Summary
Developmental venous anomalies (DVA) drain normal neural tissue and are mostly discovered incidentally. We describe a young patient with a left hemisphere superficial to deep DVA and right hemisphere venous outflow restriction presenting with a seizure. The right hemisphere drainage variation is not typical of a DVA but represents another drainage pattern on the border of normality.
Key words: developmental venous anomaly, symptoms, seizure
Introduction
Developmental venous anomaly (DVA) is the term used by Lasjaunias and his team in 1986 when they first described this condition 1. Formerly DVA was referred to as venous angioma. Lasjaunias et al. defined these lesions as having the classical features of dilated venous medullary channels that drain into an extra-parenchymatous collector.
Normal neural tissue is drained by these medullary veins, but with an absent normal anatomical venous pathway that would usually drain it. DVAs would account for about 2.5% of all intracranial vascular anomalies found on routine cerebral imaging, making it the most common vascular anomaly.
DVA is usually asymptomatic as it is a benign vascular lesion. However, some literature published since 1986 does attribute symptoms to a DVA 2-4. These symptoms are mostly headaches, seizures and intracerebral hemorrhage, with less commonly also trigeminal neuralgia, hemifacial weakness and tinnitus noted. An important caveat noted in the literature is that DVAs should not be assumed to be the cause of any symptom or sign without proper investigations. This case study briefly reviews the literature. We also describe a case study of an interesting variation in a young healthy male who presented with seizures.
Case Report
A 17-year-old male was admitted to our hospital with a history of generalized tonic-clonic seizures at irregular intervals for a three-year period. He was only recently started on anti-epileptic medication (sodium valproate) and referred for a CT brain scan. Prior to this admission he did not have any other hospital admissions. He had no allergies or other medical, or surgical history of note. There was no history of cranial trauma.
The young man is not a smoker and there is no history of alcohol or recreational drugs used. There is also no family history known of any neurovascular diseases or events.
On examination the patient was a healthy-looking young male. There were no focal neurological deficits. Normal gait was noted with grossly normal intellect for his age. No cutaneous stigmata were noted. Power and coordination and gross sensory examination were normal. Fundoscopy and the remainder of his cranial nerve examination were normal. The laboratory findings showed normal renal function and no signs of infection. There were no signs of metabolic, or inflammatory illness.
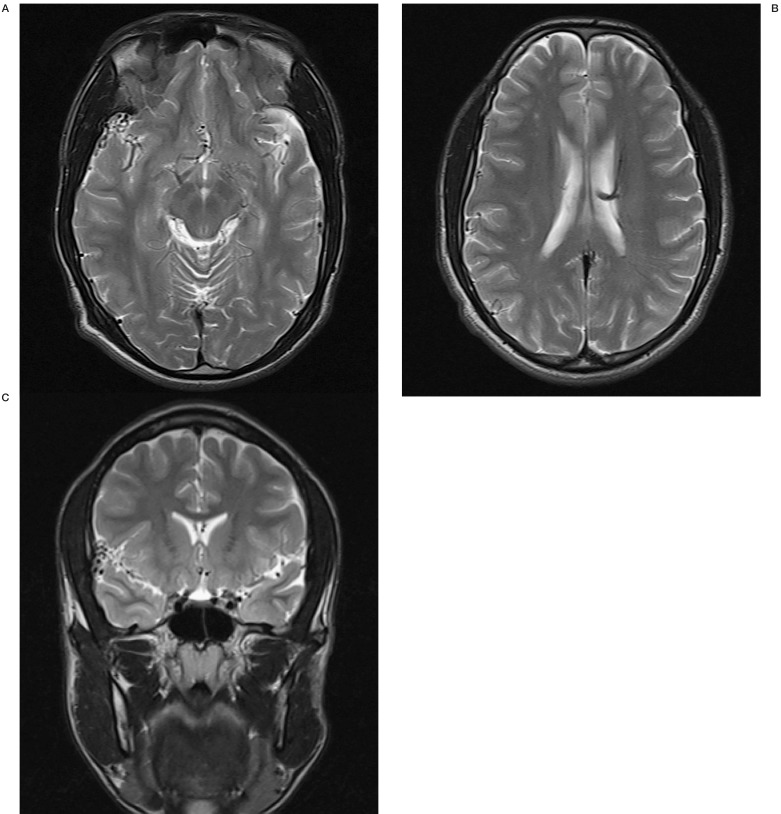
Brain computed tomography (CT) was largely insignificant, except for a small area of increased density against the dura in the right sylvian fissure. There was no mass effect visible. After the administration of contrast, dilated and tortuous vessels were then noted in the right sylvian fissure. This was confirmed on CT angiogram and was suspected to be an arteriovenous malformation because of the dilated and tortuous nature of the vessels. An MRI was performed and again a small leash of vessels in the right sylvian fissure was noted. This was associated with prominence of a right middle cerebral artery branch, but there was no dilated draining vein noted. There were also features of a superficial to deep DVA of the left hemisphere. There was no T2 intensity change of cortex or white matter and no cortical dysplasia or other structural abnormality (Figure 1).
Figure 1.
T2-weighted MRI scans showing vessel prominence in the right sylvian subarachnoid space and features of a left hemisphere DVA.
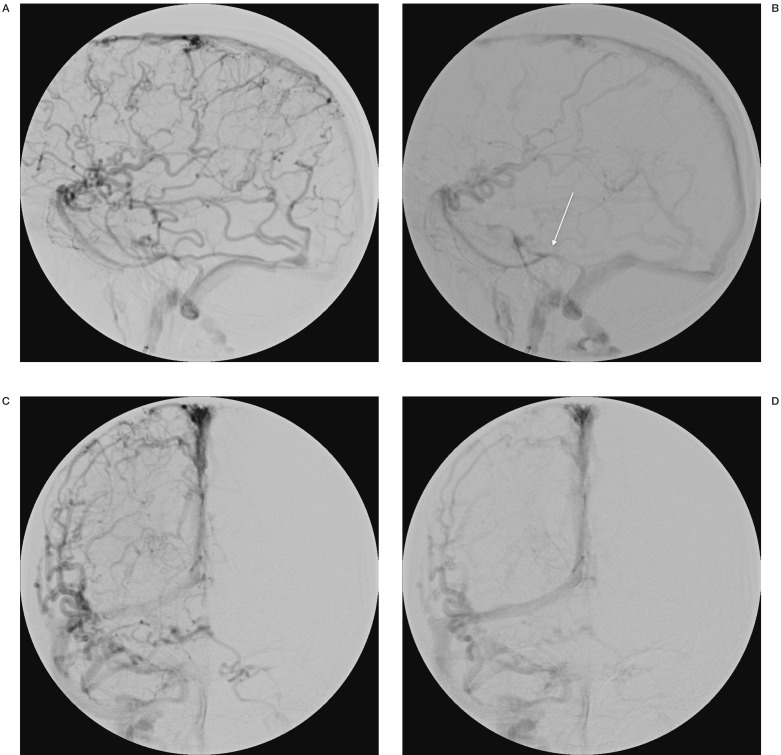
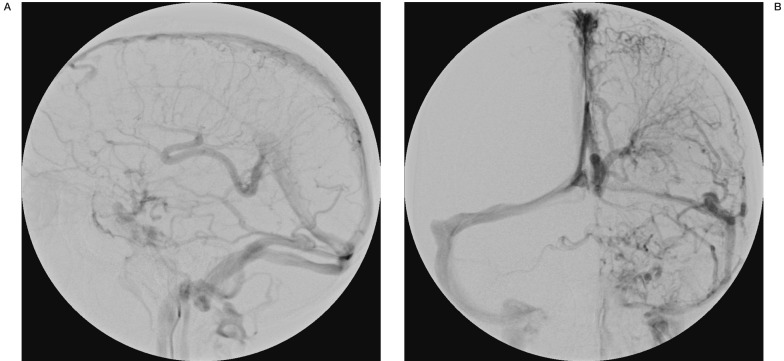
Digital subtraction angiography was performed. On formal angiography the left and right arterial circulation was noted to be normal. There were no signs of arteriovenous malformations. The vertebral circulation was also normal. Of note was the delayed venous emptying bilaterally. There was a predominant cortical drainage pattern for the whole right hemisphere with only a small area of deep drainage noted. Predominant drainage through sylvian veins into Brechet's sinus (sphenoparietal sinus) was seen. Drainage from there was into a restricted tentorial channel and then the lateral sinus. (Figure 2). On the left side there was a large deep to superficial system developmental venous anomaly (DVA) noted with classical features of DVA, such as caput medusae. There was no restricted flow noted although the angiographic venous phase was longer than expected (Figure 3). No signs of dural sinus thrombosis were noted and the posterior circulation was normal.
Figure 2.
Right cerebral hemisphere venous phase on DSA with the white arrow showing the outflow restriction of the sylvian veins at the tentorial sinus.
Figure 3.
Left cerebral hemisphere venous phase demonstrating a large superficial to deep developmental venous anomaly.
Discussion
DVA's are classified as deep or superficial depending on the location of the draining veins - either subependymal or cortical-pial. Around 70% of DVAs will drain into the superficial system, 20% into the deep system and 10% can have combined drainage. DVA is a benign anomaly where normal cerebral tissue is drained by alternative venous drainage pathways. It should be kept in mind that DVA is a diagnosis with typical imaging and histological characteristics 5. It is usually an incidental finding on imaging and most patients are asymptomatic.
The diagnosis of DVA is classically first suspected on CT imaging. Here the investigator will see a linear or curvilinear focus of enhancement. This typically courses from deep white matter to a cortical vein, deep vein or dural sinus. Further investigation with magnetic resonance imaging (MRI) usually reveals a transhemispheric flow void on both the T1 and T2 images. On gadolinium-enhanced MRI there is significant enhancement of the caput medusae appearance of the medullary veins as well as in the venous collector. Digital Subtraction angiography (DSA) will confirm the above imaging features of the caput medusae in the early to middle venous phase. There is a normal arterial phase with absence of early venous filling.
A case series published by Roccatagliata et al. mentions seven patients in whom a DVA was thought to be the cause of their symptoms (epilepsy, headaches or hemorrhage 4. They noticed a peculiar capillary stain on the DSA of these patients. The specific symptoms of these patients could be localized to the area of particular drainage involved by the DVA. They also mentioned that all the patients had perilesional hyperintense brain tissue on T2 MRI. The capillary stain is thought to represent a network of fine vessels that forms a blush in the arterial phase of the DSA. We are of opinion that this resembles the straining of the brain due to the hemodynamic imbalance produced by the DVA. The venous ischemia then leads to increased angiogenesis that shows up as a capillary stain on the DSA.
Histologically DVAs are composed of thin-walled vessels that are spread in normal neural parenchyma, draining into a larger calibre vein with a thicker wall. There is a marked absence of a smooth muscle layer and an elastic lamina 6. DVAs do not have proliferative potential, there are no associated arteriovenous shunts and the parenchyma is normal in between the dilated veins.
The presumed origin of DVAs is intrauterine venous obstruction or thrombosis that leads to variations of normal venous drainage pathways or a primary dysplasia of capillaries and small transcerebral draining veins. This then leads to the dilated medullary veins draining into a large transcerebral collector, or the persistence of intrinsic venous anastomosis and absence of normal draining veins 7. The DVA therefore is a compensatory mechanism as there is no other pathway of venous drainage.
There is some reference in the literature to decreased perfusion in the area of brain that is drained by the DVA. This can then lead to venous hypertension as a cause of symptoms in patients 8,9.
Pereira et al. divided the pathogenesis of symptoms from DVAs into the following groups:
– Mechanical (obstructive)
– Flow-related mechanisms
– Increased inflow into the DVA
– Reduced outflow from the DVA
– Increased venous pressure
In the largest review done to date by Pereira et al., they evaluated 80 patients combined from the literature and their own unit with proven symptomatic DVAs 2. In this series 14 patients had mechanical causes of their symptoms (hydrocephalus n=7; nerve symptoms like tinnitus or trigeminal pain n=6). The cause of hydrocephalus was related to compression of the aqueduct in all their cases. In 49 cases the cause of symptoms was flow-related.
When calling a DVA symptomatic it is important to note that associated causes of the symptoms need to be excluded such as:
– Hemorrhage - mostly caused by associated cavernoma
– Epilepsy - secondary to cortical dysplasia
– Pseudotumoral effects - Lasjaunias et al. used the term suspected to be caused by lymphatic abnormalities.
In our patient no other cause for epilepsy could be found other than impaired venous drainage of the brain. While there is clearly a large deep to superficial DVA of the left hemisphere the venous drainage of the right hemisphere is also anomalous although not typical of a DVA. On the right there is no caput medusae, capillary blush or collection by a single channel, but there is a dominance of cortical veins which converge on the sylvian veins and outflow is delayed by tentorial restriction. Cortical venous congestion of either hemisphere could be the cause of the patient's epilepsy. As with any DVA the right hemisphere drainage represents an extreme pattern of normal drainage and no therapeutic intervention beyond anticonvulsants is contemplated.
Conclusion
Seizures without other cause have been reported in patients with DVA and are mostly thought to be related to reduced blood outflow from the DVA. This case demonstrates a large left deep to superficial DVA and delayed venous drainage of the right hemisphere related to cortical venous dominance with convergence of veins towards a restricted tentorial sinus. Both hemispheres demonstrate delayed venous outflow.
References
- 1.Lasjaunias P, Burrows P, Planet C. Developmental venous anomalies (DVA): the so-called venous angioma. Neurosurg Rev. 1986;9:233–242. doi: 10.1007/BF01743138. [DOI] [PubMed] [Google Scholar]
- 2.Pereira VM, Geibprasert S, Krings T, et al. Pathomechanisms of symptomatic developmental venous anomalies. Stroke. 2008;39:3201–3215. doi: 10.1161/STROKEAHA.108.521799. [DOI] [PubMed] [Google Scholar]
- 3.Walsh M, Parmar H. Developmental venous anomaly with symptomatic thrombosis of the draining vein. J Neurosurg. 2008;109:1119–1122. doi: 10.3171/JNS.2008.109.12.1119. [DOI] [PubMed] [Google Scholar]
- 4.Roccatagliata L, Berg R, Soderman M, et al. Developmental venous anomalies with capillary stain: a subgroup of symptomatic DVAs? Neuroradiology. 2011;54:475–480. doi: 10.1007/s00234-011-0890-y. [DOI] [PubMed] [Google Scholar]
- 5.Valavanis A, Wellauer J, Yasargil MG. The radiological diagnosis of cerebral venous angioma: cerebral angiography and computed tomography. Neuroradiology. 1983;24:193–199. doi: 10.1007/BF00399770. [DOI] [PubMed] [Google Scholar]
- 6.Abe M, Hagihara N, Tabuchi K, et al. Histologically classified venous angiomas of the brain: a controversy. Neurol Med Chir (Tokyo) 2003;43:1–10. doi: 10.2176/nmc.43.1. [DOI] [PubMed] [Google Scholar]
- 7.Okudera T, Ohta T, Huang YP, et al. Developmental and radiological anatomy of the superficial cerebral convexity vessels in the human fetus. J Neuroradiol. 1988;15:205–224. [PubMed] [Google Scholar]
- 8.Matsuda H, Terada T, Katoh M, et al. Brain perfusion SPECT in a patient with a subtle venous angioma. Clin Nucl Med. 1994;19:785–788. doi: 10.1097/00003072-199409000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Tomura N, Inugami A, Uemura K, et al. Multiple medullary venous malformations decreasing cerebral blood flow: case report. Surg Neurol. 1991;35:131–135. doi: 10.1016/0090-3019(91)90264-a. [DOI] [PubMed] [Google Scholar]
- 10.Abla A, Wait SD, Uschold T, et al. Developmental venous anomaly, cavernous malformation, and capillary telangiectasia: spectrum of a single disease. Acta Neurochirurg. 2008;150:487–489. doi: 10.1007/s00701-008-1570-5. discussion 489. [DOI] [PubMed] [Google Scholar]
- 11.Hon JML, Bhattacharya JJ, Counsell CE, et al. The presentation and clinical course of intracranial developmental venous anomalies in adults: a systematic review and prospective, population, based study. Stroke. 2009;40:1980–1985. doi: 10.1161/STROKEAHA.108.533034. [DOI] [PubMed] [Google Scholar]
- 12.Rammos SK, Maina R, Lanzino G. Developmental venous anomalies: current concepts and implications for management. Neurosurgery. 2009;65:20–29. doi: 10.1227/01.NEU.0000347091.06694.3E. discussion 9-30. [DOI] [PubMed] [Google Scholar]
- 13.Ruíz DS, Yilmaz H, Gailloud P. Cerebral developmental venous anomalies: current concepts. Ann Neurol. 2009;66:271–283. doi: 10.1002/ana.21754. [DOI] [PubMed] [Google Scholar]