Abstract
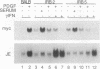
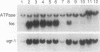
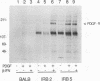
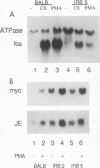
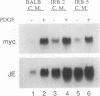
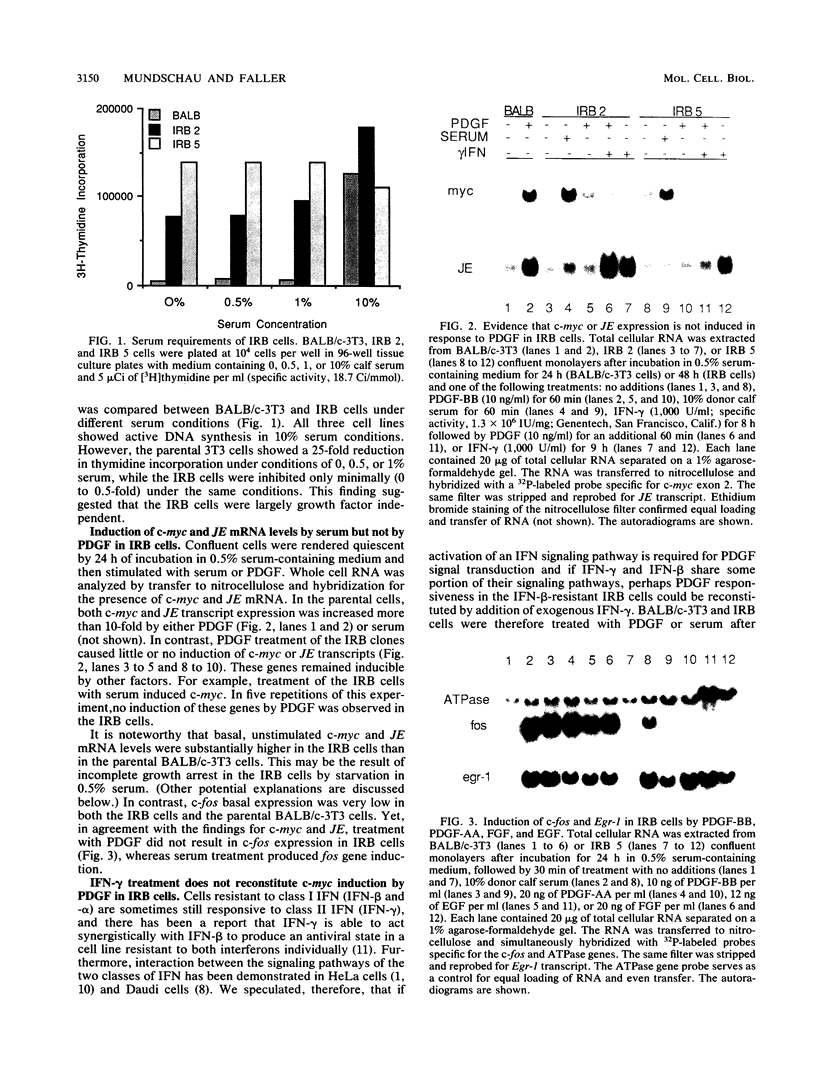
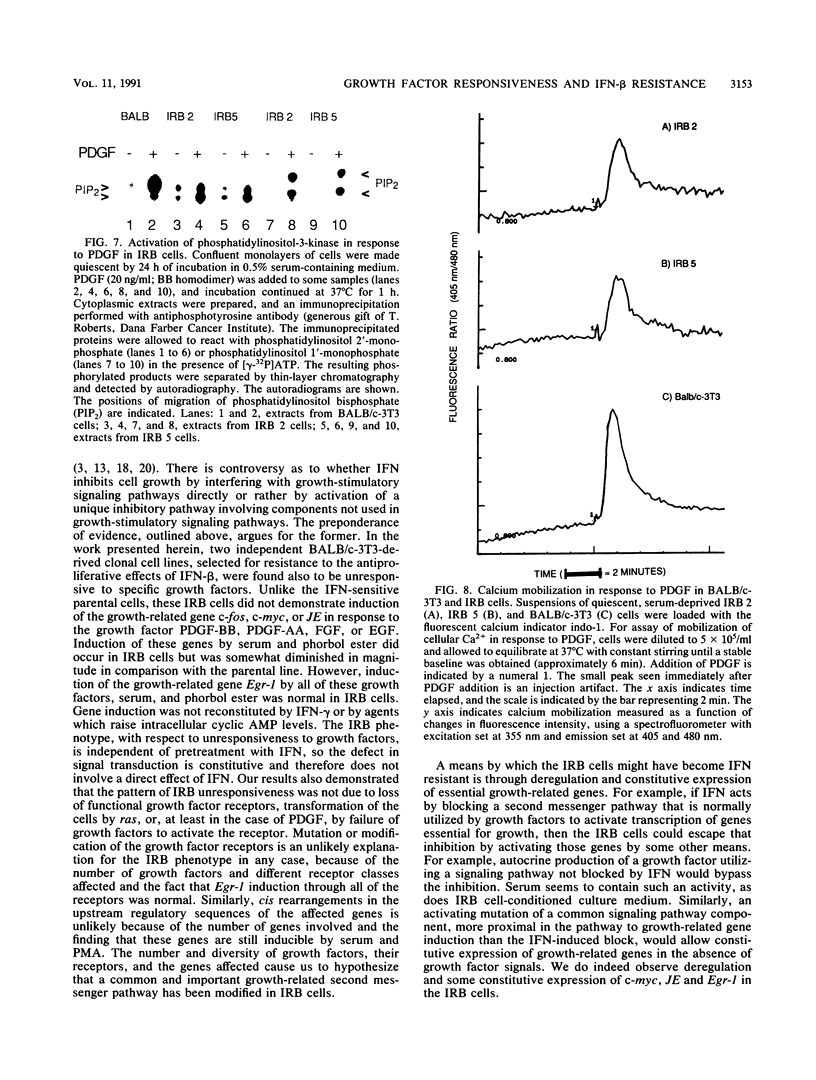
Several lines of evidence now exist to suggest an interaction between the platelet-derived growth factor (PDGF) growth-stimulatory signal transduction pathway and the beta interferon (IFN-beta) growth-inhibitory signal transduction pathway. The most direct examples are inhibition of PDGF-mediated gene induction and mitogenesis by IFN-beta and the effects of activators and inhibitors of the IFN-inducible double-stranded RNA-dependent eIF2 kinase on expression of PDGF-inducible genes. To further investigate the nature of this PDGF/IFN-beta interaction, we selected BALB/c-3T3 cells for resistance to growth inhibition by IFN-beta and analyzed the phenotypes of resulting clonal lines (called IRB cells) with respect to PDGF signal transduction. Although selected only for IFN resistance, the IRB cells were found to be defective for induction of growth-related genes c-fos, c-myc and JE in response to PDGF. This block to signal transduction was not due to loss or inactivation of PDGF receptors, as immunoprecipitation of PDGF receptors with antiphosphotyrosine antibodies showed them to be present at equal levels in the BALB/c-3T3 and IRB cells and to be autophosphorylated normally in response to PDGF. Furthermore, treatment with other peptide growth factors (PDGF-AA, fibroblast growth factor, and epidermal growth factor) also failed to induce c-fos, c-myc, or JE expression in IRB cells. All of these growth factors, however, were able to induce another early growth-related gene, Egr-1. The block to signaling was not due to a defect in inositol phosphate metabolism, as PDGF treatment induced normal calcium mobilization and phosphotidylinositol-3-kinase activation in these cells. Activation of protein kinase C by phorbol esters did induce c-fos, c-myc, and JE in IRB cells, indicating that signalling pathways distal to this enzyme remained intact. We have previously shown that IFN-inducible enzyme activities, including double-stranded RNA-dependent eIF2 kinase and 2',5'-oligoadenylate synthetase, are normal in IRB cells. The finding that the induction of multiple growth-related genes by several independent growth factors is inhibited in these IFN-resistant cells suggests that there is a second messenger common to both growth factor and IFN signaling pathways and that this messenger is defective in these cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandyopadhyay S. K., Kalvakolanu D. V., Sen G. C. Gene induction by interferons: functional complementation between trans-acting factors induced by alpha interferon and gamma interferon. Mol Cell Biol. 1990 Oct;10(10):5055–5063. doi: 10.1128/mcb.10.10.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Einat M., Resnitzky D., Kimchi A. Close link between reduction of c-myc expression by interferon and, G0/G1 arrest. Nature. 1985 Feb 14;313(6003):597–600. doi: 10.1038/313597a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Forsberg K., Paulsson Y., Westermark B. Effect on platelet-derived growth factor-induced mitogenesis of double-stranded RNA: evidence for an autocrine growth inhibition mediated by interferon-beta. J Cell Physiol. 1988 Aug;136(2):266–272. doi: 10.1002/jcp.1041360208. [DOI] [PubMed] [Google Scholar]
- Garcia-Blanco M. A., Lengyel P., Morrison E., Brownlee C., Stiles C. D., Rutherford M., Hannigan G., Williams B. R. Regulation of 2',5'-oligoadenylate synthetase gene expression by interferons and platelet-derived growth factor. Mol Cell Biol. 1989 Mar;9(3):1060–1068. doi: 10.1128/mcb.9.3.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. J., Jones S. D., Kaplan D. R., Whitman M., Rollins B. J., Stiles C. D. Evidence for a novel signal transduction pathway activated by platelet-derived growth factor and by double-stranded RNA. Mol Cell Biol. 1989 Apr;9(4):1705–1713. doi: 10.1128/mcb.9.4.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler D. S., Pine R., Pfeffer L. M., Levy D. E., Darnell J. E., Jr Cells resistant to interferon are defective in activation of a promoter-binding factor. EMBO J. 1988 Dec 1;7(12):3779–3783. doi: 10.1002/j.1460-2075.1988.tb03262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi A., Resnitzky D., Ber R., Gat G. Recessive genetic deregulation abrogates c-myc suppression by interferon and is implicated in oncogenesis. Mol Cell Biol. 1988 Jul;8(7):2828–2836. doi: 10.1128/mcb.8.7.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. E., Lew D. J., Decker T., Kessler D. S., Darnell J. E., Jr Synergistic interaction between interferon-alpha and interferon-gamma through induced synthesis of one subunit of the transcription factor ISGF3. EMBO J. 1990 Apr;9(4):1105–1111. doi: 10.1002/j.1460-2075.1990.tb08216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. A., Huq A., Shan B. Beta and gamma interferons act synergistically to produce an antiviral state in cells resistant to both interferons individually. J Virol. 1989 Nov;63(11):4569–4578. doi: 10.1128/jvi.63.11.4569-4578.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermann M. K., Faller D. V. Autocrine induction of major histocompatibility complex class I antigen expression results from induction of beta interferon in oncogene-transformed BALB/c-3T3 cells. Mol Cell Biol. 1989 May;9(5):1969–1977. doi: 10.1128/mcb.9.5.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleszak E. Inhibition of mitogenic activity of PDGF, EGF, and FGF by interferon-gamma. Exp Cell Res. 1988 Dec;179(2):575–580. doi: 10.1016/0014-4827(88)90295-9. [DOI] [PubMed] [Google Scholar]
- Olinger P. L., Benjamin C. W., Gorman R. R., Connor J. A. Cyclic AMP can partially restore platelet-derived growth factor-stimulated prostaglandin E2 biosynthesis, and calcium mobilization in EJ-ras-transformed NIH-3T3 cells. J Cell Physiol. 1989 May;139(2):335–345. doi: 10.1002/jcp.1041390216. [DOI] [PubMed] [Google Scholar]
- Petryshyn R., Chen J. J., London I. M. Growth-related expression of a double-stranded RNA-dependent protein kinase in 3T3 cells. J Biol Chem. 1984 Dec 10;259(23):14736–14742. [PubMed] [Google Scholar]
- Prpic V., Yu S. F., Figueiredo F., Hollenbach P. W., Gawdi G., Herman B., Uhing R. J., Adams D. O. Role of Na+/H+ exchange by interferon-gamma in enhanced expression of JE and I-A beta genes. Science. 1989 Apr 28;244(4903):469–471. doi: 10.1126/science.2541500. [DOI] [PubMed] [Google Scholar]
- Rake J. B., Quiñones M. A., Faller D. V. Inhibition of platelet-derived growth factor-mediated signal transduction by transforming ras. Suppression of receptor autophosphorylation. J Biol Chem. 1991 Mar 15;266(8):5348–5352. [PubMed] [Google Scholar]
- Resnitzky D., Yarden A., Zipori D., Kimchi A. Autocrine beta-related interferon controls c-myc suppression and growth arrest during hematopoietic cell differentiation. Cell. 1986 Jul 4;46(1):31–40. doi: 10.1016/0092-8674(86)90857-3. [DOI] [PubMed] [Google Scholar]
- Sukhatme V. P., Cao X. M., Chang L. C., Tsai-Morris C. H., Stamenkovich D., Ferreira P. C., Cohen D. R., Edwards S. A., Shows T. B., Curran T. A zinc finger-encoding gene coregulated with c-fos during growth and differentiation, and after cellular depolarization. Cell. 1988 Apr 8;53(1):37–43. doi: 10.1016/0092-8674(88)90485-0. [DOI] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Balkwill F., Ebsworth N., Rozengurt E. Antiviral and antiproliferative effects of interferons in quiescent fibroblasts are dissociable. Virology. 1985 Dec;147(2):405–412. doi: 10.1016/0042-6822(85)90142-4. [DOI] [PubMed] [Google Scholar]
- Thomas N. S. Regulation of the product of a possible human cell cycle control gene CDC2Hs in B-cells by alpha-interferon and phorbol ester. J Biol Chem. 1989 Aug 15;264(23):13697–13700. [PubMed] [Google Scholar]
- Tiwari R. K., Kusari J., Kumar R., Sen G. C. Gene induction by interferons and double-stranded RNA: selective inhibition by 2-aminopurine. Mol Cell Biol. 1988 Oct;8(10):4289–4294. doi: 10.1128/mcb.8.10.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman M., Kaplan D. R., Schaffhausen B., Cantley L., Roberts T. M. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985 May 16;315(6016):239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]
- Zinn K., Keller A., Whittemore L. A., Maniatis T. 2-Aminopurine selectively inhibits the induction of beta-interferon, c-fos, and c-myc gene expression. Science. 1988 Apr 8;240(4849):210–213. doi: 10.1126/science.3281258. [DOI] [PubMed] [Google Scholar]
- Zullo J. N., Cochran B. H., Huang A. S., Stiles C. D. Platelet-derived growth factor and double-stranded ribonucleic acids stimulate expression of the same genes in 3T3 cells. Cell. 1985 Dec;43(3 Pt 2):793–800. doi: 10.1016/0092-8674(85)90252-1. [DOI] [PubMed] [Google Scholar]
- Zullo J. N., Faller D. V. P21 v-ras inhibits induction of c-myc and c-fos expression by platelet-derived growth factor. Mol Cell Biol. 1988 Dec;8(12):5080–5085. doi: 10.1128/mcb.8.12.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]