Abstract
Background
Mechanical ventilation has been documented to paradoxically cause lung injury. As a commonly used volatile anesthetic, sevoflurane has been proven to possess antiinflammatory and antioxidative properties. This study aims to investigate the protective effects of sevoflurane on inflammation and ventilator-induced lung injury during mechanical ventilation in healthy mice.
Methods
The adult healthy mice were divided into four groups, each consisting of ten subjects: mice in group Con-LVT and group Sev-LVT were ventilated with tidal volumes of 8 mL/kg for 4 hours, while those in group Con-HVT and group Sev-HVT were ventilated with tidal volumes of 16 mL/kg instead. Control mice (group Con-LVT and Con-HVT) were subjected to fresh air, while sevoflurane-treated mice (groups Sev- LVT and Sev-HVT) were subjected to air mixed with 1 vol% sevoflurane. After 4 hours of ventilation, the bronchoalveolar lavage (BAL) fluid was collected and analyzed for the levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and IL-10. Lung homogenates were harvested to detect the expression of nuclear factor-kappa B (NF-κB) and heme oxygenase (HO)-1 mRNA by reverse transcription-polymerase chain reaction method. Lung damage was evaluated using the modified Ventilator-Induced Lung Injury histological scoring system.
Results
Compared to group Con-LVT, the levels of TNF-α, IL-1β, IL-6, and IL-10 in BAL fluid, mRNA expressions of NF-κB and HO-1 in lung tissue, and lung injury scores were significantly increased in group Con-HVT; compared to group Con-HVT, group Sev-HVT BAL samples showed decreased levels of TNF-α, IL-1β, and IL-6; they also showed increased levels of IL-10, the downregulation of NF-κB mRNA, and HO-1 mRNA upregulation; the lung injury scores were significantly lower in group Sev-HVT than group Con-HVT.
Conclusion
Mechanical ventilation with high tidal volume might lead to lung injury, which could be significantly, but not completely, attenuated by sevoflurane inhalation by inhibiting the NF-κB-mediated proinflammatory cytokine generation and upregulating HO-1 expression.
Keywords: mechanical ventilation, sevoflurane, inflammation, lung injury
Introduction
Mechanical ventilation is an essential respiratory support approach during general anesthesia and provides a lifesaving intervention in acute respiratory failure therapy.1 However, excessive alveolar distension can lead to lung injury due to increased pulmonary vascular permeability, alteration in lung mechanics, and increased production of inflammatory mediators.2,3 Although modulation of ventilator settings such as applying low tidal volumes and low plateau pressure may help to relieve lung injury,4 ventilator-induced lung injury (VILI) remains a major problem in the long-term use of mechanical ventilation with an unacceptably high rate of morbidity and mortality.5 The underlying mechanisms of VILI are incompletely understood, but a large body of investigation indicates that proinflammatory cytokines and heme oxygenase (HO)-1 play an important role in the development of VILI.6,7 Therefore, alternative therapeutic antiinflammatory strategies to further minimize the risk of VILI need to be developed.
Sevoflurane is a widely used volatile anesthetic whose antiinflammation properties have recently gained increasing attention.8–10 Many studies have demonstrated that sevoflurane can modulate inflammatory cascades and has protective effects against ischemia/reperfusion injury.11–13 Studies by Suter et al11 demonstrated a decrease in the production of inflammatory proteins in alveolar epithelial cells with sevoflurane preconditioning in endotoxin-induced lung injury. However, as a widely used anesthetic in general anesthesia, whether sevoflurane is capable of attenuating VILI remains unknown.
Based on these recent findings, the aim of the current study was to verify the hypothesis that sevoflurane may attenuate pulmonary inflammation and VILI by upregulating HO-1 expression and inhibiting the nuclear factor-kappa B (NF-κB) mediated proinflammatory cytokine generation in an in vivo model of VILI.
Materials and methods
Animal preparation
The study strictly abided by the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All experimental procedures were approved by the Animal Ethics Committee of Wenzhou Medical College.
Adult healthy male C57BL/6N mice weighing 30 g to 35 g were obtained from the Animal Experimental Center of Wenzhou Medical College. All mice were anesthetized with 90 mg/kg of ketamine and 1 mg/kg of acepromazine intraperitoneally and placed in the supine position. Muscular relaxation was achieved by applying an intraperitoneal injection of 1.5 mg/kg of vecuronium. Anesthesia was maintained by continuous intraperitoneal administration of ketamine (45 mg/kg/hour), acepromazine (0.5 mg/kg/hour), and vecuronium (0.75 mg/kg/hour) as needed. Adequacy of anesthesia during maintenance was assessed based on hemodynamic responses. Tracheotomy was established using a 15-gauge catheter, and a polyethylene catheter was inserted into the left carotid artery for direct blood pressure monitoring and blood gas sampling. An ear vein was cannulated with a 7-gauge intravenous catheter for receiving an intravenous infusion of Ringer’s solution (5 mL · kg−1 · h−1).
Experimental design
The 40 mice were divided into four groups: (1) group Con-LVT (n = 10): the mice were ventilated with fresh air at a tidal volume of 8 mL/kg; (2) group Con-HVT (n = 10): the mice were ventilated with synthetic air at a tidal volume of 16 mL/kg; (3) group Sev-LVT (n = 10): the mice were ventilated with synthetic air supplemented with 1 vol% minimum alveolar concentration (MAC) sevoflurane at a tidal volume of 8 mL/kg; (4) group Sev-HVT (n = 10): ventilated with synthetic air supplemented with 1 vol% MAC sevoflurane at a tidal volume of 16 mL/kg. The inspiratory/expiratory ratio was 1:2, the respiratory rate was 16 bpm, the total gas flow was 1.5 L/minute, and the fraction of inspired oxygen (FiO2) was maintained for all animals and no positive end-expiratory pressure was applied. Oxygen saturation, arterial blood pressure, and electrocardiography were monitored continuously and recorded every 15 minutes. Mean airway pressure and peak inspiratory pressure were monitored continuously and recorded every 30 minutes. Arterial blood gases were analyzed hourly. The above monitoring instruments came from Hewlett–Packard Co, Ltd (Palo Alto, CA, USA).
Bronchoalveolar lavage and cytokine measurements
Mice were put to death after 4 hours of continuous mechanical ventilation, and bronchoalveolar lavage (BAL) was performed via the tracheal catheter in the right lung lobes using 0.8 mL of phosphate buffered saline; the withdrawn fluid was centrifuged, and the supernatant was snap frozen and stored at −80°C for further use. Aliquots of BAL fluid were detected in duplicate with enzyme-linked immunosorbent assay (ELISA kit offered by Glory Science Co, Ltd, Del Rio, TX, USA) kits for tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, and IL-10 according to the manufacturer’s instructions. The minimum detection limits for these kits were 3.5 pg/mL for TNF-α, 3.0 pg/mL for IL-1β, 15 pg/mL for IL-6, and 20 pg/mL for IL-10.
Measurement of NF-κB and HO-1 mRNA by RT-PCR
The left lung tissues were homogenized and total RNA was extracted using TRIzol reagent (Shanghai Sangon Biological Engineering Technology and Service Co, Ltd, Shanghai, People’s Republic of China) according to the manufacturer’s instructions. For reverse transcription-polymerase chain reaction (RT-PCR), 1 μg of total RNA from each sample was resuspended in 25 μL final volume of reaction buffer. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. The following primers were used for PCR: HO-1 forward primer 5′-TCC TCA ATG CTG TAC TGG TCC-3′, reverse primer 5′-ATG TTC TTC CTT TCC AGG TC-3′; NF-κB forward primer 5′-AGT TGA GGG GAC TTT CCC AGG C-3′, reverse primer 5′-GCC TGG GAA AGT CCC CTC AAC T-3′; and GAPDH forward primer 5′-AAT GCA TCC TGC ACC ACC AA-3′, and reverse primer 5′-GTA GCC ATA TTC ATT GTC ATA-3′.
Assessment of lung histopathology
The left lung lobe was fixed in 4% buffered formalin solution overnight at room temperature, dehydrated, embedded in paraplast, and then 4 μm-thick sections were obtained. Lung histopathology was assessed under a light microscope after hematoxylin and eosin staining. The degree of lung damage was assessed using a modified VILI histological scoring system:14 (1) thickness of the alveolar walls; (2) infiltration or aggregation of inflammatory cells; and (3) hemorrhage. Each item was graded according to the following five-point scale: 0, minimal damage; 1, mild damage; 2, moderate damage; 3, severe damage; 4, maximal damage. The degree of lung damage was assessed by the sum of scores ranging from 0 to 12.
Statistical analysis
Statistical analysis was performed with the Statistical Package for the Social Sciences version 15.0 (SPSS, IBM Corporation, Armonk, NY, USA). Data were analyzed for normality using the Kolmogorov–Smirmov method, and the normally distributed data were expressed as mean ± standard deviation. To compare normally distributed data between each group, one-way analysis of variance followed by the Student–Newman–Keuls post hoc test was employed. A P-value of less than 0.05 was considered significant.
Results
The change in hemodynamics and respiratory function
No significant differences were observed in heart rate and mean arterial pressure between or within groups (P > 0.05). Mean airway pressures and peak airway pressure were higher in group Con-HVT and Sev-HVT compared with groups Con-LVT and Sev-LVT, respectively, and there were no significant differences between the two HVT groups or LVT groups (Table 1).
Table 1.
Parameters for ventilation strategies and blood gas
| Con-HVT | Sev-HVT | Con-LVT | Sev-LVT | |
|---|---|---|---|---|
| Hemodynamics | ||||
| MAP (mmHg) | 55.6 ± 3.1 | 54.4 ± 3.3 | 56.2 ± 3.5 | 55.7 ± 2.8 |
| HR | 126 ± 4.6 | 128 ± 5.6 | 124 ± 4.0 | 127 ± 5.2 |
| Respiratory pressure | ||||
| Pmean (cm H2O) | 14.5 ± 0.6* | 15.1 ± 0.3* | 8.9 ± 0.4 | 8.7 ± 0.5 |
| Ppeak (cm H2O) | 25.7 ± 1.2* | 26.8 ± 1.8* | 12.4 ± 0.9 | 12.4 ± 0.6 |
| Blood gas | ||||
| pH | 7.48 ± 0.05* | 7.47 ± 0.04* | 7.31 ± 0.03 | 7.32 ± 0.04 |
| HCO3 (mmol/L) | 23.5 ± 1.2 | 24.2 ± 0.8 | 25.6 ± 1.4 | 25.7 ± 1.3 |
| PO2 (mmHg) | 125 ± 5.4 | 128 ± 7.9 | 124 ± 4.3 | 126 ± 6.7 |
| PCO2 (mmHg) | 35.4 ± 1.5* | 36.2 ± 1.3* | 48.7 ± 2.6 | 47.7 ± 3.1 |
Note:
P < 0.05, compared with Con- LVT or Sev- LVT.
Abbreviations: Con-HVT, high tidal volume of synthetic air ventilation; Sev-HVT, high tidal volume of air ventilation supplemented with sevoflurane; Con-LVT, low tidal volume of synthetic air ventilation; Sev-LVT, low tidal volume of air ventilation supplemented with sevoflurane; MAP, mean arterial pressure; HR, heart rate; Pmean, mean airway pressures; Ppeak, peak airway pressures.
Arterial pH in all animals was kept within the range of 7.3–7.5 during the protocol. HVT groups had significantly higher arterial pH than LVT groups (Table 1). Partial pressure of carbon dioxide (PaCO2) was within its normal range of 35–50 mmHg in all mice. Compared to the LVT groups, PaCO2 levels in HVT groups were significantly higher (Table 1). Partial pressure of arterial oxygen (PaO2) differed between any two groups.
The measurement of inflammatory cytokines in BAL fluid
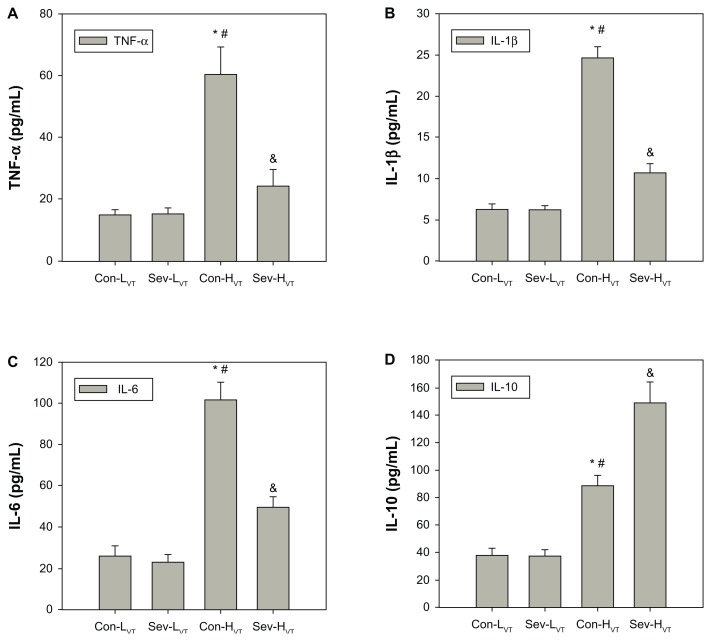
The levels of TNF-α, IL-1β, IL-6, and IL-10 in BAL fluid in the HVT groups were significantly higher than those in the LVT groups (Figure 1). The levels of TNF-α, IL-1β, and IL-6 in BAL fluid were significantly decreased, and IL-10 levels were increased in the Sev-HVT group as compared with the Con-HVT group (Figure 1).
Figure 1.
All mice were ventilated for 4 hours with low tidal volume (8 mL/kg, group Con-LVT and group Sev-LVT) or high tidal volume (16 mL/kg, group Con-HVT and group Sev-HVT). The BAL fluid was obtained from the right lung lobes and analyzed for the concentration of TNF-α (A), IL-1β (B), IL-6 (C), and IL-10 (D) by ELISA.
Notes: *P < 0.05, group Con-HVT versus Con-LVT; #P < 0.05, group Con-HVT versus Sev-LVT; &P < 0.05, group Sev-HVT versus Con-HVT.
Abbreviations: Con-LVT, low tidal volume of synthetic air ventilation; Sev-LVT, low tidal volume of air ventilation supplemented with sevoflurane; Con-HVT, high tidal volume of synthetic air ventilation; Sev-HVT, high tidal volume of air ventilation supplemented with sevoflurane; BAL, bronchoalveolar lavage; TNF, tumor necrosis factor; IL, interleukin; ELISA, enzyme-linked immunosorbent assay.
The expressions of NF-κB and HO-1 mRNA in the lung homogenates
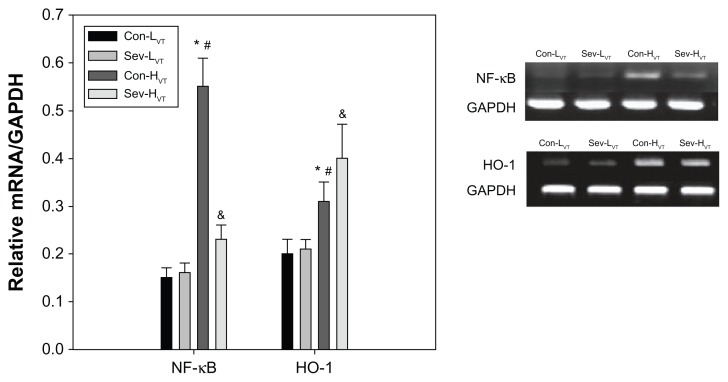
In the lung homogenates, the expressions of NF-κB and HO-1 were found to be significantly regulated in the HVT group as compared to the LVT group (Figure 2). The mRNA expression levels of NF-κB were lower, and the expression of HO-1 mRNA were higher in group Sev-HVT than group Con-HVT (Figure 2).
Figure 2.
All mice were ventilated for 4 hours with low tidal volumes (8 mL/kg, group Con-LVT and group Sev-LVT) or high tidal volumes (16 mL/kg, group Con-HVT and group Sev-HVT).
Notes: The left lung was homogenized and RNA was extracted, followed by the measurement of the expression of NF-κB and HO-1 mRNA using RT-PCR. *P < 0.05, group Con-HVT versus Con-LVT; #P < 0.05, group Con-HVT versus Sev-LVT; &P < 0.05, group Sev-HVT versus Con-HVT.
Abbreviations: Con-LVT, low tidal volume of synthetic air ventilation; Sev-LVT, low tidal volume of air ventilation supplemented with sevoflurane; Con-HVT, high tidal volume of synthetic air ventilation; Sev-HVT, high tidal volume of air ventilation supplemented with sevoflurane; GADPH, glyceraldehyde 3-phosphate dehydrogenase; NF-κB, nuclear factor-kappa B; HO-1, heme oxygenase-1; RT-PCR, reverse transcription polymerase chain reaction.
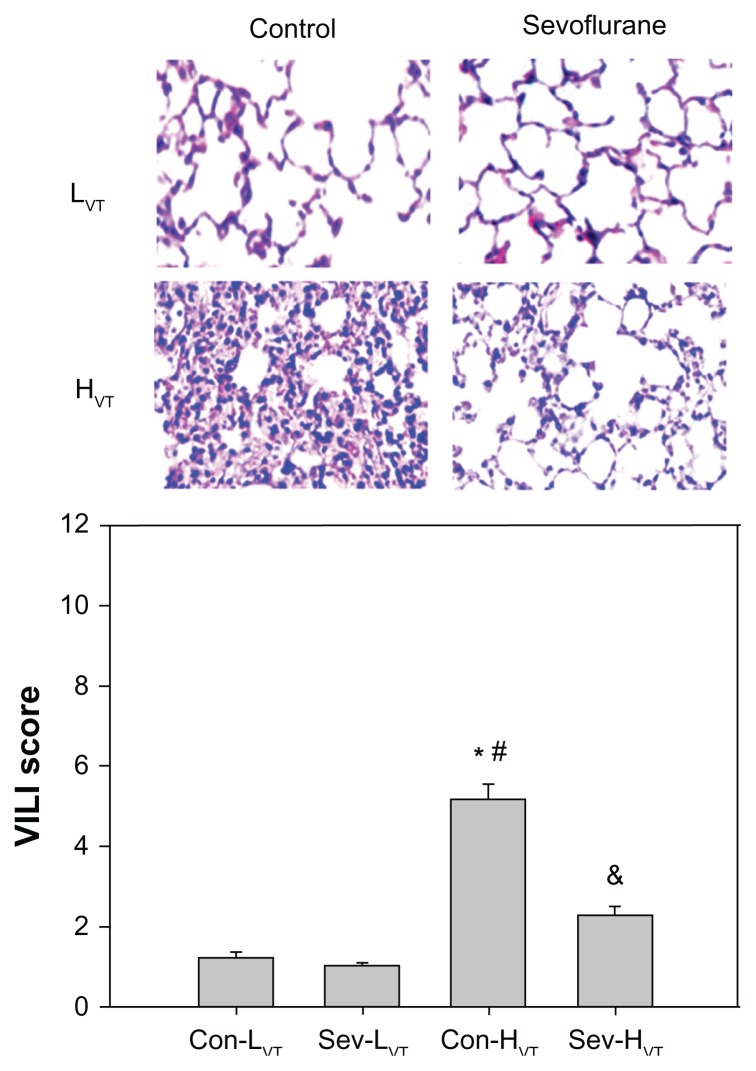
The assessment of lung histopathological changes
Normal histology in lung tissue was observed in the Con-LVT and Sev-LVT groups, while the lung presented severe lesions in the Con-HVT group, manifested as lung interstitial edema, inflammatory cell infiltration, pulmonary alveolus rupture, and hemorrhage. These histopathological changes were markedly attenuated in the group Sev-HVT (Figure 3). The lung injury scores were significantly lower in group Sev-HVT (2.32 ± 0.18) versus group Con-HVT (5.20 ± 0.34) (Figure 3).
Figure 3.
All mice were ventilated for 4 hours with low tidal volumes (8 mL/kg, group Con-LVT and group Sev-LVT) or high tidal volumes (16 mL/kg, group Con-HVT and group Sev-HVT).
Notes: The sections from the right lung lobe were hematoxylin and eosin stained. Representative pictures were shown for each experimental group (magnification × 400). The lung tissue in the HVT group demonstrated histological changes with lung interstitial mesenchymal edema, inflammatory cell infiltration, pulmonary alveolus rupture, bursts, and hemorrhage. These changes were markedly attenuated by sevoflurane administration. Lung injury scores were calculated using modified VILI scoring. *P < 0.05, group Con-HVT versus Con-LVT; #P < 0.05, group Con-HVT versus Sev-LVT; &P < 0.05, group Sev-HVT versus Con- HVT.
Abbreviations: Con-LVT, low tidal volume of synthetic air ventilation; Sev-LVT, low tidal volume of air ventilation supplemented with sevoflurane; Con-HVT, high tidal volume of synthetic air ventilation; Sev-HVT, high tidal volume of air ventilation supplemented with sevoflurane; HVT, high tidal volume; VILI, ventilator-induced lung injury.
Discussion
The main findings of our study were that sevoflurane inhalation was shown to attenuate VILI by upregulating HO-1 expression and inhibiting the NF-κB-mediated proinflammatory cytokine generation.
Many studies have shown that severe lung injury is provoked by ventilation with high tidal volumes.15–17 Although the exact underlying mechanisms of VILI remain undefined, several potential pathways have been proposed, with barotrauma appearing to be the generally accepted one. In our study, significant infiltration of neutrophils was observed on pathological examination of the lung tissue exposed to high tidal volume ventilation. Studies showed that NF-κB signaling can be rapidly induced by a large number of stimuli, which trigger the transcription of inflammatory cytokines.18,19 During the process of positive pressure ventilation with high tidal volume, baroreceptors on lung epithelial cells and alveolar macrophages are stimulated by excessive alveolar stretching, leading to serial biochemical events via a mechanical transduction mechanism, which would cause the modulation of the NF-κB signal pathway, the activation of inflammatory cells, and formation of chemical chemoattractants. With the action of chemotactic stimuli and expression of adhesion molecules, the infiltration of activated neutrophils into the lung tissue may initiate an inflammatory cascade that unavoidably results in the generation of its down-flow cytokines (eg, TNF-α, IL-1β, IL-6).20 Our study demonstrated that increased airway pressure and inflammatory cytokine release are associated with HVT mechanical ventilation. Similar results have been obtained by Chu et al.21
To avoid the influence of systemic hemodynamics, we applied 1 vol% sevoflurane. There was no significant difference among the four groups with regard to mean arterial pressure during ventilation. Lee et al12 demonstrated that volatile anesthetics reduced necrosis and inflammation. Schilling et al22 recently reported that sevoflurane suppressed local alveolar inflammatory responses during one-lung ventilation. Our studies showed that sevoflurane administration significantly attenuated the lung injury by the inhibition of TNF-α, IL-1β, IL-6, and the augmentation of IL-10. At the same time, the protein expression of the proinflammatory cytokine induction of NF-κB was also remarkably inhibited in the groups with sevoflurane. NF-κB is a pivotal inducible transcription factor that regulates the expression of many genes of inflammation, so we deduce that sevoflurane administration results in a proinflammatory shift by downregulating the mRNA expression of NF-κB. Consistent with this finding, Zhong et al23 reported that sevoflurane protected the lung from ischemia/reperfusion injury by suppressing the expression of NF-κB.
HO – the rate-limiting enzyme in heme degradation to carbon monoxide (CO), free iron, and biliverdin – is normally expressed at low levels in tissues. In the previous study, it was found that HO-1 expression could be induced by heavy metals, glutathione depletors, immunostimulants, and a variety of stressful conditions.24,25 In our studies, HO-1 expression is induced by ventilation with a tidal volume of 16 mL/kg, indicating that HO-1 expression is sensitive to induction by mechanical stimulation. The increase in HO-1 expression has been considered to be an inducible defense and a beneficial response to acute lung injury;26–28 however, the underlying mechanisms are still poorly understood. An et al29 reported the beneficial effect of HO-1 to ventilator-induced lung injury. Park et al30 found that the overexpression of HO-1 mediated the antiinflammatory response through Nrf-2, PI3K/Akt, and ERK activation. During those downstream products of HO-1, CO has been demonstrated to modulate the inflammatory pathway in a variety of experimental models, reducing the production of inflammatory cytokines, while increasing the production of antiinflammatory cytokines through interaction with the MAPK pathways.31 CO might be able to depress leukocyte adhesion through the modulation of platelet dynamics,32 but the studies of Maulik et al33 indicate that the antiinflammatory function of CO contributes to the production of nitric oxide, resulting in the indirect modulation of adhesive interaction.
Although Schwer et al6 had reported that the augmentation of HO-1 could be induced by sevoflurane in the rat liver, we provided the first proof that 1% MAC sevoflurane administration upregulated the mRNA expression of HO-1 in the lung tissue during ventilation, which reduced proinflammatory cytokine generation. We presume that this could be the underlying mechanism of sevoflurane attenuating VILI. Whether this beneficial effect is threshold or dose-dependent still remains unanswered.
Conclusion
Mechanical ventilation with high tidal volume could probably lead to lung injury, which could be significantly attenuated by sevoflurane inhalation by upregulating HO-1 expression and inhibiting the NF-κB-mediated proinflammatory cytokine generation.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Faller S, Ryter SW, Choi AM, Loop T, Schmidt R, Hoetzel A. Inhaled hydrogen sulfide protects against ventilator-induced lung injury. Anesthesiology. 2010;113(1):104–115. doi: 10.1097/ALN.0b013e3181de7107. [DOI] [PubMed] [Google Scholar]
- 2.Veldhuizen RA, Slutsky AS, Joseph M, McCaig L. Effects of mechanical ventilation of isolated mouse lungs on surfactant and inflammatory cytokines. Eur Respir J. 2001;17(3):488–494. doi: 10.1183/09031936.01.17304880. [DOI] [PubMed] [Google Scholar]
- 3.Boost KA, Hoegl S, Dolfen A, et al. Inhaled levosimendan reduces mortality and release of proinflammatory mediators in a rat model of experimental ventilator-induced lung injury. Crit Care Med. 2008;36(6):1873–1879. doi: 10.1097/CCM.0b013e3181743e63. [DOI] [PubMed] [Google Scholar]
- 4.Eastman A, Holland D, Higgins J, et al. High-frequency percussive ventilation improves oxygenation in trauma patients with acute respiratory distress syndrome: a retrospective review. Am J Surg. 2006;192(2):191–195. doi: 10.1016/j.amjsurg.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 5.Belperio JA, Keane MP, Lynch JP, 3rd, Strieter RM. The role of cytokines during the pathogenesis of ventilator-associated and ventilator-induced lung injury. Semin Respir Crit Care Med. 2006;27(4):350–364. doi: 10.1055/s-2006-948289. [DOI] [PubMed] [Google Scholar]
- 6.Schwer CI, Stoll P, Pietsch U, et al. Up-regulation of heme oxygenase-1 by sevoflurane is not dependent on Kupffer cells and associates with ERK1/2 and AP-1 activation in the rat liver. Int J Biochem Cell Biol. 2010;42(11):1876–1883. doi: 10.1016/j.biocel.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Li N, Venkatesan MI, Miguel A, et al. Induction of heme oxygenase-1 expression in macrophages by diesel exhaust particle chemicals and quinones via the antioxidant-responsive element. J Immunol. 2000;165(6):3393–3401. doi: 10.4049/jimmunol.165.6.3393. [DOI] [PubMed] [Google Scholar]
- 8.Steurer M, Schläpfer M, Steurer M, et al. The volatile anaesthetic sevoflurane attenuates lipopolysaccharide-induced injury in alveolar macrophages. Clin Exp Immunol. 2009;155(2):224–230. doi: 10.1111/j.1365-2249.2008.03807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sánchez-Conde P, Rodríguez-López JM, Nicolás JL, et al. The comparative abilities of propofol and sevoflurane to modulate inflammation and oxidative stress in the kidney after aortic cross-clamping. Anesth Analg. 2008;106(2):371–378. doi: 10.1213/ane.0b013e318160580b. table of contents. [DOI] [PubMed] [Google Scholar]
- 10.Kong HY, Zhu SM, Wang LQ, He Y, Xie HY, Zheng SS. Sevoflurane protects against acute kidney injury in a small-size liver transplantation model. Am J Nephrol. 2010;32(4):347–355. doi: 10.1159/000319623. [DOI] [PubMed] [Google Scholar]
- 11.Suter D, Spahn DR, Blumenthal S, et al. The immunomodulatory effect of sevoflurane in endotoxin-injured alveolar epithelial cells. Anesth Analg. 2007;104(3):638–645. doi: 10.1213/01.ane.0000255046.06058.58. [DOI] [PubMed] [Google Scholar]
- 12.Lee HT, Ota-Setlik A, Fu Y, Nasr SH, Emala CW. Differential protective effects of volatile anesthetics against renal ischemia-reperfusion injury in vivo. Anesthesiology. 2004;101(6):1313–1324. doi: 10.1097/00000542-200412000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Lee HT, Kim M, Jan M, Emala CW. Anti-inflammatory and antinecrotic effects of the volatile anesthetic sevoflurane in kidney proximal tubule cells. Am J Physiol Renal Physiol. 2006;291(1):F67–F78. doi: 10.1152/ajprenal.00412.2005. [DOI] [PubMed] [Google Scholar]
- 14.Nishina K, Mikawa K, Takao Y, Shiga M, Maekawa N, Obara H. Intravenous lidocaine attenuates acute lung injury induced by hydrochloric acid aspiration in rabbits. Anesthesiology. 1998;88(5):1300–1309. doi: 10.1097/00000542-199805000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Frank JA, Pittet JF, Wray C, Matthay MA. Protection from experimental ventilator-induced acute lung injury by IL-1 receptor blockade. Thorax. 2008;63(2):147–153. doi: 10.1136/thx.2007.079608. [DOI] [PubMed] [Google Scholar]
- 16.Jiang JS, Wang LF, Chou HC, Chen CM. Angiotensin-converting enzyme inhibitor captopril attenuates ventilator-induced lung injury in rats. J Appl Physiol. 2007;102(6):2098–2103. doi: 10.1152/japplphysiol.00514.2006. [DOI] [PubMed] [Google Scholar]
- 17.Kim JH, Suk MH, Yoon DW, et al. Inhibition of matrix metalloproteinase-9 prevents neutrophilic inflammation in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;291(4):L580–L587. doi: 10.1152/ajplung.00270.2005. [DOI] [PubMed] [Google Scholar]
- 18.Lutz J, Luong le A, Strobl M, et al. The A20 gene protects kidneys from ischaemia/reperfusion injury by suppressing pro-inflammatory activation. J Mol Med (Berl) 2008;86(12):1329–1339. doi: 10.1007/s00109-008-0405-4. [DOI] [PubMed] [Google Scholar]
- 19.Coornaert B, Carpentier I, Beyaert R. A20: central gatekeeper in inflammation and immunity. J Biol Chem. 2009;284(13):8217–8221. doi: 10.1074/jbc.R800032200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung HY, Cesari M, Anton S, et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8(1):18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chu EK, Whitehead T, Slutsky AS. Effects of cyclic opening and closing at low- and high-volume ventilation on bronchoalveolar lavage cytokines. Crit Care Med. 2004;32(1):168–174. doi: 10.1097/01.CCM.0000104203.20830.AE. [DOI] [PubMed] [Google Scholar]
- 22.Schilling T, Kozian A, Senturk M, et al. Effects of volatile and intravenous anesthesia on the alveolar and systemic inflammatory response in thoracic surgical patients. Anesthesiology. 2011;115(1):65–74. doi: 10.1097/ALN.0b013e318214b9de. [DOI] [PubMed] [Google Scholar]
- 23.Zhong C, Zhou Y, Liu H. Nuclear factor kappaB and anesthetic preconditioning during myocardial ischemia-reperfusion. Anesthesiology. 2004;100(3):540–546. doi: 10.1097/00000542-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Abraham NG, Kappas A. Heme oxygenase and the cardiovascular-renal system. Free Radic Biol Med. 2005;39(1):1–25. doi: 10.1016/j.freeradbiomed.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Otterbein LE, Otterbein SL, Ifedigbo E, et al. MKK3 mitogen-activated protein kinase pathway mediates carbon monoxide-induced protection against oxidant-induced lung injury. Am J Pathol. 2003;163(6):2555–2563. doi: 10.1016/S0002-9440(10)63610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donnelly LE, Barnes PJ. Expression of heme oxygenase in human airway epithelial cells. Am J Respir Cell Mol Biol. 2001;24(3):295–303. doi: 10.1165/ajrcmb.24.3.4001. [DOI] [PubMed] [Google Scholar]
- 27.Olszanecki R, Gebska A, Korbut R. The role of haem oxygenase-1 in the decrease of endothelial intercellular adhesion molecule-1 expression by curcumin. Basic Clin Pharmacol Toxicol. 2007;101(6):411–415. doi: 10.1111/j.1742-7843.2007.00151.x. [DOI] [PubMed] [Google Scholar]
- 28.Ge ZJ, Jiang GJ, Zhao YP, Wang GX, Tan YF. Systemic perfluorohexane attenuates lung injury induced by lipopolysaccharide in rats: the role of heme oxygenase-1. Pharmacol Rep. 2010;62(1):170–177. doi: 10.1016/s1734-1140(10)70254-1. [DOI] [PubMed] [Google Scholar]
- 29.An L, Liu CT, Qin XB, Liu QH, Liu Y, Yu SY. Protective effects of hemin in an experimental model of ventilator-induced lung injury. Eur J Pharmacol. 2011;661(1–3):102–108. doi: 10.1016/j.ejphar.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 30.Park SY, Park DJ, Kim YH, et al. Upregulation of heme oxygenase-1 via PI3K/Akt and Nrf-2 signaling pathways mediates the anti-inflammatory activity of Schisandrin in Porphyromonas gingivalis LPS-stimulated macrophages. Immuno Lett. 2011;139(1–2):93–101. doi: 10.1016/j.imlet.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Kirkby KA, Adin CA. Products of heme oxygenase and their potential therapeutic applications. Am J Physiol Renal Physiol. 2006;290(3):F563–F571. doi: 10.1152/ajprenal.00220.2005. [DOI] [PubMed] [Google Scholar]
- 32.Nowell SA, Leakey JE, Warren JF, Lang NP, Frame LT. Identification of enzymes responsible for the metabolism of heme in human platelets. J Biol Chem. 1998;273(50):33342–33346. doi: 10.1074/jbc.273.50.33342. [DOI] [PubMed] [Google Scholar]
- 33.Maulik N, Engelman DT, Watanabe M, et al. Nitric oxide/carbon monoxide. A molecular switch for myocardial preservation during ischemia. Circulation. 1996;94(Suppl 9):II398–II406. [PubMed] [Google Scholar]