Abstract
We used eye-tracking to examine 4.5- to 12.5-month-old infants’ (N = 92) eye-movements during 3-s presentations of upright and inverted faces. Scanning of inverted faces was statistically indistinguishable at 4.5, 6.5, 8, and 12.5 months of age; at each of these ages infants disproportionately scanned the region containing the eyes. Scanning of upright faces changed over this age range. When viewing upright faces, 4.5-month-old and 6.5-month-old infants focused disproportionately on the region containing the eyes, whereas 12.5-month-old and 8-month-old infants distributed looking more broadly, scanning more of the internal area of the faces. These results are consistent with other observed developmental differences in face processing, and provide insight into how moment-to-moment face processing changes during infancy.
Keywords: Face perception, eye-tracking, scanning
From an early age, infants are precocious face processors and show systematic preferences for particular facial characteristics (e.g. attractiveness, race) (Kelly et al., 2007; Langlois, Ritter, Roggman, & Vaughn, 1991). During the first year infants become attentive to feature combinations (Cashon & Cohen, 2004; Schwarzer, Zauner, & Jovanovic, 2007), develop selective sensitivity to the differences between human faces as compared to non-human faces (Pascalis, de Haan, & Nelson, 2002), and show the inversion effect, in which upright and inverted faces are processed differently (Bhatt, Bertin, Hayden, & Reed, 2005; Cashon & Cohen, 2004; Gallay, Baudouin, Durand, Lemoine, & Lécuyer, 2006; Rose, Jankowski, & Feldman, 2008; Turati, Sangrigoli, Ruel, & de Schonen, 2004).
These conclusions were drawn primarily from evaluations of global looking measures over tens of seconds. Clearly, such procedures have uncovered pivotal developmental transitions in infants’ face processing, but they have revealed less about how infants process faces. For example, although infants show more robust memory for upright faces than for inverted faces (Fagan, 1972) and prefer female over male faces (Quinn, Yahr, Kuhn, Slater, & Pascalis, 2002), we know relatively little about the mechanisms that underlie these differences. One possibility is that infants engage distinct visual investigation strategies for different types of faces. Such differences in visual investigation have been related to differences in learning (Amso, Fitzgerald, Davidow, Gilhooly, & Tottenham, 2010).
We examined changes in infants’ visual investigation of faces using eye-tracking procedures, which afford finer temporal and spatial resolution than global looking times and may reflect active, online processing (Karatekin, 2007). Other studies using eye-tracking procedures have revealed that whereas 1-month-old infants fixated external regions of faces, 2-month-old infants showed increased fixation to internal regions (Hainline, 1978; Haith, Bergman, & Moore, 1977; Maurer & Salapatek, 1976). More recently, eye-tracking work has revealed that although older infants continue to prefer the internal regions of faces this preference is stronger for upright than for inverted faces (Gallay, et al., 2006) and for own-race versus other race faces (Liu et al., 2011).
A focus on the internal regions of faces is potentially important face processing, as internal features are more important than external ones in identifying faces (Ellis, Shepherd, & Davies, 1979; Ge et al., 2008; Osborne & Stevenage, 2008). However, not all internal regions are equivalent. Adults fixate eyes more than other features (Heisz & Shore, 2008), and eyes and mouths are represented in social regions of the brains of both humans and non-human primates (Allison, Puce, & McCarthy, 2000). A few studies have shown infants’ scanning of the internal regions changes over age (Hunnius & Geuze, 2004; Wheeler et al., 2011). Here, in addition to evaluating infants’ scanning of internal and external regions of faces, we also examined their scanning of different internal regions (e.g., the regions containing the eyes, nose, and mouth).
We assessed 4.5- to 12.5-month-old infants’ eye-movements as they visually inspected a relatively large number of different faces, presented one at a time during brief (3-s) exposures. Our study differed from previous work in several ways. First, we used static images rather than dynamic stimuli because much of the work uncovering developmental change in face processing has used static images. Second, we used brief presentations with many different faces. This contrasts with other eye-tracking studies in which one face was presented on multiple trials of up to 15 s (Gallay, et al., 2006), or in which one or two faces were presented on single trials of tens of seconds or more (Liu, et al., 2011; Young, Merin, Rogers, & Ozonoff, 2009).
Third, whereas previous eye-tracking studies have primarily examined infants’ inspection of upright faces (but see Gallay, et al., 2006), here we examined infants’ visual investigation of upright and inverted faces. Inverted faces are commonly used as a control for comparison to upright faces because orientation does not change physical properties such as luminance or areas of light-dark contrast. Therefore, looking behaviors driven by such low-level factors will not be influenced by orientation (see Quinn, et al., 2002, for an example). But, children and adults perceive, recognize, and remember upright faces more skillfully than inverted faces (Carey & Diamond, 1977; Diamond & Carey, 1986; Mondloch & Maurer, 2008), perhaps due to upright, but not inverted faces engaging specialized face processing (e.g., Farah, Wilson, Maxwell Drain, & Tanaka, 1995; Freire, Lee, & Symons, 2000), or due to differences in experience with upright and inverted faces (e.g., Valentine, 1988). Regardless of why upright and inverted faces are processed differently, comparison of infants’ scanning of upright and inverted faces will determine whether observed development reflects face processing general to all faces (regardless of orientation), or whether visual inspection of faces differs for upright and inverted faces. In general, therefore, this investigation will complement and extend previous studies.
We chose 4.5 months as our youngest age because although face processing continues to develop well into childhood (Carey & Diamond, 1977), there is significant maturation of infants’ face processing by 4 months due both to changes in the visual system and in cortical regions responsible for processing and representing face information (M. H. Johnson, 2005), probably as a result of experience with faces (Acerra, Burnod, & de Schonen, 2002). It is therefore possible that scanning patterns may be stable by this age, and we will observe no change in eye-movements from 4 to 12 months. Alternatively, significant changes in infants’ face processing occur during the first year, presumably due to developmental processes such as “perceptual narrowing” (Nelson, 2001; Quinn, et al., 2002; Ramsey, Langlois, & Marti, 2005) or a shift from processing faces in terms of individual features to configural or holistic properties of faces (Schwarzer, et al., 2007). If these changes are reflected in patterns of visual investigation then we should see variation across age in how infants scan faces.
Method
Participants
The final sample included healthy, typically developing, and full-term infants; 24 at 4.5 months (129.38 days, SD = 8.71, 8 girls), 27 at 6.5 months (197.78 days, SD = 9.08, 12 girls), 21 at 8 months (239.90 days, SD = 13.56, 9 girls), 20 at 12.5 months (378.65 days, SD = 8.08, 5 girls). We tested 66 infants at UC Davis and 26 at Grinnell College. Seventy-two infants were Caucasian; the remaining infants were Asian, African American, mixed race, other, or race was unreported. Regardless of race, 24 infants were Hispanic. Ninety-one mothers had completed high school, and 56 mothers had completed at least a bachelor’s degree. Infants from the two institutions did not differ in SES.
We obtained infant names from state vital records or a professional list broker at UC Davis and from local newspaper announcements at Grinnell College. Infants were given a small toy or t-shirt for participating. All infants participated only once.
An additional 30 infants were tested but excluded because of an inability to calibrate (n = 4), failure to complete 16 trials (n = 21), experimenter or equipment error (n = 2), or fussiness or general inattention (i.e., refused to look at the monitor) (n = 3).
Apparatus
At both institutions, we recorded infant eye-movements using an Applied Science Laboratory (ASL) R6 Pan/Tilt remote eye tracker, fitted with a magnetic head tracker (Ascension Flock of Birds) that helped maintain focus on the infant’s eye by communicating to the eye-tracker the location and orientation of a sensor attached to the infants’ head (via an infant-sized headband). The eye-tracking camera sat below and in front of a 37” Westinghouse LCD monitor (16:9 aspect ratio) on which the stimuli were presented. A wide-angle camera was used to monitor the infants’ behavior. A Dell computer controlled the eye-camera, ran the eye-tracking software, and received input from the head tracker. A second computer presented the stimuli and sent event codes to the eye-tracking computer.
Stimuli
We used a set of 48 images from the MacBrain Face Stimulus Set (Tottenham et al., 2009) of individuals approximately in their mid-20s, of varied races (30 faces were Caucasian, 18 faces were African American or Asian), both genders (24 faces were male), depicting a (mouth closed) happy or a sad expression. The photographs were similar in luminance and contrast, clothing was masked, and none of the models wore jewelry or ornaments in the hair. Hair colors and styles were representative of the sample of faces (i.e., most women had longer hair than the men).
To attract infants’ attention to the center of the monitor between trials, we used a series of colored shapes (e.g., cross, diamond) that loomed for 800 ms, at the center of the monitor to approximately 16 by 16 visual angle, accompanied by a sound such as “boing” or “clank.”
Procedure
Infants sat on a parent’s lap, approximately 110 cm from the monitor and 60 cm from the eye-camera. An eye-tracking experimenter, seated out of sight behind a curtain, manually focused the eye-camera on the infant’s right eye and initiated the eye-tracker’s automatic procedures. Then, a stimulus-presentation experimenter, also out of sight, initiated the calibration procedure using a program created in Adobe Director to present colorful, contracting and expanding circles first at 11.5 above and to the left of fixation and then at 11.5 below and to the right of fixation. When the infant fixated each circle, the eye-tracking experimenter pressed a computer key; point-of-gaze (POG) at these known locations was used to determine where infants were looking at each subsequent sample. The calibration procedure typically took only a few minutes, and could be reinitiated at any point during the session. None of the infants included here required recalibration.
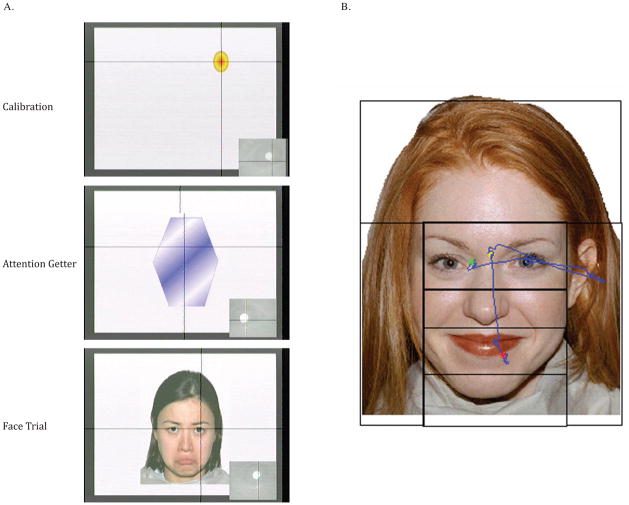
Following calibration, the stimulus-presentation experimenter initiated the attention-getter. When the infant fixated this stimulus, the stimulus-presentation experimenter initiated a 3000 ms trial with a 9.8 (h) by 8.3 (w) face presented in the center of the screen (see Figure 1A). Each trial was accompanied by a randomly chosen orchestral selection by Bach, Beethoven, Mozart, Pachelbel, Ravel, or Vivaldi (Although not often explicitly stated, in eye-tracking studies music often accompanies the presentation of visual stimuli to maintain general interest in the task). If at any time during the session the infant looked away from the monitor, the stimulus-presentation experimenter could attract his or her attention by presenting animated singing animals, short clips (2 s) from children’s television shows (e.g., teletubbies, sesame street), colored circles that loomed at 9 different locations, a set of unpredictably moving colored shapes, or babies’ faces accompanied by children’s music.
Figure 1.
Each infant was shown the faces in a different random order until he or she lost interest, or until 96 trials had been presented (each face was presented no more than twice per infant). Infants were randomly assigned to the upright face condition (10 4.5-month-old, 14 6.5-month-old, 11 8-month-old and 11 12.5-month-old infants) or the inverted face condition.
Data processing
Eye-positions were sampled on-line at 60 Hz. To reduce noise in the data, we filtered our data by computing a running average of 4 samples. We recorded X and Y coordinates of the eye position for each averaged sample, event codes indicating the start and end of each trial, and the particular face presented. We used a blink filter of 12 samples (200 ms)—if the pupil was lost for less than 12 samples, the missing samples were interpolated.
We imported eye-movement data into ILAB, a Matlab toolbox for analyzing eye-movement data (Gitelman, 2002). We evaluated the number of recorded samples that fell into several Areas of Interest (AOIs). The face was divided into an Internal AOI, containing the eyes, nose, and mouth (5.0 by 4.5 , or about 28% of the area of the face), and an External AOI (the entire region, 9.8 by 8.3 , minus this internal region, or about 72% of the area of the face). The internal region was further divided into an Upper region, which included the eyes (2.2 h by 4.6 w; 44% of the internal region), a Middle region, which included the nose (1.5 h by 4.6 w; 30% of the internal region), and a Lower region, which included the mouth (1.3 by 4.6 w; 26% of the internal region) (see Figure 1B). We created three identical sets of AOIs by shifting the internal regions up or down relative to the center. As a result, we could fit each face to a set of internal AOIs that captured the key features of interest (e.g., for each face, the upper AOI included the eyes). We did this in a way that maintained the relative sizes of the internal and external regions, although the internal AOI varied in position with respect to the center of the screen (e.g., when the internal regions were lower, there external region around the chin was smaller and around the hairline was larger).
Results
Our analyses included all trials in which we recorded at least 200 ms of looking, and all infants who had at least 16 trials that met this criterion. The analyses of the internal regions included all trials in which 200 ms was recorded to that region. This conservative criterion ensures that we included all trials in which infants made at least one clear fixation to one of the regions (although it also means that we included some trials that did not reflect infants’ systematic scanning of faces). We included infants’ only if they contributed data on 16 trials; infants who completed fewer trials were extremely variable and their responses were based on looking at very few faces. Thus, this criterion was important because our goal was to establish patterns of looking across a broad range of faces. There were no differences across age or conditions for the number of trials contributed to the analyses of the overall looking (M = 45.78 trials, SD = 18.72, range 16 to 94) or looking to the internal regions (M = 44.35 trials, SD = 18.47, range 16 to 94).
We calculated for each infant the median fixation duration to each AOI on each trial. Means are more influenced by outlier or extreme values than are medians, thus calculating median scores provided a better estimate of infants’ looking in this procedure. This is particularly important for infants who contributed relatively fewer trials. Although the individual infants’ scores were their median responding, the analyses conducted compared the means of those medians—for example, asking whether the mean of infants’ median fixation to upright and inverted faces differed.
Our first analyses compared infants’ scanning of the internal and external regions. An Analysis of Variance (ANOVA) on infants’ looking times to the internal and external regions of faces with region (internal, external) as the within-subject variable and infant age and face orientation as between-subjects variables revealed a robust and significant effect of region, F(1, 84) = 276.03, p < .001, ηp2 = .77, but no effect of age or orientation. Infants looked longer at the internal region, M = 1976.46 ms, SD = 592.13, than the external region, M = 429.93 ms, SD = 357.47 (Separate analyses conducted at each age and for each orientation confirmed this pattern held regardless of age or face orientation).
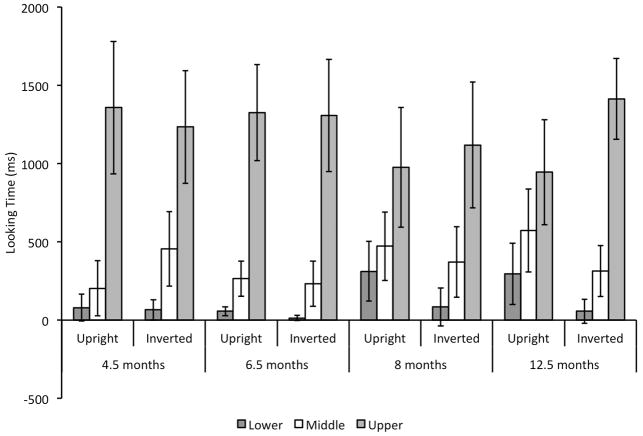
Next, we compared infants’ looking to the upper, middle, and lower internal regions. As can be seen in Figure 2, infants generally focused on upper part of the face—that part that contained the eyes. In addition, although this preference did not vary with face orientation for 4.5- and 6.5-month-old infants, it did vary with orientation for 8.5- and 12.5-month-old infants. An ANOVA conducted on duration of looking to the upper, middle, and lower AOIs with region as the within-subjects factors, and orientation and infant age as between-subject factors revealed a significant main effect of region, F(2, 168) = 165.73, p < .001, ηp2 = .66. In general infants looked most at the “upper” region, M = 1211.02 ms, SD = 556.78, and less at the middle, M = 360.68 ms, SD = 319.45, and lower, M = 117.01 ms, SD = 194.26, regions.
Figure 2.
The predicted region by age group by orientation interaction was not significant, F(6, 168) = 1.85, p = .09, ηp2 = .06, which is not surprising given the 4 levels of age. To test our predictions that face orientation would differentially influence older and younger infants’ scanning, we conducted separate ANOVAs on the upright and inverted conditions, with age as the between-subject factor and region as the within-subject factor. Each analysis revealed significant main effects of region (upright condition: F(2, 86) = 66.33, p < .001, ηp2 = .61, inverted condition: F(2, 82) = 101.41, p < .001, ηp2 = .71), but the feature by age interaction was significant only for the upright condition, F(2, 86) = 3.03, p = .01, ηp2 = .17 (inverted condition: F(6, 82) = .66, p = .70, ηp2 = .04). Thus, infants at the different ages inspected upright faces differently, but across age infants distributed their looking similarly to inverted faces.
We also conducted separate ANOVAs for each age group, with condition (upright versus inverted) as the between-subjects factor and region as the within-subjects factor. Each analysis revealed a main effect of region: F(2, 44) = 43.78, p < .001, ηp2= .67 at 4.5 months, F(2, 50) = 98.66, p < .001, ηp2= .80 at 6.5 months, F(2, 38) = 18.27, p < .001, ηp2= .49 at 8 months, and F(2, 36) = 37.18, p < .001, ηp2= .67 at 12.5 months. Only at 12.5 months did the ANOVA reveal a significant region by condition interaction F(2, 36) = 5.92, p = .006, ηp2= .25, all other F’s < 1.23. Figure 2 shows that at 12.5 months, infants looked more at the mouths of upright faces than inverted faces, and less at the eyes of upright faces than inverted faces.
Finally, we determined how infants’ looking to the three internal regions was related to the proportion that each comprised of the internal region (see Table 1). We calculated the proportion of time infants looked at each region by dividing duration of looking to that region by looking to the total internal area. For each group of infants, we conducted 3 t-tests (one for each region); to adjust for multiple comparisons, we used p = .025 as our criterion for significance.
Table 1.
Proportion looking times to internal AOIs by age and face orientation. (Standard deviations are in parentheses).
| Age | Condition | Lower | Middle | Upper |
|---|---|---|---|---|
| Proportion of Area | .30 | .26 | .44 | |
| 4.5 months | Upright | .11 (.11) *** | .17 (.09) ** | .72 (.19) *** |
| Inverted | .11 (.11) *** | .26 (.14) | .63 (.21) ** | |
| 6.5 months | Upright | .10 (.06) *** | .22 (.09) | .68 (.13) *** |
| Inverted | .05 (05) *** | .20 (.13) | .75 (.16)*** | |
| 8 months | Upright | .22 (.15) | .26 (.12) | .52 (.22) |
| Inverted | .10 (.09) *** | .24 (.12) | .66 (.20) ** | |
| 12.5 months | Upright | .19 (.13) * | .30 (.13) | .52 (.25) |
| Inverted | .06 (.05) *** | .20 (.09) | .74 (.12) *** |
Note. When compared to the relative area of the region,
p ≤ .025,
p ≤ .01,
p ≤ .001
The same pattern of looking was observed at 4.5 and 6.5 months regardless of the orientation of the face. They looked more at the upper region than expected given the relative size of that region in upright faces [4.5 months: t(9) = 4.55, p = .001, d = 1.44; 6.5 months: t(14) = 7.33, p < .001, d = 1.89] and inverted faces [4.5 months: t(13) = 3.23, p = .007, d = .86; 6.5 months: t(11) = 6.64, p < .001, d = 1.92] (recall the “upper” region contains the eyes in inverted faces). They looked less at the lower region than expected given its relative size for upright faces [4.5 months: t(9) = −5.13, p =.001, d = 1.62; 6.5 months: t(14) = −13.77, p < .001, d = 3.56] and inverted faces [4.5 months: t(13) = −6.76, p <.001, d = 1.81; 6.5 months: t(11) = −18.64, p < .001, d = 5.38]. The proportion of looking to the middle region did not differ from chance, with the exception of four-month-old infants’ looking at upright faces, [t(9) = −3.14, p = .01, d = .99; 6.5 months, upright condition: t(14) = −1.69, p = .11, d = .44; 4.5 months, inverted condition: t(13) = .16, p = .44, d = .04; 6.5 months inverted condition: t(11) = −1.51, p = .16, d = .44]. An ANOVA comparing data from 4.5- and 6.5-month-old infants yielded no significant effects or interactions with age. In general, therefore, 4.5- and 6.5-month-old infants looked disproportionately at the upper internal region regardless of face orientation.
In contrast to these younger infants, the older 8- and 12.5-month-old infants had different patterns of looking to the upright and inverted faces. When looking at upright faces, the proportion of looking to the upper, middle, and lower regions did not differ from that expected given relative size of that region, with the exception of 12.5-month-old infants’ looking at the lower region, t(10) = −2.79, p = .02, d = .84 [looking to the upper region at 8 months, t(10) = 1.11, p = .30, d = .33, and 12.5 months, t(10) = .99, p = .35, d = .30; looking to the middle region at 8 months, t(10) = .21, p = .84, d = .06, and 12.5 months t(10) = 0.99, p = .35, d = .30, and looking to the lower region at 8 months, t(10) = −1.72, p = .12, d = .52]. However, when looking at inverted faces, the proportion of looking to the upper region was significantly greater than expected given its size, [8 months: t(9) = 3.40, p = .008, d = 1.0; 12.5 months: t(8) = 7.07, p < .001, d = 2.36 ], and the proportion of looking to the lower region was significantly less than expected given its size [8 months: t(9) = −7.20, p < .001, d = 2.28; 12.5 months t(8) = −14.16, p < .001, d = 4.72]. The proportion of looking at the middle region did not differ from that expected by the relative size of that region [8 months: t(9) = −.43, p = .68, d = .14; 12.5 months t(8) = −1.73, p =. 12, d = .58]. An ANOVA comparing 8- and 12.5-month-old infants yielded no significant effects or interactions with age. Thus, 8- and 12.5-month-old infants distributed their looking disproportionately to the upper region (that contained the eyes) when looking at inverted faces, but distributed their looking across the internal region when looking at upright faces.
Discussion
During brief 3-s exposures to upright or inverted faces, infants focused on internal features more than external features, and more on the eye region than on other internal features. Consistent with other reported findings (Hunnius & Geuze, 2004; Wheeler, et al., 2011), over development infants increasingly focus on the non-eye internal regions when visually investigating upright faces. Importantly, in these other studies infants viewed a small number of dynamic faces presented over relatively long periods of time; we observed this pattern when infants examined a large number of faces that were presented for brief periods of time. Thus, the pattern is consistent across contexts, and infants’ visual inspection of upright faces develops at a time when other aspects of their face processing undergo change.
We also examined infants’ scanning of inverted faces, allowing us to determine whether the differences in scanning were general to all faces or specific to infants’ scanning of upright faces. At all ages tested, infants focused on the upper internal region of inverted faces—where the eyes are located. At the youngest ages, infants’ scanning of upright and inverted faces were statistically indistinguishable. Older infants, in contrast, scanned upright faces more broadly than they did inverted faces, and, at least by 12.5 months, infants’ scanning of upright and inverted faces were statistically different. This change in visual inspection strategy we uncovered was specific to upright faces, and corresponds periods in development of other changes in the effect of inversion on infants’ face processing (Cashon & Cohen, 2004; Schwarzer, et al., 2007).
Of course, we demonstrated that infants’ scanning changed, but not why it changed. The fact that the effect was specific to infants’ scanning of upright faces is consistent with the possibility that 6 to 8 months represents a period of time when configural or holistic face processing emerges (Cashon & Cohen, 2004; Schwarzer, et al., 2007), and the changes in scanning are the result of a shift from piecemeal to configural processing of upright faces (Carey & Diamond, 1977; Diamond & Carey, 1986; Mondloch & Maurer, 2008). This difference also may reflect increasing experience with upright faces (e.g., Valentine, 1988), just as increased experience with own-race faces seems to be related to similar changes in scanning (Wheeler, et al., 2011).
In general, the literature does not seem to be consistent with inversion effects reflecting a sudden emergence of specialized processes for representing faces holistically. Whereas we, like others, found an inversion between 6 and 8 months of age, others have found such an effect in younger infants (Gallay, et al., 2006; Rose, et al., 2008; Turati, et al., 2004). A number of factors may contribute to differences in when inversion is observed to influence face processing. When the procedure involves presenting a single face over several trials and over tens of seconds, the inversion effect is observed early in infancy. When presented with multiple faces and/or with very short presentations, the inversion effect emerges later. One possibility is that inversion effects in young infants emerge over time, and we would have observed an effect of inversion if we had presented the faces for longer durations. Another possibility is that young infants may process upright and inverted faces differently despite scanning them in the same way, scanning differences may not be directly related to differences in processing. The point is that our results do not negate previous findings of an “inversion effect” in young infants; we simply show that in 3-s trials infants’ scanning of upright faces, but not inverted faces, develops over the first year of life. Future research can address whether these differences contribute to or reflect the other documented developmental differences in face processing, or whether the effects of orientation vary with the experimental procedures used.
Documenting such changes in infants’ visual exploration of faces is important because visual exploration styles predict learning. For example, Amso and her colleagues (Amso, et al., 2010) found that the way infants scanned a face during habituation was related to their processing of the emotion of that face. Johnson, Slemmer, and Amso (2004) found that differences in how infants scanned rod-and-box displays differentiated infants who perceived the rod as whole versus broken. The developmental changes we observed here in how infants scanned faces, therefore, lead to the prediction that whereas younger infants should learn the same thing about upright and inverted faces, older infants should learn different things about faces in different orientations. This prediction has yet to be directly tested, but our results are consistent with other studies evidence of the emergence of face inversion effects on learning (Bhatt, et al., 2005; Cashon & Cohen, 2004).
Finally, it must be pointed out that our conclusions are based on infants’ looking at a set of racially diverse faces, some of which are from the infant’s own race and some of which are from other races. Other studies have shown differences between own- and other-races for infants’ recognition and discrimination of faces (Kelly et al., 2009) and their scanning of faces (Wheeler, et al., 2011). We do not have sufficient power to detect such effects in our sample, but future studies may reveal that the developmental effects we observed are influenced by the match between the infants’ race and that of the faces under investigation.
In summary, infants’ visually inspection of upright, but not inverted, faces during brief presentations develops over the first year. This developmental shift occurred at a time of other documented developmental changes in infants’ face processing. Thus, infants’ initial sampling of face images appears to contribute to and is influenced by other aspects of face processing.
Acknowledgments
This research and preparation of this manuscript were made possible by NIH grant HD56018 awarded to LMO and a grant to Grinnell College from the Andrew W. Mellon Foundation awarded to AEE. We thank Lisa Christoffer, Karinna Hurley, and Stacy McCarthy, and the undergraduate students in the Infant Cognition Laboratory at the University of California, Davis, and Meghan McDoniel and Hannah Hagen-Atwell at Grinnell College for their help with this project.
References
- Acerra F, Burnod Y, de Schonen S. Modelling aspects of face processing in early infancy. Developmental Science. 2002;5:98–117. [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: Role of the STS region. Trends in Cognitive Sciences. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Amso D, Fitzgerald M, Davidow J, Gilhooly T, Tottenham N. Visual Exploration Strategies and the Development of Infants' Facial Emotion Discrimination. Frontiers in Psychology. 2010;1:180. doi: 10.3389/fpsyg.2010.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt RS, Bertin E, Hayden A, Reed A. Face processing in infancy: Developmental changes in the use of different kinds of relational information. Child Development. 2005;76:169–181. doi: 10.1111/j.1467-8624.2005.00837.x. [DOI] [PubMed] [Google Scholar]
- Carey S, Diamond R. From piecemeal to configurational representation of faces. Science. 1977;195:312–314. doi: 10.1126/science.831281. [DOI] [PubMed] [Google Scholar]
- Cashon CH, Cohen LB. Beyond U-shaped development in infants' processing of faces: An information-processing account. Journal of Cognition and Development. 2004;5:59–80. [Google Scholar]
- Diamond R, Carey S. Why faces are and are not special: An effect of expertise. Journal of Experimental Psychology: General. 1986;115:107–117. doi: 10.1037//0096-3445.115.2.107. [DOI] [PubMed] [Google Scholar]
- Ellis HD, Shepherd JW, Davies GM. Identification of familiar and unfamiliar faces from internal and external features: Some implications for theories of face recognition. Perception. 1979;8:431–439. doi: 10.1068/p080431. [DOI] [PubMed] [Google Scholar]
- Fagan JF. Infants' recognition memory for faces. Journal of Experimental Child Psychology. 1972;14:453–476. doi: 10.1016/0022-0965(72)90065-3. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Wilson KD, Maxwell Drain H, Tanaka JR. The inverted face inversion effect in prosopagnosia: Evidence for mandatory, face-specific perceptual mechanisms. Vision Research. 1995;35:2089–2093. doi: 10.1016/0042-6989(94)00273-o. [DOI] [PubMed] [Google Scholar]
- Freire A, Lee K, Symons LA. The face-inversion effect as a deficit in the encoding of configural information: Direct evidence. Perception. 2000;29:159–170. doi: 10.1068/p3012. [DOI] [PubMed] [Google Scholar]
- Gallay M, Baudouin JY, Durand K, Lemoine C, Lécuyer R. Qualitative Differences in the Exploration of Upright and Upside-Down Faces in Four-Month-Old Infants: An Eye-Movement Study. Child Development. 2006;77:984–996. doi: 10.1111/j.1467-8624.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- Ge L, Anzures G, Wang Z, Kelly DJ, Pascalis O, Quinn PC, Lee K. An inner face advantage in children's recognition of familiar peers. Journal of Experimental Child Psychology. 2008;101:124–136. doi: 10.1016/j.jecp.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR. ILAB: A program for postexperimental eye movement analysis. Behavior Research Methods, Instruments & Computers Special Issue: Eye movement research methods. 2002;34:605–612. doi: 10.3758/bf03195488. [DOI] [PubMed] [Google Scholar]
- Hainline L. Developmental changes in visual scanning of face and nonface patterns by infants. Journal of Experimental Child Psychology. 1978;25:90–115. doi: 10.1016/0022-0965(78)90041-3. [DOI] [PubMed] [Google Scholar]
- Haith MM, Bergman T, Moore MJ. Eye contact and face scanning in early infancy. Science. 1977;198:853–855. doi: 10.1126/science.918670. [DOI] [PubMed] [Google Scholar]
- Heisz JJ, Shore DI. More efficient scanning for familiar faces. Journal of Vision. 2008;8:1–10. doi: 10.1167/8.1.9. [DOI] [PubMed] [Google Scholar]
- Hunnius S, Geuze RH. Developmental Changes in Visual Scanning of dynamic faces and abstract stimuli in infants: A longitudinal study. Infancy. 2004;6:231–255. doi: 10.1207/s15327078in0602_5. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Subcortical face processing. Nature Reviews Neuroscience. 2005;6:766–786. doi: 10.1038/nrn1766. [DOI] [PubMed] [Google Scholar]
- Johnson SP, Slemmer JA, Amso D. Where infants look determines how they see: Eye movements and object perception performance in 3-month-olds. Infancy. 2004;6:185–201. doi: 10.1207/s15327078in0602_3. [DOI] [PubMed] [Google Scholar]
- Karatekin C. Eye tracking studies of normative and atypical development. Developmental Review. 2007;27:283–348. [Google Scholar]
- Kelly DJ, Liu S, Lee K, Quinn PC, Pascalis O, Slater AM, Ge L. Development of the other-race effect during infancy: Evidence toward universality? Journal of Experimental Child Psychology. 2009;104:105–114. doi: 10.1016/j.jecp.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DJ, Quinn PC, Slater AM, Lee K, Ge L, Pascalis O. The other-race effect develops during infancy: Evidence of perceptual narrowing. Psychological Science. 2007;18:1084–1089. doi: 10.1111/j.1467-9280.2007.02029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois JH, Ritter JM, Roggman LA, Vaughn LS. Facial diversity and infant preferences for attractive faces. Developmental Psychology. 1991;27:79–84. doi: 10.1037//0012-1649.35.3.848. [DOI] [PubMed] [Google Scholar]
- Liu S, Quinn PC, Wheeler A, Xiao N, Ge L, Lee K. Similarity and difference in the processing of same- and other-race faces as revealed by eye tracking in 4- to 9-month-olds. Journal of Experimental Child Psychology. 2011;108:180–189. doi: 10.1016/j.jecp.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer D, Salapatek P. Developmental changes in the scanning of faces by young infants. Child Development. 1976;47:523–527. [PubMed] [Google Scholar]
- Mondloch CJ, Maurer D. The effect of face orientation on holistic processing. Perception. 2008;37:1175–1186. doi: 10.1068/p6048. [DOI] [PubMed] [Google Scholar]
- Nelson CA. The development and neural bases of face recognition. Infant and Child Development Special Issue: Face Processing in Infancy and Early Childhood. 2001;10:3–18. [Google Scholar]
- Osborne CD, Stevenage SV. Internal feature saliency as a marker of familiarity and configural processing. Visual Cognition. 2008;16:23–43. [Google Scholar]
- Pascalis O, de Haan M, Nelson CA. Is face processing species-specific during the first year of life? Science. 2002;296:1321–1323. doi: 10.1126/science.1070223. [DOI] [PubMed] [Google Scholar]
- Quinn PC, Yahr J, Kuhn A, Slater AM, Pascalis O. Representation of the gender of human faces by infants: A preference for female. Perception. 2002;31:1109–1121. doi: 10.1068/p3331. [DOI] [PubMed] [Google Scholar]
- Ramsey JL, Langlois JH, Marti NC. Infant categorization of faces: Ladies first. Developmental Review. 2005;25:212–246. [Google Scholar]
- Rose SA, Jankowski JJ, Feldman JF. The inversion effect in infancy: The role of internal and external features. Infant Behavior & Development. 2008;31:470–480. doi: 10.1016/j.infbeh.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer G, Zauner N, Jovanovic B. Evidence of a shift from featural to configural face processing in infancy. Developmental Science. 2007;10:452–463. doi: 10.1111/j.1467-7687.2007.00599.x. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turati C, Sangrigoli S, Ruel J, de Schonen S. Evidence of the face inversion effect in 4-month-old infants. Infancy. 2004;6:275–297. doi: 10.1207/s15327078in0602_8. [DOI] [PubMed] [Google Scholar]
- Valentine T. Upside-down faces: A review of the effect of inversion upon face recognition. British Journal of Psychology. 1988;79:471–491. doi: 10.1111/j.2044-8295.1988.tb02747.x. [DOI] [PubMed] [Google Scholar]
- Wheeler A, Anzures G, Quinn PC, Pascalis O, Omrin DS, Lee K. Caucasian infants scan own- and other-race faces differently. PLoS ONE. 2011;6(4):e18621. doi: 10.1371/journal.pone.0018621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GS, Merin N, Rogers SJ, Ozonoff S. Gaze behavior and affect at 6 months: Predicting clinical outcomes and language development in typically developing infants and infants at risk for autism. Developmental Science. 2009;12:798–814. doi: 10.1111/j.1467-7687.2009.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]