Abstract
Cerebral edema is a serious complication of ischemic brain injury. Cerebral edema includes accumulation of extracellular fluid due to leakage of the brain’s microvessel permeability barrier, and swelling of astrocytes as they absorb water from the extracellular space. Expression of matrix adhesion receptors in brain microvessels decreases in ischemic stroke; this contributes to increased microvessel permeability and detachment of astrocytes from the extracellular matrix (ECM). Since loss of the astrocyte adhesion receptor dystroglycan has been associated with disrupted polarization of ion and water channels, we hypothesized that adhesion of astrocytes to the ECM contributes to regulation of water uptake, and that disruption of matrix adhesion impairs the ability of astrocytes to direct water transport. To test this hypothesis, the capacity of astrocytes to take up water was measured using a fluorescence self-quenching assay under both oxygen/glucose deprivation (OGD) and direct antibody-mediated blockade of α-dystroglycan. Both conditions decreased the rate of water uptake. Moreover, inhibiting proteolytic cleavage of dystroglycan that occurs in OGD abrogated the effect of OGD, but not direct blockade of α-dystroglycan, indicating that interfering with dystroglycan-matrix binding itself affects water uptake. Activation of extracellular signal-related kinase (ERK) by OGD was dependent on α-dystroglycan binding, and inhibition of ERK activity with U0126 abrogated the loss of water uptake following OGD. These studies demonstrate for the first time that water uptake in astrocytes is regulated by dystroglycan-dependent signaling associated with matrix adhesion. This presents a novel potential approach to the treatment of cerebral edema.
Keywords: astrocyte, dystroglycan, water transport, homeostasis, edema, adhesion, extracellular matrix, ischemia, oxygen/glucose deprivation
1. Introduction
Cerebral edema is a serious complication of ischemic and traumatic brain injuries, and includes both the accumulation of extracellular fluid due to leakage of the brain’s microvessel permeability barrier and swelling of astrocytes as they absorb water from the extracellular space (Kahle et al., 2009). The microvessel endothelium and astrocytes are anchored to the proteins of the extracellular matrix (ECM) by adhesion receptors (integrins and dystroglycan) (Baeten and Akassoglou, 2011). Ligation of adhesion receptors activates intracellular signaling cascades, suggesting that adhesion receptors regulate cellular functions (Moore and Winder, 2010; Shattil et al., 1994). However, the roles of matrix adhesion in the cellular and molecular mechanisms underlying the development and resolution of cerebral edema are not well understood.
The expression of specific endothelial and astrocyte adhesion receptors decreases acutely in ischemic stroke (Milner et al., 2008b; Tagaya et al., 2001; Wagner et al., 1997). We recently demonstrated that antibody-mediated blockade of the adhesion receptor β1-integrin in brain microvessel endothelial cells increases permeability, indicating that adhesion receptor binding to the matrix is an essential component of microvessel integrity (Osada et al., 2011). The acute phase of focal ischemia is also marked by progressive loss of astrocyte-ECM contacts and swelling of astrocytes and their endfeet in select microvessels in the ischemic territory (Kwon et al., 2009). However, the functional consequences to the astrocyte of decreased dystroglycan and loss of adhesion are not known. Dystroglycan is a signaling scaffold for extracellular signal-related kinase (ERK, also known as p42/44 mitogen-activated protein kinase) (Spence et al., 2004), activation of which is obligatory for reactive gliosis (Mandell and VandenBerg, 1999) and is involved in regulating the expression of many proteins following ischemic injury, including ion and water channels, in astrocytes (Qi et al., 2011). In lung alveolar cells, dystroglycan functions as a mechanosensitive transducer of cell stretching via an ERK-dependent mechanism (Jones et al., 2005). It is not known whether dystroglycan has a similar mechanosensitive role in astrocytes; however, there is emerging evidence that mechanosensitive pathways involving adhesion receptors are involved in regulation of ion channel expression in the brain’s vascular system (Kurland et al., 2012).
Regulation of fluid balance in the brain extracellular space by astrocytes is accomplished in part via inward rectifying potassium channels (i.e., Kir 4.1) and aquaporins (i.e., AQP4). Polarized expression of these channels in the perivascular endfeet depends on dystroglycan (Wolburg-Buchholz et al., 2009). This suggests that acute loss of dystroglycan in ischemia may diminish the ability of astrocytes to resolve edema (Papadopoulos et al., 2004).
We hypothesized that adhesion of astrocytes to the vascular basal lamina via dystroglycan contributes to regulation of water transport by astrocytes, and that disruption of dystroglycan-laminin interaction impairs the ability of astrocytes to direct water transport. To test this hypothesis, the capacity of astrocytes to take up water was measured under experimental ischemia (oxygen/glucose deprivation, OGD) and direct blockade of dystroglycan with IIH6C4, an antibody against the extracellular (α) subunit of dystroglycan that blocks the dystroglycan-laminin binding site (Gee et al., 1994).
2. Results
2.1 IIH6C4 blocks adhesion of astrocytes to laminin
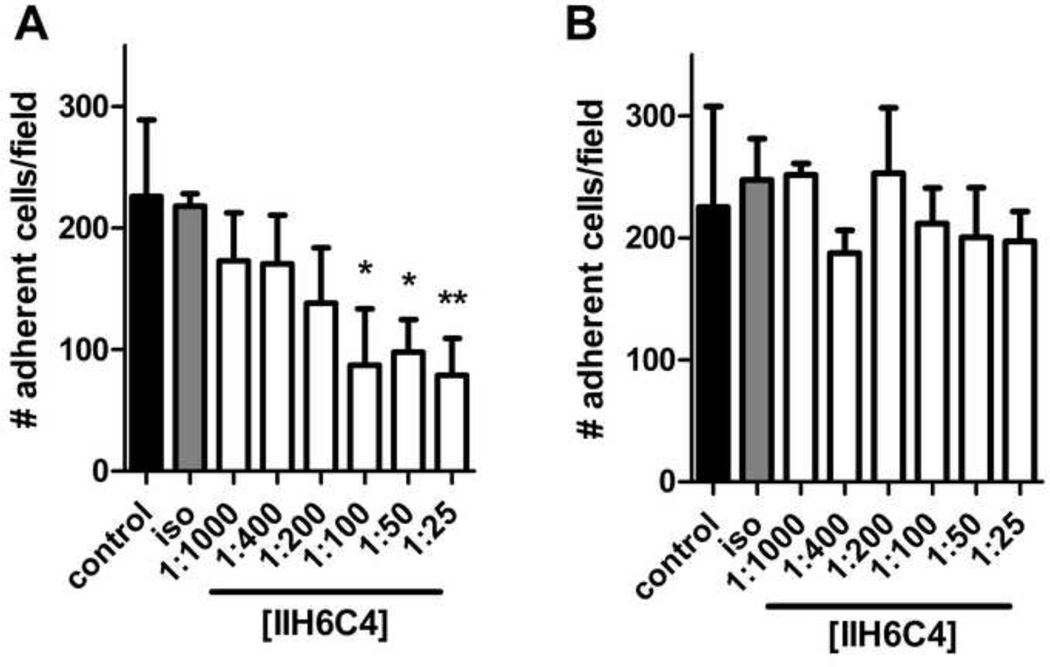
The ability of IIH6C4 to selectively block adhesion of astrocytes to laminin was confirmed with an adhesion assay (Milner et al., 2008b). Incubation of astrocytes in suspension with IIH6C4 decreased the number that adhered to a laminin-coated cell culture plate in a concentration-dependent manner (Figure 1A). The maximum effect (~40% of the number of adherent untreated cells) was observed at 1:100 dilution of the antibody, with higher concentrations of IIH6C4 having no additional effect. The isotype control antibody (diluted 1:25) had no effect on the ability of astrocytes to adhere to laminin. Furthermore, IIH6C4 had no effect on the ability of astrocytes to adhere to fibronectin, another matrix protein that is not a ligand for dystroglycan (Figure 1B). Similarly, IIH6C4 did not affect astrocyte adhesion to collagen IV (data not shown). The fact that IIH6C4 did not completely inhibit astrocyte adhesion to laminin-coated wells indicates that other matrix adhesion receptors are present that interact with laminin, and/or that some astrocytes adhere to plastic in a non-specific manner. Taken together, these data show that IIH6C4 specifically blocks the adhesion of astrocytes to laminin via dystroglycan.
Figure 1. IIH6C4 specifically blocks dystroglycan-mediated adhesion of astrocytes to laminin.
Data are mean ± S.D. adherent cells counted within a defined grid, four fields counted per condition shown, on wells coated with either laminin (A) or fibronectin (B). II6C4 led to a significant decrease in the number of adherent cells at concentrations of 1:100 or higher on laminin, but had no effect on cells plated onto fibonectin. Control: untreated cells; iso: isotype control antibody at 1:25 dilution. Significance determined by Kruskal-Wallis test with a Dunn’s Multiple Comparison test. * = p < 0.05, ** = p < 0.01 versus isotype control.
2.2 OGD decreases water uptake in astrocytes
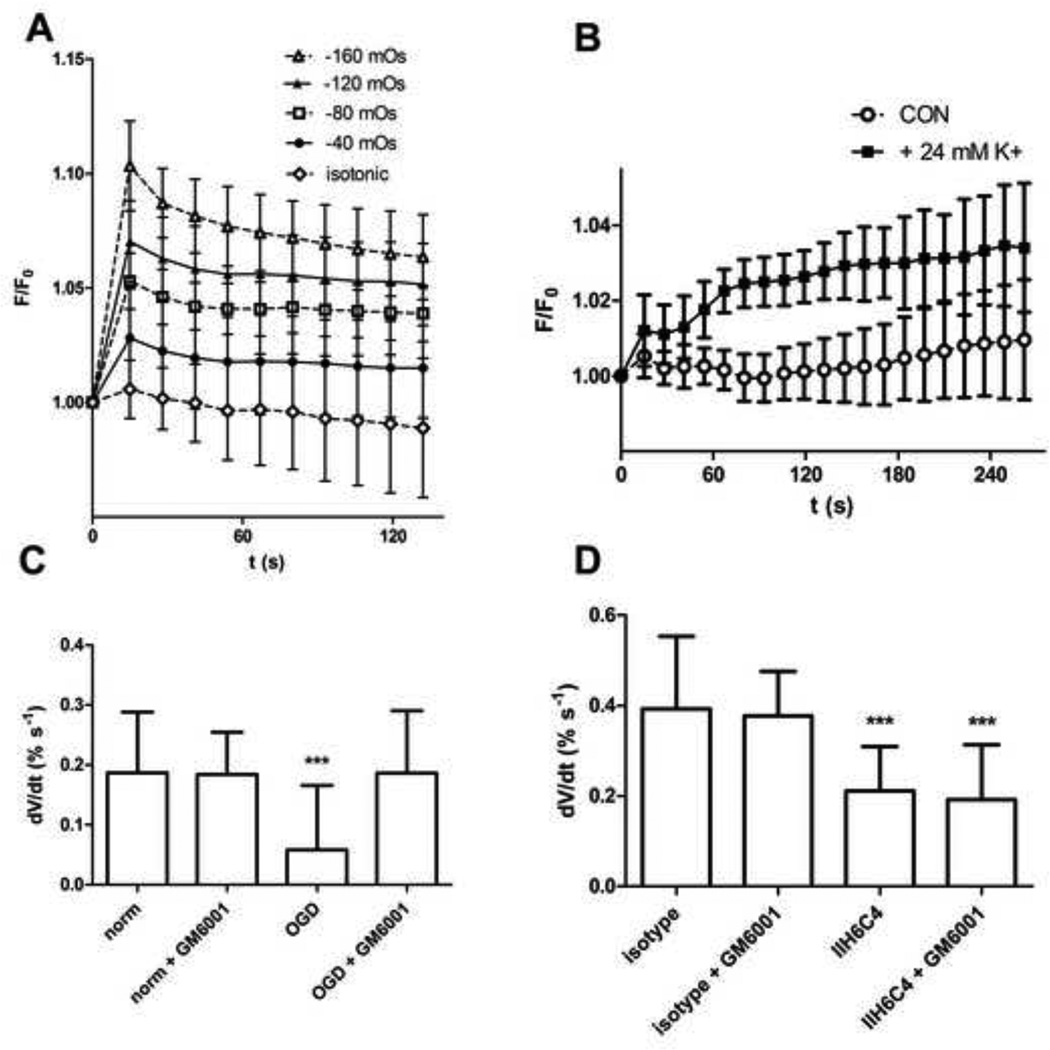
Rapid changes in cell volume can be quantified by taking advantage of calcein self-quenching, in which changes in fluorescence can be used to estimate changes in cell volume (Hamann et al., 2002; Solenov et al., 2004). Astrocytes take up the acetomethoxy derivative of calcein (calcein-AM) and metabolize it into the non-cell permeable, fluorescent form of calcein. Acute reduction of extracellular osmolarity by addition of deionized water (diH2O) to the wells resulted in a rapid and reproducible increase in cell fluorescence (Figure 2A). This reflects dilution of intracellular calcein as the cells swell, leading to decreased self-quenching. Progressively larger changes in osmolarity produced progressively larger changes in fluorescence (Figure 2A). Similarly, acute increase of extracellular osmolarity by addition of mannitol resulted in decreased cell fluorescence (data not shown).
Figure 2. Water uptake measurements in astrocytes.
A: Astrocytes respond to decreased osmolarity in the extracellular fluid with rapid and reproducible increases in calcein fluorescence (F/F0 indicates relative fluorescence). The change in osmolarity indicated was achieved by addition of diH2O (15-100 µl) to artificial cerebrospinal fluid (aCSF); isotonic controls had 100 µl of additional aCSF added. Data shown are mean ± S.D., n = 8 wells per condition. B: Addition of 24 mM K+ also leads to an increase in fluorescence. Data shown are mean ± S.D., n = 8 wells per condition. C: The initial rate of change in cell volume (dV/dt) following addition of 24 mM K+ was measured in astrocytes following 18 h OGD or normoxia with or without GM6001 (100 µM). Data are mean change in estimated cell volume ± S.D., n = 24 wells per condition, pooled from 3 independent experiments. Significance was determined by one-way ANOVA with a Bonferonni’s Multiple Comparison test. *** = p < 0.001 versus normoxia. D: dV/dt was measured following 24 h incubation with IIH6C4 or isotype control antibody with or without GM6001. Data are mean change in estimated cell volume ± S.D., n = 24 wells per condition, pooled from 3 independent experiments. Significance was determined by one-way ANOVA with a Bonferonni’s Multiple Comparison test. *** = p < 0.001 versus isotype.
Acute elevation of extracellular K+ also resulted in cell swelling reflected by an increase in cell fluorescence, which was approximately linear through the first 60 seconds following isotonic addition of 24mM K+ to the media (Figure 2B). In subsequent experiments 24mM K+ was added following treatments, and fluorescence readings for the first 60 seconds after addition of K+ were used to estimate the rate of water uptake into the cells by converting relative fluorescence (F/Fo) to relative cell volume (V/Vo) using equation (1) and determining the slope of a linear regression of V/Vo versus time.
Exposing astrocytes to OGD significantly reduced the rate of cell swelling in response to 24mM K+, indicating a diminished rate of water uptake (Figure 2C). OGD did not result in significant astrocyte cell death (propidium iodide uptake observed in < 2% of cells in all conditions). Astrocytes exposed to OGD displayed identical volume changes in response to changes in osmolarity as cells kept in normoxic conditions, and had identical background fluorescence (Supplemental Figure 1).
The decrease in astrocyte dystroglycan expression in OGD is protease-dependent, and is inhibited by the general matrix metalloprotease inhibitor GM6001 (Milner et al., 2008b). In this experiment, pre-treatment with GM6001 abolished the effect of OGD on astrocyte water uptake (Figure 2C), suggesting that protecting the surface expression of dystroglycan preserves the capacity of astrocytes to take up water.
2.3 IIH6C4 mimics the effect of OGD on water uptake
To test directly whether astrocyte water uptake is dependent on the interaction of dystroglycan with laminin, astrocytes were incubated with IIH6C4 or isotype antibody for 24 h prior to measurement of water uptake. IIH6C4 also decreased water uptake, but this effect was not blocked by GM6001 (Figure 2D). To confirm that the effect of IIH6C4 was due to its specific interaction with dystroglycan, rather than non-specific binding, these results were confirmed with A2G80, a peptide derived from the dystroglycan-binding domain of laminin (Suzuki et al., 2010). A2G80 also blocks astrocyte adhesion to laminin, and decreases water uptake by astrocytes (Supplemental Figure 2).
2.4 ERK activation in ischemic astrocytes is dependent on dystroglycan binding
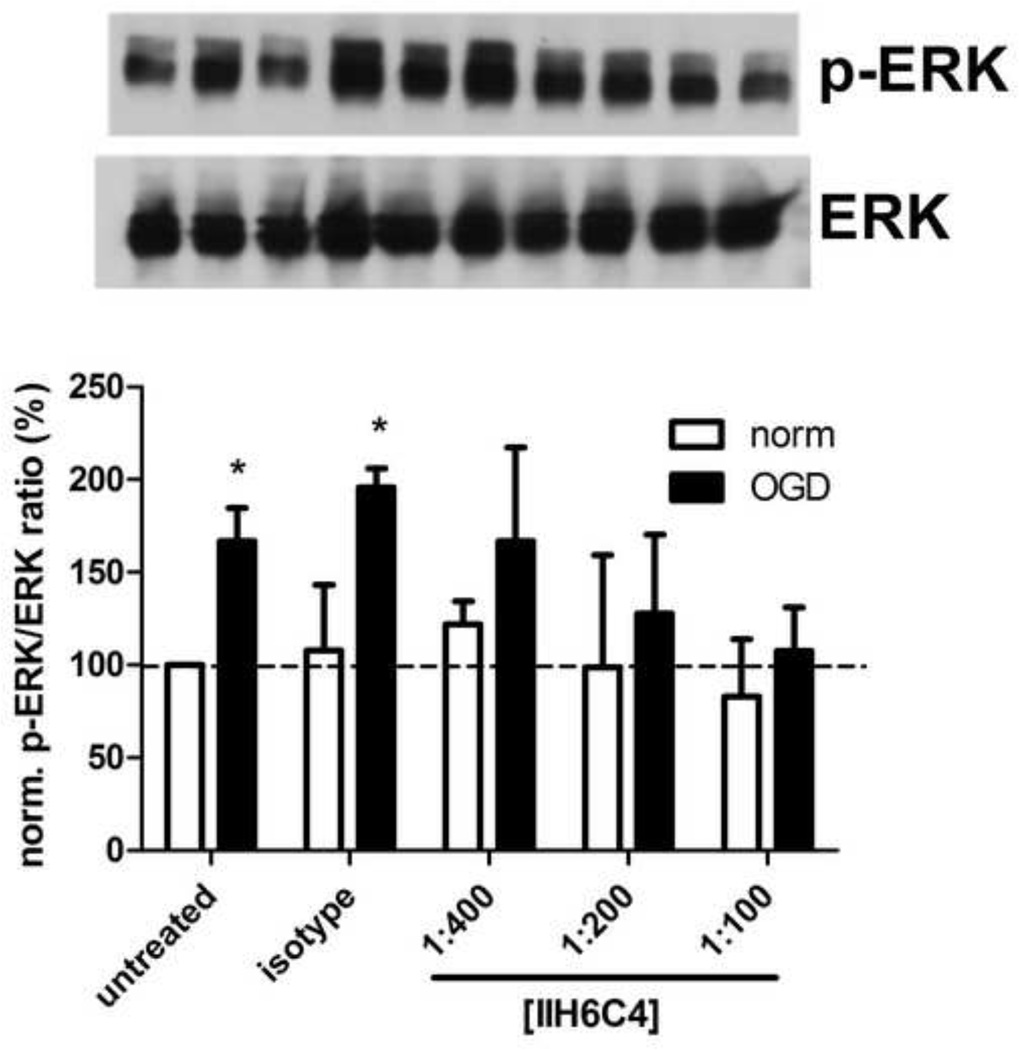
OGD increased phosphorylation of ERK in astrocytes (Figure 3). Preincubation of astrocytes with IIH6C4 blocked activation of ERK by OGD in a concentration-dependent manner. Note that the concentration of IIH6C4 at which ERK phosphorylation was abrogated is the same concentration at which maximal blockade of astrocyte binding to laminin is achieved (1:100, Figure 1A). Taken together, these data indicate that astrocyte ERK activation by OGD is dependent on dystroglycan binding to laminin.
Figure 3. ERK activation in response to OGD is dystroglycan-dependent.
Astrocytes were pre-incubated with the concentration of IIH6C4 indicated (or isotype control antibody) 24 h prior to induction of OGD or normoxia. Protein was extracted from astrocytes immediately following OGD/normoxia for immunoblot. Blots shown are a representative experiment (bands correspond to the order indicated on the bar graph below); bar graphs represent the average of 3 independent experiments. Data are mean normalized band densities (measured between 40 and 50 kDa) ± S.D. Significance was determined by two-way ANOVA with a Bonferonni post-hoc test.* = p < 0.05 vs. normoxia.
2.5 Blocking ERK signaling abrogates loss of water uptake in OGD
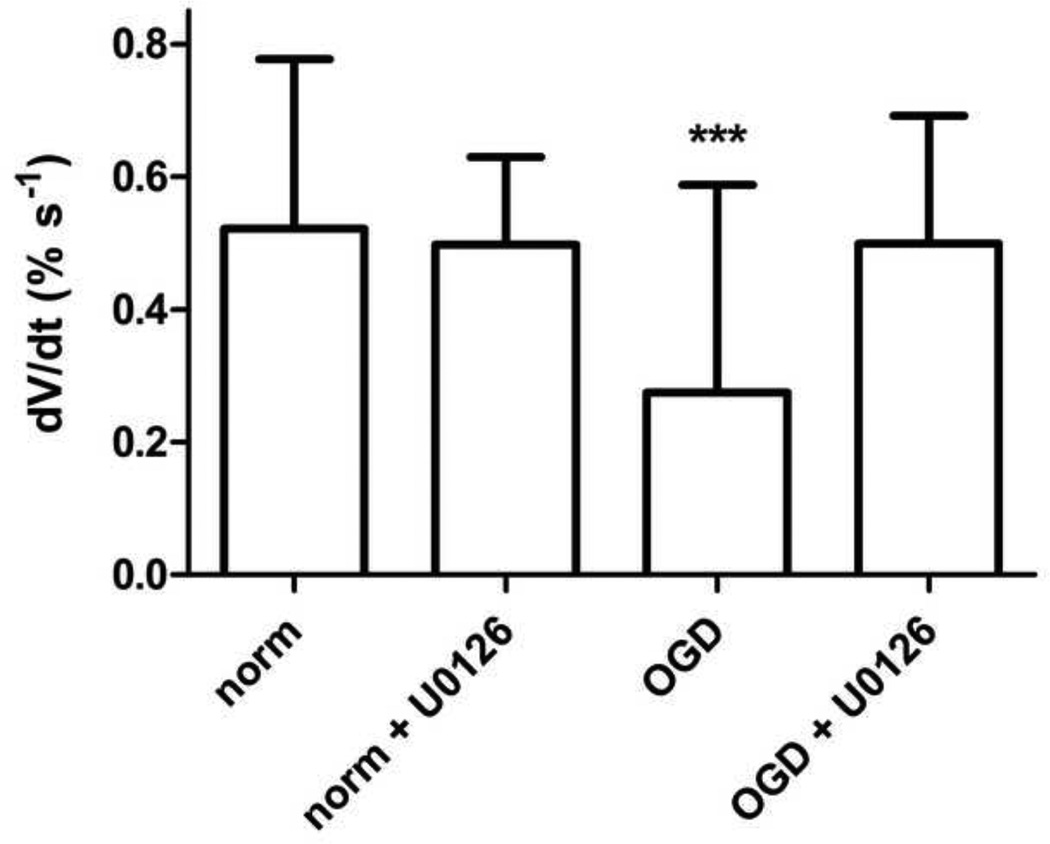
The ERK inhibitor U0126 blocked phosphorylation/activation of ERK in astrocytes by OGD (Supplemental Figure 3). Preincubation of astrocytes with U0126 also blocked the reduction in water uptake by OGD (Figure 4).
Figure 4. Inhibition of ERK blocks loss of water uptake in OGD.
Astrocytes underwent OGD or normoxia in the presence or absence of the ERK inhibitor U0126 (1 µM). Data are mean change in estimated cell volume ± S.D., n = 32 wells per condition, pooled from 4 independent experiments. Significance was determined by one-way ANOVA with a Bonferonni’s Multiple Comparison test. *** = p < 0.001 versus normoxia.
3. Discussion
Astrocyte swelling and detachment from the ECM occurs on select microvessels acutely following focal ischemia (Kwon et al., 2009), but it is not known how altered adhesion receptor-matrix interactions affects the development and resolution of cerebral edema that occurs as a result of lost microvessel integrity. The principal findings of this study are: 1) experimental ischemia (OGD) decreases the rate of water uptake in astrocytes in vitro, 2) direct blockade of α-dystroglycan on adherent cells mimics the effect of OGD on water uptake in astrocytes, 3) ERK phosphorylation in astrocytes in response to OGD is dependent on dystroglycan binding, and 4) inhibiting the proteolysis of dystroglycan or ERK activation preserves water uptake in astrocytes following OGD. These observations have a number of implications for cerebral edema formation and microvessel function.
Astrocytes are attached to the ECM via adhesion receptors that include integrins and dystroglycan (Baeten and Akassoglou, 2011; Milner et al., 2008a; Milner et al., 2008b; Paulus et al., 1993); in vivo expression of several of these receptors (α1β1 and α6β4 integrins, and dystroglycan) rapidly decreases in the microvasculature following focal ischemia (Milner et al., 2008b; Steiner et al., 2012; Tagaya et al., 2001; Wagner et al., 1997). Astrocytes are the primary cellular source of dystroglycan associated with the brain microvasculature (Milner et al., 2008b). However, the specific functional role of dystroglycan and its interaction with the ECM in astrocytes is not well understood. Dystroglycan binds to ECM components laminin, agrin, and perlecan, constituting a link between the ECM and the interior of the cell (Bozzi et al., 2009). Complete knockout of the Dag1 gene that encodes both subunits of dystroglycan leads to embryonic lethality, and brain-specific deletion of dystroglycan leads to profound structural and functional abnormalities in the CNS (Henry et al., 1998; Moore et al., 2002).
In this study, OGD led to a significant reduction in the rate at which astrocytes take up water in response to elevated extracellular K+ (Figure 2C). We have previously shown that OGD does not result in significant astrocyte cell death (Milner et al., 2008a) and confirmed this finding here by propidium iodide uptake, (observed in < 2% of cells in all conditions tested). Background fluorescence determinations were identical in astrocytes following both normoxia and OGD (Supplemental Figure 1), indicating that the osmotically inactive portions of the cells were not different under these experimental conditions (Hamann et al., 2002). Taken together, these data indicate that the decreased uptake of water following OGD was not due to cell death or pre-existing swelling of the cells. Exposure of cells to the general matrix metalloprotease inhibitor GM6001 abolished the effect of OGD on astrocyte water uptake (Figure 2C), suggesting that protection of dystroglycan from the proteolysis previously observed in OGD (Milner et al., 2008b) preserves the capacity of astrocytes to take up water.
Direct blockade of α-dystroglycan binding to laminin also decreased the rate of water uptake in astrocytes, indicating that disruption of α-dystroglycan binding to the matrix rather than decreased dystroglycan expression leads to changes in water uptake following OGD. Notably, GM6001 did not preserve water uptake in the context of α-dystroglycan blockade (Figure 2D), confirming that the effect of GM6001 on water uptake is due to protection of dystroglycan. Finally, there was no sign of astrocyte cell detachment from the wells observed via light microscopy following either OGD or incubation with IIH6C4, indicating that astrocytes remained anchored through other matrix adhesion receptors. Thus, the specific interaction of dystroglycan with laminin, rather than cell attachment per se, appears to be essential for normal water uptake function. Noel and colleagues recently demonstrated that the drug chloranil inhibits laminin-induced clustering of both dystroglycan and AQP4 in astrocyte endfeet via reactive oxygen species and generation of a metalloprotease activity that cleaves α-dystroglycan (Noel et al., 2011). That finding, together with the data presented in this study, are consistent with the hypothesis that dystroglycan specifically contributes to astrocyte-mediated fluid homeostasis.
Astrocytes rapidly swell in response to OGD, hyposmotic stress, elevated extracellular K+, oxidative stress, or traumatic (concussive) injury in vitro via signaling that involves rapid phosphorylation of intracellular kinases including ERK (Jayakumar et al., 2008; Moriyama et al., 2010; Risher et al., 2009). However, it is not known how these stressors signal to intracellular pathways in astrocytes. Matrix adhesion receptors including dystroglycan are known to function as transducers of cell stretching in lung alveolar cells (Jones et al., 2005). It is possible that dystroglycan plays a similar role in astrocytes, transducing changes in tension between the astrocyte endfeet and the ECM, initiating signaling that mediates compensatory responses such as upregulation of channel expression and/or alteration of their cellular distribution (Cai et al., 2011). In this study, ERK phosphorylation increased following OGD, an effect that was blocked when OGD was performed in the presence of the α-dystroglycan-blocking antibody IIH6C4 (Figure 3). This suggests that the persistent activation of ERK observed following OGD depends in part on interaction of α-dystroglycan with the ECM.
Interestingly, pharmacologic inhibition of ERK activation in OGD with U0126 also preserved the capacity of astrocytes to take up water following OGD (Figure 4). It is important to note that activation/phosphorylation of ERK has been reported in astrocytes in in vitro ischemic models, but this has generally been observed after 2–6 hours experimental ischemia (Qi et al., 2011). It is likely that ERK activation in response to experimental ischemia is biphasic (Alderliesten et al., 2007). Note also that U0126 reduces the amount of phosphorylated ERK to nearly undetectable levels (Supplemental Figure 3). Thus, we cannot at present know whether the effect of the U0126 on water uptake in this study is related to inhibition of acute or more long-term activation of ERK in OGD, or by reduction of constitutive ERK activity. However, given the relationship of dystroglycan-ECM interactions to ERK signaling reported here and by others (Al-Ahmad et al., 2011; Spence et al., 2004), and the involvement of dystroglycan-ECM interaction in regulating water uptake (Figure 2C and 2D), it is likely that regulation of water uptake in astrocytes by dystroglycan-ECM adhesion involves activation of ERK.
In normoxic cerebral tissues of the non-human primate, dystroglycan and AQP4 are colocalized to most microvessels (Milner et al., 2008b). AQP4-null mice have an impaired ability to clear excess water from the extracellular space and worse functional outcomes in models of vasogenic edema (Papadopoulos et al., 2004). Interestingly, earlier studies in the AQP4-null mouse had indicated that absence of AQP4 reduced edema and improved neurological outcome following focal ischemia (Manley et al., 2000). This raises the important question of whether the acute loss of dystroglycan in astrocytes (and the putatively associated disorganization of AQP4) serves to slow the formation of cell swelling in astrocytes early in ischemia, or exacerbates the effect of subsequent vasogenic edema (via impaired clearance) when the permeability barrier has been compromised, or both. Steiner and colleagues (Steiner et al., 2012) recently showed that in mice lacking the dystroglycan ligand agrin, both dystroglycan and AQP4 were less intensely expressed in astrocyte endfeet, though their total protein expression was not altered, indicating a loss of polarization. In the agrin-null mice, early edema formation following transient focal ischemia was significantly less than in wild-type controls. However, those studies were limited to 24 h following occlusion, and the loss of dystroglycan in the ischemic core has been observed to persist for at least 7 days (Li et al., 2012).
It remains to be seen whether the apparent reduction in the capacity of astrocytes to take up water observed in vitro here has a direct correlate in cerebral ischemia, and if so, whether it serves to protect tissue, as suggested by the results of Steiner et al. It is important to consider that astrocytes are an essential component of brain microvessels that contribute to both the permeability barrier (Abbott et al., 2006; Hawkins and Davis, 2005) and to neuronal regulation of microvascular function (Iadecola and Nedergaard, 2007) in the theoretical neurovascular unit (del Zoppo, 2006). We suggest that while the initial slowing of astrocyte swelling may be enabled by disaggregation of the dystroglycan complex, this disaggregation also contributes to the net formation of edema by: 1) impaired regulation of blood flow and/or impaired microvascular integrity due to reduced astrocyte-endothelial cell contact (Kwon et al., 2009), and 2) a decreased capacity for astrocytes to clear water from the extracellular space as injury evolves, as indicated by the data reported here. The diminished water uptake in astrocytes also suggests that the K+ buffering function of astrocytes is adversely affected by disaggregation of the dystroglycan complex as well, given that clustering of AQP4 and Kir4.1 is induced in astrocytes by the interaction of laminin with dystroglycan (Guadagno and Moukhles, 2004). This represents another mechanism by which loss of dystroglycan likely contributes to the evolution of ischemic brain injury.
Future studies will determine the precise molecular mechanism(s) by which dystroglycan binding signals to the water transport apparatus in astrocytes as well as the specific protein alterations involved. Further, a more detailed understanding of the timing of these cellular events in ischemia will inform the design of intervention studies in vivo. The data presented here constitute the first demonstration that dystroglycan modulates the fluid transport function in astrocytes, and suggest involvement of adhesion-dependent signaling. Targeting these processes to preserve astrocyte function and microvessel integrity represents a novel and potentially promising approach to the treatment of cerebral ischemia.
4. Experimental Procedures
4.1 Reagents
Mouse anti-α-dystroglycan (IIH6C4) and isotype control (mouse IgG2b) antibodies were obtained from Millipore (Temecula, CA). Mouse anti-ERK and rabbit anti-p-ERK antibodies were obtained from Cell Signaling Technology (Danvers, MA). HRP-conjugated anti-mouse and anti-rabbit secondary antibodies were obtained from Jackson Immunoresearch (West Grove, PA). Calcein-AM was obtained from Invitrogen (Eugene, OR). GM6001 was obtained from Millipore. U0126 was obtained from Cell Signaling Technology. All other reagents were obtained from Sigma (St. Louis, MO) unless otherwise noted.
4.2 Primary cell culture
All animal procedures were approved by the University of Washington Institutional Animal Care an use committee and conform to NIH guidelines. Pregnant female mice were purchased from Charles River Laboratories. Glial cells were cultured from the brains of neonatal (1–3 days old) mouse pups using established techniques (Milner et al., 2008b). Briefly, cerebral hemispheres were dissected from the brainstem and cerebellum, meninges and choroid plexuses removed, and the remaining tissue minced and digested in media containing papain and DNAse. Cells were pelleted, resuspended in growth media (DMEM supplemented with fetal calf serum, glutamine, and antibiotics) and grown to confluence in poly-D-lysine-coated flasks. Microglial cells and oligodendrocytes were removed by shaking prior to passage, resulting in astrocyte preparations that were > 98% pure as determined by light microscopy (Milner et al., 2008b). Astrocytes typically reached confluence in 9–12 days, and were used in the first passage only. Astrocytes were either used for adhesion assays (see below) or passaged to laminin-coated 6-well plates (for protein expression studies), or 96-well plates (for water transport studies).
4.3 Adhesion assay
25 µl of laminin or fibronectin (10 µg/ml in PBS) were applied to the center of the wells of a 24-well cell culture plate for 2 h at 37°C. The wells were then washed with PBS. Cells were gently lifted with a cell scraper and resuspended in serum-free DMEM at 7.5 × 105 cells/ml. IIH6C4 or isotype control antibody were added to aliquots of the cell suspension, and 100 µl of suspension were applied to the wells with the matrix proteins for 1 h at 37°C. Non-adherent cells were then washed away with PBS, and the cells were permeabilized with 0.1% Triton X-100 for 10 min, fixed in 3.7% formaldehyde for 10 min, and incubated with DAPI (5 µg/ml) for 5 min, followed by rinsing with PBS. Adherent cells were counted on a fluorescent microscope with a 10x objective. Results are reported as the number of adherent cells within a defined grid.
4.4 Oxygen/glucose deprivation (OGD) and antibody-mediated blockade of α-dystroglycan
Serum-containing media was removed from the cell cultures by washing with PBS before adding serum-free high glucose medium (4.5 g/L, DMEM containing 4 mM L-glutamine, penicillin, and streptomycin, supplemented with N1 medium) for normoxic controls or low-glucose medium (1 g/L, supplemented DMEM) for OGD. Cultures containing low-glucose medium were placed in a hypoxia chamber flushed with 95% N2 and 5% CO2 for 1 h, and then sealed for the duration of the experiment. O2 levels decreased to 0.1–0.4% at 4 h, and were maintained throughout the experiment (18 h). Normoxic controls were maintained in serum-free media under standard incubator conditions in parallel.
The α-dystroglycan function-blocking studies were performed in the same serum-free media as normoxic controls, with 24 h exposure to either IIH6C4 (α-dystroglycan blocking) or isotype antibodies.
4.5 Water uptake measurements
Changes in astrocyte cell volume and water transport were measured by calcein self-quenching (Hamann et al., 2002; Solenov et al., 2004). Astrocytes were washed with PBS to remove serum-containing media and placed in artificial cerebrospinal fluid (aCSF, in mM: 120 NaCl, 2.5 KCl, 25 NaHCO3, 1 NaH2PO4, 2.5 CaCl2, 1.3 MgSO4, 10 glucose) containing 5 µM calcein-AM for 1 h at 37°C. Following incubation, astrocytes were maintained in aCSF without calcein-AM for baseline fluorescence measurements and subsequent treatments and measurements. Fluorescence measurements were made on a CytoFluor fluorescence plate reader (excitation 485 nm, emission 530 nm). For a typical experiment, 5–10 measurements were taken 15 seconds apart to establish baseline fluorescence and signal drift for each well. 25 µl aCSF containing 122.5 mM KCl (replacing all NaCl, final K+ concentration is 26.5 mM) was then added and the plate immediately returned to the reader for 10 sequential measurements. Changes in relative fluorescence are related to changes in cell volume by:
| (1) |
where V/Vo and F/Fo are the relative cell volume and relative fluorescence, respectively, and fb is the background fluorescence. Background fluorescence was determined using calibration measurements taken in each experiment using decreased osmotic pressure (aCSF + diH2O) to increase cell volume (Supplemental Figure 1). The Y-intercept of a plot of relative fluorescence against the reciprocal of relative osmotic pressure yields the value for fb (Hamann et al., 2002), which typically ranged from 0.80 to 0.95. The relative rate of water uptake was estimated by the initial rate of change in volume (dV/dt) immediately following cell exposure to elevated K+.
4.6 Immunoblot
For immunoblot studies, astrocytes were washed in PBS, lifted with a cell scraper, suspended in PBS, and pelleted for 10 min at 10,000 × g. The supernatants were carefully aspirated and the tubes containing the pellets snapfrozen in liquid nitrogen and stored at −80° C for subsequent use. Pellets were resuspended in lysis buffer (CelLytic MT, Sigma) containing 0.1% sodium dodecyl sulfate with protease and phosphatase inhibitors on ice, sonicated for 20 s, and incubated 10 min on ice prior to centrifuging 10 min at 10,000 × g. Supernatants were decanted to fresh tubes and quantified for protein content using the bicinchoninic acid method. Protein samples (2 µg per lane) were loaded onto hand-cast 10% Tris-glycine gels and resolved under reduced conditions at 150 V for 1 h, then transferred to PVDF membranes at 30 V for 90 min. Both electropheresis and transfer were performed on ice. Membranes were blocked for 1 h in 3% BSA/0.1% Tween-20/PBS, and incubated overnight with anti-p-ERK antibody at 4°C with gentle agitation. Membranes were rinsed with PBS and incubated with HRP-conjugated secondary antibody for 1 h at RT, rinsed, and bands were visualized with ECL Plus (GE Healthcare, Pittsburgh, PA) onto X-ray film. After stripping and re-blocking, the membranes were re-probed for total ERK by the same protocol. All primary and secondary antibodies were diluted 1:5000 in 3% BSA/0.1% Tween-20/PBS. X-ray films were scanned and bands quantified using ImageJ software.
4.7 Statistical Analysis
All data are presented as mean ± S.D., with the numbers of replicates as indicated. Normality of data sets was tested by the D’Agostino and Pearson test. Means were compared by nonparametric tests, one-way ANOVA, or two-way ANOVA as appropriate, and significance set at p < 0.05. All analyses were carried out using GraphPad Prism v. 5.04.
Supplementary Material
Highlights.
Experimental ischemia (OGD) decreases the rate of water uptake in astrocytes in vitro
Blockade of α-dystroglycan mimics the effect of OGD on water uptake
ERK phosphorylation in response to OGD depends on dystroglycan binding
Inhibiting protease activity or ERK activation preserves water uptake in astrocytes
Acknowledgments
This study was supported in part by NIH Grants NS 053716 and NS 038710 to Dr. del Zoppo.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Brian T. Hawkins, Email: bthawk@uw.edu.
Yu-Huan Gu, Email: yhg2@u.washington.edu.
Yoshikane Izawa, Email: izaway@u.washington.edu.
Gregory J. del Zoppo, Email: grgdlzp@u.washington.edu.
References
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Al-Ahmad AJ, Lee B, Saini M, Bix GJ. Perlecan domain V modulates astrogliosis In vitro and after focal cerebral ischemia through multiple receptors and increased nerve growth factor release. Glia. 2011;52:1822–1840. doi: 10.1002/glia.21227. [DOI] [PubMed] [Google Scholar]
- Alderliesten M, de Graauw M, Oldenampsen J, Qin Y, Pont C, van Buren L, van de Water B. Extracellular signal-regulated kinase activation during renal ischemia/reperfusion mediates focal adhesion dissolution and renal injury. Am J Pathol. 2007;171:452–462. doi: 10.2353/ajpath.2007.060805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeten KM, Akassoglou K. Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev Neurobiol. 2011;71:1018–1039. doi: 10.1002/dneu.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzi M, Morlacchi S, Bigotti MG, Sciandra F, Brancaccio A. Functional diversity of dystroglycan. Matrix Biology. 2009;28:179–187. doi: 10.1016/j.matbio.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Cai L, Du T, Song D, Li B, Hertz L, Peng L. Astrocyte ERK phosphorylation precedes K(+)-induced swelling but follows hypotonicityinduced swelling. Neuropathology. 2011;31:250–264. doi: 10.1111/j.1440-1789.2010.01172.x. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ. Stroke and neurovascular protection. N Engl J Med. 2006;354:553–555. doi: 10.1056/NEJMp058312. [DOI] [PubMed] [Google Scholar]
- Gee SH, Montanaro F, Lindenbaum MH, Carbonetto S. Dystroglycan-alpha, a dystrophin-associated glycoprotein, is a functional agrin receptor. Cell. 1994;77:675–686. doi: 10.1016/0092-8674(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Guadagno E, Moukhles H. Laminin-induced aggregation of the inwardly rectifying potassium channel, Kir4.1, and the water-permeable channel, AQP4, via a dystroglycan-containing complex in astrocytes. Glia. 2004;47:138–149. doi: 10.1002/glia.20039. [DOI] [PubMed] [Google Scholar]
- Hamann S, Kiilgaard JF, Litman T, Alvarez-Leefmans FJ, Winther BR, Zeuthen T. Measurement of Cell Volume Changes by Fluorescence Self-Quenching. J Fluorescence. 2002;12:139–145. [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Henry MD, Williamson RA, Campbell KP. Analysis of the role of dystroglycan in early postimplantation mouse development. Ann N Y Acad Sci. 1998;857:256–259. doi: 10.1111/j.1749-6632.1998.tb10126.x. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Jayakumar AR, Rao KV, Panickar KS, Moriyama M, Reddy PV, Norenberg MD. Trauma-induced cell swelling in cultured astrocytes. J Neuropathol Exp Neurol. 2008;67:417–427. doi: 10.1097/NEN.0b013e31816fc9d4. [DOI] [PubMed] [Google Scholar]
- Jones JC, Lane K, Hopkinson SB, Lecuona E, Geiger RC, Dean DA, Correa-Meyer E, Gonzales M, Campbell K, Sznajder JI, Budinger S. Laminin-6 assembles into multimolecular fibrillar complexes with perlecan and participates in mechanical-signal transduction via a dystroglycan-dependent, integrin-independent mechanism. J Cell Sci. 2005;118:2557–2566. doi: 10.1242/jcs.02395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle KT, Simard JM, Staley KJ, Nahed BV, Jones PS, Sun D. Molecular mechanisms of ischemic cerebral edema: role of electroneutral ion transport. Physiology (Bethesda) 2009;24:257–265. doi: 10.1152/physiol.00015.2009. [DOI] [PubMed] [Google Scholar]
- Kurland D, Hong C, Aarabi B, Gerzanich V, Simard JM. Hemorrhagic progression of a contusion after traumatic brain injury: a review. J Neurotrauma. 2012;29:19–31. doi: 10.1089/neu.2011.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon I, Kim EH, del Zoppo GJ, Heo JH. Ultrastructural and temporal changes of the microvascular basement membrane and astrocyte interface following focal cerebral ischemia. J Neurosci Res. 2009;87:668–676. doi: 10.1002/jnr.21877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Liu F, Welser-Alves JV, McCullough LD, Milner R. Upregulation of fibronectin and the alpha5beta1 and alphavbeta3 integrins on blood vessels within the cerebral ischemic penumbra. Exp Neurol. 2012;233:283–291. doi: 10.1016/j.expneurol.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell JW, VandenBerg SR. ERK/MAP kinase is chronically activated in human reactive astrocytes. Neuroreport. 1999;10:3567–3572. doi: 10.1097/00001756-199911260-00019. [DOI] [PubMed] [Google Scholar]
- Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- Milner R, Hung S, Wang X, Berg GI, Spatz M, del Zoppo GJ. Responses of endothelial cell and astrocyte matrix-integrin receptors to ischemia mimic those observed in the neurovascular unit. Stroke. 2008a;39:191–197. doi: 10.1161/STROKEAHA.107.486134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R, Hung S, Wang X, Spatz M, del Zoppo GJ. The rapid decrease in astrocyte-associated dystroglycan expression by focal cerebral ischemia is protease-dependent. J Cereb Blood Flow Metab. 2008b;28:812–823. doi: 10.1038/sj.jcbfm.9600585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CJ, Winder SJ. Dystroglycan versatility in cell adhesion: a tale of multiple motifs. Cell Commun Signal. 2010;8:3. doi: 10.1186/1478-811X-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SA, Saito F, Chen J, Michele DE, Henry MD, Messing A, Cohn RD, Ross-Barta SE, Westra S, Williamson RA, Hoshi T, Campbell KP. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature. 2002;418:422–425. doi: 10.1038/nature00838. [DOI] [PubMed] [Google Scholar]
- Moriyama M, Jayakumar AR, Tong XY, Norenberg MD. Role of mitogen-activated protein kinases in the mechanism of oxidant-induced cell swelling in cultured astrocytes. J Neurosci Res. 2010;88:2450–2458. doi: 10.1002/jnr.22400. [DOI] [PubMed] [Google Scholar]
- Noel G, Stevenson S, Moukhles H. A high throughput screen identifies chemical modulators of the laminin-induced clustering of dystroglycan and aquaporin-4 in primary astrocytes. PLoS One. 2011;6:e17559. doi: 10.1371/journal.pone.0017559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada T, Gu YH, Kanazawa M, Tsubota Y, Hawkins BT, Spatz M, Milner R, del Zoppo GJ. Interendothelial claudin-5 expression depends on cerebral endothelial cell-matrix adhesion by beta(1)-integrins. J Cereb Blood Flow Metab. 2011;31:1972–1985. doi: 10.1038/jcbfm.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos MC, Manley GT, Krishna S, Verkman AS. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004;18:1291–1293. doi: 10.1096/fj.04-1723fje. [DOI] [PubMed] [Google Scholar]
- Paulus W, Baur I, Schuppan D, Roggendorf W. Characterization of integrin receptors in normal and neoplastic human brain. Am J Pathol. 1993;143:154–163. [PMC free article] [PubMed] [Google Scholar]
- Qi LL, Fang SH, Shi WZ, Huang XQ, Zhang XY, Lu YB, Zhang WP, Wei EQ. CysLT2 receptor-mediated AQP4 up-regulation is involved in ischemic-like injury through activation of ERK and p38 MAPK in rat astrocytes. Life Sci. 2011;88:50–56. doi: 10.1016/j.lfs.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Risher WC, Andrew RD, Kirov SA. Real-time passive volume responses of astrocytes to acute osmotic and ischemic stress in cortical slices and in vivo revealed by two-photon microscopy. Glia. 2009;57:207–221. doi: 10.1002/glia.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattil SJ, Ginsberg MH, Brugge JS. Adhesive signaling in platelets. Curr Opin Cell Biol. 1994;6:695–704. doi: 10.1016/0955-0674(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Solenov E, Watanabe H, Manley GT, Verkman AS. Sevenfold-reduced osmotic water permeability in primary astrocyte cultures from AQP-4-deficient mice, measured by a fluorescence quenching method. Am J Physiol Cell Physiol. 2004;286:C426–C432. doi: 10.1152/ajpcell.00298.2003. [DOI] [PubMed] [Google Scholar]
- Spence HJ, Dhillon AS, James M, Winder SJ. Dystroglycan, a scaffold for the ERK-MAP kinase cascade. EMBO Rep. 2004;5:484–489. doi: 10.1038/sj.embor.7400140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner E, Enzmann GU, Lin S, Ghavampour S, Hannocks MJ, Zuber B, Ruegg MA, Sorokin L, Engelhardt B. Loss of astrocyte polarization upon transient focal brain ischemia as a possible mechanism to counteract early edema formation. Glia. 2012;60:1646–1659. doi: 10.1002/glia.22383. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Hozumi K, Urushibata S, Yoshimura T, Kikkawa Y, Gumerson JD, Michele DE, Hoffman MP, Yamada Y, Nomizu M. Identification of alpha-dystroglycan binding sequences in the laminin alpha2 chain LG4-5 module. Matrix Biol. 2010;29:143–151. doi: 10.1016/j.matbio.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagaya M, Haring HP, Stuiver I, Wagner S, Abumiya T, Lucero J, Lee P, Copeland BR, Seiffert D, del Zoppo GJ. Rapid loss of microvascular integrin expression during focal brain ischemia reflects neuron injury. J Cereb Blood Flow Metab. 2001;21:835–846. doi: 10.1097/00004647-200107000-00009. [DOI] [PubMed] [Google Scholar]
- Wagner S, Tagaya M, Koziol JA, Quaranta V, del Zoppo GJ. Rapid disruption of an astrocyte interaction with the extracellular matrix mediated by integrin alpha 6 beta 4 during focal cerebral ischemia/reperfusion. Stroke. 1997;28:858–865. doi: 10.1161/01.str.28.4.858. [DOI] [PubMed] [Google Scholar]
- Wolburg-Buchholz K, Mack AF, Steiner E, Pfeiffer F, Engelhardt B, Wolburg H. Loss of astrocyte polarity marks blood–brain barrier impairment during experimental autoimmune encephalomyelitis. Acta Neuropathologica. 2009;118:219–233. doi: 10.1007/s00401-009-0558-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.