might increase the cis content and also produce Abs that would recognize endogenous tau proteins. To test this hypothesis, we used NMR analysis to determine the cis and trans contents of modified tau peptides. Indeed, a pThr231-Pip tau peptide had ~74% cis (Figure 1A and Figures S1A and S1B). When immunizing rabbits, the pThr231-Pip tau peptide produced Abs that recognized endogenous human and mouse tau (Figures 1E-1H). To separate cis- and trans-specific Abs, we synthesized a pThr231-Dmp (5,5-dimethylproline) tau peptide as a cis locked peptide because Dmp locks a peptidyl-prolyl bond in cis (An et al., 1999), and a pThr231-Ala tau peptide as a pure trans peptide because Ala adopts only trans, as confirmed by NMR (Figure 1A and Figures S1C and S1D).
After Ab purification outlined in Figure 1B, the specificity of resulting cis- and trans-specific Abs was first verified using ELISA. Both cis (Figure 1C) and trans (Figure 1D) Abs specifically recognized their respective cis and trans peptides, with essentially no cross-reactivity. Their calculated Kd values for the respective cis and trans pT231-tau peptides were ~2.1 and 1.6 nM. Importantly, they did not recognize non-phoshorylated Thr231-Pro tau peptide, but both were strongly reactive to a wild-type pThr231-Pro tau peptide, as expected from the fact that cis and trans isomers in phosphopeptides can be inter-changeable relatively easily (Pastorino et al., 2006; Zhou et al., 2000). Thus, cis and trans pThr231-Pro tau Abs are highly conformation-specific in vitro.
Next, to examine whether the cis and trans Abs recognize pThr231 in tau in vivo, we performed immunoblotting and immunostaining analyses. When tau and its T231A mutant were expressed in neuronal SY5Y cells, both Abs recognized wild-type tau, but not its T231A mutant (Figure 1E), similar to ELISA results (Figures 1C and 1D). Furthermore, robust cis and trans immunostaining signals were detected in Tau-Tg mouse brains overexpressing wild-type human tau, but not after dephosphorylation (Lu et al., 1999a) or in tau knockout mouse brains (Figure 1F). When frontal cortical sections from advanced AD patients were double immunostained with the cis or trans polyclonal Ab and monoclonal Ab recognizing total tau, all cis or trans-positive neurons were also positive for total tau, but not all total tau-positive neurons were also positive for either cis or trans p-tau (Figure 1G), indicating that some, but not all, of tau is phosphorylated on Thr231, as expected. In addition, immunostaining signals of both cis and trans pThr231-Pro Abs in AD brains were partially co-localized with those of AT180 (Figure S1E) and TG3 (Figure S1F), two Abs recognizing pT231-containing tau (Jicha et al., 1997). Thus, cis and trans Abs recognize pT231-containing tau (pT231-tau) in vivo.
Finally to determine whether these immunostaining signals are specific to cis or trans pT231-tau, we pre-absorbed the Ab with cis, trans or cis+trans pT231-tau peptide prior to immunostaining on the AD frontal cortex. Staining signals of the cis Ab were abolished by pre-absorption with the cis, but not trans, peptide and vice versa (Figure 1H). Of note, a monoclonal Ab that recognized only the cis pThr231-Dmp tau peptide, but not pThr231-Pro tau peptide in ELISA (Figure S1G) could not detect any immunostaining signal in Tau-Tg mouse brains (Figure S1H). Thus, cis and trans pT231-tau Abs are conformation- and phosphorylation-specific in vitro and in vivo.
Cis, but not Trans, pT231-tau Appears Early at MCI Brains, and further Accumulates and Localizes to Dystrophic Neurites as AD Progresses
In human AD, Thr231 phosphorylation is a very early tau phosphorylation event (Luna-Munoz et al., 2007) and its levels track disease progress (Ewers et al., 2007; Hampel et al., 2001). However, it is not known whether cis, trans or both pT231-tau conformations are elevated. Moreover, there is no information available on whether and which pT231-tau conformation might be detected in human brains with mild cognitive impairment (MCI). Generation of the cis-and trans-specific Abs provides the first opportunity to address these questions.
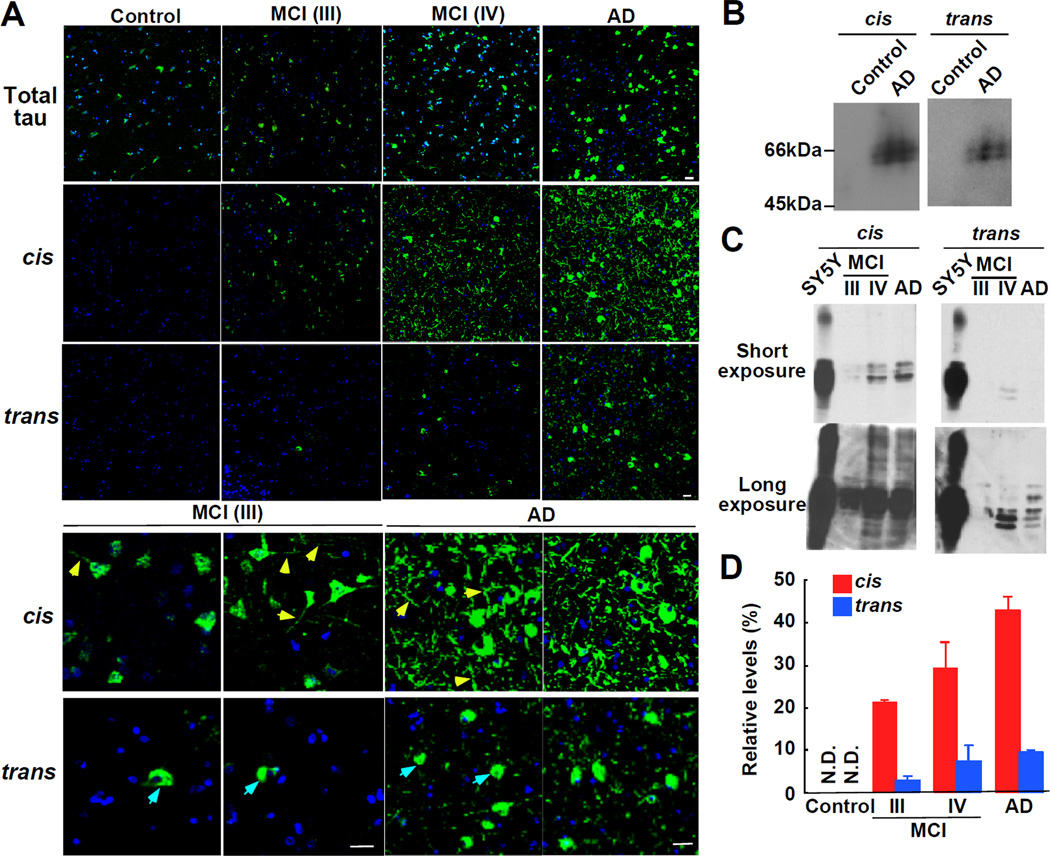
Immunostaining of human frontal cortical sections revealed that tau protein was readily detected in normal neurons and total amounts were increased with progression of neurodegeneration, especially in AD brains (Figure 2A). Although very little, if any, cis or trans pT231-tau signals were detected in 9 normal brains, they, especially cis, were dramatically increased in 11 AD brains (Figure 2A). Strong cis, but not trans, pT231-tau was detected in 4 out of 6 MCI cases (Figure 2A), with weaker, but clearly detectable cis signals in remaining 2 MCI cases (data not shown).
Figure 2. Cis, but not Trans, pT231-tau Appears Early at MCI Brains, and further Accumulates and Localizes to Dystrophic Neurites as AD Progresses.
(A) There was little, if any, cis or trans pT231-tau signal in age-matched normal brains, but they, especially cis conformation, were dramatically increased in AD brains. Strong cis, but not trans, pT231-tau was detected in MCI cases. Note, many dystrophic neurites (yellow arrows) are labeled with the cis, but not the trans, which is almost exclusively located at neuron bodies (blue arrows). Scale bars, 20 µm.
(B-D) The cis is much more abundant than the trans, especially in MCI. Frontal cortex lysates from AD and age-matched normal control (B), Braak stages III and IV (MCI) and Braak stages V and VI (AD) (C) were subjected to immunoblotting analysis with cis and trans Abs, with short and long exposures being shown in top and bottom panels, respectively. Intensities of cis or trans signals of tau-overexpressed SY5Y cells and human brain tissues at each Braak stage were semi-quantified, and the percentages of the signals of human samples relative to that of SY5Y cells were expressed as means ± SE (D).
See also Figure S2.
To confirm the above results, we performed immunoblotting analysis of the frontal cortical lysates obtained from 9 normal controls with Braak stages I and II, 6 MCI patients with Braak stages III and IV, and 11 AD patients with Braak stage V and VI to semi-quantify their cis and trans pT231-tau contents. We used cis and trans p-tau in tau-overexpressing SY5Y neuronal cells as relative standards for comparison. Again, although neither cis nor trans was detected in normal brains (Figure 2B), cis pT231-tau was significantly elevated in MCI brains and further accumulated as the Braak stage progresses, with much smaller increases in trans p-tau (Figures 2C and 2D). These results suggest that cis pT231-tau is specifically elevated in MCI and AD brains. Of note, the ability of immunostaining and immunoblotting to detect cis and trans pT231-tau conformations is not unusual because many Abs raised against human AD paired helical filaments recognize AD-specific abnormal p-tau conformations in immunostaining and immunoblotting (Davies, 2000). Furthermore, certain Ser/Thr phosphorylation causes a dramatic mobility shift in SDS gels, as shown in Cdc25C (Shen et al., 1998). Moreover, both cis and trans signals were detected by immunoblotting under denatured and native conditions (Figure S2A).
The above results suggest that cis pT231-tau might be more pathologically relevant, which is further supported by the strikingly different subcellular localization between cis and trans p-tau in MCI and AD brains. Although both p-tau conformations were found in neuron bodies in the frontal cortex, only cis pT231-tau was detected in neurites at MCI and AD brains, with more and more being accumulated in dystrophic neurites as the Braak stage increased (Figures 1G and 2A and Figure S1F, yellow arrows). Notably, p-tau in dystrophic neurites was also recognized by the pT231 Ab AT180 (Figure S2B) and total tau Ab DC25 (Figure S2C), with cis and AT180 signals being co-localized in the neurites (Figure S1E and S2C). In sharp contrast, there was almost no or very little trans pT231-tau in dystrophic neurites even in advanced AD brains (Figures 1G and 2A and Figure S1F, blue arrows). Similar results were also confirmed using Abs raised in a different rabbit (Figure S1I). This different localization pattern might be pathologically significant given that missorting of p-tau to neurites and synaptic loss in the frontal cortex are early hallmarks of AD and are highly correlated with cognitive loss in AD patients (Davies et al., 1987; DeKosky and Scheff, 1990; Scheff et al., 1990). These results not only confirm the specificity of the cis and trans Abs, but also indicate that only cis, but not trans, pT231-tau is localized to pathological relevant dystrophic neurites.
Trans, but not Cis, pT231-tau Promotes Microtubule Assembly and Pin1 Converts Cis to Trans to Restore the Ability of Cis pT231-tau to Promote Microtubule Assembly
The above results suggest that cis, but not trans, pT231-tau might be pathologically more relevant to MCI and AD. We wondered whether cis and trans p-tau conformations might have any differences in biological functions or biochemical properties relevant to tau pathology in AD, and whether they might be regulated. Pin1 is the only enzyme known to isomerize the pThr231-Pro motif in tau peptide (Zhou et al., 2000), to restore the ability of pT231-tau to promote MT assembly (Lu et al., 1999a) and to promote pT231-tau dephosphorylation and degradation (Lim et al., 2008; Liou et al., 2003; Lu et al., 1999a; Zhou et al., 2000). Finally, Pin1 is inhibited by multiple mechanisms in human MCI and AD neurons (Lee et al., 2011b). Indeed, Pin1 levels were much lower in AD brains (Figures S3A and S3B), even in MAP2-positve AD neurons (Figure S3C), suggesting that lower Pin1 is unlikely due to cell death. Thus, Pin1 might protect against tau pathology in AD by preventing the accumulation of the pathogenic cis p-tau.
To examine this possibility, we first examined whether Pin1 would increase cis to trans isomerization of pT231-tau in vitro. Both cis and trans pThr231-Pro signals were detected in tau, but not its T231A mutant (Figures 3A-3C). Importantly, adding Pin1 significantly increased trans, but reduced cis pT231-tau content. In this assay, there may be two distinct functions embodied in Pin1: acceleration of cis to trans isomerization of the pThr231-Pro motif by the PPIase domain (Pastorino et al., 2006; Yaffe et al., 1997; Zhou et al., 2000) and trans-specific binding of the pThr231-Pro motif to the WW domain (Wintjens et al., 2001). When Pin1 was added, free trans would bind to the WW domain, depleting free trans. The PPIase would greatly accelerate cis to trans isomerization to maintain their equilibrium. The overall effect of Pin1 is to increase the total amount of trans relative to cis. Consistent with this notion, WW domain mutant W34A Pin1 and PPIase domain mutant K63A Pin1 did not change the content of cis or trans (Figures S3D). Thus, Pin1 catalyzes cis to trans isomerization of pT231-tau in vitro.
Figure 3. Trans, but not Cis, pT231-tau Promotes MT Assembly and Pin1 Converts Cis to Trans to Restore the Ability of Cis pT231-tau to Promote MT Assembly.
(A-C) Addition of Pin1 to p-tau decreases cis, but increases trans pThr231-Pro levels. Recombinant tau (A, B) and its T231A mutant (B) were phosphorylated by Cdc2 or control buffer and then incubated with Pin1 before subjecting to immunoblotting analysis with conformation-specific Abs, with the relative signal intensities being quantified (C).
(D-I) Trans, but not cis, pT231-tau promotes MT assembly. FITC-labeled tubulin was treated with wild-type or T231A mutant tau, p-tau, p-tau+PP2A or p-tau+Pin1, followed by assaying MT assembly using confocal microscope (D, E). Addition of Pin1 or PP2A restored the ability of p-tau to promote MT assembly (D, G). The ability of T231A-p-tau to promote MT assembly was not affected by Pin1 addition (E, H). Incubation of Pin1-treated p-tau with the trans, but not cis Ab blocked MT assembly (F, I). MT assembly of each treatment relative to that of p-tau (G, H) and p-tau+Pin1 (I) was quantified.
See also Figure S3.
We next examined whether such Pin1-catalyzed cis to trans isomerization would affect the ability of p-tau to promote MT assembly, a major tau function that is lost in AD (Ballatore et al., 2007), using FITC-labeled tubulin, as described (Lu et al., 1999a; Nakamura et al., 2001). As expected, MT assembly was greatly increased by tau, but not Cdc2 phosphorylated tau, which was restored by PP2A (Figures 3D and 3G). Furthermore, Pin1 effectively restored the ability of p-tau to promote MT assembly (Figures 3D and 3G). In contrast, Cdc2-treated T231A tau could still promote MT assembly and its ability to affect MT function was not affected by Pin1 (Figures 3E and 3H), indicating the essential role of T231 phosphorylation for Pin1 to regulate p-tau. These results confirm the previous findings that phosphorylation of tau on Thr231 by Cdc2 disrupts its ability to promote MT assembly (Lu et al., 1999a), that Pin1 restores the MT function of pT231-tau (Lu et al., 1999a), and that the ability of Pin1 to regulate MT function depends on T231 phosphorylation (Lim et al., 2008; Zhou et al., 2000). More importantly, the ability of Pin1 to restore p-tau MT function was fully blocked by incubation of Pin1-treated p-tau with trans Ab, but not cis Ab (Figures 3F and 3I). This effect of the trans Ab was dependent on Pin1 action because application of trans or cis Ab to p-tau without Pin1 did not affect MT assembly (Figure S3E). Thus, cis, but not trans, p-tau loses normal function to promote MT assembly and Pin1 catalyzes cis to trans isomerization to restore p-tau MT function.
Cis p-Tau Is more Resistant to Protein Dephosphorylation and Degradation, and also more Prone to Protein Aggregation than the Trans
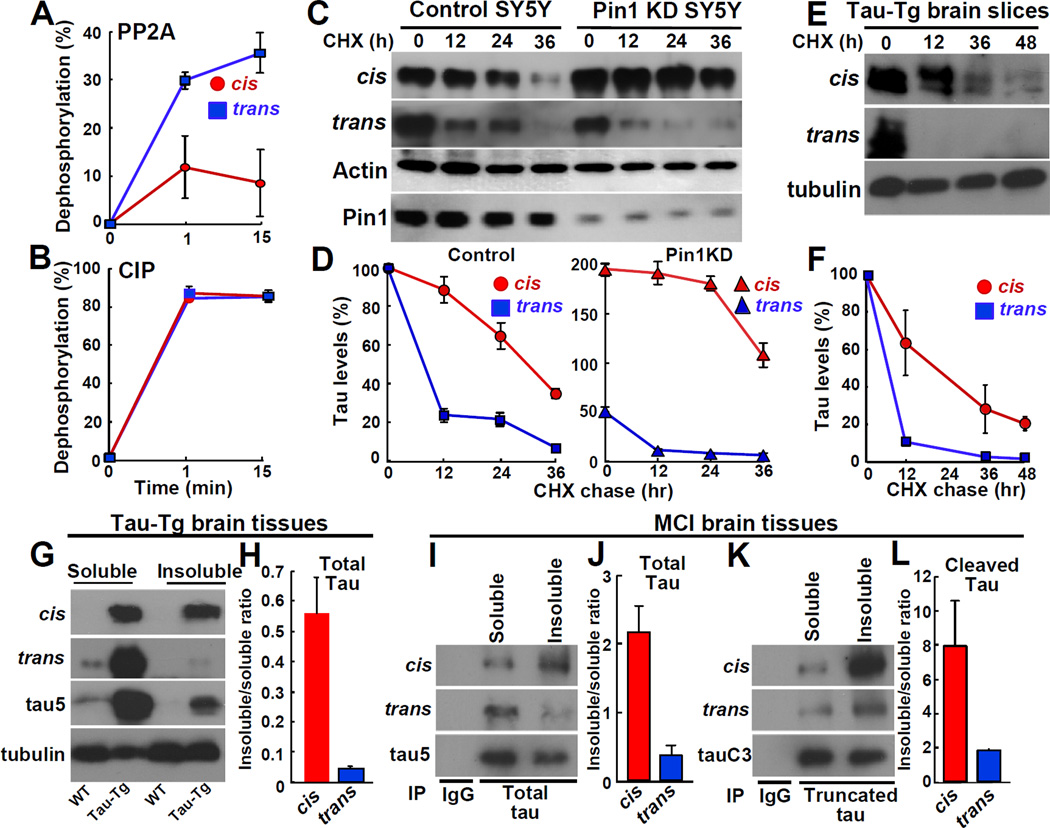
In addition to loss of tau normal function, p-tau in AD is more resistant to dephosphorylation and degradation, and more prone to protein aggregation, resulting in toxic gains-of-function (Ballatore et al., 2007). PP2A is a major Pro-directed Ser/Thr phosphatase in brain lysates that preferentially dephosphorylates pSer/Thr-Pro motifs in trans in synthetic pT231-tau peptides, as shown using chymotrypsin to chemically remove the trans conformation (Zhou et al., 2000). To directly examine whether cis pT231-tau is specifically resistant to dephosphorylation by PP2A, we used the conformation-specific Abs. Trans, but not cis, pThr231-Pro tau peptide was readily dephosphorylated by PP2A (Figure 4A), but they both were rapidly dephosphorylated by CIP (Figure 4B), a calf intestinal alkaline phosphatase that has no conformation-specificity towards its substrates (Zhou et al., 2000). Moreover, cis locked pThr231-Dmp tau peptide was much more resistant to dephosphorylation by PP2A than trans pThr231-Pro tau peptide (Figure S4A), although it was robustly dephosphorylated by CIP (Figure S4B). These results provide further evidence that PP2A is a Pro-directed phosphatase preferentially dephosphorylating pSer/Thr-Pro motifs in trans (Zhou et al., 2000).
Figure 4. Cis pT231-tau Is More Resistant to Protein Dephosphorylation and Degradation, and More Prone to Protein Aggregation than the Trans.
(A-B) Cis pT231-tau is more resistant to dephosphorylation by PP2A, but not CIP.
(C-D) Cis pT231-tau is more stable than the trans and Pin1 KD increases cis, but reduces trans in neuronal cells.
(E, F) Cis pT231-tau is more stable than the trans in brain slice cultures of Tau-Tg mice.
(G-L) Cis pT231-tau is more prone to protein aggregation than the trans in Tau-Tg mouse brains
(G, H) and human MCI brains (I-L) for total tau (tau5) (I, J), or cleaved tau (tauC3) (K, L), as determined by the sarcosyl fractionation. The amounts of cis and the trans in the insoluble fraction relative to the soluble fraction were quantified (H, J, L).
See also Figure S4.
Since Thr231 phosphorylation increases tau protein stability and aggregation (Lim et al., 2008), we next compared protein stability of cis and trans pT231-tau in neuronal cells and mouse brains using the cycloheximide chase (Lim et al., 2008). Cis p-tau was much more stable than the trans; after 12 hr, cis p-tau was not reduced, whereas trans p-tau was reduced by ~75% (Figures 4C and 4D). Given the ability of Pin1 to increase trans but decrease cis in p-tau protein (Figures 3A and 3C), we might expect that Pin1 knockdown (Pin1 KD) would increase cis, but decrease trans p-tau levels, as well as further increase cis p-tau stability. Indeed, at time 0, cis increased, but trans decreased in Pin1 KD cells, as compared to control cells (Figures 4C and 4D). Furthermore, cis was significantly more stable and had a longer half-life than trans in Pin1 KD SY5Y cells than that in control cells (Figures 4C and 4D). Essentially the same results were obtained when inhibitors of CDKs, JNKs and GSK-3, which are implicated to phosphorylate tau on Thr231, were added together with CHX to assess preexisting p-tau (Figure S4C). In brain slice cultures from Tau-Tg mice, cis p-tau had a longer half-life than the trans, being ~24 vs. ~6 hr (Figures 4E and 4F). Thus, cis p-tau is more stable than the trans in vitro and in vivo, and Pin1 converts cis to trans to promote p-tau turnover.
To examine the effects of p-tau conformations on its aggregation, we compared the contents of cis and trans pT231-tau in sarcosyl-soluble and -insoluble fractions. In Tau-Tg mouse brains, trans p-tau was almost all in the soluble fraction, with very little, if any insoluble trans (Figures 4G and 4H). However, cis p-tau was found almost equally between the insoluble and soluble fractions (Figures 4G and 4H). Similar results were also obtained in human MCI brains. As compared with trans p-tau, the cis was found much more in the insoluble fraction than in the soluble fraction for total p-tau and cleaved p-tau (de Calignon et al., 2010) (Figures 4I-4L), indicating that cis p-tau is more prone to protein aggregation.
The above results suggest that cis pT231-tau in Tau-Tg mouse brains and human AD brains might be stable under denatured condition. Indeed, cis pT231-tau in the insoluble fraction of Tau-Tg mouse brains was recognized by cis, but not trans, Ab in immunoblotting not matter SDS and boiling were used or not (Figure S4D). Furthermore, when we used cis Ab to immunoprecipitate cis pT231-tau from the insoluble fraction of AD brains, where cis pT231-tau exhibited a range of molecular weights bigger than IgG (Figures S4E and S4F), it was detected only by cis and total tau Abs, but not by trans Ab (Figure S4F). Thus, cis p-tau is more resistant to dephosphorylation, degradation and more prone to aggregation than the trans.
Cis, but not Trans, pT231-tau Is not only Correlated with Reduced Pin1 Levels, but also Fully Overlapped with Neurofibrillary Degeneration in the AD Hippocampus
The above results indicate that cis, but not trans, pT231-tau not only localizes to the pathologically relevant subcellular location, but also displays both losses of tau normal function as well as gains of toxic function. A critical question is whether Pin1 regulates the content of the cis and trans pT231-tau in neurons in vivo.
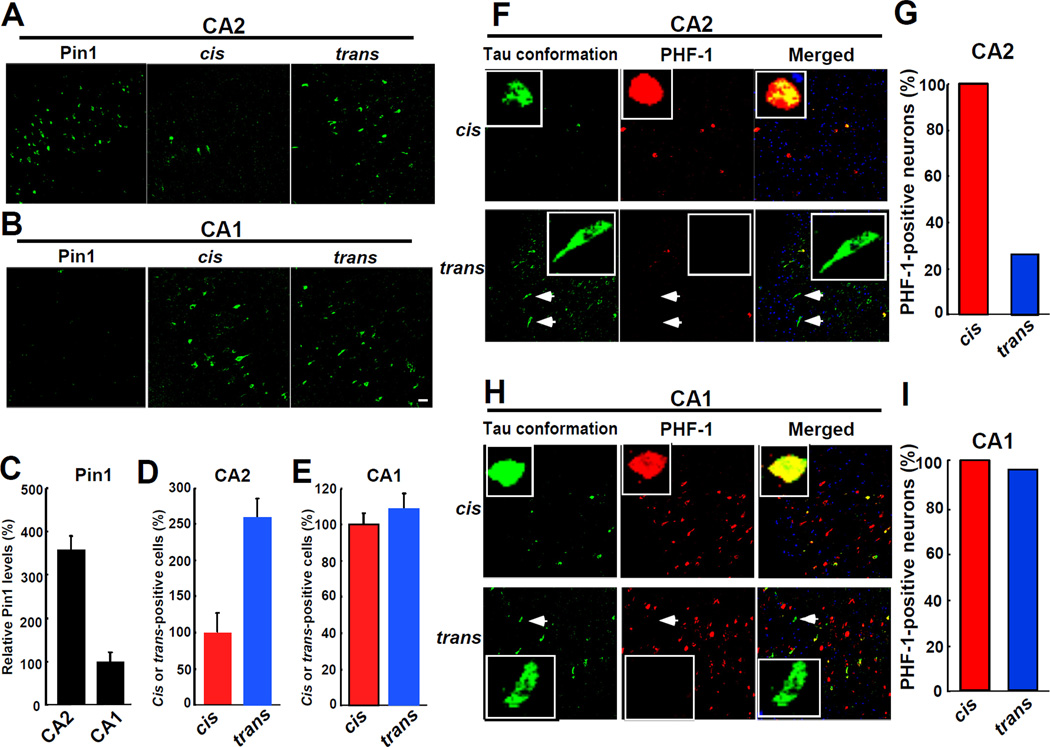
To address this question, we started with investigating the relationship between Pin1 levels and cis or trans pT231-tau levels in subregions of the AD hippocampus. As shown (Liou et al., 2003), we found that Pin1 was highly expressed in the CA2 region, but was dramatically reduced in the CA1 region (Figures 5A-5C, and Figure S5A). Furthermore, neurons that were positive for PHF-1, a solid marker of neurofibrillary neurodegeneration in AD, was prevalent in the CA1, but not CA2 region (Figures 5F and 5H and Figures S5B and S5C), as documented (Liou et al., 2003). Importantly, in the CA2 region where Pin1 was highly expressed, trans-positive neurons were dominant and only few cis-positive neurons were detected (Figures 5A and 5D and Figure S5A). However, in the CA1 region where Pin1 was barely expressed, cis-positive neurons were greatly increased with the number similar to that of trans-positive neurons (Figures 5B and 5E and Figure S5A). The Pin1-catalyzed cis-trans conversion in vivo is likely because both cis and trans forms were present in the same neurons, as demonstrated by immunostaining of two mirror sections of the AD hippocampus with cis or trans Ab and total tau Ab as a common indicator (Figure S5D).
Figure 5. Cis, but not Trans, pT231-tau Is not only Correlated with Reduced Pin1 Levels but also fully Overlapped with Neurofibrillary Degeneration in the AD Hippocampus.
(A-E) Cis, but not trans, pT231-tau is correlated with reduced Pin1 levels. CA2 (A) and CA1 (B) subregions of the AD hippocampus were immunostained with Pin1 and cis or trans Ab. Relative Pin1 levels (C) and the numbers of cis or trans positive neurons in the CA2 (D) or CA1 (E) region are quantified. In the CA2 subregion where Pin1 was highly expressed, trans-positive neurons were dominant (A, C, D), but in the CA1 subregion where Pin1 was barely expressed, both cis- and trans-positive cells were found (B, C, E).
(F-I) Cis, but not trans, pT231-tau signals are fully overlapped with PHF-1 signals. Double immunostaining images of cis or trans Ab with PHF-1 in the CA2 (F, G) and CA1 (H, I) regions showed that all cis-positive cells were also PHF-1 positive in both CA2 and CA1 regions, but 74% of trans-positive cells in the CA2 region were not positive for PHF-1. Arrows indicate trans-positive and PHF-1-negative neurons. Scale bars, 20 µm.
See also Figure S5.
More importantly, almost all cis-positive cells were also positive for PHF-1 in both CA2 (Figures 5F and 5G) and CA1 regions (Figures 5H and 5I). However, 74% of trans-positive cells in the CA2 region were negative for PHF-1 (Figures 5F and 5G), with only 26% trans-positive cells being positive, which might be expected because Thr231 is unlikely to be the only phosphorylation site that contributes to neurofibrillary neurodegeneration in AD. Thus, cis, but not trans, pT231-tau is fully overlapped with neurofibrillary degeneration and also correlated with reduced Pin1 levels in the AD hippocampus.
Whereas Pin1 Overexpression Increases Cis to Trans Conversion of the pT231-Tau, Pin1 Knockout Decreases the Conversion in AD Mouse Models
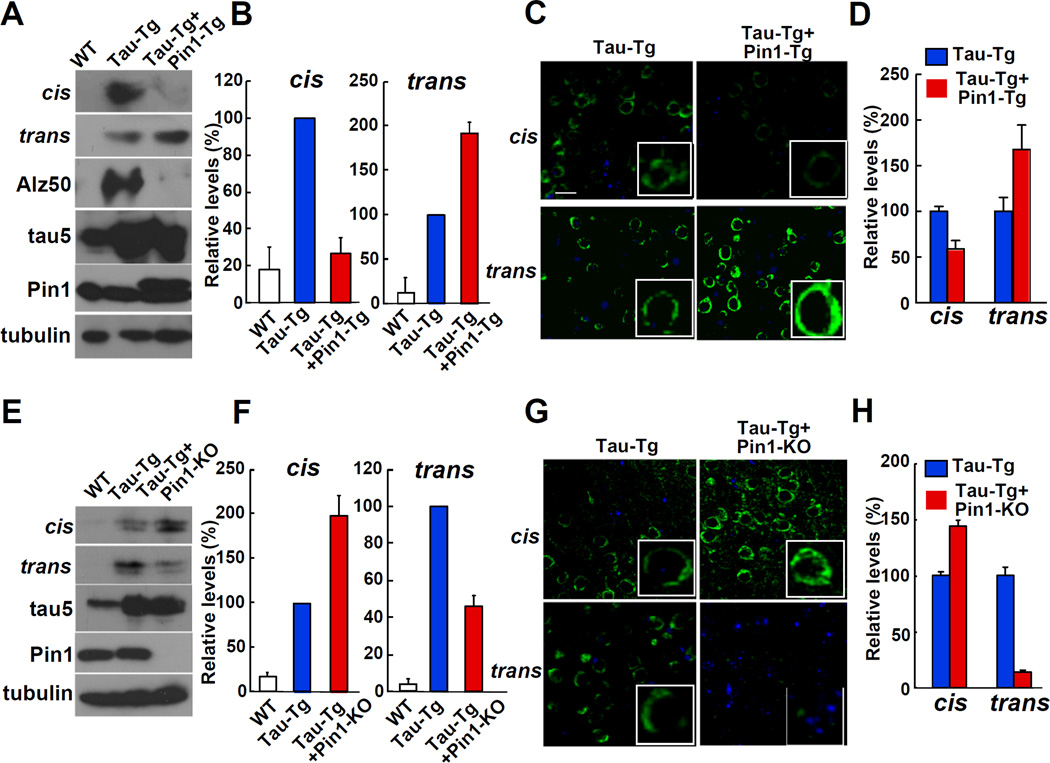
The above results suggest that Pin1 might prevent the accumulation of the pathogenic cis pT231-tau in the brain. To directly test the possibility, we examined the effects of Pin1 overexpression in postnatal neurons on the cis and trans contents of pT231-tau by crossing Thy1-Pin1 transgenic (Pin1-Tg) mice with Tau-Tg mice (Ishihara et al., 1999; Lim et al., 2008). Indeed, Pin1 overexpression significantly increased trans pT231-tau, but reduced cis pT231-tau, when compared with littermates that only overexpressed tau, as normalized using total tau (tau5 Ab) (Figures 6A and 6B) or total protein (tubulin Ab) (Figure S6A). These results were further confirmed by immunostaining on the cerebral cortex (Figures 6C and 6D). Thus Pin1 overexpression reduces cis, but increases trans pT231-tau levels in mouse brains, consistent with its ability to increase cis to trans conversion in vitro (Figures 3A and 3C) and to suppress neurofibrillary neurodegeneration in mice (Lim et al., 2008).
Figure 6. Whereas Pin1 Overexpression Increases the Cis to Trans Conversion of pT231-Tau, Pin1 Knockout Reduces the Conversion in AD Mouse Models.
(A-D) Pin1 overexpression decreases cis pThr231-Pro tau, but increases trans pT231-tau in tau transgenic mouse brains. Both immunoblotting (A, B) and immunostaining (C, D) analyses of the cerebral cortex of wild-type littermates (WT), tau transgenic (Tau-Tg), and tau and Pin1 double transgenic (Tau-Tg+Pin1-Tg) mice showed higher trans pThr231-Pro tau, but lower cis pT231-tau signals in Tau-Tg+Pin1-Tg than those in Tau-Tg mice.
(E-H), Pin1 knockout increases cis pThr231-Pro tau, but decreases trans pT231-tau in tau transgenic mouse brains. Both immunoblotting (E, F) and immunostaining (G, H) analyses of the cerebral cortex of mice revealed higher cis pThr231-Pro tau, but lower trans pT231-tau signals in Tau-Tg and Pin1 KO mice (Tau-Tg+Pin1-KO) than those in Tau-Tg mice. Scale bars, 20 µm.
See also Figure S6.
To finally determine whether endogenous Pin1 is a rate-limiting factor that controls the levels of cis and trans pT231-tau conformations in vivo, we determined the effects of Pin1 knockout on cis and trans contents of pT231-tau by crossing Pin1 knockout mice with Tau-Tg mice. Both immunoblotting (Figures 6E and 6F and Figure S6B) and immunostaining (Figures 6G and 6H) analyses showed that loss of Pin1 function significantly increased cis pT231-tau, but decreased trans pT231-tau in brains. These results are consistent with the ability of Pin1 knockout to induce tau pathology and neurodegeneration (Liou et al., 2003), directly opposite to Pin1 overexpression (Lim et al., 2008). These results were also confirmed in a condition where the intensities of the cis and trans signals are almost identical between different Tau-Tg mouse groups (Figure S6C). These results together indicate that cis, but not trans, pT231-tau is the pathogenic conformation in MCI and AD, but is converted by Pin1 to the non-pathological trans, providing the first structural evidence how the isomerase Pin1 protects against AD tau pathology.
DISCUSSION
Pin1-catalyzed cis-trans isomerization of pSer/Thr-Pro motifs is widely proposed to regulate many physiological and pathological processes, but there is no direct evidence in vivo. We have developed a novel technology to generate novel cis and trans specific Abs to provide the first evidence that cis, but not trans, pT231-tau is pathogenic in MCI and AD and that Pin1 protects against tau pathology by converting it to the non-toxic trans. These results provide the first tool to study cis-trans prolyl isomerization and their conformation-specific function and regulation, and suggest novel conformation-specific diagnoses and therapies for AD and other diseases.
Conformation-Specific Abs as a First Tool to Study Cis-Trans Prolyl Isomerization
Emerging evidence suggests that both phosphorylation-dependent and -independent cis-trans prolyl isomerization functions as a new molecular timer in many biological and pathological processes (Lu et al., 2007; Theuerkorn et al., 2011). Most of these studies are based on some structural analyses in vitro, in combination with mutational analyses in cells. Probably the most direct evidence so far to document cis- and trans-specific function is to replace the key Pro8 in the neurotransmitter 5-HT3 receptor with unnatural Pro analog having varying cis or trans preference and then assay 5-HT3 activity in Xenopus oocytes, leading the conclusion that cis-trans isomerization switches on or off the channel (Lummis et al., 2005). However, the functions of endogenous cis and trans proteins still remain to be determined.
To apply the above approach to study the conformational regulation after phosphorylation would be challenging, if not impossible, because such Pro analog motifs are unlikely to be phosphorylated in vivo to become Pin1 substrates (Brown et al., 1999). To detect Pin1-catalyzed protein conformational changes, we reasoned that Abs would only recognize either cis or trans, but not both of a pSer/Thr-Pro motif in a protein based on the completely distinct structures of these two conformations in pThr688-Pro-containing APP peptides, as revealed by NMR (Pastorino et al., 2006).
A key step to develop such conformation- and phospho-specific Abs is to increase the cis content of pSer/Thr-Pro motifs in the antigen with the smallest change possible because ~90% of pSer/Thr-Pro motifs in a synthetic peptide are in trans (Lu et al., 2007). We have here discovered that simply increasing the Pro ring by one methylene group in a pT231-tau peptide is sufficient to dramatically increases the cis content to 74%, and importantly, to generate phospho-specific Abs that recognize endogenous phosphoproteins. Moreover, we have developed cis or trans locked peptides to purify cis and trans-specific Abs. The resulting Abs turn out to be highly specific with little cross-reactivity. We have since used this technology to successfully generate conformation-specific Abs against several other proteins (data not shown). Albeit the development of an antibody-independent identification of cis and trans protein conformations in vivo will be useful, we believe that this approach can be widely used to study cis and trans specific function and regulation of a peptidyl-prolyl bond, including a non-phosphorylated one.
Conformation-Specific Localization, Biological Function and Pathological Significance in AD
We have uncovered for the first time the striking differences in the subcellular localization, biological function and pathological significance of cis and trans p-tau conformations in AD (Figure 7). Although neither cis nor trans pT231-tau is detected in the healthy brain, cis, but not trans, p-tau appears early in MCI neurons and further accumulates as AD progresses. Furthermore, cis, but not trans, p-tau is fully associated with neurofibrillary degeneration and also localizes to the pathologically relevant dystrophic neurites in AD brains. Moreover, cis, but not trans, p-tau not only loses tau normal function, but also gains tau toxic function, two major properties that are known to contribute to tau pathology in AD (Ballatore et al., 2007). These results indicate that cis, but not trans, p-T231-tau is pathogenic in AD.
Figure 7. Pin1 Prevents the Accumulation of the Pathogenic Cis pT231-tau Conformation in AD by Converting It to the Non-pathogenic Trans, and Vaccines or Abs Specifically Targeting Cis pT231-tau Might Be Developed for Early Diagnosis and Treatment of AD.
pT231-tau protein exists in the two completely distinct cis and trans conformations, as depicted in cartoons of the primary backbone structures. Cis, but not trans, pT231-tau loses normal function and also gains toxic function. Pin1 prevents the accumulation of the pathogenic cis pT231-tau conformation in AD by converts it to the non-pathogenic trans.
Conformation-specific antibodies and/or vaccines against the pathogenic cis pT231-tau might be developed for the diagnosis and treatment of AD, especially at early stages.
Pin1-Catalyzed Cis to Trans Isomerization Prevents the Accumulation of the Pathogenic p-Tau Conformation in AD
We have provided the first structural evidence that Pin1 protects against tau pathology by converting the pathogenic cis p-tau to the non-pathogenic trans (Figure. 7). These results provide the first molecular explanation why Pin1 overexpression promotes tau dephosphorylation and degradation, and inhibits tau aggregation and tau pathology, whereas Pin1 knockout has the opposite effects in model systems (Hamdane et al., 2006; Lim et al., 2008; Liou et al., 2003). These results are highly relevant to human AD because Pin1 is inhibited in MCI and AD neurons by multiple mechanisms (Lee et al., 2011b) and preventing Pin1 inhibition is associated with delaying AD onset (Ma et al., 2010). Furthermore, Pin1 is phosphorylated and catalytically inactivated by DAPK1 (Lee et al., 2011a), which is genetically linked to human AD (Li et al., 2006) and whose deletion improves learning and memory in mice (Yukawa et al., 2006). Thus, lack of sufficient Pin1 to convert cis to trans contributes to pathogenic p-tau accumulation and tau aggregation, eventually leading to tangle formation and neurodegeneration in AD.
Potential Novel Cis and Trans Conformation-Specific Disease Diagnoses and Therapies
Our exciting new insight into the role and regulation of p-tau conformations in AD also suggest novel approaches for early diagnosis and treatment of AD (Figure 7). For example, Thr231 phosphorylation is a very early phosphorylation event in human AD (Luna-Munoz et al., 2007) and its levels in cerebrospinal fluids tracks AD progression, but with large individual variations, making it difficult to become a standardized test (Ewers et al., 2007; Hampel et al., 2001). Our findings that cis, but not trans, pT231-tau appears early in MCI and is pathogenic suggest that cis pT231-tau and its ratio with trans might be a better and easier standardized biomarker, especially for early diagnosis and patient comparison. Furthermore, overexpressing Pin1 or preventing Pin1 inhibition in neurons might be a new approach to reduce the cis to trans pT231-tau ratio to prevent tau pathology at early stages. Finally, active or passive immunization against some pSer/Thr-Pro motifs in tau including the pThr231-Pro motif can reduce tau aggregates and memory deficits in mouse models (Boimel et al., 2010; Boutajangout et al., 2011; Boutajangout et al., 2010). However, our findings that only cis, but not trans, pT231-tau is pathogenic and 90% of regular synthetic pT231-tau peptides is in trans suggest that it might be more specific and effective and safer to develop conformation-specific vaccines or Abs specifically targeting cis pT231-tau for treating or even preventing AD at early stages. These studies would also further validate the conformation-specific significance in AD. Given the critical role of Pin1 and other isomerases in regulating many other proteins in physiology and disease (Lee et al., 2011b; Liou et al., 2011; Theuerkorn et al., 2011), it would be interesting to determine whether prolyl isomerization also regulates these protein conformations and whether these conformational switches might be exploited for developing novel diagnostic and therapeutic procedures.
EXPERIMENTAL PROCEDURES
Synthesis of Tau Peptides
Peptides used for the experiments are wild-type phosphorylated Thr231-Pro tau (KVAVVRpTPPKSPS), non-phosphorylated Thr231 tau (KVAVVRTPPKSPS), cis lock-in phosphorylated Thr231-Dmp tau (KVAVVRpT(5,5-dimethyl-L-proline)PKSPS), trans lock-in phosphorylated Thr231-Ala tau (P232A)(KVAVVRpTAPKSPS) and phosphorylated Thr231-Homoproline (pThr231-Pip) tau (CKKVAVVRpT(Pip)PKSPSSAK), which are synthesized by a commercial source.
NMR Spectrometry
Amide region of TOCSY spectra of tau-derived peptides (sequence KVAVVR-pT231-X232-PKSPS) were used. Pro, Pip, Dmp, or Ala was incorporated into the sequence following pThr231. 2D 1H-1H TOCSY spectra (mixing time of 70 ms) were taken and the population of the cis isomer was determined by comparing peak volumes. Peaks used included the gamma, beta, and alpha protons of pThr231 as seen from the amide proton in both the cis and trans states. Cis and trans isomers were assigned by identifying characteristic through-space NOEs between Thr231 and Xaa232 protons in 1H-1H ROESY spectra.
Production and Purification of Abs
Rabbits were immunized with pThr231-Homoproline (pThr231-Pip) tau peptide that was coupled to KLH with N-terminal Cys. The resulting sera was purified with pThr231-Homoproline (pThr231-Pip) tau peptide. For cis conformation-specific pT231-tau Abs, the resulting bound fraction was purified twice with cis pThr231-Dmp tau peptide to collect the bound fraction, followed by counter-purification with pThr231-Ala tau peptide, with the unbound fraction as cis conformation-specific pThr231-Pro tau Ab. For trans-specific pThr231-Pro tau Ab, the Ab was purified twice with wild-type pThr231-Pro tau peptide to collect the bound fraction, followed by counter-purification with pThr231-Dmp tau peptide with the unbound fraction as trans-specific pThr231-Pro tau Ab.
Human Brain Specimen
Brains from 11 patients with AD (Braak stage V and VI) (mean age ± SE: 78.4 ± 3.1 years old), 6 MCI (Braak stage III and IV) (84.4 ± 5.6) and 9 healthy controls (Braak stage I and II) (78.0 ± 6.5) were used for the analyses. All AD subjects met the clinical, and neuropathological National Institute on Aging-Reagan Institute (NIA-RI) criteria, for AD. Our studies for human samples have been approved by our Institutional Review Board.
Transgenic Overexpression and Gene Knockout Mice
Tau-Tg mice (Ishihara et al., 1999), Tau-Tg+Pin1-Tg mice and Tau-Tg+Pin1 KO mice in C57BL/6 background were generated, as described (Lim et al., 2008; Liou et al., 2003). Tau knockout mice (Dawson et al., 2001) were purchased from Jackson Laboratory.
Supplementary Material
HIGHLIGHTS.
Novel peptide chemistry enables to generate cis and trans conformation-specific antibodies Cis, but not trans, p-tau appears early, accumulates and localizes to dystrophic neurites in AD Cis, but not trans, p-tau not only loses tau normal function, but also gains toxic function in AD Pin1 accelerates cis to trans conversion to prevent pathogenic p-tau accumulation in AD
ACKNOWLEDGEMENTS
We thank Lew Cantley and Tony Hunter for insightful advice, Peter Davies for some tau Abs, Virginia Lee for tau transgenic mice, and the members of Lu/Zhou laboratories for stimulating discussions. This study was supported by NIH grants P30 AG13854 to the Northwestern Alzheimer Disease Center, R01CA122434 to X. Z. Z., R01AG029385 to L.N. and K.P.L. and R01AG039405, R01AG17870, and R01GM58556 to K.P.L
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental Information includes six supplemental figures, extended experimental procedures, and supplemental references and can be found with this article online at
REFERENCES
- An SAA, Lester CC, Peng JL, et al. Retention of the cis proline conformation in tripeptide fragments of bovine pancreatic ribonucleaase A containing a non-natural proline analogues: 5,5-dimethylproline. J Am Chem Soc. 1999;121:11558–11566. [Google Scholar]
- Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- Boimel M, Grigoriadis N, Lourbopoulos A, Haber E, Abramsky O, Rosenmann H. Efficacy and safety of immunization with phosphorylated tau against neurofibrillary tangles in mice. Exp Neurol. 2010;224:472–485. doi: 10.1016/j.expneurol.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Boutajangout A, Ingadottir J, Davies P, Sigurdsson EM. Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. J Neurochem. 2011;118:658–667. doi: 10.1111/j.1471-4159.2011.07337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau prevents cognitive decline in a new tangle mouse model. J Neurosci. 2010;30:16559–16566. doi: 10.1523/JNEUROSCI.4363-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NR, Noble ME, Endicott JA, Johnson LN. The structural basis for specificity of substrate and recruitment peptides for cyclin-dependent kinases. Nat Cell Biol. 1999;1:438–443. doi: 10.1038/15674. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Abdul HM, Opii W, Newman SF, Joshi G, Ansari MA, Sultana R. Pin1 in Alzheimer's disease. J Neurochem. 2006;98:1697–1706. doi: 10.1111/j.1471-4159.2006.03995.x. [DOI] [PubMed] [Google Scholar]
- Cruz JC, Tsai LH. Cdk5 deregulation in the pathogenesis of Alzheimer's disease. Trends Mol Med. 2004;10:452–458. doi: 10.1016/j.molmed.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Davies CA, Mann DM, Sumpter PQ, Yates PO. A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer's disease. J Neurol Sci. 1987;78:151–164. doi: 10.1016/0022-510x(87)90057-8. [DOI] [PubMed] [Google Scholar]
- Davies P. Characterization and Use of Monoclonal Antibodies to Tau and Paired Helical Filament Tau. Alzheimer's disease: Methods in Molecular Medicine. 2000;32:361–373. doi: 10.1385/1-59259-195-7:361. [DOI] [PubMed] [Google Scholar]
- Dawson HN, Ferreira A, Eyster MV, Ghoshal N, Binder LI, Vitek MP. Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J Cell Sci. 2001;114:1179–1187. doi: 10.1242/jcs.114.6.1179. [DOI] [PubMed] [Google Scholar]
- de Calignon A, Fox LM, Pitstick R, Carlson GA, Bacskai BJ, Spires-Jones TL, Hyman BT. Caspase activation precedes and leads to tangles. Nature. 2010;464:1201–1204. doi: 10.1038/nature08890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- Dolan PJ, Johnson GV. The role of tau kinases in Alzheimer's disease. Curr Opin Drug Discov Devel. 2010;13:595–603. [PMC free article] [PubMed] [Google Scholar]
- Ewers M, Buerger K, Teipel SJ, Scheltens P, Schroder J, Zinkowski RP, Bouwman FH, Schonknecht P, Schoonenboom NS, Andreasen N, et al. Multicenter assessment of CSF-phosphorylated tau for the prediction of conversion of MCI. Neurology. 2007;69:2205–2212. doi: 10.1212/01.wnl.0000286944.22262.ff. [DOI] [PubMed] [Google Scholar]
- Girardini JE, Napoli M, Piazza S, Rustighi A, Marotta C, Radaelli E, Capaci V, Jordan L, Quinlan P, Thompson A, et al. A Pin1/mutant p53 axis promotes aggressiveness in breast cancer. Cancer Cell. 2011;20:79–91. doi: 10.1016/j.ccr.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG. A century of Alzheimer's disease. Science. 2006;314:777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- Hamdane M, Dourlen P, Bretteville A, Sambo AV, Ferreira S, Ando K, Kerdraon O, Begard S, Geay L, Lippens G, et al. Pin1 allows for differential Tau dephosphorylation in neuronal cells. Mol Cell Neurosci. 2006;32:155–160. doi: 10.1016/j.mcn.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Hampel H, Buerger K, Kohnken R, Teipel SJ, Zinkowski R, Moeller HJ, Rapoport SI, Davies P. Tracking of Alzheimer's disease progression with cerebrospinal fluid tau protein phosphorylated at threonine 231. Ann Neurol. 2001;49:545–546. [PubMed] [Google Scholar]
- Ishihara T, Hong M, Zhang B, Nakagawa Y, Lee MK, Trojanowski JQ, Lee VM. Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron. 1999;24:751–762. doi: 10.1016/s0896-6273(00)81127-7. [DOI] [PubMed] [Google Scholar]
- Jicha GA, Lane E, Vincent I, Otvos L, Jr, Hoffmann R, Davies P. A conformation- and phosphorylation-dependent antibody recognizing the paired helical filaments of Alzheimer's disease. J Neurochem. 1997;69:2087–2095. doi: 10.1046/j.1471-4159.1997.69052087.x. [DOI] [PubMed] [Google Scholar]
- Lee TH, Chen CH, Suizu F, Huang P, Schiene-Fischer C, Daum S, Zhang YJ, Goate A, Chen RW, Lu KP. Death associated protein kinase 1 phosphoylates Pin1 and inhibits its prolyl isomerase activity and cellular function. Mol Cell. 2011a;22:147–159. doi: 10.1016/j.molcel.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TH, Pastorino L, Lu KP. Peptidyl-prolyl cis-trans isomerase Pin1 in aging, cancer and Alzheimer's disease. Expet Rev Mol Med. 2011b;13:e21. doi: 10.1017/S1462399411001906. [DOI] [PubMed] [Google Scholar]
- Li Y, Grupe A, Rowland C, Nowotny P, Kauwe JS, Smemo S, Hinrichs A, Tacey K, Toombs TA, Kwok S, et al. DAPK1 variants are associated with Alzheimer's disease and allele-specific expression. Hum Mol Genet. 2006;15:2560–2568. doi: 10.1093/hmg/ddl178. [DOI] [PubMed] [Google Scholar]
- Lim J, Balastik M, Lee TH, Liou YC, Sun A, Finn G, Pastorino L, Lee VM-Y, Lu KP. Pin1 has opposite effects on wild-type and P301L tau stability and tauopathy. J Clin Invest. 2008;118:1877–1889. doi: 10.1172/JCI34308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou Y-C, Sun A, Ryo A, Zhou XZ, Yu Z-X, Huang H-K, Bronson R, Bing G, Li X, Hunter T, et al. Role of the prolyl isomerase Pin1 in protecting against age-dependent neurodegeneration. Nature. 2003;424:556–561. doi: 10.1038/nature01832. [DOI] [PubMed] [Google Scholar]
- Liou YC, Zhou XZ, Lu KP. The prolyl isomerase Pin1 as a molecular switch to determine the fate of phosphoproteins. Trends Biochem Sci. 2011;36:501–514. doi: 10.1016/j.tibs.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KP, Finn G, Lee TH, Nicholson LK. Prolyl cis-trans isomerization as a molecular timer. Nature Chem Biol. 2007;3:619–629. doi: 10.1038/nchembio.2007.35. [DOI] [PubMed] [Google Scholar]
- Lu KP, Hanes SD, Hunter T. A human peptidyl-prolyl isomerase essential for regulation of mitosis. Nature. 1996;380:544–547. doi: 10.1038/380544a0. [DOI] [PubMed] [Google Scholar]
- Lu KP, Liou YC, Zhou XZ. Pinning down the proline-directed phosphorylation signaling. Trends Cell Biol. 2002;12:164–172. doi: 10.1016/s0962-8924(02)02253-5. [DOI] [PubMed] [Google Scholar]
- Lu KP, Zhou XZ. The prolyl isomerase Pin1: a pivotal new twist in phosphorylation signalling and human disease. Nat Rev Mol Cell Biol. 2007;8:904–916. doi: 10.1038/nrm2261. [DOI] [PubMed] [Google Scholar]
- Lu PJ, Wulf G, Zhou XZ, Davies P, Lu KP. The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature. 1999a;399:784–788. doi: 10.1038/21650. [DOI] [PubMed] [Google Scholar]
- Lu PJ, Zhou XZ, Shen M, Lu KP. A function of WW domains as phosphoserine- or phosphothreonine-binding modules. Science. 1999b;283:1325–1328. doi: 10.1126/science.283.5406.1325. [DOI] [PubMed] [Google Scholar]
- Lummis SC, Beene DL, Lee LW, Lester HA, Broadhurst RW, Dougherty DA. Cis-trans isomerization at a proline opens the pore of a neurotransmitter-gated ion channel. Nature. 2005;438:248–252. doi: 10.1038/nature04130. [DOI] [PubMed] [Google Scholar]
- Luna-Munoz J, Chavez-Macias L, Garcia-Sierra F, Mena R. Earliest stages of tau conformational changes are related to the appearance of a sequence of specific phospho-dependent tau epitopes in Alzheimer's disease. J Alzheimers Dis. 2007;12:365–375. doi: 10.3233/jad-2007-12410. [DOI] [PubMed] [Google Scholar]
- Ma SL, Pastorino L, Zhou XZ, Lu KP. Pin1 promotes APP protein turnover by inhibiting GSK3β kinase activity - A novel mechanism for Pin1 to protect against Alzheimer’s disease. J Biol Chem. 2011 doi: 10.1074/jbc.C111.298596. (Accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SL, Tang NLS, Tam CWC, Lui VWC, Lam LCW, Chiu HFK, Driver JA, Pastorino L, Lu KP. A functional polymorphism in Pin1 that prevents its suppression by AP4 Is associated with delayed onset of Alzheimer’s disease. Neurobiol Aging. 2010 Jun 24; doi: 10.1016/j.neurobiolaging.2010.05.018. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Zhou XZ, Kishi S, Kosugi I, Tsutsui Y, Lu KP. A specific interaction between the telomeric protein Pin2/TRF1 and the mitotic spindle. Curr Biol. 2001;11:1512–1516. doi: 10.1016/s0960-9822(01)00456-0. [DOI] [PubMed] [Google Scholar]
- Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol. 2001;2:21–32. doi: 10.1038/35048096. [DOI] [PubMed] [Google Scholar]
- Pastorino L, Sun A, Lu PJ, Zhou XZ, Balastik M, Finn G, Wulf G, Lim J, Li SH, Li X, et al. The prolyl isomerase Pin1 regulates amyloid precursor protein processing and amyloid-beta production. Nature. 2006;440:528–534. doi: 10.1038/nature04543. [DOI] [PubMed] [Google Scholar]
- Ranganathan R, Lu KP, Hunter T, Noel JP. Structural and functional analysis of the mitotic peptidyl-prolyl isomerase Pin1 suggests that substrate recognition is phosphorylation dependent. Cell. 1997;89:875–886. doi: 10.1016/s0092-8674(00)80273-1. [DOI] [PubMed] [Google Scholar]
- Scheff SW, DeKosky ST, Price DA. Quantitative assessment of cortical synaptic density in Alzheimer's disease. Neurobiol Aging. 1990;11:29–37. doi: 10.1016/0197-4580(90)90059-9. [DOI] [PubMed] [Google Scholar]
- Shen M, Stukenberg PT, Kirschner MW, Lu KP. The essential mitotic peptidyl-prolyl isomerase Pin1 binds and regulates mitosis-specific phosphoproteins. Genes Dev. 1998;12:706–720. doi: 10.1101/gad.12.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires-Jones TL, Stoothoff WH, de Calignon A, Jones PB, Hyman BT. Tau pathophysiology in neurodegeneration: a tangled issue. Trends Neurosci. 2009;32:150–159. doi: 10.1016/j.tins.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Theuerkorn M, Fischer G, Schiene-Fischer C. Prolyl cis/trans isomerase signalling pathways in cancer. Curr Opin Pharmacol. 2011;11:281–287. doi: 10.1016/j.coph.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Tun-Kyi A, Finn G, Greenwood A, Nowak M, Lee TH, Asara JM, Tsokos GC, Fitzgerald K, Israel E, Li X, et al. Essential role for the prolyl isomerase Pin1 in Toll-like receptor signaling and type I interferon-mediated immunity. Nature Immunol. 2011;12:733–741. doi: 10.1038/ni.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintjens R, Wieruszeski JM, Drobecq H, Rousselot-Pailley P, Buee L, Lippens G, Landrieu I. 1H NMR study on the binding of Pin1 Trp-Trp domain with phosphothreonine peptides. J Biol Chem. 2001;276:25150–25156. doi: 10.1074/jbc.M010327200. [DOI] [PubMed] [Google Scholar]
- Yaffe MB, Schutkowski M, Shen M, Zhou XZ, Stukenberg PT, Rahfeld J, Xu J, Kuang J, Kirschner MW, Fischer G, et al. Sequence-specific and phosphorylation-dependent proline isomerization: A potential mitotic regulatory mechanism. Science. 1997;278:1957–1960. doi: 10.1126/science.278.5345.1957. [DOI] [PubMed] [Google Scholar]
- Yuan WC, Lee YR, Huang SF, Lin YM, Chen TY, Chung HC, Tsai CH, Chen HY, Chiang CT, Lai CK, et al. A Cullin3-KLHL20 Ubiquitin ligase-dependent pathway targets PML to potentiate HIF-1 signaling and prostate cancer progression. Cancer Cell. 2011;20:214–228. doi: 10.1016/j.ccr.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Yukawa K, Tanaka T, Bai T, Li L, Tsubota Y, Owada-Makabe K, Maeda M, Hoshino K, Akira S, Iso H. Deletion of the kinase domain from death-associated protein kinase enhances spatial memory in mice. Int J Mol Med. 2006;17:869–873. [PubMed] [Google Scholar]
- Zhou XZ, Kops O, Werner A, Lu PJ, Shen M, Stoller G, Küllertz G, Stark M, Fischer G, Lu KP. Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25C and tau proteins. Mol Cell. 2000;6:873–883. doi: 10.1016/s1097-2765(05)00083-3. [DOI] [PubMed] [Google Scholar]
- Zhou XZ, Lu PJ, Wulf G, Lu KP. Phosphorylation-dependent prolyl isomerization: A novel signaling regulatory mechanism. Cell Mol Life Sci. 1999;56:788–806. doi: 10.1007/s000180050026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.