Summary
This paper reports the cost of endovascular materials used for the treatment of large-vessel ischemic stroke in the anterior circulation according to the angiographic score and clinical results at three months. From November 2009 to July 2011, 57 ischemic patients (mean age, 64.6 ±13.8 years) with anterior large vessel occlusion were included. Mean National Institutes of Health Stroke Scale (NIHSS) on admission was 18.4 ± 4.9. Mean duration of symptoms until the arterial puncture was 207±67 minutes. Recanalization was assessed using the Thrombolysis In Myocardial Infarction (TIMI) score. Patient selection was performed on a non-enhanced CT scanner. According to the TIMI final angiographic score and the modified Rankin score (mRS) at three months, we determined the cost of the material used. Complete (n=12, TIMI grade 3) or partial perfusion (n=35, TIMI grade 2) was achieved in 47 (82.5%) lesions. At three months, 33.3% (n=19) had a mRS score ≤ 2. The mean cost of the material used in the operative room was 5018±2402 euro.
Intra-arterial thrombolysis presents a substantial initial cost and the long-term economic impact has to be evaluated. Our health system has to take the price of these new technologies into account for future medical choices and urgently evaluate them in randomized controlled trials.
Key words: thrombectomy, stroke, cost
Introduction
Stroke alone accounts for 2-5% of the total health expenditure in Western countries 1-3. Cost-effectiveness studies concerning primary 4 and secondary prevention 5, diagnostic testing 6, thrombolysis 7-15, rehabilitation 16, management systems 17 and also the informal care costs of long-term care after stroke 18,19 have been published. Few studies have explored the cost-effectiveness of mechanical thrombectomy 20-22.
The goal of this article is to report the cost of the endovascular material used for patients presenting a stroke in the anterior circulation and treated by intra-arterial thrombolysis (IAT) or mechanical thrombectomy at our institution from November 2009 to July 2011. Angiographic and clinical results are also reported.
Material and Methods
From November 2009 to July 2011, a prospective analysis of the patients who underwent IAT and/or mechanical thrombectomy in the setting of acute ischemic stroke (AIS) in the anterior circulation was performed. Criteria for the endovascular procedure were: (1) NIHSS>7 at admission; (2) stroke attributable to a large vessel occlusion (extra or intracranial internal carotid artery, Sylvian artery up to M2); (3) stroke within the first six hours from symptoms onset; (4) clinical judgement of the stroke neurologist and interventional neuroradiologist on call. The pre-treatment imaging protocol was a non-enhanced CT scan in all cases. For each patient, we recorded: (1) the admission NIHSS; (2) the time between symptoms and arterial puncture; (3) all the endovascular material used in the operative room; (4) the availability of general anaesthesia; (5) the final angiographic TIMI score; (6) the 90-day modified Rankin score (mRS).
Altogether 59 consecutive patients presenting with acute stroke in the anterior circulation were treated by IAT at our institution. Two patients were excluded as no clinical data at three months were available; therefore 57 patients (mean age, 64.6 ±13.8 years; 24 women) were included in this study. The admission NIHSS was 18.4 ± 4.9. The type of occlusion and the type of endovascular material used is listed in Table 1.
Table 1.
Clinical and radiological characteristics of the 57 patients.
| Patients' demographics (n=57) Baseline stroke score |
64.6 ±13.8 years; 24 women (42%) NIHSS: 18.4 ± 4.9 |
| Type of occlusion | Tandem lesions (ICA and M1): 11 (19.3%) ICA terminal bifurcation: 10 (17.5%) MCA M1 segment: 33 (57.9%) MCA M2 segment: 3 (5.3%) |
| Thrombectomy material | Penumbra system: 44 (77.2%) MERCI system: 4 (7%) Solitaire retriever: 8 (14%) Intracranial balloon: – alone: 2 (3.5%) – with other devices: 15 (26.3%) Carotid stent: 7 (12.3%) IA rtPA alone: 3 (5.3%) |
Mean delays between initial symptoms and arterial puncture or thrombus contact were three hours 27 min ± 62 min and three hours 59 min ± 60 min respectively. In 14 cases (24.5%), general anesthesia was performed, with eight minutes of supplementary average time.
We determined prospectively the type and cost of all the intra-arterial material (stroke devices, wires, catheters, femoral introducers, carotid stents, recombinant tissue plasminogen activator) used for each thrombectomy.
Results
Complete (n=12, TIMI grade 3) or partial perfusion (n=35, TIMI grade 2) was achieved in 47 (82.5%) lesions. In ten (17.5%) patients, no residual flow (TIMI grade 0) or minimal perfusion (TIMI grade 1) was observed at the end of the procedure. Nineteen (33.3%) patients had a good outcome (mRS≤2 at 3 months), 28 (49.1%) had serious disability (3≤mRS≤5) and ten (17.5%) had died (mRS=6). Of the 11 tandem lesions, nine were associated with poor clinical outcomes (mRS≤3). Comparative clinical and angiographic data, time parameters and costs between good and poor results are presented in Table 2.
Table 2.
Comparison of clinical and radiological parameters, timing and cost of material used for deobstruction between patients with good outcomes (mRS 0-2) and poor outcomes (mRS 3-6).
| mRS | Age (yrs) | NIHSS | Time to operative room |
Tandem lesion |
TIMI 3 | Mean time for recanalization |
Cost (euro) |
|---|---|---|---|---|---|---|---|
| 0-2 (n=19; 33%) |
61.1±13.6 | 18.6±3.9 | 163±56 min | 10.5% | 37% | 106 min | 4534±2095 |
| 3-6 (n=38; 67%) |
66.4±13.7 | 18.3±5.3 | 205±58 min | 23.7% | 13% | 133 min | 5260±241 |
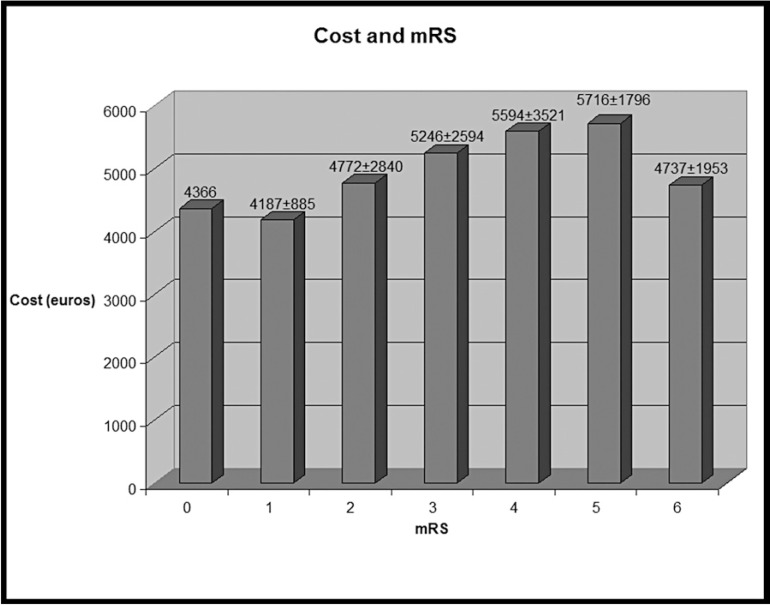
The mean cost of the material used for all the procedures was 5018±2402 euro (6936±3320 Canadian Dollars (CD); 1 CD= 0.7235 euro, average exchange rate in euro of the Paris Stock Exchange – Canada from November 2009 to July 2011 from the National Institute of Statistics and Economic Studies). The procedures with poor outcomes were more expensive (Figure 1 and Table 2).
Figure 1.
Mean cost (±SD) of the material used in the operative room for each mRS (from 0, no deficit to 6, death). The mean cost of the procedure is 5018 ± 2402 euro.
Discussion
Stroke is one of the most expensive diseases in industrialized countries and health systems are encouraged to invest money in the treatment of AIS. Economic costs include direct costs which refer to the resources used for the patient's treatment (medication, hospitalization, rehabilitation) and indirect costs which correspond to the loss of productivity, absenteeism and premature death as a long-term consequence of the stroke sequelae 7. The procedural material cost we determined in this study belongs to the direct cost. We did not take into account other costly parameters such as medical (radiologist, neurologist, anesthesiologist) and paramedical (technician, anesthetist) salaries for example.
A fundamental question could be raised: how much initial supplementary cost should be advanced in an effort to improve the clinical outcome and the long-term indirect cost? More specifically, since the recanalization rate seems to be correlated with the clinical outcome 23, should neuroradiologists keep on trying to open a vessel whatever the cost of the material used? In our experience the procedures with poor outcomes were more expensive and at the time of this study, we may have used several devices to try and open the vessel, if we could. This raises another question: when should interventional neuroradiologists stop? Procedure time depends on several parameters such as the initial clinical evaluation by the neurologist, the initial imaging, the background and the learning curve of the neuroradiologist for example.
We found that the mean cost of the material used in the operative room was about 5000 euro. At the time the study was conducted (Nov 2009-July 2011), aspiration systems (Penumbra*) were used as first line therapy where stent retrievers are more used. However, the cost difference has little impact on the average cost of the procedure (3509 euro for the aspiration system and 3175 euro for the stent retriever). Direct cost including devices and human procedures - that we did not take into account in this study - was evaluated at 10,502 euro per patient in 2003 in a Spanish center 24. This direct cost is not negligible when compared to the mean cost of hospitalization and rehabilitation. For example, the average cost per individual has been evaluated at 21,040 euro per year after a stroke in a recent Spanish study 18. To evaluate the cost-effectiveness of a treatment, we have to take into account the global associated cost of care and loss in health-related quality of life 18.
Only 14% of acute care hospitals in the US achieved primary stroke center (PSC) designation 25 and hospital-based series showed that intravenous thrombolysis (IVT) rates are low, varying between 5.7% and 21.7% of patients admitted for ischemic stroke 26,27. Consequently, as the cost-effectiveness of the initial treatment 2,20,21 and PSC for acute stroke care has been reported, it would be logical to invest money in the first steps of the curative treatment, which remains challenging when financial resources may be lacking 28.
It is difficult to evaluate the long-term financial impact of brain damage. Informal care costs due to disability represent a largely hidden burden on society 18,19. Moreover, patients and their families may also have health-related costs following stroke and these are not emphasized in economic studies 29.
Considering clinical and financial recently reported data 20-22, we can argue that it would be logical to perform the endovascular treatment of AIS in regional hospital centres with a stroke unit.
The problem is that as long as mechanical thrombectomy is not scientifically validated, we can express doubts on the medico-economic interest in developing these invasive treatments that would require a very costly initial investment (medical and paramedical training, creation of operative rooms to begin with). Another economic point of view is the absolute necessity of encouraging health policy decision-makers to keep on investing money in primary and secondary prevention.
Conclusion
Indirect costs remain very high after a stroke. We can assume that an initial costly investment such as endovascular recanalization is justified to decrease the global costs of the condition if there is a substantial improvement in the patient's long-term quality of life. Randomized trials are urgently required to establish this perceived benefit.
It is our responsibility as health professionals to participate in these trials with the understanding that such studies could lead us to save enormous amounts of money.
References
- 1.Saka O, Serra V, Samyshkin Y, et al. Cost-effectiveness of stroke unit care followed by early supported discharge. Stroke. 2009;40:24–29. doi: 10.1161/STROKEAHA.108.518043. [DOI] [PubMed] [Google Scholar]
- 2.Jung KT, Shin DW, Lee KJ, et al. Cost-effectiveness of recombinant tissue plasminogen activator in the management of acute ischemic stroke: a systematic review. J Clin Neurol. 2010;6:117–126. doi: 10.3988/jcn.2010.6.3.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergman L, van der Meulen JH, Limburg M, et al. Costs of medical care after first-ever stroke in The Netherlands. Stroke. 1995;26:1830–1836. doi: 10.1161/01.str.26.10.1830. [DOI] [PubMed] [Google Scholar]
- 4.Ebrahim S. Cost-effectiveness of stroke prevention. Br Med Bull. 2000;56:557–570. doi: 10.1258/0007142001903201. [DOI] [PubMed] [Google Scholar]
- 5.Matchar DB, Samsa GP, Liu S. Cost-effectiveness of antiplatelet agents in secondary stroke prevention: the limits of certainty. Value Health. 2005;8:572–580. doi: 10.1111/j.1524-4733.2005.00050.x. [DOI] [PubMed] [Google Scholar]
- 6.Meenan RT, Saha S, Chou R, et al. Cost-effectiveness of echocardiography to identify intracardiac thrombus among patients with first stroke or transient ischemic attack. Med Decis Making. 2007;27:161–177. doi: 10.1177/0272989X06297388. [DOI] [PubMed] [Google Scholar]
- 7.Araujo DV, Teich V, Passos RB, et al. Analysis of the cost-effectiveness of thrombolysis with alteplase in stroke. Arq Bras Cardiol. 95:12–20. doi: 10.1590/s0066-782x2010005000067. [DOI] [PubMed] [Google Scholar]
- 8.Fagan SC, Morgenstern LB, Petitta A, et al. Cost-effectiveness of tissue plasminogen activator for acute ischemic stroke: NINDS rt-PA Stroke Study Group. Neurology. 1998;50:883–890. doi: 10.1212/wnl.50.4.883. [DOI] [PubMed] [Google Scholar]
- 9.Sinclair SE, Frighetto L, Loewen PS, et al. Cost-utility analysis of tissue plasminogen activator therapy for acute ischaemic stroke: a Canadian healthcare perspective. Pharmacoeconomics. 2001;19:927–936. doi: 10.2165/00019053-200119090-00004. [DOI] [PubMed] [Google Scholar]
- 10.Mar J, Begiristain JM, Arrazola A. Cost-effectiveness analysis of thrombolytic treatment for stroke. Cerebrovasc Dis. 2005;20:193–200. doi: 10.1159/000087204. [DOI] [PubMed] [Google Scholar]
- 11.Ehlers L, Andersen G, Clausen LB, et al. Cost-effectiveness of intravenous thrombolysis with alteplase within a 3-hour window after acute ischemic stroke. Stroke. 2007;38:85–89. doi: 10.1161/01.STR.0000251790.19419.a8. [DOI] [PubMed] [Google Scholar]
- 12.Chambers MG, Koch P, Hutton J. Development of a decision-analytic model of stroke care in the United States and Europe. Value Health. 2002;5:82–97. doi: 10.1046/j.1524-4733.2002.52011.x. [DOI] [PubMed] [Google Scholar]
- 13.Sandercock P, Berge E, Dennis M, et al. Cost-effectiveness of thrombolysis with recombinant tissue plasminogen activator for acute ischemic stroke assessed by a model based on UK NHS costs. Stroke. 2004;35:1490–1497. doi: 10.1161/01.STR.0000126871.98801.6E. [DOI] [PubMed] [Google Scholar]
- 14.Stahl JE, Furie KL, Gleason S, et al. Stroke: Effect of implementing an evaluation and treatment protocol compliant with NINDS recommendations. Radiology. 2003;228:659–668. doi: 10.1148/radiol.2283021557. [DOI] [PubMed] [Google Scholar]
- 15.Moodie ML, Carter R, Mihalopoulos C, et al. Trial application of a Model of Resource Utilization, Costs, and Outcomes for Stroke (MORUCOS) to assist priority setting in stroke. Stroke. 2004;35:1041–1046. doi: 10.1161/01.STR.0000125012.36134.89. [DOI] [PubMed] [Google Scholar]
- 16.Keith RA. Rehabilitation after stroke: cost-effectiveness analyses. J R Soc Med. 1996;89:631–633. doi: 10.1177/014107689608901109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Launois R, Giroud M, Megnigbeto AC, et al. Estimating the cost-effectiveness of stroke units in France compared with conventional care. Stroke. 2004;35:770–775. doi: 10.1161/01.STR.0000117574.19517.80. [DOI] [PubMed] [Google Scholar]
- 18.Mar J, Arrospide A, Begiristain JM, et al. The impact of acquired brain damage in terms of epidemiology, economics and loss in quality of life. BMC Neurol. 11:46. doi: 10.1186/1471-2377-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Youman P, Wilson K, Harraf F, et al. The economic burden of stroke in the United Kingdom. Pharmacoeconomics. 2003;21(Suppl 1):43–50. doi: 10.2165/00019053-200321001-00005. [DOI] [PubMed] [Google Scholar]
- 20.Kim AS, Nguyen-Huynh M, Johnston SC. A cost-utility analysis of mechanical thrombectomy as anadjunct to intravenous tissue-type plasminogen activator for acute large-vessel ischemic stroke. Stroke. 42:2013–2018. doi: 10.1161/STROKEAHA.110.606889. [DOI] [PubMed] [Google Scholar]
- 21.Patil CG, Long EF, Lansberg MG. Cost-effectiveness analysis of mechanical thrombectomy in acute ischemic stroke. J Neurosurg. 2009;110:508–513. doi: 10.3171/2008.8.JNS08133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen-Huynh MN, Johnston SC. Is mechanical clot removal or disruption a cost-effective treatment for acute stroke? Am J Neuroradiol. 32:244–249. doi: 10.3174/ajnr.A2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38:967–973. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Fernandez E, Gil-Peralta A, Gonzalez A, et al. Financial analysis of intraarterial procedures in acute stroke. Neurologia. 2008;23:15–20. [PubMed] [Google Scholar]
- 25.Alberts MJ. Stroke centers, proof of concept and the concept of proof. Stroke. 41:1100–1101. doi: 10.1161/STROKEAHA.110.582148. [DOI] [PubMed] [Google Scholar]
- 26.Fischer U, Mono ML, Zwahlen M, et al. Impact of Thrombolysis on Stroke Outcome at 12 Months in a Population. The Bern Stroke Project. Stroke. 43:1039–1045. doi: 10.1161/STROKEAHA.111.630384. [DOI] [PubMed] [Google Scholar]
- 27.van Wijngaarden JD, Dirks M, Huijsman R, et al. Hospital rates of thrombolysis for acute ischemic stroke: the influence of organizational culture. Stroke. 2009;40:3390–3392. doi: 10.1161/STROKEAHA.109.559492. [DOI] [PubMed] [Google Scholar]
- 28.O’Toole LJ, Jr, Slade CP, Brewer GA, et al. Barriers and facilitators to implementing primary stroke center policy in the United State, results from 4 case study states. Am J Public Health. 101:561–566. doi: 10.2105/AJPH.2010.197954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evers SM, Struijs JN, Ament AJ, et al. International comparison of stroke cost studies. Stroke. 2004;35:1209–1215. doi: 10.1161/01.STR.0000125860.48180.48. [DOI] [PubMed] [Google Scholar]