Summary
Thrombolysis with intravenous rt-PA is the current therapy for acute ischemic stroke. Unlike other outcome factors, relatively little is known about the prognostic value of the occlusion site on treatment outcome. We compared the effectiveness and safety of intravenous thrombolysis in patients with different levels of occlusion identified by CT angiography (CTA) in anterior circulation stroke, and analyzed the influence of the occlusion site on treatment outcome in relation to other outcome factors.
We selected 71 patients from a stroke database collected between June 2007 and December 2011 at our hospital. All of the studied patients had anterior circulation stroke with intracranial occlusion detected by CTA and were treated with intravenous rt-PA. They were divided into two groups according to the site of occlusion along the middle cerebral artery course: proximal (carotid “T”, complete M1 and mild M1 occlusions) and distal (M2/M3 occlusions). Treatment effectiveness was assessed by modified Rankin Scale (mRS) at three months, considering a positive outcome a mRS value ≤ 2. Treatment safety was assessed by evaluating the rate of hemorrhagic complications seen on unenhanced CT at 24 hours. Binary logistic regression was performed to evaluate the interaction between occlusion site and other variables such as sex, age, ASPECT score on admission and baseline NIHSS value in determining treatment outcome.
The degree of effectiveness and safety differed when considering patients with proximal and distal occlusions. The percentage of successfully treated cases was 28.6% in the first group compared to 72% in the second, and the rate of hemorrhagic complications was 28.6% and 6% respectively. After adjustment for sex, age, ASPECT score on admission and baseline NIHSS value, occlusion site was the only variable significantly influencing treatment safety and, together with baseline NIHSS value, the only valid predictor of treatment effectiveness.
We demonstrated a correlation between the site of arterial occlusion and outcome of intravenous thrombolysis. By helping the choice of the best therapeutic strategy depending on the identified occlusion site, CTA could be usefully added to the examinations included in the Stroke Protocol for the baseline evaluation of patients with suspected acute stroke.
Key words: head and neck, CT-angiography, stroke, thrombolysis, occlusion site
Introduction
Fibrinolysis with intravenous rt-PA is currently the main treatment for acute ischemic stroke. The effectiveness and safety of rt-PA, approved for this use by the US Food and Drug Administration in 1996, have been extensively examined over the last two decades 1-5. The results of these studies confirmed rt-PA as more effective than placebo in achieving recanalization of the occluded vessel and clinical improvement, but also showed that the use of rt-PA is associated with an increased risk of intracerebral hemorrhage 5. Considering the benefits and potential risks associated with the use of rt-PA, since the first experimental studies special attention has been paid to the identification of prognostic factors likely to influence the probability of success and the risk of complications in order to select patients who might benefit most from rt-PA without being exposed to a high risk of adverse effects.
Many studies have been published on the predictive value of factors like time-to-treatment 6-9, severity of neurological deficits 10-12 and extension of early ischemic lesions on baseline CT scan on admission 11,13,14, emphasizing how the best results can be obtained when rt-PA is administered within three hours of symptom onset in patients with an NIHSS score ≤ 25 and an ASPECT score > 7.
However, relatively little is known about the influence of the occlusion site as a possible outcome factor after intravenous thrombolysis. A few studies showed a greater efficacy in more distal arterial occlusions such as middle cerebral artery versus internal carotid artery occlusions 1,14-19. Less information is available on different outcomes depending on the occlusion site at intracranial level 15,20-23,35-37, which is the object of our study. We compared the effectiveness and safety of intravenous thrombolysis in patients with different levels of occlusion of the middle cerebral artery identified by CT angiography (CTA) in anterior circulation stroke and analyzed the influence of occlusion site and its contribution to treatment outcome in relation to other outcome factors.
Materials and Methods
Inclusion Criteria and Treatment Protocol
This study is the result of a retrospective analysis of a prospective stroke database containing all acute stroke patients diagnosed and treated with intravenous or intra-arterial thrombolysis between June 2007 and December 2011 at our hospital. According to the Stroke Protocol, all patients underwent a first ER and neurological evaluation, including use of the National Institute of Health Stroke Scale (NIHSS) for the assessment of neurological deficit. A score ranging between 0 and 42 (normal function to maximum impairment respectively) was assigned based on evaluation of multiple items including consciousness, eye movement, visual field, motor strength, ataxia, sensory, language, dysarthria and neglect. An unenhanced CT scan was then performed to exclude hemorrhagic stroke and other possible neurological deficit causes and to quantify the extension of early ischemic changes, if present using the ASPECT score. Patients with an ASPECT score > 7 were further examined with CTA of head and neck for morphological evaluation of the supra-aortic vessels and intracranial circulation to identify the occlusion site and gain other useful information such as efficacy of collateral flow and any atheromasic carotid changes.
Patients with anterior circulation occlusion localized downstream of the M1 segment were considered eligible for intravenous thrombolysis, whereas intra-arterial thrombolysis was considered for patients with carotid “T” and M1 occlusion or with posterior circulation stroke. This protocol was introduced after a first experimental phase during which the intravenous approach was also used for some patients with carotid “T” and M1 occlusion. Before intravenous treatment, absence of exclusion criteria as established by the European Stroke Organization Guidelines 24 was verified: e.g. initial symptoms > 3 h before ER admission, age > 80 years, mild neurological deficit or TIA, increased risk of hemorrhage, epileptic seizures, deep coma, concomitant dysmetabolic diseases or severe psychiatric background.
Intravenous thrombolysis was carried out with rt-PA (Actilyse, Boehringer Ingelheim) at a dose of 0.9 mg/kg (maximum dose 90 mg), 10% of which as initial bolus and the remainder infused over 60 minutes.
Of all patients included in the database, only those with anterior circulation stroke treated intravenously were selected for the study, whereas patients who underwent intra-arterial treatment were not further analyzed.
Neuroradiologic Assessment and Identification of Occlusion Site
Unenhanced CT scan and CTA were performed with a Siemens Somatom Sensation 40 and a GE Hi-speed 4 CT scanner with the following acquisition parameters: 120 Kv/120 mA-0.5/1.
For the CTA performed with the Somatom Sensation 40 scanner, 50 ml of contrast medium (Iopamiro 370, Bracco) with 50 ml of saline were administered at 4 ml/s. For the CTA performed with the GE Hi-speed 4 CT scanner, 120 ml of the same contrast medium with 50 ml of saline were administered at the same rate. For both acquisitions, coverage was from the aortic arch to the upper part of the frontal sinuses and images were post-processed with Syngo MMVP 21/A (Siemens) software for VR, MIP and MPR reconstructions of carotid arteries and intracranial circulation.
Baseline unenhanced CT scans were evaluated according to criteria introduced by Barber et al. 25 for the calculation of ASPECT score.
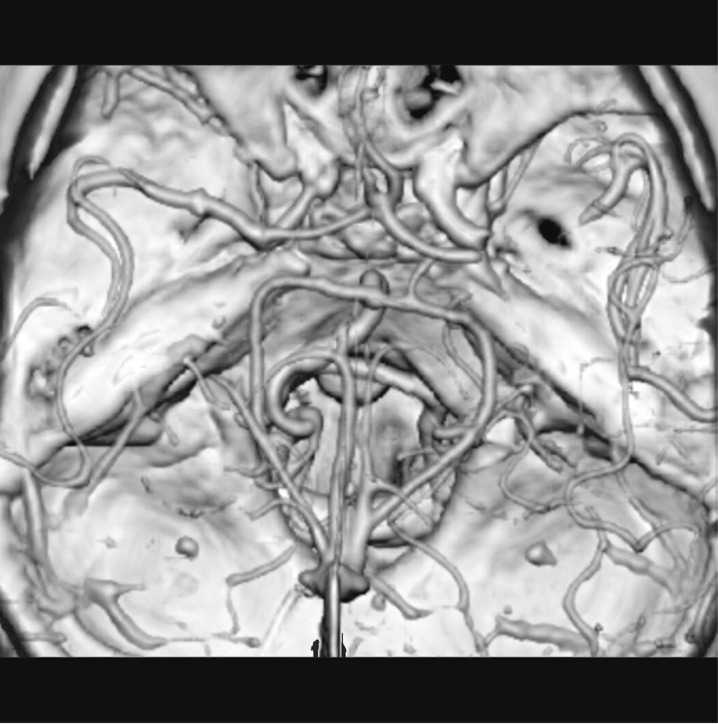
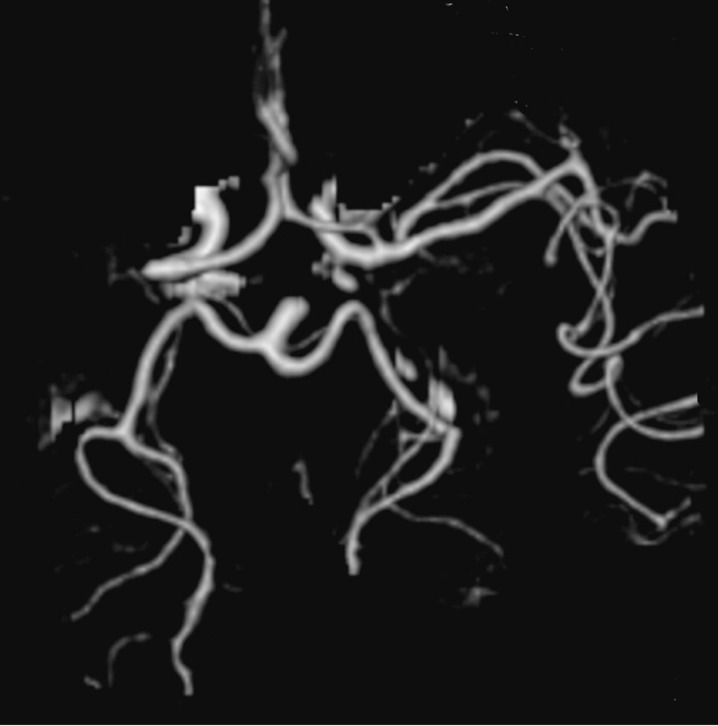
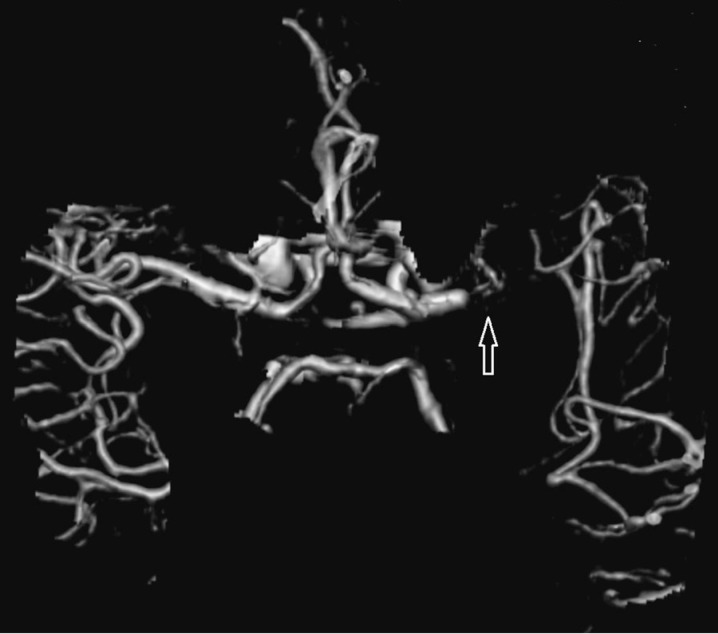
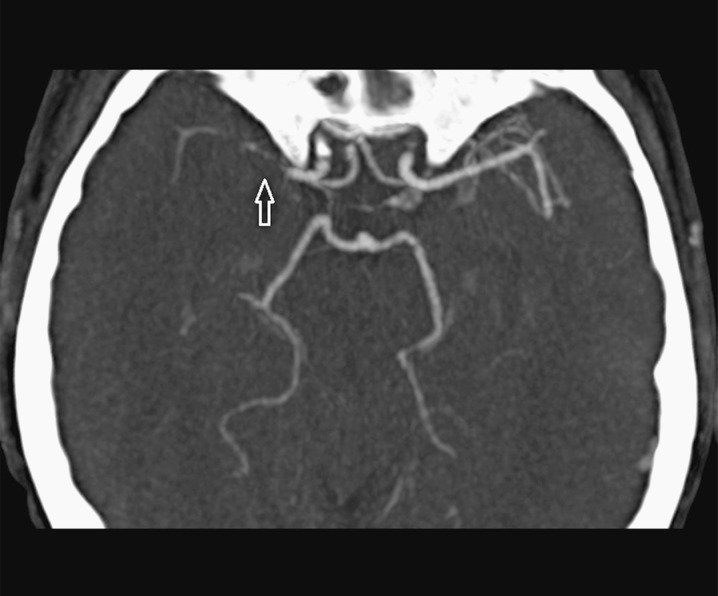
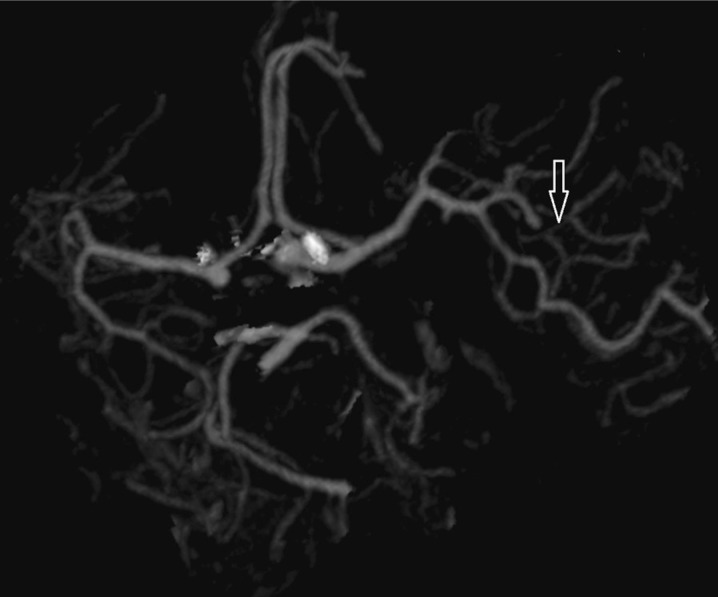
Depending on the occlusion site at intracranial level identified on CTA images, patients with anterior circulation stroke were divided into two groups: proximal (carotid “T”, complete M1 and mild M1 occlusions) and distal (M2/M3 occlusions). Carotid “T” occlusion was defined as an occlusion of the internal carotid artery at its bifurcation in the anterior cerebral artery and middle cerebral artery (Figure 1). M1 occlusion was defined as an occlusion of the middle cerebral artery proximal to the origin of its first ramification. M1 occlusions were qualified as complete (Figures 2 and 3) or mild (Figure 4) depending on the entity of the luminal-filling defect: total or partial respectively. In complete M1 occlusions, efficacy of leptomeningeal collateral flow was also evaluated according to the degree of retrograde opacification of the branches distal to the occlusion due to leptomeningeal anastomoses. M2/M3 occlusion was defined as an occlusion of the middle cerebral artery at the level of the second- or third-order ramifications (Figure 5).
Negative CTAs, i.e. no occlusions detected distal to the M3 branches, in the presence of positive clinical stroke presentation, were interpreted as an intrinsic detection limit of CTA examination. These cases were therefore treated as distal occlusions, but they were not included in the study.
Figure 1.
Right carotid T occlusion associated with ipsilateral M2/M3 occlusion in a VR reconstruction.
Figure 2.
Left complete M1 occlusion with no detectable leptomeningeal collateral flow in a VR reconstruction.
Figure 3.
Right complete M1 occlusion with good leptomeningeal collateral flow in a VR reconstruction.
Figure 4.
Right mild M1 occlusion in a MIP reconstruction
Figure 5.
Left M3 occlusion in a VR reconstruction.
Outcome Assessment
Two main parameters, effectiveness and safety, were analyzed to evaluate the outcome of intravenous thrombolysis in patients stratified by site of occlusion.
Effectiveness was assessed by quantification of residual disability with the modified Rankin Scale (mRS) at three months after stroke onset 26,27. According to earlier studies 17,19,20,22,28, good outcome was defined as a three-month mRS score ≤ 2 (no symptoms to slight disability), whereas a three-month mRS score > 2 (moderate to severe disability) or death (mRS = 6) were considered an index of poor outcome.
Incidence of hemorrhagic complications seen at unenhanced CT scan 24 hours after treatment was used for the assessment of safety. According to definitions published in previous studies 29,30, hemorrhagic events were classified as hemorrhagic infarctions (HI) and parenchymal hematomas (PH) (Figures 6 and 7). HI are defined as small (type I) or more confluent petechiae (type II) along the margins of the infarct or within the infarct area, without mass effect, and were recognized on CT scans by the presence of inhomogeneous areas of hyper- or isodensity surrounded by the low density of infarction. PH are defined as blood clots extending over < 30 % (type I) or > 30% of the infarct area (type II), with mild or significant mass effect respectively and appeared on CT as more distinct and larger homogeneous blood collections, which in more severe cases could extend beyond the original infarct area or even rupture into the ventricles. HI are relatively common and mostly asymptomatic and can be explained as an ordinary effect of reperfusion resulting from the leakage of red blood cells through small lesions of hypoxic vessel walls. Thus they were not regarded as adverse effects of thrombolysis, but rather considered evidence of treatment efficacy. Conversely, PH, which occur much less frequently, in particular PH type II, have been shown to worsen clinical outcomes 31-33 and were considered hemorrhagic complications of thrombolytic therapy.
Figure 6.
Small hemorrhagic infarction (HI) in the right basal ganglia.
Figure 7.
Large parenchymal hematoma (PH) in the deep territory of the right middle cerebral artery with mass effect on the right lateral ventricle and leftward midline shift.
Statistical Analysis
Outcome data were compared after grouping into two categories: proximal and distal occlusions. Binary logistic regression was performed to evaluate the interaction between occlusion site and other variables such as sex, age, ASPECT score on admission and baseline NIHSS value in determining treatment outcome. ASPECT scores were dichotomized into 10 vs. 8-9, i.e. no detectable ischemic lesions vs. early ischemic signs affecting up to one third of the middle cerebral artery territory. Statistical analysis was performed with IBM SPSS Statistics 21.0. Values of p < 0.05 were considered statistically significant.
Results
Between June 2007 and December 2011, 163 patients with a diagnosis of acute ischemic stroke were treated with intravenous or intra-arterial thrombolysis at our hospital. For the purpose of our study, 12 patients were excluded from this initial cohort as they had posterior circulation stroke and five other patients were also discarded due to the presence of a continuous non-thrombotic occlusion (dissection or occlusive atherosclerotic plaque) of the extracranial carotid artery. Lastly, 49 patients were excluded due to the lack of a detectable arterial occlusion on CTA scans. Of the remaining 97 patients with anterior circulation stroke and documented intracranial occlusion, 11 patients received intra-arterial rt-PA and 86 were treated with intravenous rt-PA. Among the latter group, CTA was available for 71 patients, who represented the specific object of our analysis.
This sample comprised 35 men (49.3%) and 36 women (50.7%) with a mean age of 68±13, divided into two groups according to the site of occlusion: proximal (carotid “T”, complete M1 and mild M1 occlusions) and distal (M2/M3 occlusions): carotid T occlusion in four cases (5.63%); complete M1 occlusion in eight (11.27%), five of whom with good leptomeningeal collateral flow and the remaining three with poor compensation; mild M1 occlusion in nine patients (12.68%); M2/M3 occlusion in 50 (70.42%). Overall, 21 patients had a proximal occlusion (29.58%; ten men and 11 women; mean age 68±14; mean ASPECT score on admission 9.3±0.9; mean baseline NIHSS value 16.7±5.8) and 50 patients had a distal occlusion (70.42%; 25 men and 25 women; mean age 68±12; mean ASPECT score on admission 9.5±0.7; mean baseline NIHSS value 10±5.3).
Evaluation of Treatment Effectiveness
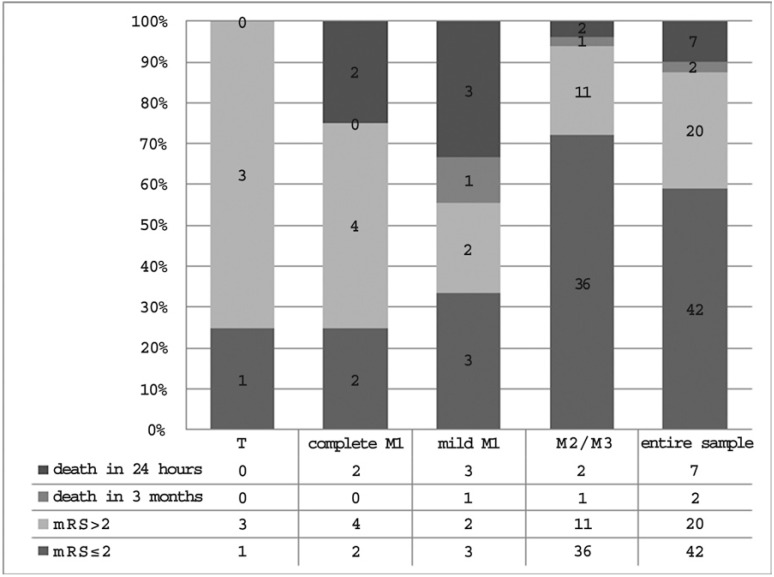
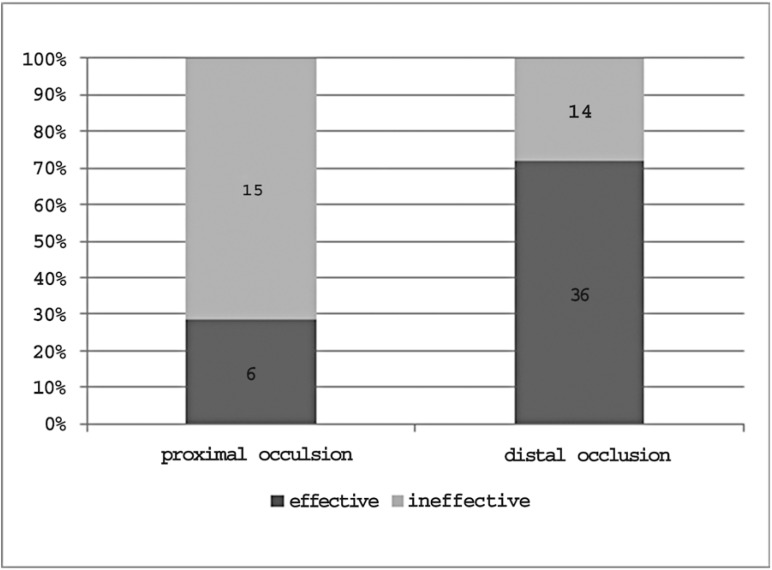
Outcome data concerning treatment effectiveness in each occlusion site and in the entire sample are shown in Figure 8. Overall, regardless of the occlusion site, i.v. thrombolysis proved effective in 42 patients (59.2%) and ineffective in the remaining 29 (40.8%) as 20 patients were assessed for moderate or severe disability, seven died in the Stroke Unit during the first 24 hours and two died within the first three months.
The percentage of therapeutic success, demonstrated by mRS values ≤ 2, differed considering each group separately: 25% in patients with carotid T occlusion, 25% in patients with complete M1 occlusion, 33.3% in patients with mild M1 occlusion and 72% in patients with M2/M3 occlusion. After grouping all sites of occlusion into proximal and distal, the rate of positive outcomes was 28.6% in the proximal group compared to 72% in the distal group (Figure 9). The role of the occlusion site - proximal or distal - in determining treatment effectiveness was further confirmed by multivariate analysis. After adjustment for sex, age, ASPECT score on admission and baseline NIHSS value, occlusion site and baseline NIHSS value were the only variables significantly influencing treatment effectiveness (p < 0.05) (Table 1).
Table 1.
Logistic regression analysis of treatment effectiveness.
| Variables in the Equation | |||||||
|---|---|---|---|---|---|---|---|
| Step 1 | B | S.E. | Wald | df | Sig. | Exp (B) | |
| Age |
.028 |
.026 |
1.150 |
1 |
.283 |
1.028 |
|
| Sex |
.168 |
.604 |
.078 |
1 |
.780 |
1.183 |
|
| ASPECTS |
-.077 |
.615 |
.016 |
1 |
.901 |
.926 |
|
| Baseline NIHSS value |
.112 |
.057 |
3.869 |
1 |
.049 |
1.118 |
|
| Occlusion site |
1.366 |
.687 |
3.952 |
1 |
.047 |
3.920 |
|
| Constant |
-5.615 |
2.131 |
6.943 |
1 |
.008 |
.004 |
|
Figure 8.
Treatment effectiveness in each occlusion site and in the entire sample.
Figure 9.
Treatment effectiveness in proximal and distal occlusions.
Evaluation of Treatment Safety
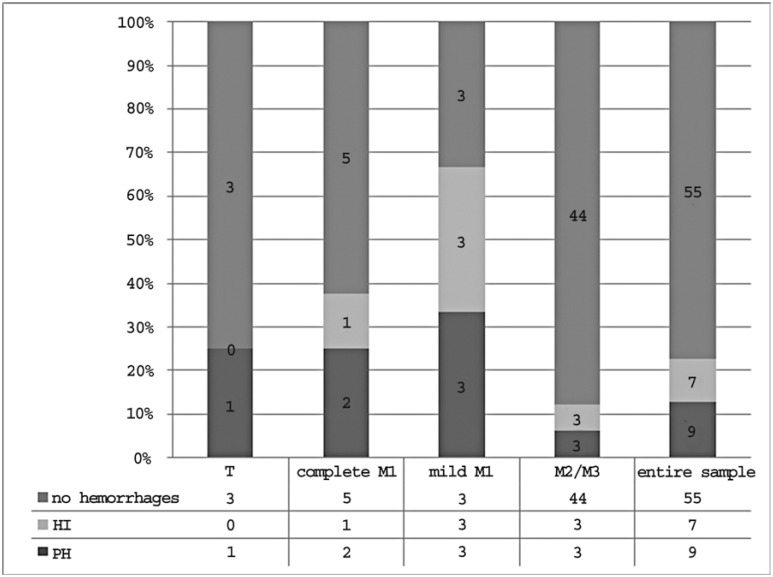
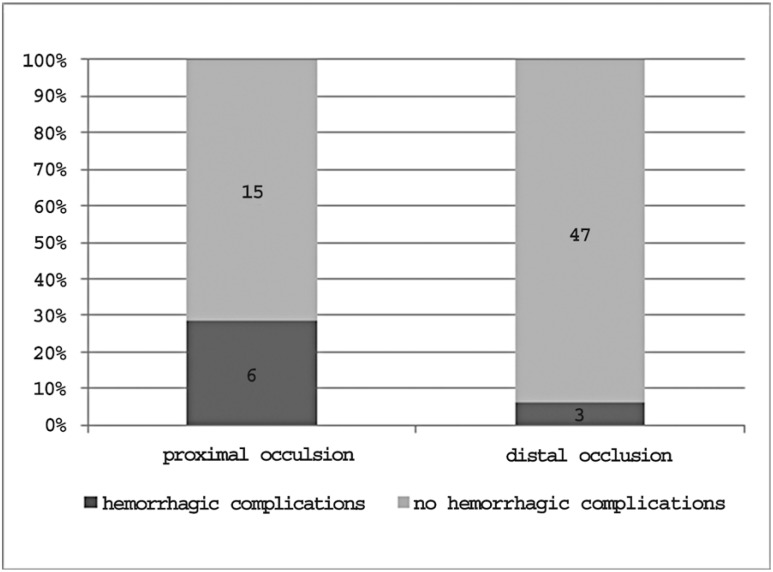
Outcome data concerning treatment safety in each occlusion site and in the entire sample are reported in Figure 10.The overall prevalence of hemorrhagic complications was 12.7%. The percentage of hemorrhagic complications varied depending on the occlusion site: 25% in patients with carotid T occlusion, 25% in patients with complete M1 occlusion, 33.3% in patients with mild M1 occlusion and 6% in patients with M2/M3 occlusion.Considering proximal and distal occlusions as a whole, the complication rates were 28.6% and 6% respectively (Figure 11).
Logistic regression analysis further confirmed the influence of occlusion site on the probability of complications after intravenous thrombolytic treatment. After adjustment for sex, age, ASPECT score on admission and baseline NIHSS value, the occlusion site was the only variable significantly correlated to treatment safety (p < 0.05) (Table 2).
Table 2.
Logistic regression analysis of treatment safety.
| Variables in the Equation | |||||||
|---|---|---|---|---|---|---|---|
| Step 1 | B | S.E. | Wald | df | Sig. | Exp(B) | |
| Age |
.006 |
.032 |
.034 |
1 |
.853 |
1.006 |
|
| Sex |
-1.105 |
.872 |
1.603 |
1 |
.205 |
.331 |
|
| ASPECTS |
-1.123 |
.991 |
1.284 |
1 |
.257 |
.325 | |
| Baseline NIHSS value |
.011 |
.076 |
.021 |
1 |
.886 |
1.011 |
|
| Occlusion site | 2.010 |
.944 |
4.535 |
1 |
.033 |
7.464 |
|
| Constant |
-2.371 |
2.505 |
.896 |
1 |
.344 |
.093 |
|
Figure 10.
Treatment safety in each occlusion site and in the entire sample.
Figure 11.
Treatment safety in proximal and distal occlusions.
Discussion
Our analysis showed a correlation between occlusion site and outcome of intravenous thrombolysis: patients with distal occlusion tended to respond more favorably to treatment than those with proximal occlusion, as demonstrated by a higher proportion of therapeutic successes and a lower occurrence of hemorrhagic complications. After adjustment for sex, age, ASPECT score on admission and baseline NIHSS value, the influence of occlusion site on treatment outcome was confirmed for both effectiveness and safety. Occlusion site, together with baseline NIHSS value, was the only factor significantly related to clinical response. Occlusion site also proved to be the only valid predictor of the risk of complications.
Our results are consistent with those reported by previous studies which also proved i.v. thrombolytic treatment to be more effective and safer in occlusions of peripheral intracranial arteries. In an angiographic study, Del Zoppo et al. 15 referred increasing rates of recanalization from proximal to distal occlusions of the middle cerebral artery (26%, 38% and 75% for M1, M2 and M3 occlusions respectively). Further evidence was provided by Sims et al. 20 who reported percentages of clinical improvement of 43% and 82% and hemorrhagic complications rates of 23% and 0% in the presence and absence of detectable occlusions in CTA images. Lastly, Saqqur et al. 21 reported recanalization rates of 30% and 44%, percentages of clinical improvement of 15.5% and 33% and prevalence of hemorrhagic complications of 12% and 4% in proximal and distal occlusions of the middle cerebral artery. Based on these and other studies, increasingly broad agreement was reached on the prognostic value of the occlusion site in determining intravenous treatment response and a new scoring system called “Clot Burden Score” was proposed, based on clot extent and location assessed by CTA 22,23. It is interesting to note that two points are attributed to extracranial carotid occlusions and M1 occlusions while only one point is assigned to M2 occlusions, confirming the different meaning of these two conditions in terms of clinical outcome and prognosis.
The most probable reason for the different outcome of intravenous thrombolysis in patients with proximal or distal occlusion in anterior circulation stroke is the different size of the occluding clot. Because of their lower surface-to-volume ratio, large clots in proximal vessels are more resistant to the lytic effect of rt-PA than small clots occluding peripheral branches of the cerebral arterial circulation. This phenomenon was evident in the first experimental studies of the early 1990s designed to evaluate the efficacy of thrombolytic drugs in animals embolized by intracarotid injection of blood clots. These studies showed that treatment success depended on the volume of the injected clots as well as their composition and density 34. These observations were subsequently confirmed also in a clinical setting 14,16,19,23.
The importance of the occlusion site as a key factor in determining treatment outcome is in line with the further observed correlation between treatment effectiveness and baseline NIHSS value. The severity of neurological deficit on admission is also related to occlusion site, as the site determines the extension of the affected brain area.
The different bleeding propensity shown by proximal and distal occlusions may be due to differences in the extension of the area affected by ischemic insult and then subjected to reperfusion injury after spontaneous or pharmacological restoration of blood flow. The occurrence of reperfusion injury is related to ischemia and subsequent cell damage with impairment of antioxidant mechanisms and accumulation of free radicals once the oxygen supply is restored. The higher incidence of parenchymal hematomas in proximal occlusions after thrombolytic treatment could thus be attributable to the greater volume of brain tissue involved.
Our study has certain limitations. First, the small number of patients studied with T and M1 occlusion -partly due to the fact that many of them were treated intra-arterially and so were excluded from analysis- which made it necessary to regroup all sites of occlusion into proximal and distal for a more significant comparison of treatment outcome.
Second, we only studied arterial occlusion through CTA whereas no CT perfusion assessment was made of infarct core vs. ischemic penumbra, which represents the portion of ischemic tissue not yet irreversibly damaged and therefore more likely to benefit from thrombolytic treatment. Thus, differences in treatment outcome may also have been a consequence of the different amount of salvageable tissue rather than only result from the different occlusion level. CTP, in addition to CTA, certainly allows a more accurate diagnosis and characterization of acute stroke and has gradually become part of the Stroke Protocol in many centers. However, CTP was not available for most of our patients, also considering that the first cases date back to 2007.
Finally, it was not possible to compare the outcomes of the studied patients with those of a non-treated group. This prevented us from determining whether the different outcome observed in proximal and distal occlusions should be attributed entirely to the different effectiveness of rt-PA depending on the occlusion site or whether it was also due to intrinsic differences in the natural history of these two conditions. If the natural history does differ, even if the use of intravenous rt-PA in patients with proximal occlusion is less effective than in patients with distal occlusion, it could still be recommended if its ability to determine a significant improvement in the clinical course could be demonstrated. However, given the ethical issues related to an eventual placebo-controlled study, “controls” could be represented by patients who could not receive thrombolytic treatment, although indicated (for example because of symptoms onset > 3 hours before ER admission), who may still be evaluated with unenhanced CT at 24 hours and three-month follow-up to compare their clinical course with that of treated patients.
Conclusions
The results of our CTA-based study demonstrated a correlation between site of arterial occlusion and outcome of intravenous thrombolysis in patients with acute ischemic stroke. Among other possible outcome factors, the occlusion site was the only variable significantly influencing treatment safety and, together with baseline NIHSS value, the only valid predictor of treatment effectiveness. These results confirm that CTA could be added to the examinations included in the Stroke Protocol for the baseline evaluation of patients with suspected acute stroke. Prolonging the duration of the diagnostic phase prior to treatment by only a few minutes, CTA allows the best therapeutic option to be chosen in each case depending on the identified occlusion site: intravenous thrombolysis for patients with distal occlusion and intra-arterial treatment for patients with proximal occlusion, where the benefits of an intravenous approach are usually more modest.
References
- 1.Mori E, Yoneda Y, Tabuchi M, et al. Intravenous recombinant tissue plasminogen activator in acute carotid artery territory stroke. Neurology. 1992;42:976–982. doi: 10.1212/wnl.42.5.976. [DOI] [PubMed] [Google Scholar]
- 2.Haley EC Jr, Brott TG, Sheppard GL, et al. Pilot randomized trial of tissue plasminogen activator in acute ischemic stroke. The TPA Bridging Study Group. Stroke. 1993;24:1000–1004. doi: 10.1161/01.str.24.7.1000. [DOI] [PubMed] [Google Scholar]
- 3.The National Institute of Neurological Disorders and Stroke rtPA Stroke Study Group Tissue Plasminogen Activator for acute ichemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 4.Hacke W, Kaste M, Fieschi C, et al. The European Cooperative Acute Stroke Study (ECASS) Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. JAMA. 1995;274((13)):1017–1025. [PubMed] [Google Scholar]
- 5.The NINDS t-PA Stroke Study Group Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke. 1997;28((11)):2109–2118. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- 6.Brott TG, Haley EC, Jr, Levy DE, et al. Urgent therapy for stroke. I. Pilot study of tissue plasminogen activator administered within 90 minutes. Stroke. 1992;23:632–40. doi: 10.1161/01.str.23.5.632. [DOI] [PubMed] [Google Scholar]
- 7.Haley EC Jr, Levy DE, Brott TG, et al. Urgent therapy for stroke. II. Pilot study of tissue plasminogen activator administered 91-180 minutes from onset. Stroke. 1992;23:641–645. doi: 10.1161/01.str.23.5.641. [DOI] [PubMed] [Google Scholar]
- 8.Marler JR, Tilley BC, Lu M, et al. Early stroke treatment associated with better outcome: the NINDS rt-PA stroke study. Neurology. 2000;55((11)):1649–1655. doi: 10.1212/wnl.55.11.1649. [DOI] [PubMed] [Google Scholar]
- 9.Hacke W, Donnan G, Fieschi C, et al. ATLANTIS Trials Investigators; ECASS Trials Investigators; NINDS rt-PA Study Group Investigators. Association of outcome with early stroke treatmen pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363((9411)):768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 10.The NINDS t-PA Stroke Study Group Generalized efficacy of t-PA for acute stroke: subgroup analysis of the NINDS t-PA Stroke Trial. Stroke. 1997;28:2119–2125. doi: 10.1161/01.str.28.11.2119. [DOI] [PubMed] [Google Scholar]
- 11.Kasim KA, Brizzi M, Petersson J, et al. Combined clinical and radiological prognostic model in acute ischemic stroke. Acta Neurol Belg. 2010;110((3)):239–245. [PubMed] [Google Scholar]
- 12.Saňák D, Herzig R, Zapletalová J, et al. Predictors of good clinical outcome in acute stroke patients treated with intravenous thrombolysis. Acta Neurol Scand. 2011;123((5)):339–344. doi: 10.1111/j.1600-0404.2010.01401.x. [DOI] [PubMed] [Google Scholar]
- 13.von Kummer R, Allen KL, Holle R, et al. Acute stroke: usefulness of early CT findings before thrombolytic therapy. Radiology. 1997;205((2)):327–333. doi: 10.1148/radiology.205.2.9356611. [DOI] [PubMed] [Google Scholar]
- 14.Trouillas P, Nighoghossian N, Derex L, et al. Thrombolysis with intravenous rtPA in a series of 100 cases of acute carotid territory stroke: determination of etiological, topographic, and radiological outcome factors. Stroke. 1998;29((12)):2529–2540. doi: 10.1161/01.str.29.12.2529. [DOI] [PubMed] [Google Scholar]
- 15.Del Zoppo GJ, Poeck K, Pessin MS, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol. 1992;32:78–86. doi: 10.1002/ana.410320113. [DOI] [PubMed] [Google Scholar]
- 16.Linfante I, Llinas RH, Selim M, et al. Clinical and vascular outcome in internal carotid artery versus middle cerebral artery occlusions after intravenous tissue plasminogen activator. Stroke. 2002;33((8)):2066–2071. doi: 10.1161/01.str.0000021001.18101.a5. [DOI] [PubMed] [Google Scholar]
- 17.Derex L, Nighoghossian N, Hermier M, et al. Influence of pretreatment MRI parameters on clinical outcome, recanalization and infarct size in 49 stroke patients treated by intravenous tissue plasminogen activator. J Neurol Sci. 2004;225((1-2)):3–9. doi: 10.1016/j.jns.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 18.Derex L, Hermier M, Adeleine P, et al. Influence of the site of arterial occlusion on multiple baseline hemodynamic MRI parameters and post-thrombolytic recanalization in acute stroke. Neuroradiology. 2004;46((11)):883–887. doi: 10.1007/s00234-004-1279-y. [DOI] [PubMed] [Google Scholar]
- 19.De Silva DA, Brekenfeld C, Ebinger M, et al. Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) Investigators. The benefits of intravenous thrombolysis relate to the site of baseline arterial occlusion in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHE. Stroke. 2010;41((2)):295–299. doi: 10.1161/STROKEAHA.109.562827. [DOI] [PubMed] [Google Scholar]
- 20.Sims JR, Rordorf G, Smith EE, et al. Arterial occlusion revealed by CT Angiography predicts NIH Stroke Score and acute outcomes after IV tPA treatment. Am J Neuroradiol. 2005;26:246–251. [PMC free article] [PubMed] [Google Scholar]
- 21.Saqqur M, Uchino K, Demchuk AM, et al. CLOTBUST Investigators. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke. 2007;38((3)):948–954. doi: 10.1161/01.STR.0000257304.21967.ba. [DOI] [PubMed] [Google Scholar]
- 22.Tan IY, Demchuk AM, Hopyan J, et al. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. Am J Neuroradiol. 2009;30((3)):525–531. doi: 10.3174/ajnr.A1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puetz V, Dzialowski I, Hill MD, et al. Calgary CTA Study Group. Intracranial thrombus extent predicts clinical outcome, final infarct size and hemorrhagic transformation in ischemic stroke: the clot burden score. Int J Stroke. 2008;3((4)):230–236. doi: 10.1111/j.1747-4949.2008.00221.x. [DOI] [PubMed] [Google Scholar]
- 24.The European Stroke Organization (ESO) Executive Committee and the ESO Writing Committee Guidelines for Management of Ischaemic Stroke and Transient Ischaemic Attack. 2008 doi: 10.1159/000131083. http://www.eso-stroke.org. [DOI] [PubMed] [Google Scholar]
- 25.Barber PA, Demchuk AM, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet. 2000;355:1670–1674. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 26.Van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 27.De Haan R, Limburg M, Bossuyt P, et al. The clinical meaning of Rankin “Handicap” grades after stroke. Stroke. 1995;26:2027–2030. doi: 10.1161/01.str.26.11.2027. [DOI] [PubMed] [Google Scholar]
- 28.Wahlgren N, Ahmed N, Dávalos A, et al. SITS-MOST investigators. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369((9558)):275–282. doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- 29.Pessin MS, Del Zoppo GJ, Estol CJ. Thrombolytic agents in the treatment of stroke. Clin Neuropharmacol. 1990;13((4)):271–289. doi: 10.1097/00002826-199008000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Wolpert SM, Bruckmann H, Greenlee R, et al. Neuroradiologic evaluation of patients with acute stroke treated with recombinant tissue plasminogen activator. The rt-PA Acute Stroke Study Group. Am J Neuroradiol. 1993;14((1)):3–13. [PMC free article] [PubMed] [Google Scholar]
- 31.Fiorelli M, Bastianello S, von Kummer R, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke. 1999;30((11)):2280–2284. doi: 10.1161/01.str.30.11.2280. [DOI] [PubMed] [Google Scholar]
- 32.Berger C, Fiorelli M, Steiner T, et al. Hemorrhagic transformation of ischemic brain tissue: asymptomatic or symptomatic? Stroke. 2001;32((6)):1330–1335. doi: 10.1161/01.str.32.6.1330. [DOI] [PubMed] [Google Scholar]
- 33.Trouillas P, von Kummer R. Classification and pathogenesis of cerebral hemorrhages after thrombolysis in ischemic stroke. Stroke. 2006;37((2)):556–561. doi: 10.1161/01.STR.0000196942.84707.71. [DOI] [PubMed] [Google Scholar]
- 34.Overgaard K. Thrombolytic therapy in experimental embolic stroke. Cerebrovasc Brain Metab Rev. 1994;6((3)):257–286. [PubMed] [Google Scholar]
- 35.Barbara C, Rossi D, Procaccianti G, et al. Quantitative determination in the CT diagnosis of ischaemic and heamorragic stroke. The Neuroradiology Journal. 2007;20(Suppl 1):110. P.39. [Google Scholar]
- 36.Simonetti L, Barbara C, Procaccianti G, et al. Is MR really essential in the diagnosis of acute carotid and vertebral dissections? The Neuroradiology Journal. 2007;20(Suppl 1):127–128. P.69. [Google Scholar]
- 37.Barbara C, Stafa A, Procaccianti G, et al. CT-Angiography and thrombolysis in acute ischemic stroke. Correlations between occlusion’s level and nihss improvement. Our experience based on 127 patients. The Neuroradiology Journal. 2009;22:771. P8:091. [Google Scholar]