Abstract
B cells exhibit a range of functional responses following TLR engagement including immunoglobulin and cytokine production, proliferation, antigen presentation and migration. However, B cell intrinsic TLR responses appear to be precisely programmed based upon the developmental stage of the cell. B cell subpopulations classified as innate immune cells including marginal zone and B-1 B cells exhibit robust responses to TLR stimulation. In contrast, activation of other B cell subsets is constrained via a variety of developmentally regulated events. In this review we provide an overview of TLR responses in murine and human B cells and specifically highlight patterns of TLR expression and developmentally regulated functional responses.
Keywords: B cell subpopulations, Marginal zone B cells, Transitional B cells, Follicular mature B cells, B-1 B cells, BAFF, TRL, MyD88, IRAK-4 deficient patients, MyD88 deficient patients, LPS, CpG, R848, Review
2. INTRODUCTION
Innate immune responses are initiated rapidly, usually within hours after antigen encounter. The receptors of the innate immune system are generally known as pattern recognition receptors (PRR), which recognize simple molecules and patterns of molecules shared by many microorganisms, so-called pathogen-associated molecular patterns (PAMPs). PRRs are germline encoded and highly conserved during evolution. Toll-like receptors are an important family of PRRs, present on many different cells of the innate immune system. In contrast, receptors of the adaptive immune system are highly antigen specific: they are clonally-rearranged and specific for a single chemical structure.
Among the cells of the immune system, B cells are unique as they express both germline-encoded Toll-like receptors and a clonally rearranged antigen-specific receptor, the B cell antigen receptor (BCR). Therefore, B cells may receive signals through both TLRs and BCR during an immune response, either simultaneously or consecutively. It is generally accepted that TLR stimulation provides a link between the innate and the adaptive immune system. Usually, TLR engagement is assumed to initiate the immune response and subsequently augment the adaptive immune response and this has been shown for B cells in several studies (1–3). However, recent studies suggest that BCR signaling can also overcome TLR non-responsiveness (4).
B cells exhibit a broad range of functional responses and most of them can be influenced by TLR signaling. The best analyzed function of B cells is immunoglobulin production. TLR stimulation can result in differentiation into plasma cells or influence class switching and affinity maturation (5). In addition, B cells can act as professional antigen presenting cells requiring expression of MHC class II which can be induced by TLR stimulation. B cells are able to produce a variety of different cytokines in response to different TLR ligands (6). Moreover, B cells can provide T cell help via co-stimulatory molecules which are up-regulated after TLR engagement (7, 8). Finally, TLR engagement can result in up- or down-regulation of different surface molecules with important modulatory functions. In case of integrin signaling these molecules are important for positioning and migration of B cells into lymphoid organs (7–9).
However, considering all these functions it must be taken into account that the B cell compartment is comprised of many different B cell subpopulations with distinct phenotypic and functional characteristics. Global analyses of B cell function therefore always represent a combination of functional responses of individual B cell subpopulations. This implies that alterations in the composition of B cell subpopulations can subsequently lead to differences in the global B cell response. This is of particular importance in interpreting in vitro assays. Over recent years, it has become increasingly clear that specific B cell subsets respond quantitatively and qualitatively differently to TLR engagement. In part, this distinction has lead to classification of Marginal zone and B-1 B cells as innate, vs. naïve mature B cells as adaptive, immune cells (10). The purpose of this review is to highlight the important differences among B cell subsets derived from both mouse and human with respect to both TLR expression and developmental and functional responses to TLR engagement.
3. THE ROLE OF TLR SIGNALING IN B CELL DEVELOPMENT, DIFFERENTIATION AND SURVIVAL
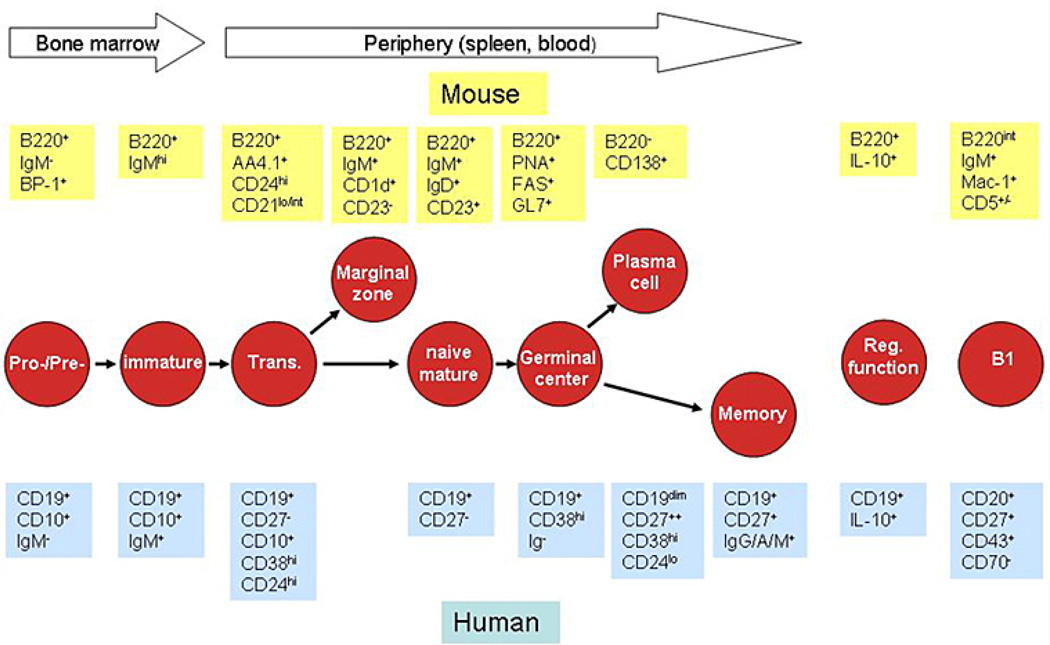
It is well known that signaling through the BCR is required for the development and maintenance of B cells. Increasing knowledge about TLR signaling has raised the question as to whether similar to the BCR, signaling through TLRs might be required for proper B cell development and survival. B cell development begins in the bone marrow (Figure 1). With the expression of CD19 or B220 pro-B cells can be first identified as committed irrevocably to the B cell lineage. Productive V(D)J recombination leads to synthesis of the membrane immunoglobulin heavy-chain protein mu, which associates with the surrogate light-chain proteins to form the pre-BCR characteristic for pre-B cells. Expression of the pre-BCR serves as a checkpoint that monitors for functional immunoglobulin H-chain rearrangement and triggers clonal expansion and developmental progression of pre-B cells into the immature B cell stage expressing cell-surface IgM. Immature B cells migrate from the bone marrow to the spleen where they further mature through so-called ‘transitional’ B cell stages into at least two distinct subsets, e.g. follicular mature (FM) and marginal zone (MZ) B cells. Upon antigen encounter, FM B cells enter the germinal center reaction where they can undergo class switching and somatic hypermutation, and differentiate either into memory or antibody producing plasma cells. In contrast to the predominant population of B-2 B cells comprising the aforementioned B cell subpopulations, B-1 B cells are a minor population of B cells that are found in multiple tissues, including the peritoneal and pleural cavities in mice. Recently, a strong candidate for the equivalent of murine B-1 B cells has been identified in humans (11). Similar to MZ B cells, murine B-1 B cells are highly responsive to TLR signaling. Whereas B-1 B cells were initially thought to be exclusively generated during fetal life, B-1 B cell specific progenitors have also been identified in adult mice although the frequency of such cells declines rapidly beyond the newborn stage (12, 13). Over the last few years, B cells with a regulatory function and referred to as regulatory B cells or B10 cells, entered the focus of interest. Cells with such functional activity have now been identified in both mice and humans (14, 15). Moreover, a putative progenitor of B cells with a regulatory function has been described within the spleen (15, 16).
Figure 1.
Characteristic markers for developmental B cell subpopulations. Schematic depiction of B cell subsets during B cell development and their characteristic phenotypic markers as mentioned in the text in both mouse and human. No well established markers exist for murine memory B cells or human marginal zone B cells.
Investigations of different transgenic or knockout mouse models have demonstrated the important role of BCR signaling for B cell development. For example, in mice targeted deletions of the immunoglobulin cytoplasmic tail (17) or the immunoglobulin mu heavy chain (muMT) (18) result in a developmental arrest at the pro- to pre- B cell checkpoint. Moreover, mice defective in Bruton’s tyrosine kinase (Btk) exhibit reduced numbers of peripheral B cells and B cell development arrests at the transitional B cell step (19–21). The corresponding mutation in humans leads to an almost complete loss of B cells in the periphery (<1% B cells of all lymphocytes) and the clinical phenotype of Bruton’s disease characterized by agammaglobulinemia in addition to absent B cells (22, 23). The necessity of BCR signaling for maintenance of mature B cells has been demonstrated by Rajewksy’s group by generating transgenic mice in which the BCR can be inducibly deleted. Ablation of the BCR led to rapid death of mature B cells indicating that basal constitutive BCR signaling is required for survival of mature B cells (24).
Finally, BCR signaling clearly impacts developmental B cell fate: Whereas distinct signals facilitate development towards MZ B cells other BCR-associated events induce FM B cell development; and development of peritoneal B1 B cells has been suggested to require strong and/or sustained BCR signals (25–27).
Over the last few years, several authors addressed the question of a requirement for TLR signals for B cell development. In particular, the maturation of those B cell subpopulations highly responsive to TLR engagement might be dependent on these signals. However, although TLR signaling can influence the maturation of specific B cell subsets, there is no direct evidence that it is required. Analyzing MyD88−/− mice, Medzhitov’s group did not find differences in the B cell compartment in naïve MyD88−/− vs wild-type mice (3). We also carefully analyzed splenic and peritoneal B cell subsets in MyD88−/−, +/− and +/+ mice, including young mice at 3 and 4 weeks of age. No difference in B cell subpopulations was found in adult or young mice between these groups or in cohorts of MyD88−/− TRIF−/− double deficient mice (AMB and DJR unpublished results). Consistent with our findings, analyses of B cells in MyD88−/−TRIF−/− double deficient mice presenting with a complete lack of all known TLR signaling (28) reported no differences in numbers of splenic transitional T1, transitional T2, MZ and FM, peritoneal B-1 and B-2 B cells, or bone marrow B cells (based on Hardy fractions A-F) in 2 month old mice. The only exception was elevated CD23 expression on splenic B cells from MyD88−/−TRIF−/− double deficient mice that did not influence the composition of these subpopulations. Consistent with these findings B cell development and in particular MZ B cell development has also been shown to be normal in germ-free mice (29) and rats (30). Together, although competitive repopulation studies or studies in non-specific pathogen-free or germ-free mice have not yet been reported, these combined data argue strongly against a role for TLR signaling in normal murine B cell development.
Recently, a study analyzing patients with autosomal recessive IRAK-4 and MyD88 deficiency has provided initial insight with respect to the potential role for TLR signaling in human B cell development (31). In this publication, clinical and immunological data from 48 IRAK-4 deficient and 12 MyD88 deficient patients were summarized. All patients showed normal percentages of B cells in peripheral blood. However, a detailed analysis of B cell subpopulations was not included in the study and further investigation is required to address this question. Of note, alterations in human TLR3 signaling pathways have also been described including both autosomal dominant TLR3 deficiency (32) and autosomal recessive UNC93B1 (an endoplasmic reticulum protein involved in TLR3, TLR7, and TLR9 activation) deficiency (33). However, as only very few individuals with these defects have been identified to date, little data regarding total lymphocyte populations and the B cell compartment, in particular, are available. Overall, these observations indicate that TLR signaling plays little or no role in pre-immune murine or human B cell development.
Notably, TLR signaling clearly influences the development of plasma and memory B cells implying that B cell-intrinsic TLR signals might be required for key differentiation steps following antigen encounter. This question has led to controversial results. In initial studies, Medzhitov and colleagues reported that generation of T cell-dependent antigen-specific antibody responses, and therefore also the differentiation of plasma cells, requires activation of TLRs in B cells (3). No specific antibody production was detected in MyD88−/− mice after immunization with the T cell-dependent antigen human serum albumin (HSA)-LPS, whereas wild-type mice generated robust antibody titers. Transfer of MyD88−/− vs. wild-type B cells into B cell deficient, muMT recipient mice and subsequent immunization with HSA-LPS lead to the conclusion that this finding represented a B cell intrinsic deficit. In the same experiment, recipients of MyD88−/− B cells barely developed germinal center B cells after immunization. The authors concluded that TLR signaling has an important role in the differentiation of B cells into germinal center cells and antibody production. This view was contrasted by studies from the groups of Bruce Butler and David Nemazee (28, 34). Antibody responses in MyD88−/−TRIF−/− double deficient mice to T cell-dependent immunization were largely unimpaired compared to wild-type mice indicating that differentiation of plasma cells was normal. We also assessed the role for B cell–intrinsic TLR signals for T cell-dependent immune responses by transferring MyD88−/− or wild-type B cells into muMT mice and subsequent immunization with 4-hydroxy-3-nitrophenylacetyl (NP) chicken gamma globulin (CGG). All recipients exhibited similar increases in NP-specific antibody titers during primary and secondary responses indirectly demonstrating that generation of plasma and memory B cells was normal (1). In addition, no difference in the generation of germinal centre (GC) B cells was observed between MyD88−/− and wild-type mice after T cell-dependent immunization with NP-CGG. An additional study reports no difference in antigen-specific IgG1, IgG2b and IgG3 after immunization with OVA in alum in MyD88−/− or wild-type mixed bone marrow chimeras whereas neither IgM or IgG2c was detected in both groups (2). However, when LPS was added to the immunogen, they demonstrated that the antigen-specific IgG2c primary response in contrast to other subclasses was absolutely dependent on MyD88 signalling. In summary, there seems to be no requirement for direct TLR-mediated stimulation of B cells during T cell-dependent immune responses with the possible exception of class-switch to IgG2c.
While there is no apparent need of TLR signaling for naïve B cell development, TLR engagement clearly influences B cell differentiation into GC and plasma B cells. This has been shown both in vitro and in vivo. In vitro stimulation of human transitional B cells with CpG results in expression of CD138 and up-regulation of activation-induced cytidine deaminase (AID) and Blimp-1 expression levels, thereby driving these cells to terminal differentiation and production of natural antibodies (35, 36). Moreover, CpG stimulation also triggers the generation of somatically mutated memory B cells from human immature transitional B cells in vitro as demonstrated by up-regulation of VH5 and other transcripts (37). The mutation frequency, range and type of substitutions observed in vitro are comparable to those found in memory B cells from the peripheral blood of patients with Hyper IgM syndrome and the spleen of normal infants. Therefore, somatic hypermutation in transitional B cells may represent a first step leading to generate memory B cells that subsequently refine their repertoire and specificity in germinal centers. Several studies demonstrated the differentiation of mature B cells into plasma B cells upon TLR ligation. Stimulation with CpG promoted differentiation of murine B-1 and MZ but not of FM B cells into mature plasma cells determined by cellular morphology, cytoplasmic immunoglobulin content and CD138 expression (38). In accordance with these findings B-1 and MZ but not FM B cells up-regulated Blimp-1 and XBP-1 expression and down-modulated Pax5 expression upon CpG stimulation. LPS stimulation was also reported to drive naïve mouse B cells into plasma cell differentiation (39) and this effect was synergistically enhanced by TACI ligation (40). Similarly, both human CD27- naïve and CD27+ B cells can differentiate into plasma cells upon CpG stimulation in conjunction with different cytokines (41, 42). Plasma cells can also be generated from human CD27+ memory B cells by TLR 9 stimulation alone (35). In vivo, addition of TLR ligands to the immunogen results in an enhanced GC reaction and antibody production (1, 3) and consistently, the lack of MyD88 in B cells impairs the germinal center response after immunization with Dinitrophenylated-ovalbumin (DNP-OVA) in incomplete Freund’s adjuvant (IFA) plus LPS (2) revealing a specific deficit in IgG2c class-switch (the isotype equivalent of IgG2a in C57/B6 mice).
TLR signaling has been also implicated to have an important role in maintenance of memory B cells. Previous work from Lanzavecchias group has suggested a model whereby memory B cells must be triggered via polyclonal stimuli including TLR signals to maintain humoral memory (42). They reported that human memory B lymphocytes proliferate and differentiate into plasma cells in response to polyclonal stimuli, including TLR engagement, and concluded that ongoing polyclonal activation of memory B cells is important to maintain serological memory for a human lifetime. In contrast to this report, we found no difference in long-term memory responses after transfer of MyD88−/− B cells into muMT mice and subsequent T cell-dependent immunization (1). Future analyses are warranted to better understand the role of TLR signaling for maintenance of memory B cells.
In summary of these different studies, there is no evidence that TLR activation is essential for bone marrow, splenic or peritoneal B cell development in unmanipulated mice. Data from human patients with defects in molecules of the TLR signaling pathway (IRAK-4, MyD88 and TLR3) have not indicated developmental defects in the B cell compartment, but more detailed studies are still needed to fully address this question. Furthermore, TLR activation is not required for the differentiation of GC and plasma B cells; but TLR signals facilitate these maturation steps. Additionally, B cell-intrinsic MyD88 signals are required for IgG2c class-switch, at least in some settings- an important observation given the key role for this isotype in triggering of activating Fc receptors (43). The role of TLR signals in maintenance of memory B cells is still controversial and not completely understood.
4. EXPRESSION OF TOLL-LIKE RECEPTORS IN B CELL SUBPOPULATIONS
While B cells have been described to express a variety of different TLRs, there are major differences in the TLR expression pattern for murine vs. human B cells. So far, 12 functional TLRs have been identified in mice and 10 in humans (44). TLR1-9 are expressed in both human and mouse cells. Murine TLR10 is not functional, whereas TLR11-13 have been lost from the human genome. TLRs can be divided into two subgroups depending on cellular localization: TLR1, TLR2, TLR4, TLR5, TLR6 and TLR11 are expressed on the cell surface and mainly recognize microbial membrane components. In contrast, TLR3, TLR7, TLR8 and TLR9 are expressed in intracellular vesicles including the endoplasmatic reticulum and lysosomes, recognizing microbial nucleic acids. Whereas many studies have analyzed mRNA expression, far fewer investigations have addressed protein expression levels. Because different responsiveness to TLR engagement might result from varying TLR expression, we will review here the literature specifically on TLR expression patterns within distinct B cell subpopulations in both humans and mice.
4.1. RP105
Responses to TLR4 are modulated by at least two co-receptors including CD14 and the TLR4 homolog, RP105 (45). Similar to other cell lineages, B cell responses to LPS do not only depend on TLR4 and previous work has identified a potential role for RP105 in B cells (46). In addition, MD-1 expression is indispensable for cell-surface expression of RP105, similar to MD-2 for TLR4. RP105 is a type 1 transmembrane protein with structural similarities to TLRs, containing an extracellular leucine rich-repeat domain. RP105 was first described in mouse B cells. Direct antibody-mediated cross-linking of RP-105 in vitro triggers strong B cell proliferation and protection from apoptosis induced by radiation or dexamethasone treatment (47). Consistent with these data, analysis of mice deficient for RP105 revealed impaired proliferative and humoral immune responses of RP105-deficient B cells to LPS (46). A homolog of RP105 was also identified in humans and is expressed on human B cells (48). Expression of RP105 has been shown to be associated with B cell activation (49). Serial analyses of B cells in SLE patients revealed that RP105-negative B cells are markedly decreased in parallel with a reduction in disease activity. A follow-up of this study demonstrated that RP105-negative B cells in SLE patients are highly activated cells that are responsible for the production of autoantibodies and polyclonal immunoglobulins (49). RP105 is also expressed on other cell lineages including dendritic cells and marcrophages (45, 50). Notably, in contrast to the stimulatory effect of RP105 in B cells, RP105 negatively regulates TLR4 responses in murine macrophages and dendritic cells (51). Recent collaborative studies (DJR, Christopher Karp-manuscript submitted) suggest that the putative positive role for RP105 in B cell TLR signals may have been misinterpreted and that this receptor plays a negative regulatory role in both murine lineages.
4.2. TLR expression on murine B cell subpopulations
Several groups have analyzed expression of TLR1-9 by PCR in murine B cell subpopulations including splenic FM and MZ B cells and peritoneal B1 B cells (38, 52–54). Expression of TLR5 and TLR8 was generally found to be low or absent in FM, MZ and B-1 B cells. Low to intermediate expression was detected for TLR3 and TLR6. No obvious differences were found in TLR2 expression between FM and MZ B cells, whereas levels in B-1 B cells were either higher (2, 38) or lower (2, 38) compared to FM and MZ B cells. TLR1 expression was significantly higher in FM compared to MZ and B-1 B cells in one report (38) and without any difference in other publications. TLR7 and TLR9 showed equivalent and strong expression patterns in FM, MZ and B1 B cells. TLR4 was higher in MZ compared to FM B cells in one report (54), lower in a different study (38), or equivalent in other reports (52, 53). One study reported the expression of TLR2, TLR4 and TLR9 in splenic immature/T1 B cells in comparison to FM and MZ B cells. Whereas mRNA levels for TLR2 and TLR4 seems to be slightly lower, no difference could be detected for TLR9 mRNA expression (36). Our own group investigated mRNA expression of several TLR signaling effectors including MyD88, Mal, TRIF, IRAK-1, IRAK-4, Tollip, TRAF6, or SIGIRR in FM, MZ and GC B cells (1). Strikingly, mRNA levels for MyD88 and Mal, but not TRIF, were increased fourfold in GC compared with FM and MZ B cells whereas no significant differences were observed in all other analyzed molecules. In summary, little consistency exists regarding the TLR expression pattern in B cell subpopulations. These variable results in previous studies likely reflect different gating strategies for B cell subsets and/or other differences in methodology.
While fewer studies exist regarding TLR protein expression in B cell subpopulations, results reported to date are more consistent. Most analysis has been restricted to TLR4 and its homolog RP105 probably due to a lack of other suitable antibodies. TLR4 complexed with MD-2 is expressed at low levels on B cells (relative to myeloid cells) and no differences are found between FM, MZ and GC B cells (1, 55). RP105 is expressed at consistently higher levels on MZ, in comparison with FM, B cells as determined by flow cytometry (1, 36, 55). RP105 was also reported to be expressed at much lower levels in splenic immature/T1 B cells (36). Contrasting results have been reported in respect to GC B cells. Whereas we found similar RP105 expression in FM and GC B cells by flow cytometry (1), other investigators were unable to identify RP105 on these populations using fluorescence microscopy (55).
4.3. TLR expression on human B cell subpopulations
Expression of TLR 1-10 on human B cells has been investigated by several groups mainly in distinct subpopulations from peripheral blood or tonsils using different techniques including PCR (56–58), northern blot (59), or microarray analysis (60). Data on protein expression of TLRs in human B cell subpopulations are very rare. Bernasconi et al analyzed TLR1-10 expression in naive, IgM+ and switched memory B cells from peripheral blood by conventional and real-time PCR, finding higher levels of TLR6, TLR7, TLR9, TLR10 and RP105 in memory compared with naïve B cells (56). TLR1-5 and TLR8 were expressed at low to undetectable levels. No significant difference was seen between expression levels in IgM+ versus switched memory B cells. Similar results were obtained in a study investigating TLR expression in naïve, memory and GC B cells from peripheral blood by real-time PCR (58). Expression of TLR 3–6 and TLR8 were low to undetectable, whereas TLR10 was strongly expressed. Levels of TLR1, TLR2 and TLR10 were similar in all three subsets. In contrast, TLR7 and TLR9 were slightly higher in memory compared with naïve and GC B cells.
Another study assessed TLR expression levels in plasma, naïve and memory B cells from tonsils and in peripheral blood plasma cells using real-time PCR (57). TLR3, TLR4 and TLR8 expression was absent in naïve and memory B cells but present in plasma cells. TLR2 and TLR5 were expressed at higher, and TLR10 at slightly lower, levels in plasma cells in comparison with naïve and memory B cells. TLR1, TLR6, TLR7 and TLR9 were expressed at similar levels in all subsets and no differences were observed between plasma cells from tonsils vs. peripheral blood.
Bourke et al. investigated TLR1-10 expression in resting versus GC tonsillar B cells using Northern blot analysis (59). Whereas expression of TLR2-5 was undetectable, TLR7 and TLR8 were expressed at similar levels in both subsets. Expression of TLR1, TLR9 and TLR10 was higher in GC compared with resting B cells. In the most recent study, TLR expression was analyzed in splenic naïve, IgM+ and switched memory B cells and plasma cells by microarray expression profiles (60). TLR1, TLR6 and TLR7 were found to be only weakly expressed in all subsets. TLR9 and TLR10 showed slightly higher expression levels in memory B cells than naïve and plasma B cells. CD180 (RP105) was highly expressed in naïve and memory B cells with about 2–5 fold higher expression in memory B cells. These data were also confirmed by flow cytometry. Protein expression levels of TLR9 and CD180 (RP105) were clearly higher on memory compared with naïve human splenic B cells with no obvious difference in expression levels between IgM+ and switched memory B cells. In contrast, RP105 was absent on plasma cells. Carsetti and colleagues evaluated TLR9 expression in transitional B cells from cord blood compared with those in naïve and memory B cells from peripheral blood (35). Surprisingly, transitional B cells showed the highest TLR9 expression levels among these subsets.
It is difficult to reconcile these divergent findings that have been based upon analyses of different B cell subsets from varying sources analyzed using distinct techniques. Overall, most studies suggest that TLR3-5 and TLR8 expression are relatively low or undetectable in human B cells. There is a tendency towards higher TLR expression in antigen-experienced (memory, plasma and GC) versus naïve B cells, in particular with respect to TLR9 and TLR10 although transitional B cells may represent an exception to this rule.
5. DISTINCT FUNCTIONS OF B CELL SUBPOPULATIONS IN RESPONSE TO TLR ENGAGEMENT
B cells fulfill a variety of different functions. Without further specific stimulation, they secrete so-called natural antibodies, mainly of the IgM isotype. After antigen encounter they clonally expand and differentiate into plasma cells secreting antigen-specific class-switched and high affinity antibodies. Alternatively, they can develop into long-living memory B cells. B cells can also act as antigen presenting cells. For antigen presentation, secretion of cytokines is important and B cells are known to produce a variety of different cytokines. B cell intrinsic TLR signals can modify many of these effecter functions and as there is some kind of division of labor, the effect also depends on the developmental stage of the B cell. We will therefore describe here what is known about TLR responses at different stages of B cell development.
5.1. B cells during bone marrow development and at the immature/transitional stage
Immature B cells both from bone marrow and spleen have been shown a variety of different responses to TLR engagement. Because a wide variety of different classification schemes and nomenclature for immature and transitional B cells exists and different purification methods are used throughout the literature we have included the phenotypic and/or other relevant characteristics of analyzed subsets for each cited reference. Several studies investigated the response of immature B cells to LPS prior to formal identification of TLRs (61). Early studies indicated that immature B cells obtained from spleens of 5–7 day old mice proliferated in response to LPS stimulation (62) and that LPS treatment promoted both proliferation and survival of immature splenic B cells purified from 2–3 day old mice (63). In addition, LPS stimulation prevented apoptosis and allowed proliferation of a subset of immature B cells in response to subsequent BCR engagement. These experiments suggested that LPS, although not required for development, might induce maturation of immature B cells, an idea that has been followed up by Alberto Nobrega’s group over the last few years (64, 65). In vitro stimulation of bone marrow derived B220+IgM− B cell precursors with the TLR4 agonists LPS or lipid A resulted in up-regulation of CD23 thereby increasing the percentage of CD23+ B cells in the culture system (64). This effect depended on TLR4 expression and was not achieved in response to the TLR2 ligand Pam3Cys. Follow-up studies including IgMlowCD23− (termed immature) and IgMhgihCD23− (termed transitional) B cells isolated from bone marrow demonstrated up-regulation of IgD and CD21 in addition to down-modulation of the immaturity marker AA4.1 (CD93) (65). Functional maturation of in vitro generated IgM+CD23+CD93+ B cells was confirmed by a greater proliferative response to anti-CD40 plus IL-4 compared with IgM+CD23−CD93+ B cells. Finally, administration of LPS to wild-type mice increased the absolute number of mature B cells in the bone marrow within 20 hrs suggesting that TLR4 signaling might direct B cell maturation in vivo. It is important to note, however, that based on data cited above, there is no requirement for cell intrinsic TLR4 signaling during B cell development.
Recent detailed characterization of the transitional B cell compartment has included investigation of TLR responses of transitional B cell subsets. Based on AA4.1 expression, Allman et al. separated transitional B cells into transitional T1 (AA4.1+CD23−IgMhigh), T2 (AA4.1+CD23+IgMhigh) and T3 (AA4.1+CD23+IgMlow) (20) B cells. Whereas all transitional subsets exhibited a low to medium proliferative response following LPS stimulation (compared with mature B cells), the level of LPS-induced proliferation were consistently lower in T1 B cells than T2 and T3 subsets. Using a different characterization scheme separating transitional T1 (CD24hiCD21low) and T2 (CD24hiCD21int) B cells, we reported robust proliferation in T2 B cells in response to LPS, similar to that in MZ B cells (66). In contrast, T1 B cells failed to proliferate following TLR4 engagement.
In addition to LPS, stimulation with the TLR9 ligand, CpG, has been analyzed in early B cell subsets. In our studies, all splenic B cell subsets proliferate robustly in response to CpG including T1 and FM, as well as MZ B cells (AMB, DJR unpublished data). CpG stimulation, in contrast to BCR engagement, also lead to up-regulation of the NFκB target genes A1, c-myc and Bcl-xL in purified T1 B cells reaching levels identical to FM B cells (67). Upon activation with CpG or LPS, immature/T1 B cells not only proliferate but also produce both IgM and IgG (36). After transfer of immature/T1 B cells (B220+AA4.1+) and subsequent immunization with bacterial antigen, transferred cells expressed CD138 consistent with TLR-mediated differentiation into plasma cells. Another group demonstrated that CpG stimulation protects immature bone marrow B cells (B220+IgM+IgD−) from negative selection by preventing apoptosis and receptor editing (68) in vitro and in vivo- a functional response that may promote activation of autoreactive cells and production of autoantibodies in specific settings.
Carsetti has reported comparable results using human transitional B cells (35): CpG stimulation of CD24hiCD38hiCD27− transitional B cells derived from both adult peripheral blood or cord blood led to proliferation of a subpopulation of these cells and differentiation into CD27bright plasma cells. Plasma cells expressed IgM as well as other isotypes, and consistent with this finding, transitional B cells produced both IgM and IgG antibodies upon CpG stimulation in vitro. Low levels of IgM and IgG production have also been detected in human ‘transitional 2’ B cells (CD24hiCD38hiCD21hi) from peripheral blood following stimulation with CpG plus IL-21 (69). Interestingly, the CpG response in transitional B cells was altered in a 12-year old patient suffering from recurrent bacterial infection (70). Immunological evaluation in this individual revealed a specific deficiency in antibodies to polysaccharide antigens despite normal immunoglobulin levels and specific antibody titers to tetanus toxoid. Almost all peripheral B cells had the transitional phenotype (CD24hiCD38hiCD27−), did not proliferate and failed to secrete immunoglobulin upon in vitro CpG stimulation.
Overall, both human and mouse transitional B cells are capable of proliferating and differentiating into effecter B cells producing immunoglobulin upon CpG stimulation. While transitional B cells die in response to BCR engagement, both TLR4 and TLR9 stimulation can lead to improved survival and differentiation of this B cell subset which is specifically programmed for negative selection in response to BCR engagement. Thus, TLR signals at the immature and transitional B cell stage may be capable of activating and rescuing autoreactive cells that are normally eliminated by negative selection.
5.2. Mature B cells in mice
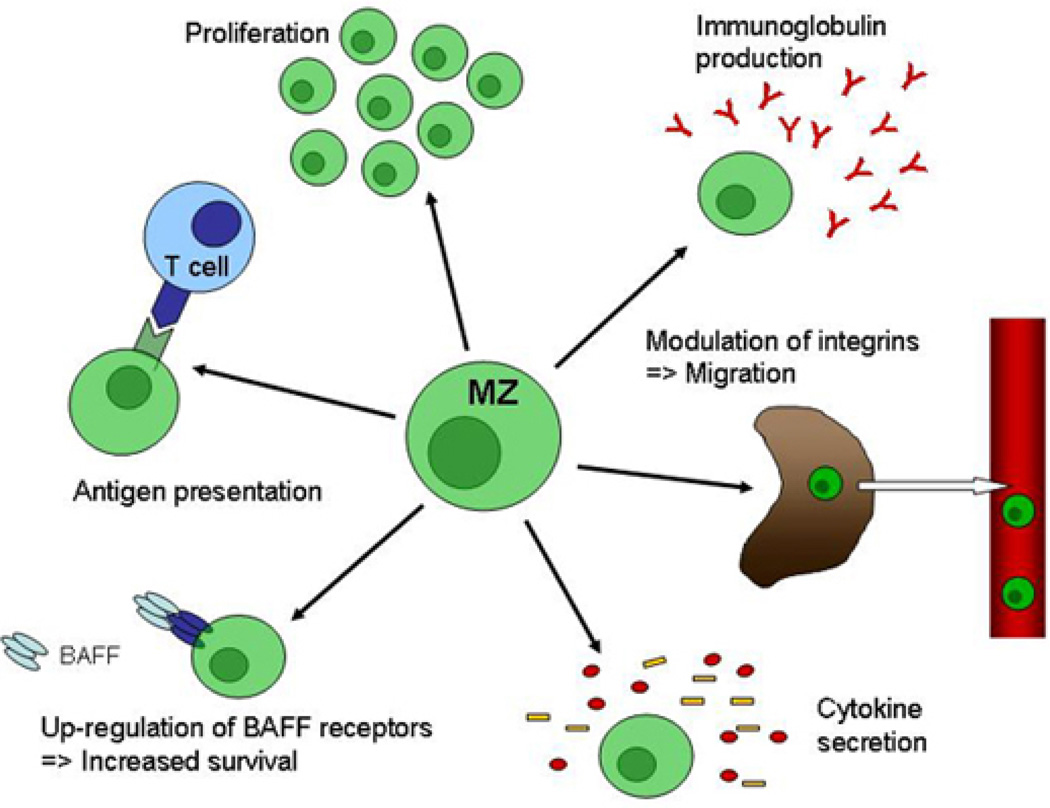
In mice, the mature B cell compartment is comprised of two major B cell subpopulations: MZ and FM B cells. These subsets can be divided based on topographic, phenotypic, gene expression and functional characteristics in addition to different developmental requirements and several reviews have focused on describing these differences (71–74). MZ B cells are key players in the early phase of immune responses and participate mainly in T cell-independent immune responses. Consistent with their classification as innate immune cells (10), they exhibit strong responses to receptors of the innate immune system including TLRs (Figure 2). In contrast, FM B cells belong to the adaptive arm of the immune system and are primarily activated through their antigen-specific BCR. This difference is reflected in a simple in vitro proliferation assay: Whereas MZ B cells robustly proliferate in response to LPS, FM B cells barely enter cell cycle. In contrast, FM B cells rapidly divide following BCR engagement, but MZ B cells undergo apoptosis in response to stimulation via the BCR (38, 75, 76). MZ B cells also exhibit stronger activation and immunoglobulin production in response to TLR signaling.
Figure 2.
Functional responses of splenic marginal zone B cells to Toll-like receptor engagement. MZ B cells are generally considered as belonging to the innate immune system. They are the main players during T cell-independent immune responses and are crucial during the early phase of immune responses. Consistent with their characterization as innate immune cells MZ B cells exhibit multiple functional responses to TLR stimulation. They rapidly can start to proliferate and secrete immunoglobulins and cytokines. Up-regulation of BAFF receptors can result in prolonged survival. Through modulation of integrins on their surface MZ B cells can leave the marginal zone and migrate to different locations. Finally, MZ B cells can act as professional antigen presenting cells, thereby providing efficient T cell help.
In addition to their role in humoral immunity, B cells can act as professional antigen-presenting cells (APC) and this ability can be induced via TLR stimulation (77). Both MZ and FM B cells were shown to rapidly up-regulate levels of B7.1 and B7.2 as well as MHC class I and II upon LPS stimulation. To further evaluate their potential as APCs, FM and MZ B cells were irradiated, co-cultured with allogeneic T cells and stimulated. Whereas purified unstimulated B cells were unable to induce T cell proliferation, LPS stimulated MZ but not FM B cells induced vigorous T cell proliferation. This indicates that MZ B cells, activated by LPS for a brief time, preferentially up-regulate key co-stimulatory molecules that help to induce allogenic T cell proliferation thus demonstrating their function as APCs.
Similar as other APCs, B cells can also produce cytokines upon activation. Barr et al analyzed the secretion of IL-6, IL-10 and INF-gamma in MZ and FM B cells after stimulation with different TLR ligands, alone or in combination (53). MZ B cells were the main source of IL-10 in response to Pam3CDK4, LPS and CpG. Maximal IL-10 secretion was found after combined stimulation with all three TLR ligands. This triple in vitro stimulus also induced a small amount of IL-10 production in FM B cells whereas no IL-10 secretion was seen after stimulation with single TLR ligands. Conversely, only FM B cells were capable of IFN-gamma production after TLR stimulation. Low levels were detectable after stimulation with single TLRs, but combined stimulation through TLR2, TLR4 and TLR9 elicited high levels of production. This triple combination also resulted in low levels of IFN-gamma production from MZ B cells. Finally, IL-6 was produced at similar levels by both subsets upon TLR engagement. A different study characterized MZ B cells as important producers of IL-10 (78). Investigating several murine lupus strains, B cells were found to be the major source of IL-10 secretion upon TLR9 stimulation. More detailed analyzes showed that IL-10 was mainly derived from MZ B cells. In addition to MZ B cells, IL-10 production has also been demonstrated in MZ B cell precursors that are phenotypically identified as B220+CD21hiCD23+ B cells initially described by the group of David Allman (79). Similar percentages of MZ and MZ-precursor B cells from MRL/lpr mice produced IL-10 in response to CpG or LPS determined by intracellular FACS staining (80). In contrast, only few FM B cells developed into IL-10 secreting cells in response to these stimuli.
A variety of surface molecules are up-regulated in response to TLR engagement including the receptors for the B cell activating factor of the TNF family (BAFF) and APRIL. BAFF is the most important B cell survival factor in the periphery and can bind to three different receptors: BAFF-R, TACI and BCMA (81, 82). In 2007, two independent groups investigated the effect of TLR4 and TLR9 stimulation on BAFF receptor expression in splenic B cells (54, 83). Both studies showed that stimulation via TLR4 or TLR9 strongly up-regulates TACI expression, whereas BAFF-R was only up-regulated upon TLR9 engagement. Both MZ and FM B cells up-regulate TACI expression in response to CpG stimulation. However, only MZ B cells and a subset of FM B cells responded to TLR4 engagement (54). These observations suggest a mechanism whereby the mode of B cell activation during immune responses can fine-tune the sensitivity and downstream outcomes of BAFF signaling, thus influencing B cell survival.
It has been well known for a long time that in vivo treatment with LPS promotes MZ B cells to migrate from the MZ into the splenic white pulp (84–86). However, the mechanisms involved in this process have only recently become clear. Jason Cyster’s group identified sphingosine-1-phosphate receptor 1 (S1P1) as an important factor for localization of MZ B cells in the MZ (7). MZ B cells express high levels of S1P1 and targeting this receptor induced migration of MZ B cells into follicles. Three hours after in vivo administration of LPS, MZ B cells down-regulated S1P1 below its level in FM B cells. Consistent with the reduced S1P1 receptor expression, LPS-exposed MZ B cells showed reduced chemotactic responsiveness to S1P in a migration assay. A subsequent study demonstrated that not only LPS stimulation, but also TLR2, TLR3 and TLR7 engagement leads to a rapid release of MZ B cells from the MZ (9). Interestingly, whereas in vivo treatment with TLR2, TLR4 and TLR7 ligands resulted in down-modulation of all S1P receptor mRNA levels, no change was seen after stimulation via TLR3. Thus, TLR engagement in vivo can alter chemokine expression thereby directing MZ B cell migration and localization. This effect might influence the amplitude of both B cell-specific and B cell-modulated immune responses.
Our group began to analyze the mechanisms accounting for differences in the proliferative response to LPS in MZ versus FM B cells (76). While in both subsets the NFkappaB and mTOR signaling cascades were similarly activated by LPS stimulation, almost no induction of ERK and Akt activation was detectable after LPS stimulation. However, MZ B cells exhibited higher basal levels of phospho-Akt and phospho-S6, consistent with a preactivated status. These results are in agreement with previously published data from another group (87). We further found that both basal and LPS activation-induced cmyc expression was markedly reduced in FM versus MZ B cells. Analysis of c-myc transgenic mice showed that enforced c-myc expression restored the defective proliferative response in FM B cells. In contrast, no significant difference in LPS-driven cycling was observed in MZ B cells from c-myc transgenic compared with wildtype mice. Interestingly, overexpression of c-myc in FM B cells also resulted in an increase in IgM production in vitro, thereby suggesting an increased capacity to participate in T cell-independent immune responses similar to the functional responses of MZ B cells.
5.3. B-1 B cells
B-1 B cells belong to a developmental lineage distinct from B-2 B cells (12) and are found in multiple tissues, including the peritoneal and pleural cavities in mice. Together with MZ B cells they play a crucial role during early immune responses to a range of pathogens participating most prominently in T cell-independent immune responses. This subset is also the primary producer of natural IgM. Accordingly, B-1 B cells are considered to be part of the innate immune system (10, 88). Based on their expression of CD5 B-1 B cells can be further subdivided into CD5+ B-1a and CD5− B-1b B cells. B-1a and B-1b B cells have been shown to possess complimentary but also distinct functions (89). While several human B cell subpopulations have been suggested to represent human B-1 B cell equivalents (90, 91) the most compelling data were very recently presented by the group of Thomas Rothstein identifying human B-1 cells as a small population of CD20+CD27+CD43+CD70− B cells present in both umbilical cord and adult peripheral blood (11).
In vitro, B-1 B cells proliferate and produce antibody in response to TLR engagement. Genestier et al investigated the response of B-1 B cells after stimulation with different TLR ligands including Pam3CSK (TLR1/TLR2), MALP2 (TLR2/TLR6), polyIC (TLR3), LPS (TLR4), flagellin (TLR5) and R848 (TLR7/TLR8) (38). All TLR agonists except flagellin and polyIC induced B-1 B cell proliferation. Proliferation of B-1, MZ and FM B cells was similar in response to R848, Pam3CSK and MALP2. Proliferation of B-1 B cells in response to LPS was stronger than in FM B cells, but less robust in response to CpG. Importantly, CpG, LPS, R848 and Pam3CSK each induced differentiation of B-1 and MZ, but not FM, B cells into immunoglobulin secreting cells producing IgM, IgG and IgA. Interestingly, B-1 B cells were the only subpopulation that produced larger amounts of IgA. A comparison of B-1a versus B-1b B cells revealed that while both subsets produced similar amounts of IgM to TLR engagement, IgG and IgA was mostly secreted by B-1b B cells. Similar results were reported in a different study, showing good proliferative responses and the induction of immunoglobulin to a variety of TLR agonists in B-1 B cells (52). Again, IgA was most abundantly secreted by B-1 B cells when compared with FM and MZ B cells. TLR-induced proliferation and antibody production upon CpG and LPS stimulation of B-1 B cells required p110delta activity and activated the Akt pathway (92). In addition, CpG stimulation of B-1 B cells induced up-regulation of Blimp-1 and XBP-1 (38).
The phenotype of human B-1 B cells was determined by testing sort-purified B cell fractions for fundamental B-1 B cell functions based on mouse studies. CD20+CD27+CD43+ B cells both from umbilical cord and adult peripheral blood spontaneously secreted IgM and efficiently stimulated T cells. In addition, they exhibited tonic intracellular signalling as determined by phosphor-flow analysis of phosphorylated PLC-gamma2 and Syk.
In vivo, murine B-1 B cell migration is regulated by TLR signals similar to MZ B cells (93). Injection of live bacteria into the peritoneal cavity resulted in egress of B-1 B cells, and this response required TLR4 expression. Upon LPS stimulation both in vitro and in vivo, B-1 B cells down-modulated surface integrins including alpha1, alpha6 and beta1 and CD9, a surface receptor important for adhesion to local matrix- changes that permitted more rapid movement of cells in response to chemokines. Other in vivo studies using a range of infectious models including S. pneumoniae (94), Borrelia hermsii (95), Cryptococcus neoformans (96), influenza virus (97) and Toxoplasmagondii (98) have shown the requirement of B-1 B cells during the induced immune response. Many of these pathogens probably involve TLR signals for activation of B-1 B cells although a direct proof is often missing. Another contribution within this issue provides a detailed description of the role of B-1 B cells and TLR signaling during infection.
5.4. Antigen-experienced cells in mice
Only limited data exist directly investigating TLR responses in antigen experienced murine B cells. This relative lack of data is probably due to difficulties in identifying and handling these B cell subpopulations. While GC B cells can be readily identified by peanut agglutinin (PNA), GL7 and/or FAS expression, GC B cells survive poorly in vitro, thereby limiting detailed analyses. In contrast to human studies, no single surface marker has been defined that reliably identifies murine memory B cells and permits rapid purification of these cells. Instead, multiple surface markers are needed for their identification (99). Alternatively, memory B cells have been identified by antigen-specificity in association with long-term BrdU labeling. Thus, this limitation has hampered analysis of TLR responses in memory B cells.
After isolation of GC B cells based on B220+PNA+ expression from mice immunized with the T cell-dependent antigen sheep red blood cells, we analyzed their proliferative response to TLR ligation in vitro (1). Because GC B cells die rapidly ex vivo, we used short-term stimulation (20 hrs). GC B cells proliferated similarly to MZ B cells in response to LPS whereas FM B cells did not proliferate at this time point. Co-stimulation of GC B cells with an antibody to CD40 and LPS led to a further increase in proliferation that was less robust in MZ B cells. GC B cells also proliferated in response to TLR2 and, less robustly, to TLR3 ligands, Pam3 and polyIC, respectively. In vivo, impaired B cell activation and GC formation were seen in MyD88−/− mice that were immunized with OVALPS in alum (3). Interestingly, GC B cells (in this study identified as GL7+ B cells) from immunized MyD88−/− mice exhibited lower expression of Blimp-1, whereas expression of BCL-6 was higher when compared with GC B cells from immunized wild-type mice. Another study reported the influence of B cell intrinsic TLR signaling on the formation of germinal centers by analyzing bone marrow chimeric mice in which the entire B cell compartment lacked the capacity to express the MyD88 gene (2). Both the number and size of germinal centers were decreased in mixed bone marrow chimeras receiving MyD88−/− BM cells after immunization with DNP-OVA. Furthermore, activation via TLR4 resulted in recruitment of B cells into ongoing GC reactions as demonstrated in vivo (100).
In a study by Wenxia Song’s group, memory B cells were isolated from mice immunized with NP-keyhole limpet hemocyanin by antigen specificity, based on B220+NP+CD138− expression and further separated into IgM+/IgD+ unswitched and IgM−IgD− NP-specific memory B cells (101). Stimulation with CpG or LPS resulted in NP-specific high-affinity IgG production from NP-specific memory B cells and NP-specific low affinity IgM secretion from unswitched memory B cells. These observations suggest that TLR agonists can directly activate murine memory B cells in vitro. However, neither CpG nor LPS alone were sufficient to activate memory B cells in vivo in this study. In contrast, we found that LPS promotes a B cell–intrinsic MyD88-dependent increase in specific antibody titers (1): B cell deficient muMT mice were adoptively transferred with wild-type or MyD88−/− B cells, immunized with NP-CGG in alum and subsequently challenged with NP-CGG in PBS. Recipient animals were injected 5 month later with 5 μg LPS. NP-specific IgM and IgG titers increased significantly in recipients of adoptively transferred MyD88 wild-type B cells, but not in recipients of MyD88−/− B cells. Our study further demonstrated that while B cell-intrinsic MyD88 signals were not required for T-cell dependent immune responses such signals can clearly amplify these responses, including, in particular, early antigen-specific IgM production. Similar results were reported by Barr et al. analyzing the role of B cell intrinsic TLR signaling during T cell-dependent immunization with DNP-OVA (emulsified in IFA) with the addition of LPS in mixed bone marrow chimeras (53). As noted above, while most antigen-specific primary responses were independent of MyD88 signaling, class-switching to IgG2c required MyD88 signaling in B cells. However, when co-cultured with T cells, MyD88−/− B cells were able to switch to IgG2c demonstrating that MyD88 is not an absolute requirement for switching to IgG2c. Of note, lower levels of IgG2c have been reported in preimmune MyD88−/− and MyD88−/−TRIF−/− double deficient mice (3, 28). Thus, although not required, B cell intrinsic TLR signals directly impact GC formation and subsequent activation of memory B cells both in vitro and in vivo.
5.5. Human naïve vs. memory B cells
As in mice, the naïve human B cell compartment comprises both follicular/naive mature and MZ B cells (102). However, as MZ B cells are only found in the spleen few direct analyses exist investigating this subpopulation, and we did not find any study regarding functional responses to TLRs. Several years ago, it was suggested that IgM+ memory B cells, present in the peripheral blood, represent a peripheral, circulating human equivalent of splenic marginal zone B cells (103, 104). This view, however, remains highly controversial (105). In this review, we have therefore focused only on comparisons of CD27− naïve mature vs. CD27+ memory B cells and have not addressed this ongoing discussion. Memory B cells can be readily subdivided into IgM+IgD+ unswitched and IgG+ or IgA+ switched subsets. Recent publications also provide data about CD27− memory B cells, however, we will not further discuss this subset (106, 107).
While no major differences have been observed between switched vs. unswitched memory B cells, overall, human memory B cells respond to a greater extend to TLR engagement than naïve mature B cells (Table 1). Lanzavecchia’s group investigated the response of naïve, IgM+ and switched memory B cells to CpG stimulation (42, 56). While naïve B cells did not divide, both memory B cell subsets underwent several divisions, and this effect was slightly more pronounced in unswitched IgM+ memory B cells. Addition of IL-15, IL-2 or IL-10 resulted in significantly increased proliferation of memory B cell subsets, and also induced weak proliferation in naïve B cells. Interestingly, although naïve B cells did not proliferate in response to CpG, this subset up-regulated expression of CD69 and CD86. This indicates that human naïve B cells are sensitive towards TLR engagement but do not enter cell cycle, an effect similar to that observed in murine FM B cells (see above; (76)). Memory B cells also differentiated into IgM, IgA and IgG antibody-producing cells upon CpG stimulation, an effect that was increased with IL-15, IL-2 or IL-10 co-stimulation. In contrast, naïve B cells failed to produce immunoglobulin under these conditions. Only co-stimulation of naive B cells with both TLR and BCR ligands resulted in proliferation and immunoglobulin production. The inability of naïve B cells to produce immunoglobulins in response to CpG stimulation was recently confirmed by a different group (69). In response to the TLR7 agonists R848 or loxoribine, naïve B cells exhibited minimal proliferation. Proliferation, however, could be induced by the addition of type I interferon (INF) (108). In contrast, memory B cells proliferated in response to TLR7 alone and addition of INF-alpha further enhanced this response. Memory B cells, and to a lesser extend naïve B cells, also produced IL-6, IgM and IgG in response to R848. Addition of INF-alpha synergistically enhanced interleukin and immunoglobulin production.
Table 1.
Comparison of known functional responses in human naive versus IgM+ and switched memory, B cells
| TLR ligand | Response | B cell subpopulation | Reference | ||
|---|---|---|---|---|---|
| naive | IgM+ mem | sw mem | |||
| Pam3CSK4 | Cytokine production | + | ++ | 110 | |
| R848/Loxoribine | Proliferation | − | ++ | 108 | |
| Imiquimod | Cytokine production | + | ++ | 110 | |
| CpG | Proliferation | − | +++ | ++ | 42, 56 |
| Secretion of Ig's | − | + | + | 42, 56, 69 | |
| Cytokine + chemokine production | + | ++ | 110 | ||
| Up-regulation of CD69 and CD86 | + | + | + | 42, 56 | |
| Up-regulation of TACI | ++ | ++ | ++ | 60, 109 | |
| Up-regulation of BCMA | − | (+) | (+) | 60, 109 |
Functional data comparing human B cell subpopulations are still relatively rare. The table shows which functional responses to different TLR ligation in human B cell subsets have been investigated so far.
Abbreviations: IgM+ mem: IgM+ memory B cells; sw mem: switched memory B cells
In addition to proliferation and immunoglobulin secretion, naïve, IgM+ and switched memory B cells up-regulated TACI expression after TLR stimulation using CpG (60, 109). However, because basal TACI expression is much higher in memory versus naïve B cells, comparing the extent of up-regulation between these subsets is difficult. CpG also induced low level expression of BCMA on memory, but not naïve, B cells, while expression of BAFF-R was unaffected. Agrawal et al. investigated the production of cytokines and chemokines in naïve and memory B cells after Pam3CSK4 (TLR1/TLR2), Imiquimod (TLR7) and CpG (TLR9) stimulation (110). All three TLR ligands stimulated both naïve and memory B cells to produce IL-1alpha, IL-1beta, IL-6, TNF-alpha, IL-13 and IL-10, but memory B cells secreted significantly greater amounts of cytokines in most cases. Similarly, chemokines (MCP-1, MIP-1alpha and MIP-1beta) were produced from both subsets in response to these stimuli. However, TLR1/TLR2 engagement resulted in significantly greater amounts of MIP1alpha and MIP1beta production by memory compared with naïve B cells.
It remains unclear whether human plasma cells can respond to TLR stimulation. Theoretically, this terminally differentiated population might be expected not to increase the rate of antibody production. However, one report exists describing that TLR triggering of plasma cells isolated from tonsils augments immunoglobulin production (57). No investigation regarding human germinal center B cells and their TLR responsiveness could be found. The overall greater responsiveness of memory B cells towards TLR signaling might have an important role in activating these cells during memory immune responses or to maintain memory as suggested by Lanzavecchia (42); and is consistent with data from murine cell transfer studies using control vs. MyD88−/− B cells (1).
5.6. B cells with a regulatory function
B cells with a regulatory function have been characterized in both mouse and human (111–113). They are characterized by secretion of IL-10 and based on their function also termed regulatory B cells (Breg) (14, 114) or B10 B cells (115). Several studies have shown that the absence of B cells can result in exacerbation of inflammatory responses. Fillatreau et al. provided the first evidence that B cells regulate autoimmunity in a murine model of experimental autoimmune encephalomyelitis (EAE) by provision of IL-10 (113). Subsequent studies demonstrated that CD19+CD21hiCD23hiCD1dhi MZ-precursor B cells can prevent induction of collagen induced arthritis (112). Tedder and colleagues identified a regulatory B cell subset with a CD1dhiCD5+ phenotype controlling T cell-dependent inflammatory responses (116) and also described putative progenitors for this population (115). Recently, B cells with a regulatory function have also been identified in humans by two independent groups (111, 117). Overall, this population is defined predominantly by the capacity to generate high levels of IL-10. As IL-10 production can be triggered via a variety of stimuli- it remains unlikely that such cells comprise a distinct B cell developmental subset.
Although not required for their development (116), TLR stimulation with CpG or LPS has been shown to promote differentiation and expansion of B cells with a regulatory function in vitro (115, 118, 119). The suppressor function of B cells required MyD88 expression as demonstrated in an in vivo model of EAE (120). Moreover, stimulation with LPS induced IL-10 expression in so-called B10 cells and this was dependent on the MyD88 pathway (115). Thus, B cells with a regulatory function can differentiate and exert their suppressor function mainly by IL-10 production upon TLR signaling. Because a whole article about this subsets and their TLR responsiveness is provided later in this issue by Simon Fillatreau, we will not further go into detail at this point.
6. CONCLUSION AND PERSPECTIVES
Our understanding of the effect of TLR signaling on distinct B cell subpopulations has strikingly increased over the last few years. In particular, our knowledge regarding murine B cell subsets and their TLR responsiveness has grown dramatically. Similar data using human B cell subsets remain less well investigated and understood. In mice, it has been convincingly demonstrated that in contrast to the BCR, signals via TLRs are not required for B cell development. While data from patients with primary immunodeficiency caused by defects in the TLR signaling pathways so far do not indicate any significant deficits in B cell maturation more detailed analyses are warranted. Although not required for development, B cell differentiation can be directed by TLR signals as has been shown for the generation of antibody-secreting plasma cells, both in vitro and in vivo. However, the hypothesis that cell intrinsic TLR signals are required for survival of B cells, in particular of memory B cells, as first suggested by Lanzavecchia’s group is still under debate and not substantiated via murine studies to date.
TLR engagement induces many different functional responses in B cells including immunoglobulin and cytokine secretion, antigen presentation, proliferation and modulation of several surface molecules, and these functional responses clearly vary with the developmental B cell stage. Immature or transitional B cells, both from mouse and human, have been demonstrated to respond to TLR ligation. In mice, MZ B cells together with B-1 B cells are generally more prone to TLR signals compared with FM B cells. In addition to immunoglobulin and cytokine secretion, TLR engagement leads to modulation of cell surface integrin expression and clearly impacts B cell migration leading to movement of cells from their usual topographic localization into the periphery or various effector tissue sites. Comparing human B cell subpopulations, memory B cells show overall stronger TLR responsiveness than naïve B cells. The mechanisms accounting for these differences in TLR responsiveness, however, have been only minimally addressed to date and are unlikely to be only explained by differences in TLR expression in B cell subpopulations.
Detailed insight into the impact of TLR signals on distinct B cell subsets is crucial for a better understanding of the role of TLR signaling in different disease entities including primary immunodeficiency, autoimmunity and response to infection. In patients with primary immunodeficiency caused by defects in the TLR signaling pathway or with defects in B cell development including, for example, patients with common variable immunodeficiency, it might be possible to better understand or predict their risk for specific infections. Similarly, our understanding of the immune responses in newborns or in patients early after stem cell transplantation with a more immature immune system will be improved. Finally, the onset of autoimmune disease or disease flares have been proposed to be triggered by TLR ligation. Because of their differences in TLR responsiveness, distinct B cell subpopulations might be of particular importance in this process.
Currently, most of our data regarding B cells and TLR responses have originated from detailed murine studies. In the future, it will be important to extend the knowledge about human B cell subpopulations and TLR signaling using a range of experimental modalities and models in order to gain insight into the above questions and to identify relevant translational modalities.
ACKNOWLEDGMENTS
This work was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft) grant ME2709/2-1 and by the European Union Marie-Curie International Re-integration Grant IRG 256372 (to AMB) and NIH grants HD037091 and AI071163 (to DJR). We apologize to any colleagues whose work we have overlooked, or whose work we were not able to cite due to space limitations.
Abbreviations
- AMP
pathogen-associated molecular patterns
- PRR
recognition receptors
- BCR
B cell antigen receptor
- FM
follicular mature
- MZ
marginal zone
- HSA
human serum albumin
- NP
4-hydroxy-3-nitrophenylacetyl
- CGG
chicken gamma globulin
- GC
germinal center
- AID
activation-induced cytidine deaminase
- DNP-OVA
Dinitrophenylated-ovalbumin
- IFA
incomplete Freund’s adjuvant
- APC
antigen-presenting cells
- BAFF
B cell activating factor of the TNF family
- S1P1
sphingosine-1-phosphate receptor 1
- PNA
peanut agglutinine
- INF
interferon
REFERENCES
- 1.Meyer-Bahlburg A, Khim S, Rawlings DJ. B cell intrinsic TLR signals amplify but are not required for humoral immunity. J Exp Med. 2007;204:3095–3101. doi: 10.1084/jem.20071250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barr TA, Brown S, Mastroeni P, Gray D. B cell intrinsic MyD88 signals drive IFN-gamma production from T cells and control switching to IgG2c. J Immunol. 2009;183:1005–1012. doi: 10.4049/jimmunol.0803706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 4.Poovassery JS, Vanden Bush TJ, Bishop GA. Antigen receptor signals rescue B cells from TLR tolerance. J Immunol. 2009;183:2974–2983. doi: 10.4049/jimmunol.0900495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bekeredjian-Ding I, Jego G. Toll-like receptors-- sentries in the B-cell response. Immunology. 2009;128:311–323. doi: 10.1111/j.1365-2567.2009.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol. 2008;20:332–338. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cinamon G, Matloubian M, Lesneski MJ, Xu Y, Low C, Lu T, Proia RL, Cyster JG. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat Immunol. 2004;5:713–720. doi: 10.1038/ni1083. [DOI] [PubMed] [Google Scholar]
- 8.Lu TT, Cyster JG. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science. 2002;297:409–412. doi: 10.1126/science.1071632. [DOI] [PubMed] [Google Scholar]
- 9.Rubtsov AV, Swanson CL, Troy S, Strauch P, Pelanda R, Torres RM. TLR agonists promote marginal zone B cell activation and facilitate T-dependent IgM responses. J Immunol. 2008;180:3882–3888. doi: 10.4049/jimmunol.180.6.3882. [DOI] [PubMed] [Google Scholar]
- 10.Bendelac A, Bonneville M, Kearney JF. Autoreactivity by design: innate B and T lymphocytes. Nat Rev Immunol. 2001;1:177–186. doi: 10.1038/35105052. [DOI] [PubMed] [Google Scholar]
- 11.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 13.Dorshkind K, Montecino-Rodriguez E. Fetal Bcell lymphopoiesis and the emergence of B-1-cell potential. Nat Rev Immunol. 2007;7:213–219. doi: 10.1038/nri2019. [DOI] [PubMed] [Google Scholar]
- 14.Mauri C, Ehrenstein MR. The 'short' history of regulatory B cells. Trends Immunol. 2008;29:34–40. doi: 10.1016/j.it.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Matsushita T, Tedder TF. Identifying regulatory B cells (B10 cells) that produce IL-10 in mice. Methods Mol Biol. 2011;677:99–111. doi: 10.1007/978-1-60761-869-0_7. [DOI] [PubMed] [Google Scholar]
- 16.Klein LC, Yeung KP, Berman JN. Cladribine inhibits a diltiazem-induced increase in red blood cell purine nucleotide concentrations in a zebrafish model. Biomarkers. 2009;14:554–559. doi: 10.3109/13547500903131698. [DOI] [PubMed] [Google Scholar]
- 17.Torres RM, Flaswinkel H, Reth M, Rajewsky K. Aberrant B cell development and immune response in mice with a compromised BCR complex. Science. 1996;272:1804–1808. doi: 10.1126/science.272.5269.1804. [DOI] [PubMed] [Google Scholar]
- 18.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 19.Loder F, Mutschler B, Ray RJ, Paige CJ, Sideras P, Torres R, Lamers MC, Carsetti R. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med. 1999;190:75–89. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 21.Rawlings DJ, Saffran DC, Tsukada S, Largaespada DA, Grimaldi JC, Cohen L, Mohr RN, Bazan JF, Howard M, Copeland NG. Mutation of unique region of Bruton's tyrosine kinase in immunodeficient XID mice. Science. 1993;261:358–361. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- 22.Ochs HD, Smith CI. X-linked agammaglobulinemia. A clinical and molecular analysis. Medicine (Baltimore) 1996;75:287–299. doi: 10.1097/00005792-199611000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Rawlings DJ. Bruton's tyrosine kinase controls a sustained calcium signal essential for B lineage development and function. Clin Immunol. 1999;91:243–253. doi: 10.1006/clim.1999.4732. [DOI] [PubMed] [Google Scholar]
- 24.Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 25.Cariappa A, Tang M, Parng C, Nebelitskiy E, Carroll M, Georgopoulos K, Pillai S. The follicular versus marginal zone B lymphocyte cell fate decision is regulated by Aiolos, Btk, CD21. Immunity. 2001;14:603–615. doi: 10.1016/s1074-7613(01)00135-2. [DOI] [PubMed] [Google Scholar]
- 26.Martin F, Kearney JF. Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk. Immunity. 2000;12:39–49. doi: 10.1016/s1074-7613(00)80157-0. [DOI] [PubMed] [Google Scholar]
- 27.Niiro H, Clark EA. Regulation of B-cell fate by antigen-receptor signals. Nat Rev Immunol. 2002;2:945–956. doi: 10.1038/nri955. [DOI] [PubMed] [Google Scholar]
- 28.Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei B, Su TT, Dalwadi H, Stephan RP, Fujiwara D, Huang TT, Brewer S, Chen L, Arditi M, Borneman J, Rawlings DJ, Braun J. Resident enteric microbiota and CD8+ T cells shape the abundance of marginal zone B cells. Eur J Immunol. 2008;38:3411–3425. doi: 10.1002/eji.200838432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumararatne DS, MacLennan IC, Bazin H, Gray D. Marginal zones: the largest B cell compartment of the rat spleen. Adv Exp Med Biol. 1982;149:67–73. doi: 10.1007/978-1-4684-9066-4_10. [DOI] [PubMed] [Google Scholar]
- 31.Picard C, von Bernuth H, Ghandil P, Chrabieh M, Levy O, Arkwright PD, McDonald D, Geha RS, Takada H, Krause JC, Creech CB, Ku CL, Ehl S, Marodi L, Al-Muhsen S, Al-Hajjar S, Al-Ghonaium A, Day-Good NK, Holland SM, Gallin JI, Chapel H, Speert DP, Rodriguez-Gallego C, Colino E, Garty BZ, Roifman C, Hara T, Yoshikawa H, Nonoyama S, Domachowske J, Issekutz AC, Tang M, Smart J, Zitnik SE, Hoarau C, Kumararatne DS, Thrasher AJ, Davies EG, Bethune C, Sirvent N, de Ricaud D, Camcioglu Y, Vasconcelos J, Guedes M, Vitor AB, Rodrigo C, Almazan F, Mendez M, Arostegui JI, Alsina L, Fortuny C, Reichenbach J, Verbsky JW, Bossuyt X, Doffinger R, Abel L, Puel A, Casanova JL. Clinical features and outcome of patients with IRAK-4 and MyD88 deficiency. Medicine (Baltimore) 2010;89:403–425. doi: 10.1097/MD.0b013e3181fd8ec3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang SY, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, Picard C, Chapgier A, Plancoulaine S, Titeux M, Cognet C, von Bernuth H, Ku CL, Casrouge A, Zhang XX, Barreiro L, Leonard J, Hamilton C, Lebon P, Heron B, Vallee L, Quintana-Murci L, Hovnanian A, Rozenberg F, Vivier E, Geissmann F, Tardieu M, Abel L, Casanova JL. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- 33.Casrouge A, Zhang SY, Eidenschenk C, Jouanguy E, Puel A, Yang K, Alcais A, Picard C, Mahfoufi N, Nicolas N, Lorenzo L, Plancoulaine S, Senechal B, Geissmann F, Tabeta K, Hoebe K, Du X, Miller RL, Heron B, Mignot C, de Villemeur TB, Lebon P, Dulac O, Rozenberg F, Beutler B, Tardieu M, Abel L, Casanova JL. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science. 2006;314:308–312. doi: 10.1126/science.1128346. [DOI] [PubMed] [Google Scholar]
- 34.Nemazee D, Gavin A, Hoebe K, Beutler B. Immunology: Toll-like receptors and antibody responses. Nature. 2006;441:E4. doi: 10.1038/nature04875. discussion E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Capolunghi F, Cascioli S, Giorda E, Rosado MM, Plebani A, Auriti C, Seganti G, Zuntini R, Ferrari S, Cagliuso M, Quinti I, Carsetti R. CpG drives human transitional B cells to terminal differentiation and production of natural antibodies. J Immunol. 2008;180:800–808. doi: 10.4049/jimmunol.180.2.800. [DOI] [PubMed] [Google Scholar]
- 36.Ueda Y, Liao D, Yang K, Patel A, Kelsoe G. Tindependent activation-induced cytidine deaminase expression, class-switch recombination, and antibody production by immature/transitional 1 B cells. J Immunol. 2007;178:3593–3601. doi: 10.4049/jimmunol.178.6.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aranburu A, Ceccarelli S, Giorda E, Lasorella R, Ballatore G, Carsetti R. TLR ligation triggers somatic hypermutation in transitional B cells inducing the generation of IgM memory B cells. J Immunol. 2010;185:7293–7301. doi: 10.4049/jimmunol.1002722. [DOI] [PubMed] [Google Scholar]
- 38.Genestier L, Taillardet M, Mondiere P, Gheit H, Bella C, Defrance T. TLR agonists selectively promote terminal plasma cell differentiation of B cell subsets specialized in thymus-independent responses. J Immunol. 2007;178:7779–7786. doi: 10.4049/jimmunol.178.12.7779. [DOI] [PubMed] [Google Scholar]
- 39.Rui L, Healy JI, Blasioli J, Goodnow CC. ERK signaling is a molecular switch integrating opposing inputs from B cell receptor and T cell cytokines to control TLR4- driven plasma cell differentiation. J Immunol. 2006;177:5337–5346. doi: 10.4049/jimmunol.177.8.5337. [DOI] [PubMed] [Google Scholar]
- 40.Ozcan E, Garibyan L, Lee JJ, Bram RJ, Lam KP, Geha RS. Transmembrane activator, calcium modulator, and cyclophilin ligand interactor drives plasma cell differentiation in LPS-activated B cells. J Allergy Clin Immunol. 2009;123:1277–1286. doi: 10.1016/j.jaci.2009.03.019. e1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huggins J, Pellegrin T, Felgar RE, Wei C, Brown M, Zheng B, Milner EC, Bernstein SH, Sanz I, Zand MS. CpG DNA activation and plasma-cell differentiation of CD27- naive human B cells. Blood. 2007;109:1611–1619. doi: 10.1182/blood-2006-03-008441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 43.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 44.Kawai T, Akira S. The role of patternrecognition receptors in innate immunity: update on Tolllike receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 45.Divanovic S, Trompette A, Petiniot LK, Allen JL, Flick LM, Belkaid Y, Madan R, Haky JJ, Karp CL. Regulation of TLR4 signaling and the host interface with pathogens and danger: the role of RP105. J Leukoc Biol. 2007;82:265–271. doi: 10.1189/jlb.0107021. [DOI] [PubMed] [Google Scholar]
- 46.Ogata H, Su I, Miyake K, Nagai Y, Akashi S, Mecklenbrauker I, Rajewsky K, Kimoto M, Tarakhovsky A. The toll-like receptor protein RP105 regulates lipopolysaccharide signaling in B cells. J Exp Med. 2000;192:23–29. doi: 10.1084/jem.192.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miyake K, Yamashita Y, Hitoshi Y, Takatsu K, Kimoto M. Murine B cell proliferation and protection from apoptosis with an antibody against a 105-kD molecule: unresponsiveness of X-linked immunodeficient B cells. J Exp Med. 1994;180:1217–1224. doi: 10.1084/jem.180.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miura Y, Miyake K, Yamashita Y, Shimazu R, Copeland NG, Gilbert DJ, Jenkins NA, Inazawa J, Abe T, Kimoto M. Molecular cloning of a human RP105 homologue and chromosomal localization of the mouse and human RP105 genes (Ly64 and LY64) Genomics. 1996;38:299–304. doi: 10.1006/geno.1996.0632. [DOI] [PubMed] [Google Scholar]
- 49.Koarada S, Tada Y, Ushiyama O, Morito F, Suzuki N, Ohta A, Miyake K, Kimoto M, Nagasawa K. B cells lacking RP105, a novel B cell antigen, in systemic lupus erythematosus. Arthritis Rheum. 1999;42:2593–2600. doi: 10.1002/1529-0131(199912)42:12<2593::AID-ANR12>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 50.Akashi-Takamura S, Miyake K. TLR accessory molecules. Curr Opin Immunol. 2008;20:420–425. doi: 10.1016/j.coi.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 51.Divanovic S, Trompette A, Atabani SF, Madan R, Golenbock DT, Visintin A, Finberg RW, Tarakhovsky A, Vogel SN, Belkaid Y, Kurt-Jones EA, Karp CL. Inhibition of TLR-4/MD-2 signaling by RP105/MD-1. J Endotoxin Res. 2005;11:363–368. doi: 10.1179/096805105X67300. [DOI] [PubMed] [Google Scholar]
- 52.Gururajan M, Jacob J, Pulendran B. Toll-like receptor expression and responsiveness of distinct murine splenic and mucosal B-cell subsets. PLoS One. 2007;2:e863. doi: 10.1371/journal.pone.0000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barr TA, Brown S, Ryan G, Zhao J, Gray D. TLR-mediated stimulation of APC: Distinct cytokine responses of B cells and dendritic cells. Eur J Immunol. 2007;37:3040–3053. doi: 10.1002/eji.200636483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Treml LS, Carlesso G, Hoek KL, Stadanlick JE, Kambayashi T, Bram RJ, Cancro MP, Khan WN. TLR stimulation modifies BLyS receptor expression in follicular and marginal zone B cells. J Immunol. 2007;178:7531–7539. doi: 10.4049/jimmunol.178.12.7531. [DOI] [PubMed] [Google Scholar]
- 55.Nagai Y, Kobayashi T, Motoi Y, Ishiguro K, Akashi S, Saitoh S, Kusumoto Y, Kaisho T, Akira S, Matsumoto M, Takatsu K, Miyake K. The radioprotective 105/MD-1 complex links TLR2 and TLR4/MD-2 in antibody response to microbial membranes. J Immunol. 2005;174:7043–7049. doi: 10.4049/jimmunol.174.11.7043. [DOI] [PubMed] [Google Scholar]
- 56.Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: upregulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–4504. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- 57.Dorner M, Brandt S, Tinguely M, Zucol F, Bourquin JP, Zauner L, Berger C, Bernasconi M, Speck RF, Nadal D. Plasma cell toll-like receptor (TLR) expression differs from that of B cells, and plasma cell TLR triggering enhances immunoglobulin production. Immunology. 2009;128:573–579. doi: 10.1111/j.1365-2567.2009.03143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mansson A, Adner M, Hockerfelt U, Cardell LO. A distinct Toll-like receptor repertoire in human tonsillar B cells, directly activated by PamCSK, R-837 and CpG-2006 stimulation. Immunology. 2006;118:539–548. doi: 10.1111/j.1365-2567.2006.02392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bourke E, Bosisio D, Golay J, Polentarutti N, Mantovani A. The toll-like receptor repertoire of human B lymphocytes: inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood. 2003;102:956–963. doi: 10.1182/blood-2002-11-3355. [DOI] [PubMed] [Google Scholar]
- 60.Good KL, Avery DT, Tangye SG. Resting human memory B cells are intrinsically programmed for enhanced survival and responsiveness to diverse stimuli compared to naive B cells. J Immunol. 2009;182:890–901. doi: 10.4049/jimmunol.182.2.890. [DOI] [PubMed] [Google Scholar]
- 61.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 62.Brines RD, Klaus GG. Inhibition of lipopolysaccharide-induced activation of immature B cells by anti-mu and anti-delta antibodies and its modulation by interleukin-4. Int Immunol. 1992;4:765–771. doi: 10.1093/intimm/4.7.765. [DOI] [PubMed] [Google Scholar]
- 63.Wechsler-Reya RJ, Monroe JG. Lipopolysaccharide prevents apoptosis and induces responsiveness to antigen receptor cross-linking in immature B cells. Immunology. 1996;89:356–362. doi: 10.1046/j.1365-2567.1996.d01-749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hayashi EA, Akira S, Nobrega A. Role of TLR in B cell development: signaling through TLR4 promotes B cell maturation and is inhibited by TLR2. J Immunol. 2005;174:6639–6647. doi: 10.4049/jimmunol.174.11.6639. [DOI] [PubMed] [Google Scholar]
- 65.Hayashi EA, Granato A, Paiva LS, Bertho AL, Bellio M, Nobrega A. TLR4 promotes B cell maturation: independence and cooperation with B lymphocyte-activating factor. J Immunol. 2010;184:4662–4672. doi: 10.4049/jimmunol.0903253. [DOI] [PubMed] [Google Scholar]
- 66.Meyer-Bahlburg A, Andrews SF, Yu KO, Porcelli SA, Rawlings DJ. Characterization of a late transitional B cell population highly sensitive to BAFFmediated homeostatic proliferation. J Exp Med. 2008;205:155–168. doi: 10.1084/jem.20071088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Andrews SF, Rawlings DJ. Transitional B cells exhibit a B cell receptor-specific nuclear defect in gene transcription. J Immunol. 2009;182:2868–2878. doi: 10.4049/jimmunol.0802368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Azulay-Debby H, Edry E, Melamed D. CpG DNA stimulates autoreactive immature B cells in the bone marrow. Eur J Immunol. 2007;37:1463–1475. doi: 10.1002/eji.200636878. [DOI] [PubMed] [Google Scholar]
- 69.Suryani S, Fulcher DA, Santner-Nanan B, Nanan R, Wong M, Shaw PJ, Gibson J, Williams A, Tangye SG. Differential expression of CD21 identifies developmentally and functionally distinct subsets of human transitional B cells. Blood. 2010;115:519–529. doi: 10.1182/blood-2009-07-234799. [DOI] [PubMed] [Google Scholar]
- 70.Plebani A, Lougaris V, Soresina A, Meini A, Zunino F, Losi CG, Gatta R, Cattaneo G, Nespoli L, Marinoni M, Capolunghi F, Vivarelli M, Quinti I, Carsetti R. A novel immunodeficiency characterized by the exclusive presence of transitional B cells unresponsive to CpG. Immunology. 2007;121:183–188. doi: 10.1111/j.1365-2567.2006.02556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lopes-Carvalho T, Kearney JF. Development and selection of marginal zone B cells. Immunol Rev. 2004;197:192–205. doi: 10.1111/j.0105-2896.2004.0112.x. [DOI] [PubMed] [Google Scholar]
- 72.Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat Rev Immunol. 2009;9:767–777. doi: 10.1038/nri2656. [DOI] [PubMed] [Google Scholar]
- 73.Martin F, Kearney JF. B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a "natural immune memory". Immunol Rev. 2000;175:70–79. [PubMed] [Google Scholar]
- 74.Allman D, Pillai S. Peripheral B cell subsets. Curr Opin Immunol. 2008;20:149–157. doi: 10.1016/j.coi.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oliver AM, Martin F, Gartland GL, Carter RH, Kearney JF. Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur J Immunol. 1997;27:2366–2374. doi: 10.1002/eji.1830270935. [DOI] [PubMed] [Google Scholar]
- 76.Meyer-Bahlburg A, Bandaranayake AD, Andrews SF, Rawlings DJ. Reduced c-myc expression levels limit follicular mature B cell cycling in response to TLR signals. J Immunol. 2009;182:4065–4075. doi: 10.4049/jimmunol.0802961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oliver AM, Martin F, Kearney JF. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J Immunol. 1999;162:7198–7207. [PubMed] [Google Scholar]
- 78.Lenert P, Brummel R, Field EH, Ashman RF. TLR-9 activation of marginal zone B cells in lupus mice regulates immunity through increased IL-10 production. J Clin Immunol. 2005;25:29–40. doi: 10.1007/s10875-005-0355-6. [DOI] [PubMed] [Google Scholar]
- 79.Srivastava B, Quinn WJ, 3rd, Hazard K, Erikson J, Allman D. Characterization of marginal zone B cell precursors. J Exp Med. 2005;202:1225–1234. doi: 10.1084/jem.20051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blair PA, Chavez-Rueda KA, Evans JG, Shlomchik MJ, Eddaoudi A, Isenberg DA, Ehrenstein MR, Mauri C. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J Immunol. 2009;182:3492–3502. doi: 10.4049/jimmunol.0803052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mackay F, Figgett WA, Saulep D, Lepage M, Hibbs ML. B-cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunol Rev. 2010;237:205–225. doi: 10.1111/j.1600-065X.2010.00944.x. [DOI] [PubMed] [Google Scholar]
- 82.Woodland RT, Schmidt MR, Thompson CB. BLyS and B cell homeostasis. Semin Immunol. 2006;18:318–326. doi: 10.1016/j.smim.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 83.Katsenelson N, Kanswal S, Puig M, Mostowski H, Verthelyi D, Akkoyunlu M. Synthetic CpG oligodeoxynucleotides augment BAFF- and APRILmediated immunoglobulin secretion. Eur J Immunol. 2007;37:1785–1795. doi: 10.1002/eji.200636800. [DOI] [PubMed] [Google Scholar]
- 84.Groeneveld PH, Erich T, Kraal G. In vivo effects of LPS on B lymphocyte subpopulations. Migration of marginal zone-lymphocytes and IgD-blast formation in the mouse spleen. Immunobiology. 1985;170:402–411. doi: 10.1016/S0171-2985(85)80064-4. [DOI] [PubMed] [Google Scholar]
- 85.Kraal G. Cells in the marginal zone of the spleen. Int Rev Cytol. 1992;132:31–74. doi: 10.1016/s0074-7696(08)62453-5. [DOI] [PubMed] [Google Scholar]
- 86.Martin F, Kearney JF. Marginal-zone B cells. Nat Rev Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 87.Donahue AC, Fruman DA. Distinct signaling mechanisms activate the target of rapamycin in response to different B-cell stimuli. Eur J Immunol. 2007;37:2923–2936. doi: 10.1002/eji.200737281. [DOI] [PubMed] [Google Scholar]
- 88.Montecino-Rodriguez E, Dorshkind K. New perspectives in B-1 B cell development and function. Trends Immunol. 2006;27:428–433. doi: 10.1016/j.it.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 89.Alugupalli KR, Gerstein RM. Divide and conquer: division of labor by B-1 B cells. Immunity. 2005;23:1–2. doi: 10.1016/j.immuni.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 90.Youinou P, Renaudineau Y. The paradox of CD5-expressing B cells in systemic lupus erythematosus. Autoimmun Rev. 2007;7:149–154. doi: 10.1016/j.autrev.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 91.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Durand CA, Hartvigsen K, Fogelstrand L, Kim S, Iritani S, Vanhaesebroeck B, Witztum JL, Puri KD, Gold MR. Phosphoinositide 3-kinase p110 delta regulates natural antibody production, marginal zone and B-1 B cell function, and autoantibody responses. J Immunol. 2009;183:5673–5684. doi: 10.4049/jimmunol.0900432. [DOI] [PubMed] [Google Scholar]
- 93.Ha SA, Tsuji M, Suzuki K, Meek B, Yasuda N, Kaisho T, Fagarasan S. Regulation of B1 cell migration by signals through Toll-like receptors. J Exp Med. 2006;203:2541–2550. doi: 10.1084/jem.20061041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 95.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 96.Subramaniam KS, Datta K, Marks MS, Pirofski LA. Improved survival of mice deficient in secretory immunoglobulin M following systemic infection with Cryptococcus neoformans. Infect Immun. 2010;78:441–452. doi: 10.1128/IAI.00506-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Choi YS, Baumgarth N. Dual role for B-1a cells in immunity to influenza virus infection. J Exp Med. 2008;205:3053–3064. doi: 10.1084/jem.20080979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen M, Mun HS, Piao LX, Aosai F, Norose K, Mohamed RM, Belal US, Fang H, Ahmed AK, Kang HK, Matsuzaki G, Kitamura D, Yano A. Induction of protective immunity by primed B-1 cells in Toxoplasma gondii -infected B cell-deficient mice. Microbiol Immunol. 2003;47:997–1003. doi: 10.1111/j.1348-0421.2003.tb03460.x. [DOI] [PubMed] [Google Scholar]
- 99.Anderson SM, Tomayko MM, Ahuja A, Haberman AM, Shlomchik MJ. New markers for murine memory B cells that define mutated and unmutated subsets. J Exp Med. 2007;204:2103–2114. doi: 10.1084/jem.20062571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hwang IY, Park C, Harrison K, Kehrl JH. TLR4 signaling augments B lymphocyte migration and overcomes the restriction that limits access to germinal center dark zones. J Exp Med. 2009;206:2641–2657. doi: 10.1084/jem.20091982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Richard K, Pierce SK, Song W. The agonists of TLR4 and 9 are sufficient to activate memory B cells to differentiate into plasma cells in vitro but not in vivo. J Immunol. 2008;181:1746–1752. doi: 10.4049/jimmunol.181.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu Rev Immunol. 2009;27:267–285. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- 103.Kruetzmann S, Rosado MM, Weber H, Germing U, Tournilhac O, Peter HH, Berner R, Peters A, Boehm T, Plebani A, Quinti I, Carsetti R. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med. 2003;197:939–945. doi: 10.1084/jem.20022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, Plebani A, Kumararatne DS, Bonnet D, Tournilhac O, Tchernia G, Steiniger B, Staudt LM, Casanova JL, Reynaud CA, Weill JC. Human blood IgM "memory" B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–3654. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tangye SG, Good KL. Human IgM+CD27+ B cells: memory B cells or "memory" B cells? J Immunol. 2007;179:13–19. doi: 10.4049/jimmunol.179.1.13. [DOI] [PubMed] [Google Scholar]
- 106.Sanz I, Wei C, Lee FE, Anolik J. Phenotypic and functional heterogeneity of human memory B cells. Semin Immunol. 2008;20:67–82. doi: 10.1016/j.smim.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nicholas MW, Dooley MA, Hogan SL, Anolik J, Looney J, Sanz I, Clarke SH. A novel subset of memory B cells is enriched in autoreactivity and correlates with adverse outcomes in SLE. Clin Immunol. 2008;126:189–201. doi: 10.1016/j.clim.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bekeredjian-Ding IB, Wagner M, Hornung V, Giese T, Schnurr M, Endres S, Hartmann G. Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J Immunol. 2005;174:4043–4050. doi: 10.4049/jimmunol.174.7.4043. [DOI] [PubMed] [Google Scholar]
- 109.Darce JR, Arendt BK, Wu X, Jelinek DF. Regulated expression of BAFF-binding receptors during human B cell differentiation. J Immunol. 2007;179:7276–7286. doi: 10.4049/jimmunol.179.11.7276. [DOI] [PubMed] [Google Scholar]
- 110.Agrawal S, Gupta S. TLR1/2, TLR7, and TLR9 Signals Directly Activate Human Peripheral Blood Naive and Memory B Cell Subsets to Produce Cytokines, Chemokines, and Hematopoietic Growth Factors. J Clin Immunol. 2011 doi: 10.1007/s10875-010-9456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2011;32:129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 112.Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, Mauri C : Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178:7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- 113.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 114.Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006;176:705–710. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- 115.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T celldependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 117.Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM, Williams AD, Hall RP, St Clair EW, Tedder TF. Characterization of a rare IL-10- competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tian J, Zekzer D, Hanssen L, Lu Y, Olcott A, Kaufman DL. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J Immunol. 2001;167:1081–1089. doi: 10.4049/jimmunol.167.2.1081. [DOI] [PubMed] [Google Scholar]
- 119.Brummel R, Lenert P. Activation of marginal zone B cells from lupus mice with type A(D) CpGoligodeoxynucleotides. J Immunol. 2005;174:2429–2434. doi: 10.4049/jimmunol.174.4.2429. [DOI] [PubMed] [Google Scholar]
- 120.Lampropoulou V, Hoehlig K, Roch T, Neves P, Calderon Gomez E, Sweenie CH, Hao Y, Freitas AA, Steinhoff U, Anderton SM, Fillatreau S. TLR-activated B cells suppress T cellmediated autoimmunity. J Immunol. 2008;180:4763–4773. doi: 10.4049/jimmunol.180.7.4763. [DOI] [PubMed] [Google Scholar]