Abstract
Nanotechnology is an up-and-coming branch of science that studies and designs materials with at least one dimension sized from 1–100 nm. These nanomaterials have unique functions at the cellular, atomic, and molecular levels.1 The term “nanotechnology” was first coined in 1974.2 Since then, it has evolved dramatically and now consists of distinct and independent scientific fields. Nanotechnology is a highly studied topic of interest, as nanoparticles can be applied to various fields ranging from medicine and pharmacology, to chemistry and agriculture, to environmental science and consumer goods.3 The rapidly evolving field of nanomedicine incorporates nanotechnology with medical applications, seeking to give rise to new diagnostic means, treatments, and tools. Over the past two decades, numerous studies that underscore the successful fusion of nanotechnology with novel medical applications have emerged. This has given rise to promising new therapies for a variety of diseases, especially cancer. It is becoming abundantly clear that nanotechnology has found a place in the medical field by providing new and more efficient ways to deliver treatment. Ophthalmology can also stand to benefit significantly from the advances in nanotechnology research. As it relates to the eye, research in the nanomedicine field has been particularly focused on developing various treatments to prevent and/or reduce corneal neovascularization among other ophthalmologic disorders. This review article aims to provide an overview of corneal neovascularization, currently available treatments, and where nanotechnology comes into play.
Introduction
Corneal neovascularization (NV) is the formation of new vascular structures in areas that were previously avascular. The mechanisms that may be involved in NV regulation are vasculogenesis—the formation of new blood vessels from bone marrow–derived angioblasts (mainly during embryogenesis)—and angiogenesis—the formation of new vessels from preexisting vascular structures.4 Corneal angiogenesis results from a wide variety of diseases and conditions, but most of these pathologies are associated with hypoxia, inflammation, and/or limbal barrier function.5,6 Therefore, it is no surprise that the most affected populations are patients who wear contact lenses, have had corneal transplants, or suffer from an infectious disease of the eye. In general, patients who have sustained a traumatic eye injury (physical insult, infectious disease, or inflammation) are at risk of developing corneal angiogenesis, which can have detrimental effects on the patient's vision. In 2010, 42,642 corneal transplants were performed in the United States alone compared to just over 12,000 in the two preceding years,7 indicating the increasing prevalence of the operation. As of 2007, there were also 35 million patients wearing contact lenses.8 From these statistics alone, one can see the large size of the population at risk of developing corneal NV. Currently, the treatments for corneal NV include corticosteroids and nonsteroidal anti-inflammatory eye drops, photodynamic therapy, photocoagulation, and intravitreal/subconjunctival injections of antibody against vascular endothelial growth factor-A (VEGF-A).9 While useful, these therapies face an array of obstacles based on the unique physiological composition of the eye. As a result, the efficacy of these therapies is limited. The difficulties associated with current treatment options highlight the need for newer drugs that are not subject to the same physiological barriers. This need has prompted researchers to study the use of nanoparticles in the treatment of eye-related diseases intensively. In the future, these therapies could become mainstays for the treatment of corneal angiogenesis as well as a host of other non-ocular diseases, such as cancer, Parkinson's disease, and Alzheimer's disease.10,11 This review examines the current body of literature for the ways in which ocular disorders are currently treated and considers how nanotechnology might offer new therapeutic options for the management of corneal NV as well as other diseases.
Angiogenesis in the Anterior Segment
Angiogenesis is the result of an upregulation of angiogenic factors with concurrent downregulation of antiangiogenic factors owing to some kind of injury or inflammation within the eye.9 These factors help shape vascular growth by inducing endothelial cell migration and proliferation and promoting the formation of a capillary tube as part of the wound-healing process.12,13 Therefore, it is no surprise that an injury to the cornea engenders the same response that occurs in the rest of the body. However, in the case of the cornea, NV leads to severe disturbances in vision. Before delving into how NV is treated and how nanomedicine can help shape new therapies, one must first understand the basic principles of corneal angiogenesis.
Corneal NV is one of the most common sources of vision loss and blindness,14 underscoring the importance of effective therapies for treating patients that display corneal NV. To treat corneal NV effectively, the therapy must target the source. In the case of the cornea, NV occurs as a result of the presence of members of a family of related proteins termed vascular endothelial growth factors (VEGFs) and other potential players, such as platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), and interleukin (IL)-1β and IL-8.15 VEGF-A in particular is a potent factor for angiogenesis. This angiogenic factor binds the receptors, VEGFR-1 (Ftl-1) and VEGFR-2 (KDR/Flk-1), stimulating autophosphorylation of specific receptor tyrosine residues.5 These autophosphorylated receptors trigger a signaling cascade that ultimately results in the transcription of genes that promote vascular endothelial cells to mobilize, grow, and divide. This process leads to angiogenesis within the cornea.14 Another important angiogenic factor is bFGF. In the cornea, this growth factor is normally found only in epithelial cells, but is absent from the corneal epithelial cell basement membrane. However, in the case of an injury to the cornea, the ruptured cells release bFGF, which binds to bFGF receptors (FGFRs)—also tyrosine kinases—in fibroblasts located near the basement membrane. This stimulates a process similar to that initiated by VEGF, resulting in transduction of a transcription signal.14 In both cases, the end result is the promotion of angiogenesis. A third class of factors important in corneal angiogenesis is the matrix metalloproteinases (MMPs).16–18 MMPs cleave the basement membrane, allowing vascular endothelial cells to mobilize toward the site of injury.14 All of these factors provide possible targets for therapeutic intervention.
Diseases Related to Corneal NV
Various disease states, such as infection, inflammation, and traumatic injury, can lead to corneal NV. Neovascular patterns can be categorized into three clinical groups.5,6,9,19 The first is deep NV that overlies Descemet's membrane, as seen in herpetic and interstitial keratitis. The second is stromal NV, which can result from stromal keratitis or alkaline injury. The third is vascular pannus, a consequence of ocular surface disorders. The most common culprit in corneal NV is infection. For example, infectious keratitis frequently leads to corneal NV and is usually caused by infection by members of the herpes virus family. Not much is known about the mechanisms by which these viruses induce NV and VEGF upregulation, but it is hypothesized that IL-6 and MMP-9 play a role.15 Viruses are not the only culprits responsible for keratitis; bacterial and fungal infections can also produce this pathology. A bacterial infection brought on by chlamydia trachomatis is the world's leading infectious cause of blindness.20 Inflammation and trauma to the eye can also occur with contact lens overuse, chemical burns, and limbal stem cell deficiency, all of which lead to corneal NV. Corneal NV may also present secondary to autoimmune diseases, such as rheumatoid arthritis and Sjörgen's syndrome. For example, keratoconjunctivitis sicca due to Sjörgen's syndrome is the most common ophthalmic manifestation of rheumatoid arthritis and leads to corneal NV.21 Other degenerative diseases, such as pterygium and Terrien's marginal degeneration, can also lead to corneal NV.15 Recently, ectrodactyly-ectodermal dysplasia-cleft lip and palate (EEC) syndrome was linked to corneal NV. EEC is a rare autosomal-dominant form of multiple congenital anomaly with variable expression and reduced penetrance. A study conducted by Felipe et al. described corneal changes in three unrelated patients with EEC syndrome.22
Allograft rejection after corneal transplantation is one major issue arising from corneal NV in these patients. Transplantations are termed “high risk” when grafting onto a vascularized corneal bed. Corneal transplantation has an impressive 90% success rate and is one of the most performed graft procedures worldwide. However, in cases where the surgery is high risk, the success rate is drastically reduced to 50%.23 Minimization of the inflammatory response reduces corneal NV after corneal transplantation and increases graft survival.
As noted above, another major source of corneal NV is hypoxia caused by extended use of contact lenses. In this setting, hypoxia induces the translocation of the transcription factor hypoxia-inducible factor 1α (HIF-1α) into the nucleus and binding to HIF-1β to form HIF-1. HIF-1 is then able to bind to the hypoxia response element (HRE) in the promoters of VEGF genes.24 Binding of HIF-1 to the HRE region then activates transcription of the VEGF genes, increasing the levels of these potent activators of corneal angiogenesis. A study by Chen et al. demonstrated that VEGF and HIF-1α expression are upregulated after extended contact lens wear in a mouse model,24 providing further evidence that HIF-1α and VEGF cooperate in the induction of corneal NV. However, this group also noted that inhibiting HIF-1α altered the expression of several other genes related to angiogenesis. In particular, they showed that knocking down HIF-1α using short hairpin RNA (shRNA) decreased the expression of IL-1β as well as MMP-2/9 compared to negative control mice administered only saline. Similar to the pattern described above, this pathway involves production of IL as a byproduct of the inflammatory process as well as MMPs as critical angiogenic factors. The importance of this observation is that hypoxia causes NV through two processes: one involving the expression of VEGF, and the other involving the expression of inflammatory factors.
Regardless of the source, corneal NV represents a major complication in transplant rejection, infection, and injury. Understanding the etiology of corneal NV has been hampered by the complexity of the multiple overlapping pathways that may be at work. Nonetheless, elucidating these pathways allows researchers to develop drugs that inhibit angiogenesis by targeting the factors involved in the process. In the following section, the current treatments and their limitations as anti-angiogenic factors in relation to anterior segment disorders is examined, laying the groundwork for understanding the importance of developing novel therapeutic options.
Current Treatment Options for Corneal NV
Ocular anterior segment disorders are restricted to the structures anterior to the vitreous humor, which includes the anterior and posterior chambers and the aqueous humor filling them. The major treatment for ocular anterior segment disorders is drug therapy. However, ocular therapy faces numerous obstacles that impede adequate drug delivery and efficacy. Such obstacles may include various cellular barriers in the eye, such as the blood–aqueous and blood–retinal barriers. The most common therapy for diseases of the anterior segment are topical drugs in the form of eye drops.25 These are the most accessible and the least invasive form of treatment for ocular disease. Unfortunately, regardless of the drugs they carry, many eye drops are limited in their ability to treat these diseases to their fullest potential due to the lipophilic and hydrophilic nature of the cornea, which acts as a protective barrier.26–29 Further, eye drops are washed away by tear fluid, limiting the bioavailability of the drugs they carry.25 Because of these physiological barriers, topical eye drops must be applied multiple times throughout the day. They also irritate the eye and produce temporary blurred vision after application, causing discomfort to the patient and leading to poor patient compliance.30,31 The physiological barriers that limit the efficacy of eye drops also produce limitations for drugs delivered via subconjunctival and intravitreal injections. In addition, these methods can be more invasive and expensive. Current treatments for corneal NV can be divided into anti-VEGF-A treatments and non-VEGF-A treatments.
Anti-VEGF treatments
Numerous anti-VEGF-A treatments have demonstrated positive results in the treatment of corneal NV. Whether of traumatic, inflammatory, or hypoxic origin, corneal diseases are associated with upregulation of a number of cytokines and growth factors that induce infiltration of neutrophils, macrophages, and lymphocytes.32 This inflammatory response, which can be coupled to trauma or hypoxia, is often accompanied by an angiogenic response. As mentioned above, studies have also shown that VEGF is a major inducer of corneal NV in both experimental models and human corneas. Therefore, antiangiogenic therapies that target VEGF-A or other VEGF-related molecules are becoming the “gold standard” in corneal NV treatment. Anti-VEGF-A and other VEGF-related treatments use topical drops, subconjunctival injections, and intravitreal injections to deliver the therapeutic agent.19
A study by Hashemian et al. comparing bevacizumab (Avastin) with betamethasone (a corticosteroid) showed that a single subconjunctival injection of bevacizumab prevented the formation and promoted the regression of major vessels compared to a single subconjunctival injection of betamethasone in a rat model of corneal NV.32 The study employed male Sprague–Dawley rats (n=100) whose corneas were chemical cauterized using silver nitrate/potassium nitrate sticks. The rats were randomly divided into 10 treatment groups. Groups 1–5 were treated immediately after cauterization, and groups 6–10 were treated 7 days after cauterization. The numbers of major vessels originating from the limbus and reaching the corneal scar were counted 7 days after cauterization in groups 1–5 and 14 days after cauterization in groups 6–10. Animals in groups treated with the highest two doses of bevacizumab (5 and 25 mg/mL) exhibited a significant reduction in the number of major vessels whether treated immediately after cauterization or 7 days after cauterization. Similarly, a recent study conducted by Sener et al. investigated the inhibitory effects of subconjunctival application of various VEGF antibodies, including bevacizumab, ranibizumab, and pegaptanib, as well as the Human Epidermal Growth Factor Receptor (HER)-2 antibody, trastuzumab, in a rat model of experimental corneal NV.33 These VEGF antibodies were all found to be effective in reducing corneal NV. However, bevacizumab was found to be the most effective of the group. With its high success rate, bevacizumab has moved into clinical trials, with various studies currently ongoing. For example, Bock et al. investigated the ability of bevacizumab eye drops to inhibit corneal NV.34 Five patients (aged 42±14 years) with aggressive corneal NV who were unresponsive to conventional therapy were treated with bevacizumab eye drops (5 mg/mL) five times daily for 0.6 to 6 months. Bevacizumab was well tolerated and caused no obvious corneal side effects, and all patients showed a reduction in the area of NV. Although numerous studies have found bevacizumab to be the most effective therapy, this is not a universal finding. For instance, a comparative study of ranibizumab (Lucentis) and bevacizumab conducted by Stevenson et al. suggested that ranibizumab may be modestly more effective than bevacizumab in terms of both onset of action and efficacy.20 However, ranibizumab (also a monoclonal antibody Fab fragment derived from the same parent mouse antibody as bevacizumab) is more costly.
The use of small interfering RNA (siRNA) to silence VEGF genes is another anti-VEGF therapeutic approach. siRNA is double-stranded, and after processing by Dicer (an RNAase III enzyme), these double-stranded RNA fragments insert into the RNA-induced silencing complex, resulting in sequence-specific degradation of the target mRNA. The unique advantage of siRNA over anti-VEGF antibodies is that siRNA is capable of traversing cellular boundaries and inhibiting post-translational processing. In this way, siRNA can target both extracellular and intracellular VEGF and its receptors, something anti-VEGF antibodies cannot do. However, siRNA may only have a transient effect on mammalian cells because it is not replicated. At present, siRNA therapeutic agents such as bevasiranib and siRNA-207 are being tested.35,36 However, siRNA may have some limitations, as it may be too short to be recognized by Dicer, and it may require a vehicle to be delivered into the cell and modification to bypass the TLR-3 innate immunity receptors to which it binds nonspecifically.37,38
It is important to keep in mind that the counterbalancing mechanism that regulates angiogenesis is the overproduction of angiogenic stimulators (VEGF) and the underproduction of angiogenic inhibitors (e.g., angiostatin, endostatin).15,39–41 Because this balance is so delicate, therapeutic alternatives targeting angiogenic inhibitors are as essential as those targeting angiogenic stimulators. In one study of angiogenic inhibitors, Zhang et al. examined the effects of plasminogen kringle 5 (K5), a proteolytic fragment of plasminogen that functions as a potent angiogenic inhibitor by preventing the proliferation of endothelial cells. In this study, which employed a rabbit model of alkali (NaOH)-burn–induced corneal angiogenesis, these authors showed that subsequent topical application of K5 (eye drops, four times daily) inhibited the development of both corneal NV and inflammation.42 Using a slit lamp microscope to monitor corneal NV (including the length of vessels in the cornea) and inflammation, they found that K5 delayed the onset of corneal NV and decreased NV areas in a dose-dependent manner. Moreover, K5 induced the regression of newly formed vessels and decreased the inflammatory index in corneas at different time points after the alkali burn. This study further showed that K5 inhibited VEGF-induced endothelial cell proliferation. Thus, topical application of K5 eye drops inhibited and reduced corneal NV. This was achieved in these animal models without a detectable immune response or toxicity. VEGF expression, as examined by immunohistochemical and Western blot analyses, was increased in the cornea. Apoptosis of corneal endothelial cells, as assessed using the TUNEL assay, was also increased by K5. The effects of K5 were also examined in primary bovine aortic endothelial cells (BAECs). Using MTT assays, flow cytometry, transmission electron microscopy, and DNA fragmentation assays, these authors showed that K5 inhibited proliferation and induced apoptosis in BAECs.42
Antisense oligonucleotides with a base sequence complementary to a specific mRNA are able to modulate the expression of specific genes containing the corresponding sequence. Antisense oligonucleotides have been used to target insulin receptor substrate (IRS-1), a cytosolic adapter protein involved in the recruitment of proteins to surface receptors that does not contain intrinsic kinase activity. In addition, IRS proteins have the ability to associate with integrins, which may intervene in the regulation of cell growth. The use of antisense oligonucleotides targeting IRS-1 has an antiangiogenic effect that can also be used therapeutically to restore homeostasis when the pro- and anti-angiogenic balance has been disturbed. Using this approach, Andrieu-Soler et al. treated rat eyes containing neovessels with subconjunctival injections of IRS-1 Antisense oligonucleotides, IRS-1 sense oligonucleotide, or PBS for 4 to 9 days and then evaluated corneas on day 10 for corneal NV.43 Western blot analyses revealed that IRS-1, VEGF, and IL1-β mRNA expression were modulated 8 and 24 hours after the first subconjunctival injection. Importantly, this study showed that inhibition of IRS-1 expression using a specific phosphorothioate antisense oligonucleotide efficiently reduced the growth of neovessels in the corneal model of NV. The use of antisense oligonucleotides has already moved into a Phase II clinical trial with studies of gene signal (GS)-101, an antisense oligonucleotide that inhibits the expression of IRS-1 and is designed only to target currently growing neovessels. In this randomized, double-blind, multicenter, dose-determining study, four groups of 10 patients were treated with three doses of GS-101 (43, 86, and 172 μg/day total) or placebo administered twice daily in eye drops. The results showed that GS-101 eye drops were well tolerated at an optimal dose of 86μg/day, which specifically inhibited and promoted the regression of NV, providing an effective and noninvasive approach for the treatment of active corneal angiogenesis.43
The problem with the use of any of these therapies, both VEGF related and non-VGF related, is that the mechanism by which the drugs are introduced into the eye is not efficacious. Suspensions eye drops are quickly cleared, intravitreal and subconjunctival injections may lead to inflammation, and systemic dosing may lead to cytotoxicity. These limitations have prompted researchers to develop novel agents for the treatment of ocular disorders. The solution to the problems caused by the physiological barriers of the eye may be solved through the use of nanotechnology.
Non-VEGF treatments
The term “non-VEGF-related treatment” is used here to refer to any treatment of corneal angiogenesis that does not directly inhibit the VEGF protein (e.g., an antibody against VEGF-A). These treatments can be generally grouped into anti-inflammatory agents, proteinaceous factors, and laser therapy.
Since 1950, the antiangiogenic effects of steroids have been well documented.44 Because steroids inhibit inflammation, it is reasonable to surmise that angiogenesis is also related to inflammation. Indeed, this has been shown to be true. The list of commonly used steroids includes cortisone, dexamethosone, prednisolone, and methylprednisolone, among others. The overall antiangiogenic effect of steroids results from multiple properties, including inhibition of chemotaxis, modulation of proteolytic functions of vascular endothelial cells, and inhibition of proinflammatory cytokines. Furthermore, steroids also inhibit prostaglandin production, which, as will be shown below, is a regulator of VEGF expression.44 Clinical studies have shown that the use of steroids both pre- and postkeratoplasty decreases the rate of rejection due to corneal NV.45
It has been established that the inflammatory response plays an important role in angiogenesis, as evidenced by the concurrent upregulation of cyclooxygenase (COX)-2 and prostaglandins (PGs) with angiogenesis. Studies have shown that COX-2 and PGs regulate VEGF expression by inducing transcription of VEGF genes and also regulate genes necessary for the inflammatory response. A proposed mechanism for the role of PGE2 in angiogenesis posits that PGE2 induces expression of the chemokine receptor CXCR4, which functions in endothelial cell migration. In any case, it is clear that components of the inflammatory response play an important role in angiogenesis.46 Knowing this, researchers have examined nonsteroidal anti-inflammatory drugs (NSAIDs) as a means for reducing corneal angiogenesis. For example, Monnier et al. established that NSAIDs and COX-2 inhibitors effectively reduced corneal angiogenesis in a mouse model.46 In a clinical setting, this could mean that NSAIDs in conjunction with other treatments might help improve patient outcome.
In addition to the anti-inflammatory treatments available, other proteinaceous factors have shown promise in inhibiting angiogenesis in an indirectly VEGF-related manner. One example is a recent study of netrin-1 on corneal angiogenesis. Netrin-1 is expressed in many different tissues in mammals, including mammary glands, pancreas, lungs, kidneys, intestines, liver, and spleen.47 Historically, the role of netrin-1 in angiogenesis has been a source of debate, with some studies showing that netrin-1 is an antiangiogenic factor and others showing that it has proangiogenic properties. In a study by Han et al., mouse corneas were subjected to alkali burns and the effect of topically administered netrin-1 on corneal angiogenesis was examined. These authors established that the netrin-1 receptor, UNC5B, is highly expressed in corneal stroma after wounding and showed that corneal edema and corneal scarring were reduced in mice treated with netrin-1 eye drops compared to control (PBS-treated) mice 24 days after alkali burn. More importantly, netrin-1 reduced both the area and length of new blood vessels formed through angiogenesis. Armed with the knowledge that angiogenesis is the result of an imbalance between angiogenic and antiangiogenic factors, these researchers then looked at netrin-1's effect on the expression of VEGF and the natural antiangiogenic compound, pigment epithelium-derived factor (PEDF). Western blot analyses revealed that the levels of VEGF on day 24 were reduced and those of PEDF were increased in netrin-1 treated mice, effects not observed in PBS-treated controls. Taken together, these results showed that netrin-1 effectively reduced corneal NV in vivo and demonstrated its potential use in suspension eye drops.
A similar study evaluated the effects of SERPINA3K, a natural antiangiogenic protein,48 on corneal angiogenesis. Similar to the experiment described above, mice were subjected to an alkali burn and then treated with either a PBS control or SERPINA3K eye drop solution. Eight days after alkali burn, the area of corneal NV was smaller in mice treated with SERPINA3K, and the cornea appeared more transparent and with less edema compared to control mice.48 A Western blot analysis of the cornea performed 8 days after alkali burn showed that mice treated with the SERPINA3K expressed much lower levels of VEGF and higher levels of PEDF.48 From these data, it is clear that netrin-1 and SERPINA3K are two factors that show considerable promise in the inhibition of corneal angiogenesis.
In addition to drug treatments, more invasive interventions can be used to treat corneal NV in a VEGF-independent manner. One such technique is the use of an argon laser. In this technique, laser light is used to ablate new blood vessels in the cornea. The premise of this technique is that the absorption rate of argon energy by hemoglobin is high enough to cause coagulation of the blood in the vessels.44 One clinical study observed that rejection episodes were reversed using argon laser therapy. The patients in the study suffered from corneal NV related to transplant rejection that was unresponsive to steroid treatment.44,49 Photodynamic therapy, a similar process, uses a laser to activate a photosensitizing compound that is preferentially targeted to the neovasculature. The activated compound generates free radicals, which are cytotoxic to vascular endothelial cells, thereby destroying blood vessels.44 Although both techniques have shown success in a clinical setting, they both suffer from the same flaw: high-energy lasers can result in activation of the inflammatory response, which can exacerbate the problem.
Another surgical technique that can be used is fine-needle diathermy. In this procedure, a needle is inserted within the lumen of, or adjacent to the neovasculature and an electrical or thermal current is used to cauterize the vessels. In a pioneering clinical study, Pillai et al. treated 14 patients with an established history of corneal NV.50 In 8 of the 14 patients, the surgeons achieved successful occlusion of all of the neovasculature; in four patients, 75% of the vessels were occluded; and in two patients, 50% of vessels were occluded. Used in conjunction with anti-VEGF therapy, the full occlusion rate improved to 11 of 16 patients. Despite these promising results, this technique may also evoke an inflammatory response, as seen with laser therapy.51
Nanotechnology Drugs and Targeting
The need to develop new drugs for diseases that have been notoriously difficult to treat has led to the use of nanotechnology in medical applications, an emerging field known as nanomedicine. This field utilizes small particles with a size less than 100 nm to treat various disorders.52 Nanomedicine offers several advantages over traditional therapies. Figure 1 summarizes the different types of nanocarriers and how they compare in size to other biological components. The unique multifunctional properties of nanomaterials make them suitable for therapeutic use and enable them to overcome many physiological barriers. The versatility in nanomaterial synthesis can also provide opportunities for structural modification to impart properties such as biodegradability, stimuli-responsiveness, and attachment of targeting and imaging agents. Nanotechnology applications are capable of achieving enhanced drug permeation, controlled drug release, and drug targeting.53 For example, nanocarriers such as polymer–drug conjugates have demonstrated better biodistribution profiles and longer blood circulation times compared to the free drug, especially with the use of targeting moieties that home the therapeutic agent to its target tissue.52–54 This provides a solution to the rapid clearance of free drugs, which often results in a rapid loss of therapeutic efficacy.55
FIG. 1.
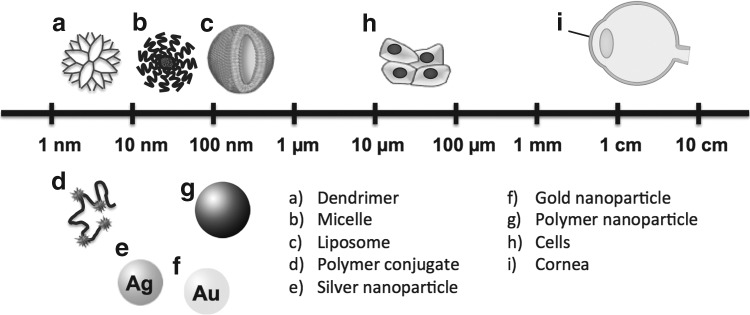
Scale comparison of various nanocarriers to cells and a cornea.
Nanomedicine has also found applications in ophthalmology, by providing safer, less invasive, and cheaper treatment options. For example, chitosan nanoparticles have been shown to be beneficial in certain ocular surgeries by treating and preventing postsurgical scarring.56
The primary goal of using nanotechnology for the treatment of ocular diseases is to achieve targeted delivery, controlled release, enhanced pharmacokinetics, and improved efficacy.57 The bioavailability of a drug in the ocular environment is one of the major barriers to effective drug therapy. Unlike conventional topical treatments, nanocarriers can interact with the unique chemical composition of the cornea, allowing for longer residence times and resistance to ocular clearance mechanisms. As reported by Bhatta et al., natamycin-encapsulated licithan/chitosan nanoparticles prolonged ocular exposure to the drug by 1.5 times and decreased clearance by a factor of 7.4 compared to commercially available suspensions.58 A separate study using polyanhydride nanoparticles to deliver mamantine into the eye showed that nanoparticles sustained drug release for 15 days following an initial burst release.59 This suggests that drug therapy using these types of nanocarriers allows for extended, continuous release of the drug, which overcomes another obstacle facing conventional treatment options. In another study, 20-nm gold nanoparticles (Au-NPs) were able to pass through the blood–retinal barrier and associate with retinal-epithelial cells, retinoblastoma cells, and astrocytes without any observed toxicity.60 The mechanism for this passage was not determined, but it was hypothesized that the size of the nanoparticles as well as their shape and chemical composition could play important roles in this process. This study demonstrates the ability of such nanoparticles to bypass the blood–retinal barrier, a critical step in drug delivery and yet another barrier in conventional ocular drug treatments. Moreover, the lack of cytotoxicity means that a therapy utilizing Au-NPs would not produce unwanted side effects, which can also occur with current therapeutic options.60 The three studies summarized above are just a few of many that have demonstrated the great potential of different types of nanocarriers in this area.57
Nanotechnology for Ocular Delivery
A number of nanocarriers, including polymeric nanoparticles, micelles, liposomes, and polymer–drug conjugates, among others, have been developed to deliver drugs for various therapeutic applications. The materials that constitute these systems are often decorated with targeting moieties and imaging tags to aid in the delivery of therapeutic payloads to target tissues. An ideal nanocarrier for ocular delivery should meet several criteria in order to achieve an optimal outcome. For example, particle size should be small to minimize irritation. Moreover, the nanocarrier itself should be sufficiently bioavailable to provide adequate treatment. It should ideally be in a suspension form to ensure high therapeutic efficacy, and it should be either biodegradable or biocompatible to avoid producing unwanted side effects such as cytotoxicity.57
Nanoparticles have been typically administered via intravitreal injections into the eye. However, this procedure is invasive and has a high degree of risk. An alternative that satisfies the need for less invasive and safer delivery methods is to combine nanotechnology with contact lenses, which are commonplace devices used by millions of people every day.8 The goal in using contact lenses for drug delivery is to increase the amount of time drugs reside on the corneal surface without being cleared via the nasolacrimal system. On the basis of modeling studies, it has been postulated that 50% of a drug released from contact lenses could be absorbed by the cornea. However, in vivo studies are necessary to confirm this mathematical prediction. In this context, it has been shown that contact lenses coated with poly(lactide-co-glycolic acid) (PLGA), an FDA-approved biodegradable polymer that is widely used for various biomedical studies,61 are able to control drug release kinetics.62 However, these nanoparticle-coated contact lenses for drug delivery still face challenges, including the requirement that they be worn for a longer period of time, causing discomfort; the fact that drugs are released in the same pre-lens tear film, leaving them susceptible to clearance; and the potential for the drug to become trapped in the hybrid hydrogel matrix of the contact lenses due to its slow release from the nanoparticles, highlighting the importance of sufficient drug shelf life to maintain its efficacy.8
Polymeric micelles are nanoparticles that self-assemble as a result of amphiphilic interactions.63 An important characteristic of micelles is their core–shell structure. The core contains the hydrophobic constituents of the nanoparticle matrix and typically constitutes the depot for therapeutic drug, whereas the hydrophilic shell provides interactions with solvents, thereby stabilizing and prolonging the half-life of the therapeutic drug.64,65 Furthermore, polymeric micelles are biodegradable and biocompatible, thus preventing adverse effects. Polymeric micelles have attracted considerable interest in the drug-delivery field and offer a number of benefits in ocular delivery.8 In a series of in vivo studies, Di Tommaso et al. demonstrated that polymeric micelles have the ability to overcome ocular surface barriers, thus providing a drug reservoir in the cornea that leads to effective prevention of corneal graft rejection.23 Studies have also shown that polymeric micelles increase the ocular permeability of various drugs.66 Thus, polymeric micelles as a delivery mechanism, promises to improve both drug stability and function, thereby prolonging drug efficacy.
Microemulsions have proven to be an interesting alternative because of their intrinsic properties and specific structures. Microemulsions are clear, stable mixtures of water, oil, and surfactant in combination with a cosurfactant to help stabilize the system.67,68 They can be administered in suspensions, whereby they help minimize the limitations presented by typical eye drops. When so administered, the presence of surfactant and cosurfactant molecules enhances membrane permeability, thereby increasing drug uptake and passage through the corneal membrane. Adding to the benefits of microemulsions is their transparency, thermodynamic stability, and small droplet size in the dispersed phase.57 Although there is no physical difference between micelles and microemulsions, a distinction in nomenclature has arisen because of an industrial aspect. Microemulsions have a more complicated composition, which can include up to five components: water, oil, a surfactant, a cosurfactant, and salt.57
Liposomes are composed of a lipid bilayer and function as artificial vesicles. Liposomes have a biphasic nature reflected in their lipophilic lipid bilayer and hydrophilic interior aqueous compartment. Thus, liposomes can encapsulate hydrophilic and/or lipophilic therapeutic agents.69 The surface charge of liposomes is a characteristic of special importance to the cornea. The corneal surface is negatively charged and therefore favors positively charged liposomes over negatively charged and neutral liposomes. Moreover, current research suggests that absorption of encapsulated drugs across corneal membranes can be enhanced by positively charged liposomes.70 Liposomes have shown enhancement in precorneal retention, sustained drug release, and transcorneal permeation. Despite these advantageous properties, liposomes still face challenges in application due to their short shelf life and limited drug-loading capacity.8,57
Niosomes are nonionic surfactant vesicles. Like liposomes, niosomes have a biphasic nature that enables them to entrap lipophilic and hydrophilic drugs.71,72 Preference is given to niosomes for topical ocular delivery for various reasons: they are chemically more stable; they are less toxic due to their nonionic nature; they improve drug performance through better bioavailability and controlled delivery at specific sites; and they are nonimmunogenic, biodegradable, and biocompatible.8,57
Dendrimers are nanometer-size, three-dimensional, hyperbranched, monodisperse, multifunctional macromolecular structures that have been widely explored for various biomedical applications.73,74 Dendrimers, which are typically 1–10 nm in size, are effective therapeutic drug carriers for various reasons. Notably, the highly branched structure of the dendrimer allows easy functionalization of its surface.75,76 Dendrimers have also been used to encapsulate drugs in their cores in the form of host–guest inclusion complexes.75,76 Poly(amidoamine) (PAMAM) dendrimers, which are among the most widely investigated family of dendrimers for biological applications, have been shown to improve ocular residence time. On the downside, dendrimers have been reported to cause blurred vision in some animal models.8
Cyclodextrins (CDs) are a series of cyclic oligosaccharides capable of forming inclusion complexes with therapeutic drugs, thus improving their pharmacokinetic properties.77,78 Ophthalmic formulations co-administered with CDs enhance corneal penetration, ocular absorption, and efficacy of poorly water-soluble drugs, such as dexamethasone, cyclosporin, and acetazolamide. Moreover, cytotoxicity studies have shown that orally administered CDs are practically nontoxic,8 highlighting another positive aspect of CDs. CDs, as well as other nanocarrier systems described above, represent highly promising ocular drug-delivery systems that merit further research.
Gold and silver nanoparticles (Au-NPs and Ag-NPs respectively) conjugated with a heparin derivative have demonstrated efficacy as anti-angiogenesis agents.79 Studies of both cancer- and ocular-therapy applications have shown that Au-NPs and Ag-NPs conjugated with a heparin derivative are effectively delivered to their target, where they bind VEGF receptors to inhibit the actions of VEGF via different signaling pathways and ultimately inhibit angiogenesis. Thus, both Au-NPs and Ag-NPs conjugated with antiangiogenic factors clearly have antiangiogenic properties that make them excellent candidates for biomedical use. The unique properties of these metal particles also confer excellent stability at physiological salt concentrations, making them useful tools for coupling antiangiogenic factors in ocular drug therapy, among other biomedical applications.
Nanotechnology in Corneal Angiogenesis
Currently, one of the main treatments for angiogenesis-related blindness is monthly, intravitreal injection of bevacizumab (Avastin), an antibody that targets VEGF-A.80 The drug functions by directly inhibiting the angiogenic factors that vascularize the cornea, which in turn inhibits corneal angiogenesis.81 Anti-VEGF injections are expensive, and their efficacy is limited by the bioavailability of the drug. By improving bioavailability, nanotechnology may offer a solution, providing effective inhibition of the angiogenic factors that can lead to blindness.
Table 1 outlines the various nanocarrier systems used for corneal NV treatment. In a study using polymeric nanoparticles for antiangiogenesis therapy by Singh et al., PLGA-based nanoparticles were used for targeted nonviral retinal gene delivery for the management of choroidal NV.82 Seemingly unconventional by current standards, this approach involved the generation of an anti-VEGF intraceptor plasmid termed Flt23K encoding a synthetic construct composed of the VEGF-binding domains 2 and 3 of VEGFR-1. These domains were then coupled to the KDEL endoplasmic reticulum-retention sequence, which functions in sequestering proteins within the endoplasmic reticulum. The nanoparticles were then coated with linear sequences of arginine-glycine-aspartic acid (RGD) peptides, tranferrin or both, and tested in a rat model of choroidal NV. Upon intravenous administration, nanoparticles exhibited targeted delivery to the neovascular eye but not the control eye. In general, the presence of transferrin, RGD peptide, or both resulted in increased retinal delivery of the nanoparticles and subsequent gene expression compared to nonfunctionalized nanoparticles. Moreover, choroidal NV areas were reduced by 73.3%, 56.5%, and 46.7% in rats treated with RGD, transferrin, and dual-targeted nanoparticles, respectively, compared to rats treated with nontargeted nanoparticles.82 Thus, these nanoparticles allowed targeted gene delivery to the neovascular eye after intravenous administration and inhibited the progression of choroidal NV. Importantly, the nanoparticles did not accumulate in organs other than the eye, reducing the possibility of cytotoxicity to other tissues. The authors attributed the high efficiency of the RGD nanoparticles in particular to the fact that RGD binds to integrin αvβ3,82 which is overexpressed in ocular NV. This study demonstrates that therapies based on nanotechnology have the capacity to target unique components of the angiogenesis cycle in a very different manner than conventional therapies. For this reason, new therapies should aim to target different aspects of the angiogenesis pathway. For example, the activator, receptor, signaling pathway, and downstream gene expression are all suitable targets for novel therapies.
Table 1.
Advances in Nanotechnology Research for Corneal Neovascularization Therapy
| Nanomaterial | Formulation | Administration | Ref. |
|---|---|---|---|
| Dendrimers | Dendrimer porphyrins (DP) | Intravenous injection | 86 |
| Micelles | DP-encapsulated PEG-PLL micelles | Intravenous injection | 86 |
| PEG-b-P[Asp(DET)] encapsulating sflt-1 | Subconjunctival injection | 85 | |
| PEG-PCL encapsulating celastrol | Subconjunctival injection | 87 | |
| Nanoparticles (NPs) | Albumin NPs encapsulating Flt23K | Intrastromal injection | 88 |
| PLGA NPs encapsulating Flt23K | Intrastromal injection | 82 | |
| PLGA NPs encapsulating VEGF-A shRNA | Intrastromal injection | 89 |
PEG-PLL, polyethylene glycol-b-poly(L-lysine); PEG-b-P[Asp(DET)], polyethylene glycol-b-poly(N-(2-aminoethyl)-2-aminoethyl aspargine; PEG-PCL, polyethylene glycol-b-poly(ɛ-caprolactone); PLGA, polylactide-co-glycolide.
One problem with conventional gene therapy in the treatment of corneal NV is the lack of highly efficient vectors with low toxicity. Adenoviral vectors can cause toxicity and evoke immune/inflammatory responses.78 Adeno-associated viruses (AAVs) and gutless adenoviruses have emerged as an attractive alternative to adenoviral vectors due to the significant reduction in immunogenicity and good tissue penetration in the case of AAVs.83 Intrastromal injection of naked DNA, while safe and possibly effective, is not a viable option for repeated use because it is invasive and can lead to edema and inflammation.84 Therefore, nonviral vectors appear to be the most useful alternatives.
In a recent study of gene therapy in corneal angiogenesis, Iriyama et al. designed micellar nanovectors as nonviral gene delivery vectors.85 These micelles were composed of polyethylene glycol (PEG)-b-polycation copolymers containing ethylenediamine units in their side chains. The copolymers formed a polyplex micelle by complexing with plasmid DNA containing an expression construct for soluble VEGFR-1 (sFlt-1). By acting as a sink for VEGF, exogenously expressed sFlt-1 prevented activation of the signal cascade that triggers angiogenesis. The micelle nanovectors harboring the sFlt-1 gene were stably introduced in vivo via subconjunctival injection and exhibited prolonged sFlt-1 expression, as demonstrated by reverse transcription-polymerase chain reaction (RT-PCR). Compared to control mice, nanovector-treated mice showed a 45% decrease in experimentally induced vascularization of the cornea, indicating that sFlt-1 was able to inhibit corneal NV significantly. From this study, it is clear that treatments for corneal NV are not limited to the use of conventional therapeutic agents. By using nanoparticles and other classes of nanomaterials in conjunction with factors that prevent angiogenesis in vivo, it is possible to design novel therapies that are not subject to the common pitfalls of available treatments.
Conclusions
Ocular angiogenesis results from the upregulation of proangiogenic and downregulation of antiangiogenic factors, which occurs due to wound healing following a traumatic injury to the eye. This process is primarily mediated by the VEGF family of proteins, and current therapies are aimed at disrupting the various steps in this pathway. Within the cornea, NV can have detrimental effects on the patient's vision. The logic behind current treatment options is that, by preventing angiogenesis, physicians can negate these adverse effects. Considerable research has demonstrated the advantages and disadvantages of available treatment options. Notably, physiological barriers within the eye, such as tears, prevent adequate treatment of ocular disorders by limiting the bioavailability of applied drugs. Repeated intraocular injections of drugs that inhibit angiogenesis, while proven useful, also carry a risk of inflammation, edema, and short-term side effects, including retinal detachment. These shortcomings of pharmacological treatments have motivated researchers to develop drugs that can overcome these challenges while remaining nontoxic and efficient. Specifically, researchers have investigated the use of different classes of nanomaterials in the treatment of corneal NV and other ocular diseases.
It is clear that nanotechnology has vast therapeutic potential in biomedical applications. As the field of medicine moves forward, it remains to be seen what role nanotechnology will play in the treatment of various diseases. The technology is available, and its promise has been demonstrated, but where this exciting field of research will lead is ultimately dictated by human ingenuity. Clearly, advances in nanotechnology show powerful potential for patients suffering from corneal NV disorders. However, the current lack of nanodrugs approved for clinical use represents a major challenge. Hopefully, in the coming years, novel treatments based on nanotechnology will emerge as the standard of care for corneal NV because such new treatments are badly needed to improve therapeutic efficacy.
Acknowledgments
This work was partially supported by National Institutes of Health grants UL1TR000050 and EY001792 and EY021886 (JHC) and an unrestricted grant from Research to Prevent Blindness, New York, NY.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Basavaraj K.H. Nanotechnology in medicine and relevance to dermatology: present concepts. Indian J Dermatol. 2012;57:169–174. doi: 10.4103/0019-5154.96186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taniguchi N. On the basic voncept of nanotechnology. Proc. ICPE Tokyo. 1974;2:18–23. [Google Scholar]
- 3.Brakmane G. Winslet M. Seifalian A.M. Systematic review: the applications of nanotechnology in gastroenterology. Aliment Pharmacol Ther. 2012;36:213–221. doi: 10.1111/j.1365-2036.2012.05179.x. [DOI] [PubMed] [Google Scholar]
- 4.Azar D.T. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2006;104:264–302. [PMC free article] [PubMed] [Google Scholar]
- 5.Menzel-Severing J. Emerging techniques to treat corneal neovascularisation. Eye (Lond) 2012;26:2–12. doi: 10.1038/eye.2011.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tshionyi M. Shay E. Lunde E., et al. Hemangiogenesis and lymphangiogenesis in corneal pathology. Cornea. 2012;31:74–80. doi: 10.1097/ICO.0b013e31821dd986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan D.T. Dart J.K. Holland E.J., et al. Corneal transplantation. Lancet. 2012;379:1749–1761. doi: 10.1016/S0140-6736(12)60437-1. [DOI] [PubMed] [Google Scholar]
- 8.Liu S. Jones L. Gu F.X. Nanomaterials for ocular drug delivery. Macromol Biosci. 2012;12:608–620. doi: 10.1002/mabi.201100419. [DOI] [PubMed] [Google Scholar]
- 9.Chang J.H. Gabison E.E. Kato T., et al. Corneal neovascularization. Curr Opin Ophthalmol. 2001;12:242–249. doi: 10.1097/00055735-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Ding H. Portilla-Arias J. Patil R., et al. The optimization of polymalic acid peptide copolymers for endosomolytic drug delivery. Biomaterials. 2011;32:5269–5278. doi: 10.1016/j.biomaterials.2011.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding H. Inoue S. Ljubimov A.V., et al. Inhibition of brain tumor growth by intravenous poly (beta-L-malic acid) nanobioconjugate with pH-dependent drug release [corrected] Proc Natl Acad Sci U S A. 2010;107:18143–18148. doi: 10.1073/pnas.1003919107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stromblad S. Cheresh D.A. Integrins, angiogenesis and vascular cell survival. Chem Biol. 1996;3:881–885. doi: 10.1016/s1074-5521(96)90176-3. [DOI] [PubMed] [Google Scholar]
- 13.Lee H.S. Chung S.K. The effect of subconjunctival suramin on corneal neovascularization in rabbits. Cornea. 2010;29:86–92. doi: 10.1097/ICO.0b013e3181ae91e3. [DOI] [PubMed] [Google Scholar]
- 14.Shakiba Y. Mansouri K. Arshadi D., et al. Corneal neovascularization: molecular events and therapeutic options. Recent Pat Inflamm Allergy Drug Discov. 2009;3:221–231. doi: 10.2174/187221309789257450. [DOI] [PubMed] [Google Scholar]
- 15.Ellenberg D. Azar D.T. Hallak J.A., et al. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog Retin Eye Res. 2010;29:208–248. doi: 10.1016/j.preteyeres.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onguchi T. Han K.Y. Chang J.H., et al. Membrane type-1 matrix metalloproteinase potentiates basic fibroblast growth factor-induced corneal neovascularization. Am J Pathol. 2009;174:1564–1571. doi: 10.2353/ajpath.2009.080452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato T. Kure T. Chang J.H., et al. Diminished corneal angiogenesis in gelatinase A-deficient mice. FEBS Lett. 2001;508:187–190. doi: 10.1016/s0014-5793(01)02897-6. [DOI] [PubMed] [Google Scholar]
- 18.Han K.Y. Fahd D.C. Tshionyi M., et al. MT1-MMP modulates bFGF-induced VEGF-A expression in corneal fibroblasts. Protein Pept Lett. 2012 doi: 10.2174/092986612803521639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang J.H. Garg N.K. Lunde E., et al. Corneal neovascularization: an anti-VEGF therapy review. Surv Ophthalmol. 2012;57:415–429. doi: 10.1016/j.survophthal.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevenson W. Cheng S.F. Dastjerdi M.H., et al. Corneal neovascularization and the utility of topical VEGF inhibition: ranibizumab (lucentis) vs bevacizumab (avastin) Ocul Surf. 2012;10:67–83. doi: 10.1016/j.jtos.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson M. Eadie S. Kerato-conjunctivitis sicca and rheumatoid arthritis. Ann Rheum Dis. 1956;15:21–25. doi: 10.1136/ard.15.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felipe A.F. Abazari A. Hammersmith K.M., et al. Corneal changes in ectrodactyly-ectodermal dysplasia-cleft lip and palate syndrome: case series and literature review. Int Ophthalmol. 2012 doi: 10.1007/s10792-012-9585-6. [DOI] [PubMed] [Google Scholar]
- 23.Di Tommaso C. Bourges J.L. Valamanesh F., et al. Novel micelle carriers for cyclosporin A topical ocular delivery: in vivo cornea penetration, ocular distribution and efficacy studies. Eur J Pharm Biopharm. 2012;81:257–264. doi: 10.1016/j.ejpb.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Chen P. Yin H. Wang Y., et al. Inhibition of VEGF expression and corneal neovascularization by shRNA targeting HIF-1alpha in a mouse model of closed eye contact lens wear. Mol Vis. 2012;18:864–873. [PMC free article] [PubMed] [Google Scholar]
- 25.Gaudana R. Jwala J. Boddu S.H., et al. Recent perspectives in ocular drug delivery. Pharm Res. 2009;26:1197–1216. doi: 10.1007/s11095-008-9694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Govindarajan B. Gipson I.K. Membrane-tethered mucins have multiple functions on the ocular surface. Exp Eye Res. 2010;90:655–663. doi: 10.1016/j.exer.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albertsmeyer A.C. Kakkassery V. Spurr-Michaud S., et al. Effect of pro-inflammatory mediators on membrane-associated mucins expressed by human ocular surface epithelial cells. Exp Eye Res. 2010;90:444–451. doi: 10.1016/j.exer.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gipson I.K. The ocular surface: the challenge to enable and protect vision: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2007;48(4390):4391–4398. doi: 10.1167/iovs.07-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gipson I.K. Distribution of mucins at the ocular surface. Exp Eye Res. 2004;78:379–388. doi: 10.1016/s0014-4835(03)00204-5. [DOI] [PubMed] [Google Scholar]
- 30.Rawas-Qalaji M. Williams C.A. Advances in ocular drug delivery. Curr Eye Res. 2012;37:345–356. doi: 10.3109/02713683.2011.652286. [DOI] [PubMed] [Google Scholar]
- 31.Lee S.S. Hughes P.M. Robinson M.R. Recent advances in drug delivery systems for treating ocular complications of systemic diseases. Curr Opin Ophthalmol. 2009;20:511–5119. doi: 10.1097/ICU.0b013e328330ccb9. [DOI] [PubMed] [Google Scholar]
- 32.Hashemian M.N. Moghimi S. Kiumehr S., et al. Prevention and treatment of corneal neovascularization: comparison of different doses of subconjunctival bevacizumab with corticosteroid in experimental rats. Ophthalmic Res. 2009;42:90–95. doi: 10.1159/000224783. [DOI] [PubMed] [Google Scholar]
- 33.Sener E. Yuksel N. Yildiz D.K., et al. The impact of subconjuctivally injected EGF and VEGF inhibitors on experimental corneal neovascularization in rat model. Curr Eye Res. 2011;36:1005–10013. doi: 10.3109/02713683.2011.601840. [DOI] [PubMed] [Google Scholar]
- 34.Bock F. Konig Y. Kruse F., et al. Bevacizumab (Avastin) eye drops inhibit corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2008;246:281–284. doi: 10.1007/s00417-007-0684-4. [DOI] [PubMed] [Google Scholar]
- 35.Dykxhoorn D.M. Novina C.D. Sharp P.A. Killing the messenger: short RNAs that silence gene expression. Nat Rev Mol Cell Biol. 2003;4:457–467. doi: 10.1038/nrm1129. [DOI] [PubMed] [Google Scholar]
- 36.Fire A. Xu S. Montgomery M.K., et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 37.Cho W.G. Albuquerque R.J. Kleinman M.E., et al. Small interfering RNA-induced TLR3 activation inhibits blood and lymphatic vessel growth. Proc Natl Acad Sci U S A. 2009;106:7137–7142. doi: 10.1073/pnas.0812317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarallo V. Hirano Y. Gelfand B.D., et al. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell. 2012;149:847–859. doi: 10.1016/j.cell.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han K.Y. Azar D.T. Sabri A., et al. Characterization of the interaction between endostatin short peptide and VEGF receptor 3. Protein Pept Lett. 2012 doi: 10.2174/092986612802084465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang J.H. Javier J.A. Chang G.Y., et al. Functional characterization of neostatins, the MMP-derived, enzymatic cleavage products of type XVIII collagen. FEBS Lett. 2005;579:3601–3606. doi: 10.1016/j.febslet.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 41.Lin H.C. Chang J.H. Jain S., et al. Matrilysin cleavage of corneal collagen type XVIII NC1 domain and generation of a 28-kDa fragment. Invest Ophthalmol Vis Sci. 2001;42:2517–2524. [PubMed] [Google Scholar]
- 42.Zhang Z. Ma J.X. Gao G., et al. Plasminogen kringle 5 inhibits alkali-burn-induced corneal neovascularization. Invest Ophthalmol Vis Sci. 2005;46:4062–71. doi: 10.1167/iovs.04-1330. [DOI] [PubMed] [Google Scholar]
- 43.Andrieu-Soler C. Berdugo M. Doat M., et al. Downregulation of IRS-1 expression causes inhibition of corneal angiogenesis. Invest Ophthalmol Vis Sci. 2005;46:4072–4078. doi: 10.1167/iovs.05-0105. [DOI] [PubMed] [Google Scholar]
- 44.Gupta D. Illingworth C. Treatments for corneal neovascularization: a review. Cornea. 2011 doi: 10.1097/ICO.0b013e318201405a. [DOI] [PubMed] [Google Scholar]
- 45.Cursiefen C. Wenkel H. Martus P., et al. Impact of short-term versus long-term topical steroids on corneal neovascularization after non-high-risk keratoplasty. Graefes Arch Clin Exp Ophthalmol. 2001;239:514–521. doi: 10.1007/s004170100313. [DOI] [PubMed] [Google Scholar]
- 46.Monnier Y. Zaric J. Ruegg C. Inhibition of angiogenesis by non-steroidal anti-inflammatory drugs: from the bench to the bedside and back. Curr Drug Targets Inflamm Allergy. 2005;4:31–38. doi: 10.2174/1568010053622975. [DOI] [PubMed] [Google Scholar]
- 47.Han Y. Shao Y. Lin Z., et al. Netrin-1 simultaneously suppresses corneal inflammation and neovascularization. Invest Ophthalmol Vis Sci. 2012;53:1285–1295. doi: 10.1167/iovs.11-8722. [DOI] [PubMed] [Google Scholar]
- 48.Liu X. Lin Z. Zhou T., et al. Anti-angiogenic and anti-inflammatory effects of SERPINA3K on corneal injury. PLoS One. 2011;6:e16712. doi: 10.1371/journal.pone.0016712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nirankari V.S. Baer J.C. Corneal argon laser photocoagulation for neovascularization in penetrating keratoplasty. Ophthalmology. 1986;93:1304–1309. doi: 10.1016/s0161-6420(86)33581-4. [DOI] [PubMed] [Google Scholar]
- 50.Pillai C.T. Dua H.S. Hossain P. Fine needle diathermy occlusion of corneal vessels. Invest Ophthalmol Vis Sci. 2000;41:2148–53. [PubMed] [Google Scholar]
- 51.Koenig Y. Bock F. Kruse F.E., et al. Angioregressive pretreatment of mature corneal blood vessels before keratoplasty: fine-needle vessel coagulation combined with anti-VEGFs. Cornea. 2012 doi: 10.1097/ICO.0b013e31823f8f7a. [DOI] [PubMed] [Google Scholar]
- 52.Peer D. Karp J.M. Hong S., et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 53.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 54.Duncan R. Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer. 2006;6:688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- 55.Gref R. Minamitake Y. Peracchia M.T., et al. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263:1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 56.Shao T. Li X. Ge J. Target drug delivery system as a new scarring modulation after glaucoma filtration surgery. Diagn Pathol. 2011;6:64. doi: 10.1186/1746-1596-6-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sahoo S.K. Dilnawaz F. Krishnakumar S. Nanotechnology in ocular drug delivery. Drug Discov Today. 2008;13:144–151. doi: 10.1016/j.drudis.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 58.Bhatta R.S. Chandasana H. Chhonker Y.S., et al. Mucoadhesive nanoparticles for prolonged ocular delivery of natamycin: in vitro and pharmacokinetics studies. Int J Pharm. 2012 doi: 10.1016/j.ijpharm.2012.04.060. [DOI] [PubMed] [Google Scholar]
- 59.Prieto E. Puente B. Uixera A., et al. Gantrez AN nanoparticles for ocular delivery of memantine: in vitro release evaluation in albino rabbits. Ophthalmic Res. 2012;48:109–117. doi: 10.1159/000337136. [DOI] [PubMed] [Google Scholar]
- 60.Kim J.H. Kim J.H. Kim K.W., et al. Intravenously administered gold nanoparticles pass through the blood–retinal barrier depending on the particle size, and induce no retinal toxicity. Nanotechnology. 2009;20:505101. doi: 10.1088/0957-4484/20/50/505101. [DOI] [PubMed] [Google Scholar]
- 61.Loo S.C. Tan Z.Y. Chow Y.J., et al. Drug release from irradiated PLGA and PLLA multi-layered films. J Pharm Sci. 2010;99:3060–3071. doi: 10.1002/jps.22079. [DOI] [PubMed] [Google Scholar]
- 62.Ciolino J.B. Hoare T.R. Iwata N.G., et al. A drug-eluting contact lens. Invest Ophthalmol Vis Sci. 2009;50:3346–3352. doi: 10.1167/iovs.08-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kataoka K. Harada A. Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev. 2001;47:113–131. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 64.Aliabadi H.M. Lavasanifar A. Polymeric micelles for drug delivery. Expert Opin Drug Deliv. 2006;3:139–162. doi: 10.1517/17425247.3.1.139. [DOI] [PubMed] [Google Scholar]
- 65.Croy S.R. Kwon G.S. Polymeric micelles for drug delivery. Curr Pharm Des. 2006;12:4669–4684. doi: 10.2174/138161206779026245. [DOI] [PubMed] [Google Scholar]
- 66.Civiale C. Licciardi M. Cavallaro G., et al. Polyhydroxyethylaspartamide-based micelles for ocular drug delivery. Int J Pharm. 2009;378:177–186. doi: 10.1016/j.ijpharm.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 67.Lawrence M.J. Rees G.D. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 2000;45:89–121. doi: 10.1016/s0169-409x(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 68.Sultana Y. Maurya D.P. Iqbal Z., et al. Nanotechnology in ocular delivery: current and future directions. Drugs Today (Barc) 2011;47:441–55. doi: 10.1358/dot.2011.47.6.1549023. [DOI] [PubMed] [Google Scholar]
- 69.Torchilin V.P. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 70.Thrimawithana T.R. Young S. Bunt C.R., et al. Drug delivery to the posterior segment of the eye. Drug Discov Today. 2011;16:270–277. doi: 10.1016/j.drudis.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 71.Shatalebi M.A. Mostafavi S.A. Moghaddas A. Niosome as a drug carrier for topical delivery of N-acetyl glucosamine. Res Pharm Sci. 2010;5:107–17. [PMC free article] [PubMed] [Google Scholar]
- 72.Kazi K.M. Mandal A.S. Biswas N., et al. Niosome: a future of targeted drug delivery systems. J Adv Pharm Technol Res. 2010;1:374–380. doi: 10.4103/0110-5558.76435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yao C. Wang W. Zhou X., et al. Effects of poly(amidoamine) dendrimers on ocular absorption of puerarin using microdialysis. J Ocul Pharmacol Ther. 2011;27:565–569. doi: 10.1089/jop.2010.0196. [DOI] [PubMed] [Google Scholar]
- 74.Spataro G. Malecaze F. Turrin C.O., et al. Designing dendrimers for ocular drug delivery. Eur J Med Chem. 2010;45:326–334. doi: 10.1016/j.ejmech.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 75.Shi X. Wang S.H. Lee I., et al. Comparison of the internalization of targeted dendrimers and dendrimer-entrapped gold nanoparticles into cancer cells. Biopolymers. 2009;91:936–942. doi: 10.1002/bip.21279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Majoros I.J. Williams C.R. Tomalia D.A., et al. New dendrimers: synthesis and characterization of POPAM–PAMAM hybrid dendrimers. Macromolecules. 2008;41:8372–8379. doi: 10.1021/ma801843a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Siefert B. Pleyer U. Muller M., et al. Influence of cyclodextrins on the in vitro corneal permeability and in vivo ocular distribution of thalidomide. J Ocul Pharmacol Ther. 1999;15:429–438. doi: 10.1089/jop.1999.15.429. [DOI] [PubMed] [Google Scholar]
- 78.Davis M.E. Brewster M.E. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov. 2004;3:1023–1035. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]
- 79.Kemp M.M. Kumar A. Mousa S., et al. Gold and silver nanoparticles conjugated with heparin derivative possess anti-angiogenesis properties. Nanotechnology. 2009;20:455104. doi: 10.1088/0957-4484/20/45/455104. [DOI] [PubMed] [Google Scholar]
- 80.Kim J.H. Kim M.H. Jo D.H., et al. The inhibition of retinal neovascularization by gold nanoparticles via suppression of VEGFR-2 activation. Biomaterials. 2011;32:1865–1871. doi: 10.1016/j.biomaterials.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 81.Oner V. Kucukerdonmez C. Akova Y.A., et al. Topical and subconjunctival bevacizumab for corneal neovascularization in an experimental rat model. Ophthalmic Res. 2012;48:118–123. doi: 10.1159/000337139. [DOI] [PubMed] [Google Scholar]
- 82.Singh S.R. Grossniklaus H.E. Kang S.J., et al. Intravenous transferrin, RGD peptide and dual-targeted nanoparticles enhance anti-VEGF intraceptor gene delivery to laser-induced CNV. Gene Ther. 2009;16:645–659. doi: 10.1038/gt.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaplitt M.G. Leone P. Samulski R.J., et al. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994;8:148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- 84.Stechschulte S.U. Joussen A.M. von Recum H.A., et al. Rapid ocular angiogenic control via naked DNA delivery to cornea. Invest Ophthalmol Vis Sci. 2001;42:1975–1979. [PubMed] [Google Scholar]
- 85.Iriyama A. Usui T. Yanagi Y., et al. Gene transfer using micellar nanovectors inhibits corneal neovascularization in vivo. Cornea. 2011;30:1423–1427. doi: 10.1097/ICO.0b013e318206c893. [DOI] [PubMed] [Google Scholar]
- 86.Sugisaki K. Usui T. Nishiyama N., et al. Photodynamic therapy for corneal neovascularization using polymeric micelles encapsulating dendrimer porphyrins. Invest Ophthalmol Vis Sci. 2008;49:894–899. doi: 10.1167/iovs.07-0389. [DOI] [PubMed] [Google Scholar]
- 87.Li W. Feng S.S. Guo Y. Block copolymer micelles for nanomedicine. Nanomedicine (Lond) 2012;7:169–172. doi: 10.2217/nnm.11.182. [DOI] [PubMed] [Google Scholar]
- 88.Jani P.D. Singh N. Jenkins C., et al. Nanoparticles sustain expression of Flt intraceptors in the cornea and inhibit injury-induced corneal angiogenesis. Invest Ophthalmol Vis Sci. 2007;48:2030–2036. doi: 10.1167/iovs.06-0853. [DOI] [PubMed] [Google Scholar]
- 89.Qazi Y. Stagg B. Singh N., et al. Nanoparticle-mediated delivery of shRNA.VEGF-a plasmids regresses corneal neovascularization. Invest Ophthalmol Vis Sci. 2012;53:2837–2844. doi: 10.1167/iovs.11-9139. [DOI] [PMC free article] [PubMed] [Google Scholar]


